Abstract
During eye development, surface ectoderm cells that express PAX6 differentiate into corneal, limbal and conjunctival epithelia. However, several aspects of this differentiation process -- such as the developmental origin of the limbal epithelium and the mechanisms that underlie PAX6-mediated lineage specification -- are not properly understood. To explore these issues, we used single-cell RNA sequencing to study ocular surface epithelial cells derived from human induced pluripotent stem cells. Our analysis reveals that the corneal and conjunctival epithelial cell lineages originate from the surface ectoderm, and that the conjunctival lineage contributes to limbal epithelial cell populations. We also show that primordial conjunctival epithelial cells express limbal epithelial markers before lineage bifurcation. Finally, the activity and expression of PAX6 are highest in corneal epithelial cells, followed by those of the limbal epithelium and conjunctival epithelium, suggesting that lineage-specific differentiation is regulated by levels of PAX6 activity. These findings provide deeper understanding of the early-stage human ocular surface development.
Subject terms: Differentiation, Data integration
PAX6-dependent epithelial differentiation and developmental trajectories of the ocular surface are elucidated through single-cell RNA sequencing of hiPSC-derived ocular organoids.
Introduction
The surface of the human eye is covered by an ocular surface comprising conjunctival, limbal, and corneal epithelium. These epithelial cells originate from the surface ectoderm (SE). During the initial stages of ocular development, optic vesicles bilaterally contact SE, and the lens placode is induced at the sites of cellular contact. The lens placode then invaginates to form the lens vesicle1,2. The SE above the lens vesicle, referred to as ocular surface ectoderm (OSE), is characterized by the co-expression of PAX6 and TP633,4. Corneal and conjunctival epithelium derive from the OSE after the lens vesicle formation5. Then, limbal epithelium develops between the corneal and conjunctival epithelium and harbors limbal stem cells (LSCs)6–8 that provide corneal epithelial cells.
Genetic lineage-tracing studies showed that Pax6-positive progenitor cells contribute to the corneal epithelium development9, suggesting that Pax6-positive SE are the source of LSCs, but their embryonic origin remains elusive. The stem cell region of the conjunctival epithelium has been reported in the fornix, limbus, bulbar conjunctiva, palpebral conjunctiva, and mucocutaneous junction (MCJ), but the origin of conjunctival stem cells is unclear10. Clarifying the embryological origin is essential to understand the stem cell biology of ocular surface epithelium. However, how the SE differentiates into lineage-specific cells of the corneal, conjunctival, and limbal epithelium through specific gene expression changes is unknown.
PAX6 is an essential transcription factor (TF) for eye development11 that acts as a multi-level regulator during ocular morphogenesis. Earlier and broader inactivation of Pax6 in the OSE resulted in corneal and limbal epithelial cells adopting the conjunctival keratin expression12. Furthermore, PAX6 and TP63, a marker of stratified epithelial stem/progenitor cells, regulate LSC maintenance and differentiation9. Beyond the ocular surface, PAX6 is an essential eye field transcription factor (EFTF) that is crucial for eye development, interacting with RAX, SIX3, PAX6, LHX2, and SIX6 to induce retinal development and with SOX2 and SIX3 to drive lens formation. Furthermore, PAX6 has a dose-dependent role in lens development13. Given its critical role in the ocular surface and other eye structures, PAX6 expression levels may influence ocular surface epithelial differentiation. However, whether a comparable dose-dependency of PAX6 affects ocular surface epithelial development is not established.
Research into early human embryonic development has traditionally relied upon animal models and human fetal tissues. Recently, organoids that mimic their corresponding human tissues have been developed, providing important insights into the complex biological mechanisms underlying developmental processes14,15. In the context of ocular development, various ocular organoids such as corneal, lens, retinal, and lacrimal gland organoids have been successfully established, enabling disease modeling and functional studies of the respective tissues16. Beyond these applications, scRNA-seq has been used to explore developmental trajectories within cornea17, retina18, lacrimal gland19 organoids, revealing insights into lineage specification and cell maturation. In previous studies, we successfully generated a self-formed ectodermal autonomous multizone (SEAM) from human induced pluripotent stem cells (hiPSCs) as an ocular developmental organoid3. SEAM mimics the ocular developmental process, as demonstrated by its well-defined differentiation trajectory from hiPSCs to ectodermal ocular lineages20,21, proving its value as a tool for studying ocular development. Additionally, SEAM culture has enabled the generation and isolation of functional epithelial cell sheets from corneal and conjunctival epithelial progenitor cells, with KGF and EGF signaling promoting their differentiation3,22,23. This epithelial induction process potentially contains the lineage for ocular surface epithelial cells.
In this study, we aimed to investigate the lineage of early-stage ocular surface development by employing scRNA-seq to analyze surface epithelial cells derived from SEAMs and the differentiation landscape across the developmental transition from surface ectoderm to corneal, limbal, and conjunctival epithelial cells. Additionally, we investigated PAX6 expression and activity in these cells and identified the TF networks involved in the development of specialized ocular surface epithelia.
Results
Ocular development analysis combining SEAM and scRNA-seq
The establishment of reporter hiPSCs that can trace the OSE is important for understanding the development of the ocular surface. First, we generated PAX6-EGFP/TP63-tdTomato reporter hiPSCs to trace the lineages of the OSE (Supplementary Fig. 1a–c); we confirmed that they had the potential to differentiate into the SEAM using our established differentiation protocol (see Methods)3,22 (Fig. 1a). The SEAM forms four concentric zones from the center outwards, namely zones 1, 2, 3, and 4, corresponding to the neuroectoderm, optic vesicle cup, OSE, and SE, respectively. PAX6 is expressed in zones 1, 2, and 3, while TP63 is expressed in zones 3 and 4. After 4 weeks of reporter iPSC differentiation, we confirmed the co-localization of PAX6 and the enhanced green fluorescence protein (EGFP) signal across zones 1 to 3, as well as the co-localization of TP63 and tdTomato across zones 3 and 4 (Supplementary Fig. 1d). Thus, we successfully generated reporter iPSCs capable of tracking the OSE. Next, we differentiated SEAMs from the reporter iPSCs and subjected the iPSC-derived ectodermal cells to fluorescence-activated cell sorting (FACS) at 0, 2, 4, 6, 8, and 10 weeks (Fig. 1b). TP63-tdTomato-positive cells were observed at 2 weeks, with an increase in the number of PAX6 and TP63 double-positive cells observed at 4 weeks. Removing cell populations in zones 1 and 2 at 7 weeks through manual pipetting led to an increase in the number of TP63-positive cells by week 8. By week 10, an increase in the population of cells co-expressing PAX6 and TP63 was observed. Thus, tracking ocular surface ectoderm/epithelium differentiation from iPSCs was feasible.
Fig. 1. Experimental workflow from dual reporter iPSCs to SEAM and ocular surface epithelial cells for adaptation to 10x scRNA-seq.
a Schematic detailing the differentiation protocol from hiPSC colonies to SEAM. Differentiation is induced through media changes and growth factor treatments, leading to the expression of PAX6-EGFP and TP63-tdTomato. b Sequential imaging of hiPSC differentiation into ocular surface epithelial cells across 10 weeks, with PAX6-EGFP and TP63-tdTomato fluorescence indicating maturation, and FACS plots revealing TP63-positive cell frequency during KGF treatment. First corresponds to zone 1, second to zone 2, third/fourth to zones 3/4, third to zone 3, and fourth to zone 4. Representative images and FACS data from three independent biological replicates are shown. Scale bars: 100 μm. c The analysis workflow of post-differentiation includes sorting TP63-positive cells, 10x scRNA-seq, and bioinformatic integration to map cellular differentiation across time points (2w, 4w, and 8w) and identify distinctive cell subpopulations. DM differentiation medium, ODM ocular differentiation medium, OEM ocular epithelial medium, KGF keratinocyte growth factor, EGF epidermal growth factor, w week(s).
To perform a detailed analysis of the cells constituting the SEAM, we conducted scRNA-seq on unsorted total cells at 2 and 4 weeks after differentiation (Supplementary Fig. 2a). At 2 weeks, we identified six distinct cell clusters: optic vesicle cup, neuroepithelial cells, SE, neurons, residual iPSCs, and differentiating neuroepithelial cells (Supplementary Fig. 2b, c and Supplementary Data 1). The optic vesicle cup cluster was marked by the high expression of PAX6, SIX3, SIX6, LHX2, RAX, OTX2, VSX2, and MITF. Neuroepithelial cells exhibited a signature of high expression of PAX624, FOXG125,26, NES27, MSI128, SOX129,30, and SOX231. Differentiating neuroepithelial cells showed increased expression levels of neuroepithelial markers, as well as those of the early neuronal differentiation markers CUX1 and CUX232. Neurons exhibited high expression levels of neuronal markers such as TUBB333, MAP234, DCX35, and NEUROD136. TP63 expression was exclusive to the SE. Residual hiPSCs exhibited high POU5F137 and LIN28A expression38. To confirm the distribution of each cell cluster, immunohistochemistry was performed on cultured SEAMs (Supplementary Fig. 2d). TUBB3 and FOXG1 were localized to zone 1, RAX and VSX2 were restricted to zone 2, and MITF showed weak expression in both zones. These results indicate that neuroepithelial cells, differentiating neuroepithelial cells, and neurons corresponded to zone 1, the optic vesicle cup corresponded to zone 2, and SE corresponded to zones 3 and 4. To evaluate the activity of TFs in each cluster, we performed single-cell regulatory network inference and clustering (SCENIC), an analytical method that identifies TFs and their target genes39. PAX6 showed the highest regulon activity in the optic vesicle cup (Supplementary Fig. 2e). Supplementary Data 2 lists cluster-specific regulons, and Supplementary Data 3 presents PAX6 targets with enriched motifs or expression correlation. To systematically elucidate the interrelationships between TFs in the clusters, we conducted a TF regulon activity correlation network analysis (Supplementary Fig. 2f and Supplementary Data 2). The optic vesicle cup formed a network comprising optic vesicle markers such as SIX3, SIX6, VSX2, and PAX6. Based on similar gene expression profiles at 2 weeks, we identified five clusters comprising the optic vesicle cup, neuroepithelial cells, neurons, differentiating neuroepithelial cells, and SE from the analysis of total-cell scRNA-seq data at 4 weeks (Supplementary Fig. 2g, h and Supplementary Data 1). Additionally, a new cluster characterized by the expression of PAX2 and VAX1 was identified as the optic stalk. TUBB3 and FOXG1 were confined to zone 1 per immunohistochemistry results, while RAX and VSX2 were exclusive to zone 2. MITF was detected mainly in zone 2 and sporadically in zone 1 (Supplementary Fig. 2i). The PAX6 regulon activity decreased in the neural clusters compared with that of the optic vesicle cup cluster (Supplementary Fig. 2j and Supplementary Data 2, 3). This observation agrees with previous findings that Pax6 activity peaks during the neural progenitors' commitment and decreases as neurogenesis progresses40. TF regulon activity correlation network analysis demonstrated that the optic vesicle cup cluster formed a network characterized by optic vesicle markers such as SIX6, VSX2, and PAX6 (Supplementary Fig. 2k and Supplementary Data 2). Meanwhile, the optic stalk cluster showed high VAX1 activity. In vivo, VAX1 and PAX2 inhibit the expression of PAX6 and partition the retina from the optic stalk during ocular development41. At 2 weeks, PAX2-positive cells within the SEAM overlapped with PAX6-positive cells. By 3 and 4 weeks, PAX2-positive cells were mainly localized at the border between zones 1 and 2, exhibiting low PAX6 expression (Supplementary Fig. 2l). This transition from co-expression of PAX2 and PAX6 to a mutually exclusive distribution resembles the optic stalk development.
