Abstract
Objectives
This study aimed to measure gingival blood flow under different orthodontic forces using laser speckle contrast imaging (LSCI).
Methods
Forty eligible individuals were recruited and randomly assigned into 2 groups: 50 g group and 150 g group. According to the groups, forces of 50 g or 150 g were applied from the maxillary canines to the first molars on the left or right side of each participant. Four regions of interest (ROIs) were selected on the gingiva of the maxillary canine: ROI 1 and ROI 2 were located on the marginal and attached gingiva, respectively, while ROI 3 and ROI 4 were positioned on the mesial and distal interdental papillae. All ROIs was measured using LSCI at different time intervals (baseline, 30 minutes, 1, 3, 7, and 14 days).
Results
In the resting state without force loading, statistically significant differences in blood flow were observed among the marginal gingiva, attached gingiva and interdental papilla. The marginal gingiva exhibited lower blood flow compared to other regions. Evaluation of blood flow between the groups revealed significant differences at ROI 1 at 1 day and 3 days, and at ROI 4 at 1 day and 3 days. Intra-group comparisons showed significant differences in blood flow within each group for each region at each time point (P < .05). After applying orthodontic forces, blood flow reached its nadir within 30 minutes to 1 day. The 150 g group exhibited a more significant decrease compared to the 50 g group. Blood flow peaked at 3 days in the 50 g group and at 7 days in the 150 g group. Both groups finally returned to baseline at day 14.
Conclusions
Laser speckle contrast imaging is a reliable tool for monitoring gingival blood flow in orthodontic treatment. Gingival blood flow decreased after force application, reaching peak values in 3 to 7 days and eventually returning to baseline within 14 days. Although heavier forces induced more severe ischaemia, blood flow returned to baseline within 2 weeks. Given the lack of comparison with other validated methods, emphasis of this study placed on force effects, regional differences and the non-invasive advantages of LSCI.
Clinical Significance
In orthodontic treatment, LSCI can ensure the safety of orthodontic diagnosis and therapy by monitoring changes in gingival blood flow. Furthermore, compared to previous technologies, LSCI offers numerous advantages, including rapid assessment and low technical sensitivity. The findings of this study provide important guidance for assessing periodontal tissue health and designing optimal, personalised orthodontic force levels during orthodontic treatment.
Trial registration
The trial was registered in Chinese Clinical Trial Registry (ChiCTR2400082438) on 29 March 2024.
KEY WORDS: Laser speckle contrast imaging, Gingival blood flow, Orthodontic force, Periodontal health
Introduction
Orthodontic treatment of malocclusion is a complex process that involves several responses in the dental and periodontal tissues, such as the periodontal ligament (PDL), gingiva, dental pulp and alveolar bone.1, 2, 3 The resorption and deposition of alveolar bone and remodelling of the PDL make teeth move effectively.4,5 During tooth movement, inflammatory response is a common and essential element because it involves vasodilation, increased vascular permeability and blood flow, fluid exudation and leukocyte migration.6,7 The degeneration of endothelial cells and changes in blood flow, vascular calibre and vascular permeability in gingiva may be the first signs of the onset of pathological events.8, 9, 10, 11, 12 The capillary network beneath the gingival epithelium primarily originates from small arteries within the lamina propria's vascular network.11, 12, 13, 14 These capillary units are among the initial blood vessels affected by inflammation. During the provision of treatment, orthodontists must identify the optimal force, considering the temporal characteristics and magnitude with the maximum patient comfort and without damage of tissue. Real-time monitoring of gingival microcirculation during orthodontic treatment can significantly contribute to assessing periodontal tissue health and optimising orthodontic force magnitude during clinical practice.
Laser doppler flowmetry (LDF) is currently the predominant method for monitoring gingival blood flow, but it has several limitations such as restricted temporal resolution, shallow measurement depth, sensitivity to movement, and a limited measurement range.15, 16, 17 Additionally, LDF can only be performed at a single site with poor repeatability of sensor placement due to its small probe diameter.18,19
Laser speckle contrast imaging (LSCI) is a full-field optical imaging technology that uses the spatial and temporal statistical characteristics of laser speckle intensity to monitor blood flow in living tissues.20 It offers several advantages over traditional methods, namely simple equipment, non-invasive operation, no need for contrast agents, fast imaging speed, high resolution and the ability for continuous measurement. LSCI excels at monitoring blood flow in shallow tissues, providing superior imaging and analysis of parameters like blood flow rate, blood perfusion index and vessel morphology.21, 22, 23, 24 It has been used to monitor blood flow in transplanted flaps and wound healing during periodontal surgery.25, 26, 27 Additionally, high-resolution LSCI has shown good repeatability, reproducibility and short-term/long-term reliability for human gingival blood flow assessment.28, 29, 30 It has been proven that LSCI was a promising tool for evaluating gingival microcirculation over clinical follow-up periods.30,31 In addition to monitoring gingival blood flow, Julio et al.32 used an excised human adult molar as a phantom to monitor dental pulp blood flow, while Luciano et al. used a raw image obtained by digital photography, based on the difference between the speckle pattern of a carious lesion tooth surface area and that of a sound area to monitor early caries.33 These results strongly suggested the potential of LSCI to assess the changes of pulpal blood flow in teeth and diagnose white spot lesions (carious lesions in its early stages) in a clinical environment. LSCI monitoring uses a low-power (70 mW) solid-state laser with a wavelength of 785 nm to measure gingival blood flow, the scanning head operates without contacting the monitored tissue, and following laser irradiation, a camera automatically captures and processes the reflected light signals.34,35 The data are then used to generate colour-coded images that display the spatial distribution of blood flow within the tissue. Compared to LDF, LSCI offers advantages in terms of superior image quality and faster measurement times.19,36
Currently, there is a dearth of research investigating the application of this technique during orthodontic treatment. Therefore, this study aimed to apply LSCI technique for monitoring changes in gingival microcirculation under orthodontic treatment. In addition, we studied the differences in gingival microcirculation caused by different forces of magnitudes and durations.
