Abstract
Porous organic polymers (POPs) with nitrogen-enriched structures are the type of materials with the advantages of porosity, excellent stability, and wide synthetic pathways, which possesses the great potential for application in the proton conduction. Herein, we report the synthesis of a novel type of pyrrole-based POPs that exhibit high surface areas and nitrogen-rich skeletons. The fabricated pyrrole-linked POPs render the high potential for the proton conducting application. In order to achieve precise modulation of the polymer structures, a benzothiadiazole moiety was introduced to increase the active site for forming strong hydrogen bonds with phosphoric acid. This modification allowed for precise manipulation of the monomers, which achieves a proton conductivity of 38.0 mS cm–1 at 180 °C. This work demonstrates a new type of nitrogen-rich POPs for proton conduction applications.


1. Introduction
Porous organic polymers (POPs) have emerged as a prominent class of functional materials, attracting considerable interest due to their highly tunable porous structures, low density, and diverse synthetic methodologies. − Typically, POPs are constructed with building units using light elements such as C, H, N, B, O, which are interconnected via covalent bonds. , The different elements in POPs determine their applications in different fields, such as electrode materials, gas capture and so on. It is worth mentioning that nitrogen-containing POPs can enhance the polarity, conjugation effect and interaction with guest molecules due to the advantages of heteroatomic effects, and thus have potentials in the fields of photocatalysis , and proton conduction. In particular, the lone pair electrons of nitrogen atoms make them exhibit a certain alkaline, which show a strong interaction with the proton carrier to promote the proton dissociation.
In recent years, significant advancements have been made in the study of POPs within the realm of proton conduction. The progress can be attributed not only to the advantageous nitrogen-rich composition of POPs but also to their high surface area, pore structure, and notable acid stability. For instance, Jiang et al. synthesized five hexagonal covalent organic frameworks (COFs) with varying pore sizes, which transitioned from microporous to mesoporous structures and exhibited a remarkable increase of proton conductivity. We demonstrated a method for the direct cyclotrimerization of aromatic aldehydes into covalent triazine frameworks (CTFs) by incorporating the nitrogen source. This approach enabled the synthesis of CTFs with a high fluoride and nitrogen content, resulting in high proton conduction at 150 °C. Similarly, Du et al. introduced a strategy to enhance proton conductivity by modulating the interlayer interactions of COFs. By incorporating triazine units to improve interlayer interactions and the binding affinity with phosphoric acid, the conductivity of COFs increased approximately 10-fold.
Although nitrogen-containing POPs hold significant potential for applications in proton conduction, the diversity of these materials remains quite limited. Currently, nitrogen-containing heterocyclic polymers are restricted to a few types, such as CTFs, polybenzimidazoles, and polyimides. It is of great interest to expand the diversity of the nitrogen heterocyclic based POPs. In the term, pyrrole is highly valued in materials science due to its facile synthesis and robust structural integrity. For example, linear polypyrrole is renowned for its superior electrical conductivity, and tetrapyrrole-based porous polymers are extensively studied in photoelectric technologies.
Herein, we report the synthesis of a novel class of pyrrole-based POPs using a one-pot method that incorporates pyrrole as the linking unit. This synthesis process is characterized by its simplicity and mild conditions. Furthermore, the polymer’s various properties can be optimized through strategic functional group modification, thereby enhancing proton conductivity. Notably, the pyrrole ring’s alkaline nitrogen atoms act as proton carriers, enabling efficient binding with phosphoric acid and resulting in exceptional proton conductivity. The incorporation of benzothiadiazole groups further amplifies the polymer’s specific surface area and the density of active sites, enhancing its interaction with phosphoric acid. As a result, the proton conductivity of the phosphoric acid-loaded polymer achieves 38.0 mS cm–1 at 180 °C. This research not only extends categories of POPs but also opens new possibilities for the development of advanced materials in proton conduction applications.
2. Experimental Section
2.1. Materials
1,4-Diacetylbenzene (98%), p-Phenylenediamine (99%), Iodine (99.8%), o-dichlorobenzene (o-DCB, 99%), N,N-dimethylformamide (DMF, 99%) and ethanol (99.5%) were obtained from Aladdin Co. 4,7-bis(4-aminophenyl)-2,1,3-benzothiadiazole was supplied by Yanshen Technology Co., Ltd. (Jilin, China) and the dilute hydrochloric (HCl, 37%) acid was purchased from China National Medicines Corporation Ltd.
