Summary
Studying prey hunting behavior in mice provides an ideal opportunity to identify mechanisms that control visual stimulus detection and orienting in the mammalian brain. Emerging work has shown that visual orienting varies during development in a way that reflects the maturity of the underlying visual system. However, it was unknown whether mice varied in visual orienting and prey hunting during adolescence when many underlying motivational and attentional systems are in flux. We therefore quantified visual orienting in freely moving adolescent and adult mice of both sexes during prey capture versus when presented visual motion only. Robust sex differences in innate visual orienting and predatory aggression first emerge during adolescence when females are more likely to approach visual motion and males more likely to arrest, yet, females display the least predatory aggression. Thus, females and males display opposing visual orienting and predatory bias, which are dissociated during adolescence in mouse.
Subject areas: Behavioral neuroscience, Developmental neuroscience
Graphical abstract
Highlights
-
•
Adolescent mice innately approach insects more than adults
-
•
Adolescent mice show sex differences in hunger-driven predatory aggression
-
•
Sex differences in innate visual orienting emerge during adolescence
-
•
Sex differences in visual orienting bias are age and visual feature specific
Behavioral neuroscience; Developmental neuroscience
Introduction
Prey capture is a ubiquitous natural behavior where animals integrate specific sensory cues, motivational states, and motor actions to successfully procure highly rewarding food.1,2 Under natural conditions, most animals successfully utilize a range of specific multisensory cues to reliably recognize and capture natural prey.1,3 Importantly, many natural behaviors vary by sex in mammals as they may be differentially beneficial between the sexes for survival and reproductive success, yet prey capture is a natural context in which both sexes benefit from this form of aggression.2,4 Specific prey “cues” are therefore considered a key part of an organism’s sensory ecology that has shaped the evolution of the structure and function of their senses over time.5,6,7 Many species rely on vision to detect, identify and localize palatable prey.8 There is behavioral, cellular, and physiological evidence that sensitivity to prey motion specifically has driven the evolution of eyes and visuo-motor behavior throughout the mammalian lineage.5,8 Recent studies in the mouse have also revealed specialized circuit pathways that link specific motion cues to positive orienting behaviors that ultimately facilitate effective prey foraging behavior in adult animals,9,10,11,12 while other circuit-level studies have been performed in only adult males.13 Yet, studying the development of prey capture behaviors in both sexes in the most widely studied strain of mouse will allow us to better understand how “generalizable” recent circuit level findings are within this species.
While innate orienting behaviors may imply a relative “hard-wiring” of underlying neural circuits linking stimulus to response, there is ample evidence of context-dependent regulation of prey-related visual orienting responses and function of underlying neural circuits throughout the animal kingdom. In zebrafish, hunger state robustly alters innate behavioral and underlying neuronal responses to moving visual objects.14 Insect-naive adult mice may either avoid or attack live insects without food-deprivation,2,13 and show more consistent predatory aggression once food-restriction begins.6 Further, successful prey capture experience reinforces approach responses toward both prey and visual object motion, reducing variability in behavioral responses.6,15 Similarly, “small object” motion stimuli presented to a nocturnal species of primate facilitates accurate spatial orienting responses in both appetitive and aversive contexts.16 Thus, multiple agents of selection appear to have reinforced the conservation of prioritizing and responding to the presence of small moving objects in the visual field, while allowing for context-dependent flexibility in an animal’s overt behavioral response to that motion.
Studying the ontogeny of responses to visual motion as it relates to prey pursuit behavior in the mouse presents an opportunity to identify developmentally controlled mechanisms that flexibly gate visually driven orienting responses in the mammal.15,17 Mammals go through several life stages with distinct physiological needs that exert control over dietary requirements.18 Thus, the immediate value of pursuing prey/small moving objects during development may be distinct from that present in the adult. As adults, mice and other mammals flexibly utilize specific sensory cues to optimize food foraging strategies.19 However, independent and successful foraging behavior must first emerge robustly after fully weaning off mother’s milk and near the onset of adolescence in mice, starting at postnatal day 30 (P30). While the onset of adolescence is associated with a wide variety of change in adaptive behaviors, intense competition for food between conspecifics is one of the most critical environmental forces facing newly adolescent weanlings.20 For example, the territorial dispersal that requires advanced navigation skills to facilitate the establishment of new breeding territory and breeding behaviors themselves do not emerge until late or post adolescence in both sexes of mice.21 Moreover, it has been documented in lab strains of house mice, that adolescents consume more food each day than young adult mice,22 while feeding behaviors between the sexes of adult mice is not significantly different until 20 weeks of age.23 We thus reasoned that mice could be highly tuned toward visual cues indicating nutritious insect prey in early adolescence. Robust developmental changes characteristic of adolescence could enable a suite of sensory processing and behavioral shifts that facilitate effective and independent prey foraging to offset the potential costs related to increasing their risk of predation.
