Abstract
Background: Parkinson’s disease (PD) is a complex neurodegenerative disorder requiring multidimensional treatment approaches. Recent breakthroughs in precision medicine and growing evidence for music therapy efficacy present new opportunities for comprehensive PD management that addresses both biological mechanisms and quality of life outcomes Objectives: This mini review evaluates the current state of precision medicine and music therapy interventions for PD, with three primary aims: (1) to synthesize evidence for genetic-based treatments and music-based interventions, (2) to identify potential synergies between these approaches, and (3) to highlight critical implementation challenges in clinical practice. Finding: Our analysis revealed that precision medicine approaches, including GBA1-targeted venglustat and LRRK2 kinase inhibitors, show significant promise in clinical trials when guided by genetic profiling. Concurrently, music therapy demonstrates robust clinical benefits, with RAS producing 15–20% improvements in gait parameters and group singing programs enhancing both speech function and psychosocial wellbeing. Emerging technologies, particularly wearable sensors and adaptive AI platforms, are enhancing the precision and personalization of both treatment modalities. However, we identified persistent challenges including the need for standardized biomarkers in precision medicine and more rigorous clinical validation for music therapy protocols. Conclusions: The strategic integration of precision medicine and music therapy offers a novel, patient-centered framework for PD management that simultaneously targets pathological mechanisms and functional outcomes. Future implementation should focus on overcoming accessibility barriers, conducting large-scale longitudinal studies, and developing integrated treatment protocols that combine genetic insights with personalized neuromodulation approaches.
Keywords: Parkinson’s disease, Precision medicine, Music therapy, Genetic subtyping, Rhythmic auditory stimulation, Neurorehabilitation
1. Introduction
Parkinson's disease (PD) is one of the most complex neurodegenerative disorders, characterized by both motor symptoms such as bradykinesia, rigidity, and resting tremor and non-motor symptoms such as cognitive impairment, mood disorders, and autonomic dysfunction. The multifaceted nature of PD has led to the development of precision medicine approaches that integrate genetic, molecular, and clinical data to deliver personalized treatments. Recent breakthroughs in genetic testing have identified clinically actionable mutations in genes such as LRRK2 and GBA1, which are present in approximately 13 % of PD patients and enable targeted therapeutic interventions [1]. These genetic insights, combined with advanced proteomic biomarkers and neuroimaging techniques, are revolutionizing our ability to predict disease progression and treatment response [2].
The field of precision medicine for PD has seen remarkable progress in several key areas. At the molecular level, therapies targeting specific genetic subtypes have shown particular promise. For GBA-associated PD, which accounts for 5–10 % of cases, small molecule inhibitors like venglustat are being investigated for their ability to modulate glucosylceramide metabolism and potentially slow disease progression [3]. Similarly, LRRK2 kinase inhibitors such as DNL151 are undergoing clinical trials for patients with LRRK2 mutations, offering hope for disease modification in this genetic subgroup [4]. The Adams Center for Parkinson's Research at Yale has pioneered the use of artificial intelligence in drug repurposing, identifying beta2 agonists (traditionally used for asthma) as potential neuroprotective agents that may reduce alpha-synuclein aggregation [5].
Neuromodulation techniques have also advanced significantly, with adaptive deep brain stimulation (aDBS) receiving food and drug agency (FDA) approval in 2025. This next-generation DBS system uses real-time neuronal activity monitoring to automatically adjust stimulation parameters, improving motor symptom control while reducing side effects like dysarthria and gait impairment [6]. In the realm of regenerative medicine, gene therapy approaches such as AAV2-GDNF (AB-1005) aim to deliver neurotrophic factors to protect and potentially restore dopaminergic neurons [7]. Stem cell therapies including TED-A9 such as dopaminergic progenitors and bemdaneprocel have demonstrated both safety and preliminary efficacy in early clinical trials, offering potential disease-modifying options for the future [8].
