Abstract
Background
Tracheobronchomegaly, also known as Mounier-Kuhn syndrome (MKS), is a rare congenital condition characterized by significant dilation of the trachea and main bronchi along with an abnormal wall structure. Diagnosis can be confirmed through computed tomography, pulmonary function tests, and diagnostic bronchoscopy. Currently, there is no curative treatment for MKS; thus, symptomatic and supportive care remain the primary therapeutic approaches. Early diagnosis, effective infection control, and individualized management are crucial for improving patient outcomes.
Methods
This case report describes a middle-aged woman who presented with chronic cough, expectoration, and wheezing. She had been misdiagnosed with chronic obstructive pulmonary disease (COPD) at a local hospital for an extended period and was subsequently referred to our institution for fiberoptic bronchoscopy, which confirmed the diagnosis of MKS. By reviewing the literature via PubMed, we conducted a retrospective analysis of 29 previously reported cases of MKS, including the present case, totaling 30 cases (21 males and 9 females), predominantly middle-aged and elderly individuals.
Conclusions
Based on our literature review, the misdiagnosis rate of MKS remains high, often accompanied by significant diagnostic delays. Additionally, the proportion of secondary MKS cases has increased, challenging the traditional notion that MKS is exclusively congenital. Despite its rarity, clinicians should consider MKS in patients presenting with recurrent lower respiratory tract infections, abnormal tracheobronchial morphology., poor response to antibiotic therapy, or refractory COPD-like symptoms. Early imaging and bronchoscopic evaluations are essential to confirm the diagnosis and prevent delayed treatment.
Keywords: Tracheobronchomegaly, Mounier-Kuhn syndrome, COPD, Relapsing polychondritis, Tracheobronchomalacia
Introduction
MKS is a rare congenital or acquired airway disease characterized by abnormal dilatation (more than three times the normal diameter) of the trachea and main bronchi, leading to structural weakness and dysfunction of the airway wall. Currently, most data on MKS are derived from case reports, with a lack of comprehensive epidemiological studies. Congenital cases predominantly occur in newborns or infants and may be associated with developmental abnormalities such as tracheal cartilage defects. In adults, MKS may be linked to genetic factors, chronic airway inflammation, infections, or connective tissue diseases, such as relapsing polychondritis. Among patients with relapsing polychondritis, tracheomalacia is present in 100% of those with type 1 disease, and concurrent tracheomalacia/bronchomalacia is common in those with type 2 disease [1]. Some cases are associated with genetic mutations (e.g., in the CACNA1H calcium-channel gene), which result in abnormalities of tracheal smooth muscle or chondrogenesis [2]. Bronchiectasis (observed in 30% of cases), emphysema, or recurrent pneumonia are frequent complications, and atelectasis may develop due to impaired mucus clearance [3, 4]. In adults, chronic obstructive pulmonary disease (COPD) may worsen, particularly when there is a mismatch between airway dimensions and lung volumes [5, 6]. Bronchoscopy, which enables direct visualization of airway collapse and luminal dilatation, remains the gold standard for diagnosis [7, 8]. Clinically, MKS can easily be mistaken for COPD or bronchiectasis. Herein, we present a case of MKS that was misdiagnosed as COPD to highlight the importance of reducing misdiagnosis and mistreatment of this condition.