SE differentiation and the PAX6-associated TF network at 2 weeks of development
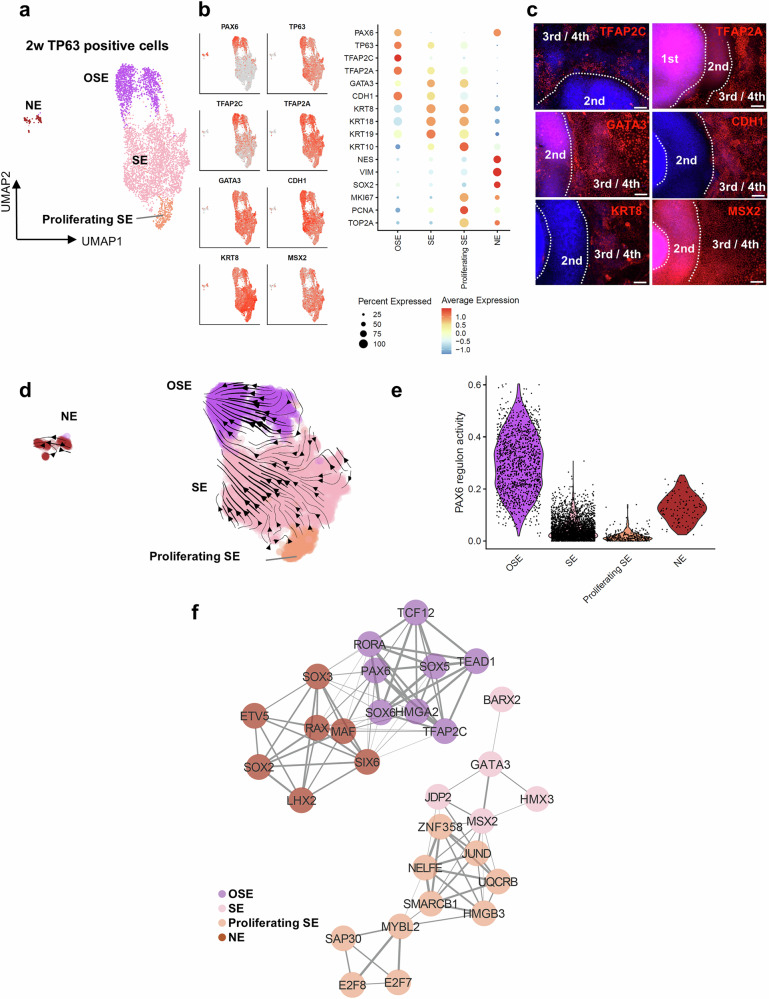
To investigate the progression of ocular surface epithelial cell differentiation, we conducted analyses at 2, 4, and 8 weeks. These time points were determined based on established developmental stages in the SEAM differentiation system. Previous reports indicate that TP63 becomes detectable around 2 weeks, marking the onset of epithelial lineage specification. At 4 weeks, colonies exhibit an autonomously formed four-layered structure, and major ocular anlagen are established at this stage. By 8 weeks, KRT12 expression is observed, indicating corneal epithelial commitment3. At these points, we isolated TP63-positive cells for scRNA-seq (Fig. 1c). Based on the gene expression profiles from the scRNA-seq data of TP63-positive cells at 2 weeks, we identified four distinct cell clusters (SE, OSE, proliferating SE, and neuroectoderm [NE]) (Fig. 2a, b and Supplementary Data 1). SE markers, including TFAP2C, TFAP2A GATA3, CDH1, KRT8, KRT18, and KRT1942–44, were ubiquitously expressed in the SE, OSE, and proliferating SE clusters. The OSE showed high expression of PAX6, TP63, and TFAP2C, while SE and proliferating SE exhibited low PAX6 expression. Proliferating SE exhibited high expression of MKI67, PCNA, and TOP2A, and NE exhibited high expression of NES, VIM, SOX2, and PAX6. We investigated the protein expression of SE markers, including TFAP2C, TFAP2A, GATA3, CDH1, KRT8, and MSX2, within the SEAM at 2 weeks (Fig. 2c). At this stage, distinguishing between zones 3 and 4 was difficult due to sporadic PAX6 expression in zone 3. Consequently, we classified TP63-positive zones as zones 3/4 (Fig. 1b) and observed significant SE marker expression in these zones. Although SE markers were observed in zones 3/4, TFAP2C expression was mainly confined to the inner layer of zones 3/4. These results indicated that zones 3/4 exhibited characteristics of the SE, and OSE was located in the inner layer of zones 3/4. Then we explored RNA velocity to analyze the differentiation dynamics of TP63-positive cells. The RNA velocity flow indicated a developmental trajectory from proliferating SE to SE, and from SE to OSE (Fig. 2d). Subsequently, we investigated PAX6 regulon activity (Fig. 2e and Supplementary Data 2, 3) and conducted TF network analysis on TP63-positive cells at 2 weeks (Fig. 2f and Supplementary Data 2). PAX6 showed the highest regulon activity in OSE, which was characterized by a network including PAX6 and TFAP2C. The ectodermal markers MSX2 and GATA3 were activated in SE and correlated with each other. Additionally, MSX2 was correlated with JUND in proliferating SE. E2F7 and E2F8, proliferation markers inherently associated with the squamous epithelial differentiation pathways45,46, were specifically activated in proliferating SE cells. In summary, during the early stages of eye development, TP63-positive cells were mainly composed of SE and OSE differentiated from SE, both forming distinct TF networks.
Fig. 2. Identification of a TF network involving PAX6, focusing on OSE and SE at 2 weeks of development.
a UMAP plot categorizing TP63-positive cells at 2 weeks into clusters corresponding to OSE, SE, proliferating SE, and NE. b UMAP and bubble plots of TP63-positive cells at 2 weeks showing the expression levels of key markers with an emphasis on OSE, SE, and NE. c Immunofluorescence staining of SEAM zones (first zone 1, second zone 2, third/fourth zone 3/4), highlighting the localization of markers (TFAP2C, TFAP2A, GATA3, CDH1, KRT8, and MSX2). Scale bar: 100 μm. Representative images from three independent biological replicates are shown. d RNA velocity analysis showing the dynamic transition of TP63-positive cells at 2 weeks, indicating the direction of cellular differentiation. e Violin plot depicting the PAX6 regulon activity in each cell cluster of TP63-positive cells at 2 weeks. f Network diagram from SCENIC analysis, illustrating the TF regulon activities specific to each cluster within the TP63-positive cells at 2 weeks. Node colors and edge thickness represent clusters and coefficients, respectively. scRNA-seq analyses (a, b, d–f) were performed using one biological replicate. OSE ocular surface ectoderm, SE surface ectoderm, NE neuroectoderm, w week(s).