Material and methods
Trial design, sample size, participants and eligibility criteria
This was a parallel-group randomised controlled trial with a 1:1 allocation ratio. There were no changes to the study protocol after trial commencement. A power analysis using GPower (version 3.1.9.7; Franz Faul University) with alpha (α) level of 0.05 (5%) and beta (β) level of 0.2 (i.e., power = 80%) was conducted based on preliminary data that derived from pre-experiment. The effect size was set to 0.229, determined by a sample size of 16 participants per group. The sample size was increased by 25% to compensate for possible dropout during different follow-up intervals; a total of 40 participants were recruited for this study.
Forty eligible individuals (20 females, 20 males) who needed orthodontic treatment were recruited for this study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hefei Stomatological Hospital of China (ethics number: Y20230105). The study recruited participants meeting the following criteria: (1) age 18-30 with good oral hygiene, (2) complete dentition, (3) no medications affecting periodontal blood flow taken in the past 3 months, and (4) dentition crowding of 0-3 mm. (The difference between what the length of the dental arch should be and the existing length of the dental arch before the maxillary first molar, performed on the patient’s oral model.) Conversely, those with (1) periodontal or systemic disease, (2) tooth caries or ongoing dental treatment, (3) a history of orthodontics, (4) vascular disease or (5) pregnancy/smoking were excluded.
All participants underwent a comprehensive periodontal examination, received oral hygiene education and had supragingival scaling performed. After 1 week, participants who met the inclusion criteria were selected. Their oral hygiene index (OHI), gingival index (GI) and probing depth (PD) were then documented. All participants were informed of the methods and provided written informed consent.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hefei Stomatological Hospital of China (ethics number: Y20230105). The trial was registered in Chinese Clinical Trial Registry (ChiCTR2400082438) on 29 March 2024. All participants were informed of the methods and provided written informed consent. Participants were informed that they could discontinue participation at any time.
Randomization and blinding
The design of this randomised clinical trial was a parallel-group, 2-arm trial with a 1:1 allocation ratio. Eligible participants were asked to pick an envelope from a stack of opaque sealed envelopes. The envelopes were evenly divided into two types: 50g and 150g. Afterwards, each participant drew lots to determine the orthodontic force applied on the left or right side of the maxillary arch. This allocation was done by one of the operators. In this study, 50 g or 150 g of force was applied to the participants’ teeth by the same orthodontist, and it was not possible to blind this orthodontist. Participants were not aware of the magnitude of orthodontic force applied on their maxillary arch. Blood flow measurements were performed by a single investigator who was blinded to the magnitude of the orthodontic force applied and was not involved in the placement of the buttons. All data were recorded and statistically analysed by 1 investigator who was unaware of the magnitude of orthodontic force applied.
Interventions
Two buttons (Shinye) were bonded to the centre of the clinical crown of the labial surfaces on the maxillary canine and first molar teeth of participants using a tension spring and a dynamometer (Tiantian dental) for precise application. The design of this model was based on previous research (Figure 1).37
Fig. 1.
Application of 50 g or 150 g.
In the hour preceding each blood flow measurement, participants were instructed to abstain from oral hygiene practices (brushing, gargling), food and beverage consumption, and to remain in a relaxed, reclined position for at least 15 minutes.
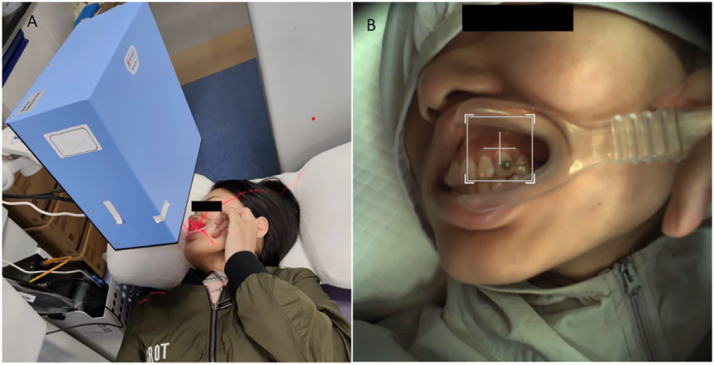
A mouth opener was used to gently retract the corners of the mouth, minimising movement of the mucosal surface near the monitoring site. Blood flow was measured by LSCI (785 nm PeriCam PSI HR System, Perimed AB). The LSCI probe was positioned perpendicular to the keratinised gingiva above the left or right maxillary canine, with a resolution set at 60 μm/pixel. The monitoring distance was set to 15 cm and the sampling frequency at 21 Hz (frames per second) with medium sampling density. The LSCI instrument was connected to a computer, and the measured values were displayed and recorded with a software application (PimSoft, Perimed AB). The instrument was set to take snapshots of each area. Each snapshot was constructed by averaging 20 images in 2 seconds to average out pulsatile variation in the blood flow. The blood flow value was defined as the average of all the pixel perfusion values in the selected area. Blood flow was expressed in an arbitrary value called Laser Speckle Perfusion Unit (LSPU). All measurements were conducted at room temperature (26 °C) between 7:00 AM and 10:00 AM. Every measurement was repeated 3 times. Blood flow values were recorded before force application (baseline, T0) and at subsequent time intervals after force application: 30 minutes (T1), 1 day (T2), 3 days (T3), 7 days (T4) and 14 days (T5). Mean arterial pressure (MAP) of participants was recorded both before and after each blood flow–monitoring session (Figure 2).