2.2. Characterization Methods
Fourier transform infrared spectroscopy (FT-IR) was conducted using the Mattson Alpha-Centauri spectrometer (Nicolet iS50, Thermo Scientific). Solid-state 13C nuclear magnetic resonance (NMR) spectra were acquired using the JNM-ECZ600R spectrometer. Powder X-ray diffraction (PXRD) analyses were performed with the D8 Advance (Bruker), equipped with Cu Kα radiation (λ = 1.54056 Å). X-ray photoelectron spectroscopy (XPS) was analyzed using the Kratos Analytical Axis Ultra DLD instrument. Scanning electron microscopy (SEM) images were obtained with the MAIA3 LMH. Thermogravimetric analysis (TGA) was carried out in a nitrogen atmosphere using the STA 449F5 from PerkinElmer Instruments, over a temperature range of 30–800 °C. After degassing in a vacuum at 120 °C for 12 h, nitrogen adsorption and desorption isotherms at 77 K were measured using a BSD-16 analyzer. The specific surface areas of the PYR–POP-1 and PYR–POP-2 samples were determined by the Brunauer–Emmett–Teller (BET) method, while the pore size distributions were calculated by the BJH method.
2.3. Preparation of PYR–POPs
2.3.1. Synthesis of PYR–POP-1
PYR–POP-1 was synthesized by a simple polycondensation route. p-phenylenediamine (0.4 mmol, 43.2 mg), 1,4-diacetyl benzene (0.8 mmol, 129.8 mg) and iodine (0.8 mmol, 203.1 mg) were dissolved in 15 mL o-DCB in a 50 mL round-bottomed flask. The mixture was heated and stirred in a magnetically heated stirrer at 40 °C for 24 h, 80 °C for 24 h, 120 °C for 24 h, and 160 °C for 24 h. After cooling to room temperature, the filter obtained solid, and then the filter was washed several times with an appropriate amount of DMF, dilute hydrochloric acid solution, water and anhydrous ethanol, and then the black solid was obtained by vacuum drying at 100 °C for 12 h. (120 mg, 85.1% yield).
2.3.2. Synthesis of PYR–POP-2
4,7-bis(4-Aminophenyl)-2,1,3-benzothiadiazole (0.3 mmol, 95.5 mg) and 1,4-diacetyl benzene (0.6 mmol, 97.3 mg) and iodine (0.6 mmol, 152.3 mg) were dissolved in 15 mL o-DCB in a 50 mL round-bottomed flask. The mixture was heated and stirred in a magnetically heated stirrer at 40 °C for 24 h, 80 °C for 24 h, 120 °C for 24 h, and 160 °C for 24 h. The filter solidified after cooling to room temperature. It was then repeatedly washed with an appropriate amount of DMF, dilute hydrochloric acid solution, water and anhydrous ethanol. Finally, the deep red solid was obtained by vacuum drying at 100 °C for 12 h. (154 mg, 91.2% yield).
2.4. Preparation of H3PO4@PYR–POPs
Using PYR–POP-1 as an example. The preparation process begins with activating PYR–POP-1 in a vacuum at 150 °C for 12 h. Next, commercial phosphoric acid (85%) is diluted with methanol in a 1:1 volume ratio. Subsequently, 100 mg of PYR–POP-1 is added to 5 mL of the diluted phosphoric acid and stirred at 25 °C for 24 h. The resulting filter product is then rinsed with 10 mL of water and dried under vacuum at 120 °C for 12 h, yielding approximately 187 mg of H3PO4@PYR–POP-1, which contains 87% H3PO4. H3PO4@PYR–POP-2 is prepared using the same procedure, achieving a phosphoric acid loading rate of 114%.
2.5. Proton Conductivity Measurement
The powdered H3PO4@PYR–POPs samples were compacted into a quartz glass mold with a diameter of 5 mm and a length of approximately 3 mm, then tightly pressed between two electrodes. The proton conductivity (σ, S/cm) of H3PO4@PYR–POPs was derived from the electrochemical impedance spectroscopy (EIS) curve, measured in the absence of water. The AC impedance spectrum was recorded using a CHI600E electrochemical workstation. Temperature-dependent proton conductivity was evaluated in the range of 100 to 180 °C under dry air conditions within a closed oven. Prior to the conductivity measurements, the sample was vacuum-dried at 120 °C for 12 h to eliminate residual moisture. The proton conductivity can be calculated as follows
For the tested sample, L represents the distance (cm) between the platinum electrodes, which is approximately 3 mm. R denotes the resistance at a given temperature, and A is the cross-sectional area of the test die (cm2). The activation energy (E a) can be calculated using the following formula
where σ and σ0 represent the conductivity and pre-exponential factors, respectively. T denotes the absolute temperature (in Kelvin), E a represents the activation energy, and k is the Boltzmann constant.
3. Results and Discussion
3.1. Synthesis and Structural Characterization of PYR–POPs
As illustrated in Figure , we have successfully synthesized the pyrrole-based porous organic polymers, that are PYR–POP-1 and PYR–POP-2, through an iodine-mediated catalytic cascade condensation reaction, building on the methodologies described in previous studies (Figure a). The condensation polymerization mechanism, as illustrated in Figure a, is proposed to proceed through the following steps: (i) aniline undergoes reaction with acetophenone to form a ketoimine (product I) via dehydration. (ii) The methyl group within the ketoimine undergoes iodination, yielding product II. (iii) Subsequently, products I and II engage in a methylation reaction, resulting in the formation of a quaternary ammonium iodide salt (product III). (iv) Finally, product III undergoes deamination and elimination, leading to the formation of the final product, 1,2,4-triarylpyrroles, through an aromatization process. Notably, this represents the first instance of synthesizing porous organic polymers interconnected by pyrrole rings (Figure b). During the optimization of experimental conditions, we discovered that the reaction temperature plays a crucial role in both the yield and the specific surface area of the resulting polymers. Specifically, an excessively high initial reaction temperature led to the sublimation of the iodine catalyst, adversely affecting the outcome. To confirm the structure of the synthesized polymers, we employed several characterization techniques, including FT-IR, solid-state CP-MAS 13C NMR and XPS.