Bifurcation of the corneo-conjunctival epithelial lineage and its associated TF network at 4 weeks
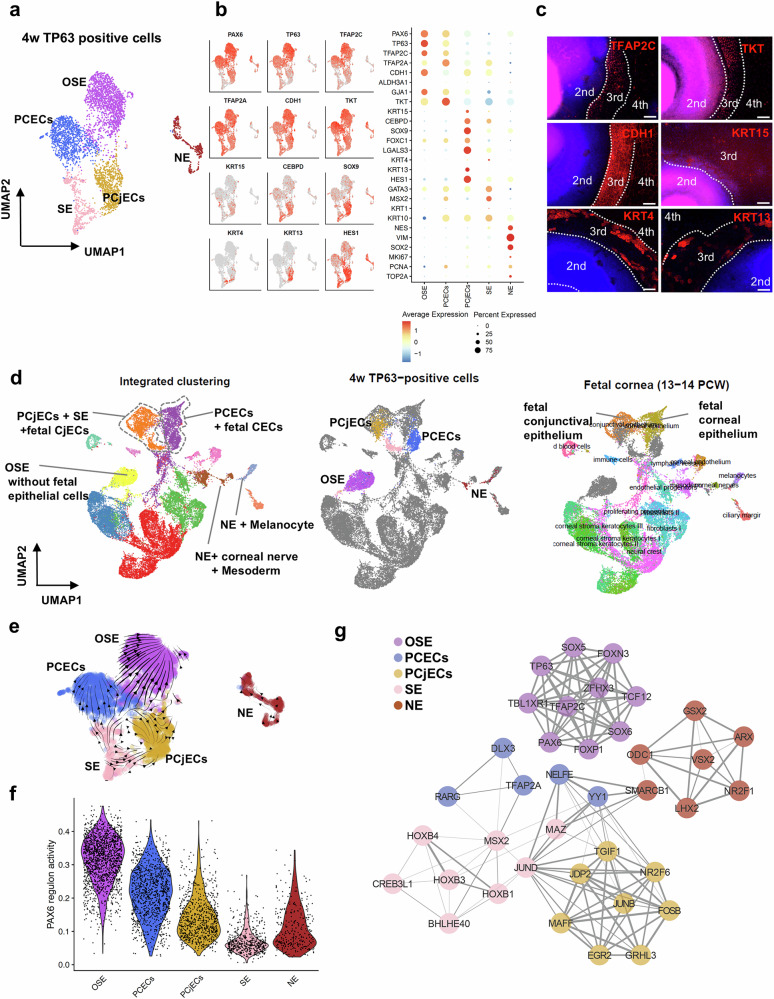
At 4 weeks, TP63-positive cells formed five distinct clusters: OSE, primordial corneal epithelial cells (PCECs), primordial conjunctival epithelial cells (PCjECs), SE, and NE (Fig. 3a, b and Supplementary Data 1). Both OSE and PCECs exhibited high expression levels of PAX6 and TP63, as well as SE markers such as TFAP2C and TFAP2A. These cells also expressed corneal epithelial markers such as GJA147 and TKT48, although they did not express KRT349 or KRT1249,50, which mark terminally differentiated corneal epithelial cells. PAX6 expression was intermediate in PCjECs and lower in SE. PCjECs expressed conjunctival epithelial markers (KRT4 and KRT1351), the limbal epithelial marker KRT15, and limbal stem/progenitor markers such as CEBPD52, SOX953, FOXC154, and LGALS355. PCjECs also expressed HES1, which is expressed in the conjunctival epithelium of embryonic mice56. The SE showed an increased expression of SE markers, such as MSX2 and GATA3. Additionally, the SE showed relatively higher expression of KRT10, an epidermal marker, while exhibiting scarce expression of another epidermal marker, KRT1. The NE exhibited high expression of NES, VIM, and SOX2. Then, we evaluated the protein expression levels of several SEAM markers at 4 weeks, including the SE markers TFAP2C and CDH1, the corneal marker TKT, the limbal marker KRT15, and conjunctival markers KRT4 and KRT13 (Fig. 3c). CDH1 expression was primarily observed in zone 3 with sporadic expression of KRT15 in this zone. TFAP2C and TKT were expressed in the inner layer of zone 3, whereas KRT4 and KRT13 were mainly distributed in the outer layer.
Fig. 3. Identification of primordial ocular surface epithelial cells and their TF network at 4 weeks of development.
a UMAP plot categorizing TP63-positive cells at 4 weeks into clusters corresponding to OSE, PCECs, PCjECs, SE, and NE. b UMAP and bubble plots of TP63-positive cells at 4 weeks showing the expression levels of key markers. The UMAP plots emphasize the differential expression of markers for OSE, PCECs, PCjECs, and SE. c Immunofluorescence staining of SEAM zones (first zone 1, second zone 2, third/fourth zone 3/ 4) at 4 weeks, displaying key markers for SE and conjunctival epithelium, indicated in red. Scale bar: 100 μm. Representative images from three independent biological replicates are shown. d Integrated UMAP of TP63-positive cells at 4 weeks with fetal corneal and conjunctival data from 13–14 post-conception weeks (PCW). Left: Clustering of the integrated datasets. Cells were classified into 12 groups. Middle: TP63-positive cells at 4 weeks are highlighted. Right: Fetal corneal and conjunctival cells are highlighted. e RNA velocity analysis showing the dynamic transition of TP63-positive cells at 4 weeks, indicating the direction of cellular differentiation. f Violin plot depicting the PAX6 regulon activity in each cell cluster of TP63-positive cells at 4 weeks. g Network diagram from SCENIC analysis, illustrating the TF regulon activities specific to each cluster within the TP63-positive cells at 4 weeks. Node colors and edge thickness represent clusters and correlation coefficients, respectively. scRNA-seq analyses (a, b, d–g) were performed using one biological replicate. OSE ocular surface ectoderm, SE surface ectoderm, PCECs primordial corneal epithelial cells, PCjECs primordial conjunctival epithelial cells, NE neuroectoderm, w week(s).
To determine the similarity between these cells and their in vivo counterparts, we integrated our scRNA-seq data on TP63-positive cells at 4 weeks with the publicly available scRNA-seq data on the human fetal cornea and adjacent conjunctiva at 13–14 post-conception weeks (PCWs)7 (Fig. 3d). The OSE cluster remained distinct and did not overlap with the fetal cells. PCECs clustered with the fetal corneal epithelium, while PCjECs and SE clustered with the fetal conjunctival epithelium. NE clustered with the fetal corneal nerves and melanocytes. These results indicate that PCECs and PCjECs mimic the fetal corneal and conjunctival epithelial cells, respectively. Then, we utilized RNA velocity to further analyze the differentiation dynamics at the 4-week time point (Fig. 3e). The RNA velocity analysis indicated a developmental trajectory from SE to PCECs and PCjECs. The flow from SE to OSE was less distinct than the RNA velocity observed at 2 weeks. Next, we evaluated PAX6 regulon activity in each cluster and constructed a TF network. PAX6 regulon activity gradually decreased in the order of OSE, PCECs, PCjECs, and SE (Fig. 3f and Supplementary Data 2, 3). In the TF network at 4 weeks (Fig. 3g and Supplementary Data 2), PAX6 was not among the top ten regulons, but was analyzed to confirm its correlation with other TFs. OSE was characterized by a network composed of distinct TFs, including PAX6 and the ectodermal markers TFAP2C and TP63. The ectodermal marker TFAP2A and DLX3, involved in keratinocyte differentiation57, were activated in PCECs. The correlation networks of JUND and MSX2 within SE, and that of NELFE in PCECs, were observed; the correlation among these three TFs was evident in SE and proliferating SE at 2 weeks. Additionally, JUND formed a network with PCjEC-specific TFs, including AP-1-related TFs such as JUNB and FOSB. Due to their undifferentiated state, PCECs showed a scarcity of corneal epithelium-specific TFs. However, PCjECs featured the basal corneo-conjunctival epithelial marker JUNB58. Thus, we identified distinct epithelial cell clusters, including OSE, PCECs, PCjECs, and SE, and observed their differentiation trajectory, the gradational distribution of PAX6 regulon activity, and their inferred TF network. Notably, PCjECs, which resembled fetal conjunctival epithelium, expressed limbal epithelial markers.
Identification of a TF network involving PAX6 in ocular surface epithelial cell types at 8 weeks
At 8 weeks, TP63-positive cells were organized into eight clusters: corneal epithelial cells (CECs), proliferating CECs, limbal epithelial cells (LECs), proliferating LECs, conjunctival epithelial cells (CjECs), CjECs with MCJ phenotype, residual OSE, and neural cells (Fig. 4a and Supplementary Data 1). The expression of corneal, limbal, conjunctival, and epidermal epithelial cell markers was projected onto uniform manifold approximation and projection (UMAP) (Fig. 4b). CECs expressed corneal epithelial markers, including ALDH3A159, GJA1, TKT, and CLU. Moreover, sporadic KRT12 and ANGPTL7 60 expression was exclusively observed in CECs. CjECs, including those with the MCJ phenotype, exhibited high expression of conjunctival epithelial markers, including KRT4, KRT13, KRT7, and BST223. CjECs with the MCJ phenotype exhibited the highest expression of KRT1 and KRT10. This biphasic character is also evident in the MCJ of the eyelid61. LECs exhibited high expression of KRT15, CEBPD, SOX9, and FOXC1. Proliferating CECs and LECs exhibited high expression of proliferative markers such as MKI67 and TOP2A. Residual OSE exhibited moderate PAX6 expression and the highest levels of TP63. Although KRT15, KRT4, and KRT13 were weakly detected in Residual OSE, this was the case for other specific markers of ocular surface epithelium. Thus, compared with 4 weeks, differentiation markers for corneal, limbal, and conjunctival epithelial cells were specifically expressed in each cluster. Meanwhile, Residual OSE remained with few specific differentiation markers.
Fig. 4. Identification of a TF network including PAX6 in ocular surface epithelial cells at 8 weeks.
a UMAP plot categorizing TP63-positive cells at 8 weeks into clusters corresponding to CECs, proliferating CECs, LECs, proliferating LECs, CjECs, CjECs with MCJ phenotype, residual OSE, and neural cells. b UMAP plots showing the expression levels of marker genes relevant to corneal, limbal, conjunctival, and epidermal epithelial cells, as well as proliferating and neural markers. c Scatter plots showing the expression levels of PAX6 (x-axis) and conjunctival epithelial cell markers KRT4, KRT13, and KRT7 (y-axis). Each dot color represents the cluster. Pearson correlation coefficients between the two genes are shown above the plots. d Immunofluorescence images of SEAM zones at 8 weeks showing the localization of KRT13, KRT4, MUC4, KRT12, KRT10, and KRT15 in PAX6-positive or -negative cells. Scale bar: 100 μm. Representative images from three independent biological replicates are shown. e Integrated UMAP of TP63-positive cells at 8 weeks with fetal corneal and conjunctival data from 16 post-conception weeks (PCW). Left: Clustering of the integrated datasets. Cells were classified into nine groups. Middle: TP63-positive cells at 8 weeks are highlighted. Right: Fetal corneal and conjunctival cells are highlighted. f Violin plot depicting the PAX6 regulon activity in each cell cluster of TP63-positive cells at 8 weeks. g Network diagram from SCENIC analysis, illustrating the TF regulon activities specific to each cluster within the TP63-positive cells at 8 weeks. Node colors and edge thickness represent clusters and correlation coefficients, respectively. scRNA-seq analyses (a–c, e–g) were performed using one biological replicate. OSE ocular surface ectoderm, CECs corneal epithelial cells, LECs limbal epithelial cells, CjECs conjunctival epithelial cells, MCJ mucocutaneous junction, TF transcription factor, PCW post-conception w, week(s).
