Abstract
Introduction
Oral zinc supplementation is a well-known intervention, widely used to prevent infectious diseases. However, it is also necessary to demonstrate that it achieves the maximum benefit at the lowest possible cost in pneumonia in children, particularly in low- and middle-income countries. This economic evaluation aims to estimate the cost-utility and cost-effectiveness of zinc supplementation for the prevention of community-acquired pneumonia in children aged 1 to 6 years in Colombia.
Materials and methods
Using the decision tree analysis, we estimated the expected benefits and costs of zinc supplementation versus no supplementation based on data on effectiveness and cost previously published in Colombia. The perspective of the present economic evaluation is the perspective of society with a time horizon of 1 year.
Results
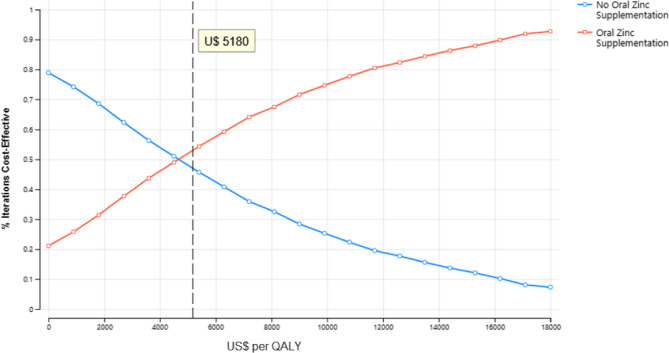
The net monetary benefit (NMB) with oral zinc supplementation was US$ 5,063.3 (95% UI: 5,061–5,065), compared to US$ 5,062.6 (95% UI: 5,060–5,064) without supplementation. The estimated cost effectiveness ratio was U$ 4 873 per QALY and U$ 10 324 per year lived gained. In the probabilistic sensitivity analysis, oral zinc supplementation was the most cost-effective therapy in the 53.4% of replications at willingness-to-pay (WTP) of U$ 5180 values.
Conclusion
In conclusion, oral zinc supplementation in children aged 1 to 6 years in Colombia appears to be a potentially cost-effective strategy for the prevention of community-acquired pneumonia compared to non-supplementation. These findings should be interpreted with caution given the small absolute difference in benefits and the marginal incremental net monetary benefit observed. This result becomes an input for the construction of public health policies aimed at reducing the burden of disease generated by respiratory infections in Colombia, as well as serving as an example and motivation to assess the cost and utility of these strategies in other developing and developed countries.
Introduction
Pneumonia stands as the leading cause of mortality among paediatric populations in comparison to other infectious diseases. Globally, pneumonia contributes to over 700,000 fatalities in children under the age of five annually, equivalent to approximately 2,000 deaths per day [1]. The rate of decline in pneumonia-related mortality within this age has considerably trailed the progress witnessed in addressing mortality caused by other infectious diseases [2]. The disease burden associated with paediatric pneumonia exhibits a strong correlation with socio-economic factors, encompassing issues such as malnutrition, deficient breastfeeding practices, limited access to safe drinking water, exposure to indoor and outdoor air pollution, and insufficient healthcare access [3]. Moreover, pneumonia occurrences in children under the age of six entail substantial out-of-pocket expenses in developing nations, thereby imposing a substantial economic burden on society as a whole [4].
Among the factors mentioned above, one of the potentially modifiable ones is nutritional problems. Zinc supplementation in children as a preventive strategy has been shown to reduce the incidence of diarrhoea and pneumonia in addition to correcting zinc deficiency in malnourished children [5]. A meta-analysis of six controlled clinical trials with 5193 participants showed that zinc supplementation reduced the incidence of pneumonia by 13% (RR 0.87; CI 95% 0.81 to 0.94), and the prevalence of pneumonia by 41% (RR 0.59; CI 95% 0.35 to 0.99) [6]. Another meta-analysis incorporating 28 randomized controlled trials with 237,068 participants revealed that zinc supplementation significantly reduced the risk of all-cause mortality by 16% in children (RR: 0.84; CI 95% 0.74, 0.96) [7].
