Abstract
Huntington disease (HD) is a common autosomal dominant neurodegenerative disease with early adult–onset motor abnormalities and dementia. Many studies of HD show that huntingtin (CAG)n repeat–expansion length is a sensitive and specific marker for HD. However, there are a significant number of examples of HD in the absence of a huntingtin (CAG)n expansion, suggesting that mutations in other genes can provoke HD-like disorders. The identification of genes responsible for these “phenocopies” may greatly improve the reliability of genetic screens for HD and may provide further insight into neurodegenerative disease. We have examined an HD phenocopy pedigree with linkage to chromosome 20p12 for mutations in the prion protein (PrP) gene (PRNP). This reveals that affected individuals are heterozygous for a 192-nucleotide (nt) insertion within the PrP coding region, which encodes an expanded PrP with eight extra octapeptide repeats. This reveals that this HD phenocopy is, in fact, a familial prion disease and that PrP repeat-expansion mutations can provoke an HD “genocopy.” PrP repeat expansions are well characterized and provoke early-onset, slowly progressive atypical prion diseases with an autosomal dominant pattern of inheritance and a remarkable range of clinical features, many of which overlap with those of HD. This observation raises the possibility that an unknown number of HD phenocopies are, in fact, familial prion diseases and argues that clinicians should consider screening for PrP mutations in individuals with HD-like diseases in which the characteristic HD (CAG)n repeat expansions are absent.
Huntington disease (HD [MIM 143100]) is an autosomal dominant neurodegenerative disease characterized by chorea, rigidity, and progressive dementia. A worldwide study of HD revealed (CAG)n repeat–expansion length in the huntingtin protein (GenBank accession number AAB38240) to be a sensitive and specific marker for the disease (Kremer et al. 1994). However, at least one study has reported cases of HD without CAG expansions (Andrew et al. 1994), suggesting the presence of HD phenocopies (MIM 603218). The possibility that mutations in other genes are capable of provoking an illness similar to HD has important implications for genetic testing.
Investigation of a pedigree, affected with an HD phenocopy, that lacked the characteristic HD (CAG)n expansion revealed autosomal dominant inheritance and linkage (LOD 3.01) to a 2.7-cM interval on chromosome 20p12 (Xiang et al. 1998). This region encodes the prion protein (PrP [MIM 176640; GenBank accession number BAA00011]) and contains a number of other plausible candidate genes, which were screened and were initially found to be normal in this family. Nevertheless, this region remains a candidate locus for an HD-like disease, and it has been suggested that studies involving HD phenocopies should examine this region for linkage (Lesperance and Burmeister 2000).
The recent discovery of a PrP-like gene, doppel or Prnd (MIM 604263; GenBank accession number NP_036541]), 16 kb downstream of the PrP gene in mice (Prnp) and humans (PRNP) (Moore et al. 1999), suggested a new candidate gene for this HD phenocopy. We therefore examined this region in the family with this HD phenocopy for mutations in the PRND coding region. We also sequenced a region containing the terminal exon of PrP, together with the splice sites and 3′ UTR to screen for novel noncoding mutations in PRNP.
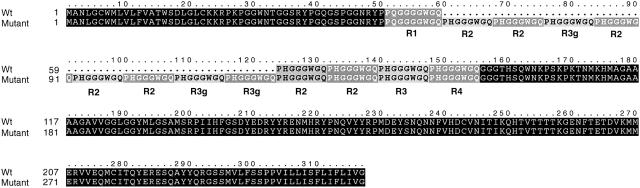
We examined both genes in four affected and one unaffected members of the family. The PRND open reading frame (ORF) was normal; however, analysis of the PRNP ORF revealed a 192-bp in-frame octapeptide repeat expansion in all affected family members examined. In all cases, the mutation was on an allele encoding methionine at PrP residue 129. All affected members were heterozygous, consistent with the autosomal dominant pattern of inheritance in this family. The mutation was the result of an expansion within the N-terminal repeat region of PrP. Although the wild-type PrP allele consists of five repeats between codons 51 and 91, the mutant allele is predicted to generate an elongated PrP with a total of 13 N-terminal repeats (fig. 1). These repeats have been classified into four main types, and the normal sequence is R1-R2-R2-R3-R4 (Kretzschmar et al. 1986), in which R1 encodes the nonapeptide PQGGGGWGQ and R2–R4 encode the octapeptide PHGGGWGQ. On the basis of this classification scheme, the repeats in the elongated PrP from the mutant allele had the sequence R1-R2-R2-R3g-R2-R2-R2-R3g-R3g-R2-R2-R3-R4, in which repeat R3g contains a silent nucleotide substitution.