Case description
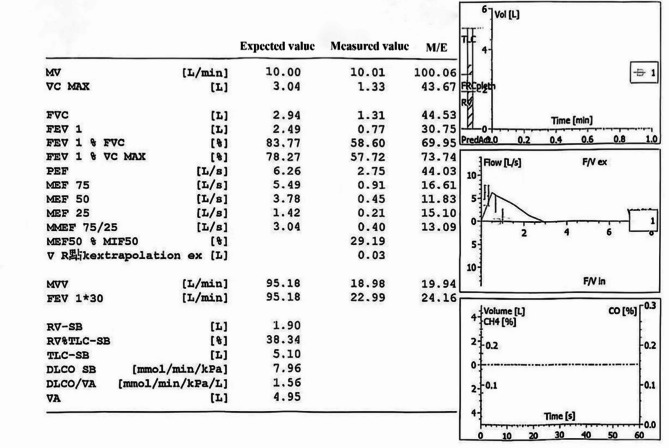
A 57-year-old female patient was admitted to the hospital with the chief complaint of “repeated cough, expectoration, and wheezing for more than 10 years, exacerbated over the past half month.” Over a decade ago, the patient began experiencing intermittent cough and expectoration without any obvious trigger. The sputum was white and viscous, accompanied by chest tightness and shortness of breath. Despite receiving anti-infection treatment at a local hospital, her symptoms did not significantly improve. Half a month prior to admission, she experienced worsening cough, expectoration, and wheezing without an apparent cause. The sputum was described as white and foamy, difficult to expel, and was associated with chest tightness. Imaging revealed partial pleural thickening on the left side. Pulmonary function testing demonstrated extremely severe mixed ventilatory dysfunction, with all parameters of the maximal expiratory flow-volume curve showing significant reduction. Maximal ventilatory capacity was severely diminished, and the bronchodilation test was negative (pulmonary function report shown in Table 1). Conventional treatments including anti-infection, expectorant, asthma control, and other therapies administered at another hospital failed to significantly alleviate her symptoms. She was subsequently referred to our hospital for further diagnosis and management. The patient had no significant medical history or family history of similar conditions. Physical examination revealed cyanosis of the lips, decreased breath sounds bilaterally, and wheezing in both lungs.
Table 1.
Laboratory test results
| Check Items | 2025-1-7 | 2025-1-12 | 2025-1-15 | Reference Range |
|---|---|---|---|---|
| Blood Routine | ||||
| White Blood Cell Count (WBC) | 10.77*10^9 | 6.46*10^9 | (3.50–9.50)*10^9/L | |
| Hemoglobin (HGB) | 156 | 162 |
130–175 g/L (Male) 120–160 g/L (Female) |
|
| Neutrophil Percentage (NEUT) | 73.6 | 69.7 | 40–75% | |
| Lymphocyte Percentage (LYM) | 17.7 | 20.3 | 20–50% | |
| Monocyte Count (MONO) | 0.82*10^9 | 0.40*10^9 | 0.10–0.60/L | |
| Inflammation Indicators | ||||
| Interleukin-6 (IL-6) | 20.386 | 0-5.90 pg/mL | ||
| C-Reactive Protein (CRP) | 2.85 | 14.2 | 0–6 mg/L | |
| Erythrocyte Sedimentation Rate (ESR) | 4.00 |
0–15 mm/h (Male) 0–20 mm/h (Female) |
||
| Procalcitonin (PCT) | <0.05 | <0.10 | <0.10ng/mL | |
| Novel Coronavirus Nucleic Acid Detection | Negative | Negative | ||
| Six Respiratory Pathogen Nucleic Acid Detection | Negative | Negative | ||
| Urine Analysis | Negative | Negative | ||
| Stool Routine | Negative | Negative | ||
| Blood Culture | Negative | Negative | ||
| ANA Profile | Negative | Negative | ||
| Acid-Fast Bacilli, Tuberculin DNA, TB Gene-xpert, Respiratory Pathogen Nucleic Acid, Fungi & NGS (Bronchoscopy Lavage Fluid) | Negative | Negative (Lavage Fluid) | Negative | |
After admission, a chest CT scan showed dilation and irregularity of the main tracheal lumen, diverticula of the trachea, airway obstruction in the left upper lobe and basal segment of the lower lobe with corresponding atelectasis, inflammation in the left lung and right middle lobe, widening of the main pulmonary artery and right pulmonary artery trunk, and mild left-sided pleural effusion (CT findings shown in Fig. 1). Bronchoscopy results are presented in Fig. 2, and additional laboratory test results are summarized in (Table 1 ) (Fig. 3).
Fig. 1.
Pulmonary function report
Fig. 2.