1.
(a) Proposed reaction mechanism for synthesis of 1,2,4-Triarylpyrrole. (b) Schematic diagram of reaction synthesis of PYR–POPs.
Further analysis of the FT-IR spectrum of PYR–POP-1 reveals several characteristic absorption peaks (Figure a). The CC stretching vibrations of the benzene ring and pyrrole are observed at 1600 and 1515 cm–1, respectively. The C–N bond stretching vibration of the pyrrole is identified at 1266 cm–1. Additionally, the CO stretching vibration corresponding to unreacted components is observed at 1680 cm–1. A relatively intense peak at 830 cm–1 corresponds to the C–H bending vibration in the benzene ring. In comparison, the FT-IR spectrum of PYR–POP-2 shows a characteristic N–S stretch in the benzothiadiazole moiety at 1480 cm–1. , Further analysis of the 13C NMR spectrum revealed that the carbon peaks corresponding to the benzene ring were observed at 122.7, 125.7, 127.4, and 129.5 ppm. The characteristic peaks of the pyrrole ring appeared at 107.3, 118.0, and 134.5 ppm, while the peaks corresponding to the carbon atoms at the junction between the benzene and pyrrole rings were concentrated at 133.4, 135.7, and 136.6 ppm, as shown in Figure b. , Additionally, the structural integrity of the polymers was assessed using XPS. As depicted in Figure c, the primary peak at 400.1 eV is consistent with the nitrogen atoms within the pyrrole ring. Figure d shows two distinct peaks at 400.0 and 399.2 eV, which are assigned to the nitrogen atoms in pyrrole and thiadiazole units, respectively. Collectively, these comprehensive characterization techniques confirm the successful synthesis and structural validation of the pyrrole-linked porous organic polymers. Subsequently, nitrogen adsorption–desorption analyses were conducted on both polymers. The isotherms for PYR–POP-1 and PYR–POP-2 reveal limited nitrogen uptake in the low-pressure region (P/P 0 < 0.1), suggesting that these polymers exhibit a relatively small amount of microporosity (Figure e,f). The BET specific surface areas for PYR–POP-1 and PYR–POP-2 were determined to be 128.7 and 240.8 m2 g–1, respectively, indicating that both materials possess a discernible porous structure. Additionally, the adsorption–desorption curves display characteristic H3-type hysteresis loops, indicative of mesoporous structures. Regarding pore size distribution, the data align well with the BET surface area results (Figures S2 and S3). The pore size distribution for PYR–POP-1 predominantly centers around 3.1 nm, whereas PYR–POP-2 shows a peak at approximately 2.5 nm. The presence of various pore sizes in the materials suggests that imperfections during the polymerization process contribute to the formation of both micropores and macropores. Figure S1 illustrates the PXRD patterns for both PYR–POP-1 and PYR–POP-2, which reveal the absence of significant diffraction peaks. This indicates that both polymers are amorphous.
2.
(a) FT-IR spectra of PYR–POPs. (b) solid-state 13C CP-MAS NMR spectra of PYR–POP-1. (c,d) N 1s XPS Spectra of PYR–POP-1 and PYR–POP-2. (e,f) N2 adsorption and desorption isotherms (77 K) curves of PYR–POP-1 and PYR–POP-2.
