Abstract
Fragile-X syndrome is caused by an unstable CGG trinucleotide repeat in the FMR1 gene at Xq27. Intermediate alleles (51–200 repeats) can undergo expansion to the full mutation on transmission from mother to offspring. To evaluate the effectiveness of a fragile-X carrier–screening program, we tested 14,334 Israeli women of child-bearing age for fragile-X carrier status between 1992 and 2000. These women were either preconceptional or pregnant and had no family history of mental retardation. All those found to be carriers of premutation or full-mutation alleles were offered genetic counseling and also prenatal diagnosis, if applicable. We identified 207 carriers of an allele with >50 repeats, representing a prevalence of 1:69. There were 127 carriers with >54 repeats, representing a prevalence of 1:113. Three asymptomatic women carried the fully mutated allele. Among the premutation and full-mutation carriers, 177 prenatal diagnoses were performed. Expansion occurred in 30 fetuses, 5 of which had an expansion to the full mutation. On the basis of these results, the expected number of avoided patients born to women identified as carriers, the cost of the test in this study (U.S. $100), and the cost of lifetime care for a mentally retarded person (>$350,000), screening was calculated to be cost-effective. Because of the high prevalence of fragile-X premutation or full-mutation alleles, even in the general population, and because of the cost-effectiveness of the program, we recommend that screening to identify female carriers should be carried out on a wide scale.
Introduction
Fragile-X syndrome is an X-linked genetic syndrome now recognized as the second leading identifiable cause of mental retardation, after Down syndrome. Its frequency in males is estimated to be ∼1:4,000 (Murray et al. 1996; Turner et al. 1996). It is caused by an unstable CGG trinucleotide-repeat sequence mutation found in the fragile-X mental retardation gene (FMR1 [MIM 309550]) at the chromosomal locus Xq27 (Kremer et al. 1991; Oberlé et al. 1991; Verkerk et al. 1991; Yu et al. 1991). Routine clinical testing for mutations in the FMR1 gene determines the trinucleotide-repeat number and/or gene-methylation status, which are constant in each individual, so that each person needs to undergo the test only once. The following types of alleles are observed: normal (6–50 CGG repeats, usually 29–30), premutation (or “intermediate”) (51–200 CGG repeats), and full mutation (>200 CGG repeats) (Fu et al. 1991). Full-mutation alleles are disease causing, whereas premutation alleles are not associated with clinical symptoms but can undergo expansion to the full mutation on transmission from mothers to offspring. Because of this, prenatal testing in premutation or full-mutation carriers should be offered in every pregnancy. In the presence of the full mutation, the CpG island at the 5′ of the FMR1 gene is hypermethylated, and the transcription of the gene is shut down (Oberlé et al. 1991; Pieretti et al. 1991).
The clinical features of males with the full mutation are mental retardation, long face, large ears, prominent jaw, postpubertal macroorchidism, strabismus, mitral-valve prolapse, hyperactivity, and autistic behavior. Similar, but milder, clinical features have been reported in ∼50%–70% of females heterozygous for the full mutation (Rousseau et al. 1991; de Vries et al. 1996). Females who are premutation carriers have a 50% risk in each pregnancy of transmitting the abnormal allele to the offspring. The risks of expansion of a premutation allele to the full mutation in the offspring have been calculated on the basis of premutation repeat size and have been summarized elsewhere (Warren and Nelson 1994; Nolin et al. 1996). The likelihood of repeat instability increases with increasing repeat number. There is no expansion of the premutation allele to the full mutation if it is transmitted to the offspring by males (Oberlé et al. 1991).
Premutation (>54 repeats) carriers can be detected only by direct molecular analysis of the FMR1 gene repeat number, because they lack abnormal clinical features. The prevalence of premutation carriers has been found previously to be between 1:152 and 1:259 (Rousseau et al. 1995; Murray et al. 1997; Ryynänen et al. 1999; Pesso et al. 2000).
In a retrospective study performed by Rousseau et al. (1995), the prevalence of premutation allele (>54 repeats) carriers was found to be 1:259, and a meta-analysis of five studies carried out by Murray et al. (1997) established a premutation allele frequency of 1:273. A smaller prospective study found a carrier frequency of 1:246 for alleles with >60 repeats among 1,477 pregnant women tested for the fragile-X premutation in the Finnish population (Ryynänen et al. 1999), and, in a comprehensive study conducted by Pesso et al. (2000), the premutation carrier frequency among Israeli women was found to be 1:70 for alleles with ⩾52 or more repeats and 1:152 for alleles with >54 repeats.
The aim of the present study was to evaluate the effectiveness of a fragile-X carrier–screening program, by studying the FMR1 gene CGG-repeat size and the pregnancy outcome in the premutation and full-mutation carriers in a group of 14,334 Israeli women of child-bearing age from different ethnic groups and with no family history of mental retardation.
Subjects and Methods
Between January 1, 1992, and October 31, 2000, a total of 14,334 preconceptional or pregnant women were tested at the Rabin Medical Center, Israel. They applied for testing on their own initiative or on the advice of their physician, on a self-pay basis. They each completed a questionnaire to ascertain any family history of mental retardation, and all women with such a family history were excluded from the study. The ethnic origins of the women screened were established by randomly sampling the data from 1 out of every group of 10 consecutive women each year between 1995 and 2000. Details about the women during the years 1992–1994 were missing, so we assumed the same ethnic distribution as for the other years.
All the women who were found to be carriers of premutation alleles were offered genetic counseling. Those who were already pregnant, and the preconceptional women in the event of their becoming pregnant, were provided with information about prenatal diagnosis by amniotic-fluid analysis or chorionic villus sampling (CVS), which would be financed by the Israeli Ministry of Health.
