Abstract
A family with a (1;11)(q42;q14.3) translocation significantly linked to a clinical phenotype that includes schizophrenia and affective disorders is described. This translocation generates a LOD score of 3.6 when the disease phenotype is restricted to schizophrenia, of 4.5 when the disease phenotype is restricted to affective disorders, of 7.1 when relatives with recurrent major depression, with bipolar disorder, or with schizophrenia are all classed as affected. This evidence for linkage is among the strongest reported for a psychiatric disorder. Family members showed no distinctive features by which the psychiatric phenotype could be distinguished from unrelated cases of either schizophrenia or affective disorders, and no physical, neurological, or dysmorphic conditions co-occurred with psychiatric symptoms. Translocation carriers and noncarriers had the same mean intelligence quotient. Translocation carriers were similar to subjects with schizophrenia and different from noncarriers and controls, in showing a significant reduction in the amplitude of the P300 event-related potential (ERP). Furthermore, P300 amplitude reduction and latency prolongation were measured in some carriers of the translocation who had no psychiatric symptoms—a pattern found in other families with multiple members with schizophrenia, in which amplitude of and latency of P300 appear to be trait markers of risk. The results of karyotypic, clinical, and ERP investigations of this family suggest that the recently described genes DISC1 and DISC2, which are directly disrupted by the breakpoint on chromosome 1, may have a role in the development of a disease phenotype that includes schizophrenia as well as unipolar and bipolar affective disorders.
Schizophrenia (MIM 181500), bipolar disorder (MIM 125480), and recurrent major depression (i.e., unipolar disorder) are among the most prevalent causes of disability worldwide (Lopez and Murray 1998). Family and twin research has established the importance of inherited factors, and several chromosomal regions likely to harbor susceptibility genes have been identified during 10 years of extensive linkage and association studies (Potash and DePaulo 2000; Riley and McGuffin 2000). At several chromosomal locations—including 13q, 18p, and 22q—linkage has been reported to both schizophrenia and bipolar disorder, raising the possibility that some genetic risk factors contribute to a range of psychotic symptoms and give rise to phenotypes that cross the traditional diagnostic boundaries of schizophrenia and of affective disorders (Wildenauer et al. 1999; Berrettini 2000). However, it is not clear, given the large number of linkage reports for both disorders, that the number of regions with positive results with regard to schizophrenia and bipolar disorder exceeds chance expectation. Whereas most family and twin studies do not support coaggregation of these disorders, some family studies have reported overlap of predisposition to schizophrenia and affective disorders (Maier et al. 1993). We describe the clinical phenotypes found in a large family in which a balanced translocation—(1;11)(q42;q14.3)—that disrupts two novel brain-expressed genes cosegregates with major psychiatric disorders. Clinical diagnoses in family members were based on DSM-IV criteria derived from a standard interview and from a case-note review. The phenotype was also investigated in a subgroup of family members, by measurement of intelligence quotient (IQ) and the auditory P300 event-related potential (ERP), which has been shown to be abnormal in patients with schizophrenia and in their unaffected relatives and is a putative trait marker of risk for schizophrenia (Blackwood et al. 1991; Sham et al. 1994; Weisbrod et al. 1999).
This family was first ascertained by Jacobs et al. (1970), who reported the translocation in the propositus, who had adolescent conduct disorder, and in members of four generations of the propositus's extended family. Follow-up of the family over 20 years revealed an increased incidence of major psychiatric disorders, including schizophrenia and recurrent major depression, among relatives with the translocation and found no cases of these disorders in relatives with a normal karyotype (St. Clair et al. 1990). It was proposed that disruption of one or more genes at or near one of the translocation breakpoints increased the risk of development of psychosis in this family. Over the past 10 years, members of this family have been systematically followed up by direct interview, regular contact with general practitioners, or hospital case-note review. The study has been approved by the local Ethics of Research Committee, and family members have given informed, written consent. Interviews were conducted by experienced psychiatrists (D.H.R.B., W.J.M., and D.St.C.) using the Schedule for Affective Disorders and Schizophrenia L (Endicott and Spitzer 1978); consensus diagnoses blind to carrier status and based on DSM-IV criteria are shown in figure 1. Of the 87 members of the family who were karyotyped, 37 carried the translocation. Of the 50 noncarriers, 18 were married-in relatives. Living relatives were interviewed directly by a psychiatrist, and deceased members were included in the analysis if adequate hospital and general-practice case records were available. A psychiatric diagnosis was reached for 29 carriers, 38 noncarriers, and the 2 founders (who were not karyotyped).
