Plasma lipoproteins are important determinants of atherosclerosis, a disease of the large arteries that underlies most deaths in industrialized nations (Lusis 2000). Atherogenesis begins in response to an arterial injury, which leads to a series of inflammatory, proliferative, and apoptotic cellular events that produce complicated arterial-wall plaques (Ross 1993, 1999). Some plaques are vulnerable to rupture and thrombosis, leading to arterial occlusion, whose location specifies the clinical presentation, usually a heart attack or stroke (Ross 1993, 1999). Genetic susceptibility plays a key role in atherosclerosis, particularly when clinical end points strike early in life. Some risk factors—such as dyslipidemia, diabetes and hypertension—themselves have complex genetic determinants. Other risk factors—such as smoking, poor diet, inactivity, and stress—can modulate expression of the genetic susceptibility.
Study of monogenic dyslipidemias has exposed key metabolic mechanisms. The exemplar was the autosomal dominant (AD) form of familial hypercholesterolemia (FH) (MIM 143890), whose characterization led to the discovery of receptor-mediated endocytosis (RME) via the LDL receptor (Brown and Goldstein 1986). This in turn led to development of statin drugs, which reduce LDL cholesterol and coronary heart disease (CHD) mortality (4S Investigators 1994) and which are now used by tens of millions of people. However, LDL-cholesterol reduction does not always prevent CHD (Superko 1996), and many patients with CHD do not have elevated LDL cholesterol as their primary dyslipidemia (Genest et al. 1992). Recent delineation of the molecular basis of other monogenic dyslipidemias therefore has the potential to reveal new pathways that could lead to additional downstream public-health benefits for CHD reduction.
Nongenetic and nonhuman experiments have revealed many fascinating gene products for lipoprotein metabolism. This review will focus on monogenic lipoprotein disorders, summarized in table 1. Before 1999, most mutations for monogenic lipoprotein disorders were identified on the basis of the function of the gene product. Since 1999, positional cloning has revealed novel genes—such as those for Tangier disease, sitosterolemia, and autosomal recessive (AR) hypercholesterolemia (ARH). Rather than describing in depth any particular pathway, this review will attempt to position newer genetic findings within the context of prior knowledge of plasma lipoprotein metabolism.
Table 1.
Monogenic Disorders Affecting Plasma Lipoproteins
| Lipoprotein Profile and Gene | Gene Product | Disease(s) |
| Cholesterol: | ||
| LDL: | ||
| Increased: | ||
| LDLR (MIM 143890) | LDL receptor | AD FH |
| APOB (MIM 107730) | apo B | FDB |
| ARH (MIM 603813) | ARH | AR FH |
| LIPA (MIM 278000) | Lysosomal lipase | Wolman disease, cholesteryl-ester–storage disease |
| Decreased: | ||
| MTP (MIM 157147) | MTP | ABL |
| APOB (MIM 107730) | apo B | Hypobetalipoproteinemia |
| SLC10A2 (MIM 601295) | ASBT | Primary BA malabsorption |
| HDL: | ||
| Decreased: | ||
| APOA1 (MIM 107680) | apo AI | Analphalipoproteinemia |
| LCAT (MIM 245900) | LCAT | Fish-eye disease, familial LCAT deficiency |
| ABCA1 (MIM 600046) | ABCA1 | Tangier disease, familial hypoalphalipoproteinemia |
| Increased: | ||
| CETP (MIM 118470) | Cholesteryl-ester–transfer protein | CETP deficiency |
| LIPC (MIM 151670) | HL | HL deficiency |
| Triglycerides: | ||
| APOC2 (MIM 207750) | APOC2 | APOC2 deficiency, hyperchylomicronemia |
| LPL (MIM 238600) | LPL | LPL deficiency, hyperchylomicronemia |
| Remnants and IDL: | ||
| APOE (MIM 107741) | apo E | Dysbetalipoproteinemia |
| LIPC (MIM 151670) | HL | HL deficiency |
| Sitosterol: | ||
| ABCG5 (MIM 605459) | ATP-binding cassette, subfamily G, member 5 | Sitosterolemia |
| ABCG8 (MIM 605460) | ATP-binding cassette, subfamily G, member 8 | Sitosterolemia |
Plasma Lipids and Lipoproteins
The most clinically relevant plasma lipids are cholesterol and triglyceride (TG). Cholesterol in particular has captivated generations of researchers: cholesterol-related discoveries serve as scientific milestones for the 20th century (Vance and van den Bosch 2000). Cholesterol is a structural component of cell membranes and is the precursor of steroid hormones and vitamin D and also of oxysterols and bile acids (BA), which activate nuclear-hormone receptors involved in sterol metabolism (Russell 1999). Cholesterol is also required for activation of sonic hedgehog, which is involved with forebrain patterning (Parisi and Lin 1998). But cholesterol also has a dark side. For instance, cholesterol entrapped within arterial-wall macrophages leads to foam-cell formation and plaque growth. In men, a rise in total plasma cholesterol from 5.2 to 6.2 mmol/liter (from 200 to 240 mg/dl) is associated with a threefold-increased risk of death from CHD (Stamler et al. 2000).
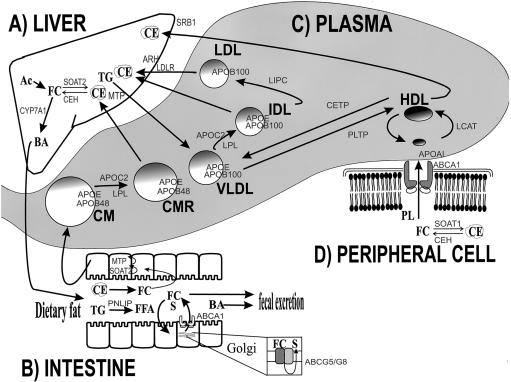
Lipoproteins are spherical macromolecules that transport cholesterol and TG—in addition to phospholipid (PL), fat-soluble antioxidants and vitamins, and cholesteryl ester (CE)—through plasma, from sites of synthesis and absorption to sites of uptake. Lipoproteins have a nonpolar lipid core with a hydrophilic surface coat containing free cholesterol (FC), PL, and apolipoprotein (apo) molecules. The main TG-carrying lipoproteins are chylomicrons (CM) and very-low-density lipoprotein (VLDL). The main cholesterol-carrying lipoproteins are LDL and HDL. Lipoproteins are distinguished from each other on the basis of size, density, electrophoretic mobility, lipid and protein content, composition, and function (table 2). Lipoprotein metabolism is a complex network of biochemical processes, including assembly, secretion, processing, and catabolism, as shown in figure 1. The liver (fig. 1A), intestine (fig. 1B), and peripheral cells (fig. 1D) are important determinants of plasma lipoproteins (fig. 1C).
Table 2.
Features of Primary Plasma Lipoproteins
|
Cholesterol(% wt) |
|||||||||
| Lipoprotein Class | Size(nm) | Density(g/ml) | Electrophoretic Mobility | TG(% wt) | PL(% wt) | Free | Esterified | Protein(% wt) | Primary Apolipoprotein(s) |
| CM | 75–1,200 | .94 | Origin (cathode) | 80–95 | 3–6 | 1–3 | 2–4 | 1–2 | A-I, A-IV, B-48, C-I, C-III, E |
| VLDL | 30–70 | .94–1.006 | Pre-β | 45–65 | 15–20 | 4–8 | 16–22 | 6–10 | B-100, E, C-I, C-II, C-III |
| LDL | 18–30 | 1.019–1.063 | β | 4–8 | 18–24 | 6–8 | 45–50 | 18–22 | B-100 |
| HDL | 5–12 | 1.063–1.21 | α | 2–7 | 26–32 | 3–5 | 15–20 | 45–55 | A-I, A-II, E |
Figure 1.
Four compartments important in plasma lipoprotein metabolism: liver (A), intestine (B), plasma (C), and peripheral cell (D). The main plasma lipoproteins—namely, CM (and its remnant, CMR), VLDL (and its remnant, IDL), LDL, and HDL—are represented as spheres within the shaded plasma compartment (C). Incoming arrows to the plasma compartment represent secretion from tissues into plasma, and outgoing arrows from the plasma represent uptake into tissues. Within the plasma space, arrows between lipoproteins represent processing reactions. In the liver (A) and peripheral cell (D), arrows between lipid constituents—such as the cellular pools of FC, CE, TG, acetyl coenzyme A (Ac), BA, and PL—indicate intracellular processes. In the intestinal lumen (B), arrows between lipids indicate processing and/or progress through the intestinal tract. In the intestine (B), outgoing and incoming arrows involving enterocytes indicate, respectively, secretion into and absorption from the lumen of lipids (B), including FFA, TG, FC, BA, and sitosterol (S). Gene products are shown, throughout the figure, in a lighter font. Gene products involved in lipoprotein secretion and catabolism, in intracellular lipid processing, and in intestinal lipid transport are shown beside the appropriate arrow. Intestinal intracellular ABCG5 and ABCG8 are indicated (B). Apolipoproteins are shown on the surfaces of the plasma lipoproteins (C). Abbreviations are defined in table 1 and in the text. Earlier studies of the molecular basis of monogenic dyslipidemias—such as mutant LDLR in AD FH, mutant genes resulting in abnormal apolipoproteins (APOB, APOE, APOC2, and APOAI), plasma enzyme deficiencies (LPL, LIPC, CETP, and LCAT), and ABL (MTP)—provided key insights into plasma lipoprotein metabolism. The recent positional cloning–based discoveries of genes for three other monogenic dyslipidemias—ARH in AR FH (A), ABCG5 and ABCG8 in sitosterolemia (B), and ABCA1 in Tangier disease (B and D)—have added new routes to the lipoprotein metabolic road map.
A Bird’s-Eye View of Lipoprotein Metabolism
The Liver
In the liver (and also in extrahepatic tissues), FC (fig. 1A) is synthesized from acetyl coenzyme A (CoA) (fig. 1A, Ac) by a multistep synthetic pathway, with the last committed step catalyzed by 3-hydroxy-3-methylglutaryl (HMG) CoA reductase. FC can also be (re-)esterified to CE (fig. 1A) by an acyl CoA:cholesterol acyltransferase (ACAT), also called “sterol O-acyltransferase” (SOAT2) (fig. 1A), for incorporation into cytosolic lipid-storage droplets or lipoprotein assembly. Lipoprotein-derived CE can be hydrolyzed to release FC by lysosomal CE hydrolase (CEH) (fig. 1A), also called “lysosomal acid lipase,” and a distinct neutral CEH hydrolyzes cytoplasmic CE. Hepatic FC can be diverted to BA (fig. 1A) synthetic pathway by hydroxylation, for which cholesterol 7-α-hydroxylase (CYP7A1) (MIM 118445) (fig. 1A) is rate limiting. Fat or carbohydrate not required by the liver for energy or synthesis is converted to TG (fig. 1A) by several biosynthetic pathways. The microsomal TG-transfer protein (MTP) (fig. 1A) in hepatocytes directs assembly of CE and TG together with apo B-100 and apo E, to produce VLDL (fig. 1C and table 2) for secretion into plasma.
