Abstract
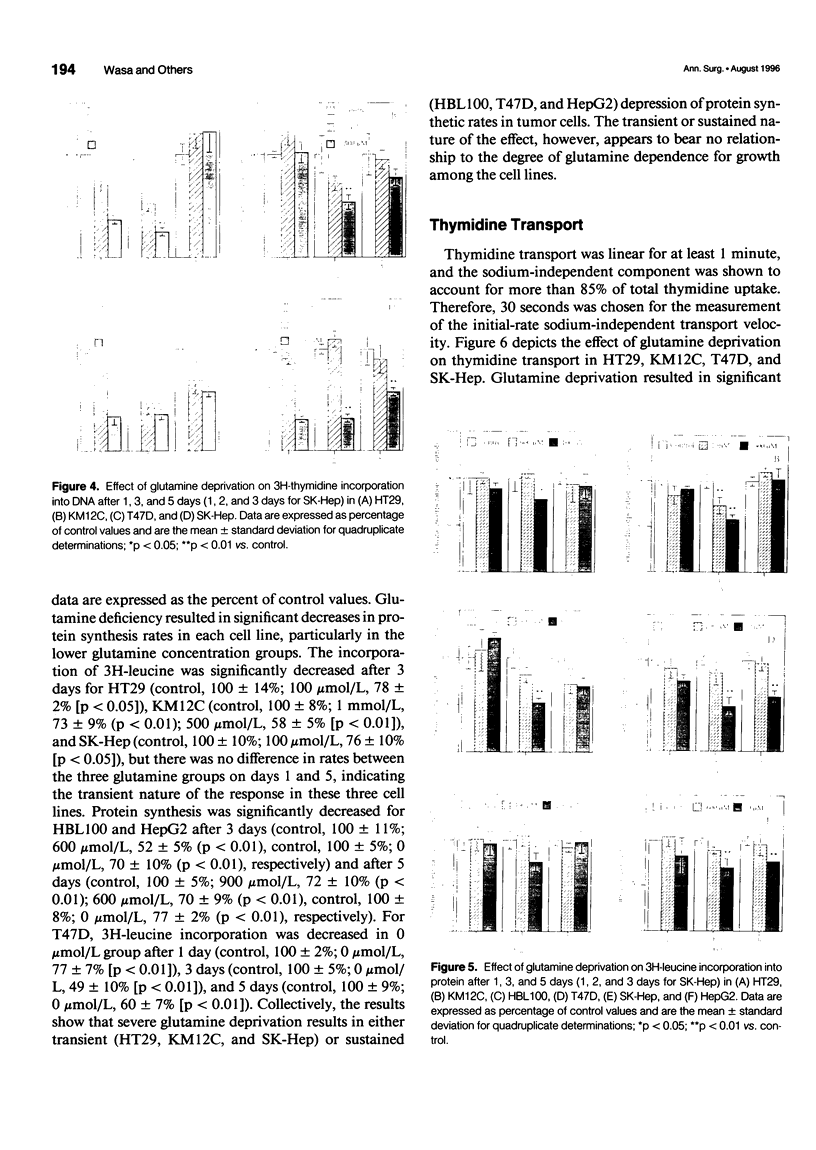
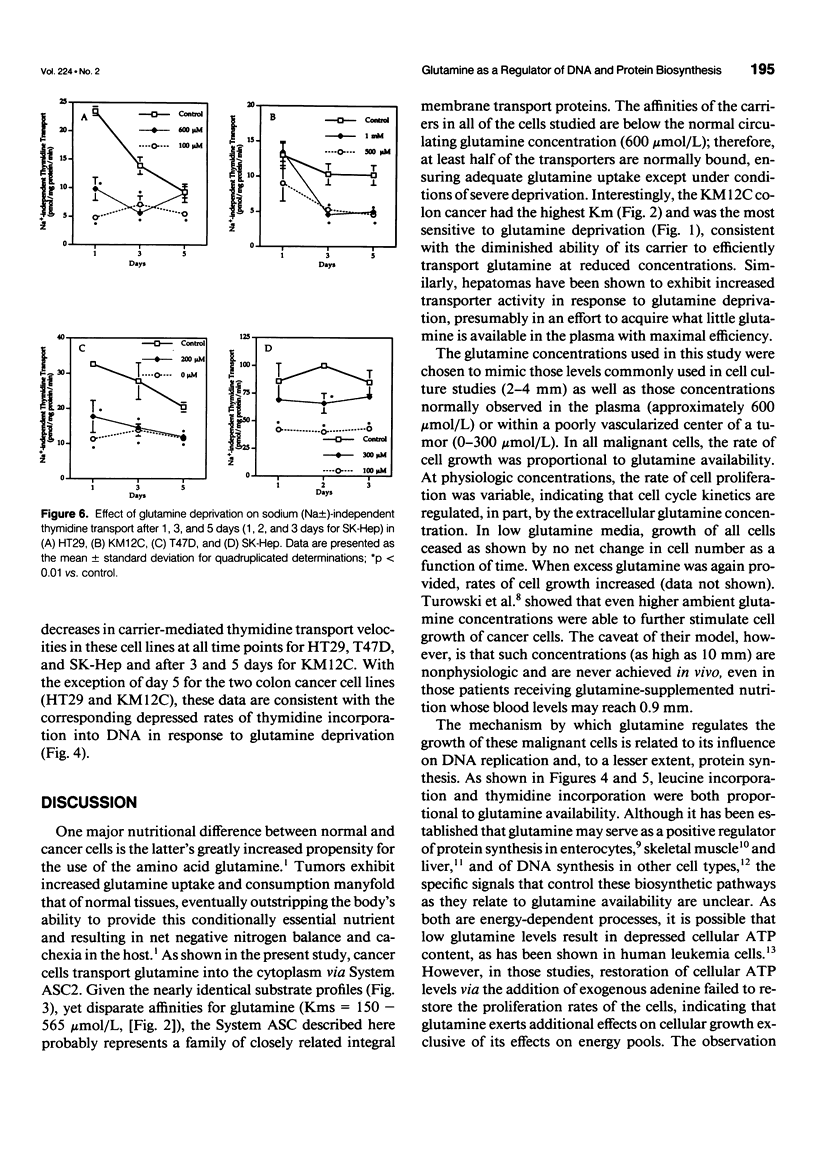
OBJECTIVE: The transport of glutamine by six different human solid tumor-derived cell lines (e.g., breast, colon, liver) was characterized and the impact of glutamine deprivation on rates of tumor cell proliferation and DNA and protein synthesis was assayed. SUMMARY BACKGROUND DATA: Glutamine is added routinely to cell culture media and its importance for cellular growth has been established. However, carrier-mediated glutamine transport by solid tumors has not been studied extensively, and the mechanisms by which glutamine contributes to cell growth regulation require further investigation. METHODS: In a panel of different human solid tumor-derived cells, sodium-dependent glutamine transport was characterized in vitro and rates of cell proliferation, protein and DNA synthesis, as well as thymidine transport, were correlated with glutamine concentrations in the culture media. RESULTS: In all cells, regardless of tissue origin, sodium-dependent glutamine transport was mediated almost exclusively by a single carrier. There was a range of Michaelis constants (Km) and maximal transport velocities (Vmax) for the glutamine transporter in each cell type, but the amino acid inhibition profiles were nearly identical, consistent with uptake by the System ASC family of transporters. Rates of cell growth, DNA and protein synthesis, and thymidine transport correlated with the glutamine concentration in the culture media, indicating the central role of this amino acid in regulating cellular proliferation. CONCLUSIONS: These data indicate that glutamine transport by all solid tumors is mediated by the System ASC family of transporters. The variation in Km values suggests that some cancers may be better suited to survive in a low glutamine environment than others. The mechanism by which glutamine supports cell proliferation and regulates cell cycle kinetics involves its modulation of DNA and protein biosynthetic rates.