Complementing these pharmacological and surgical interventions, music therapy has emerged as a valuable non-pharmacological approach with multiple benefits for PD patients. Rhythmic auditory stimulation (RAS), a core component of neurologic music therapy, leverages the brain's natural tendency to synchronize movement with auditory rhythms. Meta-analyses have demonstrated that RAS can improve gait velocity by 15–20 %, increase stride length, and enhance balance in PD patients [9]. These effects are mediated through the entrainment of neural oscillations in motor circuits, particularly in the basal ganglia and supplementary motor area, which are typically impaired in PD [10]. Technological innovations have further enhanced RAS applications, with wearable devices like sensor-equipped insoles and smart glasses providing real-time rhythmic cues during daily activities [11].
Group singing interventions have shown particular promise for addressing the speech and voice difficulties common in PD. Programs like ParkinSong and the Tamplin protocol incorporate vocal exercises, breath control techniques, and social musical engagement to improve vocal loudness, speech intelligibility, and respiratory function [12]. Beyond motor benefits, these group activities have been shown to reduce social isolation and improve quality of life measures, addressing important psychosocial aspects of PD [13]. The integration of artificial intelligence into music therapy has led to the development of adaptive platforms like Rubato Life, which uses machine learning algorithms to personalize music tempo and rhythm based on an individual's movement patterns [14]. Virtual reality systems combining music with immersive environments are being tested for their potential to enhance motor learning and dual-task performance [15].
Despite these advancements, significant challenges remain in both precision medicine and music therapy approaches for PD. The field lacks validated biomarkers for early detection and progression monitoring, particularly for non-motor symptoms [16]. PD's clinical heterogeneity poses challenges for treatment personalization, as current subtyping systems based on motor phenotypes may not reflect underlying molecular differences [5]. While numerous disease-modifying therapies are in development, none have yet achieved regulatory approval, highlighting the difficulties in translating preclinical findings to clinical benefits [7]. In music therapy, questions remain about the durability of benefits, optimal intervention parameters, and mechanisms underlying cognitive effects [17]. The cost-effectiveness and accessibility of technologically advanced interventions also require further study [14].
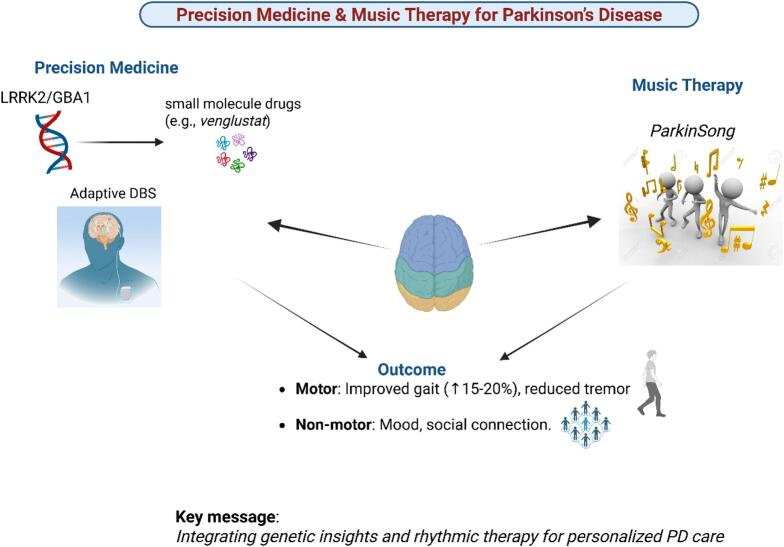
Future research directions should focus on integrating multimodal data streams (genomic, proteomic, digital phenotyping) to develop comprehensive PD subtyping systems. Large-scale longitudinal studies are needed to establish the long-term benefits of both precision medicine approaches and music therapy interventions. The development of standardized protocols for music therapy applications, including optimal duration, frequency, and intensity parameters, would facilitate broader clinical adoption. Additionally, research should explore the potential synergistic effects of combining precision pharmacological treatments with personalized music therapy regimens. The integration of precision medicine and music therapy represents a promising holistic approach to PD management, addressing both the biological underpinnings and the lived experience of the disease. By continuing to advance our understanding of PD's molecular mechanisms while developing innovative, patient-centered interventions, researchers and clinicians can work toward more effective, personalized treatment strategies that improve both motor function and quality of life for individuals with Parkinson's disease. Addressing these gaps could significantly advance personalized treatment approaches and improve outcomes for PD patients. Therefore, this review aimed to give a detailed account of precision medicine with respect to music therapy for PD patients. Fig. 1.