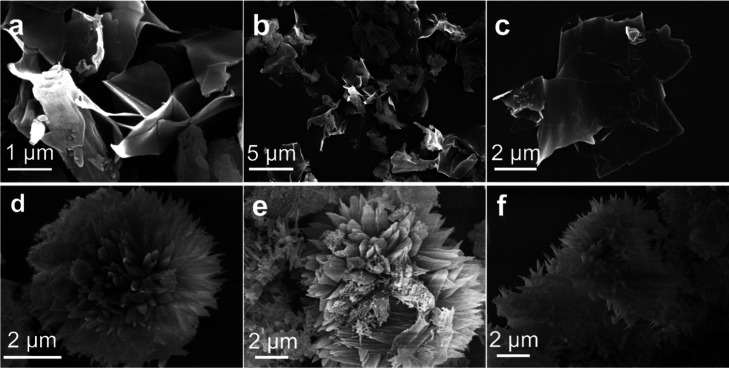
The SEM analysis elucidated distinct morphologies for the two polymers under investigation. As illustrated in Figure a–c, PYR–POP-1 exhibits a pronounced two-dimensional lamellar structure. This morphology is markedly different from the commonly reported rod-like, spheroidal, or blocky structures seen in mesoporous materials. The SEM images reveal that PYR–POP-1 forms well-defined nanosheets with transverse dimensions extending to several micrometers. This layered, sheet-like morphology is indicative of a stacking behavior akin to that observed in polypyrrole synthesized via the soft template method, despite PYR–POP-1 being an amorphous material. Rigid para-monomers facilitate the planar extension of the molecular structure during polymerization, providing the foundation for lamellar morphology formation. At temperatures between 40 and 80 °C, oligomers are formed, which self-assemble into small lamellar aggregates via hydrogen bonding and π–π stacking. At higher temperatures (120 to 160 °C), these oligomers further polymerize into sheet-like structures, which then stack into layers through π–π interactions and van der Waals forces. Additionally, the polarity of o-DCB may enhance the solubilization of aromatic monomers and reduce the interfacial tension, aiding in lamellar formation. Conversely, PYR–POP-2 displays an unconventional sea urchin-like morphology as shown in Figure d–f. The SEM images reveal that the material is composed of short, variable-length fibers arranged radially, mimicking the appearance of sea urchins. This structure emerged without the use of any template during synthesis, suggesting a self-assembly mechanism that resulted in the formation of the sea urchin-like morphology. Elemental analysis (EA) of both materials indicated that their actual elemental compositions closely match the theoretical values predicted (Table S1). Notably, the incorporation of the thiadiazole group in PYR–POP-2 results in a higher nitrogen content compared to PYR–POP-1. The TGA further demonstrates that PYR–POP-2 exhibits high thermal stability, with negligible weight loss up to 300 °C. In contrast, PYR–POP-1 begins to decompose at approximately 200 °C, signifying that PYR–POP-2 is more resistant to thermal degradation compared to PYR–POP-1 (Figure S4).
3.
(a–c) SEM images of PYR–POP-1. (d–f) SEM images of PYR–POP-2.
3.2. Proton Conductivity Test
To investigate the proton conductivity of PYR–POPs loaded with 85% phosphoric acid, we regulated various functional groups as proton carriers. Proton conductivity was assessed using EIS conducted at an electrochemical workstation. As illustrated in Figure a,b, the conductivity measurements of H3PO4@PYR–POPs at different temperatures were measured. These results demonstrate a consistent enhancement in proton conductivity with increasing temperature. In comparison, PYR–POP-2 exhibited superior proton conductivity due to its higher specific surface area and increased number of alkaline nitrogen sites. At 180 °C, H3PO4@PYR–POP-2 achieved a maximum proton conductivity of 38.0 mS cm–1 (Figure d), indicating the pivotal role of nitrogen atoms in enhancing proton conduction. The stability of H3PO4@PYR–POPs was assessed by subjecting the materials to a 12 h exposure at 180 °C. It was found that H3PO4@PYR–POP-2 retained 83.3% of its proton conductivity, while H3PO4@PYR–POP-1 retained 82.5% (Figures S5 and S6). These results demonstrate that both materials exhibit significant stability in high-temperature proton conductivity, indicating their potential for reliable performance in high-temperature applications.
4.
(a,b) Nyquist plots of H3PO4@PYR–POP-1 and H3PO4@PYR–POP-2; (c,d) the temperature-dependent proton conductivity of H3PO4@PYR–POPs. (e,f) Arrhenius diagram of H3PO4@PYR–POPs tested at different temperatures.
3.3. Analysis of the Proton Conduction Mechanism
To gain deeper insights into the differences between the two materials, the FT-IR was employed to analyze the phosphoric acid-loaded materials, revealing a pronounced PO absorption peak at 950 cm–1, which confirms the successful incorporation of phosphoric acid in both materials (Figures S7 and S8). Further, BET surface area analysis indicated that H3PO4@PYR–POP-2 exhibited a complete loss of surface area, dropping to 0 m2 g–1, signifying full saturation with phosphoric acid. In contrast, H3PO4@PYR–POP-1 maintained a specific surface area of 22 m2 g–1 (Figures S9 and S10). To elucidate the proton conduction mechanisms, activation energy (E a) was calculated using the Arrhenius equation. The Ea values for H3PO4@PYR–POP-1 and H3PO4@PYR–POP-2 were determined to be 0.255 and 0.276 eV, respectively (Figure e,f). These Ea values, which are less than 0.4 eV, indicate that the Grotthuss-type hopping mechanism is followed by the proton conduction process in both materials. −
To elucidate the mechanism underlying proton conduction, we investigated the interaction between phosphoric acid and PYR–POPs using XPS. The P 2p XPS spectra revealed two distinct peaks at 134.4 and 135.2 eV, which are attributed to H2PO4 – and H3PO4, respectively (Figure a,b). , We calculated the peak area ratio of H2PO4 – in the XPS spectra following the incorporation of phosphoric acid into the PYR–POPs. The ratios obtained were found to be 53.2% (for H3PO4@PYR–POP-1) and 58.3% (for H3PO4@PYR–POP-2). These findings suggest that the proportion of H2PO4 – plays a critical role in determining the activation energy for proton conduction. The activation energy for proton transport in phosphoric acid-supported polymers is strongly influenced by the energy barrier associated with the H3PO4 → H2PO4 – transition. Since this transition represents a lower energy barrier for proton conduction, a higher proportion of H2PO4 – results in a reduced activation energy for proton transport. Consequently, the data indicate that a greater proportion of H2PO4 – enhances the efficiency of proton conduction, aligning with the observed experimental results.
