Abstract
Otosclerosis due to abnormal bone homeostasis of the otic capsule is a frequent cause of hearing loss in adults. Usually, the hearing loss is conductive, resulting from fixation of the stapedial footplate, which prevents normal ossicular vibration in response to sound. An additional type of sensorineural hearing loss may be caused by otosclerotic damage to the cochlea. The etiology of the disease is unknown, and both environmental and genetic factors have been implicated. Autosomal dominant inheritance with reduced penetrance has been proposed, but large families are extremely rare. To elucidate the pathogenesis of the disease, identification of the responsible genes is essential. In this study, we completed linkage analysis in a Belgian family in which otosclerosis segregates as an autosomal dominant disease. After excluding linkage to a known locus on chromosome 15 (OTSC1), we found linkage on chromosome 7q, with a multipoint LOD score of 3.54. Analysis of key recombinant individuals maps this otosclerosis locus (OTSC2) to a 16-cM interval on chromosome 7q34-36 between markers D7S495 and D7S2426.
Hearing impairment is one of the most frequent handicaps in the Western world. It can be conductive (caused by abnormalities in the conduction of sound in the outer or middle ear) or sensorineural (resulting from defects in the perception of sound in the inner ear) or both (mixed). Otosclerosis is a common bone disorder of the otic capsule, unique to the endochondral layer of the temporal bone and known to affect human beings only (Declau and Van de Heyning 1996). Otosclerosis leads to a progressive hearing impairment characterized by conductive or mixed hearing loss. The conductive hearing loss is due to a fixation of the stapedial footplate in the oval window, and cochlear otosclerotic foci are thought to cause a sensorineural component in some cases. Although stapedial microsurgery has proved to be a successful means of reducing the conductive hearing impairment, otosclerosis gives rise to considerable morbidity (Somers et al. 1997).
Otosclerosis is divided into histological and clinical types. The term “histological otosclerosis” is used to describe otosclerosis in the absence of clinical symptoms or manifestations. This type of disease, which is discovered by routine sectioning of temporal bones at autopsy, has a prevalence of 6%–7% (Morrison and Bundey 1970). Clinical otosclerosis (MIM 166800), in contrast, implies the presence of hearing impairment (Shambaugh 1949). Its prevalence is 0.2%–1% among white adults, and the age at onset is usually 20–40 years (Gordon 1989). In ∼85% of patients, both ears are involved (Somers et al. 1994).
The etiology of otosclerosis is complex, and it probably involves an interaction between genes and environmental factors. Epidemiological studies support autosomal dominant inheritance with reduced penetrance (Albrecht 1922; Larsson 1962; Morrison 1967; Causse and Causse 1984; Ben Arab et al. 1993), although a viral involvement also has been suggested (Niedermeyer et al. 1994; Arnold et al. 1996; McKenna et al. 1996). Because the etiology of otosclerosis remains poorly characterized, no effective medical therapy has been developed to prevent or stabilize the disease. Large families of ⩾10 affected persons are difficult to ascertain, and other factors, such as reduced penetrance and a high frequency of phenocopies, complicate efforts at linkage analysis. At present, only one locus for otosclerosis, OTSC1 on chromosome 15q25-26, has been identified, and the responsible gene has not been cloned (Tomek et al. 1998). Otosclerosis is therefore one of the last important types of hearing impairment for which a genetic cause remains to be elucidated.
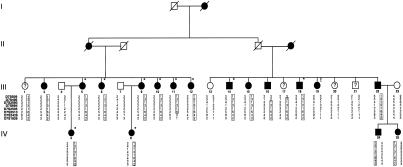
The Belgian family presented here (fig. 1) displays autosomal dominant otosclerosis and was ascertained through the Saint-Augustinus Hospital. We performed pure-tone audiometry for all family members, using air conduction at 250, 500, 1000, 2000, 4000, and 8000 Hz and bone conduction at 500, 1000, 2000, and 4000 Hz. Ten persons with surgically confirmed otosclerosis were considered affected. For the patients who had not had surgery, the clinical diagnosis of otosclerosis was based on audiological data, which we analyzed using an algorithm described elsewhere (Govaerts et al. 1998). In brief, the audiometric curves of these persons were compared with age- and sex-dependent data according to the International Organization for Standardization 7029 standard (1984). Using analysis of variance, we determined the frequency that was the most sensitive indicator of otosclerosis in this family, and we ranked family members according to their hearing loss, expressed as hearing standard deviations (HSD) at this frequency. In addition, we measured tympanic membrane compliance and ipsilateral and contralateral stapedial reflex decay. Seven additional persons, who had a conductive or mixed hearing loss that exceeded three HSDs together with absent or immeasurable stapedial reflexes, were classified as affected. Because the age at onset of otosclerotic hearing loss varies, only family members who were >60 years of age and had normal hearing (<2 HSDs) were considered unaffected. Only one family member (1.7 HSDs at age 70 years) and three relatives by marriage were labeled unaffected. The status of the remaining four persons was considered uncertain. Information on deceased the deceased was obtained by questioning living family members.