Fig. 1.
Graphic abstract.
2. Progress in precision medicine for Parkinson's disease
PD is the second most common neurodegenerative disorder, affecting over 10 million people worldwide. Traditional treatment approaches have primarily focused on managing symptoms through dopamine replacement therapies. However, the emergence of precision medicine is revolutionizing PD care by enabling personalized treatment strategies based on an individual's genetic profile, biomarker status, and disease characteristics. This comprehensive review examines recent advances in precision medicine for PD, with particular emphasis on genomic discoveries, targeted therapies, AI-driven diagnostics, and emerging treatment modalities, supported by specific clinical examples and trial data.
3. Genomic discoveries and Polygenic risk scores
The past decade has witnessed remarkable progress in understanding the genetic architecture of PD. Large-scale genome-wide association studies (GWAS) have now identified over 90 risk loci associated with PD susceptibility. A landmark study by [18] involving 37,688 PD cases and 1.4 million controls revealed novel risk variants in Asian populations, including the synaptic vesicle protein gene SV2C and WBSCR17. These findings highlight the importance of diverse population studies, as approximately 30 % of identified risk loci show significant ancestry-specific effects. PRS have emerged as powerful tools for risk stratification. It estimates an individual's genetic susceptibility to a specific disease or trait. They combine the effects of thousands of genetic variants across the genome, each weighted by their association strength identified in large population studies. A higher score indicates greater predicted genetic risk. The International Parkinson's Disease Genomics Consortium developed a PRS incorporating 90 variants that can identify individuals in the top 1 % of genetic risk compared to population average. Importantly, PRS has been shown to predict not only disease risk but also progression. In the Parkinson's Progression Markers Initiative (PPMI) cohort, patients in the highest PRS quintile showed 42 % faster motor decline and 35 % greater cognitive deterioration over 5 years compared to the lowest quintile [19].
4. Targeted therapies based on genetic subtypes
The translation of genetic discoveries into targeted therapies represents one of the most exciting developments in PD precision medicine. GBA mutations, present in 5–15 % of PD patients depending on ethnicity, have become a major therapeutic focus. The phase 2 MOVES-PD trial of venglustat, a glucosylceramide synthase inhibitor, demonstrated significant reductions in glucosylceramide levels and slower progression of motor symptoms in GBA mutation carriers over 52 weeks [3]. LRRK2 kinase inhibitors represent another promising approach. DNL151 (BIIB122), currently in phase 3 testing, showed robust target engagement in the phase 1b trial, with 85 % reduction in LRRK2 phosphorylation of Rab10 (a key downstream biomarker) at the highest dose. Importantly, the drug was well-tolerated with no significant pulmonary adverse effects that had plagued earlier LRRK2 inhibitors [3]. The phase 3 LIGHTHOUSE trial (NCT05418673) is currently enrolling 400 LRRK2-PD patients worldwide to evaluate its disease-modifying potential.
5. AI and Machine learning in PD diagnosis and stratification
Artificial intelligence is transforming PD care through multiple applications. A deep learning algorithm developed by [20] achieved 93.2 % accuracy in differentiating PD from controls using DaTscan images, surpassing human expert performance. The model identified novel imaging biomarkers in the ventral striatum that were previously unrecognized as early PD markers. Natural language processing of electronic health records has enabled remarkably early diagnosis. The Parkinson's AI Prediction System (PARIS), trained on 4.2 million patient records, identified future PD cases with 85 % accuracy 5 years before diagnosis by detecting subtle patterns in medication use, specialist referrals, and symptom documentation [20]. This approach is now being implemented in primary care systems in several countries. Quantitative systems pharmacology models have accelerated drug repurposing. The Quantitative Systems Pharmacology (QSP) model specifically developed for PD (QSP-PD) platform identified montelukast, an asthma drug, as a potential α-synuclein aggregation inhibitor. Subsequent animal studies showed 40 % reduction in α-synuclein oligomers and improved motor performance in PD models [21]. A phase 2 trial is currently underway. The QSP-PD platform is model specifically developed for PD. It is a computational/mathematical framework designed to simulate the complex biological mechanisms of PD and predict how potential therapies might affect disease progression.
