Abstract
This study investigates the skin-whitening potential of 4-Hydroxyacetophenone (4-HAP), a compound commonly used as a preservative in cosmetics. Despite its widespread use, its whitening activity has not been extensively reported. Our preliminary research identified several commercial cosmetic formulations exhibiting whitening effects without known whitening agents. We hypothesized that 4-HAP might be a key ingredient. To test this, we employed a mushroom tyrosinase model, B16 mouse melanoma cells, and zebrafish to systematically evaluate the whitening activity of 4-HAP. Our findings demonstrate that 4-HAP is a potent tyrosinase inhibitor, significantly reducing melanin content in vitro and in vivo. Additionally, molecular docking and dynamics analyses revealed that the stable binding of 4-HAP to tyrosinase is crucial for its whitening effect. In formulations containing 2% 4-HAP, human skin models showed nearly complete recovery from UV-induced pigmentation after 4 weeks, while control groups exhibited persistent UV marks. These results confirm the efficacy of 4-HAP as a stable whitening agent in cosmetic formulations, highlighting its potential for further development in skin lightening products. This study has confirmed that 4-HAP holding the potential to become an effective whitening ingredient in the cosmetics field.


1. Introduction
Human skin pigmentation and melanin synthesis vary greatly and are influenced by genetics, ultraviolet radiation, and certain medications. Pigmentation not only affects an individual’s appearance but can also impact psychological health and social functioning. For instance, melasma, a skin condition caused by abnormal pigmentation, is a significant concern. Therefore, the inhibition of melanin is crucial from both cosmetic and medical perspectives. Melanin is primarily produced by melanocytes, which are mainly distributed in the stratum corneum of the skin and the basal layer of hair follicles, with their core function being the generation of melanin. The biosynthesis of melanin can be initiated by l-tyrosine or l-dihydroxyphenylalanine (l-DOPA), and the oxidation of l-DOPA to dopaquinone is a common step in the pathways for the production of eumelanin and pheomelanin. After a series of oxidation–reduction reactions, intermediates such as dihydroxyindole (DHI) and DHI carboxylic acid (DHICA) are produced, which polymerize to form the two types of melanin. In the melanin biosynthetic pathway, tyrosinase catalyzes the rate-limiting first two steps, the reactions of l-tyrosine and l-DOPA, thus inhibiting tyrosinase can prevent the accumulation of active intermediates from the source, reducing the production of melanin. Given the significant biological importance of tyrosinase, and the well-established structure and functional motifs, the inhibition of tyrosinase has always been a primary target in the research aimed at reducing pigmentation.
With the development of the economy and the enhancement of personal skincare awareness, the demand for cosmetics is continuously increasing. Among the many effects of cosmetics, whitening effects are particularly a focus and have become one of the hotspots in cosmetic research and development in Asia. 4-Hydroxyacetophenone (4-HAP), also known as pinusol, is a natural component derived from the stems and leaves of Asteraceae plants such as Artemisia scoparia, as well as from plants like Artemisia capillaris Thunb. and the roots of Asclepiadaceae plants like Tylophora kerrii Craib, but the 4-HAP used in industrial production is mainly obtained synthetically. As a commonly used cosmetic ingredient, 4-HAP not only has soothing and oil-controlling effects, stabilizes emulsions, and possesses antioxidant properties, but also has preservative and bacteriostatic functions. Ingredients added to cosmetics must be mild and have low potential for irritation and sensitization, and 4-HAP meets these requirements. As an alternative to traditional preservatives, the bacteriostatic ability of 4-HAP comes from its phenolic hydroxyl group, which can provide hydrogen-donating capacity, form a phenoxy radical with electronegativity, and exhibit strong reductive properties. Studies have shown that 4-HAP can damage bacterial cellular components through free radicals or reactive oxygen species (ROS), exerting inhibitory effects on bacteria such as fungi and Aspergillus niger. 4-HAP demonstrates good stability under different pH and temperature conditions, can extend the shelf life of other active ingredients in cosmetics, and enhance the effectiveness of other preservatives. Moreover, 4-HAP is compatible with other cosmetic ingredients and packaging materials, leading to its increasingly widespread application in cosmetics.
The molecular structure of 4-HAP includes a benzene ring with substituents of a phenolic hydroxyl group and a ketone carbonyl group, thus possessing the chemical properties of both phenols and aromatic ketones. Compared with common whitening active structures, 4-HAP is structurally similar to compounds such as hydroquinone and β-Arbutin, all of which contain a phenoxy group with a phenolic hydroxyl group at the para position. These compounds can act as substrate analogs of tyrosinase, competitively inhibiting its activity. ,
During testing of cosmetics, we unexpectedly found that although some products did not contain known whitening active ingredients, they exhibited a certain degree of whitening effect. This suggests that there may be unknown whitening components in cosmetics. Based on the analysis of the formulations of these products, we speculate that 4-HAP may possess whitening activity.
Thus, this study aims to investigate the whitening efficacy of 4-HAP employing the following methodologies: (1) The whitening activity of 4-HAP will be assessed using mushroom tyrosinase, B16 mouse melanoma cells, and zebrafish models. (2) The whitening activity of 4-HAP and its structural analogs will be evaluated using the zebrafish model. (3) The whitening mechanism of 4-HAP will be explored through molecular docking and molecular dynamics simulations. (4) 4-HAP will be incorporated into cosmetic formulations, and its stability, safety, and whitening activity will be verified through zebrafish model and human clinical trials.
