Abstract
Recurrent miscarriage due to sporadic chromosomal abnormalities may simply be a consequence of the dramatic increase of trisomic conceptions with increased maternal age. However, it is also possible that some couples are at increased risk of abnormalities as a result of gonadal mosaicism, factors affecting chromosome structure and segregation, increased sperm aneuploidy in the male partner, or accelerated “aging” of the ovaries. We report cytogenetic and molecular findings from 122 spontaneous abortions (SAs) from 54 couples who were ascertained as having two or more documented aneuploid or polyploid SAs. The distribution of abnormalities in this group was similar to those from 307 SAs that involved chromosome abnormalities and were diagnosed at the same center but did not involve documented recurrent aneuploidy/polyploidy. Although recurrence of the same abnormality was observed in eight families, this number was equal to that expected by chance, indicating that gonadal mosaicism is rarely the explanation for recurrence. The origin of the abnormality was determined in 37 SAs from 23 of the couples in the study. A maternal meiotic origin was involved in 30 trisomies and in 1 triploid SA; 3 additional maternal trisomies were of possible somatic origin. A paternal origin was found in the remaining two trisomies and in one triploid SA. In addition, one double trisomy was the consequence of both a maternal and a paternal meiotic error. These results confirm that the etiology of trisomy is predominantly a result of meiotic errors related to increased maternal age, regardless of whether the couple has experienced one or multiple aneuploid SAs. Furthermore, this is true even when a second SA involves the same abnormality. Nonetheless, these data do not exclude some population variability in risk for aneuploidy.
Introduction
Approximately 15% of all clinically recognized pregnancies are spontaneously aborted before 20 weeks of gestation, and approximately half of these are attributable to detectable chromosome abnormalities (Hassold and Jacobs 1984). The most common abnormality observed is trisomy (∼30% of all losses), although sex-chromosome monosomy and polyploidy account for the majority of the remaining chromosome abnormalities found (each contributing to ∼10% of total losses). However, the frequency of specific abnormalities depends strongly on the age of the study group members, because risks of most trisomies increase dramatically with increasing age of the mother, whereas sex-chromosome monosomy and polyploidy do not (Hassold and Chiu 1985) . From the latter study, it is estimated that >40% of clinically detected pregnancies end in spontaneous abortion (SA) among women >40 years of age, with autosomal trisomy accounting for >60% of the total SAs in this group.
Increasing age is the overwhelming risk factor for trisomy, and any evidence of greater recurrence risk of a trisomic SA after a first trisomic SA is generally found to be weak (Morton et al. 1987) or nonsignificant (Warburton et al. 1987) after correction of data for differences in maternal age. Nonetheless, a variety of studies suggest the existence of risk factors in addition to maternal age, and thus trisomy risk may be considered to be a multifactorial trait, with a major factor (age) and a number of minor factors contributing to its etiology. Recently, reduced total follicular number in the ovaries has been associated with increased risk for trisomy (Freeman et al. 2000; Kline et al. 2000). An altered risk for trisomy in some individuals may also be the consequence of variants in proteins affecting DNA methylation or chromosome segregation during meiosis. For example, polymorphisms in genes involved in folic acid metabolism, as well as differences in folic acid intake, are possible maternal risk factors associated with Down syndrome (trisomy 21) (Hobbs et al. 2000), although they were not found to be increased among women who had SAs involving trisomy (Hassold et al. 2001). Mutations in hMSH2, a mismatch repair gene, have been associated with a significant increase in chromosomally abnormal sperm (Martin et al. 2000). Environmental factors, such as caffeine intake, have also been implicated as modifying risk for trisomy 21 (Torfs and Christianson 2000).
Gonadal mosaicism for a chromosome abnormality in phenotypically normal individuals can also contribute to recurrent trisomy. Reports of gonadal mosaicism have so far been limited to recurrence of trisomy 21 (Down syndrome) or trisomy 18 (Kohn and Shohat 1987; Nielsen et al. 1988; Gersdorf et al. 1990; Sachs et al. 1990; Pangalos et al. 1992; Tseng et al. 1994; Satge et al. 1996). Although it is possible that these trisomies are more likely to arise or to persist in germ cells, it is also likely that germline mosaicism for other types of abnormalities would rarely be detected, because karyotyping is not performed on most early SAs. Thus, germline mosaicism for other trisomies could occur but is unlikely to be ascertained.
Reports of an increase of chromosome abnormalities in sperm from the male partners of women experiencing recurrent miscarriage suggest that paternal factors could be important in this subgroup (Giorlandino et al. 1998; Rubio et al. 1999). An increase in sperm abnormalities has also been reported in the fathers of females with Turner syndrome (X-monosomy) (Martinez-Pasarell et al. 1999). Nonetheless, the frequency of disomic sperm is generally low (<5%) in fertile men (Shi and Martin 2000), and men with structural abnormalities that are associated with increased rate of nondisjunction, such as Robertsonian translocations, may more commonly experience reduced fertility due to oligospermia (De Braekeleer and Dao 1991).
To further evaluate the basis for recurrent SA as a consequence of chromosome abnormalities, we collected data on cytogenetic and molecular findings from couples with two or more documented aneuploid (e.g., trisomy or 45,X) or polyploid (e.g., triploidy or tetraploidy) SAs at <20 weeks of gestation). The distribution of abnormalities found and the frequency of recurrence of the same abnormality were compared with other SAs examined by karyotyping at the same center. The frequency of trisomy was also stratified on the basis of the number of previous SAs, using both the recurrent and sporadic aneuploidy/polyploidy data. The origin of the error was determined for trisomic cases, to determine whether there was an increased incidence of paternal errors, as well as to try to identify alleles not present in either parent (as can occur in some cases of gonadal mosaicism).