Figure 1.
Pedigree of the Belgian family with autosomal dominant otosclerosis, showing the most likely haplotypes for the chromosome 7 markers. The haplotype linked to otosclerosis is boxed. Only family members whose DNA was analyzed are numbered. A question mark (?) inidicates family members with an atypical or limited hearing impairment; an asterisk (*) indicates persons with surgically confirmed otosclerosis; and an exclamation point (!) indicates the phenocopy (person III-19).
After the 25 participants gave informed consent, blood samples were obtained and were used as a source of genomic DNA, which was isolated by standard techniques. All these 25 DNA samples (from family members numbered in fig. 1) were used in subsequent LOD score calculations. LOD scores were calculated using the FASTLINK computer program (Cottingham et al. 1993). The linkage parameters were chosen in compliance with the body of older studies (Larsson 1960; Morrison 1967; Gapany-Gapanaviscius 1975; Donnell and Alfi 1980; Causse and Causse 1984), which suggest that otosclerosis is inherited as an autosomal dominant disease with reduced penetrance. As standard linkage parameters, the frequency of the otosclerosis gene was set at .0001, and the disease was assumed to be autosomal dominant and 90% penetrant. To allow for possible phenocopies, this chance was set at 1%, because without surgical exploration it is often difficult to exclude hearing impairment of other origins. Equal recombination frequencies between males and females were assumed. For each marker, the number of alleles in the LOD score calculations was set at the observed number of alleles in the pedigree (N); allele frequencies were set at 1/N.
First, linkage to the known otosclerosis locus on chromosome 15q25-q26 was investigated using the linked markers D15S652, D15S1004, and D15S657 (Tomek et al. 1998). This locus was excluded with negative two-point LOD scores (table 1). A genome search was then performed on 25 DNA samples, using genetic markers from the Cooperative Human Linkage Center screening set (Weber, version 6). This set contains 391 microsatellite markers covering the complete genome, with an average intermarker spacing of 10 cM and an average heterozygosity of 76%. Because we did not detect linkage with these markers, we screened another 66 markers from a second genomewide microsatellite set for fluorescence-based, semiautomated genome mapping (Reed et al. 1994) and 120 markers from the Généthon genetic linkage map (Dib et al. 1996). Although this second analysis did not generate a two-point LOD score >3, there were five regions with a positive two-point LOD score >1. At this time, 75% of the complete genome had been excluded, with negative two-point LOD scores <−2; two-point LOD scores <−1 were reached for 92% of the genome.
Table 1.