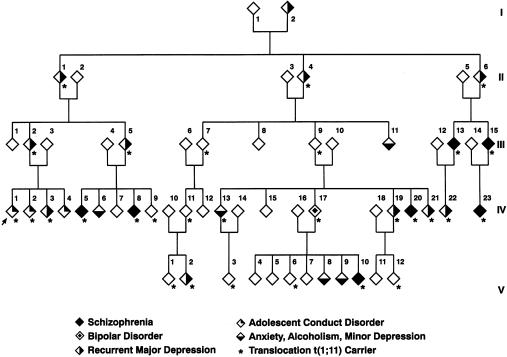
Figure 1.
Part of the family with a (1;11)(q42;q14.3) translocation. Karyotype analysis has been performed on 87 members of this family, and clinical psychiatric data were obtained from 69 of those family members. Shown are 58 of the family members for whom carrier status is known and whose psychiatric phenotype has been defined through follow-up by direct interview, general-practice contact, or hospital case-note review.
Figure 1 shows the family members for whom psychiatric assessment was possible and for whom karyotype status was available. To preserve the anonymity of family members, 11 individuals with a normal karyotype and without a psychiatric diagnosis (all were siblings of carriers) have been omitted from the figure but were included in the linkage analysis.
Nine new cases of major psychiatric disorder have been diagnosed during the follow-up period over the past 10 years. Of note is a relative (IV:17) with bipolar disorder whose child (V:10) developed a chronic schizophrenic disorder. For the 29 subjects with the translocation, the following diagnoses were made: schizophrenia (in 7 cases), bipolar disorder (in 1 case), recurrent major depression (in 10 cases), adolescent conduct-and-emotional disorder (in 2 cases), and minor depression (in 1 case). The propositus and two siblings were the only members of the extended pedigree who were diagnosed with severe adolescent conduct-and-emotional disorder, and, because one of these siblings had a normal karyotype, adolescent disorder appears to be unrelated to the translocation. No major psychiatric disorder was diagnosed in 38 relatives with a normal karyotype. In this group, psychiatric diagnoses were as follows: adolescent conduct-and-emotional disorder (in one case), minor depression (in three cases), and alcoholism (in one case). A maximum LOD score of 7.1 confirmed close linkage of the translocation to the clinical phenotype that included schizophrenia, bipolar disorder, and recurrent major depression (table 1). Linkage between the translocation and major affective disorder (LOD score 4.5) was also confirmed in an analysis in which only the cases of recurrent major depression and the single case of bipolar disorder were coded as affected and in which the seven cases of schizophrenia were coded as unknown. Linkage with schizophrenia alone was also significant (LOD score 3.6) in an analysis that included the seven cases of schizophrenia and that coded other disorders as unknown. These are among the highest LOD scores reported, to date, for linkage to either schizophrenia or affective disorders.
Table 1.
Linkage of t(1;11) to Schizophrenia and Affective Disorders
|
LOD Score at θ = |
||||||
| Phenotypea | Gene Frequencyb | Penetrance | 0 | .05 | .1 | .2 |
| Model 1 | .004 | .2 | 3.6 | 3.3 | 3.0 | 2.3 |
| Model 2 | .03 | .3 | 4.5 | 4.0 | 3.6 | 2.7 |
| Model 3 | .03 | .5 | 7.1 | 6.6 | 5.9 | 4.6 |
| Model 4 | .1 | .6 | 1.9 | 2.5 | 2.6 | 2.4 |
Linkage analysis was performed by MLINK, under the assumption of dominant inheritance, for four definitions of the disease phenotype (Terwilliger and Ott 1994): model 1 = 7 cases of schizophrenia (all other diagnoses coded as unknown); model 2 = 11 cases of recurrent major depression and 1 case of bipolar disorder (schizophrenia and other diagnoses coded as unknown); model 3 = schizophrenia, recurrent major depression, and bipolar disorder; model 4 = all diagnoses, including alcoholism, minor depression, anxiety, and adolescent conduct-and-emotional disorder.