We performed Southern blotting to ascertain the presence of normal, premutation (intermediate), or full-mutation alleles (Rousseau et al. 1991). In those women who were suspected carriers of premutation alleles, we performed PCR analysis (Brown et al. 1993) to determine the exact number of CGG repeats in the abnormal allele.
A cost-benefit study was recently carried out to examine whether, from a financial point of view, a screening program for fragile-X carrier status of the entire female population of Israel is justified. This is described in the Appendix.
Results
Among 14,334 normal healthy women screened for FMR1 allelic expansion, we found 207 carriers of an allele with >50 repeats, representing a prevalence of 1:69 (confidence interval [CI] 1:62 to 1:83). When taking 55 CGG repeats as the lower limit for premutation-carrier status, the prevalence of such carriers was 1:113 (CI 1:96 to 1:136) (127 women). Three women were found to carry the fully mutated allele. Table 1 details the number of premutation carriers for each range of the number of CGG repeats.
Table 1.
Number of Premutation Carriers for Each Range of the Number of CGG Repeats
| CGG-Repeat Range | No. (%)of Women(n=207) |
| 50–54 | 80 (39) |
| 55–60 | 62 (30) |
| 61–65 | 15 (7.2) |
| 66–70 | 25 (12) |
| 71–75 | 4 (1.9) |
| 76–80 | 9 (4.3) |
| 81–200 | 9 (4.3) |
| >200 | 3 (1.5) |
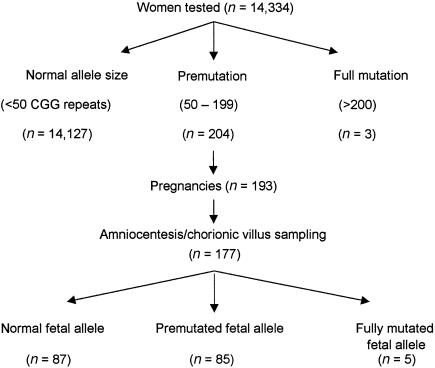
Of the women in the premutation/full-mutation carrier group, 173 were pregnant. Of these, 2 had miscarriages, 14 refused prenatal diagnosis, 16 had either two pregnancies each (8 women) or twins (8 women), and 2 had three pregnancies each, so that out of the total of 193 pregnancies, 177 (91.7%) prenatal diagnosis procedures were performed (fig. 1).
Figure 1.
Flowchart of fragile-X screening results
Table 2 details the transmission of the FMR1 allele to the fetus. In 90 pregnancies (50.8%), the allele containing >50 repeats was transmitted, and 5 of these expanded to the full mutation.
Table 2.
Transmission of FMR1 from Mother to Fetus
|
Fetal Alleles |
|||
| MaternalRepeatNumberinAbnormalAllele | Non-PremutationAlleleTransmitted(<50 Repeats) | PremutationAlleleTransmitted(50–200 Repeats) | FullMutationAlleleTransmitted(>200 Repeats) |
| 50–55 | 36 | 39 | 0 |
| 56–60 | 22 | 22 | 0 |
| 61–65 | 7 | 8 | 0 |
| 66–70 | 10 | 10 | 2 |
| 71–75 | 2 | 3 | 0 |
| 76–80 | 3 | 2 | 1 |
| 81–200 | 6 | 1 | 1 |
| >200 | 2 | 0 | 1 |
| Total | 87 | 85 | 5 |
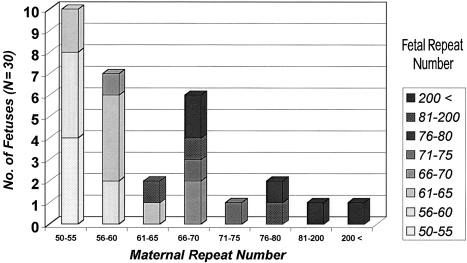
Figure 2 shows the results of prenatal diagnosis in the group of fetuses with expansion of the abnormal allele, according to the maternal premutation allele size. There were 30 fetuses (16.9%) in which expansion occurred, 5 of which (16.7%) had an expansion to the full mutation. All these five pregnancies were terminated after confirmation of the prenatal diagnosis. No full mutation was found in the fetuses of mothers with <70 CGG repeats.
Figure 2.
Maternal allele expansion in the fetus
In Israel, there are many diverse ethnic groups in a relatively small population. Table 3 depicts the ethnic distribution among the women screened and among the premutation carriers. There were no ethnic differences among premutation carriers, compared with the general population, and no statistically significant differences were found between any of the groups studied.
Table 3.
Ethnic Distribution among Population Screened and among Fragile-X Premutation Carriers
|
No. (%) in Ethnic Group |
|||||||||
| Population | AshkenaziJews | Iranianand IraqiJews | NorthAfricanJews | BalkanJews | YemeniteJews | SyrianJews | Non-JewishIsraelis | Othera | Total |
| Population screened in: | |||||||||
| 1996 | 143.0 | 23.0 | 29.0 | 16.0 | 10.5 | 4.5 | 0.0 | 2.0 | 228.0 |
| 1997 | 165.5b | 28.0 | 30.5 | 11.0 | 8.5 | 3.0 | 3.0 | 5.5 | 255.0 |
| 1998 | 88.0 | 24.0 | 15.5 | 9.5 | 10.0 | .5 | 5.0 | 2.5 | 155.0 |
| 1999 | 96.0 | 23.0 | 21.5 | 6.0 | 7.5 | 3.0 | 1.0 | 2.0 | 160.0 |
| 2000 | 52.0 | 7.5 | 22.0 | 3.0 | 5.0 | 3.0 | 2.0 | 5.5 | 100.0 |
| Total | 544.5 (60.65) | 105.5 (11.75) | 118.5 (13.2) | 45.5 (5.1) | 41.5 (4.6) | 14.0 (1.55) | 11.0 (1.2) | 17.5 (1.95) | 898.0 (100) |
| Premutation carriersc | 113.5b (54.85) | 21.0 (10.15) | 24.0 (11.6) | 8.5 (4.1) | 8.0 (3.85) | 1.0 (.5) | 0.0 (0) | 6.0 (2.9) | 207.0 (100) |
Including subjects from India, Georgia, and Kurdistan.
