Abstract
Background and Objective
Denosumab is a fully human monoclonal antibody (IgG2) k subclass that targets and binds with high affinity and specificity to receptor activator of nuclear factor-κB ligand (RANKL). Gedeon Richter’s denosumab RGB-14-P and RGB-14-X are proposed biosimilar drug products to the reference medicinal products Prolia® and Xgeva® (marketing authorisation holder: Amgen Europe B.V. in the European Union [EU] and Amgen Inc. in USA, respectively). The present study demonstrates the structural, physico-chemical and functional similarity between RGB-14 and reference drug products marketed in the EU and US.
Methods
Using an extensive, state-of-the-art analytical and functional panel of 38 methods ensured the comprehensive characterisation of the biosimilar and reference drug products. To assess biosimilarity, physico-chemical and biological functional tests were performed using multiple orthogonal techniques, in addition to the in-depth comparison of the primary and higher-order structures of the therapeutic proteins.
Results
It has been demonstrated that the primary and higher order structures of RGB-14-P and RGB-14-X drug products are identical or highly similar to those of EU/US Prolia® and Xgeva®. The purity profiles of the biosimilar and reference products were similar. Only minor differences were observed in glycosylation patterns and charge variant profiles. A wide range of bioassays was used demonstrating similarity in terms of potency, ligand and receptor binding. Additionally, during comprehensive analysis of the reference product data as the function of expiry dates, shifts were revealed in certain quality parameters, although these did not impact the biological activity of the products.
Conclusion
The extensive analytical and functional similarity assessment study provides robust evidence that the structure and function of RGB-14-P and RGB-14-X are highly similar to those of EU/US Prolia® and Xgeva®.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40259-025-00738-w.
Key Points
| A comprehensive and detailed analytical and functional similarity study was carried out for Gedeon Richter’s biosimilar denosumab RGB-14-P and RGB-14-X products against the EU- and US-licensed reference drug products Prolia® and Xgeva®. |
| On the basis of the comparative assessment, RGB-14-P versus Prolia® and RGB-14-X versus Xgeva® are highly similar drug products. |
Introduction
RGB-14-P and RGB-14-X are proposed biosimilar drug products to the denosumab reference medicinal products Prolia® and Xgeva®. Denosumab is a fully human monoclonal antibody (immunoglobulin [Ig]G2-subclass k-subclass) that binds to the receptor activator of nuclear factor-κB ligand (RANKL). RANKL induces osteoclastogenesis by binding with the receptor activator of nuclear factor-κB (RANK) expressed in bone marrow macrophages [1]. Denosumab inhibits osteoclastogenesis by decreasing RANKL/RANK binding, thus reducing bone resorption [2]. Denosumab was approved by the European Medicines Agency and U.S. Food and Drug Administration as Prolia® [3, 4] for the treatment of postmenopausal osteoporosis in women, and as Xgeva® for the treatment of giant cell tumour [5, 6].
A biosimilar medicinal product is a highly similar version of the already authorised reference product in terms of structural, functional and clinical characteristics. The primary goal in biosimilar development is to ensure a high degree of similarity to the reference product, particularly in physico-chemical and functional properties. A comprehensive similarity study covering all structural, physico-chemical and biological-functional critical quality attributes of the active pharmaceutical ingredient should be conducted to assess biosimilarity.
The basic principles of biosimilar development should be compliant with the US [7] and EU [8–10] biosimilar guidelines. To align with the totality-of-evidence approach, it is essential to define Critical Quality Attributes (CQAs) in accordance with the requirements of ICH guidance documents (ICHQ8–11) [11–14], which outline the principles of pharmaceutical development relevant to Quality by Design (QbD). A fundamental aspect of biosimilar development involves the comprehensive characterisation of multiple batches of the reference products to establish quality ranges (QR).
The current biosimilarity study focuses on the analytical and functional similarity evaluation of RGB-14-P (proposed biosimilar to Prolia®), RGB-14-X (proposed biosimilar to Xgeva®) and Prolia®, Xgeva® reference drug products. Additionally, the study includes an evaluation of the distribution of the reference product quality data as a function of expiry dates.
Materials and Methods
Evaluation Strategy for the Determination of Quality Ranges (QR)
In the final similarity study, a total of 49 EU Prolia®, 34 US Prolia®, 40 EU Xgeva® and 25 US Xgeva® batches were analysed alongside 8 RGB-14-P and 6 RGB-14-X drug product batches. These RGB-14 batches were produced from independent drug substance batches using the final commercial process and scale. QR were determined by measuring the reference product batches over several years using available analytical and functional methods.
Compared with the biosimilarity literature [15–21], an outstanding number of methods was used. The descriptions of the major and critical methods and the obtained results are given in this publication, whilst the remaining descriptions and data are provided in the Supplementary Material in Table 1. For the quantitative results, the QR calculation was based on the formula mean ± X × SD (standard deviation of the method), determined from the results of the analysis of available EU and US Prolia®, Xgeva® batches. For the EU market, a fixed standard deviation multiplier X = 3 was applied (i.e. mean ± 3 × SD) across all quantitative methods. In contrast, for US Prolia® and Xgeva®, a more rigorous range approach was used with parameter-specific standard deviation multipliers according to FDA requirements [7]. The value of ‘X’ was determined on the basis of the criticality of the quality attribute, the specificity of the parameter (how specific is the evaluated parameter for the attribute being considered) and the precision of the test method (RSD%). The ratings can be high, moderate or low, and scores between 2 and 3 were assigned to them according to Table 1. The precision of the method parameters was considered according to the RSD% values derived from the intermediate precision results of the method qualification or validation. The method precision is considered ‘high’ if the RSD% of the parameter is ≤ 1.0%, ‘moderate’ if it is 1.0–5.0% and ‘low’ if it is ≥ 5.0%. The contributions of each factor are considered independently: (a) methods with lower precision (i.e. high SD) were assigned a lower multiplier (to avoid excessive widening of the QR), (b) quality attributes with higher criticality were assigned a lower factor (to ensure a tighter range) and (c) low parameter specificity was assigned a higher factor. The final score (proposed QR multiplier) of a parameter was calculated as a weighted linear combination of the scores related to the three factors. Factor weights are defined in Table 1.
Table 1.
Factor ratings, corresponding scores and factor weightings
| Factor | Precision | Criticality | Specificity |
|---|---|---|---|
| Rating | |||
| High | 3.0 | 2.0 | 2.0 |
| Moderate | 2.5 | 2.5 | 2.5 |
| Low | 2.0 | 3.0 | 3.0 |
| Weighting | 1.5 | 3.0 | 0.5 |
The determination of the quality ranges was performed using a rigorous range approach and the quantitative results of the measurements were compared with the quality ranges obtained with the parameter-specific standard deviation multipliers as described above (specific multiplier values are listed in Supplementary Table 2).
Definitions for the evaluation of quantitative parameters were the following: highly similar, if more than 90% of the RGB-14 batches fall within the QR; similar, if at least 50% of the RGB-14 batches are within the QR, though some may fall outside; and different, if more than 50% of the RGB-14 batches fall outside the QR.
The results of structural characterisation and identity methods were evaluated either through parallel analyses or by comparison with theoretical values. No multiplier was defined for negative functional assays, certain biophysical and sub-visible particle methods.
Materials
The RGB-14-P and Prolia® drug products are available as 60 mg/mL concentrate for solution for injection in pre-filled syringe, whilst RGB-14-X and Xgeva® drug products as 120 mg/1.7 mL concentrate for solution for injection in vial. Prolia® contains 17 mM acetate and 4.7% sorbitol, whilst Xgeva® has 18 mM acetate and 4.6% sorbitol [3–6]. Gedeon Richter applied the same Xgeva® formulation buffer composition for both RGB-14-P and RGB-14-X qualitatively and quantitatively; they only differ in their final protein concentration and container closure system. All reference product samples were stored under prescribed conditions.
Analytical Methods
Intact Molecular Mass Analysis and Intact Glycation Analysis by Liquid Chromatography–Mass Spectrometry (LC-MS)
Intact mass analysis was performed after a dilution step using MilliQ water and the samples were analysed on a Shimadzu Nexera X2 ultra-high-performance liquid chromatography (UHPLC) instrument coupled to a Bruker maXis II Q-TOF high resolution mass spectrometer. The online desalting of intact samples was performed on a Waters Acquity BEH C4 column (2.1 × 100 mm, 1.7 µm, 300 Å) at 80 °C with a flow rate of 0.4 mL/min using water/acetonitrile gradient with 0.1% trifluoroacetic acid (TFA) as eluent additive. The eluents were the followings: eluent A: H2O = 100 (V/V%), eluent B: H2O:acetonitrile (ACN) = 10:90 (V/V%) and eluent C: H2O:cc. TFA = 100:1 (V/V%). The same chromatographic conditions were applied for the intact glycation analysis where the samples were diluted and additionally treated with PNGaseF (New England BioLabs) and CPB (Sigma) enzymes.
The spectra of the detected peaks were averaged and deconvoluted using the BioPharma Compass software (version 2.0.1, Bruker). During the evaluation, the deconvoluted mass spectra and the measured average mass values were compared. In case of the glycation analysis, the relative intensity of the mono-glycated peak was determined.
Reduced and Non-reduced Peptide Mapping Analysis by Liquid Chromatography–Mass Spectrometry (LC-MS) with Lys-C Enzyme
For reduced peptide mapping, the samples were first denatured by guanidine hydrochloride under reducing conditions (using dithiothreitol) at neutral pH and 37 °C, then diluted and digested by Lys-C enzyme (Fujifilm Wako Pure Chemical Corporation). Finally, the disulfide bridges were reduced by tris-(2-Carboxyethyl)phosphine (TCEP), and the digestion was stopped by the addition of acetic acid.
As a first step of the non-reduced peptide mapping, the samples were denatured by guanidine hydrochloride at neutral pH and 37 °C. The free thiol groups were labelled by N-ethylmaleimide. The denatured samples were diluted and digested by Lys-C enzyme (Fujifilm Wako Pure Chemical Corporation). Finally, the digestion was stopped by the addition of acetic acid.
The resulting peptides were separated on a Waters Acquity BEH Phenyl column (1.7 μm, 2.1 × 150 mm, 130 Å) at 58 °C with a flow rate of 0.3 mL/min using a gradient of water/acetonitrile with 0.1% TFA as eluent additive. The eluents were the followings: eluent A: H2O:TFA = 100:0.1 (V/V%) and eluent B: H2O:ACN:TFA = 50:50:0.1 (V/V%). The peptide mapping analysis was performed by liquid chromatography-electrospray ionisation-tandem mass spectrometry (LC-ESI-MS/MS) with a Shimadzu Nexera X2 ultra-performance liquid chromatography (UPLC) instrument coupled to an Orbitrap Fusion Tribrid instrument (Thermo Fisher Scientific). Data evaluation of the MS and MS/MS spectra was performed by the BioPharma Finder (2.0) and Xcalibur (4.1) software (Thermo Fisher Scientific). A semi-quantitative analysis of post-translational modification (PTM) levels was also included in the data evaluation procedure: the relative area% of a given modified peptide was calculated by dividing the extracted ion chromatogram (EIC) peak area of the modified peptide by the sum of the EIC peak areas of all modified and native peptides of that specific peptide. The same charge states were chosen for both the modified peptide and its native form.
Reduced Peptide Mapping Analysis by LC-MS (with Chymotrypsin Enzyme)
To achieve 100% sequence coverage of the protein at the peptide level, a digestion with chymotrypsin (Promega) enzyme was also performed. After the denaturation of the protein by guanidine hydrochloride under reducing conditions (using dithiothreitol) at neutral pH and 37 °C, the samples were diluted and digested by chymotrypsin enzyme and the disulfide bridges were reduced by TCEP, and digestion was stopped by the addition of acetic acid. The resulting peptides were separated on a Waters Acquity UHPLC Peptide CSH C18 column (1.7 μm, 2.1 × 150 mm, 130 Å) at 55 °C with a flow rate of 0.3 mL/min using a gradient of water/acetonitrile with 0.1% formic acid (FA) as eluent additive. The eluents were the followings: eluent A: H2O:FA = 100:0.1 (V/V%) and eluent B: H2O:ACN:FA = 50:50:0.1 (V/V%). A Shimadzu Nexera X2 UPLC coupled to an Orbitrap Fusion Tribrid instrument (Thermo Fisher Scientific) was used for the measurements.