Gene frequencies were compatible with the assumption of a lifetime risk of .006 for schizophrenia or of .05 for major affective disorders; the translocation was assumed to be rare in the population, with frequency .00001.
Single-family studies are important for the identification of the group of psychoses in which single-gene loci may have a major effect on illness risk. However, findings may be unique to one family, and, because the translocation is rare, it is important to investigate possible phenotype differences between members of this family and unrelated individuals with either schizophrenia or affective disorders. Direct interviews as well as hospital and general-practice case-note reviews confirmed that the translocation was not associated with any physical or neurological illnesses, developmental abnormalities, or dysmorphisms, and the IQs of the family members, measured by the National Adult Reading Test, were within the normal range. There was no difference in the mean IQ of 12 relatives with the translocation (mean IQ 100.3) and 8 relatives with a normal karyotype (mean IQ 100.5). During the follow-up period, the auditory P300 ERP of 12 relatives with the translocation, 10 noncarriers, 20 unrelated subjects with schizophrenia, and 26 controls—all of age 18–60 years—was recorded, to compare the phenotypes in members of this family and unrelated subjects. Prolonged latency of and reduced amplitude of P300 ERP, which have consistently been found in patients with schizophrenia and in their relatives, are thought to indicate deficits in the speed and the efficiency of the processing of stimuli in short-term memory and may be considered trait markers of risk (Schreiber et al. 1992; Blackwood 2000). Changes in the latency and the amplitude of P300 are also found in patients with bipolar disorder (Muir et al. 1991), but there are differences between patients with schizophrenia and patients with affective disorders, in the topography of the amplitude reduction (Salisbury et al. 1999). P300 is normal in recurrent major depression, although, in families with multiple members with schizophrenia, relatives who have recurrent major depression may show the same P300 abnormalities as do their relatives with schizophrenia (Blackwood et al. 1991). As described by Souza et al. (1995), recordings were made from three midline sites (frontal, central, and parietal [Pz]) and left and right temporal sites (T3 and T4) by use of linked ear electrodes as a reference while subjects performed a two-tone–discrimination task. The electroencephalogram was amplified by 10,000× and had a bandwidth of 0.16–30 Hz (−3 dB), and data were sampled at a rate of 250 Hz by an analog-to-digital converter with 12-bit resolution. The data sweep was 244 ms prestimulus and 752 ms poststimulus. Tone pips of 20 ms duration at 1,000 Hz (frequent) and 1,500 Hz (rare), with a probability (frequent:rare) of 10:1, were delivered binaurally at 75 dB (sound-pressure level) and interstimulus interval of 1.1 s. Subjects were instructed to count silently the high-pitched tones. The P300 latency (in ms) was measured via the least-mean-squares (LMS) method (Glabus et al. 1994), and amplitude (in μV) was defined as the highest point in a predefined window (280–550 ms). The Pz site was chosen for all analyses. Table 2 shows that translocation carriers were similar to patients with schizophrenia but differed significantly from noncarriers and controls, in measures of P300 amplitude and of P300 latency. Analysis of P300 amplitude showed the following: controls > patients with schizophrenia, P=.007; controls > relatives with the translocation, P=.01; relatives with a normal karyotype > patients with schizophrenia, P=.007; and relatives with a normal karyotype > relatives with the translocation, P=.009. Analysis of P300 latency showed the following: controls < patients with schizophrenia, P=.02; and relatives with a normal karyotype < patients with schizophrenia, P=.007. P300 amplitudes <2 SD below the control mean were recorded in 7 of the 12 relatives with the translocation, and the diagnoses for these 7 subjects were depression (in three cases), schizophrenia (in two cases), and no symptoms (in two cases). Similarly, for P300 latency, the two translocation carriers with latency >2 SD above the control mean were a relative with no psychiatric diagnosis and a relative with schizophrenia. Significant changes in the amplitude and the latency of P300 in translocation carriers were therefore not restricted to subjects with a psychiatric diagnosis. The findings are consistent with previous findings in other families with multiple members with schizophrenia, in which the amplitude of and the latency of P300 appear to be trait markers of risk, rather than state markers reflecting the presence of symptoms (Blackwood et al. 1991; Sham et al. 1994).