Full text
PDF


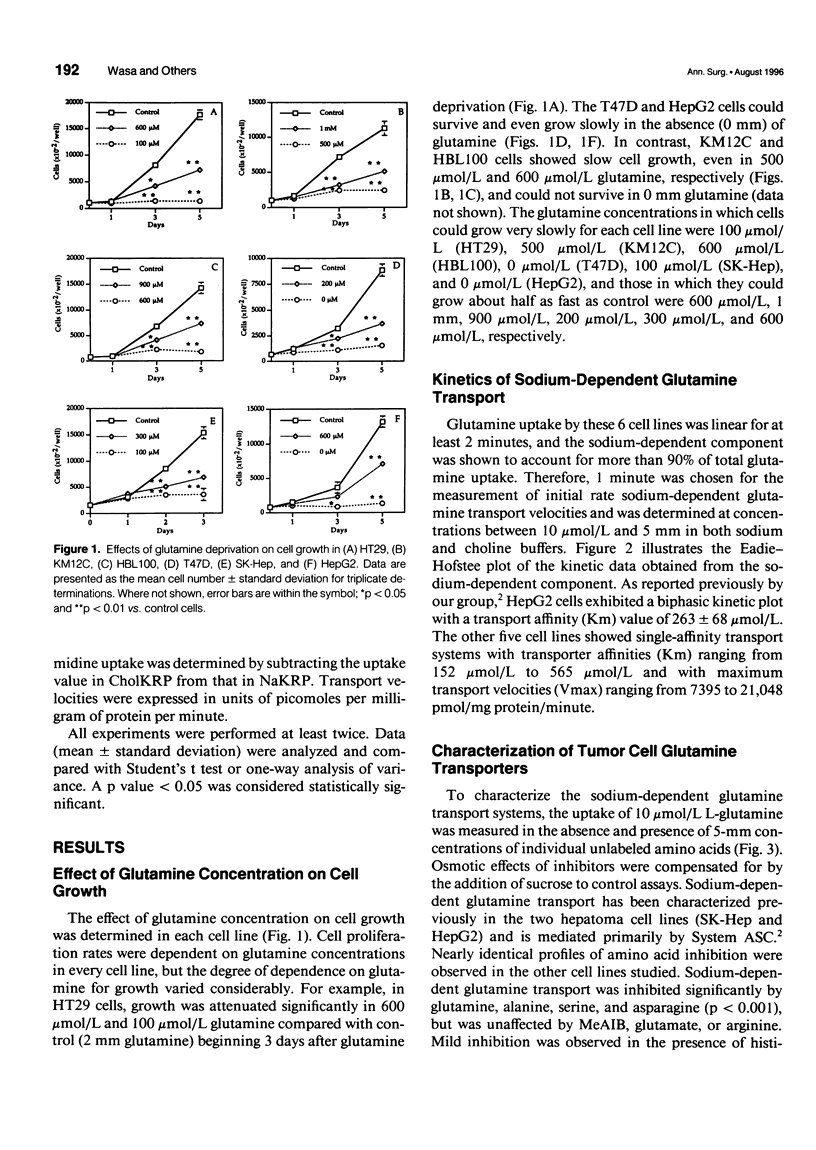
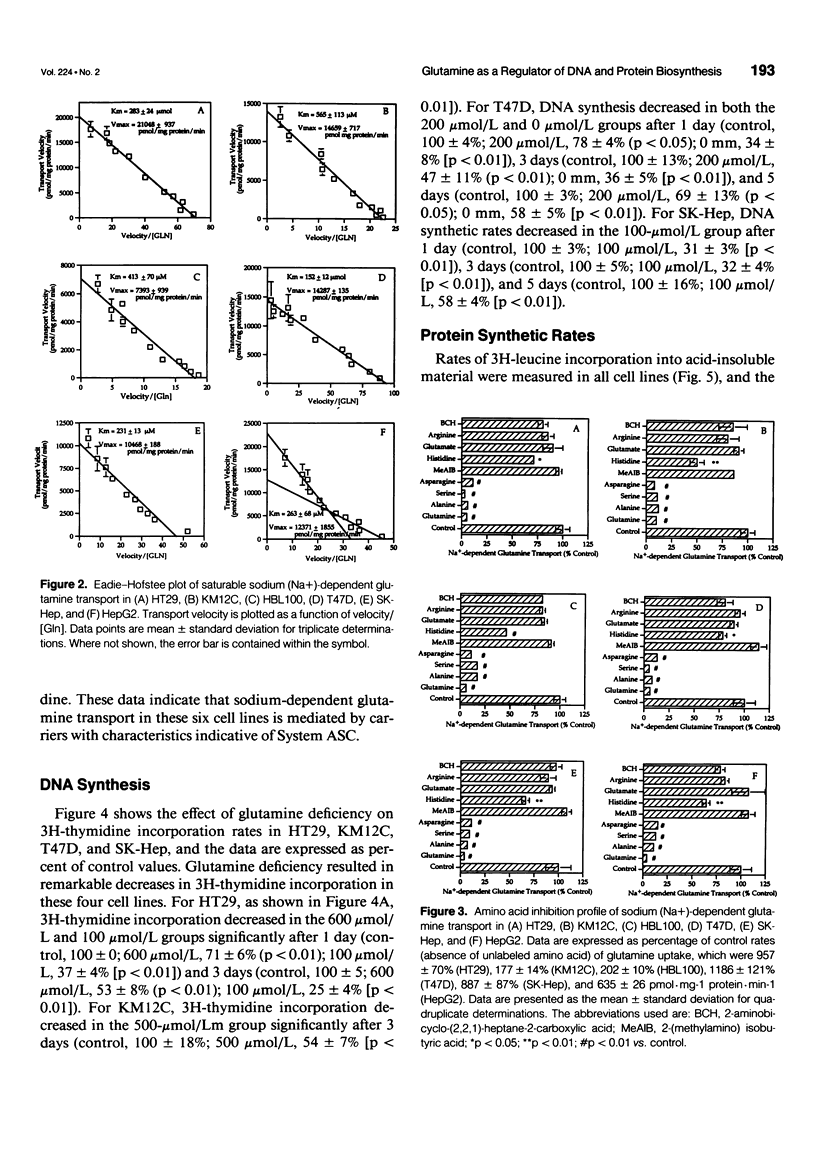


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austgen T. R., Dudrick P. S., Sitren H., Bland K. I., Copeland E., Souba W. W. The effects of glutamine-enriched total parenteral nutrition on tumor growth and host tissues. Ann Surg. 1992 Feb;215(2):107–113. doi: 10.1097/00000658-199202000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode B. P., Kaminski D. L., Souba W. W., Li A. P. Glutamine transport in isolated human hepatocytes and transformed liver cells. Hepatology. 1995 Feb;21(2):511–520. [PubMed] [Google Scholar]
- Cai J. W., Hughes C. S., Shen J. W., Subjeck J. R. Induction of heat-shock proteins by glutamine. The 'feeding effect'. FEBS Lett. 1991 Aug 19;288(1-2):229–232. doi: 10.1016/0014-5793(91)81041-6. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Fischer J. E., Chance W. T. Total parenteral nutrition, glutamine, and tumor growth. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):86S–89S. doi: 10.1177/0148607190014004101. [DOI] [PubMed] [Google Scholar]
- Gammon M. T., Isselbacher K. J. Neoplastic potentials and regulation of uptake of nutrients. I. A glutamine independent variant of polyoma BHK with A very high neoplastic potential. J Cell Physiol. 1976 Dec;89(4):759–764. doi: 10.1002/jcp.1040890438. [DOI] [PubMed] [Google Scholar]
- Higashiguchi T., Hasselgren P. O., Wagner K., Fischer J. E. Effect of glutamine on protein synthesis in isolated intestinal epithelial cells. JPEN J Parenter Enteral Nutr. 1993 Jul-Aug;17(4):307–314. doi: 10.1177/0148607193017004307. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Hutson R. G., Laine R. O. Amino acid-regulated gene expression in eukaryotic cells. FASEB J. 1994 Jan;8(1):13–19. doi: 10.1096/fasebj.8.1.8299885. [DOI] [PubMed] [Google Scholar]