Hotspot Peptide Mapping Analysis by Reversed Phase–Ultra-High-Performance Liquid Chromatography with UV Detection (RP-UHPLC-UV), with Lys-C Enzyme
The extent of heavy chain (HC) M253 oxidation was measured with reversed-phase chromatography. Samples were first denatured by guanidine hydrochloride under reducing conditions (using dithiothreitol) at neutral pH and 37 °C, then diluted and digested by Lys-C enzyme (Wako). The disulfide bridges were reduced by TCEP, and digestion was stopped by the addition of acetic acid. The oxidised and native HC 250–289 peptides were separated using the same chromatographic conditions as used in the reduced Lys-C peptide mapping analysis by LC-MS on a Waters Acquity H-Class UPLC. The relative amounts of the peptides containing the single oxidised and the non-oxidised (native) forms of the M253 were compared by area% evaluation of the chromatogram acquired at 215 nm.
Free Thiol Content by Ellman’s Assay
The number of free thiol groups (sulfhydryl, -SH) per protein molecule was determined quantitatively using 5,5′-dithiobis-[2-nitrobenzoic acid] (DTNB, Ellman’s reagent). In the rapid and stoichiometric reaction one mole 2-nitro-5-thiobenzoate (TNB2-) is released per one mole thiol. The amount of the released TNB2- was quantified using an absorbance plate reader (MTP Reader Infinite Series, Tecan). Free thiol concentrations of test samples were calculated from the N-acetyl-l-cysteine standard calibration curve.
Disulfide Isoform Determination by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
The composition of disulfide isoforms was determined with reversed-phase chromatography. Denosumab has four disulfide bonds in the hinge region, and the disulfide isoforms can be distinguished on the basis of the different positions of these disulfide bridges. The sample was diluted with formulation buffer and the measurement was performed using a HALO C4 Protein 1000 Å (2.7 μm 2.1 × 150 mm) column (Halo) on a UPLC (Shimadzu Nexera) with UV detection. Chromatographic parameters: column temperature: 80 °C, flow rate: 0.7 mL/min, gradient elution (eluent A%: isopropanol [IPA]:H2O:TFA = 2:98:0.1 [V/V%] and eluent B%: IPA:ACN:H2O:TFA = 70:20:10:0.1 [V/V%]). Data were acquired and processed by quantitative integration using Empower™3 (Waters) or LabSolutions CS 6.88 SP1 (Shimadzu) software. As a final result, the relative amount (area%) of the different isoforms (B, AB, A1, A2) was obtained.
N-Glycosylation Profile by Hydrophilic Interaction Chromatography with Fluorescence Detection (HILIC-UHPLC-FL)
Firstly, N-glycans were released (PNGase, GlycoPrep® Kit, Agilent Technologies) from the denatured proteins and derivatised using an Instant Procaine (InstantPCTM, GlycoPrep® Kit, Agilent Technologies) dye, followed by the removal of the excess dye (manufacturer’s recommendations were followed). Secondly, the purified N-glycan mixture was analysed using an Acquity UPLC BEH Glycan (1.7 μm 2.1 × 150 mm) column (Waters) on a UPLC/UHPLC (Shimadzu or Waters) with fluorescent (FLR) detection (excitation wavelength [λEX] = 285 nm, emission wavelength [λEM] = 345 nm). Chromatographic parameters: column temperature: 45 °C, flow rate: 0.5 mL/min, gradient elution (eluent A: 50 mM ammonium formate, pH = 4.4, eluent B: ACN). Data were acquired and processed using Empower™3 (Waters) or LabSolutions CS 6.88 SP1 (Shimadzu) software. As a final result, the relative amount (area%) of the different N-glycan forms was obtained.
Analysis of Bisecting GlcNAc and Gal-α-1,3-Gal Forms by HILIC-UHPLC-FL/ESI-MS/MS with Exoglycosidase Digestions
Samples were prepared according to the HILIC-UHPLC-FL released glycan protocol divided to aliquots and digested with different combination of exoglycosidases. For the identification of bisecting GlcNAc forms the results of the α(2-3,6,8,9)-sialidase A, β(1-4)-galactosidase and β-N-acetylhexosaminidase digested aliquots were compared with the α(2-3,6,8,9)-sialidase A and β(1-4)-galactosidase digested samples. For the identification of galactose-α(1-3)-galactose linkage containing forms, the α(2-3,6,8,9)-sialidase A, β(1-4)-galactosidase and α(1-3,4,6)-galactosidase digested aliquots were compared with the α(2-3,6,8,9)-sialidase A and β(1-4)-galactosidase digested samples. The samples were analysed on a Shimadzu Nexera X2 UHPLC instrument coupled to a Bruker maXis II Q-TOF mass spectrometer. The same chromatographic conditions were applied as in the HILIC-UHPLC-FL analysis. N-glycans were identified on the basis of their measured mass, relative retention time, MS/MS spectra and the results of the exoglycosidase digestions using DataAnalysis (4.3) and BioPharma Compass (2.0.1) software (Bruker).
Sialic Acid Content by RP-HPLC-FL
N-Acetylneuraminic acid (NANA) and N-glycolylneuraminic acid (NGNA) contents were determined in two steps. Firstly, the sialic acids were released from the sugar chains by acidic hydrolysis (HCl) from a 10 mg/mL protein solution, and derivatised using the fluorescent dye 1,2-diamino-4,5-methylenedioxybenzene (DMB, Takara Bio Inc.). Secondly, sialic acids were separated using a Phenomenex Kinetex C8 column (2.1 × 100 mm, 2.6 µm) on a UPLC/UHPLC (Shimadzu or Waters) instrument with FL detection (λEX = 373 nm, λEM = 448 nm). Chromatographic parameters: column temperature: 35 °C, flow rate: 0.35 mL/min, gradient elution (eluent A%: MeOH:H2O = 15:85 [V/V%]; eluent B%: MeOH:H2O:TFA = 70:30:0.1 [V/V%]). Data based on NANA and NGNA standard (Sigma) calibration curves were acquired and processed by Empower™3 (Waters) or LabSolutions CS 6.88 SP1 (Shimadzu) software.
Non-glycosylated Heavy Chain (NgHC) Fragment by Reducing Capillary Gel Electrophoresis Sodium Dodecyl Sulphate (R-CE-SDS)
Non-glycosylated heavy chain was measured on a Maurice S. (ProteinSimple) CE instrument. The fragments subjected to analysis (NgHC, HC, LC) were generated by reducing the disulfide bridges of the antibody by β-mercaptoethanol together with sodium dodecyl sulphate (Maurice CE-SDS Plus 1x Sample Buffer, ProteinSimple). The length and diameter of the capillary was 150 mm and 50 μm, respectively. At the beginning of a 25-min run the sample load was performed electrokinetically, and the compounds were detected at 220 nm. Compass for iCE software (ProteinSimple v 2.1.0) was used.
Hydrogen/Deuterium Exchange Mass Spectrometry (HDX-MS)
The samples were prepared by a two-step dilution, first with H2O-buffer, then with D2O-buffer and were subjected to deuteration for different time periods. Following quenching with a low-pH buffer, the samples were placed in an ice bath before being injected into the HDX-manager module of the Waters M-Class liquid chromatography system coupled with a Waters Synapt XS® ion mobility quadrupole Q-TOF mass spectrometer (Waters Corporation, Milford, MA, USA). The protein was digested online by a Waters Enzymate® BEH Pepsin column (30 mm × 2.1 mm, 5 µm), the peptides were first trapped on a Waters Acquity® UPLC BEH C18 VanGuard Pre-column (5 mm × 2.1 mm, 1.7 µm). Then they were separated on a Waters Acquity® UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 µm) before finally being eluted into the mass spectrometer. Chromatographic parameters were as follows: digestion column temperature 20 °C; analytical column temperature 0.1 °C; digestion eluent flow rate 100 μL/min; analytical eluent flow rate 40 μL/min; gradient elution (eluent A%: H2O:cc. FA = 100:0.2 [V/V%]; eluent B%: ACN:cc. FA = 100:0.2 [V/V%]). The mass spectrometer was set to positive ion—HDMSE (ion mobility) mode. The acquired HDX-MS data were analysed in two stages: denosumab-derived peptides were identified using Waters PLGS 3.0.3 and subsequently, Waters DynamX 3.0 software was used to calculate the absolute and relative deuteration results for the identified peptides.
Nuclear Magnetic Resonance Spectroscopy (2D NMR)
Two-dimensional 1H–13C heteronuclear single quantum coherence (2D HSQC) NMR spectra of intact protein samples were recorded. The samples were prepared for NMR measurements under conditions replicating the Prolia® formulation, with the formulation solution supplemented with 5% v/v D2O. The protein concentration was standardised to a nominal value of 40 mg/mL. All NMR data were collected at 320 K on a Bruker Avance III HDX 800 MHz spectrometer, equipped with a 5-mm cryogenically cooled triple-resonance Z-pulsed field gradient TCI probe (Bruker Corporation, Billerica, MA, USA). Parameters of a gradient-selected sensitivity-enhanced HSQC pulse sequence were optimised for methyl groups. 2D methyl-HSQC spectra were compared visually, as well as by a chemometric approach: sets of combined chemical shift difference (CCSD) values of pairwise comparisons of peak lists were evaluated statistically [22].
High Molecular Weight Species (HMWs) by Size Exclusion Chromatography (SE-HPLC)
Chromatographic resolution was achieved with an AdvancedBio SEC 300 Å column (7.8 × 300 mm, 2.7 µm, Agilent) on a Shimadzu Nexera HPLC system. Chromatographic parameters: column temperature: 30 °C, flow rate: 0.7 mL/min, isocratic flow (eluent: 5 V/V% ACN and 300 mM KCl containing 50 mM K3PO4 buffer pH = 6.8), FL detection: (λEX = 280 nm, λEM = 350 nm). Data were acquired and processed by Empower™3 (Waters) or LabSolutions CS 6.88 SP1 (Shimadzu) software.
Low Molecular Weight Species (LMWs) by Non-reducing Capillary Electrophoresis Sodium Dodecyl Sulphate (NR-CE-SDS)
Maurice S. (ProteinSimple) CE instrument was applied using a Compass for iCE software (ProteinSimple v 2.1.0). Samples were denatured with sodium dodecyl sulphate (Maurice CE-SDS Plus 1x Sample Buffer, ProteinSimple) and acetylated by iodoacetamide. The length and diameter of the capillary was 150 mm and 50 μm, respectively. At the beginning of a 35-min run the sample load was performed electrokinetically, and the compounds were detected at 220 nm.
Charge Variants by Imaged Capillary Isoelectric Focusing (cIEF) with Native Treatment and CPB Digestion
Charge variants were separated in 0.5 mg/mL protein solution either with native treatment or after carboxypeptidase B (Sigma) enzyme digestion in ampholyte (Pharmalyte 3-10, Sigma) and 0.6 M urea containing matrix using a Maurice S. CE instrument (ProteinSimple) with dedicated cIEF cartridge. Maurice’s cIEF cartridge contains a 100 µm ID × 5 cm fluorocarbon-coated capillary, which is pressure-filled with 0.5% methyl cellulose. During the 6.5-min cIEF run, separation is monitored in real time using absorbance detection at 280 nm. The basis for the assessment of identity was the isoelectric points of the tested samples, which were determined on the basis of the position of the pI markers (pI 7.05 and pI 9.50 from ProteinSimple). Using as a purity method, area% data of the main peak and sum of acidic and basic variants were evaluated. Data were acquired and processed by Compass for iCE software (ProteinSimple v 2.1.0).
Functional Assays
Target (RANKL) Binding by Enzyme-Linked Immunosorbent Assay (ELISA)
Binding of test samples to RANKL was assessed by indirect ELISA. Recombinant human soluble RANKL (sRANKL) was coated onto a 96-well plate, followed by adding serial dilution of test samples, after a blocking step. The binding was detected using HRP-conjugated anti-human IgG (Fc-specific) secondary antibody followed by the 3,3′,5,5′-tetramethylbenzidine (TMB) reagent. The emerging enzymatic reaction was stopped by adding sulfuric acid. The dose-response data were fitted to a 4PL curve using PLA software (Stegmann System), and the relative binding was calculated on the basis of the half maximal effective concentration (EC50) values compared with the reference standard.