Comparison of Markers for Chromosome 15q25-26 and the Known Otosclerosis Locus (OTSC1)
|
Two-Point LOD Scores at θ = |
|||||||
| Marker | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D15S652 | -3.54 | −3.16 | −2.65 | −2.18 | −1.12 | −.48 | −.14 |
| D15S1004 | −5.08 | −3.83 | −2.35 | −1.56 | −.72 | −.27 | −.06 |
| D15S657 | −4.52 | −3.50 | −1.94 | −1.17 | −.51 | −.20 | −.05 |
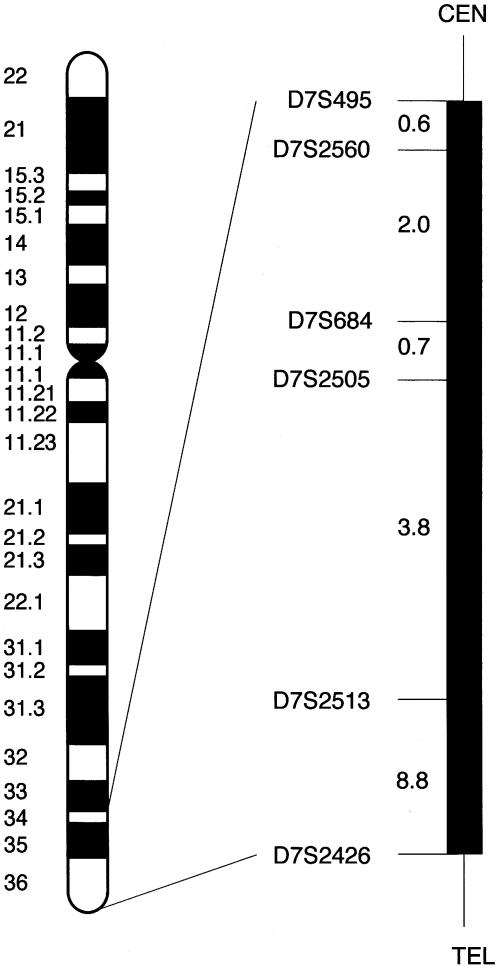
The five candidate regions were examined using additional markers to reconstruct haplotypes. Four of the regions with two-point LOD scores >1 were excluded, but one region was also positive when tested with additional markers. This region on chromosome 7 includes marker D7S684, which gave a two-point LOD score of 1.88 at recombination fraction (θ) .05 (table 2). In the D7S2560–D7S2513 interval, multipoint calculations were performed using five-point rolling LODs. A maximum multipoint LOD score of 3.54 was obtained when standard linkage parameters were used. When the disease gene frequency was varied, the penetrance and the phenocopy rate had little effect on the LOD score results (table 3), showing that the LOD score calculations were robust to changes in linkage parameters. An eight-marker haplotype was constructed (fig. 1). Flanking markers were defined by recombinations in individuals III-11 (D7S2426, telomeric) and III-16 (D7S495, centromeric). A genetic map of the candidate region is presented in figure 2. The key recombinant individuals place the gene for otosclerosis in the 16-cM interval flanked by markers D7S495 and D7S2426.
Table 2.
Comparison of Markers for Otosclerosis and Chromosome 7 (OTSC2)
|
Two-Point LOD Scores at θ = |
|||||||
| Marker | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D7S509 | −.54 | −.06 | .59 | .76 | .64 | .35 | .1 |
| D7S495 | −.26 | .02 | .44 | .57 | .48 | .27 | .09 |
| D7S2560 | 1.21 | 1.18 | 1.07 | .94 | .68 | .44 | .22 |
| D7S684 | 1.52 | 1.72 | 1.88 | 1.76 | 1.29 | .74 | .27 |
| D7S2505 | 1.15 | 1.35 | 1.53 | 1.44 | 1.03 | .56 | .16 |
| D7S2513 | 1.29 | 1.49 | 1.64 | 1.52 | 1.02 | .45 | .05 |
| D7S2426 | .11 | .60 | 1.26 | 1.44 | 1.28 | .83 | .27 |
| D7S1826 | .15 | .52 | 1.07 | 1.25 | 1.14 | .77 | .30 |
Table 3.
Maximum Chromosome 7 Multipoint LOD Scores for Various Linkage Parameters
| Penetrance (%) | Phenocopy Rate (%) | Disease Gene Frequency | Maximum LOD Score |
| 90 | 1 | 1/1,000 | 3.50 |
| 90 | 1 | 1/10,000 | 3.54 |
| 90 | 1 | 1/100,000 | 3.54 |
| 90 | .2 | 1/10,000 | 3.44 |
| 90 | 5 | 1/10,000 | 3.65 |
| 40 | 1 | 1/10,000 | 3.39 |
Figure 2.
Localization of OTSC2 on chromosome 7q34-36. The black bar indicates the region on the Généthon genetic map (Dib et al. 1996) that contains the gene for otosclerosis. The distances between the markers are represented in centimorgans.
Although subject III-19 received the clinical diagnosis of otosclerosis on the basis of audiometric testing, she did not inherit the disease haplotype, and reevaluation of the clinical data revealed no obvious discrepancies. This could reflect an unrecognized double crossover, the likelihood of which is ∼0.8% between D7S2513 and D7S2426, ∼0.3% between D7S2505 and D7S2513, and ∼0.07% between D7S495 and D7S684 (the affected mother of III-19 is not informative at D7S2560). Alternatively, because there are numerous causes of acquired conductive or mixed hearing loss, the finding could reflect a phenocopy. For example, incudo-malleolar fixation and tympanosclerosis can result in identical audiometric profiles, and distinguishing these etiologies from otosclerosis requires surgical exploration of the middle ear.