The Intestine
In the intestine, dietary fat is processed prior to absorption; for instance, pancreatic lipase (PNLIP) (MIM 246600) (fig. 1B) hydrolyzes dietary TG to liberate free fatty acid (FFA) (fig. 1B), which is absorbed both passively and actively. BA (fig. 1A) from liver is secreted and subsequently reabsorbed through specific mediators. BA permits absorption of lumenal FC (fig. 1B). The ABCG5 and ABCG8 half-transporters (fig. 1B) may also re-excrete absorbed FC, in addition to plant sterols such as sitosterol (fig. 1B, S). Intestinal ABCA1 (fig. 1B) also mediates re-excretion of absorbed FC into the bowel lumen. There is likely to be redundancy between the ABCA1, ABCG5, and ABCG8 pathways for intestinal FC absorption. Within enterocytes, processing by SOAT2 (fig. 1B) and TG biosynthetic pathways prepare CE and TG, respectively, for MTP-mediated assembly (fig. 1B) with apo B-48 and apo E into CM (fig. 1C), which is the main lipoprotein carrying fat of exogenous origin secreted into lymph and plasma.
Plasma
Within the capillaries of adipose tissue and muscle, CM and VLDL core TG are hydrolyzed to FFA by endothelial-bound lipoprotein lipase (LPL) (fig. 1C), which uses apo CII (APOC2) (fig. 1C) as a cofactor. FFAs are either re-esterified and stored as TG in fat cells or oxidized to provide energy in muscle. CM and VLDL are remodeled into the short-lived, smaller, denser, more CE-rich CM remnants (CMR) (fig. 1C) and intermediate-density lipoprotein (IDL) (fig. 1C), respectively. CMR and some IDL are cleared by apo E-mediated endocytosis through hepatic-remnant receptors (fig. 1A), contributing to the hepatic CE pool (fig. 1A). IDL that is not cleared is then hydrolyzed by hepatic lipase (HL) (fig. 1C, LIPC), making smaller, CE-rich LDL particles (fig. 1C).
Approximately 70% of total plasma cholesterol is partitioned into LDL (fig. 1C). LDL is actually a spectrum of particles whose main lipid is CE and whose defining protein moiety is a single molecule of apo B-100 (table 2). LDL is principally responsible for cholesterol transport into peripheral tissues. LDL has a plasma half-life of ∼3 d and is cleared by the binding of apo B-100 to hepatocyte LDLR (fig. 1A), clustering in coated pits, internalization by RME, and degradation in lysosomes. The ARH product (fig. 1A) probably interacts with the LDLR. After RME, apo B-100–containing lipoproteins are processed through lysosomes, and freed cholesterol enters the cellular pool. As hepatic FC increases, LDLR transcription is suppressed, RME is reduced, and plasma LDL rises. Chronic excess LDL alters arterial endothelial function. LDL that does not undergo regulated RME is taken up in an unregulated manner by scavenger receptors (such as CD36 and SRA-I/II; not shown in fig. 1) on arterial-wall macrophages. Entrapped LDL lipids can become oxidized, generating toxic intermediates that induce cytokine production and chemotaxis of inflammatory cells (Ross 1999). Arterial-wall macrophages can become engorged with cholesterol from LDL, creating foam cells, which are a key component of atherogenic plaques (Ross 1993).
Apolipoproteins provide plasma lipoproteins with structural stability and solubility. They also serve as ligands for receptors and/or as activators for enzymes. Apo B-100 of VLDL, IDL, and LDL (fig. 1C) is synthesized in the liver, whereas apo B-48 of CM and CMR (fig. 1C) is synthesized in the small intestine. Both apo B-100 (4,356 amino acids) and apo B-48 (2,152 amino acids) are APOB (MIM 107730) products. Apo B-48 mRNA is produced by editing of the apo B-100 mRNA (Hodges and Scott 1992), mediated by a 27-kD editase (APOBEC1) (MIM 600130). Other apolipoproteins represented in figure 1C are APOC2 (a cofactor for LPL), apo E (a ligand for RME), and apo AI (an activator of LCAT).
“Reverse Cholesterol Transport” (RCT)
RCT describes cholesterol transport from peripheral cells back to the liver, for secretion in bile (Glomset 1980). The liver and small intestine produce nascent HDL particles (fig. 1C), which attract excess FC both from extrahepatic cells and from other circulating lipoproteins. Within peripheral cells, SOAT1 and CEH (fig. 1D) maintain the balance between FC and CE. PL (fig. 1D) and FC (fig. 1D) that accumulate in the intimal layer of the arteries are transferred to apo AI (fig. 1C) of nascent HDL, a process mediated by the ABCA1 (fig. 1D). Using apo AI as a cofactor, plasma lecithin:cholesterol acyltransferase (LCAT) (fig. 1C) converts FC to CE, providing a source of core lipid and thus supporting plasma HDL levels. Plasma CE-transfer protein (CETP) (fig. 1C) and PL-transfer protein (PLTP) (MIM 172425) (fig. 1C) modify HDL by shuttling CE and PL between HDL and TG-rich lipoproteins (fig. 1C, VLDL and CM), and these lipoproteins are rapidly cleared by the liver, as described above. HL (encoded by LIPC, as shown in fig. 1D) hydrolyzes HDL TG, thus reducing HDL size. HDL delivers cholesterol to the liver, and scavenger-receptor BI (fig. 1A, SRBI) mediates selective uptake of lipids. Macrophages ingest large amounts of lipoprotein-derived cholesterol and cannot down-regulate uptake in response to cholesterol loading (Brown and Goldstein 1983). Macrophage receptors for oxidized LDL (such as CD36 and SR-AI/II; not shown in fig. 1) can be regulated, but not by cholesterol (Nicholson et al. 2000; van Berkel et al. 2000). Consequently, to prevent lipid accumulation, macrophages depend on cholesterol efflux through transfer to HDL. HDL particles can be defined on the basis of either size (table 2) or protein composition (Leroy et al. 1993). In addition to RCT, HDL might (1) suppress cytokine-induced adhesion of endothelial cells, (2) protect LDL from oxidation, and/or (3) have anticoagulant effects (Lusis 2000; Tall and Wang 2000).
Plasma Lipoproteins and Risk of CHD
An enormous body of literature indicates that risk of CHD is directly related to plasma total- and LDL-cholesterol concentration (Stamler et al. 2000) and inversely related to plasma HDL-cholesterol concentration (Rhoads et al. 1976; Stamler et al. 2000). HDL cholesterol <0.9 mmol/liter (<35 mg/dl) is an independent risk factor for CHD: low HDL is the most common dyslipidemia in patients with CHD who are age <60 years (Genest et al. 1992). Plasma LDL-cholesterol and apo-B concentration are correlated (Sniderman 1988), as are plasma HDL-cholesterol and apo-AI concentration (Moll et al. 1989). The relationship between plasma TG and CHD is more difficult to prove, since elevated plasma TG is often accompanied by low HDL cholesterol. However, elevated plasma TG, usually due to excess VLDL and/or remnant particles, appears to be independently associated with increased risk of CHD (Austin 1999; Yarnell et al. 2001), especially in familial hypertriglyceridemia (Austin et al. 2000). Grossly elevated plasma TG, due to excess CM, is associated with increased risk of pancreatitis (Santamarina-Fojo 1998).
Disorders Affecting Plasma LDL
Monogenic Disorders with LDL Excess
The archetypal monogenic disorder of LDL excess is AD FH, which results from one of >600 reported LDLR mutations that reduce LDLR number and/or activity (see The Low Density Lipoprotein Receptor (LDLR) Gene in Familial Hypercholesterolemia web site). In most populations, FH heterozygotes and homozygotes have frequencies of ∼1:500 and ∼1:106, respectively, with higher frequencies in some ethnic groups, due to founder effects. Because of the gene-dosage relationship with phenotypic severity, FH can also be considered as an autosomal codominant disorder. FH homozygotes have up to eightfold-increased plasma LDL, with prominent tissue deposits of cholesterol (xanthomata), and CHD as early as childhood. FH heterozygotes have approximately threefold-increased plasma LDL, with xanthomata, and greatly increased risk of CHD in mid adulthood (Simon Broome Register Group 1991). DNA analysis may complement clinical and biochemical diagnosis of FH (Williams et al. 1993). Environment (Williams et al. 1986; Sijbrands et al. 2001) and other genes (Emi et al. 1991) can modulate the clinical severity of FH.
A second monogenic AD disorder causing elevated LDL cholesterol, called “familial defective apo B-100” (FDB), results from missense mutations in APOB that affect binding to LDLR (Myant 1993). In Europeans, heterozygous FDB has a prevalence of ∼1:1,000 and is clinically milder than heterozygous FH (Myant 1993).
A third monogenic disorder causing elevated LDL cholesterol, ARH, has been characterized recently (Garcia et al. 2001). ARH is distinct from homozygous FH, because parents have normal plasma LDL cholesterol, unlike the parents of FH homozygotes, who have elevated LDL. Children and adolescents with ARH have severe hypercholesterolemia, with skin findings and early CHD. ARH was initially mapped to chromosome 1p35 (Zuliani et al. 1995, 1999). Mutant ARH (MIM 605747) encodes a putative adapter protein, which might facilitate LDLR movement into coated pits (fig. 1A) (Goldstein and Brown 2001). Interestingly, mutant ARH was found in one family in which a locus on 15q25-q26 previously had been demonstrated (Ciccarese et al. 2000).
Additional genetic heterogeneity for elevated LDL cholesterol has been suggested by reports of a new locus for AD FH (Haddad et al. 1999; Hunt et al. 2000). Also, sequencing of LDLR in 60 subjects with clinical AD FH has indicated that ∼40% had neither an LDLR mutation nor an FDB mutation (Wang et al. 2001), a finding consistent with genetic heterogeneity. Finally, mutant LIPA encoding lysosomal cholesterol hydrolase (lysosomal acid lipase) underlies both Wolman disease (MIM 278000) and the milder CE-storage disease (CESD) (Anderson et al. 1994), both of which have elevated plasma LDL, which is also consistent with genetic heterogeneity for the elevated-LDL-cholesterol phenotype.
Monogenic Disorders with LDL Deficiency
The archetypal disorder of LDL deficiency is abetalipoproteinemia (ABL) (MIM 200100), a very rare (frequency ∼1:106) AR disease defined by the absence of apo B-100–containing lipoproteins, giving very low plasma cholesterol and TG (Rader and Brewer 1993). ABL was described in patients with acanthocytosis, spinocerebellar degeneration, and atypical retinitis pigmentosa (Bassen and Kornzweig 1950). Mutations in MTP (MIM 157147) cause ABL (Wetterau et al. 1992; Narcis et al. 1995). MTP in the liver (fig. 1A) and intestine (fig. 1B) is required for assembly and secretion of apo B–containing lipoproteins (Wetterau et al. 1991). MTP is a heterodimer composed of the multifunctional enzyme protein disulfide isomerase (PDI) and a 97-kD subunit. PDI appears necessary to maintain the structural integrity of MTP; however, no mutations in PDI have been reported.
Homozygous familial hypobetalipoproteinemia (FHBL1) (MIM 107730) is another AR disorder of LDL deficiency, and it is caused by APOB mutations affecting the integrity of the LDL particle (Schonfeld 1995). FHBL1 is distinct from ABL because parents have half-normal plasma apo B-100 and LDL cholesterol, unlike the parents of ABL homozygotes, who have no lipoprotein abnormalities. In both ABL and FHBL1, subjects develop fat malabsorption and fat-soluble vitamin deficiencies. High oral doses of fat-soluble vitamins can arrest the neuropathy and retinopathy (Muller et al. 1985). Interestingly, heterozygosity for both mutant APOB, causing FHBL1, and mutant LDLR, causing FH, was associated with normal plasma lipoproteins (Emi et al. 1991).