Table 2.
P300 Latency and P300 Amplitude in the Family with the Translocation, in Patients with Schizophrenia, and in Controls[Note]
|
Mean (SD) of P300 |
||
| Group | Latency(ms) | Amplitude(μV) |
| Relatives: | ||
| With translocation (n=12) | 366.2 (41.8) | 6.8 (3.4) |
| With normal karyotype (n=10) | 338.3 (34.5) | 12.1 (3.5) |
| Patients with schizophrenia (n=20) | 391.3 (47.2) | 7.2 (4.0) |
| Controls (n=26) | 354.8 (26.5) | 11.0 (3.1) |
Note.— Results were determined by multivariate analysis of variance. There was a group effect for latency (F=5.62, df=3, P=.002) and for amplitude (F=8.62, df=3, P=.0001). Scheffe post hoc comparisons of means showed significant differences between groups, in P300 latency (controls < patients with schizophrenia, P=.02; relatives with a normal karyotype < patients with schizophrenia, P=.007) and in P300 amplitude (controls > patients with schizophrenia, P=.007; controls > relatives with the translocation, P=.01; relatives with a normal karyotype > patients with schizophrenia, P=.007; relatives with a normal karyotype > relatives with the translocation, P=.009).
We have described elsewhere two genes, disrupted in schizophrenia 1 (DISC1 [MIM 605210]) and disrupted in schizophrenia 2 (DISC2), that are directly disrupted by the breakpoint on chromosome 1, and a third gene, translin-associated factor X (TSNAX [MIM 602964]), that is in very close proximity (Millar et al. 2000). These and other functionally related genes are strong candidates for a role in the development of psychiatric disorders. There are no genes close to the breakpoint on 11q14.3, making this the less-likely site for genes contributing to psychosis in the family, although we cannot entirely rule out the possibility that translocation has long-range positional effects on gene expression.
Single large families with multiple affected members have important advantages for the study of genetically complex disorders such as the major psychoses, when substantial locus heterogeneity is probable. A disadvantage is that findings may be either rare or unique to one family and, thus, not relevant to the disease itself. There are, however, independent reports of suggestive linkage, for both schizophrenia and bipolar disorder, with markers mapped close to the breakpoint on 1q42. The strongest independent support for linkage to the 1q42 breakpoint region is from two studies of Finnish families with schizophrenia. A genomewide scan of 134 affected sibling pairs with schizophrenia revealed a maximum LOD score of 2.6 with the marker D1S439, which lies <500 kb from the translocation breakpoint (Ekelund et al. 2000). In a second study, of 20 families with schizophrenia that are from an isolated Finnish subpopulation, Hovatta et al. (1999) reported a maximum LOD score of 3.7 at 1q32-41, and there is overlap of the regions showing linkage in these two Finnish populations. Linkage between the microsatellite D1S103, which maps <1 Mb from the breakpoint, and bipolar disorder has been reported in two separate studies: Gejman et al. (1993) found suggestive linkage (LOD score 2.4) with D1S103 in 1 of 19 families with bipolar disorder, and LaBuda et al. (1996) reported increased allele sharing (P<.0001), under one weighting function, in Old Order Amish families with bipolar disorder. Detera-Wadleigh et al. (1999), reported, in a genomewide scan of 22 families with bipolar disorder, elevated identical-by-descent sharing (maximum LOD score 2.3) in a 30-cM region spanning 1q25-q42 and extending to the breakpoint region; it is noteworthy that, in 15 of these 22 families with bipolar disorder, at least one individual was diagnosed with either schizophrenia or schizoaffective disorder. The breakpoint on 1q42 is ∼60 cM from the locus on 1q21-q22, where linkage to schizophrenia has also been reported (Brzustowicz et al. 2000; Gurling et al. 2001), and this breakpoint appears to be a separate locus related to schizophrenia.