2. Results and Discussion
2.1. Cosmetics without Whitening Ingredients Possess Whitening Effects
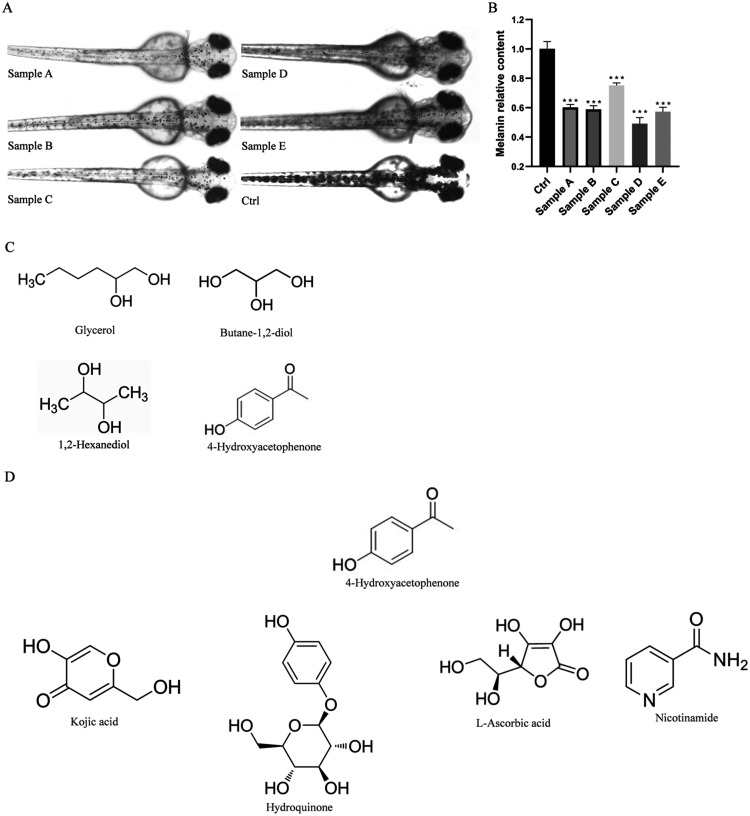
After treating zebrafish embryos with five samples at their previously determined maximum safe concentrations from 24 to 72 hpf, the results showed a varying degree of melanin reduction in the embryos treated with each sample, indicating a certain whitening effect. Randomly selecting 5 embryos per well, photographs of their melanin generation were taken and imported into ImageJ for the quantification of melanin area. The results indicated that the melanin content in all five samples was significantly lower than that in the control group, thereby validating the whitening activity of these samples (Figure A,B). Among the formulations of these five samples (Table ), we identified common components excluding water and found that the preservative 4-HAP shared a structural similarity with common whitening ingredients, both featuring a cyclic structure and functional groups with hydroxyl or ketone carbonyl groups (Figure C,D). Therefore, we suspect that 4-HAP is responsible for the observed whitening effect.
1.
Cosmetics without known whitening ingredients possess whitening effects. (A) Surface melanin of 24 hpf zebrafish embryos treated with five over-the-counter samples without known whitening ingredients and the control group for 48 h, (B) Relative melanin content, (C) Common ingredients among the five over-the-counter samples without known whitening ingredients, excluding water, (D) Common whitening ingredients. Data are expressed as mean ± SEM (n = 6). ***P < 0.001, versus Ctrl.
1. Ingredients of the Samples.
| sample | ingredients |
|---|---|
| A | water (aqua); glycerol; dipropylene glycol; ethanol; betaine; 4-hydroxybenzophenone; phenoxyethanol; phenyl trimethicone; octyl dodecyl polyethylene-16; PEG-60 hydrogenated castor oil; carbomer; saccharide isomerate; butane-1,2-diol; ammonium, butyrate, and glycyrrhizic acid; hexylene glycol; butanediol (1,2-hexanediol) |
| B | water (aqua); butane-1,2-diol; yeast/bacillus/black tea ferment; salicylic acid; glycerol; 1,2-hexanediol; 4-hydroxybenzophenone; hydroxyethylpiperazine ethane sulfonic acid; propylene glycol; hydroxyethyl urea; panthenol; sodium hydroxide |
| C | water (aqua); butane-1,2-diol; erythritol; medicinal Fomes officinalis (mushroom) extract; 4-t-butylcyclohexanol; Schisandra extract; gray lupine seed extract; Sargassum fusiforme extract; flax seed extract; red clover flower extract; citrus fruit extract; Lactobacillus/black mustard seed ferment filtrate; caffeine; biotin; betaine; Coffea arabica seed extract; 1,2-hexanediol; 4-hydroxybenzophenone; malt extract; 10-hydroxydecanoic acid; glycerol; 1,2-pentanediol; branched starch; glyceryl glucoside; 1,3-propanediol; xanthan gum; cyclodextrin; isopentyl diglycerol; PEG-40 hydrogenated castor oil; fragrance; ammonium, butyrate, and glycyrrhizic acid; lactic acid bacteria ferment extract |
| D | water (aqua); panthenol; butane-1,2-diol; glycerol; PEG/PPG-14/7 dimethicone; 1,2-hexanediol; 4-hydroxybenzophenone; Portulaca oleracea (purslane) extract; arginine; sodium hyaluronate |
| E | water (aqua); butane-1,2-diol; glycerol; dipropylene glycol; glycereth-26; panthenol; 1,2-hexanediol; 4-hydroxybenzophenone; xanthan gum |
2.2. 4-HAP Exhibits Whitening Activity in Both the Zebrafish In Vivo Model and the In Vitro Models of Mushroom Tyrosinase and B16 Mouse Melanoma Cells
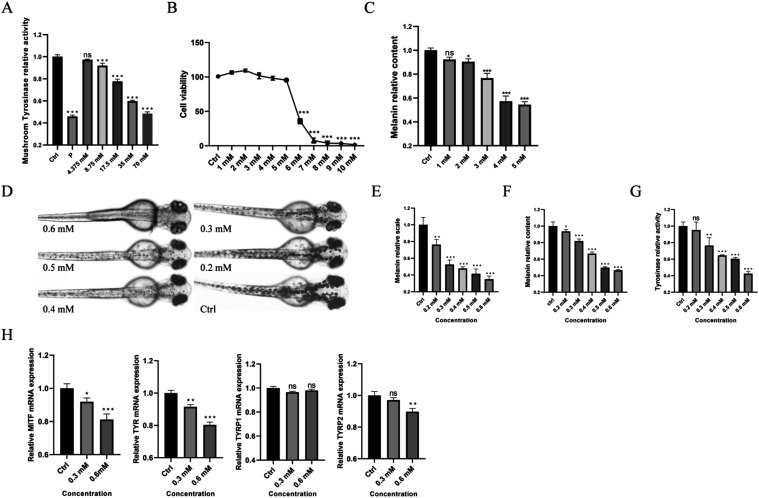
In experiments utilizing the mushroom tyrosinase model, we observed a decreasing trend in tyrosinase activity with increasing concentrations of 4-HAP (Figure A). At a concentration of 8.75 mM 4-HAP, tyrosinase activity was significantly inhibited compared to the control group. At the maximum concentration of 70 mM, 4-HAP exhibited more than 50% inhibition of tyrosinase activity.
2.
