Unsubstantiated, false, or misleading health claims can lead consumers to use products not tested for safety and effectiveness. This paper highlights misleading cessation claims for two products: KickNic, a disposable vaping device containing a “nicotine substitute,” and YELLO, a nicotine pouch.1,2 Both manufacturers violate the Federal Food, Drug, and Cosmetic Act (FDCA) by making misleading therapeutic claims. In the United States (US), the Food and Drug Administration’s (FDA’s) Center for Drug Evaluation and Research (CDER) oversees therapeutic claims.3,4 Drugs must demonstrate safety and effectiveness—in this case, as nicotine cessation aids—for CDER approval.3,4 Alternatively, the FDA’s Center for Tobacco Products (CTP) authorizes reduced risk claims for commercial tobacco and nicotine products.5,6 No e-cigarettes or nicotine pouches have been authorized to make reduced risk claims or approved as cessation aids by the FDA.7–9
KickNic resembles a disposable e-cigarette but contains “NoNic6,” which is described as a “non-nicotine compound that provides users an experience similar to traditional nicotine liquids.”10 Since KickNic does not contain nicotine, it does not meet the federal definition of a tobacco product and is outside of CTP’s jurisdiction.11 KickNic’s “Vape Kit” includes four devices with different NoNic6 concentrations, which the company claims helps consumers “quit nicotine in 4 weeks” by “gradually reduc[ing] the nicotine substitute” (Figures 1 and 2).10,12 Because CDER has not approved KickNic as a cessation aid, these claims violate the FDCA.3,4 Additionally, little is known about the safety and effects of NoNic6 and other substances marketed as nicotine substitutes (e.g., nicotine analogs, nicotinamide) that are increasingly common in e-cigarettes and oral pouch products, and CTP’s lack of authority over these products raises concerns about product safety13–15 While unauthorized cessation claims for e-cigarettes are well-documented, the extent to which vape products containing “nicotine substitutes” make similar claims is unclear.16,17
Figure 1:

Screenshots of questions and answers from KickNic’s website (December 5, 2024). Highlighting added by the authors to point out explicit cessation claims.
Figure 2:

A KickNic Vape Kit in the Cool Mint flavor option, with a red box added by the authors to highlight an explicit cessation claim (“to help you quit”). SFN (underlined in red by the authors) stands for “substitute for nicotine” (referring to the NoNic6 in the product, which the company describes as a “non-nicotine compound that provides users an experience similar to traditional nicotine liquids”). Image obtained from the Midwest Goods, Inc. website (a vape wholesaler website).