Methods
Case Ascertainment
SAs were ascertained (1) through screening of cytogenetic records from all karyotyped SAs for the year 2000 at British Columbia's Children’s and Women's Hospital and Health Centre (BCCWHHC) and (2) through the Recurrent Pregnancy Loss Clinic at BCCWHHC. Some of the latter cases overlap with another study on karyotype results from patients with recurrent SA (Stephenson et al. [in press]). In total, 54 couples were identified as having recurrent aneuploidy/polyploidy (see table 1). The mean number of SAs was 3.7, and the mean number of live births was 0.9 in this group; 41 couples had 2, 12 couples had 3, and 1 couple had 4 documented aneuploid or polyploid SAs. Parental karyotypes were normal in the 40 individuals for whom this information was available, with the exception of one woman who was a carrier of a balanced 15;Y translocation (details of this family have been published elsewhere [Rajcan-Separovic et al. 2001]). The mean maternal age at the time of SA with chromosomal abnormality was 37.9 years (range 19–46 years).
Table 1.
Characteristics of Study Population
| Type ofAneuploidy/Polyploidyand Ascertainmenta | No. ofCouples(No. of SAs) | Mean No. ofSAs per Couple | Mean No.of Term Birthsper Couple | Mean MaternalAge(years) |
| Recurrent: | ||||
| Cytogenetics | 17 (41) | 3.6 | 1.4 | 38.5 |
| RPL clinic | 37 (81) | 3.7 | .7 | 37.6 |
| Overall | 54 (122) | 3.7 | .9 | 37.9 |
| Single: | ||||
| Cytogenetics | 179 | 2.3 | 1.0 | 36.5 |
| RPL clinic | 128 | 4.0 | 1.1 | 34.6 |
| Overall | 307 | 3.1 | 1.0 | 35.7 |
RPL = recurrent pregnancy loss.
The distribution of abnormalities in the recurrent aneuploidy/polyploidy group was compared with that from SAs identified at the same center that occurred in couples without any documented history of other SAs with chromosomal abnormality (table 1). These cases are referred to in this article as the “single aneuploidy/polyploidy group.” There was no difference in maternal age (Student t test) or abnormalities found (χ2 contingency test), depending on the ascertainment source, although the mean number of SAs was higher among cases ascertained through the Recurrent Pregnancy Loss Clinic. Because the most common indication for karyotyping an abortus is a history of recurrent SA, most of these couples have had multiple nonkaryotyped SAs and may well have experienced other aneuploidies that were not documented. We therefore also compared distribution of abnormalities conditional on number of previous SAs.
Molecular Studies
DNA studies were performed in cases for which a surgical pathology specimen from the abortus was available. The specimens were generally samples from chorionic villi that had been frozen at −70°C at the Embryopathology Laboratory at British Columbia’s Children's Hospital. Samples of parental blood were taken after signed informed consent was obtained, according to a protocol approved by the University of British Columbia ethics review board. Material from the abortus and one or both parents was available for DNA analysis from 37 aneuploid or polyploid SAs from 23 of the couples identified as experiencing recurrent aneuploidy/polyploidy.
Methods used for determining origin of trisomy are described elsewhere (Robinson et al. 1995, 1997, 1999). In brief, parental origin of the trisomy was determined by comparing inheritance of microsatellite markers in the pathology specimen with those of the parents. The observation of a marker that amplifies three distinct alleles from trisomic tissue provides clear confirmation of a meiotic origin of the extra chromosome. We attempted to make each case informative for at least two markers before concluding a meiotic origin. Likewise, parental origin was assigned only when at least two clear informative results were present. In 16 SAs, paternal DNA was unavailable, but a maternal origin was considered likely if all typed markers (6–12 per abnormality) along the involved chromosome were compatible with transmission of two maternal alleles. Stage of meiotic origin was indicated in cases in which a marker <10 cM from the centromere was informative or in which two markers <30 cM apart bounded the centromere on either side and indicated the same origin. When many markers that completely span the chromosome pair show reduction to homozygosity, it is assumed that the extra chromosome has arisen from a postzygotic mitotic (somatic) error.
Results
Distribution of Abnormalities
In total, 96 (80%) of the 120 chromosome abnormalities identified in the recurrent aneuploidy/polyploidy group involved an autosomal trisomy. The most common abnormalities were trisomy 16 (18 cases), trisomy 15 (17 cases), triploidy (16 cases), and trisomy 21 (10 cases). There were nine cases of double trisomy plus one case of 46,−X,+21. When results were stratified by maternal age, contingency-table analysis showed that the frequency of trisomy was not different from that observed for the single aneuploidy/polyploidy group, even when other age groupings were used (table 2). There was an overall deficiency of monosomy in the recurrent aneuploidy/polyploidy group; however, sample sizes were too small to consider monosomy and polyploidy separately when subdivided by age. To test further for possible association, binary logistic regression (SPSS statistical package) was used. Frequencies of trisomy, monosomy, or triploidy were tested separately as outcome variables, and maternal age at SA and ascertainment source (recurrent or single aneuploidy/polyploidy group) were used as the independent variables. No effect of source was found after accounting for age effects, even when data were limited to younger subsets of women (e.g., those <35 years of age). There was an overall increase of trisomy, with a corresponding decrease in monosomy and polyploidy, in both the single and recurrent group when compared with a previously published report of cytogenetic diagnoses performed during 1978–1989 at this same center (Kalousek et al. 1993). However, mean maternal age was not reported in the latter study and seems likely to have increased over time. No significant differences in the distribution of trisomy, monosomy, and polyploidy were detected by age-stratified contingency-table analysis between the present Vancouver study and published reports of sporadic aneuploidies from Honolulu (Hassold and Chiu 1985) (data not shown).
Table 2.