Despite these benefits, when the cost-effectiveness of zinc supplementation in infectious diseases has been evaluated, conflicting results have been obtained. In Colombia, pneumonia accounts for approximately 16% of mortality in children under 5, with an estimated incidence of 76 per 1,000 children annually. A national cost-of-illness study reported that each episode can cost over US$1,000, highlighting a substantial economic impact on both families and the healthcare system [8, 9]. While studies have been published that have shown that such zinc supplementation is cost-effective in preventing acute diarrheal disease [10], recent studies in developing countries, including Colombia, this cost-effectiveness has not been demonstrated [11, 12]. On the other hand, the fact that it is a potentially low-cost intervention, with extensive experience of safety and use at the population level; makes zinc supplementation a very attractive health policy for reducing the burden of disease generated by any disease. However, beyond demonstrating its effectiveness and safety, it is also essential to show that zinc supplementation is an efficient intervention—delivering maximum benefit at the lowest possible cost [13–17]. This is where economic evaluations play an important role in the construction of public health policy [18]. The objective of this economic evaluation is to estimate the cost-utility and cost-effectiveness of zinc supplementation for the prevention of community-acquired pneumonia in children aged 1 to 6 years in Colombia.
Materials and methods
Using the decision tree analysis, we estimated the expected benefits and costs of zinc supplementation versus no supplementation. This technique was used since estimates of the costs and benefits of this intervention were made for an acute episode of community-acquired pneumonia, and not recurrent events over time. This decision tree has two branches that follow the first decision node: the zinc supplementation strategy and the zinc non-supplementation strategy, see Fig. 1. The model simulates a hypothetical cohort of children aged 1–6 years, entering the model before pneumonia onset, allowing estimation of the preventive effect of zinc. Then in each branch, a probability node is opened with two possible events: the occurrence or not of pneumonia. For each of these events, a probability node is opened again with two possible events death from pneumonia or general cause or survival. The expected benefits of supplementation were estimated in quality-of-life adjusted life years (QALYs).
Fig. 1.
Decision tree
The intervention evaluated was oral zinc supplementation versus no supplementation, in children aged 1 to 6 years without chronic diseases (congenital heart disease, malnutrition, chronic lung disease, congenital diseases). This economic evaluation was developed in the context of the Colombian health system. Zinc supplementation is currently funded by private and public health insurers in Colombia. However, such supplementation is not currently indicated as a preventive strategy within the Colombian management guidelines for childhood pneumonia.
The time horizon was 1 year. A 1-year time horizon was selected because the model addresses a first acute episode of community-acquired pneumonia. Although shorter time frames capture the acute nature of the illness, a 12-month horizon allows for a more comprehensive assessment of costs and outcomes, without modelling recurrences or long-term sequelae, which were beyond the scope of this study. Given this time horizon, no discount rates were applied to the expected costs or benefits. The perspective of the present economic evaluation is the perspective of society since direct medical costs, out-of-pocket expenses, and costs associated with loss due to labour productivity were included.
As shown in Fig. 1, the decision tree requires the probability of community-acquired pneumonia, pneumonia mortality, and overall cause; as well as the relative risks of Zinc supplementation for clinical outcomes such as incidence of pneumonia, pneumonia mortality, and risk of all-cause mortality.
The incidence of pneumonia in children aged 1 to 6 years of age was extracted from the study published by Camacho et al., Table 1 [8]. In this study, the authors report the mortality and incidence of community-acquired pneumonia in the City of Bogotá confirmed by radiological diagnosis in this age group. The probability of death from general causes in children under 5 years of age was extracted directly from the national statistics reported by DANE [19]. All the probabilities extracted were annual, and since the time horizon of the model is one year there was no need to modify them. We used different meta-analyses for different clinical outcomes to reflect the most robust and specific evidence available. For pneumonia incidence, we selected the Lassi et al. meta-analysis [6], which focused on that outcome. For pneumonia mortality, we used Rouhani et al. [7], which provided the most updated pooled estimate for mortality outcomes in children. The Lassi et al. meta-analysis included six randomized controlled trials involving 5193 children aged 1 to 6 years from the general community or attending a community clinic for acute diarrhoea not complicated. Children with malnutrition, diarrhoea for more than 7 days, or suspected chronic diseases were excluded from these clinical trials. These clinical trials evaluated doses between 10 and 35 mg of oral zinc every 24 h between 12 and 24 months. Zinc supplementation reduced the incidence of pneumonia (diagnosis based on age-specific rapid breathing and confirmed by chest examination or chest x-ray) by 21% RR 0.79[95% CI 0.71,0.88] I2 = 0%. The Rouhani P, et al. meta-analysis [7] included twenty-eight clinical trials conducted in children who are healthy or have acute diarrhoea with no complications. The studies evaluated supplementation with doses between 5 and 20 mg of zinc orally every 24 h for 12 months. Zinc supplementation significantly reduced the risk of death from pneumonia (Diagnosis based on age-specific rapid breathing and confirmed by chest examination or chest x-ray) (RR 0.70: 95% CI 0.64, 0.98), I2 = 21.2%). Utilities were extracted from a utility assessment study of parent preferences for paediatric health outcomes made in 4016 participants [20]. Utilities represent health-related quality of life on a scale from 0 (death) to 1 (perfect health). They were used to calculate QALYs by multiplying utility by time spent in a health state. These utilities were assumed constant without any change during the time horizon of our estimation.