Bronchoscope: From left to right, the trachea carina, right main bronchus, right upper lobe, right lower lobe, left main bronchus, left tongue lobe, left lower lobe bronchus, left upper lobe, and the basal segment of the left lower lobe are observed. It is noted that the protuberance appears as soft as a line. The main bronchi on both sides are enlarged; the membrane is thin with multiple diverticula present. Additionally, the cartilage rings are slender and exhibit dynamic collapse
Fig. 3.
High-resolution CT of the chest reveals that the main tracheal cavity is not fully dilated, and there is a presence of a tracheal diverticulum. The airway of the left upper lobe and the basal segment of the left lower lobe are occluded, resulting in atelectasis of the corresponding lung tissue. Additionally, inflammation is observed in both the left lung and the middle lobe of the right lung. Furthermore, there is dilation of both the main pulmonary artery and the trunk of the right pulmonary artery, with inner diameters measuring approximately 3.7 cm and 3.2 cm, respectively
The diagnosis and treatment plan included low-flow oxygen therapy, mechanical assistance for expectoration, anti-infection treatment, nebulization, bronchodilation, asthma control, expectoration facilitation, and vasodilation. Chest CT revealed abnormal dilation of the main bronchus, prompting fiberoptic bronchoscopy, which confirmed MKS. Following the aforementioned treatment regimen, the patient’s symptoms improved, and she was discharged. Follow-up is ongoing.
Literature review
The search formula “(((((((Tracheobronchomegalies[Title/Abstract]) OR (Mounier-Kuhn Syndrome[Title/Abstract])) OR (Mounier Kuhn Syndrome[Title/Abstract]))) OR (Congenital Tracheobronchomegaly[Title/Abstract]))) OR (Congenital Tracheobronchomegalies[Title/Abstract]))) OR (Tracheobronchomegalies, Congenital[Title/Abstract]))) OR (Tracheobronchomegaly, Congenital[Title/Abstract]))) OR (“Tracheobronchomegaly“[Mesh])” was used to retrieve articles in PubMed. A total of 298 articles were identified, the majority of which were case reports (n = 224). Thirty case reports published in the last five years were retrospectively analyzed. Only patients aged 18 years and older were included, while non-English literature and studies lacking clear measurements of tracheal or main bronchial diameters were excluded. Ultimately, 27 articles were reviewed, reporting a total of 28 cases of MKS. Including the present case, a total of 29 cases were documented, comprising 20 males and 9 females. A meticulous review of all articles was conducted to eliminate duplicates and ensure comprehensive inclusion of relevant information. Data were systematically extracted and presented in tabular form. Case reports, reviews, and research papers over the years indicate that most MKS patients are middle-aged or elderly, with a male predominance. The primary clinical manifestations include chronic cough, expectoration, and recurrent respiratory tract infections; some patients also experience wheezing or dyspnea. Computed tomography (CT) is the primary diagnostic tool, often revealing abnormal dilation of the trachea and main bronchi, diverticulum formation, and dynamic collapse in certain cases. Bronchoscopy serves as a valuable diagnostic method for directly observing airway morphology and dynamic changes. Treatment focuses on physical sputum clearance methods (e.g., high-frequency chest wall oscillation, postural drainage), and antibiotic prophylaxis can reduce the risk of acute exacerbations by approximately 50%. Lung transplantation may be considered for patients with end-stage respiratory failure, with a reported 5-year survival rate of 68%. Notably, 23% of asymptomatic patients do not require intervention but should undergo regular monitoring of lung function and radiographic progression.
From the literature published over the years, we can draw the following conclusions: (1) The misdiagnosis rate of MKS remains high, emphasizing the need for radiologists to pay attention to tracheal diameter measurements. (2) This disease is frequently associated with delayed diagnosis (typically ranging from 5 to 20 years). It is recommended that airway diameter be measured in patients presenting with unexplained chronic expectoration. (3) The proportion of secondary MKS has increased, with 22% of recent literature linking the condition to connective tissue diseases, challenging the traditional perception of MKS as exclusively congenital. Despite clear diagnostic criteria, the nonspecific clinical manifestations of MKS often lead to misdiagnosis. This case represents a typical example of prolonged misdiagnosis as chronic obstructive pulmonary disease (COPD), a scenario occasionally mentioned in existing literature. However, the detailed diagnostic process and differential diagnosis of this case are particularly noteworthy. (The specific literature is summarized in Table 2).