Kinetic Analysis of RANKL, Neonatal Fc Receptor (FcRn), Fcγ Receptor (FcγRI/CD64, FcγRIIa/CD32a, FcγRIIIa/CD16a) and the Complement Component 1q (C1q) Binding by Biolayer Interferometry (BLI)
The kinetic constants were determined by BLI using Octet RED96e instrument (Sartorius). As ligands, RANKL (R&D Systems) was immobilised on AR2G biosensors (Amine Reactive 2nd Generation) using covalent amine-coupling, whilst His-tagged human FcRn (Sino Biological), CD64 (Sino Biological), CD32a 131H (R&D Systems) and CD16a 158V (Acro Biosystems) were immobilised on HIS1K biosensors (Anti-Penta-HIS, Sartorius), respectively, and varying concentrations of test samples were used as analyte. In case of C1q binding, test samples were immobilised on FAB2G biosensors (Anti-Human Fab-CH1 2nd Generation, Sartorius) as ligands, and varying concentrations of human C1q (Merck) were used as analyte. During each kinetic analysis, the sensorgrams were collected and processed using the integrated Octet Data Analysis HT software (Sartorius). The kinetic parameters of the interactions (equilibrium dissociation constant [KD], association rate constant [ka] and dissociation rate constant [kdis]) were determined by fitting Langmuir 1:1 model to the kinetic curves.
Membrane Bound RANKL (mbRANKL) Binding by Cell-Based Flow Cytometry
The relative binding affinity to a recombinant Jurkat T-cell line stably and highly expressing human RANKL on its surface was assessed in a flow-cytometry-based assay. The amount of denosumab bound to the cell surface can be detected by indirect immunofluorescence staining using a fluorescent dye-conjugated anti-human IgG F(ab’)2 fragment. The mean fluorescent intensity (MFI) signal is directly proportional to the amount of denosumab bound to the cell surface. The reference standard and test samples are diluted in the same way and the biological activity of the test samples is calculated relative to the reference standard which represents 100% biological activity.
Inhibition of RANKL/RANK Signalling by Cell-Based Neutralisation Reporter Assay
Neutralising ability of test sample against RANKL was determined using DiscoverX PathHunter® U2OS RANK-IkB Functional Assay kit. Cells were cultured using AssayComplete™ Cell Culture Kit-103 (DiscoverX) before seeded into a 96-well assay plate. Serial dilutions of reference standard and test samples were prepared and mixed with sRANKL (PeproTech) solution for its neutralisation, then added to cells and incubated for 24 h. The generated chemiluminescence signal was detected by using Bioassay ED Detection kit (DiscoverX). The dose-response data were fitted to a 5PL curve using PLA software (Stegmann System), and the relative biological activity was calculated on the basis of the EC50 values compared with the reference standard.
Inhibition of Osteoclast Differentiation by Cell-Based TRAcP5b ELISA
The assay exploits the ability of the precursor cell line RAW264.7 (ATCC) to differentiate into mature osteoclasts that secrete active tartrate-resistant acid phosphatase 5b (TRAcP5b) in response to sRANKL stimulation. RAW264.7 cells were seeded on a plate and then treated with mixture of sRANKL and varying concentrations of reference and test samples. The amount of TRAcP5b secreted into the supernatant is detected by Mouse TRAP (TRAcP 5b) ELISA (Immunodiagnostic Systems), using multi-mode plate reader (BioTek). Dose-response curves of reference and test samples were fitted with a 5PL model and compared using the PLA software (Stegmann Systems). Relative biological activity is calculated from EC50 values.
Results
The majority of the applied methods provided quantitative data, on the basis of which QRs or at least min–max ranges could be established for the different critical and non-critical quality attributes to evaluate the similarity of RGB-14-P and RGB-14-X proposed biosimilars to the reference products EU/US Prolia® and Xgeva®.
Structural Characterisation
Primary Structure
Characterisation methods as intact mass analysis with or without PNGase F digestion, non-reduced and reduced LC-MS peptide mapping (including glycosylation site analysis), Ellman’s assay for free thiol determination, release methods as oxidation hotspot, N-glycan analysis extended by exoglycosidase digestion and MS-based peak characterisation were used to assess the analytical similarity of the primary structure of the biosimilar and reference drug products.
Intact mass spectra measured without the removal of N-glycans (Fig. 1) show the same denosumab isoforms both in biosimilar and reference drug product batches. The measured average mass values of the identified isoforms in RGB-14-P and RGB-14-X drug products conform both to the theoretical average masses and the results obtained for Prolia® and Xgeva® (Table 2).
Fig. 1.
Deconvoluted intact mass spectra obtained from the online RP-HPLC/ESI-MS analysis of denosumab in EU/US Prolia®, RGB-14-P, EU/US Xgeva® and RGB-14-X drug product batches
Table 2.
Summary of the intact mass analysis data obtained from the on-line RP-HPLC/ESI-MS analysis of denosumab in EU/US Prolia®, RGB-14-P, EU/US Xgeva® and RGB-14-X drug product batches (C-terminal lysine is absent)
| Form/sample | Theoretical average molecular mass [Da] | Measured average molecular mass [Da] | |||||
|---|---|---|---|---|---|---|---|
| EU Prolia® | US Prolia® | RGB-14-P | EU Xgeva® | US Xgeva® | RGB-14-X | ||
| Man5/Man5 | 146,894.9 | 146,898.5 | 146,898.5 | 146,898.2 | 146,899.5 | 146,899.7 | 146,897.7 |
| G0F/Man5 | 147,123.1 | 147,126.5 | 147,127.3 | 147,125.1 | 147,128.5 | 147,127.4 | 147,127.7 |
| G0F/G0 | 147,205.2 | 147,209.5 | 147,210.4 | 147,209.3 | 147,211.2 | 147,210.9 | 147,209.3 |
| G0F/G0F | 147,351.4 | 147,355.8 | 147,355.8 | 147,354.6 | 147,355.9 | 147,355.7 | 147,354.8 |
| G0F/G1F | 147,513.5 | 147,517.2 | 147,517.3 | 147,516.4 | 147,518.0 | 147,518.0 | 147,516.7 |
| G0F/G2F and G1F/G1F | 147,675.6 | 147,680.5 | 147,680.5 | 147,678.6 | 147,680.1 | 147,679.3 | 147,678.8 |
| G1F/G2F | 147,837.8 | 147,841.8 | 147,843.6 | 147,840.9 | 147,841.4 | 147,842.5 | 147,841.7 |
Combination of LC-MS/MS peptide mapping methods applying proteases with complementary cleavage preferences was used to verify the entire amino acid sequence of denosumab in the biosimilar and reference product batches. The isobaric amino acids (Leu/Ile) were also verified by multistage LC-MS/MS methods (methods and data not shown). Total ion chromatograms of the Lys-C (Fig. 2a) and base peak chromatograms of the chymotrypsin (Supplementary Fig. 1) digested samples under reducing conditions show similar peak pattern for RGB-14-P and RGB-14-X as compared with the chromatograms of Prolia® and Xgeva®.
Fig. 2.
Total ion chromatograms of EU/US Prolia®, RGB-14-P, EU/US Xgeva® and RGB-14-X drug product batches obtained during the online RP-HPLC/ESI-MS/MS analysis of the a Lys-C digested denosumab under reducing conditions and b Lys-C digested denosumab under non-reducing conditions
All the theoretical disulfide bridges of the major disulfide isoforms (A, A/B and B) of denosumab protein (Table 3) were verified by Lys-C LC-MS peptide mapping under non-reducing conditions (Fig. 2b). The results confirmed that the disulfide bridge connectivity is identical for denosumab molecules both in the biosimilar and reference products for all the three major disulfide isoforms. The levels of cysteine related variants, including free thiols, LC-HC and HC-HC trisulfide bonds, were comparable between the biosimilar and the reference products (Table 4).
Table 3.
Intra- and interchain disulfide bridges of A, A/B and B isoforms of denosumab (disulfide bonded peptides used for the confirmation of the disulfide bridges are listed in the Supplementary Table 3)
| Type of disulfide bridge | Positions of connected cysteine residues | ||
|---|---|---|---|
| Isoform A | Isoform A/B | Isoform B | |
| Intrachain disulfide bridges in the heavy chain (HC) |
Cys22-Cys96 Cys149-Cys205 Cys262-Cys322 Cys368-Cys426 |
Cys22-Cys96 Cys149-Cys205 Cys262-Cys322 Cys368-Cys426 |
Cys22-Cys96 Cys149-Cys205 Cys262-Cys322 Cys368-Cys426 |
| Intrachain disulfide bridges in the light chain (LC) |
Cys23-Cys89 Cys135-Cys195 |
Cys23-Cys89 Cys135-Cys195 |
Cys23-Cys89 Cys135-Cys195 |
| Interchain disulfide bridges between the light chain (LC) and the heavy chain (HC) | 2x Cys136 (HC)–Cys215 (LC) |
Cys136 (HC)–Cys215 (LC) Cys224 (HC)–Cys215 (LC) |
2x Cys224 (HC)–Cys215 (LC) |
| Interchain disulfide bridges between the heavy chains |
Cys224-Cys224 Cys225-Cys225 Cys228-Cys228 Cys231-Cys231 |
Cys136-Cys224 Cys225-Cys225 Cys228-Cys228 Cys231-Cys231 |
2x Cys136-Cys225 Cys228-Cys228 Cys231-Cys231 |
Table 4.