Table 1.
Variables defined in the base case
| Variable | Deterministic análisis* | Probabilistic analysis * | Reference | |||
|---|---|---|---|---|---|---|
| Value | Range | Distributión | Parameters | |||
| incidence of pneumonia | 0.076 | ± 25% | Beta | α = 14,75 | β = 187,41 | (1) |
| Pneumonia mortality | 0.0027 | ± 25% | Beta | α = 14 | β = 640 | (1) |
| All-causes mortality | 0.002 | ± 25% | Beta | α = 8147 | β = 3,903,808 | (2) |
| Cost episode pneumonia | 1008 | ± 25% | Pharameters | α = 18 | λ = 0,0178 | (9) |
| Cost_mg_Oral_Zinc | 0.009 | ± 25% | Gamma | α = 16 | λ = 1777 | |
| RR for pneumonia mortality | 0.803 | 0.64–0.98 | LogNormal | µ=−0,35 | ds = 0,108 | (6) |
| RR for incidence of pneumonia | 0.791 | 0.71–0.88 | LogNormal | µ=−0,23 | ds = 0,054 | (6) |
| RR for all causes of mortality | 0,842 | 0.74–0.96 | LogNormal | µ=−0.174 | ds = 0.066 | (7) |
| Disutility associated with pneumonia | 0.06 | ± 25% | Beta | α = 16 | β = 266.66 | (20) |
*source of the parameter values used in the deterministic and probabilistic model
The cost of the pneumonia episode was extracted from a cost-of-illness study conducted in Colombia by Moyano Ariza et al. [9], which estimated direct and indirect costs using primary data collected from 88 hospitalized children and 90 caregivers. This study estimated the direct costs of out-of-pocket expenses, costs associated with loss of labour productivity. The estimation of direct medical costs was made from information obtained from 88 cases of children hospitalized by pneumonia. The out-of-pocket expenses (included private insurance or public health insurance) and costs associated with loss of labour productivity were estimated on 90 parents or caregivers of children hospitalized for pneumonia. Since these costs were estimated in 2018, they were all adjusted by the inflation rate. For zinc supplementation, the annual cost per child was calculated based on a 10 mg daily dose using unit costs from SISMED 2021 [21]. The threshold used in the net monetary benefit formula refers to the willingness-to-pay per QALY gained, set at US$5180 as recommended in recent economic evaluations in Colombia [22]. Since utilities and relative risks do not come from the Colombian population, they were subjected to probabilistic sensitivity analysis as detailed below as recommended by Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement [23]. Drug costs were taken from the National Drug Price Information System (SISMED, 2021) [21]. All cost costs were transformed to 2022 costs using official inflation data in Colombia. We used US dollars (Currency rate: US$ 1.00 = COP$ 3,900) to express all costs in the study [19]. The incremental cost-effectiveness ratio (ICER) was calculated using the following formulae:
 |
Also, we estimated the net monetary benefit (NMB). NMB represents the value of an intervention in monetary terms [24]. NMB is calculated as (incremental benefit x threshold) – incremental cost. Incremental NMB measures the difference in NMB between alternative interventions, a positive incremental NMB indicates that the intervention is cost-effective compared with the alternative at the given willingness-to-pay threshold.
Sensitivity analysis
Deterministic sensitivity analysis was presented in the tornado graph. Likewise, for those variables in which a threshold value was identified, they were presented with one-way sensitivity graphs. Probabilistic sensitivity analysis was performed using Monte Carlo simulation with 10,000 iterations of each model parameter. For the probabilistic sensitivity analysis of the probabilities, a theoretical beta distribution was assumed in each of them [25, 26]. For the probabilistic sensitivity analysis, it was assumed that the costs have a gamma distribution and the relative risks a normal theoretical LogRank distribution. The ranges of cost and utilities presented in the results come from Monte Carlo simulations based on variability in primary data (costs, probabilities, utilities), not statistical inference from sample means. Cost-effectiveness calculations, in the base case and all sensitivity analyses, were performed in the TreeAge Pro 2023 software.