YELLO—a global nicotine pouch brand present in the US and United Kingdom—comes in two strengths and five flavors (Figure 3).2 YELLO contains nicotine, subjecting it to CTP’s jurisdiction; however, YELLO has not been authorized by CTP to be marketed in the US.7 YELLO’s website violates the FDCA by explicitly referring to YELLO nicotine pouches as nicotine replacement therapy (NRT) and “stop smoking products” that are “clinically proven to be effective in managing withdrawal symptoms and smoking cravings” (Figure 4).2,18,19 YELLO’s website claims their products are “safe and secure options approved by the Medical Product Agency,” likely referring to Sweden’s Medical Products Agency.18–20 While it is unclear if YELLO pouches are approved in Sweden, CDER has not approved YELLO as a safe and effective NRT.7–9 Although unauthorized cessation claims for nicotine pouches have been observed, such claims are typically more implicit (e.g., “alternatives” to other tobacco products), while explicit cessation claims—such as those on YELLO’s website—are infrequent.21
Figure 3:
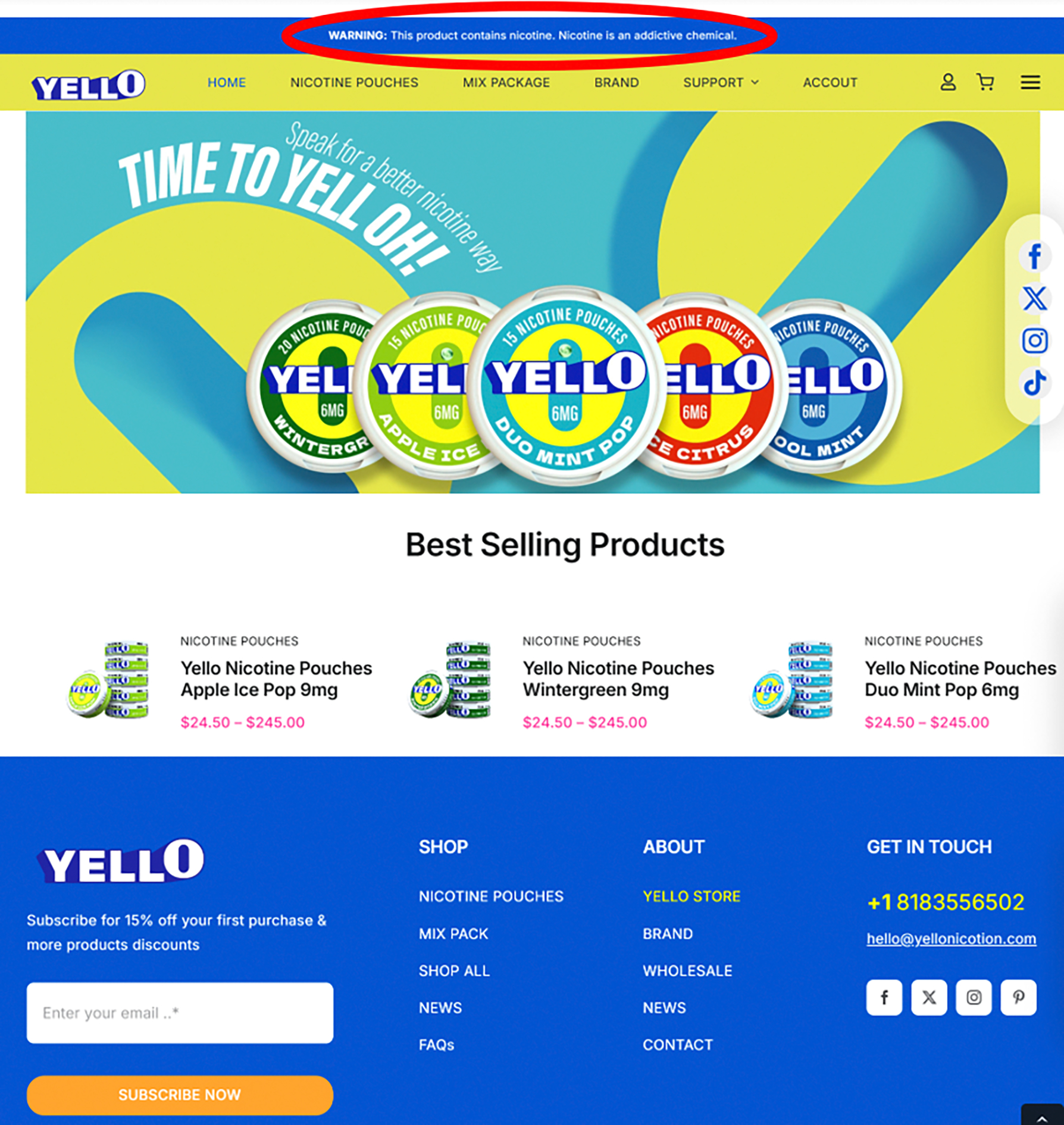
Screenshot showing portions of the yellonicotine.com homepage (April 17, 2025) that suggest this is intended to be a US-facing website: nicotine addiction warning (circled in red by the authors), USD currency, and a US-based phone number. This product is not authorized by the US FDA to be marketed in the US.
Figure 4:

YELLO FAQ’s shown under product listings and on the Contact page of yellonicotine.com. Highlighting added by the authors to point out explicit cessation claims; underlining added by the authors to show errors indicating that YELLO’s FAQ content was copied from Zonnic’s website (i.e., references to Zonnic and to nicotine sprays, which YELLO does not carry).
Both KickNic and YELLO make numerous misleading and unsubstantiated claims that violate the FDCA. Absent legal consequences, such conduct may become pervasive, potentially exposing consumers to health risks and leading them astray from evidence-based treatment. Public health researchers and professionals can support the FDA’s enforcement efforts by monitoring and reporting such conduct. State and local law enforcement authorities also have the authority to protect public health and support the FDA. For example, given KickNic and YELLO’s unauthorized claims, state Attorneys General offices may find sufficient cause to file a civil lawsuit based on violations of the FDCA, consumer protection laws, or other courses of action. Furthermore, products containing nicotine analogs are currently outside of CTP’s regulatory authority. Recognizing this, California expanded its jurisdiction to cover nicotine analog products.22 Congress can similarly expand CTP’s authority, as it did when synthetic nicotine emerged.
Funding:
This work was funded in part by grants U01CA278695 and U54CA229973 from the National Cancer Institute and the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official view of NIH or FDA.
Footnotes
Competing interests: The authors have no competing interests to declare.