- Kitoh T., Kubota M., Takimoto T., Hashimoto H., Shimizu T., Sano H., Akiyama Y., Mikawa H. Metabolic basis for differential glutamine requirements of human leukemia cell lines. J Cell Physiol. 1990 Apr;143(1):150–153. doi: 10.1002/jcp.1041430120. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Nwokedi E., Hutchins L. F., Pappas A. A., Lang N. P., Broadwater J. R., Read R. C., Westbrook K. C. Glutamine facilitates chemotherapy while reducing toxicity. JPEN J Parenter Enteral Nutr. 1992 Nov-Dec;16(6 Suppl):83S–87S. doi: 10.1177/014860719201600609. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Pappas A. A., Nwokedi E., Jensen J. C., Broadwater J. R., Lang N. P., Westbrook K. C. Effect of supplemental dietary glutamine on methotrexate concentrations in tumors. Arch Surg. 1992 Nov;127(11):1317–1320. doi: 10.1001/archsurg.1992.01420110063013. [DOI] [PubMed] [Google Scholar]
- MacLennan P. A., Brown R. A., Rennie M. J. A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. FEBS Lett. 1987 May 4;215(1):187–191. doi: 10.1016/0014-5793(87)80139-4. [DOI] [PubMed] [Google Scholar]
- Manchester K. L., Tyobeka E. M. Influence of individual amino acids on incorporation of [14C]leucine by rat liver ribosomes. J Nutr. 1980 Feb;110(2):241–247. doi: 10.1093/jn/110.2.241. [DOI] [PubMed] [Google Scholar]
- Medina M. A., Sánchez-Jiménez F., Márquez J., Rodríguez Quesada A., Núez de Castro I. Relevance of glutamine metabolism to tumor cell growth. Mol Cell Biochem. 