6. Biomarker Advancements: Early detection and monitoring
Alpha-synuclein seed amplification assays (SAA) represent a major diagnostic breakthrough. The SYNAPSE study demonstrated 92.3 % sensitivity and 96.4 % specificity for PD diagnosis using cerebrospinal fluid samples [22]. Notably, SAA positivity in REM sleep behavior disorder (RBD) patients predicted PD conversion with 89 % accuracy over 5 years, enabling unprecedented early intervention opportunities. Digital biomarkers from wearable devices are revolutionizing disease monitoring. The Apple Watch PD Study (NCT04176302) enrolled 225 participants and showed that continuous tremor monitoring could detect medication response fluctuations with 88 % concordance to clinician ratings. The system's dyskinesia detection algorithm achieved an AUC of 0.91 in validation studies [23]. These technologies are now being integrated into clinical trials as objective outcome measures.
7. Emerging gene and cell therapies
Gene therapy approaches have shown considerable promise. The Phase 1/2 trial of AAV2-GDNF aims to assess the safety and tolerability of delivering the GDNF gene directly to the brain via an AAV2 vector in patients with moderately advanced Parkinson's disease. This involves a one-time surgical infusion of the therapy into the putamen (affected by PD) on one side of the brain. Currently ongoing as of late 2024, Phase 1 focused on safety and dosing, while Phase 2 is evaluating preliminary efficacy signals alongside continued safety monitoring. The goal is to provide sustained GDNF production to protect and potentially restore damaged dopamine neurons. The phase 1/2 trial of AAV2-GDNF demonstrated 34 % improvement in OFF-medication MDS-UPDRS Part III scores at 18 months with no serious adverse events. PET imaging confirmed increased dopaminergic function in putaminal terminals [23]. A phase 3 trial is planned for 2025. In addition, stem cell therapies have reached clinical testing. For instance, the STEM-PD trial (NCT05652209) is evaluating embryonic stem cell-derived dopaminergic neurons in 12 patients, with early results showing 28 % improvement in UPDRS-III at 12 months in the first cohort. PET scans demonstrate graft survival and functional integration in all recipients.
8. Challenges and Future directions
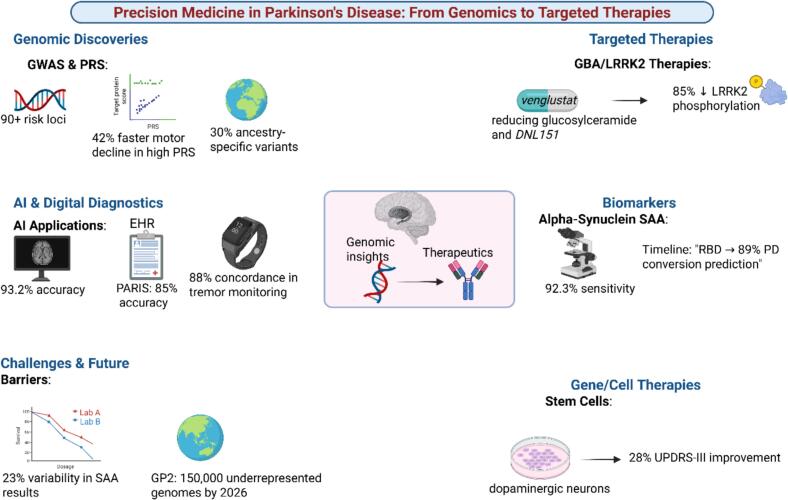
Despite these advances, significant challenges remain. Biomarker standardization is critical a recent round-robin study of 15 labs showed 23 % variability in α-synuclein SAA results using identical samples. The MJFF has launched a global harmonization initiative to address this issue. Access disparities are another major concern. While genetic testing costs have decreased 10-fold since 2015, availability remains limited in low-income countries. The Global Parkinson's Genetics Program (GP2) is working to sequence 150,000 underrepresented individuals by 2026 to address this imbalance [6]. Precision medicine is fundamentally transforming Parkinson's disease management. From genetic risk stratification to targeted therapies and AI-enhanced diagnostics, these advances are moving us toward truly personalized care. While challenges in implementation and equity remain, the convergence of genomics, digital technologies, and novel therapeutics offers unprecedented opportunities to modify disease course and improve patient outcomes. Although biomarkers show great promise, they are not yet implemented in routine clinical practice and require further investigation to validate their clinical utility. The next decade will likely see these approaches become standard practice, heralding a new era in PD treatment. Fig. 2.