Numbers denote the ethnic origin of the carrier according to the ethnic origins of each of her parents. If a woman has a parent from one ethnic group and another from a different ethnic group, she adds 0.5 to each parental origin group.
No data were available for 25 premutation carriers (12.05%).
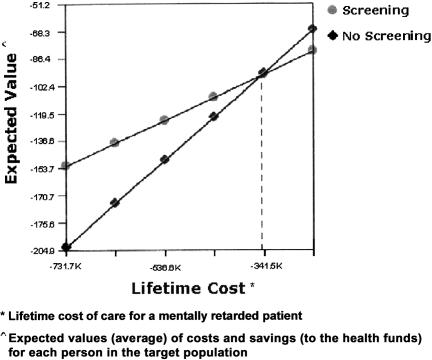
Figure 3 shows the cost-benefit analysis of running a screening program. The financial analysis concluded that the cut-off point for the cost of lifetime care of a mentally retarded person is approximately U.S. $350,000, so that if the cost in Israel is higher than this amount, the screening program is financially worthwhile. As described in the Appendix, the estimated cost of lifetime care for a mentally retarded person in Israel is $680,000, which is well above the cut-off point calculated.
Figure 3.
Cost-effectiveness of the screening program
Discussion
To date, there have been only a few reports of the estimated frequency of premutation allele carriers in the general population (Rousseau et al. 1995; Murray et al. 1997; Ryynänen et al. 1999; Pesso et al. 2000). A meta-analysis of five studies established a premutation allele frequency of 1:273 (Murray et al. 1997). In the retrospective study performed by Rousseau et al. (1995), a total of 10,624 unselected women were evaluated and the prevalence of premutation allele carriers (>54 repeats) was found to be 1:259. Haplotype analysis of polymorphic markers in the vicinity of the FMR1 gene in this study showed a strong association of the mutation with one specific haplotype, especially among individuals of French Canadian origin. It was suggested that the founder effect was partly responsible for the high carrier frequency found in this population. In a comprehensive study conducted by Pesso et al. (2000), the premutation carrier frequency among Israeli women was found to be 1:70 for alleles with ⩾52 repeats and 1:152 for alleles with >54 repeats. A smaller prospective study found a carrier frequency of 1:246 for alleles with >60 repeats, among 1,477 pregnant women tested for the fragile-X premutation in the Finnish population (Ryynänen et al. 1999). Our findings of a carrier frequency of 1:113 for women with >54 repeats and 1:220 for women with >60 repeats are comparable (table 4).
Table 4.
Studies Evaluating Prenatal Fragile-X Screening in the Population of Childbearing Women
| Study | No. ofWomenTested | PremutationCarrierFrequency | SmallestRepeatExpandedto FullMutation | No. ofAmniocenteses | No. ofFetuseswith FullMutation | No. ofPregnancyTerminations | No. ofFetalLossesDue toCVS/ACa |
| Pesso et al. 2000 | 9,459 | 1:152b | 62 | 108 | 9 | 9 | 0 |
| Ryynänen et al. 1999 | 1,477 | 1:246c | 70 | 24 | 2d | … | … |
| Our study | 14,334 | 1:113b | 70 | 177 | 5 | 5 | 0 |
CVS = chorionic villus sampling; AC = amniocentesis.
Carrier frequency for women with >54 repeats.
Carrier frequency for women with >60 repeats.
One fetus showed mosaicism.
Fragile-X syndrome has been reported in both white and black populations (Schwartz et al. 1988). In Israel, among patients with fragile X, overrepresentation of families of Tunisian Jewish origin has been described; all of these share a common haplotype that lacks AGG interruptions (Dar et al. 1995; Falik-Zaccai et al. 1997). Conversely, however, no common founder haplotype was detected in the Ashkenazi Jewish patients with fragile-X syndrome (Pesso et al. 1997). In the present study, the ethnic distribution of the premutation carriers was found to be similar to that in the general population.
An extremely important part of any genetic screening program is the availability of appropriate genetic counseling. If a premutation carrier is identified, prenatal diagnosis should be offered not only to the individual carrier but also to any relatives who might be at risk, in order to identify fetuses carrying an expansion of the premutation to the full mutation. Therefore, the benefits of undergoing the screening test are not limited to the woman’s current pregnancy (i.e., the one during which she was tested) but are also relevant for all her subsequent pregnancies and those of any carrier relatives who were identified through her. No specific treatment exists for this condition, but it is preventable by termination of affected pregnancies.
There is no clear distinction between normal and premutation alleles; the alleles with 45–60 CGG repeats are considered to be a “gray zone” because of their ability to undergo expansion on transmission to the fetus. In previous studies, instability in the transmission of the gray-zone alleles has been observed in 25% of alleles with 50–60 repeats but in <8% of those with 40–49 repeats (Nolin et al. 1996). No expansion of the premutation to the full mutation has been described for alleles containing <59 repeats (Sherman et al. 1994; Nolin et al. 1996), and, in the present study, there was no expansion of the premutation to the full mutation for alleles containing <70 repeats. Similar results were found by Ryynänen et al. (1999) and Pesso et al. (2000) (table 4). Carriers of a low borderline allele size should be reassured that the risk for having a child with fragile-X syndrome is extremely low.
