Abstract
Congenital cataract is a clinically and genetically highly heterogeneous eye disorder, with autosomal dominant inheritance being most common. We investigated a large seven-generation family with 74 individuals affected by autosomal dominant congenital cataract (ADCC). The phenotype in this family can be described as “central pouchlike” cataract with sutural opacities, and it differs from the other mapped cataracts. We performed linkage analysis with microsatellite markers in this family and excluded the known candidate genes. A genomewide search revealed linkage to markers on chromosome 15, with a maximum two-point LOD score of 5.98 at θ=0 with marker D15S117. Multipoint analysis also gave a maximum LOD score of 5.98 at D15S117. Multipoint and haplotype analysis narrowed the cataract locus to a 10-cM region between markers D15S209 and D15S1036, closely linked to marker D15S117 in q21-q22 region of chromosome 15. This is the first report of a gene for a clinically new type of ADCC at 15q21-22 locus.
Congenital cataract is a frequent cause of hereditary visual impairment in infants and accounts for 10%–40% of incidence of legal blindness (Eckstein et al. 1996). It is highly heterogeneous clinically and shows considerable inter- and intrafamilial variability (Francois 1982; Scott et al. 1994). In nearly one-third of the cases, congenital cataract has been reported as a familial trait (Vanita et al. 1999). So far, >15 independent loci have been identified for autosomal dominant congenital cataract (ADCC) on different chromosomes, including five genes coding for crystallins (CRYAA [MIM 123580], CRYBA3/A1 [MIM 123610], CRYBB2 [MIM 123620], CRYGC [MIM 123680], and CRYGD [MIM 123690]), two for gap junctional channel proteins (GJA3 [MIM 121015] and GJA8 [MIM 600897]), one for the regulatory factor (PITX3 [MIM 602669]), and one for beaded-filament structural protein-2 (BFSP2 [MIM 603212]) (review by Padma et al. 1995; Francis et al. 1999; Héon et al. 1999; Conley et al. 2000). Genetic heterogeneity of cataract is evident, since more than one gene has been reported to cause the same phenotype (Héon et al. 1999; Gill et al. 2000). On the other hand, an identical mutation can result in different phenotypes (Litt et al. 1997; Vanita 1998; Gill et al. 2000).
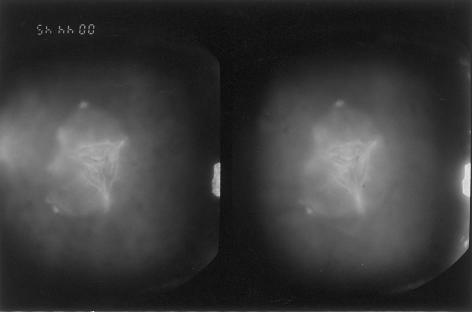
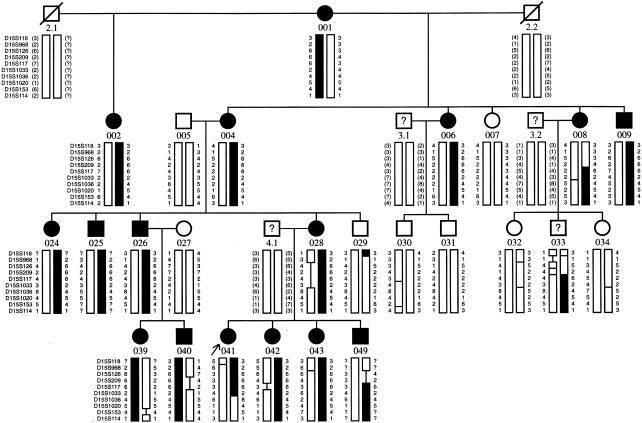
We analyzed, clinically and genetically, a large family (CC-51), of Indian origin, affected by autosomal dominant congenital cataract. The proband, an 8-year-old child, was diagnosed as having phenotypically unique “central pouchlike” cataract, with sutural opacities (fig. 1). A detailed family history was taken, and, on the basis of the information collected, 74 individuals were ascertained as affected (fig. 2). Detailed ophthalmological examination, including slit-lamp examination and photography of lens to record the cataract type, performed on 24 family members, revealed 16 members to be affected with cataract bilaterally; the remaining 8 were unaffected. Cataract was of a progressive nature, and cataractous changes were more prominent in affected older individuals.