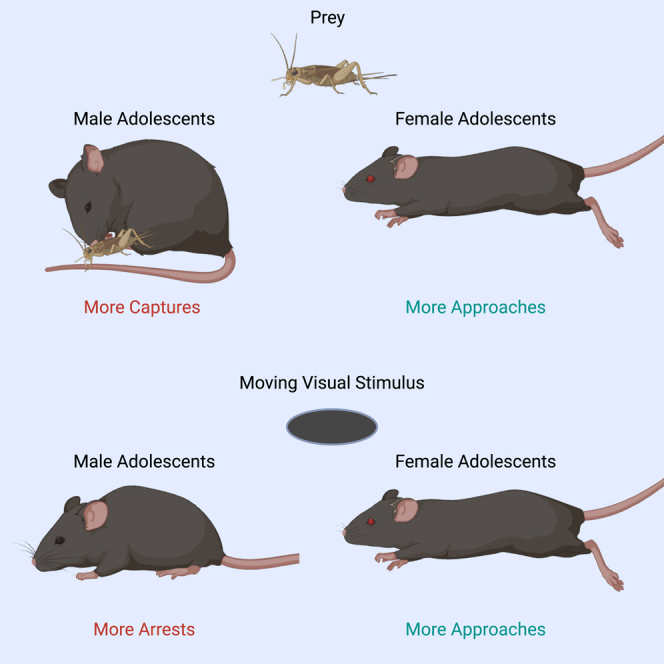
To test this idea, we quantified cricket capture and visual orienting evoked by computer generated sweeping visual motion stimuli in adult and adolescent mice of both sexes.15 We found robust differences in orienting behavior in both the natural predatory and purely visually driven context that depended on age and sex. Adolescent mice of both sexes approached and stayed in proximity to live insect prey more than adults without attacking as often, exhibiting significantly less avoidance behaviors than has been described previously for adult male mice presented insects in their home cage.13 When tested on naturalistic and visual sweeping motion stimuli that was comparable in size to live crickets, female and male adolescent mice displayed opposing biases in orienting toward visual motion. Female adolescent mice approached prey and specific speeds of visual motion stimuli robustly and more frequently than all other groups. They were also the least likely to exhibit predatory aggression toward live crickets without and with food restriction. On the other hand, the adolescent males exhibited robust arrest responses that were stimulus speed specific, and they exhibited significantly more heightened predatory aggression once food restriction began relative to all other groups. Male adults then showed enhanced arrest responses evoked by faster moving stimuli relative to all other groups. Our work therefore revealed unexpectedly that a natural sexual dimorphism in visual orienting arises during adolescence in mice and is also observed in adults depending on the speed of motion of the sweeping stimulus. Further, we provide evidence that predatory aggression and approaches toward visual motion are initially dissociated during adolescence in the mouse suggesting that attraction toward, and salience of, visual motion emerges first during development and is motivating independently of hunger and predatory aggression. Hunger, restricting the availability of food, then subsequently modifies the robustness of predatory aggression behavior.12,24,25 Thus, sexually dimorphic physiological states first emerging in adolescence in mice leads to significant differences in “innate” orienting responses toward natural prey species and visual object motion.
Results
Orienting versus predatory aggression toward crickets varies by age and sex
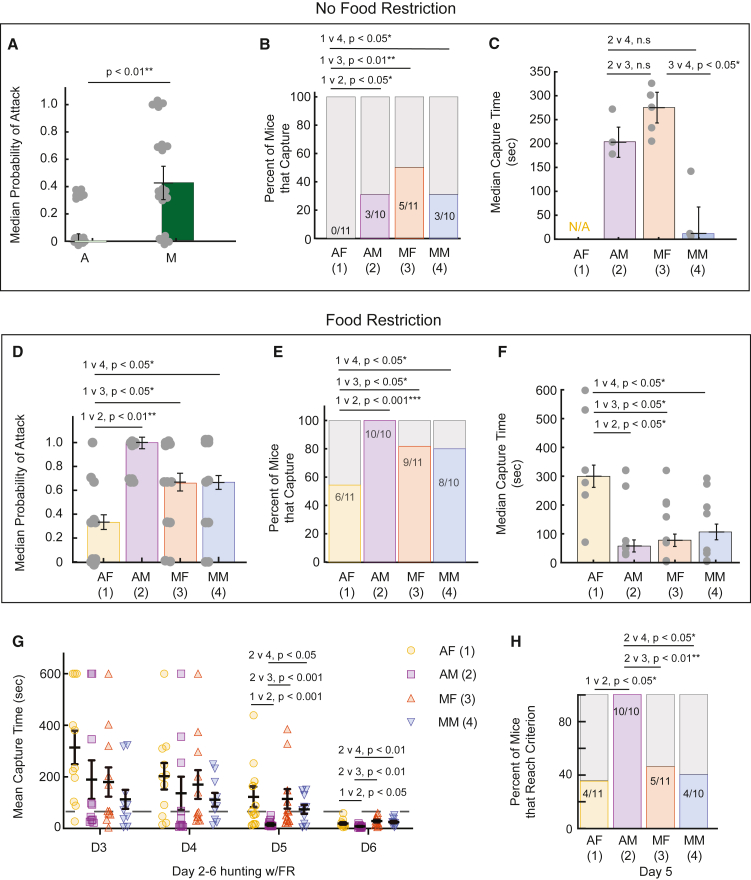
Given key changes in adolescent physiology that may serve to motivate food foraging behaviors, we hypothesized that responses toward “prey-like” stimuli would be heightened in adolescent mice (Figure 1A). To start to address this, we measured the time to first approach potential cricket “prey” when crickets were first introduced to them in a familiar environment (Figure 1B). We discovered no sex differences in this measure within each age and thus pooled the time to first approach data by age and still found no significant difference in time to approach (Figure 1B and Table S1). Next, we measured the total number of approaches toward the cricket made in the first 5-min period of interaction to determine the relative saliency of the cricket as an appetitive target to mice of different ages (Figure 1C). We noticed a dramatic increase in the number of overall approaches made toward the crickets by adolescents, and by adolescent females most significantly, relative to adults of both sexes which were similar to each other (Figure 1C and Table S1). This enhanced behavioral response along with elevated contact duration with the cricket exhibited by adolescents of both sexes (Figure 1D), indicated that adolescent mice do exhibit elevated “engagement” behaviors directed toward potential insect prey. Further, female mice showed the strongest tendency to approach and persist in approaching live insects (Videos S1, S2, S3, and S4). Importantly, the accuracy of visually guided orienting was similar to adults (Figures 1E–1G) which indicated that the differences in approach response frequency was not related to the ability to use binocular visual cues which we showed previously was immature in P21 mice.17 Notably, for this first exposure to crickets, mice were not food restricted and thus response times and approach frequency reflected innate responses dissociated from overt hunger manipulation. We also included large and comparable numbers of male and female mice of both ages to be able to detect whether behaviors varied significantly by sex as well (see Table S1). In addition, previous studies of adolescent mice had indicated elevated levels of initial spontaneous locomotion which could interact with our key measures of prey interaction.22 As time to first approach crickets (Figure 1B) did not significantly differ between groups, it is unlikely that age-dependent differences in movement and/or area locomotion explain the main differences in prey interaction behaviors that we observed. As further evidence against this possibility, we measured the general exploratory behavior of our subjects after two days of handler and environmental habituation relative to the number of cricket approaches ultimately exhibited by that animal (Figure S1). We found that general exploratory behavior of the arena prior to cricket introduction was not significantly different between all groups (Figure S1A), nor did it predict how many approaches each individual ultimately made toward the presented cricket (Figure S1B). Taken together, adolescent mice showed more innate, positive engagement with crickets than adults and it is clear that behaviors dependent upon the processing of binocular visual cues are mature-like at adolescence.