Results
The main results are presented in Table 2. The Montecarlo simulation showed that oral zinc supplementation was the least costly strategy and produced the greatest life years (LYs) gained and QALYs. The expected annual cost per patient with oral zinc supplementation was US$ 87.42 (95% uncertainty interval (95% UI) 86,0–88,8 14 772) and US$ 76.03 (95% UI 74.4–77.7) for no supplementation. The QALYs estimated with oral zinc supplementation were 0.994 (95% UI % 0.9942–0.9944) and 0.992 (UI 95% 0.9918–0.9921) for no supplementation. The net monetary benefit with oral zinc supplementation was US$ US$ 5063.3; (95% UI 5061–5065) and US$ 5062.6; (95% UI 5060–5064) for no supplementation. The estimated cost-effectiveness ratio was U$ 4 873 per QALY and U$ 10 324 per LYs, Table 3. The INMB was positive US$ 0.718 (95% UI US$−0.3 to US$1.7).
Table 2.
MonteCarlo probability distribution statistic
| Statistic | No Oral Zinc Supplementation | Oral Zinc Supplementation | INMB (U$) | ||||
|---|---|---|---|---|---|---|---|
| Cost (U$) | Effectiveness (QALYs) | NMB (U$) | Cost (U$) | Effectiveness (QALYs) | NMB (U$) | ||
| Mean | 76,0 | 0,992 | 5062,6 | 87,4 | 0,994 | 5063,3 | 0,7 |
| Std Deviation | 26,3 | 0,002 | 32,8 | 22,4 | 0,001 | 26,5 | 16,3 |
| Minimum | 18,3 | 0,979 | 4935,0 | 37,6 | 0,985 | 4958,0 | −71,6 |
| Median | 72,4 | 0,992 | 5067,2 | 85,3 | 0,994 | 5065,4 | 1,1 |
| Maximum | 164,3 | 0,996 | 5139,0 | 171,1 | 0,998 | 5123,4 | 59,9 |
| Variance | 689,6 | 0,000 | 1076,4 | 503,6 | 0,000 | 700,5 | 266,5 |
| 95%% Lower Bound | 74,4 | 0,992 | 5060,6 | 86,0 | 0,994 | 5061,7 | −0,3 |
| 95%% Upper Bound | 77,7 | 0,992 | 5064,6 | 88,8 | 0,994 | 5065,0 | 1,7 |
Table 3.
Montecarlo cost effectiveness ranking report
| Estrategia | Cost (U$) | Incremental cost (U$) | Effectiveness (QALYs) | Incremental effectiveness (QALYs) |
ICER | NMB |
|---|---|---|---|---|---|---|
| No oral Zinc supplementation | 76,03 | 0,9920 | 5062,60 | |||
| Oral Zinc supplementation | 87,42 | 11,39 | 0,9943 | 0,0023 | 4873 | 5063,32 |
Sensitivity analysis
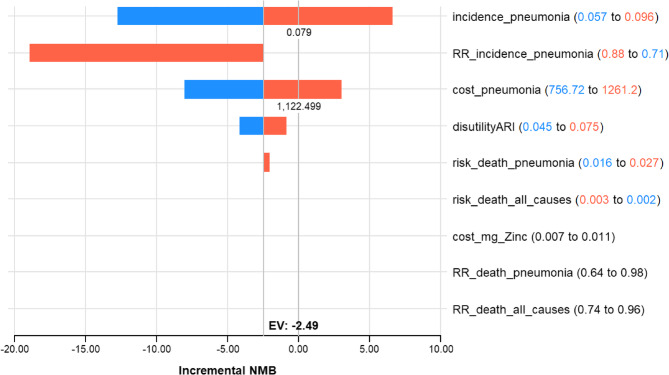
Extensive sensitivity analyses found the results to be robust to changes in most model assumptions. In the sensitivity analyses, our base case results were robust to variations in disabilities, most of the transition probabilities, and cost. Figure 2, Tornado Diagram showing variables with the greatest impact on the incremental net monetary benefit (INMB) between zinc supplementation and no supplementation at a WTP threshold of $5180. We identify two thresholds in the analysis. If the incidence of pneumonia is 0.79 or higher; the incremental net monetary benefit will be positive and oral Zinc supplementation would also be cost-effective compared to no supplementation; and if the cost of an episode of pneumonia is U$ 1 222 the incremental net monetary benefit will be positive and oral Zinc supplementation would also be cost-effective compared to no supplementation, Fig. 2.
Fig. 2.