Figure 1.
Slit-lamp photograph, under direct focal illumination, of an 8-year-old patient showing central pouchlike cataract with sutural opacities. Viewed in three dimensions, the cataract appears as a six-sided central pouch, on which are seen anterior and posterior sutural opacities, with increased prominence at their ends. One limb of the anterior Y suture ends in a dumbbell-shaped opacity, whereas the other two limbs end in knobs. Of the three limbs of the posterior Y suture, one ends in a small dumbbell-shaped opacity, the second ends in a knob, and the third is not visible. At the junction of three limbs of the anterior Y suture, the Y suture itself has split apart. The lens matter surrounding the central pouch also shows generalized opacifications.
Figure 2.
Detailed pedigree of CC-51 family with disease segregating in seven generations
After informed consent, linkage analysis with microsatellite markers was done on the DNA samples of all 24 ophthalmologically examined individuals and 1 individual (033) with affected but unconfirmed clinical status (fig. 3). Microsatellite markers and their distances were from the Généthon linkage map (markers kindly provided by Gene Mapping Centre, Max-Delbrück Centre). Microsatellites were amplified by “Touch down PCR” (MJ-Research), by means of fluorescently labeled primers following standard methods. PCR products were pooled, were denatured at 95°C for 5 min, and were electrophoresed on 4% denaturing polyacrylamide gels on an automatic DNA sequencer (ABI-Prism 377). Data were collected and analyzed by GENESCAN, version 2.1, and genotyping was done with the use of GENOTYPER 2.0 software. Autosomal dominant inheritance, with a disease-gene frequency of .0001 and full penetrance of the trait, was considered. Recombination values (θ) between males and females were considered to be equal. Two-point and multipoint analysis was performed by means of the LINKAGE program package.
Figure 3.
Analyzed portion of family CC-51 showing haplotypes for markers on chromosome 15. Sequence of markers is from centromere to telomere. Blackened bars indicate the affected haplotype. Inferred haplotypes are in parentheses. Uninformative markers are indicated by a vertical line in the haplotype bar. Recombination in individuals 008, 033, 049, and 041 places the disease locus between marker D15S209 and D15S1036.
Initially, 65 microsatellite markers from candidate gene regions on chromosomes 1, 2, 3, 10, 12, 13, 14, 16, 17, 19, 21, and 22 were analyzed, and no linkage was observed at these loci. Subsequently, in a genomewide search with 360 markers, linkage was identified to markers on chromosome 15 (table 1). Further analysis with more markers showed a maximum LOD score of 5.98 (θ=0) with marker D15S117. Multipoint analysis with chromosome 15 markers also supported linkage with a maximum LOD score of 5.98 at D15S117 (fig. 4).
Table 1.
Two-Point LOD Scores for Linkage between Cataract Locus and Chromosome 15 Markers
|
LOD Scores at θ= |
||||||||||||
| Marker | IMDa(cM) | .00 | .001 | .01 | .05 | .10 | .15 | .20 | .30 | .40 | Zmax | θmax |
| D15S118 | 7.8 | − α | −3.294 | −1.322 | −.049 | .389 | .567 | .633 | .579 | .360 | .633 | .20 |
| D15S968 | 5.5 | −.323 | 1.677 | 2.633 | 3.124 | 3.114 | 2.934 | 2.662 | 1.933 | 1.022 | 3.124 | .05 |
| D15S126 | 2.1 | −3.322 | −.284 | 1.604 | 2.678 | 2.859 | 2.768 | 2.549 | 1.879 | 1.001 | 2.859 | .10 |
| D15S209 | 3.2 | −3.628 | −.590 | 1.303 | 2.395 | 2.600 | 2.534 | 2.342 | 1.731 | .921 | 2.600 | .10 |
| D15S117 | 1.2 | 5.979 | 5.970 | 5.887 | 5.513 | 5.023 | 4.504 | 3.955 | 2.747 | 1.387 | 5.979 | .00 |
| D15S1033 | 5.7 | 1.930 | 1.934 | 1.959 | 1.979 | 1.897 | 1.752 | 1.564 | 1.096 | .548 | 1.979 | .05 |
| D15S1036 | 3.2 | .515 | 1.550 | 2.458 | 2.903 | 2.875 | 2.703 | 2.460 | 1.824 | 1.012 | 2.903 | .05 |
| D15S1020 | 1.2 | −.087 | .949 | 1.865 | 2.346 | 2.365 | 2.243 | 2.052 | 1.532 | .853 | 2.365 | .10 |
| D15S153 | −.725 | .157 | 1.076 | 1.643 | 1.758 | 1.724 | 1.614 | 1.244 | .709 | 1.758 | .10 | |
Intermarker distance.