Summary of the quantitative analytical data related to the protein primary structure of RGB-14-P and RGB-14-X biosimilars to the reference products EU/US Prolia® and Xgeva®
| Attribute | EU Prolia® quality range (n) | US Prolia® quality range (n) | RGB-14-P min–max range (n) | EU Xgeva® quality range (n) | US Xgeva® quality range (n) | RGB-14-X min–max range (n) |
|---|---|---|---|---|---|---|
| Primary structure | ||||||
| HC M254 oxidation by RP-HPLC (rel. area%) | 2.90–4.43 (27) | 2.91–4.50 (27) | 2.73–4.14 (8) | 2.72–4.46 (19) | 3.05–4.47 (23) | 2.61–3.47 (6) |
| Oxidation in CDR by LC-MS (rel. area%) | ||||||
| HC_ D99-K126 (M106) | 0.82–0.91 (6)* | 0.79–0.87 (10)* | 0.75–0.89 (4) | 0.86–1.01 (6)* | 0.81–1.03 (10)* | 0.72–0.99 (5) |
| Oxidation in non-CDR by LC-MS (rel. area%) | ||||||
| HC_D250-K289 (M253) | 3.99–4.38 (6)* | 3.91–4.35 (10)* | 3.68–4.45 (4) | 3.90–4.74 (6)* | 4.13–4.77 (10)* | 3.33–4.41 (5) |
| HC_D250-K289 (H269) | 0.29–0.36 (6)* | 0.35–0.39 (10)* | 0.18–0.22 (4) | 0.34–0.44 (6)* | 0.33–0.43 (10)* | 0.18–0.25 (5) |
| HC_G342-K361 (M359) | 1.47–1.58 (6)* | 1.47–1.58 (10)* | 1.60–1.67 (4) | 1.54–1.86 (6)* | 1.56–1.87 (10)* | 1.49–1.88 (5) |
| HC_T394-K410 (M398) | 1.37–1.49 (6)* | 1.32–1.53 (10)* | 1.16–1.22 (4) | 1.54–1.83 (6)* | 1.43–1.87 (10)* | 1.08–1.49 (5) |
| HC_S416-K440 (M429) | 0.99–1.11 (6)* | 0.90–1.06 (10)* | 0.97–1.64 (4) | 1.02–1.19 (6)* | 0.95–1.19 (10)* | 0.91–1.54 (5) |
| HC_S416-K440 (W418) | 0.03–0.04 (6)* | 0.03–0.04 (10)* | 0.04–0.08 (4) | 0.03–0.04 (6)* | 0.02–0.04 (10)* | 0.03–0.08 (5) |
| Hydroxylation in CDR by LC-MS (rel. area%) | ||||||
| HC_G44-K65 (K65) | 0.14–0.16 (6)* | 0.14–0.18 (10)* | 0.03–0.04 (4) | 0.15–0.21 (6)* | 0.14–0.22 (10)* | 0.03–0.05 (5) |
| Hydroxylation in non-CDR by LC-MS (rel. area%) | ||||||
| HC_D99-K126 (K126) | 3.32–3.65 (6)* | 3.48–3.72 (10)* | 0.29–0.34 (4) | 3.53–4.23 (6)* | 3.21–4.63 (10)* | 0.28–0.38 (5) |
| HC_D153-K215 (P194) | 2.49–2.99 (6)* | 2.82–3.25 (10)* | 0.90–0.93 (4) | 2.65–3.27 (6)* | 2.91–3.35 (10)* | 0.96–1.05 (5) |
| HC_G328-K335 (P330) | 0.16–0.27 (6)* | 0.16–0.23 (10)* | 0.66–0.93 (4) | 0.16–0.37 (6)* | 0.15–0.26 (10)* | 0.59–1.00 (5) |
| LC_E1-K40 (P15) | 0.03–0.04 (6)* | 0.02–0.04 (10)* | 0.17–0.20 (4) | 0.02–0.06 (6)* | 0.02–0.06 (10)* | 0.17–0.22 (5) |
| Deamidation in non-CDR by LC-MS (rel. area%) | ||||||
| HC_D153-K215 (N164/Q180) | 0.37–0.42 (6)* | 0.35–0.44 (10)* | 0.23–0.25 (4) | 0.37–0.44 (6)* | 0.39–0.45 (10)* | 0.24–0.29 (5) |
| HC_T290-K318_G0F_N298 isomer A (Q296/Q312/N316) | 0.60–0.68 (6)* | 0.60–0.74 (10)* | 0.59–0.65 (4) | 0.65–0.77 (6)* | 0.65–0.82 (10)* | 0.64–0.76 (5) |
| HC_ T290-K318_G0F_N298 isomer B (Q296/Q312/N316) | 0.28–0.32 (6)* | 0.26–0.36 (10)* | 0.27–0.30 (4) | 0.30–0.33 (6)* | 0.31–0.37 (10)* | 0.30–0.36 (5) |
| HC_ N362-K371 (N362) | 0.58–0.64 (6)* | 0.57–0.65 (10)* | 0.58–0.68 (4) | 0.61–0.75 (6)* | 0.59–0.75 (10)* | 0.58–0.74 (5) |
| HC_ G372-K393 (N385) | 3.08–3.24 (6)* | 3.07–3.30 (10)* | 2.32–2.39 (4) | 3.14–3.72 (6)* | 2.95–3.89 (10)* | 2.30–2.92 (5) |
| HC_ G372-K393 (N390+N385) | 3.04–3.16 (6)* | 3.03–3.24 (10)* | 1.92–1.97 (4) | 3.04–3.68 (6)* | 2.81–3.75 (10)* | 1.87–2.42 (5) |
| HC_ S416-K440 isomer A (N435) | 1.01–1.11 (6)* | 1.01–1.18 (10)* | 0.98–1.09 (4) | 1.10–1.30 (6)* | 1.05–1.31 (10)* | 1.06–1.27 (5) |
| HC_ S416-K440 isomer B (N435) | 0.97–1.42 (6)* | 0.98–1.40 (10)* | 0.94–1.29 (4) | 1.16–1.30 (6)* | 1.12–1.40 (10)* | 1.11–1.36 (5) |
| LC_ S128-K146 isomer A (N138) | 0.22–0.26 (6)* | 0.22–0.25 (10)* | 0.22–0.25 (4) | 0.24–0.30 (6)* | 0.22–0.31 (10)* | 0.23–0.31 (5) |
| LC_ S128-K146 isomer B (N138) | 0.18–0.20 (6)* | 0.18–0.21 (10)* | 0.18–0.19 (4) | 0.18–0.23 (6)* | 0.18–0.24 (10)* | 0.19–0.23 (5) |
| Isomerisation in CDR by LC-MS (rel. area%) | ||||||
| HC_ G44-K65 (D62) | 0.05–0.05 (6)* | 0.05–0.06 (10)* | 0.05–0.05 (4) | 0.05–0.07 (6)* | 0.04–0.08 (10)* | 0.05–0.07 (5) |
| Isomerisation in non-CDR by LC-MS (rel. area%) | ||||||
| HC_D250-K289 (D250/D266/D271/D281) | 0.35–0.39 (6)* | 0.32–0.38 (10)* | 0.28–0.33 (4) | 0.37–0.46 (6)* | 0.32–0.50 (10)* | 0.28–0.39 (5) |
| HC_T394-K410 (D400/D402) | 0.21–0.23 (6)* | 0.22–0.23 (10)* | 0.20–0.21 (4) | 0.22–0.28 (6)* | 0.21–0.30 (10)* | 0.19–0.26 (5) |
| LC_V151-K170 (D152/D168) | 0.44–0.52 (6)* | 0.39–0.54 (10)* | 0.28–0.43 (4) | 0.44–0.54 (6)* | 0.41–0.61 (10)* | 0.31–0.46 (5) |
| Succinimide formation in non-CDR by LC-MS (rel. area%) | ||||||
| HC_D250-K289 (D281) | 0.84–0.95 (6)* | 0.81–0.92 (10)* | 0.80–0.87 (4) | 0.88–0.97 (6)* | 0.85–0.99 (10)* | 0.80–0.90 (5) |
| HC_T394-K410 (D402) | 0.51–0.60 (6)* | 0.53–0.60 (10)* | 0.51–0.57 (4) | 0.51–0.68 (6)* | 0.54–0.68 (10)* | 0.53–0.64 (5) |
| Terminal variants by LC-MS (rel. area%) | ||||||
| HC_E1-K43_pyroGlu_N-term | 2.31–2.50 (6)* | 2.26–2.46 (10)* | 1.78–1.94 (4) | 2.34–2.45 (6)* | 2.21–2.68 (10)* | 1.67–1.88 (5) |
| LC_E1-K40_pyroGlu_N-term | 0.76–0.89 (6)* | 0.76–0.85 (10)* | 0.69–0.76 (4) | 0.79–0.93 (6)* | 0.75–0.96 (10)* | 0.67–0.82 (5) |
| HC_S441-G447_Lys_C-term | 0.47–1.11 (6)* | 0.42–0.83 (10)* | 1.61–2.56 (4) | 0.60–1.85 (6)* | 0.48–1.11 (10)* | 1.43–2.58 (5) |
| HC_S441-G447_Proline Amidation_C-term | 0.23–0.28 (6)* | 0.23–0.30 (10)* | 0.40–0.42 (4) | 0.23–0.31 (6)* | 0.25–0.33 (10)* | 0.34–0.45 (5) |
| Free Cysteines per protein by Ellman’s assay (mol/mol) | 0.21–0.44 (6) | 0.22–0.41 (10) | 0.23–0.41 (8) | 0.20–0.49 (6) | 0.25–0.43 (10) | 0.27–0.43 (6) |
| Cys related variants by LC-MS (rel. area%) | ||||||
| Trisulfide: HC-HC in isoform A | 1.1–2.0 (6)* | 0.8–2.0 (10)* | 1.6–2.2 (4) | 0.7–1.9 (6)* | 0.9–2.7 (10)* | 1.5–2.2 (5) |
| Trisulfide: LC-HC in isoform A and A/B | 0.3–0.4 (6)* | 0.3–0.5 (10)* | 0.4–0.4 (4) | 0.2–0.4 (6)* | 0.3–0.6 (10)* | 0.3–0.5 (5) |
| Monoglycation by LC-MS (rel. int. %) | 14.2–28.6 (18) | 6.7–32.2 (25) | 15.4–16.0 (8) | 8.5–32.7 (18) | 8.4–30.7 (22) | 15.2–15.7 (6) |
n, number of batches analysed, given in brackets after specific range values
CDR complementarity-determining region, HC heavy chain, HPLC high-performance liquid chromatography, LC light chain, LC-MS liquid chromatography-mass spectrometry, RL reporting limit, RP reversed phase, rel. int. % relative (peak) intensity %
*Min–max range is given instead of QR because of the limited number of samples analysed with the specific method
Site-specific analysis of the identified post-translational modifications was performed by the reduced Lys-C LC-MS peptide mapping method. The quantitative results for methionine and tryptophan oxidation, lysine and proline hydroxylation, asparagine and glutamine deamidation, aspartic acid isomerisation and succinimide formation, Fc glycosylation at HC N298 including the non-glycosylated form, N-terminal glutamate to pyroglutamate conversion, C-terminal lysine loss and amidation of proline after the removal of C-terminal lysine and glycine were compared amongst the RGB-14-P, RGB-14-X, EU/US Prolia® and Xgeva® drug products in Table 4.
The level of glycation was compared on the basis of the results of intact mass analysis after PNGase F and carboxypeptidase B digestion (Table 4 and Fig. 3). The clustering of the reference product data was observed for this quality attribute, as can be seen in Fig. 3.
Fig. 3.
Comparison of results for the mono-glycated form in a EU/US Prolia® versus RGB-14-P and b EU/US Xgeva® versus RGB-14-X. Red dashed lines: upper and lower limits of US QRs (Prolia®: 6.7–32.2%; Xgeva®: 8.4–30.7%); black dashed lines: upper and lower limits of EU QRs (Prolia®: 14.2–28.6%; Xgeva®: 8.5–32.7%)
Glycosylation
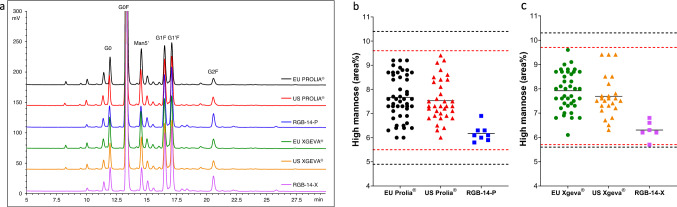
N-glycosylation pattern of denosumab was analysed by HILIC-UHPLC-FL method. Glycan peaks were identified on the basis of digestion with exoglycosidases and LC-MS/MS analysis. Profile of the FL chromatograms of RGB-14-P, RGB-14-X, EU/US Prolia® and Xgeva® drug products are compared in Fig. 4. Area% for major peaks and summed results for the galactosylated, fucosylated, afucosylated, sialylated and high-mannose forms are presented in Table 5. The relative abundance of high mannose-forms, which is a critical quality attribute due to the effect on the serum half-life of IgGs [23], is highlighted in Fig. 4. On the basis of the quantitative results, the N-glycan profile of RGB-14 products is similar to those of Prolia® and Xgeva® products.
Fig. 4.
a HILIC-UHPLC-FL chromatograms of EU/US Prolia®, RGB-14-P, EU/US Xgeva® and RGB-14-X drug product batches and comparison of the high-mannose levels in b EU/US Prolia® versus RGB-14-P and c EU/US Xgeva® versus RGB-14-X. Red dashed lines: upper and lower limits of US QRs (Prolia®: 5.5–9.6%; Xgeva®:5.7–9.7%); black dashed lines: upper and lower limits of EU QRs (Prolia®: 4.9–10.4%; Xgeva®: 5.6–10.3%)
Table 5.