4-HAP exhibits whitening activity in zebrafish melanin in vitro models as well as in mushroom tyrosinase and B16 mouse melanoma cell in vivo models. (A) Relative activity of mushroom tyrosinase, (B) Survival rate of B16 mouse melanoma cells, (C) Relative melanin content of B16 mouse melanoma cells, (D) Surface melanin of 24 hpf zebrafish embryos treated with 0.2, 0.3, 0.4, 0.5, and 0.6 mM 4-HAP and the control group, (E) Surface melanin area of zebrafish embryos, (F) Relative melanin content of zebrafish embryos, (G) Relative activity of tyrosinase in zebrafish embryos, (H) Relative gene expression levels of MITF, TYR, TYRP1, and TYRP2. Data are expressed as mean ± SEM (n = 6). Ns = nonspecifical, *P < 0.05, **P < 0.01, ***P < 0.001 versus Ctrl.
In the B16 mouse melanoma model, the maximum safe concentration of 4-HAP was determined to be 5 mM (Figure B). Quantitative melanin assays and tyrosinase activity assays revealed that melanin content and tyrosinase activity began to significantly decrease from a concentration of 2 mM 4-HAP compared to the control group (Figure C).
In the zebrafish model, the safe concentration of 4-HAP was established at 0.6 mM (Figure S1A,B). Melanin area and content assays in zebrafish embryos showed significant decreases compared to the control group, even at the lowest concentration of 4-HAP (Figure D–F). In tyrosinase activity assays of zebrafish embryos, a significant decrease in activity was observed starting from a concentration of 0.3 mM 4-HAP (Figure G). RT-qPCR results showed that the expression levels of genes MITF, TYR, TYRP1, and TYRP2, which are related to melanin production, underwent changes. At the maximum safe concentration, the expression of the other three genes, except for TYRP1, was downregulated to varying degrees. As the concentration of 4-HAP increased, the expression of these genes showed a decreasing trend (Figure H).
Collectively, these experimental results confirm that 4-HAP possesses whitening activity, with a particularly notable effect on inhibiting tyrosinase activity.
2.3. 4-HAP Analogs Do Not Possess Whitening Activity in the Zebrafish In Vivo Model
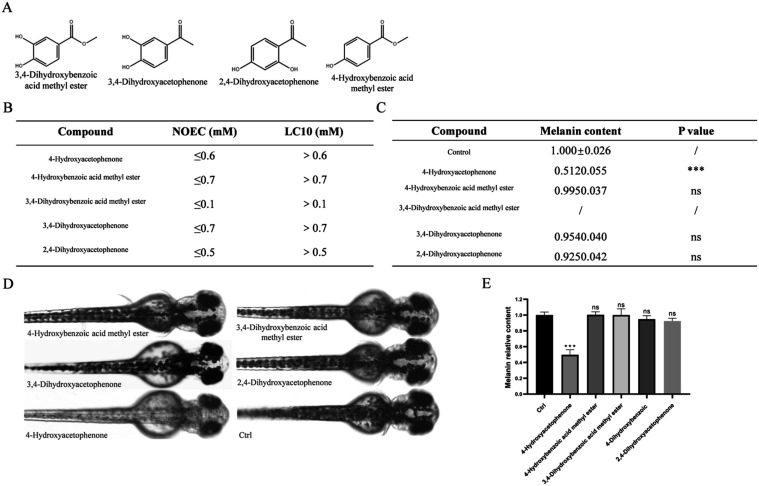
In the experiments previously mentioned, we established the whitening activity of 4-HAP. To explore whether other compounds structurally similar to 4-HAP possess similar whitening activity, this study utilized a zebrafish model to test the following 4-HAP analogs: 4-Hydroxybenzoic acid methyl ester, 3,4-dihydroxybenzoic acid methyl ester, 3,4-dihydroxyphenylacetone, and 2,4-dihydroxyphenylacetone (Figure A). The safety concentration results indicated that concentrations of 700 μM for 4-Hydroxybenzoic acid methyl ester, 100 μM for 3,4-dihydroxybenzoic acid methyl ester, 700 μM for 3,4-dihydroxyphenylacetone, and 500 μM for 2,4-dihydroxyphenylacetone resulted in 100% survival of zebrafish embryos (Figure S2). Besides, we have summarized the No Observed Effect Concentration (NOEC) and the Lethal Concentration for 10% (LC10) for these compounds (Figure B), and used these values to set the experimental concentrations for assessing their whitening activity.
3.
4-HAP analogs do not exhibit whitening activity in the zebrafish model. (A) Structural diagrams of four 4-HAP analogs, (B) LC10 and NOEC of the five compounds, (C) Zebrafish melanin content exposed to five compounds at a consistent concentration of 500 μM, (D) Surface melanin of 24 hpf zebrafish embryos treated with the highest nonlethal concentrations of 4-HAP and its analogs for 48 h, (E) Relative melanin content of 24 hpf zebrafish embryos treated with the highest nonlethal concentrations of 4-HAP and its analogs for 48 h. Data are expressed as mean ± SEM (n = 6). Ns = nonspecifical, ***P < 0.001 versus Ctrl.
First, at a consistent concentration of 500 μM, a whitening efficacy experiment was conducted for each compound. The statistical results of zebrafish melanin content are shown in (Figure C). The 4-HAP group showed a significant reduction in melanin content, while 4-Hydroxybenzoic acid methyl ester, 3,4-dihydroxyphenylacetone, and 2,4-dihydroxyphenylacetone had no significant impact on the melanin of zebrafish embryos; at a concentration of 500 μM, 3,4-dihydroxybenzoic acid methyl ester resulted in the death of all zebrafish embryos. Subsequently, whitening efficacy experiments were conducted at the maximum safe concentrations for each compound. The results indicated that, aside from the 4-HAP group which showed a significant reduction in melanin content, the other four compounds had no significant impact on the melanin of zebrafish embryos (Figure D,E). These results indicate that these 4-HAP analogs do not possess whitening activity in the zebrafish in vivo model.