As already mentioned, it is difficult to interpret the significance of DNA test results showing CGG repeat numbers in the range between normal and premutation. In the present study, the smallest premutation allele size that expanded to a full mutation was 70 repeats (table 4). Consequently, a cutoff point of 55 (or even 60) repeats can be considered as safe for making a decision about the need for prenatal testing. It has been estimated that only three invasive tests (CVS or amniocentesis) are needed to detect one full-mutation case when prenatal fetal evaluation is offered to women with ⩾60 CGG repeats (Murray et al. 1997).
In 2000, we published our results regarding selective transmission of the premutation allele from carrier females to their fetuses (Drasinover et al. 2000). Among the first 10,587 women studied, we found a significant increase in the transmission of the abnormal allele by mothers who had 51–60 repeats, but we found no increase in transmission by mothers with >61 repeats. Interestingly, with the addition of a further 3,747 women, this has been found not to be the case, and the transmission of each allele was approximately equal. The numbers are shown in table 2.
Until now, broad population screening for fragile-X syndrome has not been recommended as a routine policy by any country, although guidelines for fragile-X testing were proposed by the American College of Medical Genetics (Park et al. 1994). It was recommended that such testing should be performed in individuals with mental retardation or learning disabilities, fetuses of known carrier mothers, and individuals who have a family history of fragile-X syndrome or undiagnosed mental retardation and who are seeking reproductive counseling. The main purpose of screening for fragile-X syndrome is to reduce the incidence of the birth of affected individuals. The feasibility of population screening to detect premutation carriers has been discussed by several authors (Bonthron and Strain 1993; Bundey and Norman 1993; Howard-Peebles et al. 1993; Palomaki and Haddow 1993; Rousseau et al. 1994; Finucane 1996; Spence et al. 1996; Ryynänen et al. 1999; Tzeng et al. 1999; Wildhagen et al. 1999; Pesso et al. 2000).
If the screening program is limited only to those women with a known family history of fragile-X syndrome, several problems can occur. First, fragile-X syndrome can often appear as a new case in a family with no prior history of learning disabilities or mental retardation. Second, there may be difficulties in contacting all relatives at risk. These factors limit the sensitivity of carrier detection by a proband-based approach. It was estimated, by a microsimulation model, that at least eight consecutive generations would need to be tested in order to detect 90% of all premutation and full-mutation carriers (Wildhagen et al. 1999). Such extensive family tracing is technically difficult.
If screening of only those women who are mentally retarded or who have learning disabilities is undertaken, a significant number of the full-mutation carriers and all of the premutation carriers would be missed. Furthermore, it has been claimed that the frequency of premutation carriers was not higher among the women with a family history of mental retardation (excluding fragile-X syndrome) compared with the low-risk group (Pesso et al. 2000). Any screening program that excludes pregnant women without a known family history of mental retardation will continue to miss a significant number of fragile-X carriers.
Screening of preconceptional women will allow more time for genetic counseling and decision making about reproductive options in premutation carriers. On the other hand, such screening is difficult in practice. Therefore, the population of pregnant women appears to be the most feasible group to screen, in spite of the expected anxiety regarding the outcome of the pregnancy caused by a positive result.
Our survey included only those women who came for testing on a self-pay basis. As a result, only women from higher socioeconomic levels, who were able to afford the cost of the test, were screened, and, therefore, these women do not represent an unselected sample. The impact of this bias on the results of the simulation and its applicability to the general population are difficult to assess.
Evaluation was undertaken recently comparing the costs, effects, and benefits of prenatal, preconceptional, and school-age carrier screening (Wildhagen et al. 1998). All three screening strategies were found to have a positive cost-benefit ratio, but the authors found that prenatal screening of pregnant women would detect most carriers and would lead to the highest number of avoided fragile-X syndrome cases, and they considered that such screening was also the optimal strategy from the economic point of view.
Our calculations of the costs and benefits show that, from a financial point of view, a screening program is worthwhile. Although the actual lifetime cost of caring for a mentally retarded person in Israel is not accurately known, some previous studies have estimated the cost in other countries to be ∼$900,000 (Turner et al. 1986; Nolin et al. 1991), and others have estimated it to be between $1 million and $4 million (Finucane 1996). Since, as described above, the calculated cost of lifetime care for a mentally retarded patient in Israel ($680,000) is well above the cut-off point based on financial considerations ($350,000), the screening program is worthwhile.
The calculation based on (1) our data regarding the prevalence of the carrier status of fragile-X syndrome and the incidence of fetuses carrying the full mutation, (2) the cost, adjusted for Israel, of lifetime care for a retarded person, and (3) a 50% acceptance rate of the screening program, shows that the expected net benefit from running the program is ∼$5,500,000 per year (see Appendix). The net benefit remains positive over a wide range of acceptance rates. It is, therefore, clearly evident that the results of the cost-benefit study, from a socioeconomic point of view, justify the financing, by public funds, of a screening program in Israel for fragile-X syndrome.
Finally, scarcity of data regarding the population frequency of fragile-X premutation carriers and concerns about the cost of screening have, so far, delayed decision making. The high frequency of premutation carriers found by screening in this and other studies, including >50,000 women overall, points clearly to the appropriateness of population screening. Because of the high prevalence of fragile-X syndrome, the lack of treatment for those suffering from it, and the cost-effectiveness of the carrier-screening strategy, we propose to offer such screening to all pregnant women to minimize or even eliminate the risk of having a child with mental retardation caused by this syndrome.
Acknowledgments
We are very grateful to Dr. Tamy Shohat for performing the statistical analysis and to Professor Cyril Legum and Dr. Reuven Sharony for their helpful comments.