The OTSC2 interval includes several known genes, one of which (TIF1α [MIM 603406]), is a growth suppressor required for the growth-inhibitory activity of retinoic acid (RA). Studies have shown that all-trans RA disrupts differentiation and development of the otic capsule. This teratogen produces malformations of the epithelium-derived auditory and vestibular receptors of the inner ear and its surrounding cartilaginous capsule (Frenz and Liu 1997). PLOD3 (procollagen-lysine, 2-oxyglutarate, 5-dioxygenase 3 [MIM 603066]) also maps to the candidate region. This gene plays an important role in collagen biosynthesis and metabolism, an intriguing function in view of the pathological characteristics of otosclerosis. In vitro expression studies have shown that PLOD3 hydroxylates lysyl residues in collagen sequences in non-triple-helical conformation (Valtavaara et al. 1998). This activity is enhanced by tumor necrosis factor–α (TNFα), an important mediator in the pathogenesis of arthritis, resulting in the degradation of cartilage and the destruction of joints (Ah-Kim et al. 2000). Therefore, PLOD3 may play a role in affecting cartilage homeostasis of the inner and middle ear by interfering with chondrocytic responses to TNFα-mediated stimuli.
Cloning and completing functional and structural analysis of the OTSC2 gene may provide new insights into the molecular mechanisms of otosclerosis and may reveal targets for prevention and treatment of the disease. However, the realization of these aims will depend, in part, on the degree of heterogeneity among affected persons. In the only two completed linkage studies, two distinct loci have been identified. In both instances, the defined candidate regions are large, and it is possible that the relevant genes will not be identified until additional families are ascertained and studied.
Acknowledgments
The authors are grateful to all family members for their collaboration in this study. We thank Markus Nöthen, Thomas Wienker, and Suzanne Leal for helpful discussions. This study was supported by grants from the University of Antwerp and from the Vlaams Fonds voor Wetenschappelijk Onderzoek (FWO) (to G.V.C.) and by a grant from the American Otological Society (to R.J.H.S.). K.V.D.B. holds a predoctoral research position with the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-Technologisch Onderzoek in de Industrie, and G.V.C. holds a research position with the FWO.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Cooperative Human Linkage Center, CHLC Marker Maps, http://lpg.nci.nih.gov/html-chlc/ChlcMarkerMaps.html (for markers used for genotyping)
- Généthon, http://www.genethon.fr (for markers used for genotyping)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for otosclerosis [MIM 166800], TIF1α [MIM 603406], and PLOD3 [MIM 603066])
References
- Ah-Kim H, Zhang X, Islam S, Sofi JI, Glickberg Y, Malemud CJ, Moskowitz RW, Haqqi TM (2000) Tumour necrosis factor α enhances the expression of hydroxyl lyase, cytoplasmic antiproteinase-2 and a dual specificity kinase TTK in human chondrocyte-like cells. Cytokine 12:142–150 [DOI] [PubMed] [Google Scholar]
- Albrecht W (1922) Über der vererbung der hereditären labyrinth-schwerkörigkeit und der otosclerose. Arch Ohrenheilk Nas Kehlkopfheilk 11:244–251 [Google Scholar]
- Arnold W, Niedermeyer HP, Lehn N, Neubert W, Hofler H (1996) Measles virus in otosclerosis and the specific immune response of the inner ear. Acta Otolaryngol 116:705–709 [DOI] [PubMed] [Google Scholar]
- Ben Arab S, Bonaiti-Pellie C, Belkahia A (1993) A genetic study of otosclerosis in a population living in the north of Tunisia. Ann Genet 36:111–116 [PubMed] [Google Scholar]
- Causse JR, Causse JB (1984) Otospongiosis as a genetic disease: early detection, medical management, and prevention. Am J Otol 5:211–223 [PubMed] [Google Scholar]