Figure 1.
Measures of visual orienting behavior during live prey capture in adolescent mice (P30-45), versus mature mice (P60-P80) of both sexes during first encounter with crickets
(A) Major life-stage classification of mice from postnatal day 0 (P0) through adulthood (P75). The beginning of adolescence for mice is near P30, peaking near P35, the age range of the adolescents in our study. Darkening green indicates maturation and corresponds with data grouped by developmental stage in (A–G).
(B) Average time to first approach for each individual animal (gray circles) on the first day of the first exposure to a cricket in first 5 min. Group distributions are color coded by developmental stage (light green all adolescents, dark green all mature animals), A = adolescent, M = Mature.
(C) The average number of approaches per animal and group, yellow = adolescent female (AF), purple = adolescent male (AM), orange = mature female (MF), and blue = mature male (MM). For statistical comparison, groups were assigned a numeric value 1–4, respectively.
(D) Duration of contact data are plotted by individual, gray circles, and by age group, light versus dark green, adolescent versus mature adults, respectively.
(E) Polar plots of average cricket angles (probable view of target) of mice prior to cricket contact separated by age.
(F) Median cricket angle near the start of each approach segregated by age.
(G) Given an approach start, the probability an approach ended in contact = probability of intercept (see Videos S1, S2, S3, and S4). N = 11, 10, 11, and 10 mice, AF, AM, MF, and MM groups, respectively; Significance tested for using two-way ANOVA, sex by age, with Welch’s correction for multiple comparisons testing for identifying significant differences and to correct for multiple comparisons for data shown in A and B and G. Normality of each dataset was assessed using the Shapiro-Wilk test, Means or medians shown with overall distribution, Error bars are ± standard error of the mean or SE median derived via a bootstrapping procedure. The circular data shown in E and F were not significantly different between ages as determined by Watson’s U2 test: U2 = 0.1091 and 0.3365, respectively. Kruskal Wallis test followed by posthoc Mann-Whitney U to test for significant differences in nonparametric tests on non normal datasets. ∗ = p < 0.05, ∗∗ = p < 0.01 and ∗∗∗ = p < 0.001.
Predatory aggression differs most robustly by sex in C57BL6/J mice specifically during adolescence
To further understand how sex and age are related to hunting motivation perse vs. more fundamental sensory stimulus-response behaviors, we also measured and compared metrics directly related to predatory aggression (Figure 2). And, we specifically compared measures of predatory aggression on day one over three separate trials of cricket exposure after mice were fed ad libitum (Figures 2A–2C) versus day 2, the first day where mice were presented with three different cricket trials after food-restriction (food was removed from home cage during the preceding “dark cycle”, see STAR Methods, Figures 2D–2F). Over the first three trials of cricket exposure, there were significantly fewer trials with attacks (scored bites on cricket) in the adolescents as a group relative to the adults, no sex differences within age groups were detected for this measure (Figure 2A and Table S2). When we compared the percent of mice that captured on at least one of their three trials, we found that female adolescent mice never captured crickets compared to the small proportion of the other groups that did capture (Figure 2B). A capture was defined as biting, killing, and chewing a cricket. We then compared capture time as a proxy measure of motivation to kill between the groups that did have captures on the first day. In this case, we found that the adult males that capture and kill, did so much faster than the mature females that also captured (Figure 2C). This suggested that without overt manipulation of hunger, baseline biases that depend on age and sex exist in predatory aggression as the motivation to pursue crickets. Adolescents overall may be instead more motivated by the sensory features of the crickets themselves to approach and explore, rather than to kill or ingest. When considered with the overall increases in approaches over the first 5 min of interaction (Figure 1), this is evidence of a strong dissociation between approach drive and predatory aggression perse early in development. We next measured the same aspects of predatory aggression after all animals had been food restricted for up to 16 h as we have noted in past studies that this manipulation leads to the most significant increase in predatory aggression (rather than just exposure time or trial number), capture efficiency and killing behaviors.6,15 Continued food restriction coupled with experience then steadily leads to further increases in capture efficiency over 4–5 subsequent days of cricket presentation trials when capture efficiency plateaus. Here, we show that male adolescent mice display that largest increase in predatory aggression following food restriction with all mice in this group starting to attack crickets (Figure 2D). Similarly, the entire group of adolescent males began to capture on day 2, exhibiting a more significant percentage of the population that captured than that did not relative to all other groups (Figure 2E). Adolescent females also showed an increase in aggression following food-deprivation, as did adult females and males, but still did not display as much change in predatory aggression measures as the other groups when compared directly (Figures 2D–2F and See Table S2). Indeed, of the adolescent female mice that did hunt, their capture times were significantly higher than the other groups’ hunters (Figure 2F). This showed that the female adolescent mice were more resistant to the immediate effects of food-restriction on predatory behaviors. Importantly, all groups continue to significantly increase prey capture behavior performance over repeated days of cricket exposure with time-restricted feeding as previously reported for cohorts that were mostly male despite that both sexes were included6,15 (Figure 2G). We do show here that the adolescent male mice show the most rapid enhancement of prey capture performance over this trial structure with most mice capturing under 60 s by day 5 and the fastest prey capture times on days 5 and 6 (Figures 2H and 2G and see Table S3, fourth day exposed to crickets after food restriction). We also tested whether an individual animal’s weight predicted ultimate prey capture success, a proxy estimate of an animal’s predatory motivation, rather than age or sex by looking at the relationship between weight and prey capture performance times (Figure S2). We found no significant relationship between weight and prey capture performance (Figure S2). Indeed, outside of this specific comparison, adolescent male and female mice weigh approximately the same as each other until after adolescence,26 while adult female mice are significantly lighter than adult male mice,26 thus the sex differences described here that emerge at the same age, cannot be explained by baseline weight differences. While more in depth studies will be needed to further parse the direct causes of age and sex-dependent differences in predatory aggression in mice, and its influence by “hunger-state” our study bolsters the only existing classical study that also provided evidence for sex differences in predatory aggression in mice that was strain dependent.2 Importantly, our study adds additional insight related to sex-differences in this specific strain, C57BL6/J and developmental stage, as well as the interaction with food restriction.2
Figure 2.