Appendix: Cost-Effectiveness Analysis
By use of DATA 3.5 software (Treeage Software), a model of a decision tree was constructed, in which the decision junction was running versus not running a screening program. For each possible event, whether the decisions were made by the target population (e.g., acceptance of each step of screening, termination of a pregnancy) or by the results (carrier status, affected fetus, or miscarriage), a separate branch was made and given a probability for that event. The whole tree consisted of 132 branches representing every possible sequence of events. For each such sequence of events, a “payoff” was calculated in financial terms: the costs were assessed for each step of the screening program, including administration and publicity, blood tests, molecular-biology studies (Southern blotting and PCR), genetic counseling, invasive procedures for prenatal diagnosis (in case a screened woman was found to be a carrier), the costs of fetal loss (miscarriage) occurring as a result of invasive procedures, if taken, and the expenses for the screened woman (such as travel costs and loss of work time). Benefits taken into consideration were (1) the cost of the lifetime care of a mentally retarded person and (2) the costs saved by not performing additional fetal testing on women who would be undergoing such testing anyway, because they were at risk of having a fetus with trisomy 21 (table A1).
Table A1.
Costs and Benefits
| Factor | Benefit($) | Symbol |
| Publicity for each person in the target population | 1 | C0 |
| DNA testing and expenses for tested woman | 110 | C1 |
| Amniocentesis and karyotype | 155 | C2 |
| DNA testing of the fetus | 98 | C3 |
| Genetic counseling following fetal diagnosis | 51 | C4 |
| Iatrogenic abortion | 658 | C5 |
| Cost of genetic counseling following fetal diagnosis | 128 | C6 |
| Cost of abortion (iatrogenic or therapeutic) | 483 | C7 |
| Cost of genetic counseling following diagnosis of carrier status | 27 | C8 |
| Not running a screening program | 0 | C9 |
| Birth of a nonretarded child | 0 | B1 |
| Lifetime care of a mentally retarded person | −680,000 | B2 |
| Lost fetuses (due to either iatrogenic abortion or termination of a nonretarded female with the full fragile-X mutation) | −36,500 | B3 |
Table A2 shows the probabilities. The rates of rejection [P1] or acceptance [#(P1)] of the screening program are based on the rates of rejection or acceptance of other prenatal screening programs that are well established in Israel (Ginsberg et al. 1994, 1998). The rates, among the Israeli population, of having [#(P2)] or not having [P2] the full fragile-X mutation and of being a carrier [#(P3)] or not being a carrier [P3] of the full fragile-X mutation are based on our current study, which includes 14,334 women. The rates of acceptance [#(P4)] or rejection [P4] of genetic counseling among carriers is taken from our current study and from a recent study carried out in Finland (Ryynänen et al. 1999). The rates of the fetus having [#(P5)] or not having [P5] the full fragile-X mutation, where the mother is a carrier, are taken from our own data. The rates of performing [#(P6)] or rejecting [P6] prenatal diagnosis among carrier mothers are based on the data from our current study. The rate of finding the full fragile-X mutation in the diagnosis of the fetus [#(P7,P8,P9,P10)] is based on the data from our current study.
Table A2.
Probabilities
| Event | Probability | Symbol |
| Rejection of screening program | .5 | P1 |
| Acceptance of screening program | .5 | #(P1) |
| Not finding the full fragile-X mutation among women who rejected screening | 2,866/2,867 | P2 |
| Finding the full fragile-X mutation among women who rejected screening | 1/2,867 | #(P2) |
| Not being a carrier among women who accepted screening | 112/113 | P3 |
| Being a carrier among women who accepted screening | 1/113 | #(P3) |
| Rejection of genetic counseling among carriers | 0 | P4 |
| Acceptance of genetic counseling among carriers | 1 | #(P4) |
| A fetus that does not have the full fragile-X mutation where the mother is a carrier | .958 | P5 |
| A fetus that does have the full fragile-X mutation where the mother is a carrier | .042 | #(P5) |
| Rejection of fetal diagnosis where the mother is a carrier | .05 | P6 |
| Performing fetal diagnosis where the mother is a carrier | .95 | #(P6) |
| Finding normal fetus following fetal diagnosis | .944 | P7 |
| Iatrogenic abortion following fetal diagnosis | .01 | P8 |
| Finding somatic chromosome abnormality in the fetus at prenatal diagnosis | .002 | P9 |
| Finding sex chromosome abnormality in the fetus at prenatal diagnosis | .002 | P10 |
| Finding the full fragile-X mutation in the fetus at prenatal diagnosis | .042 | #(P7,P8,P9,P10) |
| Rejection of termination of pregnancy where there is a somatic-chromosome abnormality in the fetus | 0 | P11 |
| Termination of pregnancy where there is a somatic-chromosome abnormality in the fetus | 1 | #(P11) |
| Rejection of termination of pregnancy where there is a sex-chromosome abnormality in the fetus | 0 | P12 |
| Termination of pregnancy where there is a sex-chromosome abnormality in the fetus | 1 | #(P12) |
| Rejection of termination of pregnancy where the fetus has the full fragile-X mutation | 0 | P13 |
| Termination of pregnancy where the fetus has the full fragile-X mutation | 1 | #(P13) |
| Fetus with the full fragile-X mutation is male | .5 | P14 |
| Fetus with the full fragile-X mutation is female | .5 | #(P14) |
| Mentally retarded male with the full fragile-X mutation | 1 | P15 |
| Nonretarded male with the full fragile-X mutation | 0 | #(P15) |
| Mentally retarded female with the full fragile-X mutation | .59 | P16 |
| Nonretarded female with the full fragile-X mutation | .41 | #(P16) |
| Pregnancy is a second pregnancy | .272 | P17 |
| Pregnancy is a first or third (or more) pregnancy | .728 | #(P17) |
| Rejection of screening in second pregnancy where this was rejected in the previous pregnancy | 1 | P18 |
| Acceptance of screening in second pregnancy where this was rejected in the previous pregnancy | 0 | #(P18) |
The rate of iatrogenic abortion following prenatal diagnosis is based on the data from a study analyzing the cost-effectiveness of a screening program in the Netherlands (Wildhagen et al. 1998) and on a personal communication (R. Sharony, Genetics Institute, Meir Hospital, Sapir Medical Center, Kfar Saba, Israel). We took the rates of finding somatic-chromosome abnormalities [P9] and sex-chromosome abnormalities [P10] in the fetus from the data from a previous analysis in the Netherlands (Wildhagen et al. 1998). Finding a normal fetus following prenatal diagnosis [P7] is the complementary probability of the consequences of prenatal diagnosis. Rates of termination of pregnancy of fetuses with a somatic-chromosome abnormality [#(P11)] or a sex-chromosome abnormality [#(P12)] and of rejection of termination of pregnancy in these cases [P11 and P12] are based on past experience in Israel and on data from the Netherlands (Wildhagen et al. 1998). Rates of termination of pregnancy [#(P13)] or rejection of termination of pregnancy [P13] of a fetus with the full fragile-X mutation are based on the data of the current study, on past experience in Israel, and on data from the Netherlands (Wildhagen et al. 1998).