Additional genetic heterogeneity for low LDL cholesterol was suggested earlier (Hobbs et al. 1989), and a new locus for AD hypobetalipoproteinemia recently has been reported (Knoblauch et al. 2000). Also, APOB mutations account for a minority of cases of hypobetalipoproteinemia (Wu et al. 1999), a finding consistent with additional genetic heterogeneity. Compound heterozygosity for mutations in the apical sodium-dependent BA transporter (ASBT), encoded by SLC10A2 (MIM 601295), in a patient with steatorrhea, growth retardation, increased BA output, and low plasma LDL cholesterol (Oelkers et al. 1997) further illustrated the genetic heterogeneity of low LDL. Defining the mutation in another rare disorder, called “Anderson disease,” which is characterized by the absence of apo B-48–containing lipoproteins, might reveal a new metabolic pathway, since MTP and apolipoprotein genes are normal (Dannoura et al. 1999).
Disorders Affecting Plasma HDL
Monogenic Disorders with HDL Deficiency
Several monogenic disorders are characterized by HDL deficiency, which is also called “hypoalphalipoproteinemia.” The first molecular defects causing low HDL were discovered by sequence analysis of candidate genes in HDL metabolism; for instance, homozygosity for some mutations in APOA1 (MIM 107680) affects the assembly of HDL and has been found in patients with very low HDL cholesterol, xanthomas, and early CHD (Dammerman and Breslow 1995), and homozygosity for mutant LCAT (MIM 245900) results in impaired esterification of FE and produces many lipoprotein abnormalities, including markedly depressed HDL cholesterol, abnormal lipid deposition in tissues, anemia, renal disease, and early CHD (Kuivenhoven et al. 1997)—and certain LCAT mutations cause “fish-eye disease” (MIM 136120), which is associated with low HDL and profound corneal deposits but not with other attributes of LCAT deficiency (Kuivenhoven et al. 1997).
In 1999, three groups independently used positional cloning to show that mutant ABCA1 (MIM 600046) encoding the ABCA1 transporter was the basis of Tangier disease (TD) (Bodzioch et al. 1999; Brooks-Wilson et al. 1999; Rust et al. 1999), an extremely rare AR disease. The TD index cases had orange tonsils and undetectable HDL cholesterol (Fredrickson et al. 1961). Other findings have included hepatosplenomegaly, peripheral neuropathy, altered plasma apo B–containing lipoproteins (Herbert et al. 1978), and impaired in vitro efflux of cholesterol and PL from fibroblasts (Francis et al. 1995). Plasma HDL cholesterol is also decreased in TD heterozygotes, and risk of CHD is increased in TD homozygotes and heterozygotes (Schaefer et al. 1980; Serfaty-Lacrosniere et al. 1994; Clee et al. 2000).
ABCA1 had earlier been implicated in foam-cell metabolism (Langmann et al. 1999), with increased gene expression mediated by binding of oxysterol-activated nuclear-hormone receptors (Costet et al. 2000). ABCA1 is part of a superfamily of ∼50 transmembrane proteins that couple ATP hydrolysis to substrate transport (Schmitz et al. 2000). Mutations in ABC transporters underlie various diseases (Schmitz et al. 2000). TD mutations probably impair the ability of cell-surface ABCA1 transporters to transfer cholesterol to HDL (fig. 1D) (Lawn et al. 1999), and the lipid-poor HDL are more prone to catabolism. ABCA1 is also important in regulation of intestinal cholesterol absorption (fig. 1B) (Repa et al. 2000).
Additional genetic heterogeneity for low HDL cholesterol has been suggested by genomewide scanning (Kort et al. 2000). Also, mutations in ABCA1 and in other candidate genes account for low HDL in a minority of subjects (Brooks-Wilson et al. 1999), a finding consistent with genetic heterogeneity. Glucocerebrosidase mutations in Gaucher disease type 1 (GD1) (MIM 230800) are associated with low HDL (Pocovi et al. 1998). Finally, a subtype of Niemann-Pick disease is associated with very low HDL (Viana et al. 1990), reinforcing the importance that the Niemann-Pick type C protein (NPC1) (MIM 257220) has for intracellular cholesterol transport (Dietschy and Turley 2001).
Monogenic Disorders with HDL Excess
Rare deficiencies of two processing proteins—namely, HL and CETP—have been associated with increased plasma HDL cholesterol. In HL deficiency (MIM 151670), compound heterozygosity for mutant LIPC resulted in elevated plasma IDL, HDL, and apo AI; in TG enrichment of LDL and HDL; abnormal catabolism of remnant lipoproteins; failure to remodel HDL; and early CHD (Hegele et al. 1993). In CETP deficiency (MIM 118470), homozygosity or compound heterozygosity for CETP mutations resulted in elevated plasma HDL and apo AI, with reduced LDL and apo B. The relationship between mutant CETP and CHD is unclear (Zhong et al. 1996; Tall et al. 2000). CETP mutations are a common cause of high HDL in Japanese individuals (Inazu et al. 1990), but the molecular basis for elevated HDL in most other populations is unknown.
Disorders Affecting TG
Complete LPL deficiency is an extremely rare (frequency ∼1:106) AR disease resulting from homozygosity or compound heterozygosity for mutant LPL (MIM 238600) (Santamarina-Fojo 1998; Talmud 2001). More than 60 LPL mutations have been identified (Santamarina-Fojo 1998). Four subjects with chylomicronemia and CHD (Benlian et al. 1996) were the exception that proved the clinical rule that CM themselves do not cause CHD. Chylomicronemia also results from deficiency of the cofactor APOC2 (fig. 1C), owing to rare loss-of-function mutations in APOC2 (MIM 207750), which provided the first demonstration that a mutant apolipoprotein causes dyslipidemia (Breckenridge et al. 1978; Cox et al. 1978).
Familial Combined Hyperlipidemia (FHCL)
FHCL (Fredrickson type IIB) (MIM 144250), characterized by elevations (>90th percentile) of both plasma total cholesterol and TG in probands and affected relatives, may be the most common genetic dyslipidemia causing CHD (Goldstein et al. 1973; Nikkila and Aro 1973; Rose et al. 1973). Initially considered to have an AD pattern of transmission, FCHL was subsequently shown to have more-complex inheritance (Iselius 1981; Williams and Lalouel 1982; Cullen et al. 1994). This variability of the lipid phenotype among and within affected subjects is a formidable impediment to identification of genetic determinants. Also, the intermediate phenotypes in FCHL are heterogeneous. Although the core metabolic defect in FCHL is overproduction of apo B-100 (Kissebah et al. 1981; Cortner et al. 1991), there are several other metabolic defects (Cabezas et al. 1993; Cianflone et al. 1995; Reynisdottir et al. 1995; Aitman et al. 1997; Vakkilainen et al. 1998). In FHCL, risk of CHD is related to elevated TG (Austin et al. 2000).
Candidate-gene studies of FCHL have examined LPL and the APOA1/C3/A4 (MIM 107680/107720/107690) cluster, with conflicting results (Wojciechowski et al. 1991; Dallinga-Thie et al. 1996, 1997; Yang et al. 1996; Pajukanta et al. 1997; Wijsman et al. 1998; Coon et al. 2000). Recently, TNFRSF1B, encoding tumor necrosis-factor–receptor superfamily member 1B, was associated with FCHL in Dutch subjects (Geurts et al. 2000), but this could not be replicated in two independent Canadian samples (author's unpublished data).
More promising are the results of recent linkage studies, at least three of which, performed in independent FCHL samples, provide evidence of a locus on chromosome 1q21-q23 (Pajukanta et al. 1998; Coon et al. 2000; Pei et al. 2000). This region is syntenic to mouse chromosome 3, which harbors a murine combined-hyperlipidemia gene (Castellani et al. 1998). Other loci linked to FCHL are shown in table 3, consistent with suggestions that FCHL is genetically heterogeneous.
Table 3.
Results of Recent Selected Linkage Analyses from Genomewide Screening for Monogenic Dyslipidemias and Other Plasma Lipoprotein Variation
| Trait and Locus | LOD Score or MLS Score | Reference | Comments |
| Cholesterol: | |||
| Total: | |||
| 19p | 3.89 | Imperatore et al. (2000) | Pima Indians |
| LDL: | |||
| 1p32 | 6.8 | Hunt et al. (2000) | AD FH locus in a Utah family |
| 3 (244 cM) | 4.11 | Rainwater et al. (1999) | Quantitative-trait locus for LDL particle size |
| 3p21.1-22 | 3.35 | Yuan et al. (2000) | AD FH locus |
| 4 (126 cM) | 4.11 | Rainwater et al. (1999) | Quantitative-trait locus for LDL particle size |
| 13q | 4.8 | Knoblauch et al. (2000) | LDL lowering in AD FH and normal relatives |
| HDL: | |||
| 8 (150 cM) | 4.87 | Almasy et al. (1999) | HDL composition |
| 11q23 | 3.50 | Kort et al. (2000) | Familial hypoalphalipoproteinemia |
| 15 (62 cM) | 3.26 | Almasy et al. (1999) | HDL concentration and composition |
| Triglycerides: | |||
| 15q | 3.88 | Duggirala et al. (2000) | ∼40% of TG variation in Mexican Americans |
| apo AI: | |||
| 12 (128 cM) | 2.13 | Klos et al. (2001) | Families in Rochester, MN |
| apo AII: | |||
| 4 (22 cM) | 2.35 | Klos et al. (2001) | Families in Rochester, MN |
| 5 (79 cM) | 2.13 | Klos et al. (2001) | Families in Rochester, MN |
| TC:HDL ratio: | |||
| 17 (125 cM) | 2.48 | Klos et al. (2001) | Families in Rochester, MN |
| TG:HDL ratio: | |||
| 7q32.3-qter | 2.50 | Shearman et al. (2000) | Framingham offspring, nonstandard phenotype |
| FCHL: | |||
| 1q21-23 | 5.93 | Pajukanta et al. (1998) | Finnish families with FCHL, primary locus |
| 1q21-23 | 3.05 | Coon et al. (2000) | Independent replication, possible second locus |
| 1q21-23 | 2.60 | Pei et al. (2000) | Replicated in Chinese and German families |
| 10p11.2 | 3.20 | Pajukanta et al. (1999) | Finnish families with FCHL, high-TG subphenotype |
| 11 (62 cM) | 2.60 | Aouizerat et al. (1999) | Dutch families with FCHL, two-stage study design |
| 21q21 | 2.24 | Pajukanta et al. (1999) | Finnish families with FCHL, high–apo B subphenotype |
Disorders of Remnant Lipoproteins
Remnant lipoproteins, known collectively as “β-VLDL,” have a density of <1.006 g/ml and migrate in the β position on agarose-gel electrophoresis (Fredrickson et al. 1967). Some VLDL remnants can also be considered to be IDL. Remnant lipoproteins (fig. 1C) are normally rapidly catabolized by apo E–mediated RME, to either LDLR or the cell-surface heparan sulfate proteoglycans/LDLR-related protein complex or to heparan sulfate proteoglycans alone (Mahley 1988; Mahley et al. 1999). Remnant lipoproteins are atherogenic (Havel 2000).