Predatory attack behavior by age and sex
(A) Median Percent of trials per day w/attack response. Each mouse was exposed to 1 cricket at a time for up to 10 min constituting one trial. Each mouse then experienced up to 3, 10-min trials per day, light green is adolescent group, dark green is adult group.
(B) Percent of mice in each group that capture crickets (incidence of predatory aggression) without food restriction. Yellow = adolescent females, purple = adolescent males, orange = mature females and blue = mature males.
(C) Median capture time if a mouse captured and killed a cricket on a given trial on the first day, their capture times was averaged. If multiple capture occurred over the three trials. The adolescent female mice did not capture any crickets on the first day = N/A.
(D) Same as in A, yet all groups were first food restricted for the preceding 12–16 h and all groups displayed a significant difference in probability to attack relative to the adolescent females (Yellow).
(E) Same as B, after food restriction, all adolescent male mice now hunt.
(F) Same as in C, after food restriction, adolescent females that do capture take significantly longer to capture (latency) relative to all other groups.
(G) Mean capture times averaged across three trials per animal per day with food restriction preceding each trial day 2–6 (D2-D6).
(H) Percent of mice that reach a criterion of capturing under 60 s across three trials in one day. This criterion established from typical adult male data gathered in other studies reflecting group averages where further progress is not significantly changed. N = 11, 10, 11, and 10 mice, AF, AM, MF and MM groups, respectively. Normality of each dataset was assessed using the Shapiro-Wilk test, Non parametric tests performed on most datasets including probability data and Chi-square test performed on categorical hunting percentage and criterion reached data. Error bars are ± standard error of the median. Capture times normally distributed after the first few days, repeated measure ANOVA, sex, age and days (repeated) as analyzed factors. Welch’s correction for multipole comparisons. ∗ = p < 0.05, ∗∗ = p < 0.01 and ∗∗∗ = p < 0.001.
Innate visual orienting toward sweeping visual motion significantly differs by age and sex
Predation is a complex behavior involving multiple sensory modalities, motivations, and cross-modal sensory integration. We therefore sought to understand whether adolescent male and female mice displayed similar orienting responses to visual motion cues that can be utilized during prey capture. We employed our computerized, spontaneous perception of objects task, C-SPOT to detect and quantify innate orienting bias evoked by sweeping-motion stimuli as a function of age and sex in C57BL6/J mice.15,17 As done previously, we presented a sweeping stimulus along the bottom of the screen positioned as one of the walls of the arena for a total of 60 s to each mouse. We initially focused our analysis on orienting responses to speeds of motion shown previously to evoke the most approaches and fewest arrests in adult mice (2 cm/s).15 Consistent with live prey capture performance differences, female adolescent mice approached sweeping motion stimuli moving at speeds comparable to the movement of live insects more than all the other groups tested (speeds between 2 and 15 cm/s real world object speed, Figures 3A, 3B, S3A, and S3C; see Videos S5, S6, S7, and S8; Tables S4 and S5). In contrast, male adolescent mice were more likely to exhibit arrest responses in reaction to motion stimuli moving around 2 cm/s relative to all the other groups (Figures 3A–3E and S3A; see Videos S5, S6, S7, and S8). Unexpectedly we also observe a bias in orienting response type in adult male mice to faster moving stimuli, in which they are most likely to arrest relative to the other groups tested (Figures S3B, S3C, and S3E). We thus discovered that mice exhibit an innate bias in how they respond to sweeping visual motion stimuli moving at specific speeds that begins in adolescence and is differentially maintained in adulthood. Adult females do not retain their enhanced approach response toward visual motion that emerge during adolescence, yet the adult male still show an elevated arrest response to faster speeds of sweeping visual motion. These are unexpected observations that demonstrate that the two sexes of mice initially respond differently to sweeping visual motion that further changes from adolescence through adulthood.
Figure 3.
Innate orienting responses to sweeping visual motion in the lower field
(A) Ethograms showing the response of all four groups of mice to sweeping visual motion stimuli in the lower environment. Each row (gray line) of the ethogram is the response of an individual animal, white indicates time when animals are approaching stimulus, black is when they arrest in response to the stimulus. The magenta “9” highlights the ethogram corresponding to Videos S5, S6, S7, and S8.
(B) Mean number of approaches. Green box denotes significant difference in approach number of female adolescents.
(C) Mean number of arrests, red box denotes significant increase in arrest response in adolescent male mice.
(D) Percentage of mice in each group exhibiting at least one approach toward sweeping stimulus, white, and those that exhibit at least one arrest in response to the stimulus, black.
(E) Mean time to first response indicating their sensitivity to the stimulus at top, and likelihood that they approach the stimulus before arresting. Significance tested for using a two-way, ANOVA, sex by age, with Tukey’s HSD post hoc testing for identifying significant differences and to correct for multiple comparisons. N = 11, 10, 11, and 10 mice, AF, AM, MF, and MM groups, respectively. Normality of each dataset was assessed using the Shapiro-Wilk test, Means shown with overall distribution, Error bars are ± standard error of the mean. ∗ = p < 0.05, ∗∗ = p < 0.01 and ∗∗∗ = p < 0.001.
Discussion
In 1973, Dr Karla Butler was the first to document the idea that studying prey capture behavior in laboratory mouse strains of both sexes can provide insight in the genetic and cellular mechanisms underlying a form of motivated aggression that is ubiquitously expressed within a species.2 Moreover, the work made explicit the idea that “aggression” is not a unitary motivational process, as expressing aggressive behavior(s) depends upon ethological context, genetic background, and sex.2 We now significantly expand on this insightful foundation with behavioral data that: one, confirms Dr. Butler’s original observation that adult C57BL6 inbred strains of mice do not show significant sex-dependent differences in their initial propensity to attack crickets (Figures 2A and 2B), two, we provide direct evidence that predatory aggression in mice is indeed dissociable from visual processing that drives approach behavior, and three, surprisingly, related innate visual orienting responses driven by prey-related motion cues are robustly sexually dimorphic during adolescence in this strain. This all suggests that distinct circuit mechanisms that link sweeping visual motion to approach or arrest responses in mice are differentially affected during development to lead to adaptive behavioral choices in mature adults in the prey capture context. In other words, similar functional outcomes in terms of behavioral performance in a natural context like prey capture may be driven by distinct circuit level mechanisms in females versus males.