Fig. 2.
A, Monitoring gingival blood flow by LSCI; B, Real-time intraoral photos.
Outcomes
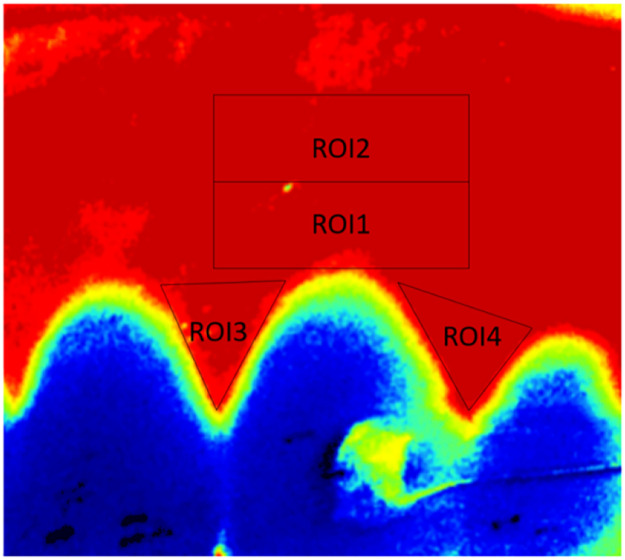
The primary outcome was the blood flow of the regions of interest (ROIs). Four ROIs were identified on the blood flow images presented on PimSoft software (Figure 3). ROI 1 (highest point of the marginal gingiva of the maxillary canine, width equal to the distance between interdental papilla tips, height of 2 mm), ROI 2 (rectangular area above ROI1 with similar dimensions), ROI 3 (mesial interdental papilla region of maxillary canine) and ROI 4 (distal interdental papilla area of maxillary canine).28 The blood flow in each ROI was calculated by averaging all pixel perfusion values within that specific region using the LSPU. Data processing and image analysis were performed by a single researcher.
Fig. 3.
ROIs selection.
The experiment lasted 14 days for all participants. We recorded measurements from the labial side of the canine gingival before, 30 minutes, 1 day, 3 days, 7 days, and 14 days after the application of force (T0, T1, T2, T3, T4 and T5, respectively). All processes were checked and calibrated 3 times.
Interim analysis and stopping guidelines
Participants were informed that they could discontinue participation at any time. No interim analysis was performed during this study.
Statistical analysis
Statistical analysis was performed using SPSS software (version 26; IBM). The categorical data of the participants in each group were assessed using χ2 test. Numerical data were presented as mean and standard deviation values. The data were analysed for normality using the Shapiro-Wilk test. Parameters showed to be normally distributed were analysed using independent-sample t-test and repeated measures analysis, otherwise using Friedman’s test and Mann-Whitney test. Further pairwise comparisons were performed using the Bonferroni post hoc test, with P values adjusted by Greenhouse-Geisser correction method. Statistical significance was defined as P < .05. In this study, gender and teeth (left canine/right canine) were tested by χ2 test; OHI, GI and PD were abnormal distribution and tested by Mann-Whitney’s U-test; and age and blood flow values at each time point in each region were normal distribution and tested by independent sample t-test and repeated measurement analysis.
Error of the method
Every measurement was repeated 3 times by one investigator to evaluate intra-observer errors. Intra-observer reproducibility was assessed using the intraclass correlation coefficient. The average intraclass correlation coefficient values was 0.92.
Results
Participant flow
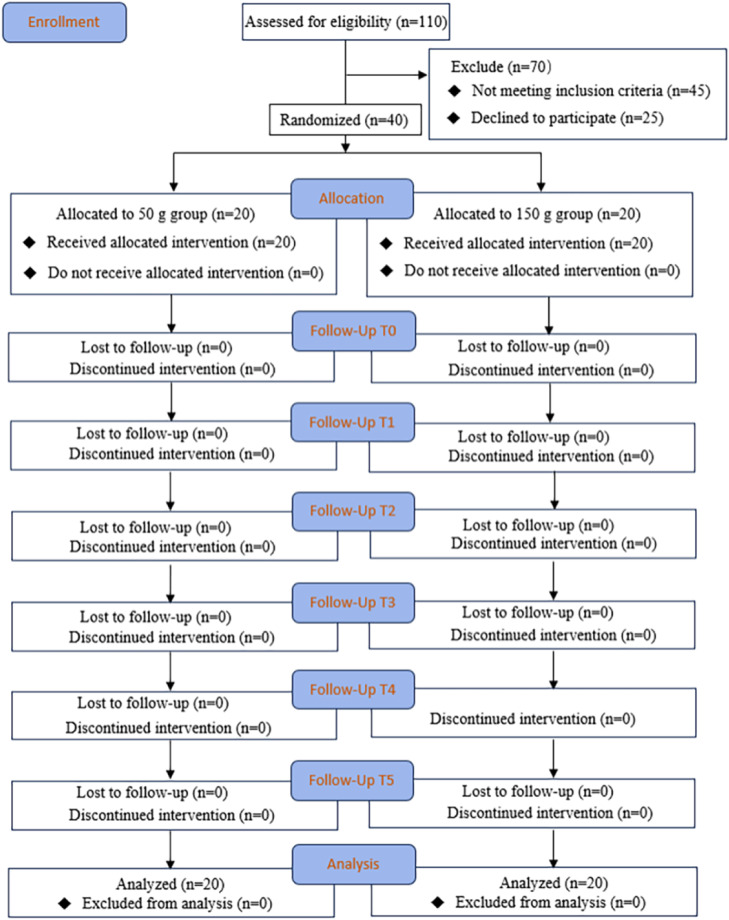
The CONSORT diagram demonstrates the flow of the participants through the trial (Figure 4).