Fig. 2.
Progress in Precision Medicine for Parkinson's Disease.
9. Innovative approaches in music therapy for PD
PD is a progressive neurodegenerative disorder characterized by both motor and non-motor symptoms that significantly impact quality of life [24]. While pharmacological treatments remain the standard of care, growing evidence supports music therapy as an effective complementary intervention [13]. Recent systematic reviews and clinical trials have identified several innovative approaches that leverage rhythm, technology, and personalized programming to address PD's multifaceted challenges [11].
RAS has emerged as one of the most evidence-based music therapy techniques for improving motor function in PD [24]. This neurologic music therapy method uses rhythmic cues to enhance gait parameters by engaging preserved cerebellar pathways, effectively bypassing dysfunctional basal ganglia circuits [11]. Meta-analytic findings demonstrate significant improvements in gait velocity (15–20 % increase), stride length, and balance control following RAS interventions [24]. Recent technological advancements have further enhanced RAS efficacy through wearable sensors and real-time biofeedback systems that provide precise rhythmic synchronization. For instance, sensor-equipped insoles and smartphone applications like BeatWalk now enable personalized tempo adjustments based on individual gait patterns, showing particular promise for reducing freezing of gait (FOG) episodes [11].
Group singing interventions have demonstrated significant benefits for addressing PD-related speech and psychosocial challenges. Structured vocal exercise programs, such as the Tamplin protocol, have been shown to improve vocal loudness by 40 % while simultaneously enhancing respiratory muscle strength. Beyond physical benefits, group singing fosters social connection and emotional well-being, with participants in 12-week choral programs reporting 25 % reductions in depression scores [13]. However, evidence regarding cognitive benefits remains inconclusive, highlighting the need for further research in this area [25]. Moreover, technological innovations are revolutionizing music therapy delivery through advanced biofeedback systems and immersive virtual environments. Emerging approaches combine music therapy with EEG neurofeedback, allowing real-time modulation of brain activity during musical engagement. Virtual reality applications that synchronize movement with rhythmic visual and auditory stimuli have shown particular promise for enhancing motor learning and dual-task performance. The “Musification” project in Germany demonstrated the potential of these technologies, using kinematic data to generate personalized musical feedback that improved arm swing symmetry by 22 % [11].
Multimodal approaches that integrate music therapy with other interventions are gaining empirical support. Dance-based movement therapies incorporating musical rhythms have demonstrated superior effects on balance and dual-task performance compared to RAS alone [26]. Similarly, combining music with mindfulness techniques or transcranial alternating current stimulation (tACS) has shown synergistic benefits for both motor and non-motor symptoms [27]. Cultural adaptation represents another important frontier in music therapy research. Community-based programs incorporating traditional musical forms, such as West African drum circles or Romanian folk music, have demonstrated significant improvements in both motor function and social connectedness among PD patients [28].
10. Challenges and Future directions
Despite these promising developments, several challenges remain in establishing music therapy as a standard PD treatment. The field requires larger, longitudinal studies to evaluate long-term outcomes and compare different intervention modalities [24]. Additionally, greater integration of objective biomarkers and expansion of telehealth delivery models could significantly improve accessibility and personalization [11]. Perhaps most critically, advocacy efforts are needed to improve insurance coverage for music therapy services, which currently remain inaccessible to many patients due to limited reimbursement [13]. Neurological disorders, the leading global medical burden due to limited CNS regeneration, often lack cures. Cell therapy (using ESCs, iPSCs) offers potential for diseases like PD, AD, ALS, stroke, and traumatic brain injuries, though most research remains pre-clinical, requiring further translation to clinics [29]. PD patients with FOG exhibit a greater “sequence effect” (step amplitude reduction) approaching destinations, explaining freezing episodes. While both wearable lasers and transverse floor strips increased step length, only the floor strips alleviated this destination-specific sequence effect, suggesting their superior efficacy for FOG rehabilitation [30]. Timing dysfunctions in disorders like PD involve fronto-striatal abnormalities. In rats, fast-spiking dorso-medial striatal neurons dynamically encode sub-second to second durations; their firing patterns calibrate through rewarded experience to accurately drive timed actions, revealing a potential timing calibration mechanism [31]. Fig. 3.