EGF has been reported to promote the differentiation of conjunctival epithelium from iPSCs22. To compare the effect of growth factors on TP63-positive cells, we conducted scRNA-seq on EGF-treated TP63-positive cells at 8 weeks (Fig. 1a and Supplementary Fig. 3a and Supplementary Data 1). TP63-positive cells isolated after EGF induction formed clusters similar to those induced by KGF, including CECs, LECs, CjECs with the MCJ phenotype, and residual OSE. Additionally, proliferating neural, retinal, and neuroepithelial cells were observed (Supplementary Fig. 3a–c). When comparing each cell type among the TP63-positive cells induced by KGF and EGF, the proportion of CECs was higher in the KGF-treated cells, while CjECs with the MCJ phenotype were more abundant in the EGF-treated cells (Supplementary Fig. 3d). Notably, PAX6 expression was lower in the EGF-treated cells. Although KRT4 was not highly expressed, KRT7 and KRT13 were highly expressed in EGF-treated cells (Supplementary Fig. 3e). Furthermore, corneal epithelium-specific genes were highly expressed in the KGF-treated cells, while there was a small but significant increase in the expression of conjunctival epithelium-specific genes in the EGF-treated cells (Supplementary Fig. 3f). These results are consistent with previous reports that indicate that EGF promotes the differentiation of iPSCs into conjunctival epithelium22,62.
Next, we focused on the relationship between PAX6 and ocular surface keratin. A significant inverse correlation was observed between the expression levels of PAX6 and those of KRT4, KRT13, and KRT7 (Fig. 4c). Regions with a low PAX6 distribution tended to exhibit high KRT13, KRT4, and MUC4 distributions according to immunohistochemical analyses (Fig. 4d). KRT12 was restricted to regions with high PAX6 distribution, while KRT10 was confined to regions with low PAX6 distribution. KRT15 was distributed in both low and high PAX6 regions. Conjunctival epithelial marker expression was negatively correlated with PAX6 expression at both transcript and protein levels.
To further investigate TP63-positive cells at 8 weeks, we integrated the scRNA-seq data on TP63-positive cells at 8 weeks with the scRNA-seq data on the human fetal cornea and adjacent conjunctiva at 16 PCW (Fig. 4e); the OSE cluster did not overlap with fetal cells. CECs, proliferating CECs, LECs, and proliferating LECs clustered with the fetal corneal epithelium and fetal limbal stem/progenitor cells. Notably, proliferating LECs and fetal limbal stem/progenitor cells were closely distributed. CjECs and CjECs with the MCJ phenotype clustered with the fetal conjunctival epithelium, while neural cells clustered with fetal melanocytes. These results suggest that TP63-positive cells differentiate into the corneal, limbal, and conjunctival epithelial cell lineages at 8 weeks.
We next evaluated PAX6 regulon activity in each cluster and constructed a TF network for TP63-positive cells at 8 weeks. PAX6 regulon activity gradually decreased in the order of CECs, LECs, CjECs, and CjECs with MCJ phenotype (Fig. 4f and Supplementary Data 2, 3). The TF network constructed from TP63-positive cells at 8 weeks showed that in CECs, PAX6 was highly correlated with the ectodermal markers TFAP2A and TFAP2C, as well as with DLX3. FOXC154, which was activated in LECs, was also correlated with PAX6 (Fig. 4g). LECs also exhibited high activity of the limbal stem cell markers FOSL263 and CEBPD52. In proliferating LECs, POU2F1, which activates ALDH3A159 and the proliferation marker E2F7, were highly correlated with TFs activated in proliferating CECs. TFs in the residual OSE formed a broad network with TFs in CECs, LECs, CjECs, and CjECs with the MCJ phenotype. TCF7L264, the mouse stem/precursor cell marker for the conjunctival epithelium, was activated in the residual OSE and correlated with TFs in CECs and LECs. Goblet cell-associated TFs, such as FOXP1 and FOXK165, were activated in the residual OSE, along with FOXQ1 in CjECs. Collectively, these results describe the TF networks that operate in differentiating ocular epithelial cells, including CECs, LECs, CjECs, CjECs with MCJ phenotype, and residual OSE. Among these, PAX6 displayed high expression and regulon activity in CECs and LECs, forming networks with their cluster-specific TFs.
Integrative trajectory analysis of ocular surface epithelial differentiation across developmental stages
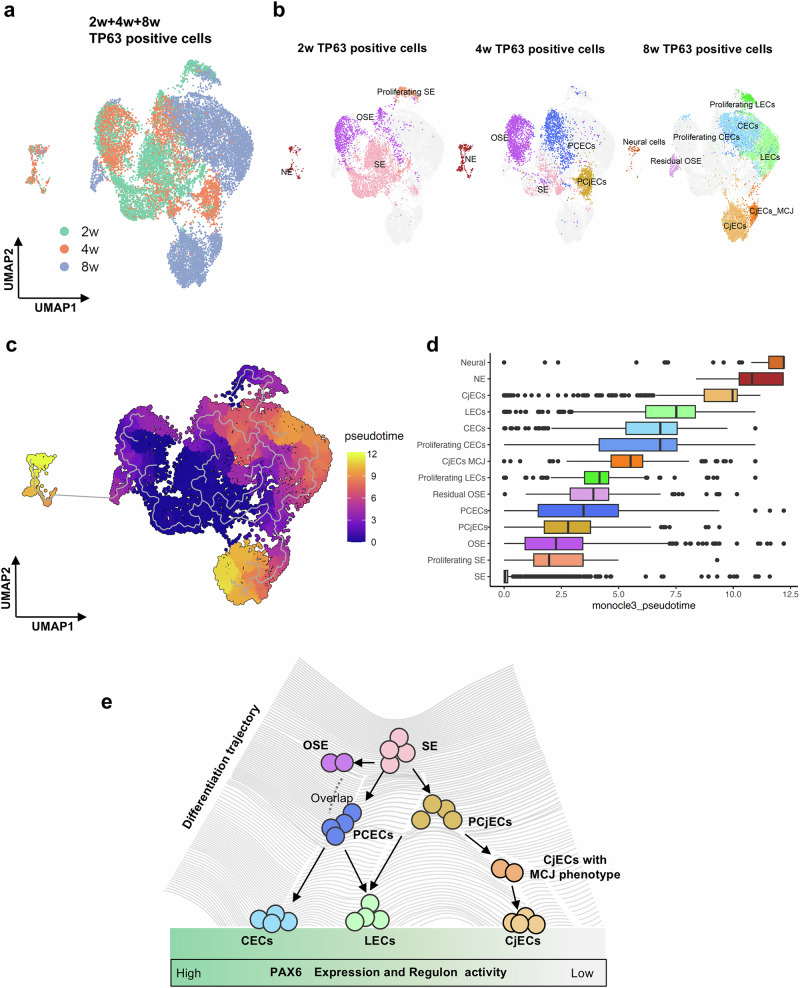
To elucidate the differentiation trajectory of TP63-positive ocular surface epithelial cells over time, we integrated SEAM-derived single-cell transcriptomic data from 2, 4, and 8 weeks (Fig. 5a). The distribution of cells previously annotated at different stages of development is shown in Fig. 5b. We employed Monocle3 to construct cellular trajectories and infer pseudotimes (Fig. 5c)66. The pseudotime ordering revealed that early cells, annotated as SE, Proliferating SE or OSE, clustered at the initial branch of the trajectory. Consistent with time-course and RNA velocity observations, these early clusters gave rise to two main transitional populations: PCECs and PCjECs. PCECs progressed to CECs and LECs. Additionally, proliferating SE differentiated into CECs via proliferating LECs. In contrast, PCjECs eventually differentiate into LECs and CjECs, via CjECs with MCJ phenotype. Examination of box plots for pseudotime distribution (Fig. 5d) confirmed that PCECs and PCjECs are positioned at intermediate trajectories, while specialized cell types, such as CECs, LECs and CjECs, appear at higher pseudotime values. Based on these results and those of PAX6 activity and expression levels at each time point, these analyses provide an integrative view of human ocular surface epithelial development in the SEAM model (Fig. 5e).
Fig. 5. Developmental lineage of ocular surface epithelial cells revealed by their similarities.
a UMAP plot showing the distribution of TP63-positive cells derived from 2, 4, and 8 weeks. b UMAP plots showing the distribution of different cell types at each time point. c UMAP plot showing pseudotime trajectory of TP63-positive cells. d Box plots showing the distribution of pseudotime values for each cell type. e A comprehensive overview of human ocular surface ectodermal development. OSE ocular surface ectoderm, SE surface ectoderm, CECs corneal epithelial cells, LECs limbal epithelial cells, CjECs conjunctival epithelial cells, PCjECs primordial conjunctival epithelial cells.
Ocular surface epithelial cell sheet identity dependent on PAX6 expression levels
PAX6 was highly expressed in CECs and LECs at 8 weeks but low in CjECs (Fig. 4b). Furthermore, PAX6 showed a tendency for a negative correlation with conjunctival epithelial markers (Fig. 4c, d). To further investigate the involvement of PAX6 in these ocular surface epithelial cells, we sorted epithelial cells based on PAX6 expression levels and generated epithelial sheets.