Fig. 4.
CONSORT diagram illustrating the participants flow in the study.
*P < .05.
Baseline data
This study included a total of 40 participants with no dropouts or exclusions. No significant differences were observed in age, gender, teeth (left canine/right canine), GI, OHI and PD at the baseline (P > .05) (Table 1). There was also no significant difference between MAP taken before each blood flow measurement (50 g group MAP 85.5 ± 2.07 mmHg; 150 g group MAP 89.6 ± 2.47 mmHg) and after each measurement (50 g group MAP 84.5 ± 2.16 mmHg; 150 g group MAP 82.8 ± 2.61 mmHg) (P > .05); furthermore, MAP values remained stable throughout the observation period.
Table 1.
Subject demographic and baseline characteristics.
| 50 g group | 150 g group | P | |
|---|---|---|---|
| Age | 23.3 ± 2.90 | 22.95 ± 2.56 | .695a |
| Gender (male) | 12 (60%) | 8 (40%) | .206b |
| Teeth (left canine) | 7 (35%) | 11 (55%) | .204b |
| GI | 0.05 ± 0.22 | 0.15 ± 0.36 | .298c |
| OHI | 0.15 ± 0.36 | 0.25 ± 0.54 | .637c |
| PD | 0.95 ± 0.47 | 1.15 ± 0.32 | .212c |
Independent-sample t-test.
χ2 test.
Mann-Whitney’s U-test.
The blood flow values in each group at T0 are listed in Table 2. Analysis of blood flow across all regions during the T0 period was done using a one-way ANOVA test and revealed significant differences among the regions (Table 3). Post hoc pairwise comparisons showed that these differences were primarily between ROI 1 and ROI 3 and ROI 1 and ROI 4: specifically, the blood flow of ROI 1 was significantly lower than that of other two regions, while the blood flow of ROI 2 was significantly lower than that of ROI 4.
Table 3.
ANOVA test for all subject BF in T0 regions.
| ROIs | BF |
|---|---|
| ROI 1 | 224.04 ± 50.92cd |
| ROI 2 | 249.69 ± 56.03d |
| ROI 3 | 270.28 ± 49.68a |
| ROI 4 | 282.10 ± 44.93ab |
| P | <.001 |
compared with ROI 1, P < .05.
compared with ROI 2, P < .05.
compared with ROI 3, P < .05.
compared with ROI 4, P < .05.
BF: gingival blood flow.
Significant (P < .05).
BF unit: LSPU.
Numbers analysed for each outcome
The average blood flow for each ROI in both the 50 g and 150 g groups at each time point (T0-T5) was collected. Inter-group comparisons revealed significant differences in blood flow between the 50 g and 150 g groups at ROI 1 and ROI 4 at both T2 and T3 (P < .05). No significant differences were observed in the other regions at any time point. Intra-group comparisons within both the 50 g and 150 g groups showed statistically significant changes in blood flow across all regions and time points (P < .05) (Table 2).
Table 2.
Comparison of intra-group and inter-group BF in ROIs.
| T0 | T1 | T2 | T3 | T4 | T5 | P* | ||
|---|---|---|---|---|---|---|---|---|
| ROI 1 | 50 g group | 223.54 ± 54.132bde | 174.41 ± 54.49a | 191.82 ± 67.00 | 241.30 ± 52.98a | 235.91 ± 53.04a | 229.07 ± 56.44 | <.001 |
| 150 g group | 224.54 ± 48.90bcdef | 143.59 ± 48.19a | 152.52 ± 47.82a | 204.63 ± 47.03a | 256.89 ± 45.71a | 249.09 ± 47.13a | <.001 | |
| P** | .951 | .066 | .039 | .026 | .188 | .231 | ||
| ROI 2 | 50 g group | 248.58 ± 57.21bcde | 213.43 ± 59.19a | 224.74 ± 60.72a | 264.87 ± 62.94a | 260.74 ± 57.43a | 253.06 ± 53.66 | <.001 |
| 150 g group | 250.80 ± 56.28bcdef | 185.99 ± 55.17a | 192.30 ± 62.48a | 236.68 ± 60.59a | 277.78 ± 55.90a | 265.98 ± 53.65a | <.001 | |
| P** | .902 | .138 | .104 | .157 | .348 | .451 | ||
| ROI 3 | 50 g group | 270.30 ± 49.08bd | 238.19 ± 53.97a | 263.86 ± 50.86 | 280.34 ± 50.97a | 276.81 ± 48.51 | 267.89 ± 53.63 | <.001 |
| 150 g group | 270.25 ± 51.55bc | 220.68 ± 56.62a | 236.62 ± 56.02a | 266.92 ± 51.49 | 277.54 ± 52.34 | 275.66 ± 50.08 | <.001 | |
| P** | .998 | .323 | .116 | .413 | .964 | .639 | ||
| ROI 4 | 50 g group | 282.71 ± 44.55bc | 242.29 ± 47.18a | 254.09 ± 48.15a | 288.56 ± 47.88 | 287.51 ± 42.06 | 283.08 ± 41.33 | <.001 |
| 150 g group | 281.48 ± 46.44bcde | 221.22 ± 48.77a | 222.47 ± 47.58a | 256.80 ± 45.44a | 300.30 ± 43.39a | 282.35 ± 42.20 | <.001 | |
| P** | .933 | .173 | .043 | .038 | .350 | .956 |
LPSU, ± s
Note: P**: Conduct an independent sample t-test between groups.