In HL deficiency, remnant lipoproteins accumulate owing to mutant LIPC, in which they are a part of the complex dyslipidemia (Hegele et al. 1993). However, the archetypal disorder of IDL excess is dysbetalipoproteinemia (Fredrickson type III) (MIM 107741) (Mahley et al. 1999). Patients with this disorder typically have both elevated cholesterol and elevated TG, to approximately equal concentrations (∼7.5 mmol/liter, or ∼300 mg/dl), an elevated CE:TG ratio in VLDL, characteristic skin findings (tuberous and/or palmar xanthomas), and early atherosclerosis. The prevalence of dysbetalipoproteinemia is ∼1:10,000 (Walden and Hegele 1994). The molecular basis is the homozygosity for the APOE E2 isoform, which differs from the common E3 isoform by a single amino acid substitution (R158C) (Walden and Hegele 1994). Because ∼1% of white individuals are E2/E2 homozygotes, secondary environmental, metabolic, or genetic factors are required for disease expression. Other rare APOE mutations produce this phenotype (Walden and Hegele 1994), but these are associated with AD inheritance and with variable clinical severity due to differences in cell-surface binding (Mahley et al. 1999).
Lipoprotein(a) (Lp[a])
Lp(a) has mesmerized researchers for 40 years (Berg 1992), but its biochemical and functional complexity have thwarted delineation of its role in atherosclerosis (Scanu and Fless 1990). Lp(a) (not shown in fig. 1) is composed of LDL, with a single molecule of apo B-100 linked to the characteristic polymorphic protein apo(a) (Scanu and Fless 1990). The structural similarity between apo(a) and plasminogen, together with the LDL moiety, suggest a prothrombotic or atherogenic role, or both (Marcovina et al. 1999; Scanu et al. 2001). Retrospective case-control studies (Rhoads et al. 1986; Jauhiainen et al. 1991; Sorrentino et al. 1992) and population-based prospective studies (Danesh et al. 2000) have shown a strong association between Lp(a) and CHD, although a direct causal role is uncertain (Morrisett 2000; Marcovina et al. 1999). Although there are no reported monogenic syndromes of Lp(a) excess, size polymorphism of the apo(a) gene on chromosome 6q26-27 is responsible for ∼90% of the interindividual variation in plasma Lp(a) concentration (Boerwinkle et al. 1992; Cohen et al. 1993). Other genes might have minor effects on plasma Lp(a) concentration (Hegele et al. 1997).
Sitosterolemia
Plant sterols consumed by humans are not utilized, and their absorption from the intestine is selectively impeded: <5% of dietary plant sterols are absorbed, compared to ∼50% of dietary cholesterol (Jones 1999). Selective absorption of animal sterols over plant sterols is defective in sitosterolemia, a rare AR disease (Lee et al. 2001b). Patients with sitosterolemia accumulate plant sterols in many tissues, have elevated plasma cholesterol, and often develop early CHD. Patel et al. (1998) mapped the sitosterolemia locus to 2p21 and then used a genetic-mapping approach to identify the causative genes, called “ABCG5” (MIM 605459) and “ABCG8” (MIM 605460) (Lee et al. 2001a). Others used genetic mapping and expression profiling to identify the sitosterolemia genes (Berge et al. 2000). These tandemly arranged ABC-family members probably synergistically re-excrete absorbed cholesterol and sitosterol from enterocytes into the gut lumen (fig. 1B) and from hepatocytes into bile (Berge et al. 2000; Lee et al. 2001b; Lu et al. 2001). Cholesterol absorption is probably increased in sitosterolemia. Two defective alleles for either ABCG5 or ABCG8, not just one defective allele for each, are required for sitosterolemia, suggesting that these transporters function as heterodimers (Lu et al. 2001).
Other Candidate Genes and Pathways for Plasma Lipoproteins
No dyslipoproteinemia mutations have been detected in most of the candidate-gene products that are directly involved in lipoprotein assembly, secretion, transport, processing, and catabolism. However, these genes are interesting functional candidates for lipoprotein phenotypes. Other indirectly associated pathways, such as BA synthesis, sterol synthesis, and fatty acid (FA) metabolism, might affect lipoproteins, although it can be difficult to predict the phenotypic impact of a mutation in a specific candidate gene.
Apolipoproteins and Receptors
No monogenic lipoprotein disorder has been shown to be due to mutant apolipoproteins AII, AIV, CI, CIII, D, H, or J. Very recently, a new apolipoprotein, called “apo AV,” has been described, which appears to be important in TG metabolism, although no mutations causing monogenic hypertriglyceridemia were found (Pennacchio et al. 2001). Similarly, no monogenic dyslipidemia mutations have been found in the LDLR-related protein LRP1 (MIM 107770). The VLDL receptor (VLDLR) (MIM 192977) was hypothesized to mediate FA entry into peripheral tissues, and it might have a role in TG metabolism (Tacken et al. 2001). One very rare VLDLR mutation was found in a subject with FCHL (Near et al. 2001). Scavenger-receptor class B type 1 (SRB1, or CLA1) (MIM 601040) is found in caveoli and promotes uptake of CE from HDL (fig. 1A) (Acton et al. 1996); however, no mutations have yet been found in SRB1. Genes for other cell-surface receptors are interesting candidates for lipoproteins (Nicholson et al. 2000; van Berkel et al. 2000).
Lipases and Processing Proteins
No disease mutation has been found in endothelial lipase (LIPG) (MIM 603684), which shares some sequence and functional similarity with other lipases (Jaye et al. 1999). PNLIP hydrolyzes dietary TG (fig. 1B), and deficiency causes fat malabsorption, failure to thrive, and low plasma lipid levels; however, no mutations were found in individuals who were PNLIP deficient (Hegele et al. 2001), suggesting that other genes are defective. PLTP is another interesting protein (Jiang et al. 1999), with no reported mutations.
BA Metabolism
BA metabolism is currently a very topical area. In addition to its role in cholesterol and dietary-fat absorption, bile is the preferred route of excretion for many drugs and xenobiotics (Stieger and Meier 1998). BA activates farnesyl X receptor (FXR), an orphan nuclear-hormone receptor that mediates BA-dependent repression of CYP7A1, the initial and rate-limiting step in BA synthesis (fig. 1A) (Russell and Setchell 1992; Repa and Mangelsdorf 2000). FXR also regulates expression of other proteins in BA metabolism (Makishima et al. 1999; del Castillo-Olivares and Gil 2000). Studies with receptor-selective agonists have revealed that oxysterol receptors and FXR are retinoid X receptor (RXR) heterodimer partners that exert complementary effects on regulation of expression of ABCA1 and CYP7A1 (Repa et al. 2000). The RXRA (MIM 180245) product is integrated into diverse physiologic pathways, such as sterol, FA, BA, and xenobiotic metabolism (Wan et al. 2000); however, no mutations have been found in RXRA (Hegele and Cao 2001). Truncated CYP7A1 has been found in an infant with cholestasis, cirrhosis, and elevated plasma 27-hydroxycholesterol (Setchell et al. 1998). Previously mentioned mutations in SLC10A2 have been found to be associated with low plasma LDL (Oelkers et al. 1997), underscoring the relationship with BA transport. Mutant CYP27A1 encoding sterol 7-hydroxylase causes cerebrotendinous xanthomatosis (MIM 213700), which is characterized by tissue lipid deposits but normal plasma lipoproteins (Cali et al. 1991).
Cholesterol Biosynthesis
The cholesterol biosynthetic pathway involves numerous enzymes and bioactive intermediates (Goldstein and Brown 1990). Multisystemic, mainly pediatric, disorders are due to defects in cholesterol biosynthesis (Kelley 2000). Developmental abnormalities, elevated serum 7-dehydrocholesterol, and low plasma cholesterol (Opitz et al. 1994) define the most common of these disorders—namely, Smith-Lemli-Optiz syndrome (SLOS) (MIM 270400). SLOS is caused by mutations in sterol delta-7-reductase (DHCR7) (MIM 602858) (Wassif et al. 1998; Waterham et al. 1998). Risk of CHD is moot, because of other systemic abnormalities, and dietary-cholesterol supplements might improve cognitive development (Kelley 1998; Nowaczyk et al. 2001). There are no reported mutations in either 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (HMGCR) (MIM 142910), which catalyzes the committed synthetic step, or cytoplasmic HMG CoA synthase (HMGCS1) (MIM 142940), which mediates an early synthetic step. However, mutant mitochondrial HMG CoA synthase (HMGCS2) (MIM 600234), the first enzyme in ketogenesis, is associated with abnormal metabolism of glucose and FA but with normal plasma lipoproteins (Bouchard et al. 2001).
Cholesterol Esterification
An essential intracellular element in the formation of CE from cholesterol is ACAT—although the preferred MIM symbol is “SOAT,” since “ACAT” has previously been given to a different enzyme with ketothiolase activity (see MIM 203750). SOAT1 (MIM 102642) and SOAT2 (MIM 601311) are members of a transferase family (Chang et al. 1993, 2001 Anderson et al. 1998; Cases et al. 1998a, 1998b; Oelkers et al. 1998). SOAT products (fig. 1A, B, and D) generate CE for storage as lipid droplets, play a role in foam-cell formation, are involved in dietary-cholesterol absorption, and supply CE for lipoprotein synthesis. Approximately 80% of newly absorbed cholesterol transported within CM enters the body as CE esterified by SOAT. Although there are no SOAT mutations, the genes’ importance has been shown by abnormal phenotypes in murine models (Meiner et al. 1996; Buhman et al. 2000).
TG Synthesis
Acyl-CoA:diacylglycerol acyltransferase (DGAT) is a microsomal enzyme that catalyzes the terminal and only committed step in TG synthesis. DGAT had been considered to be both necessary for adipose-tissue formation and essential for survival. Two groups (Cases et al. 1998a; Oelkers et al. 1998) have independently cloned DGAT (MIM 604900). Although there are no human DGAT mutations, targeted disruption produces lean mice that resist diet-induced obesity but that still synthesize TG (Smith et al. 2000), suggesting the existence of other DGAT-like enzymes.
FA Uptake and Synthesis
Several different classes of membrane proteins have been proposed as FA acceptors or transporters (Glatz and Storch 2001). FA trafficking by soluble intracellular FA-binding proteins may involve interaction with specific membrane or protein targets. No dyslipidemia results from mutations in these genes, but Bietti crystalline corneoretinal dystrophy (MIM 210370) may be due to mutations in FA-binding proteins (Jiao et al. 2000). Diseases due to mutant-FA synthetic enzymes do not feature altered plasma lipoproteins (Treem et al. 1991).
Other Regulators of Cellular Lipid Homeostasis
FA synthesis is regulated by various transcription factors, such as membrane-bound sterol regulatory element–binding protein-1 (SREBP1) (MIM 184756). Regulators of SREBP-1 include polyunsaturated FA, glucose, and insulin (Sakai and Rawson 2001). Accumulation of intracellular cholesterol suppresses the proteolytic release of the active N-terminal fragment of SREBP from the membrane-bound precursor (Sakai and Rawson 2001). Induced SREBP mutations in mice produce important metabolic phenotypes, such as lipodystrophy and insulin resistance (Shimomura et al. 1999). However, no human mutations have yet been reported, in either the genes for SREBPs, their processing enzymes, or interacting cofactors.