Some genetic risk factors for psychoses may contribute to a range of symptoms that cross traditional diagnostic boundaries, and the locus identified by the breakpoint on 1q42 appears to be implicated in families with either schizophrenia or bipolar disorder. The range of diagnoses recorded in the family with the translocation is found in other extended families with schizophrenia (St. Clair et al. 1989). Similarly, the observation that, in the family with the translocation, P300 amplitude and P300 latency are abnormal in individuals with psychoses and in their asymptomatic relatives is similar to the pattern of P300 changes in other families with multiple members with schizophrenia and may provide clues to the biological basis for the varied clinical presentations in these families. For example, reduction of P300 amplitude in patients with schizophrenia and in their relatives has been correlated with reduced regional perfusion, as measured by single-proton-emission computed tomography, in left-frontal regions (Blackwood et al. 1999) and with reduced gray-matter volume of the left-superior temporal gyrus, as measured by positron-emission tomography (O'Donnell et al. 1999). Genetic markers combined with other investigative approaches—including neurophysiology, brain imaging, and neuropsychology—may help to define subtypes of the psychoses.
Acknowledgments
This work was funded by grants from The Medical Research Council and from Akzo-Nobel (Organon). We are grateful for help received from colleagues in primary care, and, above all, we are indebted to members of the family, for their continuing support and participation in this long-term study.
Electronic-Database Information
The accession numbers and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for schizophrenia [MIM 181500], bipolar disorder [MIM 125480], DISC1 [MIM 605210], and TSNAX [MIM 602964])
References
- Berrettini WH (2000) Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry 47:245–251 [DOI] [PubMed] [Google Scholar]
- Blackwood D (2000) P300, a state and a trait marker in schizophrenia. Lancet 355:771–772 [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Glabus MF, Dunan J, O'Carroll RE, Muir WJ, Ebmeier KP (1999) Altered cerebral perfusion measured by SPECT in relatives of patients with schizophrenia: correlations with memory and P300. Br J Psychiatry 175:357–366 [DOI] [PubMed] [Google Scholar]
- Blackwood DH, St Clair DM, Muir WJ, Duffy JC (1991) Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry 48:899–909 [DOI] [PubMed] [Google Scholar]
- Brzustowicz LM, Hodgkinson KA, Chow EW, Honer WG, Bassett AS (2000) Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science 288:678–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detera-Wadleigh SD, Badner JA, Berrettini WH, Yoshikawa T, Goldin LR, Turner G, Rollins DY, Moses T, Sanders AR, Karkera JD, Esterling LE, Zeng J, Ferraro TN, Guroff JJ, Kazuba D, Maxwell ME, Nurnberger JI Jr, Gershon ES (1999) A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc Natl Acad Sci USA 96:5604–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund J, Lichtermann D, Hovatta I, Ellonen P, Suvisaari J, Terwilliger JD, Juvonen H, Varilo T, Arajarvi R, Kokko-Sahin ML, Lonnqvist J, Peltonen L (2000) Genome-wide scan for schizophrenia in the Finnish population: evidence for a locus on chromosome 7q22. Hum Mol Genet 9:1049–1057 [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL (1978) A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 35:837–844 [DOI] [PubMed] [Google Scholar]
- Gejman PV, Martinez M, Cao Q, Friedman E, Berrettini WH, Goldin LR, Koroulakis P, Ames C, Lerman MA, Gershon ES (1993) Linkage analysis of fifty-seven microsatellite loci to bipolar disorder. Neuropsychopharmacology 9:31–40 [DOI] [PubMed] [Google Scholar]
- Glabus MF, Blackwood DH, Ebmeier KP, Souza V, Walker MT, Sharp CW, Dunan JT, Muir WJ (1994) Methodological considerations in measurement of the P300 component of the auditory oddball ERP in schizophrenia. Electroencephalogr Clin Neurophysiol 90:123–134 [DOI] [PubMed] [Google Scholar]