Observed Distribution of Abnormalities in Chromosomally Abnormal SAs from the Recurrent and Single Aneuploidy/Polyploidy Groups
|
No. (Frequency) of SAs Involving |
|||
| Maternal Ageand Group(No. of Couples) | Monosomya | Polyploidy | Trisomyb |
| <30 years: | |||
| Recurrent (4) | 0 | 1 (.25) | 3 (.75) |
| Single (42) | 10 (.24) | 12 (.29) | 20 (.47) |
| 30–34 years: | |||
| Recurrent (16) | 2 (.12) | 4 (.25) | 10 (.63) |
| Single (74) | 11 (.15) | 27 (.36) | 36 (.49) |
| 35–39 years: | |||
| Recurrent (53) | 5 (.10) | 6 (.11) | 42 (.79) |
| Single (114) | 10 (.09) | 19 (.17) | 85 (.75) |
| ⩾40 years: | |||
| Recurrent (49) | 0 | 7 (.14) | 42 (.86) |
| Single (75) | 3 (.04) | 3 (.04) | 69 (.92) |
| All ages: | |||
| Recurrent (122) | 6 (.05) | 18 (.15) | 96 (.79) |
| Single (305) | 34 (.11) | 61 (.20) | 210 (.69) |
Includes three cases involving monosomy of autosomes.
Includes all single and double trisomies.
Because we did not have the power to test each individual chromosome separately in this limited data set, we categorized them according to standard cytogenetic groupings (table 3). The frequency of trisomies classified in this way appeared to correspond closely with the Vancouver single aneuploidy/polyploidy group and with published data from sporadic trisomic SAs (Hassold et al. 1984) when grouped by maternal age. There was a nonsignificant increase of trisomy 15 in the recurrent aneuploidy/polyploidy group, which was found in 17 (18%) of the 94 single autosomal trisomies and in 4 of the 9 double trisomies but was found in only 23 (11%) of 211 of the single aneuploidy/polyploidy group. There was also a greater overall increase of D-group trisomies (trisomy 13, 14, or 15) among couples with four or five SAs (30%) than among those with one or two SAs (12%) (table 4). This is most likely a chance fluctuation, because the overall distribution was not significant; however, the difference should be examined in an independent data set.
Table 3.
Distribution of Single and Recurrent Trisomies by Maternal Age
|
No. (Frequency) of Trisomies Involving Chromosomes |
||||||
| Age and Group(No. of SAs)a | 2–12 | 13–15 | 16 | 17–20 | 21,22 | XXY |
| 30–34 years: | ||||||
| Recurrentb (10) | 2 (.20) | 1 (.10) | 5 (.50) | 0 | 1 (.10) | 1 (.10) |
| Singleb (37) | 8 (.22) | 8 (.22) | 10 (.27) | 1 (.03) | 8 (.22) | 2 (.05) |
| Hassold (267) | 49 (.18) | 40 (.15) | 98 (.37) | 16 (.06) | 62 (.23) | 2 (.01) |
| 35–39 years: | ||||||
| Recurrentc (41) | 7 (.17) | 14(.34) | 9 (.22) | 0 | 11 (.27) | 0 |
| Single (80) | 8 (.10) | 21 (.26) | 21 (.26) | 10 (.13) | 20 (.25) | 0 |
| Hassold (167) | 27 (.17) | 34 (.20) | 43 (.26) | 19 (.11) | 43 (.26) | 1 (.01) |
| ⩾40 years: | ||||||
| Recurrentd (35) | 10 (.29) | 11 (.31) | 4 (.11) | 4 (.11) | 5 (.16) | 1 (.03) |
| Single (56) | 15 (.27) | 11 (.20) | 5 (.07) | 5 (.07) | 20 (.29) | 0 |
| Hassold (104) | 35 (.34) | 22 (.21) | 9 (.09) | 14 (.13) | 24 (.23) | 0 |
Data from Hassold et al. 1984 are shown for comparison.
Numbers are too small to compare.
Not significant compared with single trisomy group (χ2=7.04, df=4) or Hassold data (χ2=7.59, df=4).
Not significant compared with single trisomy group (χ2= 4.13, df=4) or Hassold data (χ2=2.1, df=4).
Table 4.
Distribution of Abnormalities (Both Single and Recurrent) Conditioned on Total Number of SAs per Couple[Note]
|
No. of SAs Involving Trisomy at |
||||||||||||
| No. ofSAs per Couple | No.ofCouples | Mean Maternal Age(years) | Polyploidy | Monosomy | All Trisomies | 2–12 | 13–15 | 16 | 17–20 | 21–22 | XXY | Double |
| 1 | 50 | 36.5 | 8 (.16) | 8 (.16) | 34 (.68) | 6 | 4 | 5 | 2 | 13 | 0 | 4 |
| 2 | 63 | 36.1 | 10 (.16) | 4 (.06) | 49 (.78) | 8 | 6 | 16 | 4 | 11 | 0 | 4 |
| 3 | 148 | 35.7 | 31 (.21) | 9 (.06) | 108 (.73) | 21 | 28 | 22 | 6 | 21 | 0 | 10 |
| 4 | 74 | 36.0 | 14 (.19) | 8 (.11) | 52 (.70) | 6 | 15 | 8 | 6 | 10 | 2 | 5 |
| 5–12 | 75 | 35.2 | 13 (.17) | 10 (.13) | 52 (.69) | 12 | 16 | 5 | 2 | 12 | 0 | 5 |
| Total | 410 | 35.8 | 76 (.19) | 39 (.10) | 295 (.72) | 53 (.13) | 69 (.17) | 56 (.14) | 20 (.05) | 67 (.16) | 2 (.005) | 28 (.07) |
Note.— No significant differences were found when frequency of monosomy, polyploidy, and trisomy (χ2=7.46, df=8, not significant) was considered or when frequency of subtypes of trisomy (χ2=23.98, df=16, P=.09) was considered. Frequency of trisomies 17–20 and 47,XXY were grouped with double trisomies because of small sample sizes.
Most of the cases in our aneuploidy/polyploidy control group had previous nonkaryotyped SAs, thereby confounding any possible difference between the recurrent and the single aneuploidy/polyploidy groups. Therefore the distribution of abnormalities was also evaluated when all the Vancouver cases (single and recurrent) were considered together and then subdivided by the total number of SAs the couple had experienced (table 4). There was no difference in mean maternal age, frequency of trisomy, or distribution of abnormalities, regardless of whether the couple had experienced only one SA or multiple SAs.