Importantly, our work provides evidence that “prey-like” visual motion is inherently salient during adolescence and is sufficient to drive rapid positive or arrest type orienting behavior in mice depending on the sex of this strain of mouse. This is consistent with previous studies demonstrating “sensory-triggered” predatory hunting controlled by specific subcortical circuits in mice.12 However, in adolescents the link between visual motion detection and aggressive predatory responses is immature and appears differentially gated in the sexes by hunger. Our data showing that adolescent males are more responsive than females to coupling their approach to attacks after just one night of food restriction supports that the two sexes differ in their baseline motivational state(s) that can be coupled to “sensory-triggered” predatory hunting perse.27 We think it is most probable that initial differences in capture success between the first and second days of hunting mostly reflect significant differences in internal motivational states related to hunger and/or baseline predatory aggression directly rather than large differences in the capability for perceptual or motor learning, but this idea now bears more detailed investigation based on the evidence of the current study. At least one other study of natural prey capture behavior, albeit in crabs, found sex-specific impacts of hunger on predatory approach behavior in males.4 Consistent with this idea generally is that male C57BL6 mice undergo a significantly steeper gain in body weight and size relative to females starting at postnatal day 35/5 weeks of age.26 Thus, early adolescence may be a unique time in the life span of mice where the two sexes are differentially motivated to meet differing metabolic demands.22,25,27 Notably, sex differences in diverse home cage behaviors such as nest building and saccharin preference are known to emerge during the onset of puberty in mice.22,25,28 Some sexually dimorphic behaviors with pubertal onset dissipate in adulthood, while others persist.22,25
Our data demonstrate that sex-differences in innate orienting responses evoked by visual motion are most robust during adolescence and indicate that each sex may go through a distinct developmental process that leads to more homogeneous behavioral responses in the adult. In females, immediately approaching object motion would have to be more strongly inhibited in the adult, while arrest responses would have to be inhibited in males to enable successful predatory approach. This idea would be consistent with a previous study showing that in male adult mice, hesitance and avoidance behaviors must be inhibited before attack and approach behaviors are optimally released in the presence of cockroaches.13 Our own previous work, while it included both sexes, was biased toward a larger male population.15 Interestingly, here, we find that adults of both sexes engage initially in alternating bouts of approach and “escape” when first encountering live crickets (Videos S1 and S2). This “back and forth” response is significantly attenuated once food restriction begins and success capturing and eating insects occurs.15 This might suggest then that females acquire their hesitance/inhibitory control over approach later in life. Further, the enhanced approach response in female adolescents that we observed is intriguing, but it remains unclear how females might specifically benefit from this bias during adolescence in nature as it is strongly uncoupled from predatory aggression itself. It is possible that it may give females an advantage in learning whether novel stimuli are threatening or rewarding early in life so that they can later quickly couple with predatory or defensive attack later in life. A field study of birds adapting to urban environments found significant sex differences in “boldness”/exploratory behaviors and avoidance behaviors that correlates with cortisol levels and survival.24 Further, one recent study shows that male and female adult mice have a different circuit configuration for responding to potential threats versus rewards routed from the ventral hippocampus to the nucleus accumbens.29 Female mice route both potential rewards and risks through the same circuit elements.29 It is fascinating to speculate that the approach bias that we observe may help females refine and tune such circuitry during encounters with ambiguous novel stimuli that raise arousal levels. In this case, we speculate that they could gather additional sensory information that can optimally balance rapid responses to potential threats versus highly rewarding stimuli in the environment. Indeed, sweeping visual motion stimuli may be inherently ambiguous alone, despite a bias that they may represent potentially nutritious food in some contexts.16 However, there is so far no field, scene statistics nor ecology data to support speculation about how this wiring or another might be used to flexibly route natural stimuli to adaptive outcomes in a sex-specific way in house mice of any strain.
Another formal possibility for the enhanced approach responses and contact seen in adolescent mice could relate to a reduction in perceived risk in the prey capture and sweeping motion stimulus context in both sexes of adolescents.30,31 Survival is not only dependent on the avoidance of threat, but of capitalizing on resources. Adolescence is characterized by an increase in risk-taking behavior.30 Mice do not disperse from their mothers until late adolescence or early sexual maturity, thus practicing hunting skills during the early to mid-adolescence stage provides them with training for adulthood without the risk of starving if their efforts are unsuccessful. It is still unclear whether the live cricket and/or the virtual stimulus were perceived as possible threats. Though the mice did not exhibit thigmotaxis nor adopt postures that typically indicate anxiety in adults, the known reduction in threat response to occur in adolescence presents a confound for interpretation of this state via behavioral analysis alone.22 Indeed, enhanced sensation seeking, exploration and impulsive responses to sensory stimuli in ambiguous or novel situations are thought to underlie human adolescent “risk-taking” behavior and are thought to be dissociable from “risk taking” behavior that emerges as a result of lacking higher-order risk assessment decision-making under “known” risk conditions.30 It is thought that one benefit that would outweigh the possible costs associated with enhanced risk-taking, regardless of its cause, at this stage of development is the ability to learn from the experiences themselves. Indeed, adolescence in mice is a period of heightened goal-directed learning and flexibility.22,31,32 It will be important in future work to understand whether female adolescent mice benefit in other learning paradigms from an enhanced approach responses toward external, salient stimuli.33
Limitations of the study
While this is the first systematic assessment of emerging sex differences in key aspects of hunting behavior and visually guided orienting during development, our subject pool encompassed the entire range of putative adolescence (∼15 days) in mice. Both sexes are matched in exact days of development as they were from the same litters, but, some specific days within this entire period may be underrepresented. This means that we do not know the exact relative moment in adolescence for each sex where differences in orienting arise, only that they do so within this range, not before P30 or after P45. Further, if the behavioral differences, as we might infer, are due to significant changes in sex-modulated hormone levels occurring during this developmental stage, we made no direct measure of hormones that significantly change during adolescence: cortisol, testosterone, and estrogen for example. However, we hypothesize that cell types in the hypothalamus or other brain nuclei known to be sexually dimorphic and differentially responsive to sex-modulated hormones in adolescent mice are candidate neural substrates to underlie our observed behavioral differences. Finally, the main finding that female adolescents approach visual stimuli most yet display the least predatory aggression does not suggest an obvious adaptive explanation in the context of prey capture perse, e.g., that they are more likely to feed well. Instead, we speculate that this enhanced “approachy-ness” may serve as a critical trait to drive experience-dependent refinement of sensory-motivational processes through increased stimulus exploring. In turn, this could lead females to better discriminate between aversive and potentially appetitive stimuli. However, we did not test these ideas directly.