P*: Repeated measures analysis of variance within ROI and groups.
compared with T0, P < .05.
compared with T1, P < .05.
compared with T2, P < .05.
compared with T3, P < .05.
compared with T4, P < .05.
compared with T5, P < .05.
A line chart was created to visualise the data (Figure 5). The data showed a significant decrease in blood flow from T1 to T2 in all regions, reaching its lowest point. Notably, the 150 g group exhibited a more pronounced decrease compared to the 50 g group. Both groups then experienced a gradual recovery in blood flow. The 50 g group reached its peak around T3, while the 150 g group peaked around T4. Finally, both groups ultimately returned to baseline by approximately T5.
Fig. 5.
Blood flow value at each time point.
Harms
There were no adverse effects reported during the study period.
Discussion
Appropriate orthodontic force is essential to ensure the success of orthodontic treatment. Due to the different tolerance of different patients to force values, it is particularly important to monitor the real-time response of teeth and periodontal tissues under different orthodontic forces. LSCI can ensure the safety of orthodontic diagnosis and therapy by monitoring changes in gingival blood flow. It offers numerous advantages, including rapid assessment and low technical sensitivity. It holds significant implications for both evaluating periodontal tissue health and determining optimal orthodontic force levels.
This study aimed to non-invasively validate the hypothesis that heavier orthodontic forces have a greater impact on gingival blood flow during the force application phase. The results demonstrated that gingival blood flow was more significantly reduced in the 150 g group compared to the 50 g group within 30 minutes to 1 day after force application. To ensure the accuracy of the findings, a carefully designed randomised, prospective clinical trial was conducted to minimise potential confounding factors, such as inter-individual variability. In addition, different ROIs were selected to assess regional differences in gingival blood flow. Drawing on previous studies regarding the physiological remodelling of teeth following orthodontic force application, we conducted a 14-day observational period and identified a periodic pattern in gingival blood flow changes.
The application of controversial forces on teeth not only significantly affects the pulpal nerve response but also induces inflammatory reactions within the periodontal tissues.38 Compared to LSCI, LDF is primarily used for monitoring pulpal blood flow through specific guiding plates.39, 40, 41 In addition, previous research has indicated that within a range of 50 g to 250 g force magnitude, there is a negative correlation between force and gingival blood flow, whereas the duration of reactive hyperaemia shows a positive correlation with the applied force.42 Because of the blood flow in the attached gingival shows spatial differences,28 we divided the blood perfusion images into 4 parts according to the location of the gingival for a better assessment of the blood flow of the gingival. The values obtained in this study were within the range reported by Molnár et al.30
In this study, forces of 50 g and 150 g were employed, applying force with a tension spring. Tensile stress elongates the periodontal ligament fibres, whereas compressive stress leads to their compression and subsequent ischaemia. Squeezing of the blood vessels may result in a decrease in the diameter of the blood vessel and an increase in vascular resistance.43 Within the first day, partial or complete occlusion of blood vessels occurs, followed by disintegration of vessel walls and degradation of red blood cells. Consequently, blood flow significantly decreases, reaching its minimum within 30 minutes to 1 day after force application. Notably, the 150 g group experienced a more pronounced decline compared to the 50 g group. As the counteracting force from adjacent teeth gradually offsets the applied force, reactive hyperaemia occurs. This phenomenon involves the re-entry of blood flow into the compressed tissue.28 Regenerative processes—marked by the proliferation of new cellular and vascular elements—become predominant, resulting in hyperaemia in the affected area. Peak hyperaemia occurred on day 3 in the 50 g group and day 7 in the 150 g group, with both groups briefly exceeding baseline levels. As the applied force diminished further, the hyperaemic response subsided, with blood flow returning to baseline in all regions by day 14. This shows that gingival blood flow can adapt the microcirculation to the new characteristics of the teeth during orthodontic movement. Similar findings have been obtained in previous studies of the periodontal ligament’s blood flow in humans.37 This means that blood circulation regeneration in the gingiva is synchronised with or precedes the regeneration cycle of tissues such as alveolar bone and periodontal fibres, usually with complete remodelling in approximately 14-21 days.37,44
Fazekas et al.28 used LSCI to demonstrate that human gingival blood supply primarily originated from coronary arteries at the apex of the gingiva, and the attached gingiva (ROI 2) exhibited high vasodilation capacity, allowing it to withstand mechanical stimulation effectively. This accounted for the absence of statistically significant differences in blood flow between the 50 g and 150 g groups at any time point in this region (ROI 2). However, the marginal gingival area (ROI 1) showed significant differences between groups at 1 day and 3 days, which can be attributed to the limited blood supply in the marginal gingiva compared to other regions16 and the 150 g force inducing a more pronounced ischaemic reaction in this area. Additionally, statistically significant differences were observed between groups in the distal interdental papilla (ROI 4) at 1 and 3 days, suggesting that the 150 g force caused a more severe ischaemic reaction in this region at these time points.