Figure 1.
Alignment of the wild-type and mutant PrP peptide sequences from a single individual from the HD phenocopy pedigree showing the eight-repeat expansion. The expanded repeats are shown below the wild-type sequence and are numbered according to silent substitutions in the DNA sequences. Wild-type human PrP contains one PQGGGGWGQ nonapeptide (encoded by R1) and four PHGGGWGQ octapeptides (encoded by R2-R4), such that the normal sequence is R1-R2-R2-R3-R4. The mutant allele has eight extra PHGGGWGQ repeats: R1-R2-R2-R3g-R2-R2-R2-R3g-R3g-R2-R2-R3-R4. Both alleles encode methionine at codon 129.
Taken together with the original study showing positive linkage (LOD 3.01) to markers D20S193–D20S895 on chromosome 20p (Xiang et al. 1998), the PrP repeat expansion described here appears to be responsible for the HD phenocopy in this family. Indeed, PrP repeat-expansion mutations involving 2, 4, 5, 6, 7, 8, 9, and 10 extra repeats have been described in other patients with progressive dementia and ataxia (Goldfarb et al. 1991; Owen et al. 1992; Duchen et al. 1993; Krasemann et al. 1995; Gambetti et al. 1999). A mutation involving an expansion of eight extra repeats that is similar to that in the family described here, with an HD-like presentation, has been described in a large French family, referred to as family “M-E,” with “prominent psychiatric features” (Laplanche et al. 1999). Comparison with the HD phenocopy family reveals that the disease presentation is similar and that many of the apparent differences may be caused by the relative emphasis placed on psychiatric versus neurological aspects of the disease. Broadly speaking, carriers of the PrP repeat insertion in family M-E presented with a disease with early adult psychiatric or behavioral disturbance, followed by neurological problems and dementia (Laplanche et al. 1999). Multiple members of the HD phenocopy kindred also developed behavioral and psychiatric disturbances, including personality change, depression, aggressiveness, and delusional thoughts (Xiang et al. 1998), often prior to or concomitant with neurological symptoms. These observations are consistent with a common PRNP repeat size of 192 bp, which encodes a PrP with eight extra octapeptide repeats on a methionine 129 allele.
Transgenic mice expressing PrP with 14 octapeptide repeats (Chiesa et al. 1998, 2000) developed an early-onset ataxia, but the disease was not demonstrated to be transmissible. However, brain homogenates from individuals with five, seven, and eight extra PrP octapeptide repeats have transmitted disease to primates (Brown et al. 1994)—strongly suggesting that PrP with repeat expansions provokes bona fide prion diseases.
The disorder in the phenocopy kindred has a number of characteristics suggestive of classical HD: an early adult onset (age range 23–41 years; mean 29.7 years), autosomal dominant syndrome consisting of personality change, cognitive decline, motor disturbance with chorea, dysarthria, and ataxia, together with atrophy of the basal ganglia. In the absence of the characteristic huntingtin (CAG)n repeat expansion, it was considered to be an HD “phenocopy.” A diagnosis of prion disease was originally considered but was excluded on the basis of a normal PrP sequence. We now know that affected members were heterozygous for a PrP repeat expansion. Although sporadic Creutzfeldt-Jakob disease (CJD) is usually considered to be a late-onset disorder with a rapid progression, familial CJD can have a significantly earlier age of onset with a more prolonged duration. This is particularly apparent in individuals with PrP repeat expansions, who tend to have an age at onset similar to that in HD. A number of similar factors, such as early age at onset and the exceptionally broad range of clinical presentations, means that familial CJD can be mistaken for HD. Chorea, although rare in sporadic CJD and most familial forms of CJD, was present in several affected members of a large English kindred with six extra PrP repeats (Collinge et al. 1992; Poulter et al. 1992). This kindred also contained at least one individual who had been diagnosed with HD. In the HD phenocopy pedigree described here, chorea seems to have been a more prominent clinical feature, being present in four of six affected individuals (Xiang et al. 1998).