- Cottingham RWJ, Idury RM, Schaffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- Declau F, Van de Heyning P (1996) Otosclerosis. In: Martini A, Read A, Stephens D (eds) Genetics and hearing impairment. Whurr, London, pp 221–230 [Google Scholar]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Donnell GN, Alfi OS (1980) Medical genetics for the otolaryngologist. Laryngoscope 90:40–46 [DOI] [PubMed] [Google Scholar]
- Frenz DA, Liu W (1997) Effect of retinoic acid on otic capsule chondrogenesis in high-density culture suggests disruption of epithelial-mesenchymal interactions. Teratology 56:233–240 [DOI] [PubMed] [Google Scholar]
- Gapany-Gapanaviscius B (1975) Otosclerosis: genetics and surgical rehabilitation. Halsted Press, New York [Google Scholar]
- Gordon MA (1989) The genetics of otosclerosis: a review. Am J Otol 10:426–438 [DOI] [PubMed] [Google Scholar]
- Govaerts PJ, De Ceulaer G, Daemers K, Verhoeven K, Van Camp G, Schatteman I, Verstreken M, Willems PJ, Somers T, Offeciers FE (1998) A new autosomal-dominant locus (DFNA12) is responsible for a nonsyndromic, midfrequency, prelingual and nonprogressive sensorineural hearing loss. Am J Otol 19:718–723 [PubMed] [Google Scholar]
- International Organization for Standardization (1984) Acoustics-threshold of hearing by air conduction as a function of age and sex for otologically normal persons [publication 7029]. International Organization for Standardization, Geneva [Google Scholar]
- Larsson A (1960) Otosclerosis: a genetic and clinical study. Acta Otolaryngol (Stockh) (Suppl) 154:1–86 [PubMed] [Google Scholar]
- Larsson A (1962) Genetic problems in otosclerosis. In: Schuknecht HF (ed) Otosclerosis. Little Brown, Boston, pp 109–117 [Google Scholar]
- McKenna MJ, Kristiansen AG, Haines J (1996) Polymerase chain reaction amplification of a measles virus sequence from human temporal bone sections with active otosclerosis. Am J Otol 17:827–830 [PubMed] [Google Scholar]
- Morrison AW (1967) Genetic factors in otosclerosis. Ann R Coll Surg Engl 41:202–237 [PMC free article] [PubMed] [Google Scholar]
- Morrison AW, Bundey SE (1970) The inheritance of otosclerosis. J Laryngol Otol 84:921–932 [DOI] [PubMed] [Google Scholar]
- Niedermeyer H, Arnold W, Neubert WJ, Hofler H (1994) Evidence of measles virus RNA in otosclerotic tissue. ORL J Otorhinolaryngol Relat Spec 56:130–132 [DOI] [PubMed] [Google Scholar]
- Reed PW, Davies JL, Copeman JB, Bennett ST, Palmer SM, Pritchard LE, Gough SCL, Kawaguchi Y, Cordell HJ, Balfour KM, Jenkins SC, Powell EE, Vignal A, Todd JA (1994) Chromosome-specific microsatellite sets for fluorescence-based, semi-automated genome mapping. Nat Genet 7:390–395 [DOI] [PubMed] [Google Scholar]
- Shambaugh GE (1949) Fenestration operation for otosclerosis. Acta Otolaryngol Suppl 79:1–101 [Google Scholar]
- Somers T, Govaerts PJ, Marquet TH, Offeciers E (1994) Statistical analysis of otosclerosis surgery performed by J Marquet. Ann Otol Rhinol Laryngol 103:945–951 [DOI] [PubMed] [Google Scholar]
- Somers T, Govaerts PJ, Janssens de Varebeke S, Offeciers E (1997) Revision stapes surgery. J Laryngol Otol 111:233–239 [DOI] [PubMed] [Google Scholar]
- Tomek MS, Brown MR, Mani SR, Ramesh A, Srisailapathy CRS, Coucke P, Zbar RIS, Bell AM, McGuirt WT, Fukushima K, Willems PJ, Van Camp G, Smith RJH (1998) Localization of a gene for otosclerosis to chromosome 15q25-q26. Hum Mol Genet 7:285–290 [DOI] [PubMed] [Google Scholar]
- Valtavaara M, Szpirer C, Szpirer J, Myllyla R (1998) Primary structure, tissue distribution, and chromosomal localization of a novel isoform of lysyl hydroxylase (lysyl hydroxylase 3). J Biol Chem 273:12881–12886 [DOI] [PubMed] [Google Scholar]