2.4. 4-HAP Achieves Whitening Activity by Binding with the Substrate of Tyrosinase
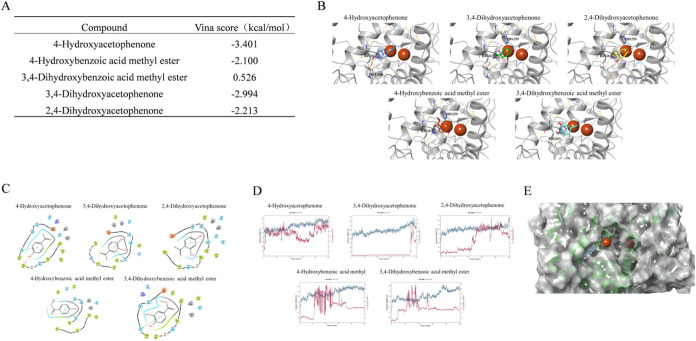
Zebrafish testing results revealed that, aside from 4-HAP, the other four structurally similar compounds had no significant impact on the melanin of zebrafish embryos. We attempted to explain this phenomenon through molecular docking and molecular dynamics simulations. Docking scores indicated that 4-HAP generally formed better interactions with tyrosinase, with lower and more stable binding energies compared to 4-Hydroxybenzoic acid methyl ester (Figure A). Specific interactions revealed that all compounds could form typical Pi-Pi conjugated interactions with the HIS amino acid residue near the active site, stabilizing the docked conformations (Figure B). 2D docking diagrams showed that active 4-HAP and structurally similar 2,4-dihydroxyphenylacetone both formed hydrogen bond interactions with MET280, which may be a key factor affecting the activity of tyrosinase inhibitors (Figure C).
4.
Molecular docking and molecular dynamics simulation suggest that 4-HAP achieves whitening activity by binding with the substrate of tyrosinase. (A) Vina score of the five compounds, (B) 3D conformation of compound docking results, (C) 2D conformation of compound docking results, (D) RMSD plot of compound dynamics simulation, (E) 4-HAP stably binds to a peripheral site after dynamics simulation.
To further quantify the stability of the docked conformations of each compound, molecular dynamics simulations were conducted using Desmond 2023.1 based on the docked structures of the complexes. The dynamics simulations were highly effective in assessing binding stability. RMSD (root-mean-square deviation) plots, which measure the structural deviation from the reference conformation at a given time, indicated that all compounds except 4-HAP gradually dissociated from the protein during the 100 ns dynamics simulation (Figure D), failing to stably bind. 4-HAP remained bound to the protein, but the binding site shifted during the dynamics simulation from the original site with dual copper ions to a peripheral site near the binding site (Figure E).
2.5. Evaluation of the Whitening Efficacy of Self-Made Cosmetics Containing 4-HAP
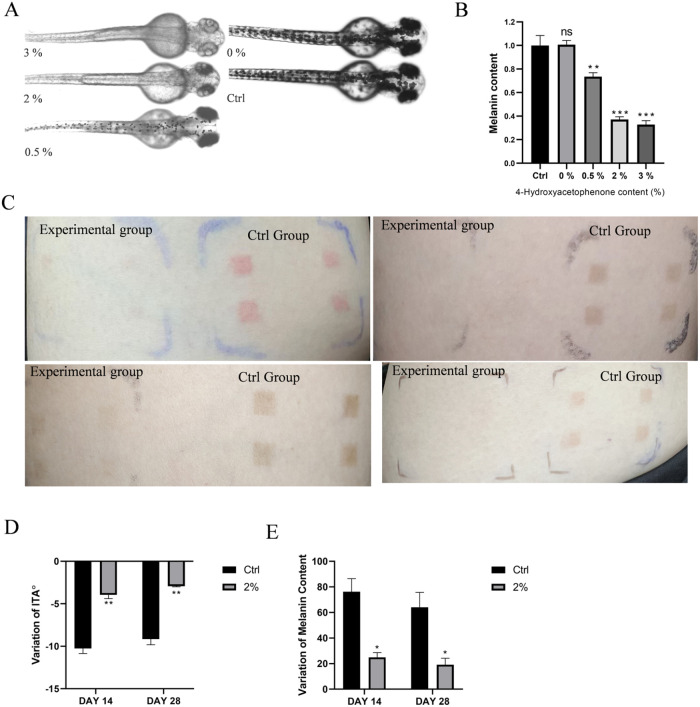
Based on the zebrafish model results, beginning with the group containing 0.5% 4-HAP, the melanin content significantly decreased with increasing concentrations compared to the control group. There was no significant difference between the group with 0% 4-HAP and the control group (Figure A,B). This indicates that 4-HAP retains its whitening effect when used alone in the samples, and this effect is not due to other ingredients in the cosmetics.
5.
Evaluation of the whitening efficacy of homemade cosmetics containing 4-HAP. (A) Surface melanin of 24 hpf embryos treated with samples containing 0, 0.5, 2, and 3% 4-HAP until 72 hpf, (B) relative melanin content of zebrafish embryos, (C) skin test area effect diagram, (D) effect of cosmetics containing 4-HAP on skin melanin, (E) impact of cosmetics containing 4-HAP on skin ITÅ values. Data are expressed as mean ± SEM (n = 6). Ns = nonspecifical, *P < 0.05, **P < 0.01, ***P < 0.001 versus Ctrl.
Due to the similar whitening effects of the samples containing 2.0 and 3.0% 4-HAP, a 2.0% 4-HAP sample was selected for human skin testing. The results of the human experiment showed that after 4 weeks, the test area of the experimental group almost returned to the pre-experiment skin condition, while the control group’s test area still clearly showed the marks left by UV exposure (Figure C). To eliminate individual differences in initial ITÅ, the change in ITÅ was used for evaluation. A larger negative change indicates a darker skin color compared to before the experiment, while values closer to 0 indicate a skin color closer to the pre-experiment condition. From day 14 to day 28, both the experimental and control groups showed a decrease in ITÅ change (Figure D), with the experimental group showing a significantly lower absolute change, approaching 0, indicating that the skin color in the test area was whiter compared to the control group and closer to the pre-experiment skin color, confirming the whitening effect of the sample. The melanin index was also evaluated using the change in values, with the melanin values on days 14 and 28 minus the values before the experiment and the first application of the sample. A larger change indicates a darker skin color compared to before the experiment, while values closer to 0 indicate a skin color closer to the pre-experiment condition. The melanin index trends were consistent with the ITÅ values. From day 14 to day 28, both the experimental and control groups showed a decrease in melanin change (Figure E), with the experimental group showing a significantly lower change, indicating that the melanin value in the test area was lower and closer to the pre-experiment skin color, further validating the sample’s ability to reduce melanin.
2.6. Discussion
4-HAP naturally occurs in Artemisia annua and is considered a natural compound with hepatoprotective and choleretic effects, demonstrating efficacy against hepatitis B and anti-inflammatory properties. The phenolic hydroxyl group in its molecular structure provides it with significant antioxidant characteristics, and its strong reducing ability can disrupt the cellular structures of bacteria such as fungi, thereby exhibiting antibacterial properties [literature citation]. In cosmetic formulations, 4-HAP is commonly used as a mild and hypoallergenic ingredient, and it has soothing and oil-controlling effects. However, to date, there have been no research reports on the relationship between 4-HAP and whitening effects or tyrosinase activity.