We followed the rates used by Wildhagen et al. (1998) in their cost-effectiveness analysis for males [P14] and females [#(P14)] among patients with the full fragile-X mutation, for mentally retarded males [P15] and females [P16] with the full fragile-X mutation, and for non–mentally retarded males [#(P15)] and females [#(P16)] with the full fragile-X mutation. The rate of second pregnancies [P17] comes from statistical data in Israel (Central Bureau of Statistics 1999). The rates of rejection [P18] or acceptance [#(P18)] of a screening program by a woman in her second pregnancy who had rejected such a program in the past is our tentative assumption.
The costs of publicity and distribution of information about the screening program are based on the costs that the Israeli Ministry of Health allocates for various screening, immunization, or early-intervention programs and is for each person in the target population (G. Ginsberg, Israeli Ministry of Health, personal communication). The cost of DNA testing of the woman [C1] consists of the current price charged for the test in Israel plus her expenses, on the basis of the calculations made for a cost-benefit analysis for prenatal diagnosis for cystic fibrosis in Israel (Ginsberg et al. 1994), and was corrected according to the change in the Consumer Price Index. The cost for amniocentesis and karyotype for fragile-X carriers [C2] is based on the cost of these tests in Israel, corrected for the number of pregnant women ⩾35 years old (Central Bureau of Statistics 1999) and for the number of women <35 years old with a positive triple test for trisomy 21 (Herman et al. 1997). The cost of the DNA test of the fetus [C3] is the same as the cost for this test in the screened woman. The cost of genetic counseling following the fetal diagnosis [C4] is calculated according to the cost for this in Israel and was corrected for the number of pregnant women ⩾35 years old (Central Bureau of Statistics 1999) and for the number of women <35 years old with a positive triple test for trisomy 21 (Herman et al. 1997). The cost of an iatrogenic abortion [C5] is the cost of a day of in-hospital stay multiplied by the average stay in hospital for this condition (1.89 d) (Ginsberg et al. 1994) and is derived from the Israeli Ministry of Health List of Prices for the year 2000. Parental costs, including traveling expenses and loss of work time, the cost of genetic counseling after the diagnosis of carrier status [C8], the cost of genetic counseling after fetal diagnosis [C6], and the cost of iatrogenic or therapeutic abortion [C7] were derived from a cost-benefit analysis for prenatal diagnosis for cystic fibrosis in Israel (Ginsberg et al. 1994) and were corrected according to the change in the Consumer Price Index. The cost of not running a screening program [C9] is, by definition, zero. However, this is not the same as the cost per woman in the target population who does not accept screening, because of the cost of the publicity of the screening program, which is constant whether or not the woman accepts screening.
The benefit per birth of an unaffected (nonretarded) child is, by definition, zero. However, the benefit of the prevention of the birth of each mentally retarded individual [B2], whether the retardation is due to the full fragile-X mutation or to other genetic causes, is the cost of the lifetime care of a mentally retarded person in Israel. Unfortunately, there is no information, or even an official estimate, of the cost of the lifetime care of a mentally retarded person in Israel. To bypass this missing information, we used the cost of the lifetime care of a male with the full fragile-X mutation in the Netherlands (Wildhagen et al. 1998), where this was calculated to be $957,000. This cost was corrected for Israel, according to the ratio between the gross national product per capita in Israel and that in the Netherlands in 1999, which was 0.71 (P. Bachrach, Royal Netherlands Embassy in Israel, personal communication), and we thus arrived at an estimate of $680,000 for the lifetime cost of caring for a mentally retarded person in Israel.
The rationale for using the costs from another country (the Netherlands) is that that country is similar in size to Israel and has a comparable system of social medicine. More importantly, the figures are only rough estimates that allowed us to run a sensitivity analysis and to find a cut-off point for Israel, so that if the cost of the lifetime care of a mentally retarded person in Israel is above this point, then the screening program will have a favorable cost-saving balance. Having arrived at this cut-off point, this figure can now be adjusted to take into consideration the existing information about the cost of the lifetime care of a mentally retarded person in Israel. The calculation—based on our data regarding the prevalence of the carrier status of fragile X, the incidence of fetuses carrying the full mutation, the cost of lifetime care for a retarded person adjusted for Israel, and the assumption of a 50% acceptance rate of the screening program—shows that the expected net benefit from running the program is ∼$5,500,000 per year.