5.
(a,b) High-resolution P 2p XPS spectra of H3PO4@PYR–POPs. (c,d) N 1s XPS spectra of H3PO4@PYR–POPs. (e,f) Schematic representation of the interaction between PYR–POPs and H3PO4. (g) Schematic representation of proton conduction in H3PO4@PYR–POP-2.
The P 2p XPS spectra alone do not fully elucidate the binding mechanisms of H3PO4 to polymers and the associated proton transport dynamics. Thus, we extended our analysis using XPS, focusing specifically on the N 1s region to gain a more comprehensive understanding. Figure c,d present the deconvoluted N 1s spectra following the incorporation of H3PO4 into the polymers. Notably, a pronounced peak around 402.3 eV was observed, marking a significant chemical shift from the original peak at approximately 400.0 eV prior to phosphoric acid loading. This shift was consistently observed for both PYR–POP-1 and PYR–POP-2, indicative of the formation of protonated pyrrole or related nitrogen species. , A distinguishing feature of PYR–POP-2 is the presence of a thiadiazole group in addition to the pyrrole units. The XPS analysis revealed a convolution peak for the protonated thiazolium nitrogen atoms at around 399.7 eV, which is a notable red shift from the initial peak at 399.2 eV, confirming successful binding of H3PO4 to these sites. Figure e,f illustrate the proposed mechanism of phosphoric acid binding to the PYRs polymers. The binding of H3PO4 to the pyrrole nitrogen atoms in both PYR–POP-1 and PYR–POP-2 involves strong hydrogen bond interactions. However, PYR–POP-2, with its additional thiadiazole nitrogen atoms, exhibits a more robust interaction with H3PO4 due to the higher density of nitrogen sitesabout twice as many as found in pyrrole nitrogen. This increased availability of binding sites not only enhances the interaction with phosphoric acid, but also facilitates greater proton dissociation, thereby improving the conductivity of PYR–POP-2 relative to PYR–POP-1, as shown in Figure g, where the proton transfer is accomplished within the polymer backbone.
4. Conclusions
In summary, we present a novel strategy for the synthesis of POPs utilizing pyrrole as the linking unit, achieved through a gradient temperature rise process with iodine serving as the catalyst. The structure of the synthesized PYR–POPs was confirmed using a wide range of characterization techniques. These polymers exhibit notable characteristics, including a substantial specific surface area, unique morphology, and a high nitrogen content. Furthermore, they demonstrate remarkable thermal stability. These unique properties suggest that PYR–POPs are promising candidates for proton conduction applications. Upon incorporation of H3PO4, PYR–POP-2 exhibited commendable proton conductivity (38.0 mS cm–1 @180 °C). This study introduces a new class of porous organic materials, expanding the diversity of these materials through the strategic incorporation and modulation of functional groups. The introduction of these groups not only diversifies the material types but also allows for the optimization of proton conductivity.
Supplementary Material
Acknowledgments
We would like to express our gratitude for the funding received from Aeronautical Science Foundation of China (no. 2023Z056070001). This work is also funded by the open fund from the Xi’an Key Laboratory of C1 Compound Bioconversion Technology. We also extend our thanks to the Analytical Testing Center of Xi’an Jiaotong University for their support in material analysis and characterization.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.5c00303.
PXRD patterns of PYR–POPs; Pore size distributions of PYR–POP-1; Pore size distributions of PYR–POP-2; Elemental contents of PYR–POPs measured by EA; TGA curves of PYR–POPs measured under nitrogen atmosphere; Proton conductivity of H3PO4@PYR–POP-1 measured at 180 °C after 12 h; Proton conductivity of H3PO4@PYR–POP-2 measured at 180 °C after 12 h; FT-IR spectra of PYR–POP-1 and H3PO4@PYR–POP-1; FT-IR spectra of PYR–POP-2 and H3PO4@PYR–POP-2; N2 adsorption and desorption isotherms (77 K) curves of PYR–POP-1 and H3PO4@PYR–POP-1; N2 adsorption and desorption isotherms (77 K) curves of PYR–POP-2 and H3PO4@PYR–POP-2 (PDF)
Qi Zhou: conceptualization, methodology, formal analysis, and writing-original draft. Lijiang Guan: formal analysis and research. Shangbin Jin and Mohamed E. El-Khouly: conceptualization, writing-review and editing, and obtaining funding.