Summary of the quantitative analytical data related to glycosylation of RGB-14-P and RGB-14-X biosimilars to the reference products EU/US Prolia® and Xgeva®
| Attribute | EU Prolia® quality range (n) | US Prolia® quality range (n) | RGB-14-P min–max range (n) | EU Xgeva® quality range (n) | US Xgeva® quality range (n) | RGB-14-X min–max range (n) |
|---|---|---|---|---|---|---|
| Glycosylation and sialylation | ||||||
| HILIC-UHPLC-FL (area%) | ||||||
| G0F | 52.9–67.2 (48) | 50.9–68.7 (34) | 54.8–57.0 (8) | 52.3–65.2 (40) | 52.1–66.6 (24) | 54.3–56.9 (6) |
| G1F | 4.7–12.0 (48) | 4.3–12.4 (34) | 10.2–12.0 (8) | 5.5–12.7 (40) | 4.7–12.3 (24) | 10.3–12.0 (6) |
| G1’F | 5.5–13.6 (48) | 5.3–13.7 (34) | 12.1–14.1 (8) | 6.2–14.6 (40) | 5.2–14.0 (24) | 12.2–14.0 (6) |
| G2F | 0.3–3.6 (48) | 0.3–3.6 (34) | 2.9–3.9 (8) | 0.8–3.7 (40) | 0.5–3.4 (24) | 3.0–3.9 (6) |
| Galactosylated | 10.8–29.1 (48) | 10.0–29.6 (34) | 25.3–30.0 (8) | 12.5–31.0 (40) | 10.5–29.7 (24) | 25.6–29.9 (6) |
| Fucosylated | 75.1–84.8 (48) | 75.7–83.4 (34) | 82.3–84.9 (8) | 75.6–85.3 (40) | 75.6–83.4 (24) | 82.5–84.8 (6) |
| Afucosylated | 2.9–7.6 (48) | 3.4–7.6 (34) | 4.3–4.8 (8) | 2.4–7.9 (40) | 3.4–7.6 (24) | 4.4–4.6 (6) |
| High mannose | 4.9–10.4 (48) | 5.5–9.6 (34) | 5.8–6.9 (8) | 5.6–10.3 (40) | 5.7–9.7 (24) | 5.7–6.89 (6) |
| Sialylated | < LOQ–0.9 (48) | < LOQ–0.7 (34) | 0.1–0.3 (8) | < LOQ–1.0 (40) | < LOQ–0.7 (24) | 0.1–0.4 (6) |
| Sialic acid content by RP-HPLC-FL (ng/mg protein) | ||||||
| NANA | 49.9–116.5 (41) | 56.7–110.0 (23) | 40.3–47.6 (8) | 37.3–125.8 (33) | 58.1–108.1(22) | 40.6–48.3 (6) |
| NGNA | < LOQ/4.7 (8) | < LOQ/4.7 (8) | < LOQ/4.7 (8) | < LOQ/4.7 (8) | < LOQ/4.7 (8) | < LOQ/4.7 (8) |
| LC-MS/glycopeptide (rel. area%) | ||||||
| Fragment | 0.2–0.3 (6)* | 0.1–0.3 (10)* | < 0.1 (4) | 0.2–0.2 (6)* | 0.2–0.2 (10)* | < 0.1 (5) |
| Pauci-mannose | 1.0–1.2 (6)* | 0.6–1.2 (10)* | ND (4) | 0.6–0.9 (6)* | 0.6–1.2 (10)* | ND (5) |
| High-mannose forms | 9.9–11.0 (6)* | 8.4–10.8 (10)* | 7.3–8.0 (4) | 8.8–9.8 (6)* | 8.2–10.5 (10)* | 7.7–8.1 (5) |
| Hybrid forms | 3.8–5.0 (6)* | 3.9–5.3 (10)* | 4.7–5.3 (4) | 3.4–4.1 (6)* | 3.9–5.6 (10)* | 4.8–5.6 (5) |
| Complex, galactosylated forms | 16.5–20.4 (6)* | 12.5–22.6 (10)* | 23.8–27.1 (4) | 20.5–26.3 (6)* | 12.1–24.7 (10)* | 25.2–30.0 (5) |
| Afucosylated forms without high-mannose | 9.6–10.1 (6)* | 8.6–10.4 (10)* | 7.6–7.9 (4) | 9.9–11.5 (6)* | 8.6–11.7 (10)* | 7.7–8.5 (5) |
| Tri-antennary forms | 0.7–0.8 (6)* | 0.6–0.9 (10)* | 0.1–0.1 (4) | 0.7–0.8 (6)* | 0.6–0.9 (10)* | 0.1–0.1 (5) |
| Sialylated forms | 0.1–0.2 (6)* | 0.1–0.2 (10)* | < 0.1 (4) | 0.2–0.3 (6)* | 0.1–0.2 (10)* | < 0.1 (5) |
| NgHC by R-CE-SDS (area%) | 0.6–1.5 (16) | 0.6–1.4 (18) | 0.4–0.6 (8) | 0.7–1.3 (14) | 0.8–1.4 (16) | 0.4–0.6 (6) |
| NgHC by LC-MS (rel. area%) | 1.3–1.7 (6)* | 1.2–1.6 (10)* | 0.6–0.7 (4) | 1.1–1.5 (6)* | 1.2–1.7 (10)* | 0.6–0.8 (5) |
n, number of batches analysed, given in brackets after specific range values
*Min–max range is given instead of QR because of the limited number of samples analysed with the specific method
Higher-Order Structure
Various state-of-the-art orthogonal methods were used to characterise the higher-order structure of the protein in the compared products.
Thermal stability was evaluated using differential scanning calorimetry (μDSC). The transition midpoint temperatures corresponding to the CH2-domain (Tm1), the Fab-fragment (Tm2) and the total enthalpy change (ΔH) results (Supplementary Fig. 2, Table 4) were highly similar between all compared sample groups (RGB-14-P, EU/US Prolia® and RGB-14-X, EU/US Xgeva®). A slightly lower level of Tm3 results (corresponding to the CH3-domain) was observed in RGB-14-P and RGB-14-X compared with Prolia® and Xgeva®: this difference can be most likely attributed to differences in disulfide-isoform composition of certain reference product batches (observed by RP-HPLC and non-reducing Lys-C LC-MS peptide mapping). It is known from the literature that the thermal stability of the B, A/B and A isoforms is different from each other [24]. These minor Tm3 differences are not expected to have an effect on biological activity and stability.
The secondary structure was characterised using far-UV circular dichroism (CD) and Fourier-transform infrared (FT-IR) spectroscopy. The resulting FT-IR and CD spectra displayed comparable profiles (Supplementary Figs. 3 and 4). These data (Supplementary Table 4) suggest a high degree of structural similarity in the secondary and tertiary conformations of denosumab across the RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva® drug products.
Hydrogen-deuterium exchange mass spectrometry (HDX-MS) was also used to investigate the higher-order structure. This technique can provide an insight into in-solution tertiary structure of proteins through the dependence of the hydrogen-deuterium exchange rate of protein backbone amides on their degree of exposure to a deuterated medium [25, 26]. The fractional deuterium uptake plots of the examined RGB-14 and reference product batches were similar to each other (example shown for the comparison of RGB-14-P and US-licensed Prolia® drug product batches in Fig. 5 for the HC and LC of denosumab, all other plots are shown in Supplementary Figs. 5–10).
Fig. 5.
Fractional deuterium uptake plot (‘butterfly-plot’) for the comparison of the deuterium uptakes of denosumab a HC and b LC peptides between the examined RGB-14-P and US-licensed Prolia® product batches. Shades of blue: RGB-14-P fractional deuterium uptake plots acquired for four different deuteration intervals; shades of red: US-licensed Prolia® fractional deuterium uptake plots acquired for four different deuteration intervals (the same as for RGB-14-P)
On the basis of the qualitative comparison of the fractional deuterium uptake plots and the quantitative evaluation of the deuterium uptake results (data not shown), it can be concluded that the higher-order structure of the denosumab protein in the compared RGB-14 and EU/US reference drug product batches show a high degree of similarity.
Structural fingerprinting based on 2D HSQC NMR [27, 28] spectroscopy was applied as an orthogonal analytical approach to HDX-MS for comparative assessment of the higher-order structure. Although methyl fingerprinting does not provide complete coverage along the primary sequence, it offers the advantage over HDX-MS of enabling structural analysis in solution under near-physiological conditions without the need for chemical modification of the intact protein. As a first criterion for spectral similarity, methyl region of the 2D HSQC spectra were compared visually (Fig. 6).
Fig. 6.
Shifted overlay view of methyl region of representative 1H–13C HSQC NMR spectra of EU/US Prolia®, RGB-14-P, EU/US Xgeva® and RGB-14-X drug product batches
As shown in Fig. 6, the compared spectra are highly similar, although some seemingly missing peaks can be observed. However, careful visual inspection using a lower intensity threshold confirmed that all protein-related peak is present in each spectrum (data not shown). NMR data were further analysed using a straightforward yet effective chemometric approach involving the statistical assessment of CCSD values [22]. Results of chemometric assessment for EU/US Prolia® batches as compared with RGB-14-P are shown in Supplementary Fig. 11.
Physico-chemical Analysis of Product-Related Variants
The product-related purity profile was investigated by determining size (SE-HPLC, NR-CE-SDS, size exclusion chromatography with multi-angle laser light scattering [SEC-MALLS], analytical ultracentrifugation [AUC]) and charge heterogeneity (cIEF), disulfide isoforms (RP-HPLC) and oxidation level (RP-UHPLC). The RGB-14-P versus EU/US Prolia® and RGB-14-X versus EU/US Xgeva® are similar in terms of all variants (Table 6).
Table 6.
Summary of the quantitative analytical data associated with size- and charge-related variants and disulfide isoforms of RGB-14-P and RGB-14-X biosimilars to the reference products EU/US Prolia® and Xgeva®
| Attribute | EU Prolia® quality range (n) | US Prolia® quality range (n) | RGB-14-P min–max range (n) | EU Xgeva® quality range (n) | US Xgeva® quality range (n) | RGB-14-X min–max range (n) |
|---|---|---|---|---|---|---|
| Size-related variants | ||||||
| SE-HPLC (area%) | ||||||
| HMW | 0.13–1.07 (41) | 0.25–0.86 (28) | 0.42–0.75 (8) | 0.14–1.51 (34) | 0.30–1.10 (23) | 0.49–0.69 (6) |
| Monomer + LMWsa | 98.93–99.87 (41) | 99.14–99.75 (28) | 99.25–99.58 (8) | 98.49–99.86 (34) | 98.90–99.70 (23) | 99.31–99.51 (6) |
| NR-CE-SDS (area%) | ||||||
| HC-HC-LC | 1.5–2.4 (16) | 1.3–2.6 (22) | 2.0–2.6 (8) | 1.4–2.3 (20) | 1.6–2.2 (17) | 2.0–2.3 (6) |
| HC-HC | < LOQ–0.6 (16) | < LOQ–0.5 (22) | N/Ab | < LOQ–0.8 (20) | < LOQ–0.5 (17) | N/Ab |
| LC | < LOQ–0.4 (16) | < LOQ–0.4 (22) | 0.4–0.4 (8) | < LOQ–0.3 (20) | 0.3–0.4 (17) | 0.4–0.4 (6) |
| ∑LMWs | 1.8–3.2 (16) | 1.6–3.4 (22) | 2.3–3.0 (8) | 1.5–3.4 (20) | 2.0–3.0 (17) | 2.3–2.7 (6) |
| Monomer | 96.8–98.2 (16) | 96.6–98.4 (22) | 97.0–97.7 (8) | 96.6–98.5 (20) | 97.0–98.0 (17) | 97.4–97.7 (6) |
| Charge-related variants | ||||||
| cIEF (area%) | ||||||
| Acidic variants | 25.9–40.0 (32) | 28.3–38.1 (30) | 30.5–32.6 (8) | 25.2–41.0 (29) | 28.5–39.4 (23) | 31.3–34.4 (6) |
| Basic variants | 1.5–12.0 (32) | 2.9–9.7 (30) | 5.4–7.1 (8) | 1.9–12.9 (29) | 3.0–10.1 (23) | 6.0–7.5 (6) |
| Main peak | 56.7–64.1 (32) | 57.1–64.1 (30) | 60.9–63.6 (8) | 55.4–63.7 (29) | 56.5–62.7 (23) | 58.5–62.3 (6) |
| cIEF (area%) with CPB digestion | ||||||
| Acidic variants | 34.0–37.7 (16) | 31.7–38.3 (24) | 32.5–35.0 (8) | 31.5–39.8 (13) | 33.5–37.3 (15) | 33.0–34.9 (6) |
| Basic variants | 4.0–5.9 (16) | 3.3–5.7 (24) | 2.2–3.7 (8) | 4.1–5.5 (13) | 4.1–5.5 (15) | 2.4–3.7 (6) |
| Main peak | 57.0–61.6 (16) | 56.6–64.5 (24) | 61.3–65.0 (8) | 55.8–63.6 (13) | 57.7–62.1 (15) | 61.8–64.4 (6) |
| pI of main peak | 8.676–8.749 (32) | 8.662–8.758 (30) | 8.676–8.736 (8) | 8.651–8.761 (29) | 8.667–8.763 (23) | 8.729–8.734 (6) |
| Disulfide isoforms | ||||||
| RP-HPLC (area%) | ||||||
| B | 43.4–83.7 (45) | 50.4–81.9 (30) | 55.7–60.6 (8) | 43.4–82.2 (39) | 54.8–79.7 (22) | 55.8–60.5 (6) |
| A/B | 4.4–32.5 (45) | 5.5–28.2 (30) | 20.5–23.4 (8) | 5.5–32.6 (39) | 6.7–25.2 (22) | 20.5–23.1 (6) |
| A1 | 3.3–14.4 (45) | 4.3–11.9 (30) | 9.7–11.7 (8) | 4.0–13.8 (39) | 5.1–10.5 (22) | 9.8–11.7 (6) |
| A2 | 7.7–10.7 (45) | 7.6–10.2 (30) | 9.0–9.3 (8) | 7.7–10.8 (39) | 8.0–10.0 (22) | 9.1–9.4 (6) |
n, number of batches analysed, given in brackets after specific range values
aMonomer and LMW peaks are integrated together
bNot detected or under LOQ
On the basis of SE-HPLC measurements, RGB-14-P and RGB-14-X batches show similar HMW and monomer + LMW content to the reference products. The monomer and LMW peaks were integrated together because of poor separation, therefore this method was not appropriate for the thorough comparison of the level of LMWs (Fig. 7a).