Tornado Diagram: Incremental NMB: Suplementacion oral con Zinc vs. No suplementacion oral con Zinc (WTP: 5180.00)
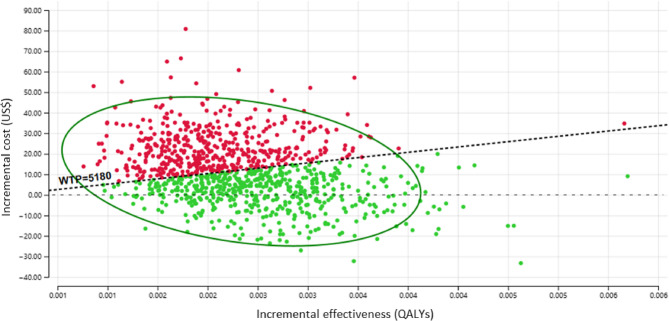
The results of the probabilistic sensitivity analysis are graphically represented in the cost-effectiveness plane, Fig. 3. The scatter plot revealed that 67% of the ICER simulations fell in quadrant I (below the WTP threshold) or quadrant IV (lower QALYs but higher cost), while only 21% were located in quadrant I above the threshold. In the probabilistic sensitivity analysis, oral zinc supplementation was the most cost-effective therapy in the 53.4% of replications at willingness-to-pay of U$ 5180 values Fig. 4.
Fig. 3.
Incremental cost-effectiveness scatterplot
Fig. 4.
Cost-effectiveness acceptability Curve
Discussion
Zinc supplementation children aged 1 to 6 years in Colombia is a cost-effective strategy for the prevention of community-acquired pneumonia compared to non-supplementation. This result becomes an input for the construction of public health policies aimed at reducing the burden of disease generated by respiratory infections in Colombia; as well as serving as an example and motivation to assess the cost and utility of these strategies in other developing and developed countries.
In our analysis, oral supplementation obtained both an ICER and NMB superior to non-supplementation when adjusting for the gain in years of life by quality. However, when measuring benefit in years of life gained (LYs), but not adjusted for quality of life, oral zinc supplementation was not cost-effective. This is explained by the differences in the magnitude of expected benefit between the two analyses. The incremental benefit was twice when benefits were measured in terms of QALYs than LYs (0.0023 vs. 0.0011), see Table 2 and this decreased the ICER and increased INMB. This result highlights the importance of adjusting the years of life for their quality for health decision-making, as recognized by health agencies such as NICE in the United Kingdom or the IETS in Colombia [27, 28]. While the incremental QALY gain with zinc supplementation is modest (0.0023), it remains significant enough to justify a cost-utility approach. If clinical equivalence were assumed, however, a cost-minimization analysis might have been appropriate. However, given the presence of a measurable gain in health outcomes, we maintain that a cost-utility analysis is the most appropriate method for this evaluation. If oral zinc supplementation were to be adopted as a national public health strategy, a comprehensive impact analysis would be essential to assess affordability and sustainability. Such an analysis could estimate the total annual cost of providing supplementation to all eligible children (e.g., those aged 1–6 years) and compare it against the potential reduction in pneumonia-related hospitalizations and medical expenditures. This would provide valuable information for policymakers on the scalability of this intervention within the Colombian healthcare system.
Sensitivity analysis demonstrates that supplementation is a cost-effective strategy, especially in settings with high incidence of community-acquired pneumonia. This result is particularly important for the implementation of this strategy within the public policy of prevention of acute respiratory infection. Directly, this intervention would have a great impact in regions far from large urban centers such as the Amazon or the Pacific, with the highest rates of morbidity due to respiratory infection in Colombia [29]. While this finding needs to be replicated in other developing countries; if it is confirmed, it could become a global strategy in regions with a high incidence of respiratory infection such as sub-Saharan Africa, South America, and Southeast Asia; or in economically depressed areas with high rates of respiratory infection in developed countries [30].
The differences in NMB between the supplementation strategy and without supplementation are small. This is primarily due to the WTP used in our study. Despite the existence of two recent WTP estimates in Colombia [22, 31, 32]. As expected, if with lower WTP like the one used here zinc supplementation is cost-effective with higher WTP like the one recommended by WHO this intervention is still cost-effective. The differences in NMB between strategies are not due to the WTP threshold per se—which is fixed—but to the differences in incremental benefits between strategies. Higher QALY gains with supplementation drive a higher NMB.