The authors declare no competing financial interest.
References
- Chen W., Chen P., Zhang G., Xing G., Feng Y., Yang Y. W., Chen L.. Macrocycle-derived hierarchical porous organic polymers: synthesis and applications. Chem. Soc. Rev. 2021;50:11684–11714. doi: 10.1039/D1CS00545F. [DOI] [PubMed] [Google Scholar]
- Wang J., Lei Y., Li S., Ma X., Li L.. Three Birds with One Sulfur: Construction of Sulfur-Bridged Porous Organic Polymers for Efficient Gold Adsorption. ACS Macro Lett. 2024;13:632–637. doi: 10.1021/acsmacrolett.4c00218. [DOI] [PubMed] [Google Scholar]
- Fritz P. W., Ashirov T., Coskun A.. Porous organic polymers with heterocyclic crown ethers for selective lithium-ion capture. Chem. 2024;10:2207–2219. doi: 10.1016/j.chempr.2024.03.014. [DOI] [Google Scholar]
- Bhattacharjee S., Tripathi A., Chatterjee R., Thapa R., Mueller T. E., Bhaumik A.. N-Heterocyclic Carbene Moiety in Highly Porous Organic Hollow Nanofibers for Efficient CO2 Conversions: A Comparative Experimental and Theoretical Study. ACS Catal. 2024;14:718–727. doi: 10.1021/acscatal.3c05576. [DOI] [Google Scholar]
- Geng K., Arumugam V., Xu H., Gao Y., Jiang D.. Covalent organic frameworks: Polymer chemistry and functional design. Prog. Polym. Sci. 2020;108:101288. doi: 10.1016/j.progpolymsci.2020.101288. [DOI] [Google Scholar]
- Li M., Liu Z., Tang J., Cheng L., Xue Y., Liu Y., Liu J.. Facile Synthesis of a Multifunctional Porous Organic Polymer Nanosonosensitizer (mHM@HMME) for Enhanced Cancer Sonodynamic Therapy. ACS Appl. Mater. Interfaces. 2024;16:28104–28117. doi: 10.1021/acsami.4c02651. [DOI] [PubMed] [Google Scholar]
- Weeraratne K. S., Alzharani A. A., El-Kaderi H. M.. Redox-Active Porous Organic Polymers as Novel Electrode Materials for Green Rechargeable Sodium-Ion Batteries. ACS Appl. Mater. Interfaces. 2019;11:23520–23526. doi: 10.1021/acsami.9b05956. [DOI] [PubMed] [Google Scholar]
- Zhang W., Li Y., Wu Y., Huang W., Wang S., Fu Y., Ma W., Li X., Ma H.. Polypyrene Porous Organic Framework for Efficiently Capturing Electron Specialty Gases. ACS Appl. Mater. Interfaces. 2023;15:29468–29477. doi: 10.1021/acsami.3c05398. [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhu H., Wang L., Lei J., Liu J.. Efficient proton conduction in porous and crystalline covalent-organic frameworks (COFs) J. Energy Chem. 2023;82:198–218. doi: 10.1016/j.jechem.2023.04.002. [DOI] [Google Scholar]
- Sahoo R., Mondal S., Pal S. C., Mukherjee D., Das M. C.. Covalent-Organic Frameworks (COFs) as Proton Conductors. Adv. Energy Mater. 2021;11:2102300. doi: 10.1002/aenm.202102300. [DOI] [Google Scholar]
- Liu M., Deng W. H., Wang X., Liu J., Jin S., Xu G., Tan B.. Hydrogen Bond Activation by Pyridinic Nitrogen for the High Proton Conductivity of Covalent Triazine Framework Loaded with H3PO4 . ChemSusChem. 2022;15:e202201298. doi: 10.1002/cssc.202201298. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Jia J., Zhi Y., Ma S., Liu X.. Porous organic polymers for light-driven organic transformations. Chem. Soc. Rev. 2022;51:2444–2490. doi: 10.1039/D1CS00808K. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang R., Lu C., Ma C., Wang F., Yang G., Zhang Y., Nie J.. Visible Light Induced Synthesis of 2-Benzoxazolecarboxamides Promoted by a Conjugated Microporous Polymer. ACS Sustain. Chem. Eng. 2024;12:16239–16248. doi: 10.1021/acssuschemeng.4c05275. [DOI] [Google Scholar]
- Li Z., Liu Z., Li H., Hasan M., Suwansoontorn A., Du G., Wang D., Zhang Y., Nagao Y.. Sulfonated Triazine-Based Porous Organic Polymers for Excellent Proton Conductivity. ACS Appl. Polym. Mater. 2020;2:3267–3273. doi: 10.1021/acsapm.0c00425. [DOI] [Google Scholar]
- Tamura M., Kishi R., Nakayama A., Nakagawa Y., Hasegawa J. Y., Tomishige K.. Formation of a New, Strongly Basic Nitrogen Anion by Metal Oxide Modification. J. Am. Chem. Soc. 2017;139:11857–11867. doi: 10.1021/jacs.7b05227. [DOI] [PubMed] [Google Scholar]