Table 2.
Summary of characteristics of 29 MKS cases
| Case report Serial number |
Literature sources | Gender/Age (years old) |
High risk factors | clinical signs and symptoms | Imaging findings and bronchoscopy | Treatment options | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Feryal et al. | Male/55 | Smoking | Cough with expectoration, difficulty breathing, and recurrent respiratory infections | Chest CT scan showed the trachea expansion, transverse Sect. 45 mm, sagittal 32 mm, gas pipe wall is irregular, tracheal diverticulum happens. The main bronchus was dilated to 26 mm on the right side and 31 mm on the left. | Resistance to infection, dilated bronchi, phlegm | Get better |
| 2 | Lu-Xia Kong et al. | Male/81 | Tuberculosis | Cough, sputum, difficulty breathing, and loss of appetite | Chest CT showed bilateral lung infection, bronchiectasis, and obvious tracheobronchial enlargement. | Give high flow oxygen therapy, antivirus, anti-infection, smooth wheezing, phlegm, nutritional support and other symptomatic treatment. | Get better |
| 3 | Marta etc. | Female/46 | Bronchial asthma | Cough, expectoration, wheezing | Chest CT showed airway anomalies expanding and associated with bronchiectasis. | Anti-inflammatory and anti-asthmatic | Get better |
| 4 | Takayuki et al. | Female/42 | Labored breathing | Chest CT tracheal bronchus enlargement with much diverticulum involving the trachea and main bronchi, cystic bronchiectasis extend to distal bronchus. | no | no | |
| 5 | Sima etc. | Male/69 | Smoking, COPD | Difficulty breathing and sweating | Chest CT: display the trachea bronchiectasis, involving the trachea and main bronchi, airway sagittal 38 mm in diameter, trachea diameter 27 mm. Fiber bronchoscope examination showed the trachea expansion, the main bronchus, cartilage ring is outstanding, mucosal atrophy. | Anti-infection and hemostasis | Slight improvement |
| 6 | Sacide S, etc. | Male/78 | COPD | Cough, expectoration, wheezing | Chest CT: left lower lobe dense consolidation with multiple nodular opacity, related to the tumor. Severe emphysematous changes with bronchiectasis of the trachea. | Concurrent tumor, Hospice care. | no |
| 7 | Sara etc. | Male/40 | Family history of smoking, asthma | Cough, fever, | Chest CT scan revealed a trachea and main bronchi expansion, trachea, right main bronchus and left main bronchial diameter of 40 mm respectively, 33 mm and 27 mm. Bronchoscopy: bronchial and bronchiectasis, mucosal atrophy, lack of muscular layer. | Conservative treatment | Get better |
| 8 | Boreum etc. | Female/94 | no | no | A chest X-ray showed marked dilatation of the trachea and main bronchi with aortic curvature and cardiac enlargement with scoliosis. 3.5 cm above the aortic arch, the longest transverse diameter of the trachea was 29.2 mm, with the diameter of the left main bronchus 15.3 mm and the diameter of the right main bronchus 20.2 mm. | no | no |
| 9 | Zhen Hua Li et al. | Male/84 | Smoking | Cough, expectoration and fever | Chest CT scan showed the trachea expansion, 62.6 mm in diameter, large bronchial diameter increased (37.9 mm) 30.9 mm on the right side, left side. Gas pipe wall muscle layer obviously thinning, show the trachea and main bronchi wall elastic fiber or smooth muscle atrophy or missing. Video bronchoscopy showed dynamic part of the narrow exhale, prompt tracheomalacia. | no | no |
| 10 | Sai-Nan Wang et al. | Male/64 | Lung cancer | Chest tightness, stabbing pain in the left lower chest | Chest CT showed a left lower lobe mass, approximately 2.8 cm in diameter, invading the pleura. Tracheal bronchus expansion and deformation, with multiple diverticulum. Tracheal before and after the largest diameter and diameter of axle is 3.12 cm and 4.47 cm respectively. Coronal plane, left main bronchus and right main bronchial diameter were 2.15 and 2.68 cm. Fiberoptic bronchoscopy showed the central airway irregular oval, muscularis mucosa thinned, diverticulum. | No specific treatment | no |