Resource availability
Lead contact
Jennifer L. Hoy (jhoy@unr.edu).
Materials availability
This study did not generate new unique reagents. However, a parts list and tips to create and run the described behavioral assays can be obtained by request from the lead contact. Any additional requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jennifer L. Hoy.
Data and code availability
-
•
Data: Raw Video files and meta data can be found at https://data.mendeley.com/datasets/xhpzwcrsmw/1.
-
•
Code: original matlab-based code from Hoy et al.6: https://github.com/nielllab/niell-lab-analysis/tree/0f4b05c537e28adf05c112ee7ba00b2aabbe553d/prey%20capture/code.
-
•
Requests for additional details of analysis methods or behavioral methods will be fulfilled by the lead contact.
Acknowledgments
We would like to thank undergraduate researchers Suvrajyoti Rout, Stetson Necaise, and Aryanna Ortega for their support in handling experimental mice and manually confirming behavioral responses to visual stimuli in mice through watching videos and manually scoring. This work funded by NIH and NEI RO1 EY032101-01A1 awarded to J.L.H.
Author contributions
R.G.-O.: conceptualization (equal); writing– original draft (equal); writing– review and editing (equal); data curation (equal); investigation (lead); validation (equal); formal analysis (equal). K.A.: formal analysis (supporting); writing – review and editing (supporting); investigation (supporting). T.F.: software (supporting); writing – review and editing (supporting); validation (supporting); project management and training (equal). J.L.H.: conceptualization (equal); writing – original draft (equal); writing – review and editing (equal); data curation (equal); software (lead); funding acquisition (lead); formal analysis (lead); methodology (lead); validation (lead); visualization (lead); project management and training (equal).
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | Jackson Labs Jax.org |
Strain #:000664 |
| Software and algorithms | ||
| Matlab_R2024b | MathWorks Matlab.mathworks.com |
N/A |
| Original Analysis Code | https://github.com/nielllab/niell-lab-analysis/tree/0f4b05c537e28adf05c112ee7ba00b2aabbe553d/prey%20capture/code | N/A |
| Raw Data (videos& metadata) | https://data.mendeley.com/datasets/xhpzwcrsmw/1 | N/A |
| GraphPad Prism 10.0 | https://www.graphpad.com/ | N/A |
Experimental model and study participant details
All animals were used in accordance with protocols approved by the University of Nevada, Reno, Institutional Animal Care and Use Committee, in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Both male and female mice were used in this study. A total of 21 P30-45 adolescent mice and 21 P60-79 mature mice were included in the data presented. The number of male and female subjects studied are similar. The specific number used in each statistical comparison is noted in figure legends. Mice were group housed in same-sex cages with up to 5 animals per cage, never less than 3 in a cage, in an on-campus vivarium with ad libitum access to water and food (Envigo, Teklad diet, 2919), except where noted that mice experienced food restriction (FR). The vivarium was maintained on a 12 hour light/dark schedule, and all testing occurred within 3 hours of the dark to light transition. Food restriction consisted of removing the food hopper from cages just before the light to dark transition (before the entry to dark phase) and testing animals just after the dark to light transition, 12–16 hours after food was removed from the home cage. After hunting, mice were allowed to return to home cage with ad libitum access to their food hopper during the rest of their light phase cycle until it was once again removed prior to entry into the dark phase of their light cycle. Body weight measurements were taken to ensure that no participants lost more than %15 of their initial baseline weights.
Method details
Apparatus
The cages of mice used for experimentation were transferred from the vivarium into a light-proof, double-walled sound dampening room in order to control environmental conditions. During all data acquisition sessions, the door to the room was closed and the lights were turned off. For each session, each mouse was individually removed from its home cage and placed into a square, white acrylic open field arena with white vinyl flooring, 60 cm long x 60 cm wide x 30 cm high. One set of opposing sides are white acrylic walls and the other two opposing sides are composed of Hewlett Packard VH240a video monitors measuring 60.5 cm diagonally, with a vertical refresh rate of 60 Hz and 1920x1080 pixel resolution. A solid white background was projected on each monitor in order to display even lighting throughout the arena which measured 250 cd/m2 of luminance. One of the monitors was used to display the visual stimulus. A Logitech HD Pro Webcam C920 digital camera was placed suspended overhead to record mouse and stimulus positions at 30 frames per second throughout each session.
Visual stimuli
Visual stimuli were generated with MATLAB Psychophysics toolbox (Brainard, 1997)34 and displayed on an LCD monitor (60 Hz refresh rate, ∼50 cd/m2 luminance) in a dark room. The computer monitors replaced two sides of a 4-sided behavioral arena. To mimic insect proportions, we displayed a small black ellipse, 2 x 1 cm, generated by a customized MATLAB Psychophysics toolbox script, which was programmed to move back and forth horizontally across the monitor with the center of the stimulus consistently 2.5 cm above the floor. Objective stimulus speed was varied over three steps between 2 cm s−1 to 50 cm s−1 and presented in a random order. Once stimuli traversed the screen, they reappeared from where they exited after 1second and traversed in the opposite direction until the full 60 s trial was complete. Mice were exposed to relative speeds varying from 2 to approximately 300 deg s−1 at the retina which was estimated based on average distance from the screen where mice either started their approach or an arrest response began as previously reported in Procacci et al.15
Behavior
Baseline mice: Prior to testing, mice were acclimated to handlers (two different experimenters in this study) and the behavior arena for 2 days: days 1 and 2 included three, 3-minute handling sessions; on day 2, mice were exposed to the behavior arena for 3 5-minute sessions each. The arena floor was cleaned thoroughly with 70% EtOH after each mouse was removed to mitigate odor distractions. Testing did not begin until the present mouse moved throughout the arena, away from walls, and demonstrated self-grooming behaviors and voluntarily entered the experimenters cupped hand(s) which indicated low levels of general anxiety and novelty in the testing environment. Mice in the cohort who did not meet these criteria by the end of the third day prior to testing received an extra day of handler and arena acclimation identical to day 2. Mice with an extra handling day did not vary by sex, age nor constituted entire home cage cohorts. Rather, extra handling was required in usually one mouse co-housed with up to 4 other cage mates that did not require additional handling when it occurred. Raw behavioral videos are accessible for observation at: “Adolescent Versus Adult Mouse Innate Visual Responses”, Mendeley Data, V1, https://doi.org/10.17632/xhpzwcrsmw.1.