- Tang X., Ma N., Xu H., Zhang H., Zhang Q., Cai L., Otake K. I., Yin P., Kitagawa S., Horike S., Gu C.. Construction of unimpeded proton-conducting pathways in solution-processed nanoporous polymer membranes. Mater. Horiz. 2021;8:3088–3095. doi: 10.1039/D1MH01147B. [DOI] [PubMed] [Google Scholar]
- Xu H., Tao S., Jiang D.. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 2016;15:722–726. doi: 10.1038/nmat4611. [DOI] [PubMed] [Google Scholar]
- Tao S., Jiang D.. Exceptional Anhydrous Proton Conduction in Covalent Organic Frameworks. J. Am. Chem. Soc. 2024;146:18151–18160. doi: 10.1021/jacs.4c06049. [DOI] [PubMed] [Google Scholar]
- Guan L., Guo Z., Zhou Q., Zhang J., Cheng C., Wang S., Zhu X., Dai S., Jin S.. A highly proton conductive perfluorinated covalent triazine framework via low-temperature synthesis. Nat. Commun. 2023;14:8114. doi: 10.1038/s41467-023-43829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Zou W., Ou Z., Zhang L., Zhang W., Wang X., Song H., Cui Z., Liang Z., Du L.. Tuning the Interlayer Interactions of 2D Covalent Organic Frameworks Enables an Ultrastable Platform for Anhydrous Proton Transport. Angew. Chem., Int. Ed. 2022;61:e202208086. doi: 10.1002/anie.202208086. [DOI] [PubMed] [Google Scholar]
- Luo H., Yang F., Li C., Zhong Y., Cheng C., Wang S., Jin S.. Polybenzimidazole-based polymers of intrinsic microporosity membrane for high-temperature proton conduction. Chem. Eng. J. 2023;476:146611. doi: 10.1016/j.cej.2023.146611. [DOI] [Google Scholar]
- Yang J., Tong L., Alsubaie A. S., Mahmoud K. H., Guo Y., Liu L., Guo L., Sun Z., Wang C.. Hybrid proton exchange membrane used in fuel cell with amino-functionalized metal–organic framework in sulfonated polyimide to construct efficient ion transport channel. Adv. Compos. Hybrid. Mater. 2022;5:834–842. doi: 10.1007/s42114-022-00469-4. [DOI] [Google Scholar]
- Zang Y., Ray S., Fung E. D., Borges A., Garner M. H., Steigerwald M. L., Solomon G. C., Patil S., Venkataraman L.. Resonant Transport in Single Diketopyrrolopyrrole Junctions. J. Am. Chem. Soc. 2018;140:13167–13170. doi: 10.1021/jacs.8b06964. [DOI] [PubMed] [Google Scholar]
- Kaiser M. R., Han Z., Wang J.. Electro-polymerized polypyrrole film for fabrication of flexible and slurry-free polypyrrole-sulfur-polypyrrole sandwich electrode for the lithium-sulfur battery. J. Power Sources. 2019;437:226925. doi: 10.1016/j.jpowsour.2019.226925. [DOI] [Google Scholar]
- Xu H., Wang F. J., Xin M., Zhang Z.. I2-Promoted Condensation/Cyclization of Aryl Methyl Ketones with Anilines for Facile Synthesis of 1,2,4-Triarylpyrroles. Eur. J. Org Chem. 2016;2016:925–929. doi: 10.1002/ejoc.201501477. [DOI] [Google Scholar]
- Mane A. T., Navale S. T., Patil V. B.. Room temperature NO2 gas sensing properties of DBSA doped PPy–WO3 hybrid nanocomposite sensor. Org. Electron. 2015;19:15–25. doi: 10.1016/j.orgel.2015.01.018. [DOI] [Google Scholar]
- Li F., Li H., Jiang H., Zhang K., Chang K., Jia S., Jiang W., Shang Y., Lu W., Deng S., Chen M.. Polypyrrole nanoparticles fabricated via Triton X-100 micelles template approach and their acetone gas sensing property. Appl. Surf. Sci. 2013;280:212–218. doi: 10.1016/j.apsusc.2013.04.132. [DOI] [Google Scholar]
- Melhi S., Ding X., Liu Z. W., Cao C. X., Han B. H.. A New Strategy to Microporous Polypyrrole Networks Based on Condensation of Pyrrole and Diketone. Macromol. Chem. Phys. 2016;217:1529–1533. doi: 10.1002/macp.201600119. [DOI] [Google Scholar]
- Jia Q., Ma X., Chen H., Li X., Huang M. H.. Unusual 3,4-Oxidative Coupling Polymerization on 1,2,5-Trisubstituted Pyrroles for Novel Porous Organic Polymers. ACS Macro Lett. 2023;12:1358–1364. doi: 10.1021/acsmacrolett.3c00439. [DOI] [PubMed] [Google Scholar]