Recurrence of the Same Trisomy
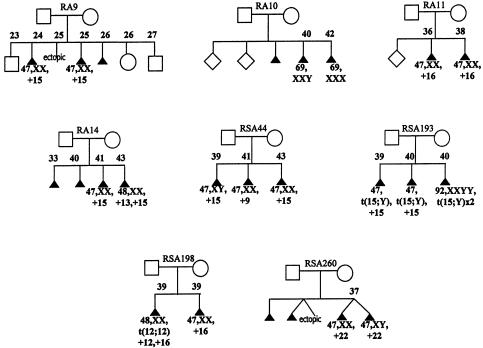
The abnormalities within any particular family generally involved different chromosomes; however, recurrence of the same trisomy (in either single or double form) did occur in eight couples (fig. 1). The abnormalities found in these families were generally not limited to these chromosomes. Four of the cases of recurrence involved trisomy 15, two involved trisomy 16, one involved trisomy 22, and one was a triploidy. In the family (RSA-260) that had two instances of trisomy 22, both instances occurred in a twin gestation after in vitro fertilization and implantation of two embryos. The discordant sex, together with molecular testing, confirmed that these two trisomy 22 embryos were the result of two distinct fertilizations. Although the molecular results were consistent with two completely different meiotic events, a somatic duplication of chromosome 22 in the germline of the mother could not be formally excluded (i.e., as discussed for a case of recurrent trisomy 21 we reported elsewhere [Bruyère et al. 2000]).
Figure 1.
Pedigrees of couples who experienced recurrence of the same abnormality. Karyotype of abortus and maternal age at the time of SA are noted when known.
To determine whether there was more recurrence than expected by chance, the expected frequency of recurrence of each abnormality encountered was calculated using the frequency observed in our total (recurrent and single aneuploidy/polyploidy groups) Vancouver data (table 5). Because we were considering each abnormality within a double trisomy as a separate occurrence for the purpose of saying an abnormality had “recurred,” we had to correct figures for this. For example, trisomy 15 occurred in 49 (10.7%) of 459 of abnormalities when each trisomy in a double trisomy was considered as a separate occurrence. The overall expected recurrence then equals the sum of the expected recurrence for the 38 couples with two aneuploidies, 10 with three aneuploidies, 5 with four aneuploidies, and 1 with five aneuploidies. It was therefore expected that trisomy 15 would occur in about one family:
Table 5.
Recurrence of the Same Abnormality within Each Family
| Abnormality | CorrectedFrequency(%)a | ExpectedRecurrenceb | ObservedRecurrence |
| 45,X | 8.1 | .66 | 0 |
| Triploidy | 14.2 | 1.95 | 1 |
| Trisomy 15 | 10.7 | 1.09 | 4 |
| Trisomy 16 | 14.6 | 1.98 | 2 |
| Trisomy 21 | 8.9 | .77 | 0 |
| Trisomy 22 | 8.7 | .73 | 1 |
| All others | … | .88 | 0 |
| Total | … | 8.09 | 8 |
Counting each trisomy from a double trisomy separately.
Based on the family structure in this data set.
Expected recurrence of trisomy 15 was
 |
Likewise, trisomy 16 would be expected to recur, on average, in approximately two couples from this data set, and this is what was observed.
Overall recurrence of the same abnormality occurred as often as expected by chance alone (table 5). Nonetheless, it is of interest that two SAs involving trisomy 15 occurred at the relatively young maternal ages of 24 and 25 years in one family (RA-9). An additional abortus from this woman was not karyotyped. No specimens were saved for molecular studies, so the possibility of gonadal mosaicism could not be investigated. Another interesting case, which has been reported elsewhere, involved a woman who carried a 15;Y translocation and had two SAs with trisomy 15 and one SA with tetraploidy (Rajcan-Separovic et al. 2001). It seems possible that the 15;Y translocation increased the risk of chromosome 15 nondisjunction, although increased maternal age likely also contributed to the SAs in this woman.
Clustering of Abnormality Types
To further determine whether certain types of abnormalities cluster within a family, the karyotype of subsequent SAs with chromosomal abnormality was evaluated, conditional on the karyotype of the first abortus (table 6). Classification of abnormalities into five categories (trisomies for acrocentric chromosomes, trisomy 16, other trisomies, monosomy, and polyploidy) revealed no obvious clustering. There were more cases of monosomy or polyploidy occurring subsequent to an SA involving trisomy (14 cases) than before an SA involving trisomy (3 cases), despite the fact that both monosomy and polyploidy are expected to become less common (relative to trisomy) with increased maternal age. Because we ascertained cases specifically on the presence of two or more chromosomally abnormal SAs, we could not compare recurrences on the basis of whether the first abortus was chromosomally normal or abnormal.
Table 6.
Karyotype of All Subsequent Aneuploid/Polyploid (Abnormal) SAs Conditional on the Karyotype of the First Abnormal SA
|
No. (Expected Frequency) of Subsequent Abnormal SAs Involvinga |
|||||
| Karyotype ofFirst Abnormal SA | Trisomy 13–15, 21, 22 | Trisomy 16 | Other Trisomyb | Monosomy | Polyploidy |
| Trisomy 13–15, 21, 22 | 19 (15.1) | 2 (4.1) | 8 (7.6) | 1 (1.8) | 6 (4.4) |
| Trisomy 16 | 2 (4.1) | 1 (1.1) | 4 (2.1) | 2 (.5) | 2 (1.1) |
| Other trisomy | 8 (7.6) | 2 (2.1) | 4 (3.8) | 0 (.9) | 3 (2.2) |
| Monosomy | 0 (1.8) | 1 (.5) | 0 (.9) | 0 (.2) | 2 (.5) |
| Polyploidy | 1 (4.4) | 1 (1.2) | 0 (2.2) | 2 (.5) | 1 (1.3) |
Expected frequencies, based on the overall frequency of each abnormality, are given in parentheses.