Structurally, 4-HAP contains a phenolic group, with a carbonyl group at the para position of its phenolic hydroxyl group, followed by a methyl group. Reviewing common known whitening agents, such as hydroquinone and β-Arbutin, their molecular structures also include a phenolic group, with a hydroxyl group at the para position of the phenol. Such structures can act as effective binding substrates for the key enzyme in melanin productiontyrosinase, covalently binding with histidine residues and interacting with copper ions in the active site of tyrosinase to competitively inhibit melanin generation. In the preliminary work of this study, we found that some cosmetics without known whitening ingredients exhibit certain whitening activity. After screening and eliminating the common components of these products, we ultimately suspect that the component responsible for the whitening effect may be the preservative 4-HAP.
Therefore, this study systematically evaluated the whitening activity of 4-HAP using mushroom tyrosinase, B16 mouse melanoma cells, and zebrafish models. The experimental results were consistent with our expectations, demonstrating that 4-HAP exhibited significant whitening effects in these models, particularly in inhibiting the activity of tyrosinase.
Given that 4-HAP demonstrated significant whitening activity in the aforementioned experiments, we hypothesize that this effect may be related to its molecular structure. Therefore, this study selected several structural analogs of 4-HAP to explore whether they also possess whitening activity. These compounds include 4-Hydroxybenzoic acid methyl ester, 3,4-dihydroxybenzoic acid methyl ester, 3,4-dihydroxyphenylacetone, and 2,4-dihydroxyphenylacetone. Their common feature with 4-HAP is the presence of a hydroxyl group on the benzene ring, which is in a para position to the carbonyl group, with differences lying in an additional hydroxyl group on the ortho or meta position of the benzene ring or a different group connected to the carbonyl group. Whitening activity tests conducted using the zebrafish model showed that these structural analogs of 4-HAP did not exhibit whitening effects in the zebrafish model. Further analysis of the binding of 4-HAP and its structural analogs to tyrosinase through molecular docking and molecular dynamics simulations revealed that 4-HAP with whitening activity has its carbonyl group deeply inserted into the protein’s active site, with the phenolic hydroxyl group forming key hydrogen bonds outside the site, gradually migrating to peripheral sites near the protein’s active site and maintaining stable binding; whereas the two dihydroxyphenylacetone compounds without whitening activity have their phenolic hydroxyl groups directed toward the copper ions and are unable to stably bind with tyrosinase. These results indicate that the whitening mechanism of 4-HAP is conferred by its unique molecular structure.
Finally, to assess the potential of 4-HAP as a whitening ingredient in cosmetic formulations, we conducted a systematic study on its whitening efficacy and stability within cosmetic formulations. Initially, we tested the whitening effects of cosmetic formulations containing 3%, 2%, 1%, 0.5%, and 0% 4-HAP in a zebrafish model. The results indicated that formulations with 2% and 3% 4-HAP demonstrated significant and comparable whitening activity. Consequently, we selected a 2% 4-HAP concentration for human skin experiments. The experimental outcomes revealed that the cosmetic formulation containing 2% 4-HAP exhibited satisfactory whitening effects in human skin trials.
3. Experimental Section
Cosmetics samples are from Symrise AG (Shanghai, China). NaOH, Butane-1,2-diol, Trolamine, Propylene glycol, Sodium Hyaluronate, Glycerol, Carbopol TR-2, 4-HAP, 4-Hydroxybenzoic acid methyl ester, 3,4-dihydroxybenzoic acid methyl ester, 3,4-dihydroxyphenylacetone, and 2,4-dihydroxyphenylacetone are from Macklin (Shanghai, China). α-Arbutin, 1,2-Hexanediol are from Teelar Biotech (Guangzhou, China). Allantoin and l-DOPA is from Aladdin.
3.1. Animals
Wild-type AB strain zebrafish (Danio rerio) were purchased from the Chinese Zebrafish Resource Center (Wuhan, China). Adult zebrafish were housed in a breeding system with a 14-h light and 10-h dark cycle. temperature ranges of 26.0–29.0 °C; water conductivity of 500–550 μS/cm; pH of 7.0–8.0. Zebrafish were fed with live brine shrimp larvae, twice a day. On the day before the experiment, after the zebrafish had been fed for 1 h, the healthy ones were selected and placed in zebrafish breeding tanks. The males and females were separated by a partition, at a ratio of 1:1. The following morning, the partition was withdrawn, so that the zebrafish were free to mate. Then, the fertilized eggs at the bottom of the tank were collected after an hour. Healthy fertilized eggs were selected and placed in an incubator at 28.5 ± 1 °C.
All zebrafish experiments complied with the Organization for Economic Co-operation and Development Test Guidelines (TG203 and TG236). All animal experimental procedures were conducted in accordance with the ethical standards of Guangdong University of Technology.
3.2. Cell Culture and Reagent
B16 mouse melanoma cells were derived from previously cryopreserved cells in our laboratory. Dulbecco’s Modified Eagle Medium (DMEM) high glucose and Roswell Park Memorial Institute (RPMI) 1640 medium were provided by Gibco (New York), while fetal bovine serum (FBS) and penicillin-streptomycin (P/S) were supplied by Zhong Qiao Xin Zhou (Shanghai, China). The Cell Counting Kit-8 (CCK8) was obtained from TransGen Biotech (Beijing, China). After resuscitation, B16 cells were cultured in RPMI-1640 complete medium under a humidified atmosphere containing 5% CO2 at 37 °C.