- Chang J. N., Shi J. W., Li Q., Li S., Wang Y. R., Chen Y., Yu F., Li S. L., Lan Y. Q.. Regulation of Redox Molecular Junctions in Covalent Organic Frameworks for H2O2 Photosynthesis Coupled with Biomass Valorization. Angew. Chem., Int. Ed. 2023;62:e202303606. doi: 10.1002/anie.202303606. [DOI] [PubMed] [Google Scholar]
- Ren X., Hou H., Liu Z., Gao F., Zheng J., Wang L., Li W., Ying P., Yang W., Wu T.. Shape-Enhanced Photocatalytic Activities of Thoroughly Mesoporous ZnO Nanofibers. Small. 2016;12:4007–4017. doi: 10.1002/smll.201600991. [DOI] [PubMed] [Google Scholar]
- Guo P., Zhao R., Zhang Z., Li J., Zhang W., Wang A., Kang T., Lian C., Guo Z., Wang J., Zhang J., Ma Y.. Droplet-Directed Anisotropic Assembly of Semifootball-Like Carbon Nanoparticles with Multimodal Pore Architectures. Adv. Funct. Mater. 2024;34:2400503. doi: 10.1002/adfm.202400503. [DOI] [Google Scholar]
- Chrzanowska A., Derylo-Marczewska A.. Mesoporous silica/protein biocomposites: Surface, topography, thermal properties. Int. J. Biol. Macromol. 2019;139:531–542. doi: 10.1016/j.ijbiomac.2019.08.025. [DOI] [PubMed] [Google Scholar]
- Li J., Jing X., Li Q., Li S., Gao X., Feng X., Wang B.. Bulk COFs and COF nanosheets for electrochemical energy storage and conversion. Chem. Soc. Rev. 2020;49:3565–3604. doi: 10.1039/D0CS00017E. [DOI] [PubMed] [Google Scholar]
- He J., Wang Y., Yuan J., Liu C., Pan C., Weng Z., Tang X., Liu Y., Yu G.. Ferrocene-integrated conjugated microporous polymer nanosheets: Active and regenerative catalysts for photomediated controlled radical polymerization. Appl. Mater. Today. 2020;18:100507. doi: 10.1016/j.apmt.2019.100507. [DOI] [Google Scholar]
- Guan L., Li Z., Wang K., Gong L., Fang Y., Yu G., Zhu M., Jin S.. Bottom-up Synthesis of Piezoelectric Covalent Triazine-based Nanotube for Hydrogen Peroxide Production from Water and Air. Angew. Chem., Int. Ed. 2024;64:e202419867. doi: 10.1002/anie.202419867. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Li C., Yang F., Guan L., Jin S.. Covalent Pyrimidine Frameworks via a Tandem Polycondensation Method for Photocatalytic Hydrogen Production and Proton Conduction. Small. 2023;19:e2204515. doi: 10.1002/smll.202204515. [DOI] [PubMed] [Google Scholar]
- Meng X., Wang H. N., Song S. Y., Zhang H. J.. Proton-conducting crystalline porous materials. Chem. Soc. Rev. 2017;46:464–480. doi: 10.1039/C6CS00528D. [DOI] [PubMed] [Google Scholar]
- Vilciauskas L., Tuckerman M. E., Bester G., Paddison S. J., Kreuer K. D.. The mechanism of proton conduction in phosphoric acid. Nat. Chem. 2012;4:461–466. doi: 10.1038/nchem.1329. [DOI] [PubMed] [Google Scholar]
- Yang Y., He X., Zhang P., Andaloussi Y. H., Zhang H., Jiang Z., Chen Y., Ma S., Cheng P., Zhang Z.. Combined Intrinsic and Extrinsic Proton Conduction in Robust Covalent Organic Frameworks for Hydrogen Fuel Cell Applications. Angew. Chem., Int. Ed. 2020;59:3678–3684. doi: 10.1002/anie.201913802. [DOI] [PubMed] [Google Scholar]
- Li J., Wang J., Wu Z., Tao S., Jiang D.. Ultrafast and Stable Proton Conduction in Polybenzimidazole Covalent Organic Frameworks via Confinement and Activation. Angew. Chem., Int. Ed. 2021;60:12918–12923. doi: 10.1002/anie.202101400. [DOI] [PubMed] [Google Scholar]
- Li S., Fried J. R., Sauer J., Colebrook J., Dudis D. S.. Computational chemistry and molecular simulations of phosphoric acid. Int. J. Quantum Chem. 2011;111:3212–3229. doi: 10.1002/qua.22702. [DOI] [Google Scholar]
- Zhang J., Kong Y.-R., Liu Y., Luo H.-B., Zou Y., Zang S.-Q., Ren X.-M.. Superprotonic Conduction of Acidified Benzimidazole-Linked Covalent Organic Framework. ACS Mater. Lett. 2022;4:2597–2603. doi: 10.1021/acsmaterialslett.2c00432. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.