After 10 weeks of culturing double knock-in hiPSC-derived ectodermal cells using the SEAM method, we isolated epithelial cells, defined as EGFP–/tdTomato+ (P1), EGFPlow/tdTomato+ (P2), EGFPmid/tdTomato+ (P3), and EGFPhigh/tdTomato+ (P4), for cell sheet fabrication (Fig. 6a). The EGFP intensity was the highest in P4, followed by P3, P2, and P1 (Fig. 6b). All cell sheets expressed TP63, but PAX6 expression was barely detectable in P1 and P2 sheets (Fig. 6c). In the qPCR analysis (Fig. 6d), PAX6 expression increased significantly from P1 to P2 and from P2 to P3. Both P3 and P4 exhibited significantly higher expression levels than those of P1 and P2. The expression of KRT12, a corneal epithelial marker, was significantly higher in P4 than those of P1, P2, and P3. MUC5AC, a conjunctival goblet cell-specific marker, was significantly higher in P3, while KRT10, an epidermal marker, showed significantly higher expression levels in P1 and P2 compared to those of P3 and P4. The transcriptional profiles of these four cell sheets were revealed by bulk RNA-seq analysis (Fig. 6e). P1 and P2 sheets predominantly exhibited higher expression levels of the epidermal markers KRT1 and KRT10 than those of P3 and P4 sheets. P3 sheets were characterized by significantly increased expression levels of the conjunctival epithelial markers, KRT4, KRT7, MUC4, and MUC5AC. In addition, the P4 sheet showed the highest expression levels of corneal markers, including KRT3, KRT12, ALDH3A1, ANGPTL7, TKT, and CLU. PAX6 expression levels sequentially increased from low in P1 and P2 sheets to moderate in P3 and high in P4 sheets. Based on these findings, P1 and P2 sheets were categorized as epidermal or conjunctival, while P3 was identified as conjunctival and P4 as corneal epithelial in phenotype. Next, we performed hematoxylin-eosin and immunofluorescence staining on P1–4 sheets (Fig. 6f), finding that the cell sheets varied in thickness, with P2 sheets being the thickest, followed by P1, P3, and P4 sheets, which had similar thicknesses. P1 and P2 sheets did not express PAX6, while P3 and P4 were positive to PAX6. Additionally, all sheets from P1 to P4 were positive for TP63 and MUC16. The P1 and P2 sheets co-expressed the conjunctival epithelial differentiation marker KRT13 and epidermal marker KRT10. In contrast, the P3 sheet did not express KRT10 but was positive for KRT13 and the goblet cell-specific marker MUC5AC. The P4 sheet showed no expression of KRT10, faint expression of KRT13, and was positive for the corneal epithelial differentiation marker KRT12. Thus, observed expression patterns of characteristic features in each epithelial cell were supported by the immunofluorescence staining data, which were consistent with the RNA-seq and qPCR data. These results indicate that ocular surface epithelial cell sheets, derived from cells that were sorted based on PAX6 expression levels, were properly generated. Furthermore, conjunctival epithelial cell sheets expressing MUC5AC require a specific level of PAX6 expression, although this level is significantly lower than that in corneal epithelial cell sheets.
Fig. 6. Ocular surface epithelial cell sheet identity dependent on PAX6 expression levels.
a Flow cytometry analysis of differentiation at 10 weeks, categorizing TP63-positive cells into four subsets (P1–P4) based on PAX6-EGFP expression levels, which were then isolated to create distinct cell sheets. b Quantification of PAX6-EGFP fluorescence intensity in subsets P1–P4, indicating varying expression levels among the isolated cell groups. n = 7 independent biological replicates. *p < 0.05. Error bars show the standard deviation. c Phase-contrast and immunofluorescence images of the P1–P4 cell sheets, demonstrating the expression of PAX6-EGFP (green) and TP63-tdTomato (magenta) after 4 weeks of culture following cell sorting. Representative images from seven independent biological replicates are shown. d qPCR analysis of gene expression in ocular surface epithelial cell sheets P1–P4. n = 7 independent biological replicates. *p < 0.05. Error bars show the standard deviation. e A heatmap of RNA-seq analysis based on the expression of differentiated corneal, limbal, and conjunctival epithelial cell markers, showing expression variability across the P1–P4 cell sheets. RNA-seq was performed on one biological replicate per group. f Hematoxylins-eosin staining and immunofluorescence of cell sheets for the identification of PAX6, TP63, and epithelial markers specific to corneal (KRT12), conjunctival (MUC16, KRT13, and MUC5AC), and epidermal (KRT10) tissues. Magenta represents marker expression, and blue indicates nuclei stained with Hoechst 33342. Scale bar: 100 µm. Representative images from three independent biological replicates are shown. Error bars represent standard deviation in (b, d). Source data are provided in Supplementary Data 4.
Discussion
In this study, we explored the differentiation landscape of SEAM-derived TP63-positive cells (Fig. 5C). These TP63-positive cells mimicked the SE differentiation during ocular development, as evidenced by gene expression profiles and similarities to the fetal ocular surface epithelium. TP63-positive cells originated from the SE and contributed to two distinct epithelial lineages: one progressing through OSE and PCECs toward CECs and LECs, and another involving PCjECs, leading to LECs and MCJ-type CjECs before reaching CjECs. This finding aligns with those of rabbit lineage-tracing studies, which also demonstrated two separate lineages for the cornea and conjunctiva67. Notably, the trajectory of PCjECs for LECs and CjECs was shown through pseudotime trajectory analysis. These PCjECs expressed limbal markers such as KRT15, CEBPD, SOX9, LGALS3, and FOXC1. In the 8.5-week human fetal eye, KRT15 is broadly expressed across the cornea, limbus, and conjunctiva, and this expression becomes restricted to the limbus by the 22nd week6. This indicates that KRT15-positive cells prior to limbal epithelial establishment may represent precursor populations of ocular surface epithelium, and PCjECs potentially correspond to these KRT15-positive cells before they become restricted to the limbus. Additionally, these PCjECs expressed conjunctival markers (KRT4 and KRT13) and showed similarity to fetal conjunctiva in our integrated scRNA-seq analysis. Therefore, PCjECs contribute to both limbal and conjunctival epithelial development. In PCjECs, AP-1-related transcription factors (JUNB and FOSB) were static TFs. JUNB expression has been previously reported in basal cells of the corneal and conjunctival epithelium in mice58. The high activity of AP-1-related factors in PCjECs may be involved in maintaining an undifferentiated state during early development. Meanwhile, CECs appear to originate from PCECs and LECs. At 8 weeks SEAMs, KRT15-positive PAX6-high cells were present, but in cell sheet formation analysis, sorting high PAX6-expressing cell populations led to the formation of corneal epithelial cell sheets with low and high KRT15 and KRT12 expression, respectively. This suggests that LECs differentiate into corneal epithelial cells through sheet formation. In adult mammals, corneal epithelial cells originate from LSC, which is harbored in LECs. Reports using KRT12-marked mice have shown that surface ectoderm-derived corneal epithelium is replaced by limbus-derived corneal epithelium68; this finding is consistent with our hypothesis that CECs derive from both PCECs and LECs.
In CECs, PAX6 achieved the highest activity and correlated with corneal epithelial-specific TFs and LSCs markers, including FOXC1 and FOSL2. The correlation between PAX6 and the LSCs markers suggests a potential involvement of PAX6 in corneal and limbal epithelial differentiation. In corneal epithelial cells, several studies have reported that the loss of PAX6 shifts the corneal epithelial cell lineage toward conjunctival or epidermal fates. For example, Sunny et al. reported the loss of KRT12 and the expression of the conjunctival marker KRT4 in the corneal epithelium of PAX6 conditional knockout mice12. Similarly, Kitazawa et al. found that the depletion of PAX6 in primary corneal epithelial cells decreased the expression of KRT3 and KRT12 while increasing those of epidermal markers such as KRT10 and IVL69. In contrast, conjunctival keratins showed a negative correlation with PAX6 at 8 weeks. Conjunctival induction with EGF is reported to reduce PAX6 expression22,62, suggesting that it promotes conjunctival keratins. In contrast, our epithelial cell sheet analysis revealed that conjunctival epithelial cell sheets expressing MUC5AC required a certain level of PAX6 expression. Similarly, in conjunctival organoids, PAX6 knockout resulted in a failure to express MUC5AC70. Thus, the coexistence of conjunctival keratins and MUC5AC is likely maintained within a restricted range of PAX6 expression during conjunctival epithelial development. An expression of PAX6 higher than this threshold appears to promote corneal epithelial differentiation, whereas lower expression promotes epidermal cell differentiation. Collectively, we propose that the expression level of PAX6 determines the developmental lineage between corneal, conjunctival, and epidermal epithelial cell fates. Cornea–conjunctiva lineage bifurcation appears to begin as early as 4 weeks. To evaluate which developmental stages in mice and humans correspond to SEAM at 4 weeks, we referred to the distribution of PAX2 and PAX6. In optic stalk formation, PAX2 is co-expressed with retinal PAX6 and becomes restricted to the optic stalk, which corresponds to E9.5-11 in mice41. At this stage in mice, optic cup formation, wherein the lens placode invaginates to form the lens vesicle, aligns with the stage of 4–5 weeks in the human eye2. A similar PAX2 and PAX6 distribution pattern is observed in SEAM at 3–4 weeks. Therefore, we demonstrate the differentiation lineage of ocular surface epithelium at a very early stage and the involvement of PAX6 in various cell types within that lineage.
This study has limitations. First, the differentiation lineage of TP63-positive cells derived from SEAM has not been confirmed with in vivo data. Although our integrated scRNA-seq analysis suggests their similarity to fetal ocular surface cells, further in vivo validation is required to fully substantiate these findings. Second, although our study provides insights into TF interactions in ocular surface development, it is based on static transcriptional activity. To determine whether PAX6 and other transcription factors directly regulate these differentiation pathways, further functional studies, such as lineage-tracing and loss-of-function experiments, are required.
In conclusion, the ocular surface differentiation landscape shaped by PAX6 and other TFs provides deeper insights into the early-stage human ocular development. The results of this study may contribute to a thorough understanding of stem cell biology and support the development of future therapeutic strategies for treating ocular diseases.