REFERENCES
- 1.KickNic. Accessed December 19, 2024. https://www.kicknicvapekit.com [Google Scholar]
- 2.YELLO. 2025. Accessed January 8, 2025. https://yellonicotine.com/ [Google Scholar]
- 3.New Drugs, 21 USC §355 (2023). Accessed January 10, 2025. https://www.govinfo.gov/content/pkg/USCODE-2023-title21/html/USCODE-2023-title21-chap9-subchapV-partA-sec355.htm [Google Scholar]
- 4.Prohibited Acts, 21 USC §331 (2023). Accessed January 10, 2025. https://www.govinfo.gov/content/pkg/USCODE-2023-title21/html/USCODE-2023-title21-chap9-subchapIII-sec331.htm [Google Scholar]
- 5.Application for Review of Certain Tobacco Products, 21 USC §387j (2023). Accessed January 10, 2025. https://www.govinfo.gov/content/pkg/USCODE-2023-title21/html/USCODE-2023-title21-chap9-subchapIX-sec387j.htm [Google Scholar]
- 6.United States Food and Drug Administration. Premarket tobacco product applications. March 27, 2024. Accessed December 19, 2024. https://www.fda.gov/tobacco-products/market-and-distribute-tobacco-product/premarket-tobacco-product-applications [Google Scholar]
- 7.United States Food and Drug Administration. Searchable tobacco products database. Accessed December 19, 2024. https://www.accessdata.fda.gov/scripts/searchtobacco/ [Google Scholar]
- 8.United States Food and Drug Administration. National drug code directory. Accessed December 19, 2024. https://dps.fda.gov/ndc [Google Scholar]
- 9.United States Food and Drug Administration. Drugs@FDA: FDA-approved drugs. Accessed December 19, 2024. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process [Google Scholar]
- 10.What is KickNic? Accessed December 19, 2024. https://www.kicknicvapekit.com/what-is-kicknic [Google Scholar]
- 11.Tobacco Products Subject to FDA Authority, Definitions. 21 CFR §1100.3 (2024). Accessed January 10, 2025. https://www.govinfo.gov/content/pkg/CFR-2024-title21-vol8/pdf/CFR-2024-title21-vol8-sec1100-3.pdf [Google Scholar]
- 12.Midwest Goods, Inc. KickNic 5K 9ML SFN nicotine free disposable vape device. 2024. Accessed December 19, 2024. https://www.midwestgoods.com/kicknic-5k-9ml-sfn-nicotine-free-disposable-vape-device-display-of-4-msrp-39-99-each/ [Google Scholar]
- 13.Erythropel HC, Jabba SV, Silinski P, et al. Variability in constituents of e-cigarette products containing nicotine analogues. JAMA. 2024;332(9):753–755. doi: 10.1001/jama.2024.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordt SE, Jabba SV. Introduction of nicotine analogue-containing oral pouch products in the United States. Tob Prev Cessat. 2024;10:10.18332/tpc/195621. doi: 10.18332/tpc/195621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabba SV, Jordt SE. Marketing of nicotinamide as nicotine replacement in electronic cigarettes and smokeless tobacco. Tob Prev Cessat. 10:35. doi: 10.18332/tpc/187767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein EG, Berman M, Hemmerich N, Carlson C, Htut S, Slater M. Online e-cigarette marketing claims: A systematic content and legal analysis. Tob Regul Sci. 2016;2(3):252–262. doi: 10.18001/TRS.2.3.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyu JC, Huang P, Jiang N, Ling PM. A systematic review of e-cigarette marketing communication: Messages, communication channels, and strategies. Int J Environ Res Public Health. 2022;19(15):9263. doi: 10.3390/ijerph19159263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.YELLO. Yello nicotine pouches appleice pop 9mg. 2025. Accessed January 8, 2025. https://yellonicotine.com/product/yello-nicotine-pouches-apple-ice-pop-9mg/ [Google Scholar]
- 19.YELLO. Contact. 2025. Accessed January 8, 2025. https://yellonicotine.com/contact/ [Google Scholar]
- 20.Government Offices of Sweden. Medical Products Agency (Läkemedelsverket, LV). Accessed December 19, 2024. https://www.government.se/government-agencies/medical-products-agency-lakemedelsverket-lv/ [Google Scholar]
- 21.Czaplicki L, Tfayli D, Spindle TR, et al. Content analysis of marketing features in US nicotine pouch ads from 2021 to 2023. Tob Control. Published online December 3, 2024:tc-2024–059010. doi: 10.1136/tc-2024-059010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cal. Health & Safety Code §104559.5 (West, Westlaw through Ch. 1017 of 2024 Reg. Sess.). [Google Scholar]