On testing day, each mouse was placed in the arena for a 1-minute habituation period. This period was used as a control trial to assess mouse behavior in the absence of a stimulus. After this period, the stimulus was presented on the display monitor described above. The Virtual stimuli were presented in random order for 60 seconds each total. For live-prey exposure, each mouse was placed into the arena with a live cricket for a total of 3 rounds, up to 10 minutes each. If the mouse caught the cricket within this period of time, it was given up to a total of 3 crickets during that 10-minute time window. If it did not catch the cricket within 10 minutes, the mouse was returned to its home cage and the arena was cleaned in preparation for the next mouse to be tested. After testing, all mice were returned to their home cages with standard food. Each session was recorded as a separate video for a total of 5 videos per mouse on test day.
Quantification and statistical analysis
DeepLabCut35 was used to digitize and extract 2-dimensional coordinates of the mouse’s nose, two ears and body center, as well as the center point of the stimulus (cricket or ellipse stimulus), throughout the video recordings at 30 frames per second. These tracks were entered into customized MATLAB scripts to extract behavioral parameters such as mouse and stimulus/cricket speed, stimulus/cricket angle, and range between mouse and stimulus/cricket.
An arrest was defined as any time the mouse’s nose and body moved less than 0.5 cm/sec for a duration of 0.5–2 seconds. Arrests that occurred in the absence of a visual stimulus, or when the stimulus was more than 130 degrees from the bearing of the nose, were excluded from analysis of visually-driven arrest responses. There were no significant differences between groups on the number of arrests exhibited without stimuli (data not shown). An approach was defined as any time the mouse’s nose came within 4 cm of the stimulus center after moving toward the stimulus for a distance of at least 5 cm, and at an average approach speed of at least 15 cm/sec. Using these definitions, we computed the percentage of stimulus trials in which each behavior was observed, as well as the number of arrests and approaches that occurred during individual trials.
Statistics were performed using MATLAB and GraphPad Prism software. Where means are reported, two-way ANOVA (sex and age) or repeated measures two-way ANOVA (sex, age and repeated days of exposure) were used to identify main effects followed by posthoc testing with correction for multiple comparisons when identifying the specific significant differences between groups. Where medians are reported, the non-parametric Kruskal Wallis test was used followed by rank sum testing between group medians. Where percentages are compared, a Chi-square test was used to detect significant differences in expected group percentages of two opposing conditions (e.g. attack versus not attack). Test results with a p-value of <0.05 were considered significant, but we note where p-values were below 0.01 and 0.001, ∗ = p < 0.05, ∗∗ = p < 0.01 and ∗∗∗ = p < 0.001. Actual p-values are listed in supplemental data tables that accompany each figure along with summaries of testing and central tendencies and whether assumptions of normality are met. No attempt was made to normalize data sets as values did not very on logarithmic scales, instead, there were significant skews towards basement values (zero/none) or ceiling (100%-max observable) measures.
Published: July 22, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113145.
Supplemental information
References
- 1.Matter F. The Neuroethology of Predation and Escape i–xvii. John Wiley & Sons, Ltd; 2016. [DOI] [Google Scholar]
- 2.Butler K. Predatory behavior in laboratory mice: strain and sex comparisons. J. Comp. Physiol. Psychol. 1973;85:243–249. doi: 10.1037/h0035008. [DOI] [PubMed] [Google Scholar]
- 3.Gomes D.G.E., Page R.A., Geipel I., Taylor R.C., Ryan M.J., Halfwerk W. Bats perceptually weight prey cues across sensory systems when hunting in noise. Science. 2016;353:1277–1280. doi: 10.1126/science.aaf7934. [DOI] [PubMed] [Google Scholar]
- 4.Salido C.A., Gancedo B.J., Tomsic D. To escape or to pursue: opposite decision making concerning a single moving object is influenced by starvation and sex. J. Exp. Biol. 2023;226 doi: 10.1242/jeb.245297. [DOI] [PubMed] [Google Scholar]
- 5.Cronin T.W. In: Ecology Of Predator-Prey Interactions. Barbosa P., Castellanos I., editors. Oxford; 2005. The Visual Ecology of Predator-Prey Interactions; pp. 105–138. [DOI] [Google Scholar]
- 6.Hoy J.L., Yavorska I., Wehr M., Niell C.M. Vision Drives Accurate Approach Behavior during Prey Capture in Laboratory Mice. Curr. Biol. 2016;26:3046–3052. doi: 10.1016/j.cub.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngo V., Gorman J.C., De la Fuente M.F., Souto A., Schiel N., Miller C.T. Active vision during prey capture in wild marmoset monkeys. Curr. Biol. 2022;32:3423–3428.e3. doi: 10.1016/j.cub.2022.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sillar K.T., Picton L.D., Heitler W.J. The Neuroethology of Predation and Escape. John Wiley & Sons, Ltd; 2016. Vision; pp. 1–34. [DOI] [Google Scholar]
- 9.Hoy J.L., Bishop H.I., Niell C.M. Defined Cell Types in Superior Colliculus Make Distinct Contributions to Prey Capture Behavior in the Mouse. Curr. Biol. 2019;29:4130–4138.e5. doi: 10.1016/j.cub.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianco I.H., Engert F. Visuomotor transformations underlying hunting behavior in zebrafish. Curr. Biol. 2015;25:831–846. doi: 10.1016/j.cub.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewert J.P. Neural correlates of key stimulus and releasing mechanism: a case study and two concepts. Trends Neurosci. 1997;20:332–339. doi: 10.1016/s0166-2236(96)01042-9. [DOI] [PubMed] [Google Scholar]