Baek et al. published in 2022 a systematic review that aimed to evaluate the quality of existing economic evaluations of child nutrition interventions in low- and middle-income countries [33]. This review reports several studies that examined zinc interventions for preventing morbidity in general. Two studies in India and two in China showed biofortification of wheat or rice with zinc is more cost-effective in terms of disability-adjusted cost per life year (DALYs) compared to no supplementation over a time horizon of 1 to 30 years. Another study in sub-Saharan Africa revealed that the cost per DALY was lower in weekly or intermittent preventive zinc supplementation interventions compared to daily preventive or therapeutic supplementation of zinc. This review reports a study in South Africa that shows that universal zinc distribution, in addition to vitamin A supplementation, could be cost-effective compared to standard care for acute diarrheal disease, especially in scenarios when the prevalence of diarrheal disease was greater than 20%. In Colombia, Mejía et al., published in 2013 an evaluation of the cost-effectiveness of zinc supplementation for the prevention of acute diarrheal disease (ADD) in children under five years of age [12]. In this evaluation, oral zinc supplementation was a cost-effective strategy for children under 5 years of age with zinc deficiencies and a high risk of presenting an episode of EDA. Martinez et al., demonstrated that daily supplementation with 5 mg of zinc for 12 months in Colombia reduces the incidence of upper respiratory infections [34] In this randomized clinical trial with 355 patients, no supplementation was associated with a 43% higher risk of presenting UTRI (IRR 1.41, 95% CI 1.07–1.87, p = 0.016) compared to those who received supplementation. All this evidence is in line with our results and promotes an increase in the evidence in low- and middle-income countries and high prevalence setting in higher income countries. Although zinc supplementation has been studied extensively in clinical trials, there is limited public data on its national coverage in LMICs, and it is not currently a WHO-recommended preventive strategy for respiratory infections.
While a 2023 Cochrane review suggests zinc supplementation results in little to no reduction in the incidence of lower respiratory tract infections globally [6], we justified the use of the 2016 Cochrane data due to its inclusion of more LMIC-relevant trials and populations similar to Colombia. Nonetheless, this discrepancy reflects evolving evidence and underscores the need for local RCTs to validate findings in this context.
Our study has the following limitations. The quality assessment of clinical trials in meta-analyses from which the relative risk of pneumonia incidence, pneumonia, and general-cause mortality was extracted was rated as low and this may increase the uncertainty of the parameters used in our model. However, this uncertainty was evaluated in both deterministic and probabilistic sensitivity analyses and the conclusion did not change, zinc continued to be cost-effective within the evaluated ranges of each variable. The incidence of low respiratory infection in children under 5 reported for Colombia in the global disease burden study is very similar to that reported by the Camacho study used in our model (0.076 vs. 0.073) [8, 35]. However, the absence of a value from a population-based study that estimates the incidence and prevalence of community-acquired pneumonia in Colombia also adds uncertainty to the model. Likewise, given this limitation, deterministic and probabilistic sensitivity analysis was performed, demonstrating that only if this probability is less than 7% could the conclusion of our evaluation change. However, in the Monte Carlo first-order simulation where 10,000 potential patients with different values and combinations of variables are evaluated individually and randomly; The conclusion was invariable regarding the cost-effectiveness of such supplementation. Our model does not incorporate the probability of new events but only the probability of a first annual episode of pneumonia; therefore, we cannot be categorical about whether this intervention is cost-effective in reducing the probability of two or more annual pneumonia events in Colombia. Our model was not designed to evaluate or contemplate whether the cost-effectiveness of zinc supplementation is differential according to the presence or absence of previous nutritional deficiencies of zinc. However, given that cost-effectiveness is present in scenarios of high prevalence of pneumonia, it could be assumed that in patients at higher risk of disease, this intervention would be cost-effective. Our studio has the following strengths. It is the first economic evaluation published internationally of zinc supplementation in the prevention of pneumonia acquired in the Community in our country. Although this implies not having similar studies that allow a direct comparison, our results have similarities with what has been published regarding this supplementation in other infectious diseases in this age group. We also carried out a deterministic and probabilistic sensitivity analysis that allowed us to identify scenarios where the conclusion of our work could change. Although a 1-year time horizon was chosen, we recognize that the costs and outcomes for pneumonia can extend beyond this period. Studies like Baek et al. [33] show that longer-term models incorporating sequelae or stratified severity can provide additional insight and should be considered in future evaluations. All costs (direct, indirect, hospitalization, outpatient) were combined into a single value. This simplification may overlook potential differences in quality-of-life impacts associated with each cost category, introducing structural uncertainty. Future models could explore disaggregated cost-utility mappings. Our model focuses on a single annual episode and does not incorporate recurrent pneumonia, which is a significant limitation considering the age group studied. This may underestimate the total potential benefit of preventive zinc, especially in children with frequent infections. However, this was partially addressed by sensitivity analyses showing robustness across a range of incidence rates. The generalizability of these results to other countries is limited. Differences in baseline pneumonia incidence, healthcare system costs, and zinc deficiency prevalence may substantially affect cost-effectiveness. Hence, local adaptations of the model are necessary before applying these findings to other setting.