We suggest that individuals presenting with HD or HD-like diseases should be screened for PrP mutations if the huntingtin (CAG)n repeat length is within the normal range, especially when clinical signs atypical of HD are present. Many of the known HD phenocopy patients have atypical features (Andrew et al. 1994), and, in retrospect, we recognize that members of the phenocopy kindred reported here had clinical features atypical of HD, such as epileptic seizures.
The PRNP ORF is encoded by a single exon (Puckett et al. 1991; Lee et al. 1998) and can be sequenced with relative ease. PCR assays capable of detecting the expanded allele can be unreliable, because there is often selective amplification of the wild-type allele so that the relatively weak signal from the enlarged allele can be overlooked easily in cases of repeat expansion. It is likely that this was responsible for the original false negative PrP screen in this family (Xiang et al. 1998). One practical solution, in the absence of a suitable positive control, is to amplify and sequence a larger region containing the entire PrP terminal exon, because (in our experience) this seems to avoid the selective amplification of the wild-type allele from such cases. Primer position is also important, as there are SNPs near the intron 1/exon 2 junction that have been shown to be responsible for the failure to amplify some rare alleles with primer mismatches (Palmer et al. 1996) in this region.
Our observation that this HD phenocopy is, in fact, a prion disease implies that an unknown proportion of patients with the HD phenocopy might have PrP mutations. Tissues from patients with HD phenocopies who have PrP mutations may therefore represent a potential source of infection to health care workers and to recipients of blood and tissue donations.
Acknowledgments
This research was supported by grants from the National Institutes of Health and by a gift from the G. Harold and Leila Y. Mathers Foundation. R.C.M. was supported by a Human Frontier Science Program Long Term Fellowship and a Research Fellowship from the Medical Faculty of the University of Edinburgh. This work was supported by the Torsten and Ragnar Söderberg Foundation, the Swedish Medical Research Council (MFR grant 03875), and the Karolinska Institute.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human prion protein [BAA00011], doppel [NP_036541], and huntingtin [AAB38240])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for HD [MIM 143100], HD phenocopy/Huntington-like neurodegenerative disorder 1 [HLN1; MIM 603218], PRNP [MIM 176640], and PRND [MIM 604263])
References
- Andrew SE, Goldberg YP, Kremer B, Squitieri F, Thielmann J, Zeisler J, Telenius H, et al (1994) Huntington disease without CAG expansion: phenocopies or errors in assignment? Am J Hum Genet 54:852–863 [PMC free article] [PubMed] [Google Scholar]
- Brown P, Gibbs CJ Jr, Rodgers-Johnson P, Asher DM, Sulima MP, Bacote A, Goldfarb LG, Gajdusek DC (1994) Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 35:513–529 [DOI] [PubMed] [Google Scholar]
- Chiesa R, Drisaldi B, Quaglio E, Migheli A, Piccardo P, Ghetti B, Harris DA (2000) Accumulation of protease-resistant prion protein (PrP) and apoptosis of cerebellar granule cells in transgenic mice expressing a PrP insertional mutation. Proc Natl Acad Sci USA 97:5574–5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R, Piccardo P, Ghetti B, Harris DA (1998) Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron 21:1339–1351 [DOI] [PubMed] [Google Scholar]
- Collinge J, Brown J, Hardy J, Mullan M, Rossor MN, Baker H, Crow TJ, Lofthouse, R, Poulter M, Ridley R (1992) Inherited prion disease with 144 base pair gene insertion. 2. clinical and pathological features. Brain 115:687–710 [DOI] [PubMed] [Google Scholar]