Fig. 7.
Size- and charge-related variants of RGB-14-P versus EU/US Prolia® and RGB-14-X versus EU/US Xgeva®: a SE-HPLC chromatograms, b NR-CE-SDS electropherograms, c cIEF electropherograms: native treatment, and d cIEF electropherograms: after CPB digestion
For the determination of ΣLMW and specific LMW contents, NR-CE-SDS was applied as an orthogonal method (Fig. 7b). Although minor differences can be observed for specific LMWS, the ΣLMW content in all products is very low and can be considered highly similar regarding RGB-14-P versus EU/US Prolia® and RGB-14-X versus EU/US Xgeva®. The SEC-MALLS analysis provided molecular weight data besides the monomer and HMW ratio values. The monomer ratio, monomer MW, HMW ratio and HMW MW data showed that RGB-14 and reference products are similar (Supplementary Table 5). Using AUC, the HMW and LMW contents were under LOQ for all products. RGB-14-P and RGB-14-X batches showed highly similar monomer content, monomer MW and sedimentation coefficient (Supplementary Table 5) to the EU/US reference products Prolia® and Xgeva®.
cIEF was applied for charge variant characterisation. The native cIEF analysis (Fig. 7c) revealed that RGB-14-P and RGB-14-X are similar in the charge variant profile to EU/US Prolia® and Xgeva®: all RGB-14 batches are well within the QRs established for all charge related parameters: main peak, acidic and basic variants. The cIEF analysis with CPB digestion revealed similarity for the acidic variants between RGB-14 and the reference products, but a slightly lower level of basic variants was obtained for the RGB-14 products compared with the reference products (Fig. 7d).
On the basis of RP-HPLC measurements of IgG2-B, IgG2-A/B, IgG2-A1 and IgG2-A2, the disulfide isoform patterns and content of RGB-14-P and RGB-14-X are similar to those of the EU/US reference products Prolia® and Xgeva® (Fig. 8).
Fig. 8.
a RP-HPLC chromatograms of disulfide isoforms B, A/B, A1 and A2 for RGB-14-P versus EU/US Prolia® and RGB-14-X versus EU/US Xgeva®, and comparison of the results obtained for the disulfide variants for (b, c) B variants, (d, e) A/B variants, (f, g) A1 variants and (h, i) A2 variants. Red dashed lines: upper and lower limits of US QRs (Prolia®: B variant: 50.4–81.9%, A/B variant: 5.5–28.2%, A1 variant: 4.3–11.9%, A2 variant: 7.6–10.2%; Xgeva®: B variant: 54.8–79.7%, A/B variant: 6.7–25.2%, A1 variant: 5.1–10.5%, A2 variant: 8.0–10.0%); black dashed lines: upper and lower limits of EU QRs (Prolia®: B variant: 43.4–83.7%, A/B variant: 4.4–32.5%, A1 variant: 3.3–14.4%, A2 variant: 7.7–10.7%; Xgeva®: B variant: 43.4–82.2%, A/B variant: 5.5–32.6%, A1 variant: 4.0–13.8%, A2 variant: 7.7–10.8%)
Stress studies of protein oxidation (H2O2, UV light) in denosumab have shown that Met253 is the amino acid most susceptible to oxidation (results not shown). The Met253 oxidation is therefore a marker for both the overall level of oxidation and the highest level of oxidation observed in RGB-14 and reference products. Under normal storage conditions, this residue is not oxidised by more than 5.0%. On the basis of the results of the Met253 oxidation level measured by RP-HPLC peptide mapping method, the RGB-14-P and RGB-14-X are similar to the reference products EU/US Prolia® and Xgeva® (Table 4).
Functional Biological Activity
Both soluble and membrane-bound RANKL are biologically capable of binding to its receptor RANK on bone marrow macrophages. RANKL-mediated oligomerisation of RANK on cell surfaces triggers the activation of intracellular signalling molecules such as NF-κB, leading to osteoclast differentiation, activation, and survival [29, 30]. It is possible that the membrane-bound form is the determinant of the spatial distribution and magnitude of bone resorption in vivo [31]. The primary mechanism of action of denosumab is the inhibition of RANKL-mediated activities through the binding and neutralisation of RANKL.
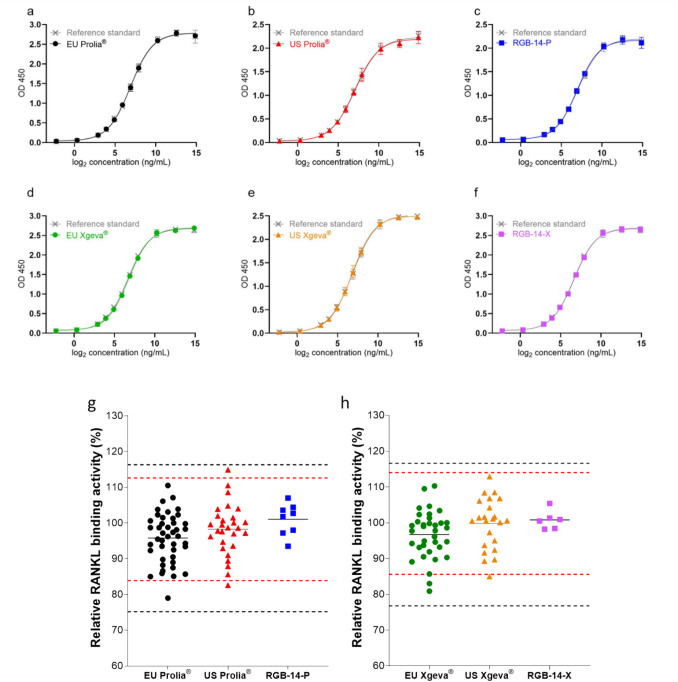
RANKL binding activity was measured using three different techniques to ensure similarity between RGB-14 and the reference products. The relative binding activity was measured by indirect RANKL ELISA assay (Fig. 9), whilst the kinetics of RANKL binding was detected with biolayer interferometry method. The orthogonal soluble RANKL binding methods demonstrate a high level of similarity between RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva®. In addition to the equilibrium dissociation constant (KD), BLI measurements also show a similar association (ka) and dissociation rate constant (kd) (Fig. 10).
Fig. 9.
sRANKL binding activity of RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva® measured by ELISA. Representative dose-response curves of a EU Prolia®, b US Prolia®, c RGB-14-P, d, EU Xgeva®, e US Xgeva® and f RGB-14-X. Scatterplot of the sRANKL binding activity of g EU/US Prolia® versus RGB-14-P and h EU/US Xgeva® versus RGB-14-X. Red dashed lines: upper and lower limits of US QRs (Prolia®: 83.9–112.6%; Xgeva®: 85.6–114.0%); black dashed lines: upper and lower limits of EU QRs (Prolia®: 75.2–116.3%; Xgeva®: 76.8–116.6%)
Fig. 10.
sRANKL binding affinity of RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva® batches measured by BLI method. Representative sensorgrams of a EU Prolia®, b US Prolia®, c RGB-14-P, d EU Xgeva®, e US Xgeva® and f RGB-14-X. KD results illustrated on scatterplot of g EU/US Prolia® versus RGB-14-P and h EU/US Xgeva® versus RGB-14-X. Red dashed lines: upper and lower limits of US QRs (Prolia®: 1.80 × 10−10 to 4.52 × 10−10 M; Xgeva®: 1.95 × 10−10 to 4.63 × 10−10 M); black dashed lines: upper and lower limits of EU QRs (Prolia®: 1.53 × 10−10 to 5.05 × 10−10 M; Xgeva®: 2.20 × 10−11 to 5.97 × 10−10 M)
Binding to membrane-associated RANKL (mbRANKL) was also measured with a cell-based flow cytometry method. The results are presented as the mean potency relative to the primary reference standard. RGB-14-P, RGB-14-X and the EU/US Prolia®, Xgeva® are considered to have similar binding activity to mbRANKL (Fig. 11).
Fig. 11.
mbRANKL binding of RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva® batches measured by flow cytometry-based assay. Representative dose-response curves of a EU Prolia®, b US Prolia®, c RGB-14-P, d EU Xgeva®, e US Xgeva® and f RGB-14-X. Scatterplot of the mbRANKL binding activity of g EU/US Prolia® versus RGB-14-P and h EU/US Xgeva® versus RGB-14-X. Black dashed lines: upper and lower limits of EU QRs (Prolia®: 83.9–112.6%; Xgeva®: 89.9–117.7%)
The comparative assessment of the potency of RGB-14 and reference products to inhibit RANKL-RANK signalling and osteoclast differentiation was performed using a DiscoverX’s PathHunter NF-κB/IκB neutralisation reporter bioassay and a RAW264.7 cell line-based TRAcP5b ELISA. Functional assay results indicate that the ability of RGB-14-P, RGB-14-X and the EU/US Prolia®, Xgeva® to inhibit RANKL signalling (Fig. 12) and osteoclast differentiation (Fig. 13) was similar.
Fig. 12.
RANKL neutralisation activity of RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva® batches measured by cell-based NF-κB/IκB neutralisation reporter assay. a EU Prolia®, b US Prolia®, c RGB-14-P, d EU Xgeva®, e US Xgeva® and f RGB-14-X. Scatterplot of the RANKL neutralisation activity of g EU/US Prolia® versus RGB-14-P and h EU/US Xgeva® versus RGB-14-X. Red dashed lines: upper and lower limits of US QRs (Prolia®: 84.2–111.9%; Xgeva®: 86.1–114.2%); black dashed lines: upper and lower limits of EU QRs (Prolia®: 79.2–116.9%; Xgeva®: 82.3–119.3%)
Fig. 13.
Inhibition activity of osteoclast differentiation of RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva® batches measured by cell-based TRAcP5b ELISA. a EU Prolia®, b US Prolia®, c RGB-14-P, d EU Xgeva®, e US Xgeva® and f RGB-14-X. Scatterplot of the inhibition activity of osteoclast differentiation of g EU/US Prolia® versus RGB-14-P and h EU/US Xgeva® versus RGB-14-X. Red dashed lines: upper and lower limits of US QRs (Prolia®: 94.0–107.9%; Xgeva®: 93.5–104.0%); black dashed lines: upper and lower limits of EU QRs (Prolia®: 94.4–102.4%; Xgeva®: 88.6–108.3%)
Several Fc-associated functions were investigated, however, apart from neonatal Fc receptor (FcRn) binding, IgG2 antibodies do not have Fc-related activity. FcRn is widely expressed on the surface of endothelial and other cells that are capable of internalising and releasing antibodies in a pH-dependent manner resulting in improved in vivo stability (half-life) and efficacy. As a result of this, the pharmacokinetic properties of recombinant human monoclonal antibody therapeutics are directly influenced by FcRn binding [32, 33]. As part of the in vitro similarity study, this is an important function to determine. The FcRn binding affinity and the kinetic parameters measured by BLI are similar for RGB-14 and reference drug products. As shown in Fig. 14g, some RGB-14-P batches fall slightly outside the US Prolia® QR, with one batch lower than the EU Prolia® QR. This deviation is attributed to the relatively narrow QR defined for the KD parameter and the higher method variability (Table 7).
Fig. 14.
FcRn binding of RGB-14-P, RGB-14-X and EU/US Prolia®, Xgeva® batches measured by BLI. Representative sensorgrams of a EU Prolia®, b US Prolia®, c RGB-14-P, d EU Xgeva®, e US Xgeva® and f RGB-14-X. KD results illustrated on scatterplot of g EU/US Prolia® versus RGB-14-P and h EU/US Xgeva® versus RGB-14-X. Red dashed lines: upper and lower limits of US QRs (Prolia®: 1.97 × 10−08 to 2.92 × 10−08 M; Xgeva®: 1.81 × 10−08 to 3.35 × 10−08 M); black dashed lines: upper and lower limits of EU QRs (Prolia®: 1.77 × 10−08 to 3.85 × 10−08 M; Xgeva®: 1.47 × 10−08 to 3.62 × 10−08 M)
Table 7.