Double trisomies were included as “other” unless both involved chromosomes were acrocentric.
Origin of Trisomy
Cases for which the origin of the abnormality could be determined are listed in table 7. Of 35 cases of trisomy, the error was of maternal origin in 32, of paternal origin in 2, and of both maternal and paternal origin in 1. One of the maternally derived trisomies occurred in the same pregnancy with a monosomy-X, which was a consequence of a missing maternal X chromosome. Molecular results from two of the maternal trisomies, involving chromosomes 5 and 16, were consistent with a postzygotic somatic origin of the trisomy. Such results, however, do not exclude a meiotic origin (i.e., a meiosis II error following no recombination between the involved chromosomes). Three previous cases of trisomy 5 from sporadic SAs have been analyzed and reported elsewhere, and all were also consistent with a somatic origin (Robinson et al. 1999). One case of triploidy (71,XXXY,+14) consisted of two maternal contributions and only one paternal contribution (i.e., the molecular results showed two different maternal and one paternal allele, except for X-linked loci where only maternal alleles were found). Another case of triploidy showed the presence of two different paternal alleles at multiple loci, an observation that was consistent with either fertilization by a diploid sperm cell (resulting from a division error during meiosis phase I) or fertilization by two different sperm cells.
Table 7.
Origin of the Abnormality in SAs from Couples with >1 Aneuploid or Polyploid SA
|
Characteristics of Studied SA |
|||||
| Family | Pregnancy Historya | Karyotype | Maternal Age(years) | ParentalOriginb | MeioticOriginc |
| RSA-1 | SA, SA, SA (47,XY,+7), SA (48,XX,+15,+16), SA (69,XXY) | 47,XY,+7 | 33 | Pat | M |
| RSA-10 | T, SA (47,XX,+15), SA, SA (47,XY,+22), T | 47,XX,+15 | 39 | Mat | M I |
| 47,XY,+22 | 41 | Mat | M | ||
| RSA-109 | SA (47,XX,+2), SA (46,XY), SA (47,XY+15), T | 47,XY+15 | 42 | Mat | M I |
| RSA-110 | SA (47,XX,+10), SA (47,XX+16), E | 47,XX,+10 | 34 | Mat | M I |
| 47,XX,+16 | 34 | Mat | Som | ||
| RSA-133 | T, SA, SA(47,XY,+14), SA(47,XY,+15), SA, SA(48,XY,+16,+18) | 47, XY+15 | 41 | Mat | M I |
| RSA-158 | T, SA, SA(47,XX,+16), SA, SA(47,XX,+9) | 47,XX,+9 | 39 | Mat | M I or II |
| RSA-189 | TA, T, SA (47, XY,+16), SA, SA, SA(46,X,+21), SA (47,XX,+16) | 47,XY,+14 | 41 | Mat | M I or II |
| 46,X,+21 | 42 | Mat (−X), Mat (21) | M I (21) | ||
| RSA-192 | TA, SA (47,XY,+8), SA, SA (71,XXXY,+14) | 71,XXXY,+14 | 38 | Mat | M I or II |
| 47,XY,+8 | 40 | Mat | M I or II | ||
| RSA-193 | T, SA (47,XY,+der(15) t(Y;15)), SA (47,XX,+der(15) t(Y;15), SA (92,XXYY) | 47,XY,+der(15) | 39 | Mat | M I |
| 47,XX,+der(15) | 40 | Mat | M I | ||
| RSA-198 | SA (48,XX+12,t(12;12)+16), SA (47,XY,+16) | 47,XY,+16 | 39 | Mat | M I |
| 48,XX,+12, t(12;12)+16 | 39 | Mat (16), Mat (12) | M I (16), Som (12) | ||
| RSA-218 | T, SA, SA (46,XX), SA (47,+17), SA (47,+15), SA (47,+16) | 47,+17 | 40 | Mat | M I |
| 47,+15 | 41 | Mat | M I | ||
| 47,+16 | 41 | Mat | M I | ||
| RSA-225 | P, T, TA, SA (47,XX,+16), SA, SA (47,XX,+12) | 47,XX,+16 | 37 | Mat | M I |
| RSA-251 | T, SA (46,XX), SA (47,XX,+4), SA (47,XY,+13) | 47,XX+4 | 35 | Mat | M I or II |
| RSA-256 | SA (47,XY,+16), T, SA (46,XX,i21q) | 47,XY,+16 | 32 | Mat | M I |
| 35 | Mat | Som | |||
| RSA-260 | SA, SA+E, SAx2(47,XY,+22;47,XX,+22) | 47,XX,+22 | 37 | Mat | M I or II |
| 47,XY,+22 | 37 | Mat | M I or II | ||
| RSA-263 | SA (+22), T (+18), T (+21) | 47,XY,+22 | 37 | Mat | M I |
| RSA-27 | SA, SA, SA (70,XXY+2), SA(46,XY and 47,XY+15) | 47,XY,+15 | 38 | Mat | M I |
| RSA-37 | T, T, SA, SA, SA (47,XY,+13), SA (47,XX,+22), T, SA (48,+14,+21), SA (47,+4) | 47,XY,+13 | 39 | Mat | M I or II |
| 47,XX,+22 | 40 | Mat | M I or II | ||
| 48,+14,+21 | 42 | Pat (14), Mat (21) | M I or II (14), M I (21) | ||
| 47,+4 | 42 | Pat | M I or II | ||
| RSA-51 | T, SA, SA (47,XY,+16), SA (47,+20), T | 47,XY,+16 | 38 | Mat | M I |
| RSA-63 | T, SA, SA, SA (47,XY,+16), SA (47,XX,+20), T | 47,XY,+16 | 40 | Mat | M I |
| RSA-66 | TA, SA, SA (70XXY,+19), SA (47,XX,+22) | 70,XXY,+19 | 34 | Pat | M I or DS |
| 47,XX,+22 | 35 | Mat | M I or II | ||
| T10-2 | SA (47,XY+10), SA, SA, T, SA (47,+21) | 47,XY,+10 | 39 | Mat | M I or II |
| T5-2 | SA, SA, SA, SA (47,XY+15), SA (47,XY,+5), SA, T | 47,XY,+5 | 35 | Mat | Som |
| T9-2 | TA, TA, SA (47,XY,+7), SA(71,XXY,+10,+11), SA(47,XY+9) | 47,XY,+9 | 40 | Mat | M I or II |
Karyotyped SAs are shown in parentheses. TA = therapeutic abortion; E = ectopic pregnancy; P = preterm live birth; T = term birth.