In conclusion, oral zinc supplementation in children aged 1 to 6 years in Colombia appears to be a potentially cost-effective strategy for the prevention of community-acquired pneumonia compared to non-supplementation. These findings should be interpreted with caution given the small absolute difference in benefits and the marginal incremental net monetary benefit observed. This result becomes an input for the construction of public health policies aimed at reducing the burden of disease generated by respiratory infections in Colombia; as well as serving as an example and motivation to assess the cost and utility of these strategies in other developing and developed countries.
Acknowledgements
None.
Authos’ contributions
J.A.B. and D.G. wrote the main manuscript text and M.A. prepared figures 1-3. All authors reviewed the manuscript.
Funding
This study was supported by the funding of authors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable. This study was only based on published data. Thus, an ethical approval or consent to participate was not necessary.
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations Children’s Fund (UNICEF). Pneumonia. UNICEF Data [Internet]. 2024 [cited 2025 Aug 4]. Available from: https://data.unicef.org/topic/child-health/pneumonia/.
- 2.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and National causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization; United Nations Children’s Fund (UNICEF). Ending preventable child deaths from pneumonia and diarrhoea by 2025 [Internet]. Geneva: WHO & UNICEF; 2013 [cited 2025 Aug 4]. Available from: https://data.unicef.org/resources/ending-preventable-child-deaths-pneumonia-diarrhoea-2025/.
- 4.Sultana M, Alam NH, Ali N, Faruque ASG, Fuchs GJ, Gyr N, et al. Household economic burden of childhood severe pneumonia in Bangladesh: a cost-of-illness study. Arch Dis Child. 2021;106(6):539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Cao D, Huang Y, Chen B, Chen Z, Wang R, et al. Zinc intakes and health outcomes: an umbrella review. Front Nutr. 2022;9: 798078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016;12:CD005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouhani P, Rezaei Kelishadi M, Saneei P. Effect of zinc supplementation on mortality in under 5-year children: a systematic review and meta-analysis of randomized clinical trials. Eur J Nutr. 2022;61(1):37–54. [DOI] [PubMed] [Google Scholar]
- 8.Camacho-Moreno G, Duarte C, Garcia D, Calderon V, Maldonado LY, Castellar L, et al. Sentinel surveillance for bacterial pneumonia and meningitis in children under the age of 5 in a tertiary pediatric hospital in Colombia– 2016. Biomedica. 2021;41(Sp 2):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyano Ariza L. Estimación de costo-enfermedad por neumonía y bronquiolitis en niños menores de 5 años en Colombia [Internet]. Bogotá: Universidad Nacional de Colombia; 2019 [cited 2025 Aug]. Available from: https://repositorio.unal.edu.co/handle/unal/75776.
- 10.Fink G, Heitner J. Evaluating the cost-effectiveness of preventive zinc supplementation. BMC Public Health. 2014;14:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saronga HP, Manji K, Liu E, Duggan CP, Menzies NA. Cost-effectiveness of zinc supplementation for prevention of childhood diarrhoea in Tanzania. Public Health Nutr. 2022;25(7):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mejia AAS, Florez ID, Sierra JM, Mejia ME, et al. Análisis de Costo efectividad Del zinc Para La prevención de La Enfermedad diarreica Aguda En Niños Menores de 5 Años En Colombia. Coyuntura Economica: Investigacion Economica Y Social. 2013;53(2):123–36. [Google Scholar]
- 13.Shiell A, Donaldson C, Mitton C, Currie G. Health economic evaluation. J Epidemiol Community Health. 2002;56(2):85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonio Buendia J, Patino DG. Cost-utility analysis of dupilumab add on therapy versus standard therapy in adolescents and adults for severe asthma in Colombia. Expert Rev Pharmacoecon Outcomes Res. 2022;22(4):575–80. [DOI] [PubMed] [Google Scholar]
- 15.Buendia JA, Rodriguez-Martinez CE, Sossa-Briceno MP. Cost-utility of Tiotropium for children with severe asthma in patients aged 1–5 years. Pediatr Allergy Immunol. 2021;32(8):1866–8. [DOI] [PubMed] [Google Scholar]
- 16.Buendia JA, Restrepo Chavarriaga GJ, Zuluaga AF. Social and economic variables related with Paraquat self-poisoning: an ecological study. BMC Public Health. 2020;20(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubovich S, Zaragoza S, Rodriguez V, Buendia J, Camargo Vargas B, Alchundia Moreira J, et al. Risk factors associated with pulmonary exacerbations in pediatric patients with cystic fibrosis. Arch Argent Pediatr. 2019;117(5):e466–72. [DOI] [PubMed] [Google Scholar]
- 18.Kim DD, Basu A. How does cost-effectiveness analysis inform health care decisions?? AMA J Ethics. 2021;23(8):E639-47. [DOI] [PubMed] [Google Scholar]
- 19.Departamento Administrativo Nacional de Estadística (DANE). Archivo Nacional de Datos (ANDA) [Internet]. Colombia: DANE; [cited 2025 Aug 4]. Available from: https://sitios.dane.gov.co/anda-index/.