- 12.Shang C., Liu A., Li D., Xie Z., Chen Z., Huang M., Li Y., Wang Y., Shen W.L., Cao P. A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nat. Neurosci. 2019;22:909–920. doi: 10.1038/s41593-019-0405-4. [DOI] [PubMed] [Google Scholar]
- 13.Rossier D., Franca V.L., Salemi T., Natale S., Gross C.T. A neural circuit for competing approach and defense underlying prey capture. Proc. Natl. Acad. Sci. USA. 2021;118:e2013. doi: 10.1073/pnas.2013411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filosa A., Barker A.J., Dal Maschio M., Baier H. Feeding State Modulates Behavioral Choice and Processing of Prey Stimuli in the Zebrafish Tectum. Neuron. 2016;90:596–608. doi: 10.1016/j.neuron.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Procacci N.M., Allen K.M., Robb G.E., Ijekah R., Lynam H., Hoy J.L. Context-dependent modulation of natural approach behaviour in mice. Proc. Biol. Sci. 2020;287 doi: 10.1098/rspb.2020.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartje V., Illemann M.J., Schmidtke D. Motion cues increase focused attention towards purely visual stimuli in a nocturnal primate and drive stimulus interaction and approach/avoidance in a context-dependent manner. Am. J. Primatol. 2021;83 doi: 10.1002/ajp.23286. [DOI] [PubMed] [Google Scholar]
- 17.Allen K., Gonzalez-Olvera R., Kumar M., Feng T., Pieraut S., Hoy J.L. A binocular perception deficit characterizes prey pursuit in developing mice. iScience. 2022;25 doi: 10.1016/j.isci.2022.105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamil A.C., Sargent D. Garland STPM Press; 1981. Foraging Behavior: Ecological, Ethological, and Psychological Approaches. [Google Scholar]
- 19.Gire D.H., Kapoor V., Arrighi-Allisan A., Seminara A., Murthy V.N. Mice develop efficient strategies for foraging and navigation using complex natural stimuli. Curr. Biol. 2016;26:1261–1273. doi: 10.1016/j.cub.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brust V., Schindler P.M., Lewejohann L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus) Front. Zool. 2015;12:S17. doi: 10.1186/1742-9994-12-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pocock M.J.O., Hauffe H.C., Searle J.B. Dispersal in house mice. Biol. J. Linn. Soc. Lond. 2005;84:565–583. doi: 10.1111/j.1095-8312.2005.00455.x. [DOI] [Google Scholar]
- 22.Moore E.M., Linsenbardt D.N., Melón L.C., Boehm S.L. Ontogenetic differences in adolescent and adult C57BL/6J and DBA/2J mice: Anxiety-like, locomotor, and consummatory behaviors. Dev. Psychobiol. 2011;53:141–156. doi: 10.1002/dev.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathod Y.D., Di Fulvio M. The feeding microstruture of male and female mice. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atwell J.W., Cardoso G.C., Whittaker D.J., Campbell-Nelson S., Robertson K.W., Ketterson E.D. Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 2012;23:960–969. doi: 10.1093/beheco/ars059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romeo R.D., Patel R., Pham L., So V.M. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci. Biobehav. Rev. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter S.H., Kästner N., Loddenkemper D.H., Kaiser S., Sachser N. A time to wean? Impact of weaning age on anxiety-like behaviour and stability of behavioural traits in full adulthood. PLoS One. 2016;11:e0167652. doi: 10.1371/journal.pone.0167652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z., Wang B., Lu J. Behavioral and physiological responses of striped field mice (Apodemus agrarius) to predator odor. Integr. Zool. 2011;6:334–340. doi: 10.1111/j.1749-4877.2011.00262.x. [DOI] [PubMed] [Google Scholar]
- 28.Reiber M., Koska I., Pace C., Schönhoff K., von Schumann L., Palme R., Potschka H. Development of behavioral patterns in young C57BL/6J mice: a home cage-based study. Sci. Rep. 2022;12:2550. doi: 10.1038/s41598-022-06395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muir J., Iyer E.S., Tse Y.C., Sorensen J., Wu S., Eid R.S., Cvetkovska V., Wassef K., Gostlin S., Vitaro P., et al. Sex-biased neural encoding of threat discrimination in nucleus accumbens afferents drives suppression of reward behavior. Nat. Neurosci. 2024;27:1966–1976. doi: 10.1038/s41593-024-01748-7. [DOI] [PubMed] [Google Scholar]
- 30.Romer D., Reyna V.F., Satterthwaite T.D. Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Dev. Cogn. Neurosci. 2017;27:19–34. doi: 10.1016/j.dcn.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin W.C., Wilbrecht L. Making sense of strengths and weaknesses observed in adolescent laboratory rodents. Curr. Opin. Psychol. 2022;45 doi: 10.1016/j.copsyc.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Xia L., Master S.L., Eckstein M.K., Baribault B., Dahl R.E., Wilbrecht L., Collins A.G.E. Modeling changes in probabilistic reinforcement learning during adolescence. PLoS Comput. Biol. 2021;17 doi: 10.1371/journal.pcbi.1008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilbrecht L., Davidow J.Y. Goal-directed learning in adolescence: neurocognitive development and contextual influences. Nat. Rev. Neurosci. 2024;25:176–194. doi: 10.1038/s41583-023-00783-w. [DOI] [PubMed] [Google Scholar]
- 34.Brainard D.H. The Psychophysics Toolbox. Spat. Vis. 1997;10:433–436. doi: 10.1038/s41583-023-00783-w. [DOI] [PubMed] [Google Scholar]
- 35.Mathis A., Mamidanna P., Cury K.M., Abe T., Murthy V.N., Mathis M.W., Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018;21:1281–1289. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: Raw Video files and meta data can be found at https://data.mendeley.com/datasets/xhpzwcrsmw/1.
-
•
Code: original matlab-based code from Hoy et al.6: https://github.com/nielllab/niell-lab-analysis/tree/0f4b05c537e28adf05c112ee7ba00b2aabbe553d/prey%20capture/code.
-
•
Requests for additional details of analysis methods or behavioral methods will be fulfilled by the lead contact.