We used the cost of the lifetime care of a male with the full fragile-X mutation in the Netherlands, since, in the way that the calculations of cost-effectiveness were made, correction has already been made for the non–mentally retarded females with the full fragile-X mutation. It should be mentioned that the value of B2 has a minus sign, since, in the way the tree is constructed, B2 represents the expense when a sequence of events leads to the birth of a retarded individual (when the birth of a nonretarded individual is given the benefit B1, which is zero).
We decided to present the cost of the loss of normal fetuses, whether due to iatrogenic abortion or to the termination of a nonretarded female with the full fragile-X mutation, on the benefit side, since these are events that are not controlled by the physician, and marked it as B3. However, since it is a loss and not a benefit, it has a minus sign. The costs for this are based on calculations made in a previously published cost-benefit analysis of prenatal screening for cystic fibrosis in Israel (Ginsberg et al. 1994) and are corrected for the change, since the original calculations were made, in the gross national product and the average income. Nevertheless, the current trend in cost-benefit analysis regarding prenatal diagnosis is to give the loss of fetuses a value of zero.
A guideline we used for the whole analysis was to remain as conservative as possible in deciding which costs and benefits to take into account; for example, we avoided giving a value of zero to the cost of lost fetuses. However, we did not take into account some “vertical” savings (such as not needing to screen women in their third or later pregnancy), as well as some expected “horizontal” savings, such as screening of female relatives of women found to be carriers during the first years of running the program.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FMR1 [MIM 309550])
References
- Bonthron D, Strain L (1993) Population screening for fragile-X syndrome. Lancet 341:769–770 [DOI] [PubMed] [Google Scholar]
- Brown WT, Houck GE Jr, Jeziorowska A, Levinson FN, Ding X, Dobkin C, Zhong N, Henderson J, Brooks SS, Jenkins EC (1993) Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA 270:1569–1575 [PubMed] [Google Scholar]
- Bundey S, Norman E (1993) Population screening for fragile-X syndrome. Lancet 341:770 [PubMed] [Google Scholar]
- Central Bureau of Statistics (1999) Israeli Statistical Year Book No. 50: 1998. Central Bureau of Statistics, Jerusalem [Google Scholar]
- Dar H, Chemke T, Schaap T, Chaki R, Bait-Or H, Cohen H, Borochowitz Z, Falik-Borenstein Z, Gelman-Kohan Z, Chemke J (1995) Ethnic distribution of the fragile X syndrome in Israel: evidence of founder chromosomes(?). Isr J Med Sci 31:323–325 [PubMed] [Google Scholar]
- de Vries BBA, Wiegers AM, Smits APT, Mohkamsing S, Duivenvoorden HJ, Fryns J-P, Curfs LMG, Halley DJJ, Oostra BA, van den Ouweland AMW, Niermeijer MF (1996) Mental status of females with an FMR1 gene full mutation. Am J Hum Genet 58:1025–1032 [PMC free article] [PubMed] [Google Scholar]
- Drasinover V, Ehrlich S, Magal N, Taub E, Libman V, Shohat T, Halpern GJ, Shohat M (2000) Increased transmission of intermediate alleles of the FMR1 gene compared with normal alleles among female heterozygotes. Am J Med Genet 93:155–157 [DOI] [PubMed] [Google Scholar]
- Falik-Zaccai TC, Shachak E, Yalon M, Lis Z, Borochowitz Z, Macpherson JN, Nelson DL, Eichler EE (1997) Predisposition to the fragile X syndrome in Jews of Tunisian descent is due to the absence of AGG interruptions on a rare Mediterranean haplotype. Am J Hum Genet 60:103–112 [PMC free article] [PubMed] [Google Scholar]
- Finucane B (1996) Should all pregnant women be offered carrier testing for fragile X syndrome? Clin Obstet Gynecol 39:772–782 [DOI] [PubMed] [Google Scholar]
- Fu Y-H, Kuhl DPA, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJMH, Holden JJA, Fenwick RG Jr, Warren ST, Oostra BA, Nelson DL, Caskey CT (1991) Variation of the CGG repeat at the Fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67:1047–1058 [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Blau H, Kerem E, Springer C, Kerem BS, Akstein E, Greenberg A, Kolumbos A, Abeliovich D, Gazit E, Yahav J (1994) Cost-benefit analysis of a national screening program for cystic fibrosis in an Israeli population. Health Econ 3:5–23 [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Tulchinsky T, Filon D, Goldfarb A, Abramov L, Rachmilevitz EA (1998) Cost-benefit analysis of a national thalassemia prevention program in Israel. J Med Screen 5:120–126 [DOI] [PubMed] [Google Scholar]
- Herman A, Maymon R, Dreazen E, Bukovsky I, Weinraub Z (1997) First trimester ultrasound screening for nuchal translucence as marker of Down’s syndrome. Harefuah 132:94–100 [PubMed] [Google Scholar]
- Howard-Peebles PN, Maddalena A, Black SH, Schulman JD (1993) Population screening for fragile-X syndrome. Lancet 341:770 [PubMed] [Google Scholar]
- Kremer EJ, Pritchard M, Lynch M, Yu S, Holman K, Baker E, Warren ST, Schlessinger D, Sutherland GR, Richards RI (1991) Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science 252:1711–1714 [DOI] [PubMed] [Google Scholar]