Pat = paternal; Mat = maternal.
M = meiosis; Som = somatic; DS = dispermy.
Discussion
Counseling a couple that has experienced multiple SAs with chromosomal abnormality is difficult, because the more losses the couple has experienced, the less likely either the involved couple or the physician will feel comfortable attributing the SAs to just “bad luck.” Thus, despite the fact that previous studies have failed to identify any clear evidence for age-independent predisposition to chromosomally abnormal SAs (Morton et al. 1987; Warburton et al. 1987; Hassold et al. 1996), we thought it was worth re-examining the etiology of abnormalities occurring specifically in couples with multiple chromosomally abnormal SAs. Overall, the present results are consistent with the hypothesis that SAs associated with numerical chromosome abnormalities are largely a consequence of apparently sporadic segregation errors that increase in occurrence with increased maternal age. The reasons for this conclusion are (1) the types of abnormalities found are similar to those found in single SAs (and historical data on sporadic SAs) when analyses are corrected for maternal age; (2) almost all trisomies arose in maternal meiosis; (3) there was not an increased recurrence of the same abnormality within a family; and (4) the mean maternal age of 38 years was high, suggesting that increased maternal age was the major predisposing factor.
Although there have been previous reports of an increase in chromosomal abnormalities in the sperm from male partners of couples experiencing recurrent pregnancy loss (Giorlandino et al. 1998; Rubio et al. 1999), the present data suggest that sperm chromosome abnormalities do not play a major role in recurrent aneuploidy/polyploidy. Only 2 of 37 abnormal SAs analyzed could be attributed solely to an error present in the sperm. The frequency of paternal errors depends on the abnormality but, overall, should account for ∼7% of meiotic-origin trisomies encountered among SAs (Robinson et al. 1999). One case (family RSA-1 [table 7]) involved a paternally derived trisomy 7. Of 27 cases of trisomy 7 analyzed and reported elsewhere, 10 were considered to be of possible “somatic origin,” and only 1 was of paternal meiotic origin (Robinson et al. 1999). The latter case was, in fact, identical to the present case, because (despite distinct ascertainment methods) a few of the present cases overlapped with those of a previous study from our laboratory on the origin of trisomy (Robinson et al. 1999). Unfortunately, specimens were not available from the two other abnormal SAs that had occurred in this couple, and we were therefore unable to determine whether all were due to paternal errors. Paternal origin seems unlikely, however, since one of the errors was a double trisomy that included trisomy 16, which is almost always maternal in origin (Hassold et al. 1995), and the other was a triploidy, which is also almost always a problem of the oocyte (either a meiotic division error in the egg or fertilization of the egg by two sperm) (McFadden and Langlois 2000; Zaragoza et al. 2000). The other two paternal errors occurred in the same family (RSA-37 [table 7]). Two other SAs from this same couple could be attributed to maternal meiotic errors that lead to trisomy 13 and trisomy 22. In addition, one of the paternal errors (trisomy 14) occurred as a double trisomy together with a trisomy 21 of maternal origin. Although it is interesting to speculate that there could be an increased rate of sperm abnormalities in the male partner, aneuploidies related to maternal age probably still accounted for the majority of the SAs, even in this family.
Although recurrent aneuploidy/polyploidy is largely a maternal age–related phenomenon, it is still possible that, in rare cases, couples experience recurrent aneuploidy/polyploidy as a consequence of gonadal mosaicism or another maternal age–independent problem. In the present data set, only two women were <31 years of age at the time of any of their aneuploid SAs. One mother had experienced four SAs before she was 20 years old, with losses occurring at 6 weeks of gestation (no karyotype), 12 weeks (no karyotype), 6 weeks (47,XX,+13), and 11 weeks (69,XXX). Another woman (family RA-9 [fig. 1]) had a pregnancy history that could be attributed to gonadal mosaicism. Her history included a term birth (at age 23 years), trisomy 15 SA at 8 weeks of gestation (at age 24 years), ectopic pregnancy (at age 25 years), trisomy 15 SA at 6 weeks of gestation (at age 25 years), SA (no karyotype) at 8 weeks of gestation (at age 26 years), preterm birth at 22 weeks of gestation (at age 26 years), and, finally, a term birth (at age 27 years). Possibly, these are exceptionally high-risk cases. The lack of difference between the abnormalities encountered in recurrent aneuploidy and those in sporadic aneuploidy does not exclude the possibility that a woman who experiences an SA involving aneuploidy, particularly at a young age, is at higher risk than a woman who never experiences an aneuploid SA. Nonetheless, it is clear that recurrence of the same trisomy in one family is rarely a result of gonadal mosaicism, because the number of observed recurrences was equal to that expected by chance alone. As yet there is no evidence of gonadal mosaicism in the etiology of recurrent SA involving any chromosome except trisomy 18 or trisomy 21. Trisomic cells may occasionally persist in the ovaries in individuals born in association with trisomy mosaicism that is confined to the placenta (Stavropoulos et al. 1998). However, it is quite possible that such aneuploid cells undergo atresia at a higher rate than do normal diploid oocytes and thus do not directly contribute to genetic errors in that individual's offspring.