- Duchen LW, Poulter M, Harding AE (1993) Dementia associated with a 216 base pair insertion in the prion protein gene: clinical and neuropathological features. Brain 116:555–567 [DOI] [PubMed] [Google Scholar]
- Gambetti P, Petersen R, Parchi P, Chen SG, Capellari S, Goldfarb L, Montagna P, Labaresi E, Piccardo P, Ghetti B (1999) Inherited prion diseases. In: Prusiner SB (ed) Prion biology and diseases. Cold Spring Harbor Press, Cold Spring Harbor, NY, pp 509–583 [Google Scholar]
- Goldfarb LG, Brown P, McCombie WR, Goldgaber D, Swergold GD, Wills PR, Cervenakova L, Baron H, Gibbs CJ, Gajdusek DC (1991) Transmissible familial Creutzfeldt-Jakob disease associated with five, seven, and eight extra octapeptide coding repeats in the PRNP gene. Proc Natl Acad Sci USA 88:10926–10930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Zerr I, Weber T, Poser S, Kretzschmar H, Hunsmann G, Bodemer W (1995) Prion disease associated with a novel nine octapeptide repeat expansion in the PRNP gene. Mol Brain Res 34:173–176 [DOI] [PubMed] [Google Scholar]
- Kremer B, Goldberg P, Andrew SE, Squitieri F, Thielmann J, Telenius H, Zeisler J, Squitieri F, Lin B, Bassett A, Almqvist E, Bird TD, Hayden MR (1994) A worldwide study of the Huntington’s disease mutation: The sensitivity and specificity of measuring CAG repeats. N Engl J Med 330:1401–1406 [DOI] [PubMed] [Google Scholar]
- Kretzschmar HA, Stowring LE, Westaway D, Stubblebine WH, Prusiner SB, DeArmond SJ (1986) Molecular cloning of a human prion protein cDNA. DNA 5:315–324 [DOI] [PubMed] [Google Scholar]
- Laplanche J-L, El Hachimi KH, Durieux I, Thuillet P, Defebvre L, Delasnerie-Laupretre N, Peoc’h K, Foncin J-F, Destee A (1999) Prominent psychiatric features and early onset in an inherited prion disease with a new insertional mutation in the prion protein gene. Brain 122:2375–2386 [DOI] [PubMed] [Google Scholar]
- Lee IY, Westaway D, Smit AFA, Wang K, Seto J, Chen L, Acharya C, Ankener M, Baskin D, Cooper C, Yao H, Prusiner SB, Hood L (1998) Complete genomic sequence and analysis of the prion protein gene region from three mammalian species. Genome Research 8:1022–1037 [DOI] [PubMed] [Google Scholar]
- Lesperance MM, Burmeister M (2000) Interpretation of linkage data for a Huntington-like disorder mapping to 4p15.3. Am J Hum Genet 67:262–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Lee IY, Silverman GL, Harrison PM, Strome R, Heinrich C, Karunaratne A, Pasternak SH, Azhar Chishti M, Liang Y, Mastrangelo P, Wang K, Smit AF, Katamine S, Carlson GA, Cohen FE, Prusiner SB, Melton DW, Tremblay P, Hood L, Westaway D (1999) Ataxia in prion protein (PrP)–deficient mice is associated with upregulation of the novel PrP-like protein doppel. J Mol Biol 292:797–817 [DOI] [PubMed] [Google Scholar]
- Owen F, Poulter M, Collinge J, Leach M, Lofthouse R, Crow T, Harding AE (1992) A dementing illness associated with a novel insertion in the prion protein gene. Mol Brain Res 13:155–157 [DOI] [PubMed] [Google Scholar]
- Palmer MS, van Leeven RH, Mahal SP, Campbell TA, Humphreys CB, Collinge J (1996) Sequence variation in intron of prion protein gene, crucial for complete diagnostic strategies. Hum Mutat 7:280–281 [DOI] [PubMed] [Google Scholar]
- Poulter M, Baker HF, Frith CD, Leach M, Lofthouse R, Ridley RM, Shah T, Owen F, Collinge J, Brown J (1992) Inherited prion disease with 144 base pair insertion. I. Genealogical and molecular studies. Brain 115:675–685 [DOI] [PubMed] [Google Scholar]
- Puckett C, Concannon P, Casey C, Hood L (1991) Genomic structure of the human prion protein gene. Am J Hum Genet 49:320–329 [PMC free article] [PubMed] [Google Scholar]
- Xiang F, Almqvist EW, Huq M, Lundin A, Hayden MR, Edstrom L, Anvret M, Zhang, Z (1998) A Huntington disease–like neurodegenerative disorder maps to chromosome 20p. Am J Hum Genet 63:1431–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]