Summary of the quantitative functional assay data of RGB-14-P and RGB-14-X biosimilars to the reference products EU/US Prolia® and Xgeva®
| Attribute | EU Prolia® quality range (n) | US Prolia® quality range (n) | RGB-14-P min–max range (n) | EU Xgeva® quality range (n) | US Xgeva® quality range (n) | RGB-14-X min–max range (n) |
|---|---|---|---|---|---|---|
| Biological activity | ||||||
| RANKL binding by ELISA | ||||||
| Biological activity (rel. %) | 75.2–116.3 (43) | 83.9–112.6 (28) | 93.5–107.0 (8) | 76.8–116.6 (35) | 85.6–114.0 (23) | 98.2–105.4 (6) |
| RANKL interaction by BLI | ||||||
| KD (M) | 1.53 × 10−10 to 5.05 × 10−10 (24) | 1.80 × 10−10 to 4.52 × 10−10 (25) | 2.83 × 10−10 to 4.06 × 10−10 (8) | 2.20 × 10−11 to 5.97 × 10−10 (16) | 1.95 × 10−10 to 4.63 × 10−10 (18) | 2.59 × 10−10 to 3.75 × 10−10 (6) |
| ka (1/Ms) | 2.81 × 10+05 to 9.04 × 10+05 (24) | 3.18 × 10+05 to 8.51 × 10+05 (25) | 4.51 × 10+05 to 6.13 × 10+05 (8) | 2.70 × 10+05 to 9.20 × 10+05 (16) | 2.45 × 10+05 to 8.98 × 10+05 (18) | 4.32 × 10+05 to 6.15 × 10+05 (6) |
| kd (1/s) | 1.09 × 10−04 to 2.71 × 10−04 (24) | 9.71 × 10−05 to 2.60 × 10−04 (25) | 1.29 × 10−04 to 2.08 × 10−04 (8) | 7.59 × 10−05 to 2.75 × 10−04 (16) | 1.13 × 10−04 to 2.51× 10−04 (18) | 1.49 × 10−04 to 2.22 × 10−04 (6) |
| Cell surface RANKL binding by flow cytometry | ||||||
| Biological activity (rel. %) | 87.6–110.7 (8) | N/A (3) | 89.9–105.1 (8) | 89.9–117.7 (7) | N/A (3) | 98.6–105.0 (6) |
| RANKL neutralisation cell-based assay | ||||||
| Biological activity (rel. %) | 79.2–116.9 (44) | 84.2–111.9 (27) | 90.0–101.3 (8) | 82.3–119.3 (34) | 86.1–114.2 (24) | 94.0–110.7 (6) |
| Inhibition of osteoclast differentiation | ||||||
| Biological activity (rel. %) | 94.4–102.4 (8) | 94.0–107.9 (12) | 96.0–105.9 (8) | 88.6–108.3 (7) | 93.5–104.0 (12) | 92.6–103.3 (6) |
| FcRn receptor binding affinity by BLI | ||||||
| KD (M) | 1.77 × 10−08 to 3.85 × 10−08 (29) | 1.97 × 10−08 to 2.92 × 10−08 (25) | 1.72 × 10−08 to 3.01 × 10−08 (8) | 1.47 × 10−08 to 3.62 × 10−08 (27) | 1.81 × 10−08 to 3.35 × 10−08 (22) | 2.16 × 10−08 to 2.72 × 10−08 (6) |
| ka (1/Ms) | 3.00 × 10+05 to 6.63 × 10+05 (29) | 3.14 × 10+05 to 6.90 × 10+05 (25) | 3.79 × 10+05 to 7.14 × 10+05 (8) | 3.35 × 10+05 to 6.83 × 10+05 (27) | 3.31 × 10+05 to 6.20E+05 (22) | 4.40 × 10+05 to 6.02 × 10+05 (6) |
| kd (1/s) | 9.76 × 10−03 to 1.69 × 10−02 (29) | 7.22 × 10−03 to 1.71 × 10−02 (25) | 9.21 × 10−03 to 1.42 × 10−02 (8) | 8.03 × 10−03 to 1.76 × 10−02 (27) | 9.57 × 10−03 to 1.45 × 10−02 (22) | 1.12 × 10−02 to 1.35 × 10−02 (6) |
n: number of batches analysed, given in brackets after specific range values
N/A not applicable, BLI biolayer interferometry, ELISA enzyme-linked immunosorbent assay, FcRn neonatal Fc receptor, RANKL receptor activator of nuclear factor-κB ligand, KD equilibrium dissociation constant, ka association rate constant, kd dissociation rate constant
Although FcγR-binding affinity can play a role in the effector functions of IgG1 and IgG4 [34], denosumab as an IgG2 subclass antibody has low affinity for FcγRIII and FcγRII, and no significant affinity to FcγRI receptor can be detected.
The two alleles of the genes that encode the human FcγRIIa and FcγRIIIa receptors result in lower- and higher-affinity variants. The polymorphisms in these genes are located at positions 131 (H/R) [35] and 158 (V/F) [36], respectively. The higher affinity variant of FcγRIIa (H131) and FcγRIIIa (V158) were used to determine the kinetic parameters of denosumab binding by BLI. Affinity (KD) results with their kinetic parameters of the two Fc-gamma receptors (FcγRIIIa, FcγRIIa) show similarity between RGB-14 and reference drug products (Supplementary Figs. 12–13, Table 6). For FcγRI, no binding activity could be detected for any product (Supplementary Fig. 14, Table 6).
Since denosumab binds less efficiently to FcγRIIIa (mean KD value: ~ 1.5 × 10−5 M), it has no significant antibody-dependent cellular cytotoxicity (ADCC), as evidenced by the negative assay performed for all products (Supplementary Fig. 15).
The C1q binding affinity and kinetic parameters of RGB-14-P and RGB-14-X measured with BLI were within the QRs (Supplementary Fig. 16, Table 6). Despite relatively strong C1q binding affinity (mean KD value: ~ 7.0 × 10−8 M), complement-dependent cytotoxicity (CDC) activity was not detected in either product (Supplementary Fig. 17).
Protein Content and Particles
Protein concentration values of RGB-14 drug products fall in the quality ranges determined for EU/US Prolia® and Xgeva®, and only a single RGB-14-P is marginally outside because of the narrow US Prolia® QR (Supplementary Table 7). On the basis of the results of MFI and RMM measurements (Supplementary Table 7), RGB-14 batches show similar or slightly lower number of subvisible particles as compared with Prolia® and Xgeva® batches.
Drifts in Quality Attributes of Reference Products Prolia® and Xgeva®
During the collection of reference sample data, we observed the clustering of certain quality attributes. This clustering appeared to correlate with the expiration dates of the samples. This phenomenon is consistent with literature findings [37, 38], where the observed significant changes in quality parameters were linked to batch expiry dates. Quality attributes of EU/US Prolia® and Xgeva® batches with expiry dates ranging from July 2017 to September 2024 were evaluated. Two distinct periods of attribute consistency were observed: one around early 2019 (referred to as the first drift) and another beginning in April–May 2020 (second drift). The exact starting date of the first drift could not be determined due to a lack of data between August 2018 and January 2019; however, data of batches expiring in early 2019 illustrate the trend. The second drift is evident for batches expiring from April to May 2020 onward. During the measurements it was found that for both EU and US Prolia® and Xgeva® batches, several physico-chemical parameters—including glycation, disulfide isoform profile, charge variant profile and N-glycosylation—showed noticeable shifts. These changes were adequately monitored by continuous ‘fingerprint’ data collection.
The results were plotted according to the batch expiry dates for each market (Fig. 15). On each graph, the X-axis represents the expiry date, and the exact location of each data point on the X-axis corresponds to the same batch across all methods. The drift phenomenon is most evident for the disulfide isoforms and glycation results. An upward shift was observed for the IgG2-B isoform, accompanied by a corresponding downward shift of the IgG2-A/B and IgG2-A1 isoforms. Interestingly, the shifts occurred simultaneously in both the EU and US markets for Prolia® and Xgeva®. Only the disulfide variant B is shown in this article (Fig. 15a). The glycation results precisely reflect the drift observed for the disulfide isoforms (Fig. 15b). However, drift 1 could not be evaluated for glycation due to the lack of data from that time. In November 2019, Amgen filed a patent application [39] aimed at modulating the glycan profile. Our results show that the two previously identified drifts appear also in the glycan profile, especially in the levels of afucosylated and high-mannose glycan species, although the magnitude of these drifts is not as significant as those observed for disulfide isoforms and glycation (Fig. 15c).
Fig 15.
Trends in disulfide isoform, mono-glycation and high-mannose quality attributes of EU/US reference products by expiry date. Grey shaded boxes show the min-max range of specific drift periods. a IgG2-B variants, b mono-glycated forms, c high-mannose glycan species and d RANKL neutralisation activity
It was examined whether the observed changes in physico-chemical quality attributes are reflected in the biological activity, however, no significant drift was found. In this article, we present the results of the RANKL neutralisation assay (Fig. 15d).
Discussion
State-of-the-art analytical and functional techniques were applied during the characterisation and similarity assessment of RGB-14-P, RGB-14-X, EU/US Prolia® and Xgeva® drug products. Mass spectrometric analysis of both intact and enzymatically digested proteins verified that the amino acid sequence of denosumab is identical between the biosimilar and reference product batches. LC-MS data were also used to compare the post-translational modification profiles of the products.
The site-specific results of N-glycosylation, oxidation, deamidation, isomerisation, succinimide formation, lysine and proline hydroxylation and N- and C-terminal variants (Tables 4 and 5) proved that the PTM profiles of RGB-14-P and RGB-14-X are highly similar to those of Prolia® and Xgeva®. Minor differences were found only in the levels of hydroxylation at K126, P194 and P330, N-terminal glutamate to pyroglutamate conversion, C-terminal lysine variant in the heavy chain and some glycopeptides. However, these parameters have no effect on antigen or FcRn binding. Moreover, the C-terminal lysine is rapidly cleaved in serum by carboxypeptidase B and is not expected to affect immunogenicity or safety [40].
The similarity of the level of high-mannose forms—a critical parameter for denosumab—was confirmed at the level of released glycans by HILIC-UHPLC-FL analysis.
The level of glycation was measured at intact level, and the results for RGB-14-P and RGB-14-X fell within the similarity ranges established by EU/US Prolia® and Xgeva® batches.
The higher-order structure of the denosumab protein in the RGB-14-P and RGB-14-X drug products was confirmed to be highly similar to that of Prolia® and Xgeva® using μDSC, far-UV CD, FT-IR, NMR and HDX-MS methods.
However, μDSC measurements revealed that the transition midpoint temperature, corresponding to the CH3-domain (Tm3) of the protein, is slightly lower in RGB-14-P and RGB-14-X compared with Prolia® and Xgeva® samples. These minor differences are most likely attributable to batch-to-batch variability in the disulfide–isoform composition of reference drug products, and these variations have no effect on biological activity and stability.
The LC-MS results from the charge variant enrichment study of RGB-14 drug substance (detailed results not shown) revealed that the basic fraction was enriched in C-terminal lysine and proline-amidated forms. This fraction also exhibited a lower rate of glycation and a predominance of disulfide isoforms B and A2. R-CE-SDS analysis indicated a slight enrichment of NgHC in the basic fraction. Despite these differences, no significant variation was observed in RANKL and FcRn binding BLI measurements of the charge variants.
The combined results from the charge variant enrichment study and the reduced peptide mapping analysis provide explanation for the differences in basic variant profiles between RGB-14 and reference products. Following CPB digestion, C-terminal lysine variants are no longer present. The slightly higher levels of basic variants of the EU/US Prolia® and Xgeva® are due to their slightly higher levels of NgHC, isomerised amino acid species and enhanced N-terminal glutamate to pyroglutamate conversion [41]. Native cIEF analysis revealed some differences in the basic variant profiles; however, the overall levels of basic variants were similar for RGB-14-P versus EU/US Prolia® and RGB-14-X versus EU/US Xgeva®. This apparent similarity can be explained by the fact that simultaneously higher level of C-terminal lysine variant and lower level of NgHC, N-terminal glutamate to pyroglutamate conversion and isomerised amino acid modifications were obtained for RGB-14-P and RGB-14-X compared with EU/US Prolia® and Xgeva®, and these differences—compensating each other—resulted in similar level of basic variants.
On the basis of the characteristics of the charge variants and the results of the biological activity assays, the observed differences in basic variants have no impact on safety and efficacy and are not considered clinically meaningful.