The mean blood flow values for each region at baseline (T0) are shown in Table 3. There was a statistically significant difference in blood flow values among these regions (P < .05), and these data were within a range similar to the values reported by Molnár et al.30 who investigated resting-state gingival blood flow in healthy individuals. Further comparison of the blood flow values at T0 revealed that ROI 1 had a significantly lower value compared to ROI 3 and ROI 4, whereas ROI 2 exhibited a significantly lower value than ROI 4. Previous studies have demonstrated spatial variations in gingival microcirculation,45,46 showing that in the resting state, blood perfusion in the marginal gingival area was comparatively lower, whereas it increased closer to the alveolar mucosa. The interdental papilla often exhibits higher blood perfusion than the marginal gingival area due to its additional supply from the periodontal membrane of adjacent teeth, and there were differences in haemodynamics between the attached gingiva and the interdental papilla16—an observation that aligns with the findings of our experiment.
Chuan et al.37 used superb microvascular imaging to explore the change of the vascular index in canine periodontal ligament under 50 g or 150 g of force and found that after applying orthodontic forces, the vascular index decreased slightly in 30 minutes, decreased to a minimum value after 1 day, increased to the maximum in 3-7 days and returned to baseline values in 14 days; the values of other vascular parameters showed similar trends. The variation trends of those vascular parameters also explained well the change of blood flow in our study.
In clinical practice, due to the naturally uneven, 3-dimensional curvature of the gingiva, achieving a perfectly perpendicular angle can be challenging. Additionally, the blood supply to the attached gingiva primarily originates from vessels within the alveolar mucosa,14 and movement of alveolar mucosa caused by a mouth opener can affect blood perfusion measurements, so selecting a monitoring area closer to the mucogingival junction will lead a less accurate measurement of gingival blood flow.30 In this study, we made meticulous adjustments to ensure precise positioning and angle alignment of both participants and the laser probe, and maintained a consistent distance between the probe and the gingiva during each measurement. These measures were crucial for obtaining reliable blood flow values. Additionally, the potential issue of individual variability in gingival blood flow must be acknowledged as an inherent property of clinical studies. At the individual level, blood flow can vary due to genetic differences, variations in periodontal conditions, aging and hormonal fluctuations, all of which contribute to diversity.47,48 We used only two force magnitudes, and the duration of the study was relatively short, which further limits the generalisability of our findings. In future research, we intend to increase the sample size, explore a broader range of force magnitudes and extend the study duration. Furthermore, as a pioneering study applying LSCI in orthodontic clinical trial, the selection of non-extraction patients as the study cohort was made after considering and referencing other valuable clinical studies.37,49 Based on the results of this study, we plan to include extraction patients (specifically first premolar extractions for orthodontic purposes) in subsequent experiments, initiating canine retraction following alignment and levelling.
Conclusion
-
-
Heavier orthodontic forces resulted in a more pronounced reduction in gingival blood flow between 30 minutes and 1 day after force application; however, these differences gradually diminished along the study.
-
-
Laser speckle contrast imaging (LSCI) offers a non-invasive and real-time method for evaluating gingival blood flow during orthodontic force application and demonstrates high sensitivity to force-induced changes in perfusion.
Authors' contributions
Clinical operation: Yin, Zhao; Conceptualisation: An, Han, Tang, Wang, Wei, Yin, Zhang, Zhao; Data analysis: Wang, Yin, Zhang, Zhao; Data collection: An, Tang, Wei, Yin, Zhao; Design: An, Han, Tang, Wang, Wei, Yin, Zhang, Zhao; Measurement: Yin, Zhao; Participant enrolment: Yin, Zhao; Writing—original draft: Yin, Zhao; Writing—review and editing: An, Han, Tang, Wang, Wei, Yin, Zhang, Zhao
Funding
This study was supported by the Hefei Municipal Health Commission Applied Medicine Research Project [grant number: Hwk2023zd019]; the Anhui Province Key Research and Development Plan Project [grant number: 2022e07020059]; and the 2023 Bengbu Medical University Graduate Research Innovation Program Project [grant number: Byycx23073].
Conflict of interests
None declared.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.identj.2025.100932.
Appendix. Supplementary materials
References