Although there appears to be little to distinguish the etiology of recurrent aneuploidy/polyploidy from that of sporadic SAs, it seems unlikely that all women are at equal risk for chromosomally abnormal SAs. Given the suggestion that some women may have ovaries that are slightly more “aged” than expected for their chronological age, it is also of interest to note that slightly more trisomies were observed in the recurrent than in the single aneuploidy/polyploidy group for women aged ⩽35 years (67% vs. 50%). Follicular number declines dramatically with increased maternal age and has been linked with trisomy risk, as well as being known as the ultimate trigger of menopause (Freeman et al. 2000; Kline et al. 2000). The fact that menopause typically occurs anywhere between 40 and 60 years of age is evidence that ovarian aging may occur at different rates in different individuals or that individuals begin life with significant differences in their ovarian reserve. Although the women in the present study are generally not having difficulties attaining pregnancy, a decline in fertility due strictly to aging of the ovaries (e.g., the depletion of ovarian follicles leading to menopause) may occur subsequent to an increase in trisomy risk. Furthermore, a large proportion of chromosomally abnormal embryos are believed to be lost before clinical detection of pregnancy, and variability could exist in the rate of preclinical versus postclinical loss of chromosomally abnormal embryos among different women. Thus, the reduced oocyte quality that occurs with increasing age may manifest as reduced fertility in some women and as more frequent occurrence of SA in others.
Many other factors could also lead to an increased risk for a trisomy in some women, without affecting etiology as assessed by the present study. Accelerated aging of oocytes could occur as a consequence of accumulation of mitochondrial mutations (Schon et al. 2000), changes in the meiotic spindle formation (Battaglia et al. 1996), or loss of replication control (Amiel et al. 2000). Centromeres and telomeres have long been thought to play important roles in mediating early pairing (Walker and Hawley 2000), and genetic variation in centromeric proteins or other proteins involved in chromatin structure could affect segregation of chromosomes.
In summary, the relatively high number of mean SAs per couple in the present study should have increased the ability to detect some etiological differences between single and recurrent aneuploid SAs if they do, in fact, exist. However, neither statistical analysis of the distribution nor molecular analysis of the origin of trisomies revealed anything unique about couples who experience recurrent aneuploidy/polyploidy. In addition, the recurrence of the same aneuploidy/polyploidy within a couple appeared generally to be the result of chance and should not be considered as strong evidence for gonadal mosaicism. Although these findings may be reassuring to couples experiencing recurrent pregnancy loss as a consequence of aneuploidy/polyploidy, it is important to point out that the findings address general etiology only and do not directly address recurrence risk.
Acknowledgments
This research was supported by Canadian Institutes of Health Research grant 15667. We would like to thank Ruby (Hong) Jiang and Laura-Jane Henderson, for technical assistance in the laboratory, Jennifer Oakes, for help in patient ascertainment, and Paul Yong, for help with logistic regression analysis and comments on the manuscript. We would also like to thank the families who kindly consented to this study.
References
- Amiel A, Reish O, Gaber E, Kedar I, Diukman R, Fejgin M (2000) Replication asynchrony increases in women at risk for aneuploid offspring. Chromosome Res 8:141–150 [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Goodwin P, Klein NA, Soules MR (1996) Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 11:2217–2222 [DOI] [PubMed] [Google Scholar]
- Bruyère H, Rupps R, Kuchinka B, Friedman JM, Robinson WP (2000) Recurrent trisomy 21 in a couple with a child presenting trisomy 21 mosaicism and maternal uniparental disomy for chromosome 21 in the euploid cell line. Am J Med Genet 94:35–41 [DOI] [PubMed] [Google Scholar]
- De Braekeleer M, Dao TN (1991) Cytogenetic studies in male infertility: a review. Hum Reprod 6:245–250 [PubMed] [Google Scholar]
- Freeman SB, Yang Q, Allran K, Taft LF, Sherman SL (2000) Women with a reduced ovarian complement may have an increased risk for a child with Down syndrome. Am J Hum Genet 66:1680–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersdorf E, Utermann B, Utermann G (1990) Trisomy 18 mosaicism in an adult woman with normal intelligence and history of miscarriage. Hum Genet 84:298–299 [DOI] [PubMed] [Google Scholar]
- Giorlandino C, Calugi G, Iaconianni L, Santoro ML, Lippa A (1998) Spermatozoa with chromosomal abnormalities may result in a higher rate of recurrent abortion. Fertil Steril 70:576–577 [DOI] [PubMed] [Google Scholar]
- Hassold T, Abruzzo M, Adkins K, Griffin D, Merrill M, Millie E, Saker D, Shen S, Zargoza M (1996) Human aneuploidy: incidence, origin and etiology. Environ Mol Mutagen 28:167–175 [DOI] [PubMed] [Google Scholar]
- Hassold TJ, Burrage LC, Chan ER, Judis LM, Schwartz S, James SJ, Jacobs PA, Thomas NS (2001) Maternal folate polymorphisms and the etiology of human nondisjunction. Am J Hum Genet 69:434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Chiu D (1985) Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 70:11–17 [DOI] [PubMed] [Google Scholar]
- Hassold T, Jacobs P (1984) Trisomy in man. Ann Rev Genet 18:69–97 [DOI] [PubMed] [Google Scholar]