Monogenic Determinants of Metabolic Conditions with Secondary Dyslipidemia
Common dyslipidemia is associated with such metabolic traits as obesity and diabetes (National Cholesterol Education Program 2001). However, dyslipidemia in monogenic forms of obesity and diabetes has not been systematically examined. This issue was addressed in Canadian kindreds with familial partial lipodystrophy of the Dunnigan type (FPLD) (MIM 151660), an AD form of insulin-resistant diabetes due to mutant LMNA (MIM 150330) encoding nuclear lamin A/C (Cao and Hegele 2000). The initial metabolic abnormality in FPLD is hyperinsulinemia due to insulin resistance, followed by dyslipidemia—that is, elevated TG and low HDL (Hegele et al. 2000a)—followed by diabetes and then premature CHD (Hegele 2001). Dyslipidemia in FPLD is thus secondary to monogenic insulin resistance. Another relatively large group that was studied are the Oji-Cree of Ontario, whose very high prevalence of type 2 diabetes was largely explained by the private G319S mutation in HNF1A (MIM 142410) encoding factor hepatic nuclear factor-1α (Hegele et al. 1999). The development of diabetes in the Oji-Cree was associated with worsened plasma lipoproteins—that is, increases both in total and LDL cholesterol and in TG, with a decrease in HDL cholesterol—but the HNF1A mutation also had an independent association with plasma lipoproteins (Hegele et al. 2000b).
Additional Loci Revealed by Genomewide Scans and Linkage Analysis
As suggested above in the “Monogenic Disorders with LDL Excess” subsection, additional heterogeneity is very likely for monogenic dyslipidemias, such as AD FH (Hunt et al. 2000), AD hypobetalipoproteinemia (Knoblauch et al. 2000), AD hypoalphalipoproteinemia (Kort et al. 2000), and FCHL. Loci identified by genomewide scanning, with either the LOD score or the maximum LOD score, are shown in table 3.
Common Genetic Polymorphisms, Plasma Lipoproteins, and CHD
Association studies using common single-nucleotide polymorphisms (SNPs) and insertion/deletion variants are a mainstay of lipoprotein research: a recent Medline search using the terms “human,” “lipids,” and “polymorphisms” identified ∼1,600 original articles dating from 1982. From a clinical perspective, only the APOE isoforms are useful for diagnosis and treatment stratification (Walden and Hegele 1994). There is fairly consistent evidence that E4 is associated with higher plasma LDL cholesterol and, also, with worsened noninvasive measurements of atherosclerosis (Smith 2000). APOE genotyping can reveal subjects with E2/E2 who are at increased risk of dysbetalipoproteinemia and also can fortuitously reveal rare APOE mutations (Hegele 1999). Nontraditional risk factors for CHD, such as APOE genotype, may help to reassign patients to higher-risk strata than those predicted by traditional risk factors. Other functional SNPs that might become clinically useful include those within LPL (Talmud and Humphries 2001), LIPC (Cohen et al. 1999), and APOC3 (Shachter 2001).
Conclusions
Early candidate-gene studies and recent positional-cloning experiments have revealed pathways underlying the monogenic disorders of lipoprotein metabolism. Genetic heterogeneity for monogenic dyslipidemias suggests that newer molecules and pathways remain to be discovered. Elucidating these pathways may provide new targets for novel intervention strategies. For patients who are not part of a kindred with a defined monogenic dyslipidemia, the clinical value of molecular diagnosis is unclear; for instance, >600 LDLR mutations underlie AD FH, but no single LDLR SNP has been associated with variation of plasma lipoproteins in the general population. Other than APOE isoforms, it is not clear, at present, whether any common genetic variant will help to predict risk of CHD in the general population, beyond estimates derived from clinical and biochemical determinations. Finally, the influence that single genes have on dyslipidemia is modulated by interactions with genetic background and/or secondary genetic effects, in addition to gender, age, hormone replacement, and diet. These modulating factors are likely to be even more important for the common polygenic dyslipidemias.
Acknowledgments
Andrea Mok and Susan Near helped with manuscript preparation. The author sincerely thanks Dr. Murray Huff, Nica Borradaile, and Dr. Geoff Pickering for their constructive comments. The author is grateful for laboratory support by grants from the Canadian Institutes for Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Genetic Diseases Network, the Canadian Diabetes Association, and the Blackburn Group. The author is a Career Investigator of the Heart and Stroke Foundation of Ontario and holds a Canada Research Chair in Human Genetics.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Low Density Lipoprotein Receptor (LDLR) Gene in Familial Hypercholesterolemia, The, http://www.ucl.ac.uk/fh/ (for LDLR number and activity)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for APOB or FHBL1 [MIM 107730], APOBEC1 [MIM 600130], FH [MIM 143890], ARH [MIM 603813], ARH [MIM 605747], Wolman disease [MIM 278000], ABL [MIM 200100], MTP [MIM 157147], SLC10A2 [MIM 601295], APOA1 [MIM 107680], LCAT [MIM 245900], fish-eye disease [MIM 136120], ABCA1 [MIM 600046], GD1 [MIM 230800], NPC1 [MIM 257220], HL [MIM 151670], CETP [MIM 118470], LPL [MIM 238600], APOC2 [MIM 207750], FHCL [MIM 144250], APOA1/C3/A4 gene cluster [MIM 107680/107720/107690], Fredrickson type III dysbetalipoproteinemia [MIM 107741], ABCG5 [MIM 605459], ABCG8 [MIM 605460], LRP1 [MIM 107770], VLDLR [MIM 192977], SRB1 or CLA1 [MIM 601040], LIPG [MIM 603684], PNLIP [MIM 246600], PLTP [MIM 172425], CYP7A1 [MIM 118445], RXRA [MIM 180245], cerebrotendinous xanthomatosis [MIM 213700], SLOS [MIM 270400], DHCR7 [MIM 602858], HMGCR [MIM 142910], HMGCS1 [MIM 142940], HMGCS2 [MIM 600234], ketothiolase activity [MIM 203750], SOAT1 [MIM 102642], SOAT2 [MIM 601311], DGAT [MIM 604900], Bietti crystalline corneoretinal dystrophy [MIM 210370], SREBP1 [MIM 184756], FPLD [MIM 151660], mutant LMNA [MIM 150330], and HNF1A [MIM 142410])
References
- Acton S, Rigotti A, Landschultz KT, Xu S, Hobbs HH, Krieger M (1996) Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518–520 [DOI] [PubMed] [Google Scholar]
- Aitman TJ, Godsland IF, Farren B, Crook D, Wong HJ, Scott J (1997) Defects of insulin action on fatty acid and carbohydrate metabolism in familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol 17:748–754 [DOI] [PubMed] [Google Scholar]
- Almasy L, Hixson JE, Rainwater DL, Cole S, Williams JT, Mahaney MC, VandeBerg JL, Stern MP, MacCluer JW, Blangero J (1999) Human pedigree-based quantitative-trait-locus mapping: localization of two genes influencing HDL-cholesterol metabolism. Am J Hum Genet 64:1686–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Byrum RS, Coates PM, Sando GN (1994) Mutations at the lysosomal acid cholesteryl ester hydrolase gene locus in Wolman disease. Proc Natl Acad Sci USA 91:2718–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL (1998) Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem 273:26747–26754 [DOI] [PubMed] [Google Scholar]
- Aouizerat BE, Allayee H, Cantor RM, Davis RC, Lanning CD, Wen PZ, Dallinga-Thie GM, de Bruin TW, Rotter JI, Lusis AJ (1999) A genome scan for familial combined hyperlipidemia reveals evidence of linkage with a locus on chromosome 11. Am J Hum Genet 65:397–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MA (1999) Epidemiology of hypertriglyceridemia and cardiovascular disease. Am J Cardiol 83:13F–16F [DOI] [PubMed] [Google Scholar]
- Austin MA, McKnight B, Edwards KL, Bradley CM, McNeely MJ, Psaty BM, Brunzell JD, Motulsky AG (2000) Cardiovascular disease mortality in familial forms of hypertriglyceridemia: a 20-year prospective study. Circulation 101:2777–2782 [DOI] [PubMed] [Google Scholar]
- Bassen FA, Kornzweig AL (1950) Malformation of the erythrocytes in a case of atypical retinitis pigmentosa. Blood 5:381–387 [PubMed] [Google Scholar]
- Benlian P, De Gennes JL, Foubert L, Zhang H, Gagne SE, Hayden M (1996) Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. N Engl J Med 335:848–854 [DOI] [PubMed] [Google Scholar]
- Berg K (1992) Lp(a) lipoprotein: an important genetic risk factor for atherosclerosis. In: Lusis AJ, Rotter JI, Sparkes RS (eds) Monographs in human genetics. Vol 14: Molecular genetics of coronary artery disease: candidate genes and processes in atherosclerosis. Karger, Basel, pp 189–207 [Google Scholar]
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH (2000) Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290:1771–1775 [DOI] [PubMed] [Google Scholar]
- Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G (1999) The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 22:347–351 [DOI] [PubMed] [Google Scholar]
- Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH (1992) Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest 90:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard L, Robert MF, Vinarov D, Stanley CA, Thompson GN, Morris A, Leonard JV, Quant P, Hsu BYL, Boneh A, Boukaftane Y, Ashmarina L, Wang S, Miziorko H, Mitchell GA (2001) Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase deficiency: clinical course and description of causal mutations in two patients. Pediatr Res 49:326–331 [DOI] [PubMed] [Google Scholar]
- Breckenridge WC, Little JA, Steiner G, Chow A, Poapst M (1978) Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med 298:1265–1273 [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Hayden MR (1999) Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 22:336–345 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL (1983) Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem 52:223–261 [DOI] [PubMed] [Google Scholar]
- ——— (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232:34–47 [DOI] [PubMed] [Google Scholar]
- Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV Jr (2000) Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med 6:1341–1347 [DOI] [PubMed] [Google Scholar]
- Cabezas MC, de Bruin TW, Jansen H, Kock LA, Kortlandt W, Erkelens DW (1993) Impaired chylomicron remnant clearance in familial combined hyperlipidemia. Arterioscler Thromb 13:804–814 [DOI] [PubMed] [Google Scholar]
- Cali JJ, Hsieh C-L, Francke U, Russell DW (1991) Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem 266:7779–7783 [PMC free article] [PubMed] [Google Scholar]
- Cao H, Hegele RA (2000) Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 9:109–112 [DOI] [PubMed] [Google Scholar]
- Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV Jr (1998a) ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase: its cloning, expression, and characterization. J Biol Chem 273:26755–26764 [DOI] [PubMed] [Google Scholar]