Figure 4.
Multipoint linkage analysis of cataract locus with chromosome 15 markers. The X-axis denotes the map of chromosome 15 markers (distance in cM), and the Y-axis indicates the LOD scores. The multipoint LOD score curve shows the highest peak value of 5.978 at locus D15S117, and the curve delimits the position of the disease gene as between markers D15S209 and D15S1036.
Haplotypes were constructed for the analyzed markers on chromosome 15 (fig. 3). Individual 033, who was ascertained as affected but could not be clinically confirmed by us personally and hence was scored as “unknown,” also showed the disease haplotype. In four affected individuals (008, 033, 041, and 049) recombination events were detected. Individuals 008, 033, and 049 showed recombination proximal to marker D15S117, whereas individual 041 showed recombination with marker distal to D15S1033. No recombination was observed, in any of the affected individuals, between markers D15S117 and D15S1033, which indicates that the disease locus was in this interval. The results of both multipoint and haplotype analyses place the cataract locus in a 10-cM region, between markers D15S209 and D15S1036 and closely linked to marker D15S117, that corresponds to the q21-22 region of chromosome 15.
There is no other report of a cataract locus in this region. This new cataract locus on human chromosome 15 shows synteny with regions of mouse chromosomes 2 and 9. Although no mouse mutants with cataract have been mapped to the relevant region of chromosome 9, the following two mouse genes are located in the critical region on chromosome 2 (J. Favor, personal communication): ENU-4022 (cataract phenotype: nuclear-zonular opacity) and BX1 (cataract phenotype: nuclear-posterior opacity). In principle, they are candidate loci for the described human cataract; however, in both cases, the underlying genes are unknown.
The genes in the proximity of the mapped locus on chromosome 15, which could be of some relevance, are FBN-1 (Fibrillin-1; associated with Marfan syndrome [MIM 134797]) and the fibroblast growth factor FGF-7/keratinocyte growth factor (KGF [MIM 148180]). FGF-7 exhibits potent mitogenic activity for epithelial cells (Liu et al. 1998). FGF-1 (MIM 131220), FGF-2 (MIM 134920), and FGF-3 (MIM 164950) are known to play a role in the development of the lens (reviewed by Francis et al. 1999). FGF-1 and 3 have been shown to bind and activate FGF receptor isoform [FGFR2 IIIb [MIM 176943]) that further induces fiber cell differentiation throughout the lens epithelium in transgenic mice (Robinson et al. 1998). Since FGF-7 has also been shown to bind and activate FGFR2 IIIb in vitro (Luo et al. 1998), there is a possibility that FGF-7 might also have some role in the lens development.
In addition, the gene for the orphan nuclear receptor ROR-alpha (RORA [MIM 600825]), which corresponds to the cataract locus mapped in the present family, has been mapped to 15q21-22 in humans. RORA activates the γF crystallin promoter and plays an important role in regulating γF crystallin gene expression in the murine lens (Tini et al. 1995). So far, the role of RORA in regulation of crystallin gene expression in humans is not known. However, its role in the regulation of crystallin expression in other animals indicates that it may have a regulatory function in crystallin gene expression in the human lens as well, making it an important candidate gene for cataract in this family. To identify the disease gene for this phenotypically distinct congenital cataract, mutation analysis in these candidate genes is in progress.