- 1.Cuoghi O.A., Faria L.P., Ervolino E., et al. Pulp analysis of teeth submitted to different types of forces: a histological study in rats. J Appl Oral Sci. 2018;26 doi: 10.1590/1678-7757-2017-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perillo L., d’Apuzzo F., Illario M., et al. Monitoring biochemical and structural changes in human periodontal ligaments during orthodontic treatment by means of micro-Raman spectroscopy. Sensors (Basel) 2020;20:497. doi: 10.3390/s20020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalajzic Z., Peluso E.B., Utreja A., et al. Effect of cyclical forces on the periodontal ligament and alveolar bone remodeling during orthodontic tooth movement. Angle Orthod. 2014;84:297–303. doi: 10.2319/032213-234.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feller L., Khammissa R.A., Schechter I., Thomadakis G., Fourie J., Lemmer J. Biological events in periodontal ligament and alveolar bone associated with application of orthodontic forces. Sci World J. 2015;2015 doi: 10.1155/2015/876509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masella R.S., Meister M. Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;129:458–468. doi: 10.1016/j.ajodo.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Timothy W.S., Pries AR. The microcirculation: physiology at the mesoscale. Physiol. 2011;1:1047–1052. doi: 10.1113/jphysiol.2010.201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roustit M., Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharm. 2013;34:373–384. doi: 10.1016/j.tips.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Verrusio C., Iorio-Siciliano V., Blasi A., Leuci S., Adamo D., Nicolo M. The effect of orthodontic treatment on periodontal tissue inflammation: a systematic review. Quintessence Int. 2018;49:69–77. doi: 10.3290/j.qi.a39225. [DOI] [PubMed] [Google Scholar]
- 9.Vandevska-Radunovic V. Neural modulation of inflammatory reactions in dental tissues incident to orthodontic tooth movement: a review of literature. Eur J Orthod. 1999;21:231–247. doi: 10.1093/ejo/21.3.231. [DOI] [PubMed] [Google Scholar]
- 10.Salles A.W.R., Salles A.M.C., Nogueira GEC. Laser Doppler blood-flow signals from human teeth during an alignment and leveling movement using a superelastic archwire. ISRN Dent. 2013;19 doi: 10.1155/2013/102816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polizzi A., Alibrandi A., Lo Giudice A., et al. Impact of periodontal microRNAs associated with alveolar bone remodeling during orthodontic tooth movement: a randomized clinical trial. J Transl Med. 2024;22(1):1155. doi: 10.1186/s12967-024-05933-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao Y., Mi S., Li X., et al. MicroRNA-155 targets SOCS1 to inhibit osteoclast differentiation during orthodontic tooth movement. BMC Oral Health. 2023;23(1):955. doi: 10.1186/s12903-023-03443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobuto T., Yanagihara K., Teranishi Y., et al. Periosteal microvasculature in the dog alveolar process. J Periodontol. 1989;60(12):709–715. doi: 10.1902/jop.1989.60.12.709. [DOI] [PubMed] [Google Scholar]
- 14.Fazekas R., Molnár E., Lohinai Z., et al. Functional characterization of collaterals in the human gingiva by laser speckle contrast imaging. Microcirculation. 2018;25(3) doi: 10.1111/micc.12446. [DOI] [PubMed] [Google Scholar]
- 15.Kralj L., Lenasi H. Wavelet analysis of laser Doppler microcirculatory signals: current applications and limitations. Front Physiol. 2023;13:2762–2777. doi: 10.3389/fphys.2022.1076445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komaki S., Ozaki H., Takahashi S., et al. Gingival blood flow before, during, and after clenching, measured by laser Doppler blood flowmeter: a pilot study. Am J Orthodont Dentofacial Orthoped. 2022;161(1):46–52. doi: 10.1016/j.ajodo.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Kuraji R., Wu Y.H., Hashimoto S., et al. Temporal and dynamic changes in gingival blood flow during progression of ligature-induced periodontitis. Oral Diseases. 2020;26(6):1292–1301. doi: 10.1111/odi.13328. [DOI] [PubMed] [Google Scholar]
- 18.Gleissner C., Kempski O., Peylo S., et al. Local gingival blood flow at healthy and inflamed sites measured by laser Doppler flowmetry. J Periodontol. 2006;77(10):1762–1771. doi: 10.1902/jop.2006.050194. [DOI] [PubMed] [Google Scholar]
- 19.Fazekas R., Molnár E., Mikecs B., Lohinai Z., Vág J. A novel approach to monitoring graft neovascularization in the human gingiva. J Vis Exp. 2019;12(143):e58535. doi: 10.3791/58535. [DOI] [PubMed] [Google Scholar]
- 20.Heeman W., Steenbergen W., van Dam G M., et al. Clinical applications of laser speckle contrast imaging: a review. J Biomed Optics. 2019;24(8):1–11. doi: 10.1117/1.JBO.24.8.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Peña R.J., Braga R.A., Jr, Pujaico-Rivera F. Diode laser reliability in dynamic laser speckle application: stability and signal to noise ratio. Optics Laser Technol. 2018;108:279–286. [Google Scholar]
- 22.Lee B., Sosnovtseva O., Sørensen C.M., Postnov DD. Multi-scale laser speckle contrast imaging of microcirculatory vasoreactivity. Biomed Opt Express. 2022;13(4):2312–2322. doi: 10.1364/BOE.451014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai L., Du Y., Fu Y., Wu X. Laser speckle contrast imaging based on spatial frequency domain filtering. Biophotonics. 2023;16(9) doi: 10.1002/jbio.202300108. [DOI] [PubMed] [Google Scholar]
- 24.Zheng S., Mertz J. Direct characterization of tissue dynamics with laser speckle contrast imaging. Biomed Opt Express. 2022;13(8):4118–4133. doi: 10.1364/BOE.462913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnár E., Molnár B., Lohinai Z., et al. Evaluation of laser speckle contrast imaging for the assessment of oral mucosal blood flow following periodontal plastic surgery: an exploratory study. BioMed Res Int. 2017;2017 doi: 10.1155/2017/4042902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bálint M., Eszter M., Réka F., et al. Assessment of palatal mucosal wound healing following connective-tissue harvesting by laser speckle contrast imaging: an observational case series study. Int J Periodont Restor Dent. 2019;39(2):64–70. doi: 10.11607/prd.3878. [DOI] [PubMed] [Google Scholar]
- 27.Jeon F.H.K., Griffin M.F., Butler P.E.M. Laser speckle contrast imaging to assess peri-oral microcirculation in systemic sclerosis. Clin Exp Rheumatol. 2020;125(3):183. [PubMed] [Google Scholar]