1992 Jul 6;113(1):1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- Poueymirou W. T., Conover J. C., Schultz R. M. Regulation of mouse preimplantation development: differential effects of CZB medium and Whitten's medium on rates and patterns of protein synthesis in 2-cell embryos. Biol Reprod. 1989 Aug;41(2):317–322. doi: 10.1095/biolreprod41.2.317. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Szondy Z., Newsholme E. A. The effect of various concentrations of nucleobases, nucleosides or glutamine on the incorporation of [3H]thymidine into DNA in rat mesenteric-lymph-node lymphocytes stimulated by phytohaemagglutinin. Biochem J. 1990 Sep 1;270(2):437–440. doi: 10.1042/bj2700437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski G. A., Rashid Z., Hong F., Madri J. A., Basson M. D. Glutamine modulates phenotype and stimulates proliferation in human colon cancer cell lines. Cancer Res. 1994 Nov 15;54(22):5974–5980. [PubMed] [Google Scholar]
- Viallard V., Denis C., Trocheris V., Murat J. C. Effect of glutamine deprivation and glutamate or ammonium chloride addition on growth rate, metabolism and differentiation of human colon cancer cell-line HT29. Int J Biochem. 1986;18(3):263–269. doi: 10.1016/0020-711x(86)90116-3. [DOI] [PubMed] [Google Scholar]
- WU C., BAUER J. M. A study of free amino acids and of glutamine synthesis in tumor-bearing rats. Cancer Res. 1960 Jul;20:848–857. [PubMed] [Google Scholar]
- Wasa M., Bode B. P., Souba W. W. Adaptive regulation of amino acid transport in nutrient-deprived human hepatomas. Am J Surg. 1996 Jan;171(1):163–169. doi: 10.1016/S0002-9610(99)80093-2. [DOI] [PubMed] [Google Scholar]
- Zetterberg A., Engström W. Glutamine and the regulation of DNA replication and cell multiplication in fibroblasts. J Cell Physiol. 1981 Sep;108(3):365–373. doi: 10.1002/jcp.1041080310. [DOI] [PubMed] [Google Scholar]