- 20.Carroll AE, Downs SM. Improving decision analyses: parent preferences (utility values) for pediatric health outcomes. J Pediatr. 2009;155(1):21–5. [DOI] [PubMed] [Google Scholar]
- 21.Ministerio de Salud y Protección Social (Colombia). Sistema de Información de Precios de Medicamentos (SISMED) 2021 [Internet]. Bogotá: MinSalud; 2021 [cited 2025 Aug 4]. Available from: www.sispro.gov.co/central-prestadores-de-servicios/Pages/SISMED-Sistema-de-Informacion-de-Precios-de-Medicamentos.aspx.
- 22.Espinosa O, Rodríguez-Lesmes P, Romano G, Orozco E, Basto S, Ávila D, Mesa L, Enríquez H. Use of cost-effectiveness thresholds in healthcare public policy: progress and challenges. Appl Health Econ Health Policy. 2024;22(6):797–804. 10.1007/s40258-024-00900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. [DOI] [PubMed] [Google Scholar]
- 24.York Health Economics Consortium (YHEC). Net Monetary Benefit [Internet]. York (UK): YHEC; 2016 [cited 2025 Aug 4]. Available from: https://yhec.co.uk/glossary/net-monetary-benefit/.
- 25.Komorowski M, Raffa J. Chapter 24: Markov models and cost effectiveness analysis: applications in medical research. In: MIT Critical Data, editor. Secondary analysis of electronic health records [Internet]. Cham (CH): Springer; 2016. Available from https://pubmed.ncbi.nlm.nih.gov/31314275/. [PubMed]
- 26.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Mak. 1993;13(4):322–38. [DOI] [PubMed] [Google Scholar]
- 27.(NICE) NIfHaCE. Developing NICE guidelines: the manual. London: National Institute for Health and Care Excellence; 2023. Available from: https://www.nice.org.uk/process/pmg20/chapter/introduction. [PubMed]
- 28.Instituto de Evaluación Tecnológica en Salud (IETS). Manual metodológico para la elaboración de evaluaciones de efectividad, seguridad y validez diagnóstica de tecnologías en salud [Internet]. Bogotá, D.C.: IETS; 2014 [cited 2025 Aug 4]. Available from:: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/INEC/IETS/manual-metodologico-elaboracion-de-evaluaciones-de-efectividad.pdf.
- 29.Así Vamos en Salud. Tasa de mortalidad por infección respiratoria aguda en menores de 5 años en Colombia 2022 [Internet]. Bogotá: Así Vamos en Salud; 2022 [cited 2025 Aug 4]. Available from: https://www.asivamosensalud.org/indicadores/enfermedades-transmisibles/tasa-de-mortalidad-por-infeccion-respiratoria-aguda-ira-en.
- 30.Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18(11):1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichon-Riviere A, Drummond M, Palacios A, Garcia-Marti S, Augustovski F. Determining the efficiency path to universal health coverage: cost-effectiveness thresholds for 174 countries based on growth in life expectancy and health expenditures. Lancet Glob Health. 2023;11(6):e833–42. [DOI] [PubMed] [Google Scholar]
- 32.Instituto de Evaluación Tecnológica en Salud (IETS). Manual metodológico para la elaboración de evaluaciones de efectividad, seguridad y validez diagnóstica de tecnologías en salud [Internet]. Bogotá, D.C.: IETS; 2014 [cited 2025 Aug 4]. Available from: https://www.iets.org.co/Lists/Documentos%20Institucionales/Attachments/24/Manual_Evaluaciones_Efectividad_Seguridad_y_Validez_Diagnostica_IETS.pdf.
- 33.Baek Y, Ademi Z, Paudel S, Fisher J, Tran T, Romero L, et al. Economic evaluations of child nutrition interventions in low- and middle-income countries: systematic review and quality appraisal. Adv Nutr. 2022;13(1):282–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Estevez NS, Alvarez-Guevara AN, Rodriguez-Martinez CE. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: a 12-month randomised controlled trial. Allergol Immunopathol (Madr). 2016;44(4):368–75. [DOI] [PubMed] [Google Scholar]
- 35.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results [Internet]. Seattle: Institute for Health Metrics and Evaluation (IHME); 2020 [cited 2025 Aug 4]. Available from: https://www.healthdata.org/research-analysis/about-gbd.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.