Experimental models and subject details
hiPSC culture
The hiPSC line 201B7 was provided by the Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan. This line was originally generated from human dermal fibroblasts of an adult healthy donor71. Pluripotency marker expression is shown in Supplementary Fig. S1. The iPSCs were cultured in StemFit medium (catalog no. AK02N; Ajinomoto, Tokyo, Japan), with iMatrix-511 silk (Nippi, Tokyo, Japan) as substrate. The cells were passaged every 7 days using 0.25 mM EDTA (Nacalai Tesque, Kyoto, Japan) and 50% TrypLE™ Express (Thermo Fisher Scientific, Waltham, MA, USA), at a density of 13,000 cells per well in a six-well plate, with the medium changed three times per week. The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Generation of PAX6-EGFP/TP63-tdTomato hiPSCs
PAX6-EGFP knock-in hiPSCs were generated using pDONOR-PAX6 (catalog no. 105239), PAX6 TALEN-L (catalog no. 109034), and PAX6 TALEN-R (catalog no. 105525) (all from Addgene, Watertown, MA, USA)42. For this procedure, hiPSCs were seeded at a density of 3×105 cells/well in an iMatrix-511 silk-coated six-well plate. The next day, the plasmids were transfected into the cells using the Stem Cell Reagent (Thermo Fisher Scientific)42, and the transfected cells were selected using 150–450 ng/mL puromycin (Nacalai Tesque, Inc., Kyoto, Japan). Homologous recombination was confirmed by genomic PCR and Sanger sequencing. The donor plasmid, TP63-tdTomato-Neo, was originally designed and supplied by GeneWiz (South Plainfield, NJ, USA). PAX6-EGFP hiPSCs were seeded at a density of 3 × 105 cells per well in six-well plates. The next day, the cells were transfected with 1.25 μg of the TP63-targeting gRNA-Cas9 plasmid (gRNA sequence: TAAGCAGGAGTGCTTTTAGGGGG) and 1.25 μg of the donor plasmid, using the Stem Cell Reagent in AK03N containing 10 μM Y-27632 (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Seven days after transfection, antibiotic selection was conducted using 50–150 μg/mL G418 (Invitrogen, Carlsbad, CA, USA) for 14 days. Selected hiPSCs were seeded onto an iMatrix-511 silk-coated 6-well cell culture plate. Homologous recombination was confirmed by means of genomic PCR and Sanger sequencing.
Method details
Differentiation of hiPSCs into the SEAM
To induce SEAM differentiation, iPSCs were seeded at densities of 3000, 1000, and 500 cells/well in 6-, 12-, and 24-well plates, respectively, in wells coated with 500 ng/mL iMatrix-511 silk, and cultured for 10 days. The medium was changed every 2–3 days. For SEAM differentiation, the cells were treated with differentiation medium (DM; G-MEM [Thermo Fisher Scientific] supplemented with 10% Knockout Serum Replacement [Thermo Fisher Scientific], 1 mM sodium pyruvate [Thermo Fisher Scientific], 1× MEM non-essential amino acids [Thermo Fisher Scientific], 55 µM monothioglycerol [Wako Pure Chemical], penicillin [Meiji Seika Pharma, Tokyo, Japan], and streptomycin [Meiji Seika Pharma] for 0–28 days. Thereafter, the cells were treated with ocular surface differentiation medium (ODM; DM and CnT-Prime without EGF and FGF [CELLnTEC, Bern, Switzerland], 1:1) containing 10 µM Y-27632 [Wako Pure Chemical], 10 ng/mL KGF [Wako Pure Chemical] or EGF for 28–56 days. Finally, the cells were treated with ocular surface epithelium maintenance medium (OEM; Dulbecco’s modified Eagle medium/F12 [Thermo Fisher Scientific]) containing B27 supplement [Thermo Fisher Scientific], 10 µM Y-27632, and 10 ng/mL KGF or EGF for 56–84 days. To prepare ocular surface epithelial cell sheets, tdTomato+ cells were sorted using FACS (SONYSH800S, Sony, Tokyo, Japan) and seeded onto iMatrix-511 silk-coated 24-well plates, with cell culture inserts containing OEM (with 10 ng/mL KGF).
qPCR
RNA was extracted from the cells using Sepasol®-RNA I Super G (Nacalai Tesque), and cDNA was synthesized using the SuperScript™ IV VILO™ Master Mix (Thermo Fisher Scientific). qPCR was performed using the TB Green® Premix DimerEraser™ reagent (Takara Bio, Kusatsu, Japan) in a 7500 Fast thermocycler (Thermo Fisher Scientific). PCR data were calculated and analyzed using the 2–ΔΔCT method. All qPCR analyses were performed using RNA from seven independent biological replicates of ocular surface epithelial cell sheets. A list of SYBR probes is provided in Table 1.
Table 1.
Q-PCR primer list
| Gene | Forward primer sequence (5’-3’) | Reverse primer sequence (5’-3’) |
|---|---|---|
| GAPDH | TCGTGGAAGGACTCATGACC | AGGCAGGGATGATGTTCTGG |
| PAX6 | TCCTTCACATCTGGCTCCATGTT | ATGCAGGAGTATGAGGAGGTCT |
| deltaNp63 | TACCTGGAAAACAATGCCCAGA | GCGCGTGGTCTGTGTTATAG |
| KRT12 | ATGAAACACGAGGAACTGGGA | TCCTGAGGTCTTCAATCAGTGG |
| KRT13 | CCCCAGGCATTGACCTGAC | GTGTTGGTAGACACCTCCTTG |
| KRT10 | TCCTACTTGGACAAAGTTCGGG | CCCCTGATGTGAGTTGCCA |
| MUC16 | AGCCACCTCATCTATTACCACA | TGTTGCTGCATTGCTTAGGGT |
| MUC4 | GGAGAGGTATCGCCCTGATAG | CCGGTGTAGCCTGTAGAACTG |
| MUC5AC | TCAGGAACAGCTTCGAGGAC | GTAGTAGGTTCCCGGCTTCAC |
Forward and reverse primer sequences (5′–3′) for each target gene are listed.
Hematoxylin and eosin staining
Cell sheets obtained from sorted PAX6-EGFP/TP63-tdTomato hiPSCs were embedded in OCT compound, cryosectioned at a thickness of 3 μm, and fixed with 10% neutral buffered formalin (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The sections were then stained with hematoxylin and eosin, dehydrated through an ethanol gradient, and mounted on glass slides. Imaging was performed using an Axio Imager.A2 microscope (Carl Zeiss, Oberkochen, Germany).
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 30 min at ~22 °C and treated with blocking buffer containing 5% normal donkey serum, 0·3% Triton™ X-100, and tris-buffered saline. Primary antibodies against NANOG, OCT-3/4, TRA-1-60, SSEA4, MITF, TFAP2C (Cell Signaling Technology, Danvers, MA, USA), PAX6 (BioLegend, San Diego, CA, USA), EGFP, RAX, TP63, KRT8, TFAP2A, CDH1, KRT4, KRT10, KRT15, VSX2, PAX2, MUC5AC (Santa Cruz Biotechnology, Dallas, TX, USA), FOXG1, KRT13, MUC16, KRT12 (Abcam, Cambridge, UK), TUBB3 (Sigma-Aldrich, St. Louis, MO, USA), and MUC4 (Millipore, Burlington, MA, USA) were used. The secondary antibodies used were Alexa Fluor™ 647-conjugated anti-mouse or rabbit-IgG antibodies. Nuclear staining was performed using Hoechst 33342 (Sigma-Aldrich). The specimens were observed under a confocal microscope (Axio Observer D1 or LSM710; Zeiss, Oberkochen, Germany) or an FV3000 microscope (Olympus, Tokyo, Japan). Details of primary antibodies used for immunofluorescence staining are provided in Table 2.
Table 2.
Primary antibodies used in this study
| Antigen | Host | Type | Clone | Company | Catalog # | RRID |
|---|---|---|---|---|---|---|
| TP63 | Mouse | Monoclonal | 4A4 | Santa Cruz Biotechnology | sc-8431 | AB_628091 |
| PAX6 | Rabbit | Polyclonal | – | BioLegend | 901301 | AB_2565003 |
| KRT12 | Rabbit | Polyclonal | – | Abcam | ab185627 | AB_2889825 |
| TKT | Mouse | Monoclonal | H-7 | Santa Cruz Biotechnology | sc-390179 | AB_2925185 |
| KRT15 | Mouse | Monoclonal | LHK15 | Santa Cruz Biotechnology | sc-47697 | AB_627847 |
| KRT13 | Mouse | Monoclonal | AE8 | Abcam | ab16112 | AB_302267 |
| MUC4 | Mouse | Monoclonal | 8G-7 | Abcam | ab52263 | AB_881163 |
| MUC5AC | Mouse | Monoclonal | CLH2 | Santa Cruz Biotechnology | sc-33667 | AB_627973 |
| KRT10 | Mouse | Monoclonal | DE-K10 | Santa Cruz Biotechnology | sc-52318 | AB_629836 |
| TFAP2A | Rabbit | Monoclonal | C83E10 | Cell Signaling Technology | 3215 | AB_2227429 |
| NANOG | Rabbit | Monoclonal | D73G4 | Cell Signaling Technology | 4903 | AB_10559205 |
| SSEA4 | Mouse | Monoclonal | MC813 | Cell Signaling Technology | 4755 | AB_1264259 |
| OCT4A | Rabbit | Monoclonal | C30A3 | Cell Signaling Technology | 2840 | AB_2167691 |
| TRA-1-60(S) | Mouse | Monoclonal | TRA-1-60(S) | Cell Signaling Technology | 4746 | AB_2119059 |
| TFAP2C | Goat | Polyclonal | – | R&D Systems | AF5059 | AB_2255891a |
| MSX2 | Mouse | Monoclonal | 786607 | R&D Systems | MAB7917 | AB_3096365 |
| GATA3 | Goat | Polyclonal | – | R&D Systems | AF2605 | AB_2108571 |
| CDH1 | Rabbit | Monoclonal | EP700Y | Abcam | ab40772 | AB_731493 |
| KRT8 | Mouse | Monoclonal | C51 | Santa Cruz Biotechnology | sc-8020 | AB_627857 |
| FOXG1 | Rabbit | Polyclonal | – | Abcam | ab18259 | AB_732415 |
| TUBB3 | Rabbit | Polyclonal | – | Sigma-Aldrich | T2200 | AB_262133 |
| VSX2 | Mouse | Monoclonal | E-12 | Santa Cruz Biotechnology | sc-365519 | AB_10842442 |
| MITF | Rabbit | Monoclonal | D5G7V | Cell Signaling Technology | 12590 | AB_2616024 |
| RAX | Mouse | Monoclonal | G-12 | Santa Cruz Biotechnology | sc-271889 | AB_10708730 |
| PAX2 | Mouse | Monoclonal | 60-P | Santa Cruz Biotechnology | sc-130387 | AB_2236656 |
| EGFP | Mouse | Monoclonal | B-2 | Santa Cruz Biotechnology | sc-9996 | AB_627695 |
| tdTomato | Rabbit | Polyclonal | – | Rockland | 600-401-379S | AB_11182807 |
Primary antibodies used for immunofluorescence staining. Host species, antibody type (monoclonal or polyclonal), clone information, company, catalog number, and RRID are listed.