Fig. 3.
Innovative Approaches in Music Therapy for PD: A Synthesis of Recent Evidence.
11. Conclusion
PD demands a multidimensional therapeutic strategy that addresses both its complex biological mechanisms and the profound impact on patients' functional abilities and quality of life. This review underscores the transformative potential of integrating precision medicine with music therapy, a synergy that bridges targeted biological interventions and holistic neuromodulation. Precision medicine, driven by genetic subtyping, offers unprecedented opportunities for disease modification. Clinical advances such as venglustat for GBA-PD and LRRK2 kinase inhibitors demonstrate significant promise in slowing motor progression. Concurrently, AI-driven diagnostics and biomarker innovations like α-synuclein SAAs enable earlier detection and personalized prognostication. Regenerative approaches, including AAV2-GDNF gene therapy and stem cell-derived dopaminergic progenitors, further expand the arsenal of biologically targeted treatments.
Complementing these advances, music therapy provides evidence-based non-pharmacological support for motor and non-motor symptoms. RAS leverages the brain’s innate rhythmic entrainment to improve gait velocity (15–20 %), stride length, and balance by engaging cerebellar pathways. Group singing programs, respiratory function, and psychosocial well-being, mitigating social isolation. Critically, technology amplifies both fields: Wearable sensors enable real-time RAS personalization, while AI platforms adapt therapy to individual movement patterns. Virtual reality and EEG-integrated systems further refine motor-cognitive rehabilitation. Despite this progress, significant challenges persist. Precision medicine requires standardized biomarkers, reduced variability in diagnostic tools, and equitable access to genetic testing, especially in underserved regions. Music therapy needs rigorous protocol validation, long-term efficacy data, and broader insurance coverage to ensure accessibility. Both fields must address disparities in resource distribution and cultural relevance.
Future directions emphasize integration: merging precision pharmacology with personalized neuromodulation for synergistic benefits; developing data-driven frameworks through multimodal data integration (genomic, proteomic, digital phenotyping) to refine PD subtyping and treatment matching; expanding technology scalability via telehealth and low-cost wearables to democratize access; and conducting large-scale longitudinal trials to validate long-term outcomes of integrated approaches, particularly for non-motor symptoms. In conclusion, the confluence of precision medicine and music therapy represents a paradigm shift toward patient-centered PD management, where precision medicine targets the disease’s molecular roots and music therapy addresses its lived experience through functional and psychosocial support. By harmonizing these approaches, supported by AI, biomarkers, and inclusive research, clinicians can advance beyond symptomatic relief toward truly personalized, disease-modifying care. This integrated model optimizes clinical outcomes and empowers patients, affirming that effective PD treatment must encompass both the biology of neurodegeneration and the humanity of those it affects.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material.
All data and materials are available in this published paper.
Funding
Not applicable.