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr (1998b) Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA 95:13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani LW, Weinreb A, Bodnar J, Goto AM, Doolittle M, Mehrabian M, Demant P, Lusis AJ (1998) Mapping a gene for combined hyperlipidemia in a mutant mouse strain. Nat Genet 18:374–377 [DOI] [PubMed] [Google Scholar]
- Chang CCY, Huh HY, Cadigan KM, Chang TY (1993) Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 268:20747–20755 [PubMed] [Google Scholar]
- Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A (2001) Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol 12:289–296 [DOI] [PubMed] [Google Scholar]
- Cianflone K, Maslowska M, Sniderman A (1995) The acylation stimulating protein-adipsin system. Int J Obes Relat Metab Disord 19 Suppl 1:S34–S38 [PubMed] [Google Scholar]
- Ciccarese M, Pacifico A, Tonolo G, Pintus P, Nikoshkov A, Zuliani G, Fellin R, Luthman H, Maioli M (2000) A new locus for autosomal recessive hypercholesterolemia maps to human chromosome 15q25-q26. Am J Hum Genet 66:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clee SM Kastelein JJ, van Dam M, Marcil M, Roomp K, Zwarts KY, Collins JA, Roelants R, Tamasawa N, Stulc T, Suda T, Ceska R, Boucher B, Rondeau C, DeSouich C, Brooks-Wilson A, Molhuizen HO, Frohlich J, Genest J Jr, Hayden MR (2000) Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J Clin Invest 106:1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Chiesa G, Hobbs HH (1993) Sequence polymorphisms in the apolipoprotein(a) gene: evidence for dissociation between apolipoprotein(a) size and plasma lipoprotein(a) levels. J Clin Invest 91:1630–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Vega GL, Grundy SM (1999) Hepatic lipase: new insights from genetic and metabolic studies. Curr Opin Lipidol 10:259–267 [DOI] [PubMed] [Google Scholar]
- Coon H, Myers RH, Borecki IB, Arnett DK, Hunt SC, Province MA, Djousse L, Leppert MF (2000) Replication of linkage of familial combined hyperlipidemia to chromosome 1q with additional heterogeneous effect of apolipoprotein A-I/C-III/A-IV locus: The NHLBI Family Heart Study. Arterioscler Thromb Vasc Biol 20:2275–2280 [DOI] [PubMed] [Google Scholar]
- Cortner JA, Coates PM, Bennett MJ, Cryer DR, Le N-A (1991) Familial combined hyperlipidemia: use of stable isotopes to demonstrate overproduction of very low density apolipoprotein B by the liver. J Inherit Metab Dis 14:915–922 [DOI] [PubMed] [Google Scholar]
- Costet P, Luo Y, Wang N, Tall AR (2000) Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem 275:28240–28245 [DOI] [PubMed] [Google Scholar]
- Cox DW, Breckenridge WC, Little JA (1978) Inheritance of apolipoprotein C-II deficiency with hypertriglyceridemia and pancreatitis. N Engl J Med 299:1421–1424 [DOI] [PubMed] [Google Scholar]
- Cullen P, Farren JS, Scott J, Farrall M (1994) Complex segregation analysis provides evidence for a major gene acting on serum triglyceride levels in 55 British families with familial combined hyperlipidemia. Arterioscler Thromb 14:1233–1249 [DOI] [PubMed] [Google Scholar]
- Dallinga-Thie GM, Bu X, van Linde-Sibenius Trip M, Rotter JI, Lusis AJ, de Bruin TWA (1996) Apolipoprotein AI/CIII/AIV gene cluster in familial combined hyperlipidemia: effects on LDL-cholesterol and apolipoproteins B and C-III. J Lipid Res 37:136–147 [PubMed] [Google Scholar]
- Dallinga-Thie GM, van Linde-Sibenius Trip M, Rotter JI, Cantor RM, Bu X, Lusis AJ, de Bruin TWA (1997) Complex genetic contribution of the apoAI-CIII-AIV gene cluster to familial combined hyperlipidemia: identification of different susceptibility haplotypes. J Clin Invest 99:953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammerman M, Breslow JL (1995) Genetic basis of lipoprotein disorders. Circulation 91:505–512 [DOI] [PubMed] [Google Scholar]
- Danesh J, Collins R, Peto R (2000) Lipoprotein(a) and coronary heart disease: meta-analysis of prospective studies. Circulation 102:1082–1085 [DOI] [PubMed] [Google Scholar]
- Dannoura AH, Berriot-Varoqueaux N, Amati P, Abadie V, Verthier N, Schmitz J, Wetterau JR, Samson-Bouma ME, Aggerbeck LP (1999) Anderson's disease: exclusion of apolipoprotein and intracellular lipid transport genes. Arterioscler Thromb Vasc Biol 19:2494–2508 [DOI] [PubMed] [Google Scholar]
- del Castillo-Olivares A, Gil G (2000) Role of FXR and FTF in bile acid-mediated suppression of cholesterol 7alpha-hydroxylase transcription. Nucleic Acids Res 28:3587–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD (2001) Cholesterol metabolism in the brain. Curr Opin Lipidol 12:105–112 [DOI] [PubMed] [Google Scholar]
- Duggirala R, Blangero J, Almasy L, Dyer TD, Williams KL, Leach RJ, O'Connell P, Stern MP (2000) A major susceptibility locus influencing plasma triglyceride concentrations is located on chromosome 15q in Mexican Americans. Am J Hum Genet 66:1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi M, Hegele RA, Hopkins PN, Wu LL, Plaetke R, Williams RR, Lalouel JM (1991) Effects of three genetic loci in a pedigree with multiple lipoprotein phenotypes. Arterioscler Thromb 11:1349–1355 [DOI] [PubMed] [Google Scholar]
- 4S Investigators (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 334:1383–1389 [PubMed] [Google Scholar]
- Francis GA, Knopp RH, Oram JF (1995) Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-1 in Tangier disease. J Clin Invest 96:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson DS, Altrocchi PH, Avioli LV, Goodman DS, Goodman HC (1961) Tangier disease: combined clinical staff conference at the National Institutes of Health. Ann Intern Med 55:1016–1031 [Google Scholar]
- Fredrickson DS, Levy RI, Lees RS (1967) Fat transport in lipoproteins—an integrated approach to mechanisms and disorders. N Engl J Med 276:273–281 [DOI] [PubMed] [Google Scholar]
- Garcia CK, Wilund K, Arca M, Zuliani G, Fellin R, Maioli M, Calandra S, Bertolini S, Cossu F, Grishin N, Barnes R, Cohen JC, Hobbs HH (2001) Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 292:1394–1398 [DOI] [PubMed] [Google Scholar]
- Genest JJ Jr, Martin-Munley SS, McNamara JR, Ordovas JM, Jenner J, Myers RH, Silberman SR, Wilson PWF, Salem DN, Schaefer EJ (1992) Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 85:2025–2033 [DOI] [PubMed] [Google Scholar]
- Geurts JM, Janssen RG, van Greevenbroek MM, van der Kallen CJ, Cantor RM, Bu X, Aouizerat BE, Allayee H, Rotter JI, de Bruin TW (2000) Identification of TNFRSF1B as a novel modifier gene in familial combined hyperlipidemia. Hum Mol Genet 9:2067–2074 [DOI] [PubMed] [Google Scholar]
- Glatz JF, Storch J (2001) Unravelling the significance of cellular fatty acid-binding proteins. Curr Opin Lipidol 12:267–274 [DOI] [PubMed] [Google Scholar]
- Glomset JA (1980) High-density lipoproteins in human health and disease. Adv Intern Med 25:91–116 [PubMed] [Google Scholar]
- Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430 [DOI] [PubMed] [Google Scholar]
- ——— (2001) The cholesterol quartet. Science 292:1310–1312 [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG (1973) Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest 52:1544–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad L, Day INM, Hunt S, Williams RR, Humphries SE, Hopkins PN (1999) Evidence for a third genetic locus causing familial hypercholesterolemia: a non-LDLR, non-APOB kindred. J Lipid Res 40:1113–1122 [PubMed] [Google Scholar]
- Havel RJ (2000) Remnant lipoproteins as therapeutic targets. Curr Opin Lipidol 11:615–620 [DOI] [PubMed] [Google Scholar]
- Hegele RA (1999) Uncovering rare mutations: an unforeseen complication of routine genotyping of APOE. Clin Chem 45:1579–1581 [PubMed] [Google Scholar]
- ——— (2001) Premature atherosclerosis associated with monogenic insulin resistance. Circulation 103:2225–2229 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Anderson CM, Wang J, Jones DC, Cao H (2000a) Association between nuclear lamin A/C R482Q mutation and partial lipodystrophy with hyperinsulinemia, dyslipidemia, hypertension, and diabetes. Genome Res 10:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele RA, Breckenridge WC, Brunt JH, Connelly PW (1997) Genetic variation in factor VII associated with variation in plasma lipoprotein(a) concentration. Arterioscler Thromb Vasc Biol 17:1701–1706 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Cao H (2001) Single nucleotide polymorphisms of RXRA encoding retinoid X receptor alpha. J Hum Genet 46:423–425 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Cao H, Harris SB, Hanley AJ, Zinman B (1999) The hepatic nuclear factor-1alpha G319S variant is associated with early-onset type 2 diabetes in Canadian Oji-Cree. J Clin Endocrinol Metab 84:1077–1082 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Cao H, Harris SB, Hanley AJ, Zinman B, Connelly PW (2000b) The private hepatocyte nuclear factor-1alpha G319S variant is associated with plasma lipoprotein variation in Canadian Oji-Cree. Arterioscler Thromb Vasc Biol 20:217–222 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Little JA, Vezina C, Maguire GF, Tu L, Wolever TS, Jenkins DJ, Connelly PW (1993) Hepatic lipase deficiency: clinical, biochemical, and molecular genetic characteristics. Arterioscler Thromb 13:720–728 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Ramdath DD, Ban MR, Carruthers MN, Carrington CV, Cao H (2001) Polymorphisms in PNLIP, encoding pancreatic lipase, and associations with metabolic traits. J Hum Genet 46:320–324 [DOI] [PubMed] [Google Scholar]
- Herbert PN, Forte T, Heinen RJ, Fredrickson DS (1978) Tangier disease: one explanation of lipid storage. N Engl J Med 299:519–521 [DOI] [PubMed] [Google Scholar]
- Hobbs HH, Leitersdorf E, Leffert CC, Cryer DR, Brown MS, Goldstein JL (1989) Evidence for a dominant gene that suppresses hypercholesterolemia in a family with defective low density lipoprotein receptors. J Clin Invest 84:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges P, Scott J (1992) Apolipoprotein B mRNA editing: a new tier for the control of gene expression. Trends Biochem Sci 17:77–81 [DOI] [PubMed] [Google Scholar]
- Hunt SC, Hopkins PN, Bulka K, McDermott MT, Thorne TL, Wardell BB, Bowen BR, Ballinger DG, Skolnick MH, Samuels ME (2000) Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol 20:1089–1093 [DOI] [PubMed] [Google Scholar]
- Imperatore G, Knowler WC, Pettitt DJ, Kobes S, Fuller JH, Bennett PH, Hanson RL (2000) A locus influencing total serum cholesterol on chromosome 19p: results from an autosomal genomic scan of serum lipid concentrations in Pima Indians. Arterioscler Thromb Vasc Biol 20:2651–2656 [DOI] [PubMed] [Google Scholar]
- Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, Takata K, Maruhama Y, Mabuchi H, Tall AR (1990) Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med 323:1234–1238 [DOI] [PubMed] [Google Scholar]