Alkaline phosphatase staining
Alkaline phosphatase staining was performed using the TRACP and ALP double-stain kits (Takara Bio). iPSCs were cultured for 7 days, treated with a fixation solution, and then stained with an alkaline phosphatase substrate. Stained cells were observed using an Axio Observer D1 microscope.
FACS analysis
Differentiated PAX6-EGFP/TP63-tdTomato hiPSCs at 2, 4, and 8 weeks were dissociated using StemPro™ Accutase™ (Thermo Fisher Scientific). The cells were analyzed and sorted using an SH800 system (Sony, Tokyo, Japan), and the collected data were sorted using the software provided with the system. Fluorescence signal intensity of EGFP was quantified based on mean fluorescence intensity, and the values were used for statistical comparisons. A detailed gating strategy is described in Supplementary Note 1.
Single-cell library preparation and sequencing
A total of eight groups were used for this analysis (total and TP63-positive cells at 2 weeks, total and TP63-positive cells at 4 weeks, KGF-treated TP63-positive cells at 8 weeks, and EGF-treated TP63-positive cells at 8 weeks). cDNA libraries were prepared as 118 bp paired-end reads using the Chromium Next GEM Single Cell 5’ Kit v2 (10x Genomics) and sequenced using a HiSeq X system (Illumina, San Diego, CA, USA).
scRNA-seq analysis
Raw FASTQ files were processed using the Cell Ranger count pipeline (version 6.1.2, 10x Genomics), with the “--include-introns” option. Reads were mapped to the human GRCh38 reference genome. The output count data were corrected for ambient RNA expression using the R package SoupX (version 1.6.2)72, and doublets were identified using the Python package scrublet (version 0.2.3)73. Corrected count matrices were processed using the R package Seurat (version 4.3.0)74. After removing the doublets, cells expressing <1700 genes, >8500 genes (10,000 genes in the total-cell datasets), or >10% mitochondrial counts were excluded. Cell cycle phase scores were calculated using the CellCycleScoring function, and data were normalized and scaled using the SCTransform function, decreasing the effects of cell cycle scores (S and G2M scores) and the percentage of mitochondrial genes. Following dimensionality reduction using the RunPCA and RunUMAP functions, clustering was performed using the FindNeighbours function with the top principal components that explain 90% of the variance and the FindClusters function with a resolution range of 0.1–0.3. For the integration analysis, the effects of total counts per cell were also repressed, and the R package Harmony (version 0.1.1)75 was used to correct batch effects in the datasets. scRNA-seq datasets for human fetal corneas were obtained from the NCBI Gene Expression Omnibus database (accession number: GSE155683)7. Corneal and conjunctival epithelium-specific genes were identified using the microarray data obtained from GSE554376. Expression scores for each cell were calculated using Seurat’s AddModuleScore function. To estimate the RNA velocities, the bam files generated from Cell Ranger were subjected to velocyto (version 0.17.17)77, and the generated loom files were further processed by scVelo (version 0.2.3)78. The UMAP coordinates and clusters identified by Seurat were merged with the velocyto outputs. The merged object was subjected to the scVelo.pp.filter_and_normalize function (min_shared_counts = 20, n_top_genes = 2000), and the moments were computed using scVelo.pp.moments. Subsequently, the velocity was calculated using the scVelo.tl.velocity function (mode=stochastic), and the velocity graph was constructed using the scVelo.tl.velocity_graph function. Finally, the RNA velocities were embedded onto the UMAP using the scVelo.pl.velocity_embedding_stream function. Gene regulatory network analysis was performed using the R package SCENIC (v1.3.1)39. Briefly, GENIE3 was used to construct a co-expression network with TFs, and RcisTarget was used to determine the TF targets (regulons) and remove indirect targets based on the TF binding motif database. The regulon activities in each cell were scored as AUCell values. Using the AUCell values, Seurat’s FindAllMarkers function with the parameter logfc.threshold = 0.005 was applied to identify the cluster-specific regulons, and the top 10 regulons based on the average log2 fold change were chosen. When TFs were specific to multiple clusters, the cluster with the highest avg_log2FC was selected. In the dataset of TP63-positive cells at 4 weeks, PAX6 was added even though it was not in the top ten regulons. Pearson’s correlation coefficients between these cluster-specific regulons were calculated, and the correlation of regulons over 0.3 was selected and visualized with Cytoscape (v3.10.0)79. Pseudotime and trajectory inference were performed using Monocle366,80. A Seurat object was converted into a Monocle-compatible format using as.cell_data_set from SeuratWrappers. A principal graph was fitted using learn_graph(), and order_cells() was applied for pseudotime ordering. The root of the trajectory was primarily determined programmatically, selecting cells from the OSE and SE clusters based on gene expression and spatial constraints.
Cell sheet fabrication
Sorted hiPSCs were plated onto iMatrix511silk-coated (0.5 μg/cm2) cell culture inserts. The cells were cultured in ocular surface epithelium maintenance medium (OEM; DMEM/F12 containing 10 μM Y-27632, 2% B27 supplement, 1% penicillin-streptomycin solution, and 10 ng/mL of KGF) at 37 °C until confluence (4 weeks).
Cell sheet RNA-seq analysis
Total RNA from EGFP-positive or EGFP-negative sorted cells was purified using an RNeasy Micro Kit with RNase-free DNase (both from Qiagen, Hilden, Germany). RNA-seq was performed at the Center of Medical Innovation and Translational Research, Osaka University, Osaka, Japan. RNA-seq libraries were prepared using the TruSeq Stranded mRNA Library Prep Kit (Illumina), and the quality and quantity of the amplicons were assessed at all steps through capillary electrophoresis (Agilent Bioanalyzer and TapeStation; Agilent, Santa Clara, CA, USA). The libraries were quantified using qPCR, immobilized, and processed onto a flow cell using a cBot device (Illumina), and then subjected to sequencing-by-synthesis using a NovaSeq 6000 S4 chemistry device on a NovaSeq 6000 platform at Macrogen (Tokyo, Japan).
The paired-end raw reads were processed with fastp (v0.19.5)81 to remove adapter sequences and low-quality reads, and the filtered reads were mapped to the human reference genome (GRCh38) with STAR (v2.7.10b)82. The read counts were quantified using RSEM (v1.3.3)83 with the rsem-calculate-expression function. The resulting transcripts per million (TPM) values were visualized as a heatmap using the R package pheatmap (v1.0.12).
Statistics and reproducibility
All data are expressed as mean ± s.d. Steel-Dwass were performed for four-group multiple comparisons (Fig. 6b, d). All statistical analyses were performed using JMP Pro (v.17.0, SAS Institute, Cary, NC, USA). Statistical significance was set at P < 0.05. Exact P values are provided in the source data (Supplementary Data 4).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank K. Imaeda, R. Kobayashi, and S. Ishino for providing technical assistance and A. Oguchi, Y. Murakawa, R. Hayashi, and M. Tsujikawa for providing technical advice. This study was supported by the Japan Agency for Medical Research and Development under grant numbers JP18gm1210004, JP22ek0109584, 22bm0804021h0003, and 24bm1123043h0002. This work was supported by JSPS KAKENHI grant numbers JP22K12817 and JP23K09043. This work was supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Author contributions
R.K. and S.H. designed the study. R.K., M.Y., S.H., A.H., A.Y., and T.T. designed and performed experiments and analyzed data. R.K. and S.H. drafted the manuscript. S.H., M.Y., T.N., S.S., K.B., L.H., A.J.Q., and K.N. provided conceptual advice regarding the study design.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Joao Valente and Pavithra Lakshminarasimhan Chavali. A peer review file is available.
Data availability
The scRNA-seq and bulk RNA-seq data of human iPSC-derived ocular cells used in this study have been deposited in the ArrayExpress database at EMBL-EBI and are available under the accession codes “E-MTAB-14019” and “E-MTAB-13992,” respectively84,85. The plasmids generated in this study are referenced at the RIKEN BioResource Research Center (BRC). Source data underlying the graphs and charts in the main figures are provided in Supplementary Data 4. All other data supporting the findings of this study are available from the corresponding author upon reasonable request.
Material availability
Unique materials produced in this study can be obtained from the lead contact following a reasonable request and after signing a material transfer agreement.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rei Kamuro, Masahito Yoshihara.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-08643-2.