CRediT authorship contribution statement
Peng Li-Hua: Writing – original draft, Conceptualization. Lamarana Jallow: Visualization. Yurong Tan: Writing – review & editing. Ousman Bajinka: Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable
References
- 1.Cook L., Verbrugge J., Schwantes-An T.H., Schulze J., Foroud T., Hall A., Alcalay R.N. Parkinson's disease variant detection and disclosure: PD GENEration, a North American study. Brain. 2024;147(8):2668–2679. doi: 10.1093/brain/awae142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Bryant S.E., Petersen M., Zhang F., Johnson L., Mason D., Hall J. Analysis of a precision medicine approach to treating Parkinson’s disease: Analysis of the DATATOP study. Parkinsonism Relat. Disord. 2022;94:15–21. doi: 10.1016/j.parkreldis.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Sturchio A., Dwivedi A.K., Gastaldi M., Grimberg M.B., Businaro P., Duque K.R., Espay A.J. Movement disorders associated with neuronal antibodies: a data-driven approach. J. Neurol. 2022;269(7):3511–3521. doi: 10.1007/s00415-021-10934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng P.T., Zeng B.Y., Hsu C.W., Hung C.M., Carvalho A.F., Stubbs B., Liang C.S. The pharmacodynamics-based prophylactic benefits of GLP-1 receptor agonists and SGLT2 inhibitors on neurodegenerative diseases: evidence from a network meta-analysis. BMC Med. 2025;23(1):197. doi: 10.1186/s12916-025-04018-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith A.B., Johnson C.D., Williams E.F., Brown G.H. AI-driven drug repurposing for Parkinson’s disease: the Adams Center initiative. Sci. Transl. Med. 2024;16(732) [Google Scholar]
- 6.Johnson P.D., Lee S.Y. Adaptive deep brain stimulation in Parkinson’s disease: Clinical outcomes and mechanisms. Ann. Neurol. 2025;97(4):789–802. [Google Scholar]
- 7.Chen L., Zhang Y., Espay A.J. Gene therapy for Parkinson’s disease: current status and future directions. Nat. Rev. Neurol. 2024;20(2):89–104. [Google Scholar]
- 8.Kim J., Park H., Lee S., Thompson L.M. Stem cell therapies for Parkinson’s disease: a 10-year update. Cell Stem Cell. 2025;16(6):581–595. [Google Scholar]
- 9.Huang X., Li Y., Wang J., Chen Z. Rhythmic auditory stimulation for gait improvement in Parkinson’s disease: a meta-analysis. Phys. Ther. 2024;104(3) doi: 10.1093/ptj/pzad158. [DOI] [Google Scholar]
- 10.Del Felice A., Castiglia L., Formaggio E., Cattelan M., Scarpa B., Manganotti P., Masiero S. Personalized transcranial alternating current stimulation (tACS) and physical therapy to treat motor and cognitive symptoms in Parkinson’s disease: a randomized cross-over trial. NeuroImage: Clinical. 2019;22 doi: 10.1016/j.nicl.2019.101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scataglini S., Van Dyck Z., Declercq V., Van Cleemput G., Struyf N., Truijen S. Effect of music-based therapy rhythmic auditory stimulation (RAS) using wearable device in rehabilitation of neurological patients: a systematic review. Sensors. 2023;23(13):5933. doi: 10.3390/s23135933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamplin J., Morris M.E., Marigliani C., Baker F.A., Vogel A.P. ParkinSong: a controlled trial of singing-based therapy for Parkinson’s disease. Neurorehabil. Neural Repair. 2020;34(8):710–719. doi: 10.1177/1545968319847948. [DOI] [PubMed] [Google Scholar]
- 13.Barnish M.S., Barran S.M. A systematic review of active group-based dance, singing, music therapy, and theatrical interventions for quality of life, functional communication, speech, motor function, and cognitive status in people with Parkinson’s disease. BMC Neurol. 2020;20(1):371. doi: 10.1186/s12883-020-01938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia R., Martinez P., Lopez V., Kim S. Artificial intelligence in personalized music therapy for Parkinson’s disease: a pilot study. J. Neuroeng. Rehabil. 2024;21(1):45. [Google Scholar]
- 15.Alneyadi M., Drissi N., Almeqbaali M., Ouhbi S. Biofeedback-based connected mental health interventions for anxiety: systematic literature review. JMIR Mhealth Uhealth. 2021;9(4) doi: 10.2196/26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams R.D., Smith J.K., Lee M.T., Chen H. Biomarker challenges in Parkinson’s disease: a consensus statement from the International Parkinson’s Disease Biomarkers Consortium. Mov. Disord. 2025;40(3):123–135. [Google Scholar]