- Gurling HM, Kalsi G, Brynjolfson J, Sigmundsson T, Sherrington R, Mankoo BS, Read T, Murphy P, Blaveri E, McQuillin A, Petursson H, Curtis D (2001) Genomewide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32.2, 5q33.2, and 8p21-22 and provides support for linkage to schizophrenia, on chromosomes 11q23.3-24 and 20q12.1-11.23. Am J Hum Genet 68:661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta I, Varilo T, Suvisaari J, Terwilliger JD, Ollikainen V, Arajarvi R, Juvonen H, Kokko-Sahin ML, Vaisanen L, Mannila H, Lonnqvist J, Peltonen L (1999) A genomewide screen for schizophrenia genes in an isolated Finnish subpopulation, suggesting multiple susceptibility loci. Am J Hum Genet 65:1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs PA, Brunton M, Frackiewicz A, Newton M, Cook PJL, Robson EB (1970) Studies on a family with three cytogenetic markers. Ann Hum Genet (Lond) 33:325–336 [Google Scholar]
- LaBuda MC, Maldonado M, Marshall D, Otten K, Gerhard DS (1996) A follow-up report of a genome search for affective disorder predisposition loci in the Old Order Amish. Am J Hum Genet 59:1343–1362 [PMC free article] [PubMed] [Google Scholar]
- Lopez AD, Murray CC (1998) The global burden of disease, 1990–2020. Nat Med 4:1241–1243 [DOI] [PubMed] [Google Scholar]
- Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O, Levinson DF (1993) Continuity and discontinuity of affective disorders and schizophrenia: results of a controlled family study. Arch Gen Psychiatry 50:871–883 [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, Arveiler B, Muir WJ, Blackwood DHR, Porteous DJ (2000) Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 9:1415–1423 [DOI] [PubMed] [Google Scholar]
- Muir WJ, St Clair DM, Blackwood DH (1991) Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med 21:867–879 [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, Niznikiewicz MA, Barnard J, Shen ZJ, Weinstein DM, Bookstein FL, Shenton ME (1999) Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology 36:388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potash JB, DePaulo JR Jr (2000) Searching high and low: a review of the genetics of bipolar disorder. Bipolar Disord 2:8–26 [DOI] [PubMed] [Google Scholar]
- Riley BP, McGuffin P (2000) Linkage and associated studies of schizophrenia. Am J Med Genet 97:23–44 [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW (1999) P300 topography differs in schizophrenia and manic psychosis. Biol Psychiatry 45:98–106 [DOI] [PubMed] [Google Scholar]
- Schreiber H, Stolz-Born G, Kornhuber HH, Born J (1992) Event-related potential correlates of impaired selective attention in children at high risk for schizophrenia. Biol Psychiatry 32:634–651 [DOI] [PubMed] [Google Scholar]
- Sham PC, Morton NE, Muir WJ, Walker M, Collins A, Shields DC, St Clair DM, Blackwood DHR (1994) Segregation analysis of complex phenotypes: an application to schizophrenia and auditory P300 latency. Psychiatr Genet 4:29–38 [PubMed] [Google Scholar]
- Souza VB, Muir WJ, Walker MT, Glabus MF, Roxborough HM, Sharp CW, Dunan JR, Blackwood DHR (1995) Auditory P300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biol Psychiatry 37:300–310 [DOI] [PubMed] [Google Scholar]
- St Clair DM, Blackwood DHR, Muir WJ, Baillie D, Hubbard A, Wright A, Evans HJ (1989) No linkage of chromosome 5q11-13 markers to schizophrenia in Scottish families. Nature 339:305–309 [DOI] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ (1990) Association within a family of a balanced autosomal translocation with major mental illness. Lancet 336:13–16 [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Ott J (1994) Handbook of human genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- Weisbrod M, Hill H, Niethammer R, Sauer H (1999) Genetic influence on auditory information processing in schizophrenia: P300 in monozygotic twins. Biol Psychiatry 46:721–725 [DOI] [PubMed] [Google Scholar]
- Wildenauer DB, Schwab SG, Maier W, Detera-Wadleigh SD (1999) Do schizophrenia and affective disorder share susceptibility genes? Schizophr Res 39:107–111 [DOI] [PubMed] [Google Scholar]