3.3. Mushroom Tyrosinase Inhibitory Assay
Prepare a 96-well plate by first adding 40 μL of 3 mM l-tyrosine solution to both the solvent reaction wells and the sample reaction wells. To the sample blank wells and sample reaction wells, add 40 μL of 4-HAP solutions at concentrations of 70, 35, 17.5, 8.75, and 4.375 mM, as well as the positive control group with 100 mM arbutin and the blank control group. Then, adjust the volume in the solvent blank wells, solvent reaction wells, sample blank wells, and sample reaction wells to 110 μL with PBS. Incubate the plate at 37 °C for 10 min. Using an 8-channel pipet, quickly add 20 μL of 500 U/mL mushroom tyrosinase (Yuanye, Shanghai, China) solution to each well, and incubate again for 15 min. Turn on the TriStar2S plate reader (Stuttgart, Germany), set the working mode to Absorbance, temperature to 37 °C, and wavelength to 475 nm, and read the absorbance values. The formula for calculating the melanin content in Mushroom tyrosinase activity is as follows
| 1 |
TA: Mushroom tyrosinase activity
ODTa: Solvent blank wells absorbance
ODTb: Solvent reaction wells absorbance
ODTc: Sample blank wells absorbance
ODTd: Sample reaction wells absorbance
3.4. Cytotoxicity Assay
B16 cells were seeded into a 96-well plate at a density of 5 × 103 cells per 100 μL, with 100 μL added per well, and incubated for 24 h to allow cell attachment. The culture medium was then removed, and 100 μL of RPMI-1640 complete medium containing various concentrations of 4-HAP (10, 9, 8,7, 6, 5, 4, 3, 2, 1, and 0 mM) was added to the wells, with 0 mM 4-HAP serving as the blank control. After incubation for 48 h, the medium was discarded, and the cells were washed twice with PBS. Then, 10 μL of CCK8 reagent diluted in RPMI-1640 at a ratio of 1:10 was added to each well and incubated for 120 min in the incubator. Place the plate in a TriStar2S plate reader and set the absorbance at 425 nm. All experiments were performed in triplicate. The RPMI-1640 complete medium was prepared with 10% FBS and 1% P/S in RPMI-1640 basal medium. The formula for calculating cell viability in B16 mouse melanoma cells is as follows
| 2 |
SR: Cell viability
ODe: Experiment groups absorbance
ODc: Control groups absorbance
ODb: Solvent background absorbance
3.5. B16 Cells BCA Assay
B16 cell total protein content was determined using a Bestbio BCA Protein Assay Kit (Shanghai, China). Total protein extraction solution, protease inhibitor, and phosphatase inhibitor were prepared at a ratio of 250:1:1. BCA Reagent A and BCA Reagent B were mixed at a ratio of 50:1. B16 cells were seeded into a 6-well plate at a density of 5 × 103 cells per 100 μL, with 2000 μL added per well, and incubated for 24 h to allow cell attachment. The culture medium was then removed, and the cells were washed twice with PBS. Subsequently, 2000 μL of DMEM complete medium containing various concentrations of 4-HAP (5, 4, 3, 2, 1, and 0 mM) was added to the wells, with 0 mM 4-HAP serving as the blank control. After 48 h of incubation, the medium was discarded, and the cells were washed twice with PBS. Then, 250 μL of the prepared total protein extraction solution was added, and the cells were gently agitated until they completely detached. The cell suspension was transferred to a 1.5 mL sterile centrifuge tube and kept at 4 °C for 20 min. A precooled 4 °C centrifuge was set to 13,500 rpm for 15 min. The supernatant was then transferred to a new centrifuge tube. Twenty microliters of the supernatant were diluted with 80 μL of pure water to prepare a 5-fold dilution and kept on ice. A 96-well plate was prepared with protein standard dilutions and pure water, with a total volume of 20 μL per well, resulting in well protein contents of 20, 19, 18, 16, 12, 8, 4, and 0 μg. The diluted total protein extracts (20 μL per well) were added to the wells, followed by the rapid addition of 200 μL of BCA working solution. The plate was incubated at 37 °C for 30 min. After the reaction, the plate was read in a TriStar2S plate reader set at 37 °C with an absorbance wavelength of 562 nm. A standard curve was plotted with protein content on the x-axis and absorbance on the y-axis, and a linear regression formula was derived to determine the actual protein concentration of the samples. All experiments were performed in triplicate. DMEM complete medium was prepared with 10% FBS and 1% P/S in DMEM basal medium.
3.6. B16 Melanin Content Assay
To each group, add the total protein extraction solution and centrifuge to obtain the pellet. Then, add 60 μL of 1 M NaOH solution to the pellet and incubate at 80 °C until the melanin is completely dissolved. Subsequently, transfer the dissolved melanin to a 96-well plate. Place the plate in a TriStar2S plate reader and set the absorbance parameter to 450 nm to measure the absorbance. All experiments were conducted independently with three replicates. The formula for calculating the melanin content in B16 mouse melanoma cells is as follows
| 3 |
MCc: Cells melanin content
ODe: Experiment groups absorbance
ODc: Control groups absorbance
ODb: Solvent background absorbance
Ce: Experimental group protein content
Cc: Control group protein content
3.7. B16 Cell Tyrosinase Activity Assay
A 96-well plate was prepared with 100 μL of 0.5 mM l-DOPA solution in each well, followed by the addition of 100 μL of total protein extracts from the aforementioned groups. The plate was incubated at 37 °C for 30 min and then read in a TriStar2S plate reader with the absorbance set to 475 nm. All experiments were conducted independently with three replicates. The formula for calculating tyrosinase activity in B16 mouse melanoma cells is as follows
| 4 |
TAc: Cells tyrosinase activity
ODe: Experiment group absorbance
ODc: Control group absorbance
ODb: Solvent background absorbance
Ce: Experiment group protein content
Cc: Control group protein content
3.8. Zebrafish Embryo Collection and Drug Treatment
Zebrafish embryos were prepared according to previously methods. Briefly, paired matings (ages 6–12 months) were used to generate zebrafish embryos, which were selected for further experiments after microscopic examination. Embryos were then incubated in Holt-Buffer at 28 ± 1 °C for 24 h.
To assess the whitening effects of five cosmetic products lacking known whitening components, zebrafish embryos were exposed to these products, and melanin content was evaluated. A 24-well plate was used with 15 embryos per well at 24 hpf, with five test groups and one control group. The concentration of each sample was based on the maximum safe concentration determined in previous 24 to 72 hpf tests. The embryos were cultured in a 28.5 °C incubator until 72 hpf, with three replicates per concentration.
To explore the whitening activity of 4-HAP, an acute toxicity test was first conducted. Embryos at 24 hpf were arranged in a 96-well plate (one embryo per well) and exposed to Holt-Buffer containing various concentrations of 4-HAP (1.5, 1.2, 1, 0.9, 0.8, 0.7, 0.6, and 0.5 mM). The embryos were cultured in a 28.5 °C incubator until 96 hpf, with observations at 48, 72, and 96 hpf. Twelve replicates were set for each concentration.