- Iselius L (1981) Complex segregation analysis of hyperglyceridemia. Hum Hered 31:222–226 [DOI] [PubMed] [Google Scholar]
- Jauhiainen M, Koskinen P, Ehnholm C, Frick MH, Manttari M, Manninen V, Huttunen JK (1991) Lipoprotein (a) and coronary heart disease risk: a nested case-control study of the Helsinki Heart Study participants. Atherosclerosis 89:59–67 [DOI] [PubMed] [Google Scholar]
- Jaye M, Lynch KJ, Krawiec J, Marchadier D, Maugeais C, Doan K, South V, Amin D, Perrone M, Rader DJ (1999) A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet 21:424–428 [DOI] [PubMed] [Google Scholar]
- Jiang XC, Bruce C, Mar J, Lin M, Ji Y, Francone OL, Tall AR (1999) Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J Clin Invest 103:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Munier FL, Iwata F, Hayakawa M, Kanai A, Lee J, Schorderet DF, Chen MS, Kaiser-Kupfer M, Hejtmancik JF (2000) Genetic linkage of Bietti crystallin corneoretinal dystrophy to chromosome 4q35. Am J Hum Genet 67:1309–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PJ (1999) Cholesterol-lowering action of plant sterols. Curr Atheroscler Rep 1:230–235 [DOI] [PubMed] [Google Scholar]
- Kelley RI (1998) RSH/Smith-Lemli-Opitz syndrome: mutations and metabolic morphogenesis. Am J Hum Genet 63:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ——— (2000) Inborn errors of cholesterol biosynthesis. Adv Pediatr 47:1–52 [PubMed] [Google Scholar]
- Kissebah AH, Alfarsi S, Adams PW (1981) Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in man: normolipidemic subjects, familial hypertriglyceridemia and familial combined hyperlipidemia. Metabolism 30:856–868 [DOI] [PubMed] [Google Scholar]
- Klos KL, Kardia SL, Ferrell RE, Turner ST, Boerwinkle E, Sing CF (2001) Genome-wide linkage analysis reveals evidence of multiple regions that influence variation in plasma lipid and apolipoprotein levels associated with risk of coronary heart disease. Arterioscler Thromb Vasc Biol 21:971–978 [DOI] [PubMed] [Google Scholar]
- Knoblauch H, Muller-Myhsok B, Busjahn A, Ben Avi L, Bahring S, Baron H, Heath SC, Uhlmann R, Faulhaber HD, Shpitzen S, Aydin A, Reshef A, Rosenthal M, Eliav O, Muhl A, Lowe A, Schurr D, Harats D, Jeschke E, Friedlander Y, Schuster H, Luft FC, Leitersdorf E (2000) A cholesterol-lowering gene maps to chromosome 13q. Am J Hum Genet 66:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort EN, Ballinger DG, Ding W, Hunt SC, Bowen BR, Abkevich V, Bulka K, Campbell B, Capener C, Gutin A, Harshman K, McDermott M, Thorne T, Wang H, Wardell B, Wong J, Hopkins PN, Skolnick M, Samuels M (2000) Evidence of linkage of familial hypoalphalipoproteinemia to a novel locus on chromosome 11q23. Am J Hum Genet 66:1845–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivenhoven JA, Pritchard H, Hill J, Frohlich J, Assmann G, Kastelein J (1997) The molecular pathology of lecithin:cholesterol acyltransferase (LCAT) deficiency syndromes. J Lipid Res 38:191–205 [PubMed] [Google Scholar]
- Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G (1999) Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun 257:29–33 [DOI] [PubMed] [Google Scholar]
- Lawn RM Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF (1999) The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest 104:R25–R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB (2001a) Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet 27:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Lu K, Patel SB (2001b) Genetic basis of sitosterolemia. Curr Opin Lipidol 12:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A, Toohill KL, Fruchart JC, Jonas A (1993) Structural properties of high density lipoprotein subclasses homogeneous in protein composition and size. J Biol Chem 268:4798–4805 [PubMed] [Google Scholar]
- Lu K, Lee MH, Hazard S, Brooks-Wilson A, Hidaka H, Kojima H, Ose L, Stalenhoef AF, Mietinnen T, Bjorkhem I, Bruckert E, Pandya A, Brewer HB Jr, Salen G, Dean M, Srivastava A, Patel SB (2001) Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet 69:278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis AJ (2000) Atherosclerosis. Nature 407:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622–630 [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Rall SC Jr (1999) Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia): questions, quandaries, and paradoxes. J Lipid Res 40:1933–1349 [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365 [DOI] [PubMed] [Google Scholar]
- Marcovina SM, Hegele RA, Koschinsky ML (1999) Lipoprotein(a) and coronary heart disease risk. Curr Cardiol Rep 1:105–111 [DOI] [PubMed] [Google Scholar]
- Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J, Farese RV Jr (1996) Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci USA 93:14041–14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll PP, Michels VV, Weidman WH, Kottke BA (1989) Genetic determination of plasma apolipoprotein AI in a population-based sample. Am J Hum Genet 44:124–139 [PMC free article] [PubMed] [Google Scholar]
- Morrisett JD (2000) The role of lipoprotein[a] in atherosclerosis. Curr Atheroscler Rep 2:243–250 [DOI] [PubMed] [Google Scholar]
- Muller DPR, Lloyd JK, Wolff OH (1985) The role of vitamin E in the treatment of the neurological features of abetalipoproteinaemia and other disorders of fat absorption. J Inherit Metab Dis 8:88–92 [DOI] [PubMed] [Google Scholar]
- Myant NB (1993) Familial defective apolipoprotein B-100: a review, including some comparisons with familial hypercholesterolaemia. Atherosclerosis 104:1–18 [DOI] [PubMed] [Google Scholar]
- Narcis TME, Shoulders CC, Chester SA, Read J, Brett DJ, Harrison GB, Grantham TT, Fox MF, Povey S, de Bruin TWA, Erkelens DW, Muller DPR, Lloyd JK and Scott J (1995) Mutations of the microsomal triglyceride-transfer-protein gene in abetalipoproteinemia. Am J Hum Genet 57:1298–1310 [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Near SE, Wang J, Hegele RA (2001) Single nucleotide polymorphisms of the very low density lipoprotein receptor (VLDLR) gene. J Hum Genet 46:490–493 [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Febbraio M, Han J, Silverstein RL, Hajjar DP (2000) CD36 in atherosclerosis: the role of a class B macrophage scavenger receptor. Annu N Y Acad Sci 902:128–131 [PubMed] [Google Scholar]
- Nikkila EA, Aro A (1973) Family study of serum lipids and lipoproteins in coronary heart-disease. Lancet 1:954–959 [DOI] [PubMed] [Google Scholar]
- Nowaczyk MJ, Nakamura LM, Eng B, Porter FD, Waye JS (2001) Frequency and ethnic distribution of the common DHCR7 mutation in Smith-Lemli-Opitz syndrome. Am J Med Genet 102:383–386 [DOI] [PubMed] [Google Scholar]
- Oelkers P, Behari A, Cromley D, Billheimer JT, Sturley SL (1998) Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J Biol Chem 273:26765–26771 [DOI] [PubMed] [Google Scholar]
- Oelkers P, Kirby LC, Heubi JE, Dawson PA (1997) Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J Clin Invest 99:1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz JM, Penchaszadeh VB, Holt MC, Spano LM, Smith VL (1994) Smith-Lemli-Opitz (RSH) syndrome bibliography: 1964–1993. Am J Med Genet 50:339–343 [DOI] [PubMed] [Google Scholar]
- Pajukanta P, Nuotio I, Terwilliger JD, Porkka KV, Ylitalo K, Pihlajamaki J, Suomalainen AJ, Syvanen AC, Lehtimaki T, Viikari JS, Laakso M, Taskinen MR, Ehnholm C, Peltonen L (1998) Linkage of familial combined hyperlipidaemia to chromosome 1q21-q23. Nat Genet 18:369–373 [DOI] [PubMed] [Google Scholar]
- Pajukanta P, Porkka KV, Antikainen M, Taskinen MR, Perola M, Murtomaki-Repo S, Ehnholm S Nuotio I, Suurinkeroinen L, Lahdenkari AT, Syvanen AC, Viikari JS, Ehnholm C, Peltonen L (1997) No evidence of linkage between familial combined hyperlipidemia and genes encoding lipolytic enzymes in Finnish families. Arterioscler Thromb Vasc Biol 17:841–850 [DOI] [PubMed] [Google Scholar]
- Pajukanta P, Terwilliger JD, Perola M, Hiekkalinna T, Nuotio I, Ellonen P, Parkkonen M, Hartiala J, Ylitalo K, Pihlajamaki J, Porkka K, Laakso M, Viikari J, Ehnholm C, Taskinen MR, Peltonen L (1999) Genomewide scan for familial combined hyperlipidemia genes in Finnish families, suggesting multiple susceptibility loci influencing triglyceride, cholesterol, and apolipoprotein B levels. Am J Hum Genet 64:1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MJ, Lin H (1998) The role of the hedgehog/patched signaling pathway in epithelial stem cell proliferation: from fly to human. Cell Res 8:15–21 [DOI] [PubMed] [Google Scholar]
- Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Miettinen TA, Grundy SM, Lee MH, Rubenstein JS, Polymeropoulos MH, Brownstein MJ (1998) Mapping a gene involved in regulating dietary cholesterol absorption: the sitosterolemia locus is found at chromosome 2p21. J Clin Invest 102:1041–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W, Baron H, Muller-Myhsok B, Knoblauch H, Al-Yahyaee SA, Hui R, Wu X, Liu L, Busjahn A, Luft FC, Schuster H (2000) Support for linkage of familial combined hyperlipidemia to chromosome 1q21-q23 in Chinese and German families. Clin Genet 57:29–34 [DOI] [PubMed] [Google Scholar]
- Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM (2001) An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 294:169–173 [DOI] [PubMed] [Google Scholar]
- Pocovi M, Cenarro A, Civeira F, Torralba MA, Perez-Calvo JI, Mozas P, Giraldo P, Giralt M, Myers RH, Cupples LA, Ordovas JM (1998) Beta-glucocerebrosidase gene locus as a link for Gaucher's disease and familial hypo-alpha-lipoproteinaemia. Lancet 351:1919–1923 [DOI] [PubMed] [Google Scholar]
- Rader DJ, Brewer HB Jr (1993) Abetalipoproteinemia. JAMA 270:865–869 [DOI] [PubMed] [Google Scholar]
- Rainwater DL, Almasy L, Blangero J, Cole SA, VandeBerg JL, MacCluer JW, Hixson JE (1999) A genome search identifies major quantitative trait loci on human chromosomes 3 and 4 that influence cholesterol concentrations in small LDL particles. Arterioscler Thromb Vasc Biol 19:777–783 [DOI] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ (2000) The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 16:459–481 [DOI] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ (2000) Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529 [DOI] [PubMed] [Google Scholar]
- Reynisdottir S, Eriksson M, Angelin B, Arner P (1995) Impaired activation of adipocyte lipolysis in familial combined hyperlipidemia. J Clin Invest 95:2161–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads GG, Dahlen G, Berg K, Morton NE, Dannenberg AL (1986) Lp(a) lipoprotein as a risk factor for myocardial infarction. JAMA 256:2540–2544 [PubMed] [Google Scholar]
- Rhoads GG, Gulbrandsen CL, Kagan A (1976) Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N Engl J Med 294:293–298 [DOI] [PubMed] [Google Scholar]