| 11 | N. Zaghba et al. | Female/67 | no | Cough, expectoration, hard breathing, fever | Chest CT showed around the trachea and main bronchi expansion, transverse diameter of 32 mm respectively, 20 mm and 17.5 mm. | Antibiotics, physical therapy, and oxygen therapy | Get better |
| 12 | Shuhan Li et al. | Male/69 | no | Cough, sputum, dyspnea, wheezing, fever | Chest CT scans, trachea, left and right main bronchus in bronchial expansion, coronal and sagittal scanning tracheal diameter were 40.1 mm and 25.3 mm, about bronchial diameter of 26.9 mm and 24.1 mm respectively. | Anti-infection, antitussive and expectorant | Partial relief |
| 13 | Z.-H. Li et al. | Male/75 | Smoking | Cough and expectoration | Chest CT tracheal expansion (34.2 mm) in diameter, large bronchial diameter increased left (right, 24.2 m, 24.8 m). Fiber bronchoscope examination showed the trachea to soften with dynamic part of the exhale, narrow wall with multiple diverticulum. | Anti-infection | Get better |
| 14 | Aasir et al. | Female/30 | HCU history | Cough, expectoration, wheezing | Chest CT showed trachea and two main bronchi abnormal expansion, accompanied by double lung bronchiectasis. | Dietitian support (and metabolic team cooperation), sputum, chest physical therapy technique, proper vaccination. | Get better |
| 15 | Aboubekr et al. | Male/59 | Type 2 diabetes | Coughing up phlegm, difficulty breathing | Chest X-ray showed tracheal permeability increases obviously, widening gap between thoracic expansion ends. | Oxygen inhalation and antibiotics were given; Inhaled corticosteroids with long-acting bronchodilator; | Get better |
| 16 | Saiara etc. | Male/89 | no | Cough, expectoration, chest pain, shortness of breath | Before and after the diameter 26.7 mm, lateral tracheal diameter 47.3 mm. Middle area columnar moniliform bronchiectasis, lower lobe. Bronchoscope examination showed the trachea expansion and deformation, membranous part of the expansion, damage of tracheal cartilage, tracheal wall before diverticulum. | Physical therapy to breathe. | Hospice care |
| 17 | Jun Xiong etc. | Male/42 | Ankylosing spondylitis | Pillow neck and lower back pain and restricted movement | CT of the lung showed an enlarged trachea 2 cm above the aortic arch. Shaft like and coronary like (right) shows the trachea diameter of 27.2 mm. Airway sagittal diameter of 22.3 mm. CT images of lung in the level of the aortic arch, tracheal the longest diameter. Lower left axial as transverse diameter of 30.5 mm. The right side of the coronal diameter of 30.4 mm. The sagittal image shows the diameter of the trachea as 27.6 mm. | Left untreated | no |
| 18 | Deborah et al. | Male/43 | History of whooping cough | Cough sputum, chest pain, difficulty breathing | Chest radiograph in trachea and main bronchi expansion, bilateral pulmonary diffuse reticular and circular shadow middle area. Chest CT: coronal CT scan, lung window: showed a significant expansion and distortion, the main bronchus right bronchial 4.25 cm long, left bronchus 3.81 cm long; Show cystic and varicose veins of bronchiectasis. Window of lung CT images, and shown the right middle ground glass opacity and bronchiectasis. | infection, cough, phlegm, bronchial Anti- dilatation, diuresis and other treatments. | Get better |
| 19 | M. Rjimati, etc. | Male/67 | Type 2 diabetes | Cough and expectoration | Chest CT cervical thoracic trachea and two main bronchiectasis. Tracheal diameter is 24.4 mm, transverse diameter of 34.7 mm, left lower lobe is a cylindrical bronchiectasis oven. | The patient’s cough was treated with BDLA, physical therapy was prescribed to clear bronchial secretions, and vaccination was planned. | Get better |