- Hassold T, Merrill M, Adkins K, Freeman S, Sherman S (1995) Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am J Hum Genet 57:867–874 [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Warburton D, Kline J, Stein Z (1984) The relationship of maternal age and trisomy among trisomic spontaneous abortions. Am J Hum Genet 36:1349–1356 [PMC free article] [PubMed] [Google Scholar]
- Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ, Pogribna M, Rozen R, James SJ (2000) Polymorphisms in genes involved in folate metabolism as maternal risk factors in Down syndrome. Am J Hum Genet 67:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek DK, Pantzar T, Tsai M, Paradice B (1993) Early spontaneous abortion: morphologic and karyotypic findings in 3,912 cases. Birth Defects Orig Artic Ser 29:53–61 [PubMed] [Google Scholar]
- Kline J, Kinney A, Levin B, Warburton D (2000) Trisomic pregnancy and earlier age at menopause. Am J Hum Genet 67:395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn G, Shohat M (1987) Trisomy 18 mosaicism in an adult with normal intelligence. Am J Med Genet 26:929–931 [DOI] [PubMed] [Google Scholar]
- Martin RH, Green J, Ko E, Barclay L, Rademaker AW (2000) Analysis of aneuploidy frequencies in sperm from patients with hereditary nonpolyposis colon cancer and an hMSH2 mutation. Am J Hum Genet 66:1149–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pasarell O, Nogues C, Bosch M, Egozcue J, Templado C (1999) Analysis of sex chromosome aneuploidy in sperm from fathers of Turner syndrome patients. Hum Genet 104:345–349 [DOI] [PubMed] [Google Scholar]
- McFadden DE, Langlois SL (2000) Parental and meiotic origin of triploidy in the fetal and embryonic period. Clin Genet 58:192–200 [DOI] [PubMed] [Google Scholar]
- Morton NE, Chiu D, Holland C, Jacobs PA, Pettay D (1987) Chromosome anomalies as predictors of recurrence risk for spontaneous abortion. Am J Med Genet 28:353–360 [DOI] [PubMed] [Google Scholar]
- Nielsen KG, Poulsen H, Mikkelsen M, Steuber E (1988) Multiple recurrence of trisomy 21 Down syndrome. Hum Genet 78:103–105 [DOI] [PubMed] [Google Scholar]
- Pangalos CG, Talbot CC Jr, Lewis JG, Adelsberger PA, Petersen MB, Serre JL, Rethore MO, de Blois MC, Parent P, Schinzel A, Binkert F, Boue J, Corbin E, Croquette MF, Gilgenkrantz S, de Grouchy J, Bertheas MG, Prieur M, Raoul O, Serville F, Siffroi JP, Thepot F, Lejeune J, Antonarakis SE (1992) DNA polymorphism analysis in families with recurrence of free trisomy 21. Am J Hum Genet 51:1015–1027 [PMC free article] [PubMed] [Google Scholar]
- Rajcan-Separovic E, Robinson WP, Stephenson M, Pantzar T, Arbour L, McFadden D, Guscott J (2001) Recurrent trisomy 15 in a female carrier of der(15)t(Y;15)(q12;p13). Am J Med Genet 99:320–324 [DOI] [PubMed] [Google Scholar]
- Robinson WP, Barrett IJ, Bernard L, Bernasconi F, Wilson RD, Best R, Howard-Peebles PN, et al (1997) A meiotic origin of trisomy in confined placental mosaicism is correlated with presence of fetal uniparental disomy, high levels of trisomy in trophoblast, and increased risk of fetal IUGR. Am J Hum Genet 60:917–927 [PMC free article] [PubMed] [Google Scholar]
- Robinson WP, Bernasconi-Quadroni F, Lau A, McFadden DE (1999) Origin of trisomy: effect of ascertainment. Am J Med Genet 84:34–42 [PubMed] [Google Scholar]
- Robinson WP, Binkert F, Bernasconi F, Lorda-Sanchez I, Werder EA, Schinzel AA (1995) Molecular studies of chromosomal mosaicism: relative frequency of chromosome gain or loss and possible role of cell selection. Am J Hum Genet 56:444–451 [PMC free article] [PubMed] [Google Scholar]
- Rubio C, Simon C, Blanco J, Vidal F, Minguez Y, Egozcue J, Crespo J, Remohi J, Pellier A (1999) Implications of sperm chromosome abnormalities in recurrent miscarriage. J Assist Reprod Genet 16:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs ES, Jahoda MG, Los FJ, Pijpers L, Wladimiroff JW (1990) Trisomy 21 mosaicism in gonads with unexpectedly high recurrence risks. Am J Med Genet Suppl 7:186–188 [DOI] [PubMed] [Google Scholar]
- Satge D, Geneix A, Goburdhun J, Lasne-Desmet P, Rosenthal C, Arnaud R, Malet P (1996) A history of miscarriages and mild prognathism as possible mode of presentation of mosaic trisomy 18 in women. Clin Genet 50:470–473 [DOI] [PubMed] [Google Scholar]
- Schon EA, Kim SH, Ferreira JC, Magalhaes P, Grace M, Warburton D, Gross SJ (2000) Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod Suppl 2 15:160–172 [DOI] [PubMed] [Google Scholar]
- Shi Q, Martin RH (2000) Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet Cell Genet 90:219–226 [DOI] [PubMed] [Google Scholar]
- Stavropoulos DJ, Bick D, Kalousek DK (1998) Molecular cytogenetic detection of confined gonadal mosaicism in a conceptus with trisomy 16 placental mosaicism. Am J Hum Genet 63:1912–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case control study. Hum Reprod (in press) [DOI] [PubMed] [Google Scholar]
- Torfs CP, Christianson RE (2000) Effect of maternal smoking and coffee consumption on the risk of having a recognized Down syndrome pregnancy. Am J Epidemiol 152:1185–1191 [DOI] [PubMed] [Google Scholar]
- Tseng LH, Chuang SM, Lee TY, Ko TM (1994) Recurrent Down's syndrome due to maternal ovarian trisomy 21 mosaicism. Arch Gynecol Obstet 255:213–216 [DOI] [PubMed] [Google Scholar]
- Walker MY, Hawley RS (2000) Hanging on to your homolog: the roles of pairing, synapsis and recombination in the maintenance of homolog adhesion. Chromosoma 109:3–9 [DOI] [PubMed] [Google Scholar]
- Warburton D, Kline J, Stein Z, Wutzler M, Chin A, Hassold T (1987) Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped spontaneous abortions. Am J Hum Genet 41:465–483 [PMC free article] [PubMed] [Google Scholar]
- Zaragoza MV, Surti U, Redline RW, Millie E, Chakravarti A, Hassold TJ (2000) Parental origin and phenotype of triploidy in spontaneous abortions: predominance of diandry and association with the partial hydatidiform mole. Am J Hum Genet 66:1807–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]