References
- 1.Casey, M. A., Lusk, S. & Kwan, K. M. Eye morphogenesis in vertebrates. Annu. Rev. Vis. Sci.9, 221–243 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Graw, J. Eye development. Curr. Top. Dev. Biol.90, 343–386 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Hayashi, R. et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature531, 376–380 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi, Y., Hayashi, R., Shibata, S., Quantock, A. J. & Nishida, K. Ocular surface ectoderm instigated by WNT inhibition and BMP4. Stem Cell Res.46, 101868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koroma, B. M., Yang, J. M. & Sundin, O. H. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Investig. Ophthalmol. Vis. Sci.38, 108–120 (1997). [PubMed] [Google Scholar]
- 6.Davies, S. B. et al. Stem cell activity in the developing human cornea. Stem Cells27, 2781–2792 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Collin, J. et al. A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells. Ocul. Surf.21, 279–298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latta, L. et al. Pathophysiology of aniridia-associated keratopathy: developmental aspects and unanswered questions. Ocul. Surf.22, 245–266 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Li, G. et al. Transcription factor PAX6 (paired box 6) controls limbal stem cell lineage in development and disease. J. Biol. Chem.290, 20448–20454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos, T., Scott, D. & Ahmad, S. An update on ocular surface epithelial stem cells: cornea and conjunctiva. Stem Cells Int. 2015, 601731 (2015). [DOI] [PMC free article] [PubMed]
- 11.Halder, G., Callaerts, P. & Gehring, W. J. New perspectives on eye evolution. Curr. Opin. Genet. Dev.5, 602–609 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Sunny, S. S., Lachova, J., Dupacova, N. & Kozmik, Z. Multiple roles of Pax6 in postnatal cornea development. Dev. Biol.491, 1–12 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Antosova, B. et al. The gene regulatory network of lens induction is wired through Meis-dependent shadow enhancers of Pax6. PLoS Genet.12, 1–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang, X. Y. et al. Human organoids in basic research and clinical applications. Signal Transduct. Target. Ther. 7, 168 (2022). [DOI] [PMC free article] [PubMed]
- 15.Bhattacharya, R. et al. Model organoids: integrated frameworks for the next frontier of healthcare advancements. Stem Cell Rev. Rep.21, 319–336 (2024). [DOI] [PubMed]
- 16.Ma, S. chao, Xie, Y. lin, Wang, Q., Fu, S. gui & Wu, H. ze. Application of eye organoids in the study of eye diseases. Exp. Eye Res.247, 110068 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Swarup, A. et al. Single-cell transcriptomic analysis of corneal organoids during development. Stem Cell Rep.18, 2482–2497 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eintracht, J. et al. Cell types of the human retina and its organoids at single-cell resolution. Front. Neuroanat.182, 2517–2528 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi, R. et al. Generation of 3D lacrimal gland organoids from human pluripotent stem cells. Nature605, 126–131 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Howard, L. et al. Single-cell transcriptomics reveals the molecular basis of human iPS cell differentiation into ectodermal ocular lineages. Commun. Biol.7, 1495 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, C. et al. Deciphering the dynamic single-cell transcriptional landscape in the ocular surface ectoderm differentiation system. Life Med.3, 1–16 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomi, K. et al. Generation of functional conjunctival epithelium, including goblet cells, from human iPSCs. Cell Rep. 34, 108715 (2021). [DOI] [PubMed]
- 23.Kitao, M. et al. Identification of BST2 as a conjunctival epithelial stem/progenitor cell marker. iScience26, 107016 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgala, P. A., Carr, C. B. & Price, D. J. The role of Pax6 in forebrain development. Dev. Neurobiol.71, 690–709 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Xuan, S. et al. Winged helix transcription factor BF-1 Is essential for the development of the cerebral hemispheres. Neuron14, 1141–1152 (1995). [DOI] [PubMed]
- 26.Martynoga, B., Morrison, H., Price, D. J. & Mason, J. O. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol.283, 113–127 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Mignone, J. L., Kukekov, V., Chiang, A. S., Steindler, D. & Enikolopov, G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol.469, 311–324 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Glazer, R. I., Vo, D. T. & Penalva, L. O. F. Musashi1: an RBP with versatile functions in normal and cancer stem cells. Front. Biosci. 17, 54–64 (2012). [DOI] [PubMed]
- 29.Venere, M. et al. Sox1 marks an activated neural stem/progenitor cell in the hippocampus. Development139, 3938–3949 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kan, L. et al. Dual function of Sox1 in telencephalic progenitor cells. Dev. Biol.310, 85–98 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victoria Graham, J. K. P. E. L. P. SOX2 functions to maintain neural progenitor identity. Neuron39, 749–765 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Nieto, M. et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J. Comp. Neurol.479, 168–180 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Katsetos, C. D., Herman, M. M. & Mörk, S. J. Class III β-tubulin in human development and cancer. Cell Motil. Cytoskeleton55, 77–96 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Dehmelt, L. & Halpain, S. The MAP2/Tau family of microtubule-associated proteins gene organization and evolutionary history. Genome Biol.6, 1–10 (2004). [Google Scholar]
- 35.Brown, J. P. et al. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol.467, 1–10 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Gao, Z. et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci.12, 1090–1092 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell126, 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Zhang, J. et al. LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell19, 66–80 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Aibar, S. et al. SCENIC: Single-cell regulatory network inference and clustering. Nat. Methods14, 1083–1086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakurela, S. et al. Mapping gene regulatory circuitry of Pax6 during neurogenesis. Cell Discov. 2, 15045 (2016). [DOI] [PMC free article] [PubMed]
- 41.Bäumer, N. et al. Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development130, 2903–2915 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Tchieu, J. et al. A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell21, 399–410.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troy, T. C. & Turksen, K. Commitment of embryonic stem cells to an epidermal cell fate and differentiation in vitro. Dev. Dyn.232, 293–300 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Qu, Y. et al. Transcriptome and proteome characterization of surface ectoderm cells differentiated from human iPSCs. Sci. Rep.6, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endo-Munoz, L. et al. E2F7 can regulate proliferation, differentiation, and apoptotic responses in human keratinocytes: Implications for cutaneous squamous cell carcinoma formation. Cancer Res.69, 1800–1808 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Thurlings, I. et al. Synergistic functions of E2F7 and E2F8 are critical to suppress stress-induced skin cancer. Oncogene36, 829–839 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen, Z., Evans, W. H., Pflugfelder, S. C. & Li, D. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells24, 1265–1273 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sax, C. M. & Piatigorsky, J. Transketolase is a major protein in the mouse cornea. J. Biol. Chem.271, 33568–33574 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Moll, R., Franke, W. W., Schiller, D. L., Geiger, B. & Krepler, R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell31, 11–24 (1982). [DOI] [PubMed] [Google Scholar]
- 50.Schermer, A., Galvin, S. & Sun, T.-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol.103, 49–62 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramirez-Miranda, A., Nakatsu, M. N., Zarei-Ghanavati, S., Nguyen, C. V. & Deng, S. X. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol. Vis.17, 1652–1660 (2011). [PMC free article] [PubMed] [Google Scholar]
- 52.Barbaro, V. et al. C/EBPδ regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol.177, 1037–1049 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menzel-Severing, J. et al. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep.8, 1–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li, M. et al. Loss of FOXC1 contributes to the corneal epithelial fate switch and pathogenesis. Signal Transduct. Target. Ther.6, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li, M. et al. The single-cell transcriptomic atlas and RORA-mediated 3D epigenomic remodeling in driving corneal epithelial differentiation. Nat. Commun. 15, 256 (2024). [DOI] [PMC free article] [PubMed]
- 56.Nakamura, T. et al. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells26, 1265–1274 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Bhattacharya, S. et al. DLX3-dependent STAT3 signaling in keratinocytes regulates skin immune homeostasis. J. Invest. Dermatol.138, 1052–1061 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirai, K. et al. Effects of the loss of conjunctival Muc16 on corneal epithelium and stroma in mice. Investig. Ophthalmol. Vis. Sci.55, 3626–3637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis, J., Davis, D., Norman, B. & Piatigorsky, J. Gene expression of the mouse corneal crystallin Aldh3a1: activation by Pax6, Oct1, and p300. Investig. Ophthalmol. Vis. Sci.49, 1814–1826 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Toyono, T. et al. Angiopoietin-like 7 is an anti-angiogenic protein required to prevent vascularization of the cornea. PLoS ONE10, e0116838 (2015). [DOI] [PMC free article] [PubMed]
- 61.Liu, S. The eyelid margin. Arch. Ophthalmol.125, 523 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Yoshihara, M. et al. High-resolution promoter map of human limbal epithelial cells cultured with keratinocyte growth factor and rho kinase inhibitor. Sci. Rep.7, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li, M. et al. Core transcription regulatory circuitry orchestrates corneal epithelial homeostasis. Nat. Commun.12, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quan, Y. et al. Tcf7l2 localization of putative stem/progenitor cells in mouse conjunctiva. Am. J. Physiol. Cell Physiol.311, C246–C254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta, D., Harvey, S. A. K., Kaminski, N. & Swamynathan, S. K. Mouse conjunctival forniceal gene expression during postnatal development and its regulation by krüppel-like factor 4. Investig. Ophthalmol. Vis. Sci.52, 4951–4962 (2011). vol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature566, 496–502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei, Z. G., Sun, T. T. & Lavker, R. M. Rabbit conjunctival and corneal epithelial cells belong to two separate lineages. Investig. Ophthalmol. Vis. Sci.37, 523–533 (1996). [PubMed] [Google Scholar]
- 68.Hayashi, Y., Watanabe, N. & Ohashi, Y. The ‘replacement hypothesis’: corneal stem cell origin epithelia are replaced by limbal stem cell origin epithelia in mouse cornea during maturation. Cornea31, 68–73 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Kitazawa, K. et al. PAX6 regulates human corneal epithelium cell identity. Exp. Eye Res.154, 30–38 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Bannier-Hélaouët, M. et al. Human conjunctiva organoids to study ocular surface homeostasis and disease. Cell Stem Cell31, 227–243.e12 (2024). [DOI] [PubMed] [Google Scholar]
- 71.Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Young, M. D. & Behjati, S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. Gigascience9, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst.8, 281–291.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner, H. C., Budak, M. T., Akinci, M. A. M. & Wolosin, J. M. Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Investig. Ophthalmol. Vis. Sci.48, 2050–2061 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.La Manno, G. et al. RNA velocity of single cells. Nature560, 494–498 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol.38, 1408–1414 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Shannon, P. et al. Cytoscape: a software environment for integrated models. Genome Res. 13, 426 (2003). [DOI] [PMC free article] [PubMed]
- 80.Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol.32, 381–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen, S., Zhou, Y., Chen, Y. & Gu, J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics12, 323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamuro, R. et al. Single-cell RNA-sequencing analysis of human iPSC-derived ocular cells [Data set]. ArrayExpress https://identifiers.org/arrayexpress:E-MTAB-14019 (2025).
- 85.Kamuro, R. et al. Characterization of human iPSC-derived ocular surface epithelial cell sheets depending on PAX6 gene expression levels [Data set]. ArrayExpress https://identifiers.org/arrayexpress:E-MTAB-13992 (2025).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The scRNA-seq and bulk RNA-seq data of human iPSC-derived ocular cells used in this study have been deposited in the ArrayExpress database at EMBL-EBI and are available under the accession codes “E-MTAB-14019” and “E-MTAB-13992,” respectively84,85. The plasmids generated in this study are referenced at the RIKEN BioResource Research Center (BRC). Source data underlying the graphs and charts in the main figures are provided in Supplementary Data 4. All other data supporting the findings of this study are available from the corresponding author upon reasonable request.
Unique materials produced in this study can be obtained from the lead contact following a reasonable request and after signing a material transfer agreement.