- Rose HG, Kranz P, Weinstock M, Juliano J, Haft JI (1973) Inheritance of combined hyperlipoproteinemia: evidence for a new lipoprotein phenotype. Am J Med 54:148–160 [DOI] [PubMed] [Google Scholar]
- Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809 [DOI] [PubMed] [Google Scholar]
- ——— (1999) Atherosclerosis--an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Russell DW (1999) Nuclear orphan receptors control cholesterol catabolism. Cell 97:539–542 [DOI] [PubMed] [Google Scholar]
- Russell DW, Setchell KD (1992) Bile acid biosynthesis. Biochemistry 26:4737–4749 [DOI] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G (1999) Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 22:352–355 [DOI] [PubMed] [Google Scholar]
- Sakai J, Rawson RB (2001) The sterol regulatory element-binding protein pathway: control of lipid homeostasis through regulated intracellular transport. Curr Opin Lipidol 12:261–266 [DOI] [PubMed] [Google Scholar]
- Santamarina-Fojo S (1998) The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 27:551–567 [DOI] [PubMed] [Google Scholar]
- Scanu AM, Fless GM (1990) Lipoprotein(a): heterogeneity and biological relevance. J Clin Invest 85:1709–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu AM, Nakajima K, Edelstein C (2001) Apolipoprotein(a): structure and biology. Front Biosci 6: D546–D554 [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Zech LA, Schwartz DE, Brewer HB Jr (1980) Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease). Ann Intern Med 93:261–266 [DOI] [PubMed] [Google Scholar]
- Schmitz G, Kaminski WE, Orso E (2000) ABC transporters in cellular lipid trafficking. Curr Opin Lipidol 11:493–501 [DOI] [PubMed] [Google Scholar]
- Schonfeld G (1995) Genetic variation of apolipoprotein B can produce both low and high levels of apo B-containing lipoproteins in plasma. Can J Cardiol 11:86G–92G [PubMed] [Google Scholar]
- Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, Smith MP Jr, Pritchard PH, Frohlich J, Lees RS (1994) Homozygous Tangier disease and cardiovascular disease. Atherosclerosis 107:85–98 [DOI] [PubMed] [Google Scholar]
- Setchell KD, Schwarz M, O'Connell NC, Lund EG, Davis DL, Lathe R, Thompson HR, Weslie Tyson R, Sokol RJ, Russell DW (1998) Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J Clin Invest 102:1690–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachter NS (2001) Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr Opin Lipidol 12:297–304 [DOI] [PubMed] [Google Scholar]
- Shearman AM, Ordovas JM, Cupples LA, Schaefer EJ, Harmon MD, Shao Y, Keen JD, DeStefano AL, Joost O, Wilson PW, Housman DE, Myers RH (2000) Evidence for a gene influencing the TG/HDL-C ratio on chromosome 7q32.3-qter: a genome-wide scan in the Framingham study. Hum Mol Genet 9:1315–1320 [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL (1999) Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401:73–76 [DOI] [PubMed] [Google Scholar]
- Sijbrands EJ, Westendorp RG, Defesche JC, de Meier PH, Smelt AH, Kastelein JJ (2001) Mortality over two centuries in large pedigree with familial hypercholesterolaemia: family tree mortality study. BMJ 322:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon Broome Register Group (1991) Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ 303:893–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD (2000) Apolipoprotein E4: an allele associated with many diseases. Ann Med 32:118–127 [DOI] [PubMed] [Google Scholar]
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV Jr (2000) Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25:87–90 [DOI] [PubMed] [Google Scholar]
- Sniderman AD (1988) Apolipoprotein B and apolipoprotein AI as predictors of coronary artery disease. Can J Cardiol 4:24A–30A [PubMed] [Google Scholar]
- Sorrentino MJ, Vielhauer C, Eisenbart JD, Fless GM, Scanu AM, Feldman T (1992) Plasma lipoprotein(a) protein concentration and coronary artery disease in black patients compared with white patients. Am J Med 93:658–662 [DOI] [PubMed] [Google Scholar]
- Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD (2000) Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 284:311–318 [DOI] [PubMed] [Google Scholar]
- Stieger B, Meier PJ (1998) Bile acid and xenobiotic transporters in liver. Curr Opin Cell Biol 10:462–467 [DOI] [PubMed] [Google Scholar]
- Superko HR (1996) Beyond LDL cholesterol reduction. Circulation 94:2351–2354 [DOI] [PubMed] [Google Scholar]
- Tacken PJ, Hofker MH, Havekes LM, van Dijk KW (2001) Living up to a name: the role of the VLDL receptor in lipid metabolism. Curr Opin Lipidol 12:275–279 [DOI] [PubMed] [Google Scholar]
- Tall AR, Jiang Xc, Luo Y, Silver D (2000) 1999 George Lyman Duff memorial lecture: lipid transfer proteins, HDL metabolism, and atherogenesis. Arterioscler Thromb Vasc Biol 20:1185–1188 [DOI] [PubMed] [Google Scholar]
- Tall AR, Wang N (2000) Tangier disease as a test of the reverse cholesterol transport hypothesis. J Clin Invest 106:1205–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmud PJ (2001) Genetic determinants of plasma triglycerides: impact of rare and common mutations. Curr Atheroscler Rep 3:191–199 [DOI] [PubMed] [Google Scholar]
- Talmud PJ, Humphries SE (2001) Genetic polymorphisms, lipoproteins and coronary artery disease risk. Curr Opin Lipidol 12:405–409 [DOI] [PubMed] [Google Scholar]
- Treem WR, Stanley CA, Hale DE, Leopold HB, Hyams JS (1991) Hypoglycemia, hypotonia, and cardiomyopathy: the evolving clinical picture of long-chain acyl-CoA dehydrogenase deficiency. Pediatrics 87:328–333 [PubMed] [Google Scholar]
- Vakkilainen J, Porkka KVK, Nuotio I, Pajukanta P, Suurinkeroinen L, Ylitalo K, Viikari JSA (1998) Glucose intolerance in familial combined hyperlipidaemia. Eur J Clin Invest 28:24–32 [DOI] [PubMed] [Google Scholar]
- van Berkel TJ, Van Eck M, Herijgers N, Fluiter K, Nion S (2000) Scavenger receptor classes A and B: their roles in atherogenesis and the metabolism of modified LDL and HDL. Ann N Y Acad Sci 902:113–126 [DOI] [PubMed] [Google Scholar]
- Vance DE, van den Bosch H (2000) Cholesterol in the year 2000. Biochim Biophys Acta 1529:1–8 [DOI] [PubMed] [Google Scholar]
- Viana MB, Giugliani R, Leite VH, Barth ML, Lekhwani C, Slade CM, Fensom A (1990) Very low levels of high density lipoprotein cholesterol in four sibs of a family with non-neuropathic Niemann-Pick disease and sea-blue histiocytosis. J Med Genet 27:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden CC, Hegele RA (1994) Apolipoprotein E in hyperlipidemia. Ann Intern Med 120:1026–1036 [DOI] [PubMed] [Google Scholar]
- Wan YJY, An D, Cai Y, Repa JJ, Chen THP, Flores M, Postic C, Magnuson MA, Chen J, Chien KR, French S, Mangelsdorf DJ, Sucov HM (2000) Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol Cell Biol 20:4436–4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Huff E, Janecka L, Hegele RA (2001) Low density lipoprotein receptor (LDLR) gene mutations in Canadian subjects with familial hypercholesterolemia, but not of French descent. Hum Mutat 18:359 [DOI] [PubMed] [Google Scholar]
- Wassif CA, Maslen C, Kachilele-Linjewile S, Lin D, Linck LM, Connor WE, Steiner RD, Porter FD (1998) Mutations in the human sterol delta-7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet 63:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Wijburg FA, Hennekam RCM, Vreken P, Poll-The BT, Dorland L, Duran M, Jira PE, Smeitink JAM, Wevers RA, Wanders RJA (1998) Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet 63:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE (1992) Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258:999–1001 [DOI] [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Laplaud PM, McLean LR (1991) Structural properties of the microsomal triglyceride-transfer-protein complex. Biochemistry 30:4406–4412 [DOI] [PubMed] [Google Scholar]
- Wijsman EM, Brunzell JD, Jarvik GP, Austin MA, Motulsky AG, Deeb SS (1998) Evidence against linkage of familial combined hyperlipidemia to the apolipoprotein AI-CIII-AIV gene complex. Arterioscler Thromb Vasc Biol 18:215–226 [DOI] [PubMed] [Google Scholar]
- Williams RR, Hasstedt SJ, Wilson DE, Ash KO, Yanowitz FF, Reiber GE, Kuida H (1986) Evidence that men with familial hypercholesterolemia can avoid early coronary death: an analysis of 77 gene carriers in four Utah pedigrees. JAMA 255:219–224 [PubMed] [Google Scholar]
- Williams RR, Hunt SC, Schumacher MC, Hegele RA, Leppert MF, Ludwig EH, Hopkins PN (1993) Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol 72:171–176 [DOI] [PubMed] [Google Scholar]
- Williams WR, Lalouel JM (1982) Complex segregation analysis of hyperlipidemia in a Seattle sample. Hum Hered 32:24–36 [DOI] [PubMed] [Google Scholar]
- Wojciechowski AP, Farrall M, Cullen P, Wilson TM, Bayliss JD, Farren B, Griffin BA, Packard CJ, Shepherd J, Scott J (1991) Familial combined hyperlipidaemia linked to the apolipoprotein AI-CIII-AIV gene cluster on chromosome 11q23-q24. Nature 349:161–164 [DOI] [PubMed] [Google Scholar]
- Wu J, Kim J, Li Q, Kwok PY, Cole TG Cefalu B, Averna M, Schonfeld G (1999) Known mutations of apoB account for only a small minority of hypobetalipoproteinemia. J Lipid Res 40:955–959 [PubMed] [Google Scholar]
- Yang WS, Nevin DN, Iwasaki L, Peng R, Brown BG, Brunzell JD, Deeb SS (1996) Regulatory mutations in the human lipoprotein lipase gene in patients with familial combined hyperlipidemia and coronary artery disease. J Lipid Res 37:2627–2637 [PubMed] [Google Scholar]
- Yarnell JW, Patterson CC, Sweetnam PM, Thomas HF, Bainton D, Elwood PC, Bolton CH, Miller NE (2001) Do total and high density lipoprotein cholesterol and triglycerides act independently in the prediction of ischemic heart disease? Ten-year follow-up of Caerphilly and Speedwell cohorts. Arterioscler Thromb Vasc Biol 21:1340–1345 [DOI] [PubMed] [Google Scholar]
- Yuan B, Neuman R, Duan SH, Weber JL, Kwok PY, Saccone NL, Wu JS, Liu KY, Schonfeld G (2000) Linkage of a gene for familial hypobetalipoproteinemia to chromosome 3p21.1-22. Am J Hum Genet 66:1699–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb JD, Tall AR (1996) Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest 97:2917–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani G, Arca M, Signore A, Bader G, Fazio S, Chianelli M, Bellosta S, Campagna F, Montali A, Maioli M, Pacifico A, Ricci G, Fellin R (1999) Characterization of a new form of inherited hypercholesterolemia: familial recessive hypercholesterolemia. Arterioscler Thromb Vasc Biol 19:802–809 [DOI] [PubMed] [Google Scholar]
- Zuliani G, Vigna GB, Corsini A, Maioli M, Romagnoni F, Fellin R (1995) Severe hypercholesterolaemia: unusual inheritance in an Italian pedigree. Eur J Clin Invest 25:322–331 [DOI] [PubMed] [Google Scholar]