After determining the safe concentration of 4-HAP, a formal experiment was conducted to evaluate its effect on embryonic melanin. A 24-well plate was used with 15 embryos per well at 24 hpf, exposed to Holt-Buffer containing 4-HAP at concentrations of 0.6, 0.5, 0.4, 0.3, 0.2 mM, and a control group, and cultured in a 28.5 °C incubator until 72 hpf. Three replicates were set for each concentration.
To investigate whether four 4-HAP analogs possess whitening efficacy, an acute toxicity test was first performed. Embryos at 24 hpf were arranged in a 96-well plate (one embryo per well) and exposed to Holt-Buffer containing various concentrations of the analogs (1, 0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, 0.1 mM). The embryos were cultured in a 28.5 °C incubator until 72 hpf, with 12 replicates per concentration.
After determining the safe concentrations of the four 4-HAP analogs, a formal experiment was conducted to evaluate their effects on embryonic melanin. A 24-well plate was used with 15 embryos per well at 24 hpf, exposed to Holt-Buffer containing the four analogs at their maximum safe concentrations, as well as a control Holt-Buffer, the remaining experimental methods are consistent with the formal experiment.
To assess the efficacy of 4-HAP in cosmetic formulations, melanin content in zebrafish embryos was evaluated. Embryos at 24 hpf were cultured in a 24-well plate with 15 embryos per well. They were exposed to control and 4-HAP sample concentrations of 0, 0.5, 2, and 3% in Holt-Buffer, the remaining experimental methods are consistent with the formal experiment.
3.9. Zebrafish Acute Toxicity Assay
At each time point (48, 72, or 96 hpf), embryos exposed to various concentrations of the test samples were observed, and survival rates were recorded. Subsequently, the lethal concentration 50% (LC50) was calculated for each sample at the respective time points.
3.10. Melanin Scale Assay
Zebrafish embryos at 72 hpf were imaged using the Zeiss Axio Imager.2 microscope imaging system (Hamburg, Germany) to document melanin distribution from a top-view perspective, with 5 embryos per group. The melanin from the head to one-quarter of the tail end was quantified using ImageJ software (National Institutes of Health). The region of interest was outlined as previously described to obtain the integrated density, and the average pixel values were compared among different treatment groups to determine the melanin area size.
3.11. Zebrafish BCA Assay
Embryos treated to 72 hpf were transferred to 1.5 mL centrifuge tubes, and 250 μL of the aforementioned total protein extraction solution was added. The embryos were then homogenized using a grinding rod until no visible tissue clumps remained. Subsequent centrifugation and other steps were performed in accordance with the previously described procedures for the B16 cells BCA assay.
3.12. Zebrafish Melanin Content Assay
To the black pellet obtained from the centrifugation, add 100 μL of 1 M NaOH solution and incubate at 85 °C until the melanin is completely dissolved. Then, transfer the dissolved melanin to a 96-well plate. Place the plate in a TriStar2S plate reader and set the absorbance parameter to 450 nm to measure the absorbance. All experiments were conducted independently with three replicates. The formula for calculating the melanin content in zebrafish is as follows
| 5 |
MCz: Zebrafish embryo melanin content
ODe: Experiment group absorbance
ODc: Control group absorbance
ODb: Solvent background absorbance
Ce: Experiment group protein content
Cc: Control group protein content
3.13. Zebrafish Tyrosinase Activity Assay
A 96-well plate was prepared by adding 150 μL of 0.5 mM l-DOPA solution to each well, followed by the addition of zebrafish total protein extracts obtained from the previous steps. The plate was incubated at 37 °C for 30 min. The absorbance parameter of the TriStar2S plate reader was set to 475 nm, and the absorbance was measured. All experiments were conducted independently with three replicates. The formula for calculating tyrosinase activity in zebrafish is as follows:
| 6 |
TAz: Zebrafish embryo tyrosinase activity
ODe: Experiment group absorbance
ODc: Control group absorbance
ODb: Solvent background absorbance
Ce: Experiment group protein content
Cc: Control group protein content
3.14. RNA Isolation and cDNA Preparation
Total RNA from embryos of each group was extracted using a Total RNA Column Extraction Kit when the embryos reached 7dpf, following the manufacturer’s protocol. The quality of the total RNA was assessed using an Agilent 2100 Bioanalyzer (Agilent, CA), agarose gel electrophoresis, and a nanophotometer. The HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme #R323) includes a genomic DNA removal module to thoroughly eliminate residual genomic DNA in the RNA template; additionally, Vazyme #R323 contains all the components required for the reverse transcription reaction, allowing the process to be completed by simply adding template RNA.
3.15. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
RT-qPCR was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) and self-designed primers (primer sequences are shown in Table ). CFX96 Touch Real-Time PCR Detection System (Bio-Rad, California) was used to perform three PCR tests for each RNA sample. Each sample contained 0.5 μL cDNA and 10 μL 2X ChamQ Universal SYBR qPCR Master Mix. Relative gene expression levels were assessed using the 2–ΔΔCt formula, and then β-actin gene expression was normalized to target gene expression. All primers were independently designed and validated.
2. Primers for Target Genes.
| primer | front primer sequence (5′-3′) | rear primer sequence (3′-5′) |
|---|---|---|
| MITF | CGAGTACAGTCACTACCAGGTTC | CAGTTGAGGGTGAGAAGGGC |
| TYR | GCGCTGGAAGGTTTTGCTAAT | GTATCCGTCGTTGTGTCCGA |
| TYRP1 | GGGAACTACAGCGGGTTTGA | GGAGAAATCCCAGAACGGCA |
| TYRP2 | CTGTGACCAATGAGGAGATT | CATAGGATTTGGGACTGTGT |
| actin | GCCGTGACCTGACTGACTAC | GGGCACCTGAACCTCTCATT |
3.16. Molecular Docking and Molecular Dynamics Simulation
Molecular docking was performed using Schrödinger 2023.1 software. The protein structure was prepared using the Protein Preparation Wizard module, which included protonation, addition of hydrogen atoms, and retention of critical bound water molecules. The ligand for docking was processed using the LigPrep module. Molecular docking was conducted using the XP mode of the Glide module, based on the binding site of the crystal ligand. Subsequent molecular dynamics simulations were performed based on the docked conformations.
Molecular dynamics simulations were conducted using Desmond. The system was solvated using the SPC water model, with the complex dissolved in a cubic box filled with water, and an appropriate amount of Na+ and Cl– ions were added to neutralize the system’s charge. A concentration of 0.15 mol/L NaCl was included to simulate a physiological protein environment. Energy minimization was performed using the OPLS4 force field. A 100 ns MD simulation was carried out at 300 K temperature and 1 atm pressure.