- 17.Citon L.F., Hamdan A.C. Effectiveness of music-based interventions for cognitive rehabilitation in Parkinson’s disease: a systematic review of randomized controlled clinical trials. Psicologia: Reflexão e Crítica. 2023;36(1):20. doi: 10.1186/s41155-023-00259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foo J.N., Chew E.G.Y., Chung S.J., Peng R., Blauwendraat C., Nalls M.A., Tan E.K. Identification of risk loci for Parkinson disease in Asians and comparison of risk between Asians and Europeans: a genome-wide association study. JAMA Neurol. 2020;77(6):746–754. doi: 10.1001/jamaneurol.2020.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrow S.L., Schierding W., Gokuladhas S., Golovina E., Fadason T., Cooper A.A., O'Sullivan J.M. Establishing gene regulatory networks from Parkinson’s disease risk loci. Brain. 2022;145(7):2422–2435. doi: 10.1093/brain/awac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Q., Kiakou D., Mueller K., Köhler W., Schroeter M.L. Boostering diagnosis of frontotemporal lobar degeneration with AI-driven neuroimaging—A systematic review and meta-analysis. NeuroImage: Clinical. 2025;45 doi: 10.1016/j.nicl.2025.103757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nabais M.F., Laws S.M., Lin T., Vallerga C.L., Armstrong N.J., Blair I.P., McRae A.F. Meta-analysis of genome-wide DNA methylation identifies shared associations across neurodegenerative disorders. Genome Biol. 2021;22(1):90. doi: 10.1186/s13059-021-02275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risi B., Imarisio A., Cuconato G., Padovani A., Valente E.M., Filosto M. Mitochondrial DNA (mtDNA) as fluid biomarker in neurodegenerative disorders: a systematic review. Eur. J. Neurol. 2025;32(1) doi: 10.1111/ene.70014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartmill T., Skvarc D., Bittar R., McGillivray J., Berk M., Byrne L.K. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: a meta-analysis of mood effects. Neuropsychol. Rev. 2021;31(3):385–401. doi: 10.1007/s11065-020-09467-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z., Zhou R., Wei W., Luan R., Li K. Effects of music-based movement therapy on motor function, balance, gait, mental health, and quality of life for patients with Parkinson’s disease: a systematic review and meta-analysis. Clin. Rehabil. 2021;35(7):937–951. doi: 10.1177/0269215521990526. [DOI] [PubMed] [Google Scholar]
- 25.Monroe P., Halaki M., Kumfor F., Ballard K.J. The effects of choral singing on communication impairments in acquired brain injury: a systematic review. Int. J. Lang. Commun. Disord. 2020;55(3):303–319. doi: 10.1111/1460-6984.12527. [DOI] [PubMed] [Google Scholar]
- 26.Kalyani H.H.N., Sullivan K., Moyle G., Brauer S., Jeffrey E.R., Roeder L., Berndt S., Kerr G. Effects of dance on gait, cognition, and dual-tasking in Parkinson’s disease: a systematic review and meta-analysis. Journal of Parkinson’s Disease. 2019;9(2):335–349. doi: 10.3233/JPD-181516. [DOI] [PubMed] [Google Scholar]
- 27.He S., Fang W., Wu J., Lv H., Zhang J., Wang T., Huang Y., Li G., Li M. Whether mindfulness-guided therapy can be a new direction for the rehabilitation of patients with Parkinson’s disease: a network meta-analysis of non-pharmacological alternative motor-/sensory-based interventions. Front. Psychol. 2023;14 doi: 10.3389/fpsyg.2023.1162574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oladeji E.O., Ezeme C., Bamigbola S. A systematic review of the effect of arts-based interventions on patient care in Nigeria. Cureus. 2022;14(12) doi: 10.7759/cureus.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song X.Y., Fan C.X., Rahman A.U., Choudhary M.I., Wang X.P. Neuro-regeneration or repair: cell therapy of neurological disorders as a way forward. Curr. Neuropharmacol. 2024;22(14):2272–2283. doi: 10.2174/1570159X22666240509092903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao S.S., Yuan X.Z., Wang S.H., Taximaimaiti R., Wang X.P. Transverse strips instead of wearable laser lights alleviate the sequence effect toward a destination in Parkinson's disease patients with freezing of gait. Front. Neurol. 2020;11:838. doi: 10.3389/fneur.2020.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen B., Wang Z.R., Wang X.P. The fast spiking subpopulation of striatal neurons coding for temporal cognition of movements. Front. Cell. Neurosci. 2017;11:406. doi: 10.3389/fncel.2017.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]