- 28.Fazekas R., Molnár E., Lohinai Z., et al. Functional characterization of collaterals in the human gingiva by laser speckle contrast imaging. Microcirculation. 2018;25(3) doi: 10.1111/micc.12446. [DOI] [PubMed] [Google Scholar]
- 29.Mikecs B., Vág J., Gerber G., Molnár B., Feigl G., Shahbazi A. Revisiting the vascularity of the keratinized gingiva in the maxillary esthetic zone. BMC Oral Health. 2021;21(1):160. doi: 10.1186/s12903-021-01445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molnár E., Fazekas R., Lohinai Z., et al. Assessment of the test-retest reliability of human gingival blood flow measurements by Laser Speckle Contrast Imaging in a healthy cohort. Microcirculation. 2018;25(2) doi: 10.1111/micc.12420. [DOI] [PubMed] [Google Scholar]
- 31.Couturier A., Bouvet R., Cracowski J L, et al. Reproducibility of high-resolution laser speckle contrast imaging to assess cutaneous microcirculation for wound healing monitoring in mice. Microvasc Res. 2022;141 doi: 10.1016/j.mvr.2022.104319. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez-San-Juan J.C., Regan C., Coyotl-Ocelotl B., Choi B. Spatial versus temporal laser speckle contrast analyses in the presence of static optical scatterers. J Biomed Opt. 2014;19(10) doi: 10.1117/1.JBO.19.10.106009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavinho L.G., Araujo S.A., Bussadori S.K., Silva J.V.P., Deana AM. Detection of white spot lesions by segmenting laser speckle images using computer vision methods. Lasers Med Sci. 2018;33(7):1565–1571. doi: 10.1007/s10103-018-2520-y. [DOI] [PubMed] [Google Scholar]
- 34.Regan C., White S.M., Yang B.Y., et al. Design and evaluation of a miniature laser speckle imaging device to assess gingival health. J Biomed Optics. 2016;21(10) doi: 10.1117/1.JBO.21.10.104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fazekas R., Molnár B., Kőhidai L., et al. Blood flow kinetics of a xenogeneic collagen matrix following a vestibuloplasty procedure in the human gingiva: an explorative study. Oral Dis. 2019;25(7):1780–1788. doi: 10.1111/odi.13163. [DOI] [PubMed] [Google Scholar]
- 36.Stewart C.J., Frank R., Forrester K.R., Tulip J., Lindsay R., Bray RC. A comparison of two laser-based methods for determination of burn scar perfusion: laser Doppler versus laser speckle imaging. Burns. 2005;31(6):744–752. doi: 10.1016/j.burns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Wu C., Liu X., Zhang H., Zhang Q., et al. Response of human periodontal ligament to orthodontic force using superb microvascular imaging. Am J Orthod Dentofacial Orthop. 2022;162(5):257–266. doi: 10.1016/j.ajodo.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Alomari F., Al-Habahbeh R., Alsakarna B. Responses of pulp sensibility tests during orthodontic treatment and retention. Int Endod J. 2011;44(7):635–643. doi: 10.1111/j.1365-2591.2011.01865.x. [DOI] [PubMed] [Google Scholar]
- 39.Ba-Hattab R., Abu Alhaija E.S., Nasrawi Y.H., Taha N., Daher H., Daher S. Leveling the curve of Spee using different sized archwires: a randomized clinical trial of blood flow changes. Clin Oral Investig. 2023;27(6):2943–2955. doi: 10.1007/s00784-023-04894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu Alhaija E.S., Taha N.A. A comparative study of initial changes in pulpal blood flow between conventional and self-ligating fixed orthodontic brackets during leveling and alignment stage. Clin Oral Investig. 2021;25(3):971–981. doi: 10.1007/s00784-020-03386-2. [DOI] [PubMed] [Google Scholar]
- 41.Alhaija E.S.A., Shahin A.Y., Badran S.A., Daher S.O., Daher HO. Pulpal blood flow changes and pain scores related to using superelastic 0.018-inch nickel titanium as the first orthodontic alignment archwire: a prospective clinical trial. J Appl Oral Sci. 2021;4(29) doi: 10.1590/1678-7757-2021-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi K., Nanda R.S. Blood flow changes in gingival tissues due to the displacement of teeth. Angle Orthodont. 1992;62(4):257–264. doi: 10.1043/0003-3219(1992)062<0257:BFCIGT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Vandevska-Radunovic V., Kristiansen A.B., Heyeraas K.J., Kvinnsland S. Changes in blood circulation in teeth and supporting tissues incident to experimental tooth movement. Eur J Orthod. 1994;16:361–369. doi: 10.1093/ejo/16.5.361. [DOI] [PubMed] [Google Scholar]
- 44.Zeno K.G., Mustapha S., Ayoub G., Ghafari JG. Effect of force direction and tooth angulation during traction of palatally impacted canines: a finite element analysis. Am J Orthod Dentofacial Orthop. 2020;157:377–384. doi: 10.1016/j.ajodo.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 45.Kerdvongbundit V., Vongsavan N., Soo-Ampon S., et al. Microcirculation and micromorphology of healthy and inflamed gingivae. Odontology. 2003;91:19–25. doi: 10.1007/s10266-003-0024-z. [DOI] [PubMed] [Google Scholar]
- 46.Kerdvongbundit V., Vongsavan N., Soo-Ampon S., et al. Microcirculation of the healthy human gingiva. Odontology. 2002;90:48–51. doi: 10.1007/s102660200007. [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Zhan Q., Bao M., Yi J., Li Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: up-date in a new decade. Int J Oral Sci. 2021;13(1):20. doi: 10.1038/s41368-021-00125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Böhl M., Kuijpers-Jagtman AM. Hyalinization during orthodontic tooth movement: a systematic review on tissue reactions. Eur J Orthod. 2009;31(1):30–36. doi: 10.1093/ejo/cjn080. [DOI] [PubMed] [Google Scholar]
- 49.Laredo-Naranjo M A, Patiño-Marín N., Martínez-Castañón G A, et al. Identification of gingival microcirculation using laser doppler flowmetry in patients with orthodontic treatment: a longitudinal pilot study. Medicina. 2021;57(10):1081. doi: 10.3390/medicina57101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.