Acknowledgments
We wish to thank the patients and their relatives, for their cooperation, and Martin Digweed, for critical reading of the manuscript. This work was supported, in part, by the Department of Biotechnology, Government of India, grant DBT/BT/IC/71/89/Pt (to J.R.S.) and the Bundesministerium für Bildung und Forschung, grant INI 331 (to K.S.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Généthon, http://www.genethon.fr/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CRYAA [MIM 123580], CRYBA3/A1 [MIM 123610], CRYBB2 [MIM 123620], CRYGC [MIM 123680], CRYGD [MIM 123690], GJA3 [MIM 121015], GJA8 [MIM 600897], PITX3 [MIM 602669], BFSP2 [MIM 603212], FBN-1 [MIM 134797], KGF/FGF-7 [MIM 148180], FGF-1 [MIM 131220], FGF-2 [MIM 134920], FGF-3 [MIM 164950], FGFR2 IIIb [MIM 176943], and RORA [MIM 600825]
References
- Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, Barnes LR, Bruchis A, Hess JF, FitzGerald PG, Weeks DE, Ferrell RE, Gorin MB (2000) A juvenile-onset, progressive cataract locus on chromosome 3q21-q22 is associated with a missense mutation in the beaded filament structural protein-2. Am J Hum Genet 66:1426–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Vijayalakshmi P, Killedar M, Gilbert C, Foster A (1996) Aetiology of childhood cataract in south India. Br J Ophthalmol 80:628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis PJ, Berry V, Moore AT, Bhattacharya S (1999) Lens biology: development and human cataractogenesis. Trends Genet 15:191–196 [DOI] [PubMed] [Google Scholar]
- Francois J (1982) Genetics of cataract. Ophthalmologica 184:61–71 [DOI] [PubMed] [Google Scholar]
- Gill D, Klose R, Munier FL, McFadden M, Priston M, Billingsley G, Ducrey N, Schorderet DF, Héon E (2000) Genetic heterogeneity of the Coppock-like cataract: a mutation in CRYBB2 on chromosome 22q11.2. Invest Ophthalmol Vis Sci 41:159–165 [PubMed] [Google Scholar]
- Héon E, Priston M, Schorderet DF, Billingsley GD, Girard PO, Lubsen N, Munier FL (1999) The γ-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH (1997) Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet 6:665–668 [DOI] [PubMed] [Google Scholar]
- Liu JJ, Shay JW, Wilson SE (1998) Characterization of a soluble KGF receptor cDNA from human corneal and breast epithelial cells. Invest Ophthalmol Vis Sci 39:2584–2593 [PubMed] [Google Scholar]
- Luo Y, Lu W, Mohamedali KA, Jang JH, Jones RB, Gabriel JL, Kan M, McKeehan WL (1998) The glycine box: a determinant of specificity for fibroblast growth factor. Biochemistry 37:16506–16515 [DOI] [PubMed] [Google Scholar]
- Padma T, Ayyagari R, Murty JS, Basti S, Fletcher T, Rao GN, Kaiser-Kupfer M, Hejtmancik JF (1995) Autosomal dominant zonular cataract with sutural opacities localized to chromosome 17q11-12. Am J Hum Genet 57:840–845 [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, Overbeek PA, Chepelinsky AB (1998) Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol 198:13–31 [DOI] [PubMed] [Google Scholar]
- Scott MH, Hejtmancik JF, Wozencraft LA, Reuter LM, Parks MM, Kaiser-Kupfer MI (1994) Autosomal dominant congenital cataract: interocular phenotypic variability. Ophthalmology 101:866–871 [DOI] [PubMed] [Google Scholar]
- Tini M, Fraser RA, Giguere V (1995) Functional interactions between retinoic acid receptor-related orphan nuclear receptor (ROR alpha) and the retinoic acid receptors in the regulation of the gamma F-crystallin promoter. J Biol Chem 270: 20156–20161 [DOI] [PubMed] [Google Scholar]
- Vanita (1998) Genetical investigations in congenital cataract cases. PhD thesis, Guru Nanak Dev University, Amritsar, India [Google Scholar]
- Vanita, Singh JR, Singh D (1999) Genetic and segregation analysis of congenital cataract in the Indian population. Clin Genet 56:389–393 [DOI] [PubMed] [Google Scholar]