- Murray J, Cuckle H, Taylor G, Hewison J (1997) Screening for fragile X syndrome: information needs for health planners. J Med Screen 4:60–94 [DOI] [PubMed] [Google Scholar]
- Murray A, Youings S, Dennis N, Latsky L, Linehan P, McKechnie N, Macpherson J, Pound M, Jacobs P (1996) Population screening at the FRAXA and FRAXE loci: molecular analyses of boys with learning difficulties and their mothers. Hum Mol Genet 5:727–735 [DOI] [PubMed] [Google Scholar]
- Nolin SL, Lewis FA III, Ye LL, Houck GE Jr, Glicksman AE, Limprasert P, Li SY, Zhong N, Ashley AE, Feingold E, Sherman SL, Brown WT (1996) Familial transmission of the FMR1 CGG repeat. Am J Hum Genet 59:1252–1261 [PMC free article] [PubMed] [Google Scholar]
- Nolin SL, Snider DA, Jenkins EC, Brown WT, Krawczun M, Stetka D, Houck G Jr, et al (1991) Fragile X screening program in New York State. Am J Med Genet 38:251–255 [DOI] [PubMed] [Google Scholar]
- Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boué J, Bertheas MF, Mandel JL (1991) Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252:1097–1102 [DOI] [PubMed] [Google Scholar]
- Palomaki GE, Haddow JE (1993) Is it time for population-based prenatal screening for fragile-X? Lancet 341:373–374 [DOI] [PubMed] [Google Scholar]
- Park V, Howard-Peebles PN, Sherman S, Taylor A, Wulfsberg E (1994) Fragile X syndrome: diagnostic and carrier testing. Am J Med Genet 53:380–3817864050 [Google Scholar]
- Pesso R, Barkai G, Ravia Y, Gak E, Frydman M, Goldman B, Friedman E (1997) No founder effect detected in Jewish Ashkenazi patients with fragile-X syndrome. Hum Genet 101:186–189 [DOI] [PubMed] [Google Scholar]
- Pesso R, Berkenstadt M, Cuckle H, Gak E, Peleg L, Frydman M, Barkai G (2000) Screening for fragile X syndrome in women of reproductive age. Prenat Diagn 20:611–614 [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL (1991) Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66:817–822 [DOI] [PubMed] [Google Scholar]
- Rousseau F, Heitz D, Biancalana V, Blumenfeld S, Kretz C, Boué J, Tommerup N, van der Hagen C, DeLozier-Blanchet C, Croquette MF, Gilgerkrantz S, Jalbert P, Voelckel MA, Oberlé I, Mandel JL (1991) Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med 325:1673–1681 [DOI] [PubMed] [Google Scholar]
- Rousseau F, Réhel R, Rouillard P, DeGranpré P, Khandjian EW (1994) High throughput and economical mutation detection and RFLP analysis using a mini-method for DNA preparation from whole blood and acrylamide gel electrophoresis. Hum Mutat 4:51–54 [DOI] [PubMed] [Google Scholar]
- Rousseau F, Rouillard P, Morel M-L, Khandjian EW, Morgan K (1995) Prevalence of carriers of premutation-size alleles of the FMRI gene–and implications for the population genetics of the fragile X syndrome. Am J Hum Genet 57:1006–1018 [PMC free article] [PubMed] [Google Scholar]
- Ryynänen M, Heinonen S, Makkonen M, Kajanoja E, Mannermaa A, Pertti K (1999) Feasibility and acceptance of screening for fragile X mutations in low-risk pregnancies. Eur J Hum Genet 7:212–216 [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Phelan MC, Pulliam LH, Wilkes G, Vanner LV, Albiez KL, Potts WA, et al (1988) Fragile X syndrome: incidence, clinical and cytogenetic findings in the black and white populations of South Carolina. Am J Med Genet 30:641–654 [DOI] [PubMed] [Google Scholar]
- Sherman SL, Maddalena A, Howard-Peebles PN, Brown WT, Nolin S, Jenkins E, Schwartz C, et al (1994) Characteristics of the transmission of the FMR1 gene from carrier females in a prospective sample of conceptuses. Am J Med Genet 51:503–506 [DOI] [PubMed] [Google Scholar]
- Spence WC, Black SH, Fallon L, Maddalena A, Cummings E, Menapace-Drew G, Bick DP, Levinson G, Schulman JD, Howard-Peebles PN (1996) Molecular fragile X screening in normal populations. Am J Med Genet 64:181–183 [DOI] [PubMed] [Google Scholar]
- Turner G, Robinson H, Laing S, Purvis-Smith S (1986) Preventive screening for the fragile X syndrome. N Engl J Med 315:607–609 [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H (1996) Prevalence of fragile X syndrome. Am J Med Genet 64:196–197 [DOI] [PubMed] [Google Scholar]
- Tzeng C-C, Cho W-C, Kuo P-L, Chen RM (1999) Pilot fragile X screening in normal population of Taiwan. Diagn Mol Pathol 8:152–156 [DOI] [PubMed] [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DPA, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, van Ommen GB, Blonden LAJ, Riggins GK, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914 [DOI] [PubMed] [Google Scholar]
- Warren ST, Nelson DL (1994) Advances in molecular analysis of fragile X syndrome. JAMA 271:536–542 [PubMed] [Google Scholar]
- Wildhagen MF, van Os TAM, Polder JJ, ten Kate LP, Habbema JDF (1999) Efficacy of cascade testing for fragile X syndrome. J Med Screen 6:70–76 [DOI] [PubMed] [Google Scholar]
- ——— (1998) Explorative study of costs, effects and savings of screening for female fragile X premutation and full mutation carriers in the general population. Community Genet 1:36–47 [DOI] [PubMed] [Google Scholar]
- Yu S, Pritchard M, Kremer EJ, Lynch M, Nancarrow J, Baker E, Holman K, Mulley JC, Warren ST, Schlessinger D, Sutherland GR, Richards RI (1991) Fragile X genotype characterized by an unstable region of DNA. Science 252:1179–1181 [DOI] [PubMed] [Google Scholar]