Clustering was observed for many physico-chemical parameters. Upon investigation, two distinct drift periods were identified: one occurring over a short interval in early 2019, and another beginning in April–May 2020. These shifts may be associated with a technology change implemented by the innovator company, Amgen. For physico-chemical attributes with long-term data availability, a characteristic correlation was observed for several attributes, including disulfide isoforms, glycation, N-glycan profiles and charge variants. The relevance of the drift phenomenon is further enhanced by structure–function relationships, as the disulfide isoforms have different antigen-binding activities [42]. Importantly, our analyses revealed that all these differences were not detectable in the biological activity of the products, a conclusion that was confirmed by multiple methods.
Conclusions
Highly similar biological activity was demonstrated between RGB-14-P, RGB-14-X and the EU/US Prolia®, Xgeva® by multiple state-of-the-art orthogonal binding and functional methods. The similarity study provides robust evidence that the structure and function of RGB-14-P, RGB-14-X are highly similar to those of EU/US Prolia® and Xgeva®.
Minor differences were observed in certain physico-chemical attributes, including the N-glycan profile and the charge variant profile obtained by CPB-digestion (notably in basic variants). High-resolution structural characterisation techniques were employed to elucidate the subtle variations detected in the physico-chemical measurements. However, these are considered clinically not meaningful. On the basis of the comprehensive evaluation of the aforementioned physico-chemical and functional similarity data, RGB-14-P, RGB-14-X and the EU/US Prolia®, Xgeva® can be regarded as highly similar denosumab drug products and are therefore anticipated to exhibit comparable efficacy and safety profiles. Furthermore, successful clinical trials on pharmacokinetics, safety, immunogenicity and efficacy (manuscript in preparation) have reinforced the biosimilarity between RGB-14-P, RGB-14-X and the reference products EU/US Prolia® and Xgeva® (publication of results in progress).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully thank the analytical scientists and technicians of Biotechnology Process Development and Analytics for method developments, qualifications and validations, measurements, data supply and figure preparation.
Declarations
Employment
All authors are employed by Gedeon Richter Plc.
Conflicts of Interest
The authors acknowledge potential conflicts of interest as current employees of Gedeon Richter Plc.
Funding
This study was funded by Gedeon Richter Plc.
Availability of Data and Material
The authors confirm that the relevant data supporting the findings of this study are available within the article and its supplementary material.
Ethics Approval
No studies with human participants or animals were carried out during this study. The suppliers of the human cell lines used in this study are indicated in the relevant sections of the article.
Consent to Participate/Publish
Not applicable.
Authors’ Contribution
Viktor Háda and Ágnes Szonja Garai developed the concept of this study. Dániel Hüse, Ádám Fizil, Zsolt Zólyomi, Gábor Lehoczki, Andrea Kis, and Zsuzsanna Kálmán-Szekeres designed and performed experiments. All authors participated in the collection, evaluation and discussion of the results and contributed text and figures to the manuscript. All authors read the manuscript and approved its publication.
Code availability
Not applicable.
References
- 1.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9:S1. 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller PD. Denosumab: anti-RANKL antibody. Curr Osteoporos Rep. 2009;7(1):18–22. 10.1007/s11914-009-0004-5. [DOI] [PubMed] [Google Scholar]
- 3.CHMP Assessment Report for Prolia® (Procedure No. EMEA/H/C/001120, 2010). https://www.ema.europa.eu/en/documents/assessment-report/prolia-epar-public-assessment-report_en.pdf. Accessed 18 June 2025.
- 4.US Food and Drug Administration. Prolia® (denosumab) injection (Reference ID: 5596256). https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/125320s219lbl.pdf. Accessed 18 June 2025.
- 5.CHMP Assessment Report for Xgeva® (Procedure No. EMEA/H/C/002173, 2011). https://www.ema.europa.eu/en/documents/assessment-report/xgeva-epar-public-assessment-report_en.pdf. Accessed 18 June 2025.
- 6.US Food and Drug Administration. Xgeva (denosumab) injection (Reference ID: 5600322). https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/125320s222lbl.pdf. Accessed 18 June 2025.
- 7.US Food and Drug Administration. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/quality-considerations-demonstrating-biosimilarity-therapeutic-protein-product-reference-product. Accessed 18 June 2025.
- 8.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf. Accessed 18 June 2025.
- 9.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active-substance-quality-issues-revision-1_en.pdf. Accessed 18 June 2025.
- 10.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products containing monoclonal antibodies—non-clinical and clinical issues. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-monoclonal-antibodies-non-clinical-and-clinical-issues_en.pdf. Accessed 18 June 2025.
- 11.ICH guideline Q8 (R2) on pharmaceutical development, EMA/CHMP/ICH/167068/2004 (22 June 2017). https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-considerations-ich-guideline-q8-r2-pharmaceutical-development-step-5_en.pdf. Accessed 18 June 2025.
- 12.ICH guideline Q9 (R1) on quality risk management, EMA/CHMP/ICH/24235/2006 Corr.2 (23 January 2025). https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-ich-guideline-q9-r1-quality-risk-management-step-5-revision-2_en.pdf. Accessed 18 June 2025.
- 13.ICH guideline Q10 on pharmaceutical quality system, EMA/CHMP/ICH/214732/2007 (September 2015). https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-guideline-q10-pharmaceutical-quality-system-step-5_en.pdf. Accessed 18 June 2025.
- 14.ICH guideline Q11 on development and manufacture of drug substances (chemical entities and biotechnological/ biological entities), EMA/CHMP/ICH/425213/2011 (November 2012). https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q11-development-and-manufacture-drug-substances-chemical-entities-and-biotechnologicalbiological-entities_en.pdf. Accessed 18 June 2025.
- 15.Liu Y, Xie J, Li Z, Mei X, Cao D, Li S, Engle L, Liu S, Ebbers HC, Liu C. Demonstration of physicochemical and functional similarity of the biosimilar BAT1806/BIIB800 to reference tocilizumab. BioDrugs. 2024;38:571–88. 10.1007/s40259-024-00662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Hong E, Lee J, Hong S, Kim J, Cho M, Kim Y, Yoo T. Characterization for the similarity assessment between proposed biosimilar SB12 and eculizumab reference product using a state-of-the-art analytical method. BioDrugs. 2023;37:569–81. 10.1007/s40259-023-00591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie L, Zhang E, Xu Y, Gao W, Wang L, Xie MH, Qin P, Lu L, Li S, Shen P, Jiang W, Liu S. Demonstrating analytical similarity of trastuzumab biosimilar HLX02 to Herceptin® with a panel of sensitive and orthogonal methods including a novel FcγRIIIa affinity chromatography technology. BioDrugs. 2020;34:363–79. 10.1007/s40259-020-00407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stüber JC, Uhland K, Reiter A, Jakob S, Wolschin F. Comparative analytical evaluation of the proposed biosimilar FYB206 and its reference medicinal product Keytruda®. Drugs R&D. 2024;24:447–64. 10.1007/s40268-024-00485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantin G, Liu Q, Shah B, Kuhns S, Wikström M, Cao S, Liu J. Analytical and functional similarity of the biosimilar candidate ABP 654 to ustekinumab reference product. Drugs R&D. 2023;23:421–38. 10.1007/s40268-023-00441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Arora T, Klakamp S, Davis J, Chandrasekher YA, Young G, Du Y, Yu B, Miller KJ. Demonstration of physicochemical and functional similarity of biosimilar adalimumab-aqvh to adalimumab. Drugs R&D. 2023;23:377–95. 10.1007/s40268-023-00437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao D, Deng C, Wang G, Mei X, Xie J, Liu Y, Liu Y, Yang Y, Li S, Liu C. Physicochemical and functional similarity assessment between proposed bevacizumab biosimilar BAT1706 and reference bevacizumab. Drugs R&D. 2023;23:267–88. 10.1007/s40268-023-00432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinson RG, Arbogast LW, Marino JP, Delaglio F. Best practices in utilization of 2D-NMR spectral data as the input for chemometric analysis in biopharmaceutical applications. J Chem Inf Model. 2020;60:2339–55. [DOI] [PubMed] [Google Scholar]
- 23.Welch J, Ausin C, Brahme N, Lacana E, Ricci S, Shultz-DePalo M. The Mannose in the mirror: a reflection on the pharmacokinetic impact of high mannose glycans of monoclonal antibodies in biosimilar development. Clin Pharmacol Ther. 2023;113(5):1003–10. 10.1002/cpt.2783. [DOI] [PubMed] [Google Scholar]
- 24.Liu-Shin LP, Fung A, Malhotra A, Ratnaswamy G. Evidence of disulfide bond scrambling during production of an antibody-drug conjugate. MAbs. 2018;10(8):1190–9. 10.1080/19420862.2018.1521128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng B, Lento C, Wilson DJ. Hydrogen deuterium exchange mass spectrometry in biopharmaceutical discovery and development—a review. Anal Chim Acta. 2016;940:8–20. 10.1016/j.aca.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Engen JR, Wales TE. Analytical aspects of hydrogen exchange mass spectrometry. Annu Rev Anal Chem. 2015;8:127–48. 10.1146/annurev-anchem-062011-143113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbogast LW, Brinson RG, Marino JP. Mapping monoclonal antibody structure by 2D 13C NMR at natural abundance. Anal Chem. 2015;87(7):3556–61. 10.1021/ac504804m. [DOI] [PubMed] [Google Scholar]
- 28.Elliott KW, Ghasriani H, Wikström M, Giddens JP, Aubin Y, Delaglio F, Marino JP, Arbogast LW. Comparative analysis of one-dimensional protein fingerprint by line shape enhancement and two-dimensional 1H,13C methyl NMR methods for characterization of the higher order structure of IgG1 monoclonal antibodies. Anal Chem. 2020;92(9):6366–73. 10.1021/acs.analchem.9b05385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–19. 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 30.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki J, Ikeda T, Kuroyama H, Seki S, Kasai M, Utsuyama M, Tatsumi M, Uematsu H, Hirokawa K. Regulation of osteoclastogenesis by three human RANKL isoforms expressed in NIH3T3 cells. Biochem Biophys Res Commun. 2004;314(4):1021–7. 10.1016/j.bbrc.2003.12.191. [DOI] [PubMed] [Google Scholar]
- 32.Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J Immunol. 2015;194(10):4595–603. 10.4049/jimmunol.1403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuber T, Frese K, Jaehrling J, Jäger S, Daubert D, Felderer K, Linnemann M, Höhne A, Kaden S, Kölln J, Tiller T, Brocks B, Ostendorp R, Pabst S. Characterization and screening of IgG binding to the neonatal Fc receptor. MAbs. 2014;6(4):928–42. 10.4161/mabs.28744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015;67(2 Pt A):171–82. 10.1016/j.molimm.2015.03.255. [DOI] [PubMed] [Google Scholar]
- 35.Warmerdam PA, Parren PW, Vlug A, Aarden LA, van de Winkel JG, Capel PJ. Polymorphism of the human Fc gamma receptor II (CD32): molecular basis and functional aspects. Immunobiology. 1992;185(2–4):175–82. 10.1016/S0171-2985(11)80639-X. [DOI] [PubMed] [Google Scholar]
- 36.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170(2):481–97. 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 2011;29(4):310–2. 10.1038/nbt.1839. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Song J, Park S, Ham S, Paek K, Kang M, Chae Y, Seo H, Kim HC, Flores M. Drifts in ADCC-related quality attributes of Herceptin®: impact on development of a trastuzumab biosimilar. MAbs. 2017;9(4):704–14. 10.1080/19420862.2017.1305530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International Application Published Under the Patent Cooperation Treaty (PCT); International Publication Number: WO 2019/213043 A1 (07.11.2019). https://patentimages.storage.googleapis.com/25/97/9b/40f1b338bb177d/WO2019213043A1.pdf. Accessed 18 June 2025.
- 40.Liu H, Ponniah G, Zhang HM, Nowak C, Neill A, Gonzalez-Lopez N, Patel R, Cheng G, Kita AZ, Andrien B. In vitro and in vivo modifications of recombinant and human IgG antibodies. MAbs. 2014;6(5):1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Valente J, Lin S, Chennamsetty N, Qiu D, Bolgar M. Cyclization of N-terminal glutamic acid to pyro-glutamic acid impacts monoclonal antibody charge heterogeneity despite its appearance as a neutral transformation. J Pharm Sci. 2019;209(10):3194–200. 10.1016/j.xphs.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Dillon TM, Ricci MS, Vezina C, Flynn GC, Liu YD, Rehder DS, Plant M, Henkle B, Li Y, Deechongkit S, Varnum B, Wypych J, Balland A, Bondarenko PV. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J Biol Chem. 2008;283(23):16206–15. 10.1074/jbc.M709988200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.