3.17. Human Skin Whitening Efficacy Experiment
3.17.1. Volunteer Recruitment
Volunteers were recruited with full disclosure of the experimental objectives and potential outcomes. Participants provided informed consent, acknowledging the risks involved in the study. Inclusion criteria included: (1) Age between 18 and 60 years, regardless of gender; (2) No skin diseases in the test area; (3) No history of photosensitivity diseases and no use of similar products recently; (4) Ability to comply with the experimental requirements and complete the study; (5) Voluntary participation and signed informed consent. Exclusion criteria included: (1) History of allergies to cosmetics or allergic diseases; (2) Women who are breastfeeding, pregnant, or within 6 months postpartum; (3) No participation in other testing programs on the test area within the past six months.
3.18. Melanin Skin Pigmentation Test
The test site was the inner thigh area of the volunteers, with eight 1 cm diameter circular regions taken from each inner thigh. Four regions on each side were designated as control and experimental groups: the control group with 0% 4-HAP and the experimental group with 2.0% 4-HAP.
Baseline data were collected from the predetermined regions on the inner thighs before the experiment. During the first week (Days 1 to 7), the test areas were treated daily with the corresponding concentrations of the test product. Data were collected on Day 7, followed by UV preirradiation to assess skin sensitivity. In the second week (Days 8 to 11), the test areas were UV-irradiated for 30 s daily and treated with the test product. From Day 12 to Day 28, the test product was applied daily. Data collection continued on Days 14, 21, and 28, completing a 28-day experimental period.
Data collection from the test areas was performed using a skin physiological parameter analyzer. The primary indicators for skin color assessment were individual type angle (ITÅ) and melanin index. ITA° was calculated based on L*, b* color space data, with higher ITÅ values indicating lighter skin and lower melanin indices suggesting lighter skin. The ITÅ calculation formula is shown as follows
| 7 |
3.19. Statistical Analysis
All analyses were performed using GraphPad Prism (version 8.0, GraphPad Software, Santiago de Chile), Origin software (version 8.0; OriginLab Corporation, Northampton, MA) and SPSS Statistics (version 26; IBM, Endicott, NY). Survival curves were plotted using Origin software. Differences between two groups were evaluated using a Student’s t test. Differences among multiple groups were analyzed using one-way analysis of variance. Differences with a P-value <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Symrise AG for providing the cosmetics samples.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.5c03812.
Survival rate of zebrafish embryos exposed to 4-HAP at 48, 72, and 96 hpf (Figure S1); Survival rate of zebrafish embryos exposed to 4-HAP analogs (Figure S2) (PDF)
#.
Q.G., Y.Z., and C.T. contributed equally to this work. Q.G.: Conceptualization, data curation, funding acquisition, methodology, project administration, supervision, writingreview and editing. Y.Z.: Formal analysis, investigation, visualization, writingoriginal draft. C.T.: Formal analysis, investigation, visualization. Q.L.: Validation. F.L.: Resources. Z.H.: Curation. C.J.: Validation. H.Z.: Conceptualization, methodology, project administration, writingreview and editing. J.X.: Validation. All authors have read and approved the final manuscript.
All animal experiments were conducted in strict accordance with the National Institutes of Health (NIH) guide for the care and use of Laboratory animals (publication No. 85-23, revised in 1985). The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Guangdong Provincial People’s Hospital, and all efforts were made to minimize suffering. The ethics approval/permit number for the use of animals in this study is S2024-849-01. Volunteers were recruited with full disclosure of the experimental objectives and potential outcomes. Participants provided informed consent, acknowledging the risks involved in the study.
The authors declare no competing financial interest.
References
- Petit A.. Skin lightening and its motives: A historical overview. Ann. Dermatol. Vénéréol. 2019;146(5):399–409. doi: 10.1016/j.annder.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Snyman M., Walsdorf R. E., Wix S. N., Gill J. G.. The metabolism of melanin synthesis-From melanocytes to melanoma. Pigment Cell Melanoma Res. 2024;37(4):438–452. doi: 10.1111/pcmr.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley P. A.. Melanin. Int. J. Biochem. Cell Biol. 1997;29(11):1235–1239. doi: 10.1016/S1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Baek N., Nam T.-g.. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib Med. Chem. 2016;31(1):1–13. doi: 10.3109/14756366.2015.1004058. [DOI] [PubMed] [Google Scholar]
- Huang T.-J., Liu S.-H., Kuo Y.-C., Chen C.-W., Chou S.-C.. Antiviral activity of chemical compound isolated from Artemisia morrisonensis against hepatitis B virus in vitro. Antiviral Res. 2014;101:97–104. doi: 10.1016/j.antiviral.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Geng C.-A., Chen H., Ma Y.-B., Huang X.-Y., Cao T.-W., He K., Wang H., Zhang X.-M., Chen J.-J.. Isolation, synthesis and anti-hepatitis B virus evaluation of p-hydroxyacetophenone derivatives from Artemisia capillaris. Bioorg. Med. Chem. Lett. 2015;25(7):1509–1514. doi: 10.1016/j.bmcl.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Chen T.. Study on local toxicity of p-hydroxyacetophenone for cosmetics % Flavour Fragrance Cosmet. 2020;(03):56–60. [Google Scholar]
- Latif N. H. A., Rahim A. A., Brosse N., Hussin M. H.. The structural characterization and antioxidant properties of oil palm fronds lignin incorporated with p-hydroxyacetophenone. Int. J. Biol. Macromol. 2019;130:947–957. doi: 10.1016/j.ijbiomac.2019.03.032. [DOI] [PubMed] [Google Scholar]
- do Nascimento A. M., Salvador M. J., Candido R. C., de Albuquerque S., de Oliveira D. C. R.. Trypanocidal and antifungal activities of p-hydroxyacetophenone derivatives from Calea uniflora (Heliantheae, Asteraceae) J. Pharm. Pharmacol. 2004;56(5):663–669. doi: 10.1211/0022357023231. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu X., Liu Z., Yu Q., Tu Y.. Analysis of raw material standard and usage of preservative in cosmetics % Flavour Fragrance Cosmet. 2022;30(08):19–29. [Google Scholar]
- Desmedt B., Courselle P., De Beer J. O., Rogiers V., Grosber M., Deconinck E., De Paepe K.. Overview of skin whitening agents with an insight into the illegal cosmetic market in Europe. J. Eur. Acad. Dermatol. Venereol. 2016;30(6):943–950. doi: 10.1111/jdv.13595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.