| 20 | Collin J et al. | Female/72 | no | Fever, cough, wheezing, difficulty breathing | Chest CT scan showed a significant increase in the trachea, bronchi, and bronchioles distal airway mucus plug. Coronary tracheal diameter measurement is 32 mm. | Resistance to infection, cough phlegm | no |
| 21 | Aswin etc. | Male/41 | no | Throat discomfort | Chest high-resolution CT showed the trachea expansion, bilateral bronchial enlargement with tracheal right posterolateral multiple diverticulum. Thoracic D1, D2 section maximum trachea diameter is 32 mm, bilateral main bronchus 20 mm diameter. - optical fiber bronchoscope examination showed expansion, tracheal tracheal posterior lateral area with multiple diverticulum, until the two main bronchi juga and expand. | Acid suppression and stomach protection | Get better |
| 22 | Saifurrahman et al. | Male/28 | Dust exposure history, smoking | Cough sputum, chest pain, difficulty breathing, breathing | High resolution chest CT shows the trachea expansion with diverticulum, Lord bronchiectasis with a wide range of bilateral cystic bronchiectasis. Bronchoscopy revealed tracheectasis (31 mm, measured 2 cm above the aortic arch), main bronchiectasis (24 mm left, 21 mm right), multiple diverticula and sacculus containing cisterna discharge. | Avoid contact with dust, smoking cessation, inhaled fluticasone/f ROM mott and chest physical therapy. | Get better |
| 23 | Ramezan et al. | Male/51 | no | Cough, fever, difficulty breathing | Chest CT: tracheal 39 mm in diameter, right main bronchus 30 mm in diameter, left main bronchus 26 mm in diameter. Double lung middle area visible ground glass opacity (GGO) and bronchiectasis, under the right lung area visible ballonet. | Mechanical ventilation, tracheotomy | Get better |
| 24 | Ben et al. | Male/43 | no | Shortness of breath | Bronchoscopy revealed severe obstructive tracheomalacia. | Surgical treatment | Get better |
| 25 | Anna et al. | Male/45 | no | Difficulty breathing, wheezing | Chest CT showed tracheal horizontal axis relative to the front axle increases; Waking in patients with flexible bronchoscope examination showed the trachea lumen narrowing, exhale airway collapse with excess membrane wall invasion and tracheal cartilage deformation. | Surgical treatment | Get better |
| 26 | Mercy corps etc. | Female/43 | no | Cough, dyspnea, chest pain | HRCT scan image display bronchiectasis (right 22.5 mm, 20.4 mm from left), bronchiectasis varicose, alveolar center, emphysema, basal fibrosis. | Positive airway pressure, were administered. | no |
| 27 | Lobna etc. | Female/46 | no | Cough, expectoration and chest pain | Chest radiograph in tracheal permeability expansion, expand bilateral basal parts bronchial lesions. Chest CT showed the expansion of the trachea and main bronchi and double bottom of cystic lung bronchiectasis. Bronchoscopy demonstrated a trachea and power gas pipe wall is irregular expansion, cough or exhale to collapse. | Given antibiotics (amoxicillin clavulanic acid) and chest physical therapy | Get better |
| 28 | Lobna etc. | Male/37 | Type 2 diabetes | Cough sputum, fever, difficulty breathing | Chest X-ray showed bilateral interstitial, bilateral pleural effusion, trachea expansion. Chest CT tracheal bronchiectasis with multiple diverticulum, diffuse interstitial lesions associated with fibrosis. Bronchoscopy showed after tracheal wall there are multiple diverticulum. | Fight infection | death |
| 29 | YuanSai etc. | Female/57 | no | Cough, expectoration, wheezing | Chest CT showed that the main tracheal cavity was dilated and not smooth, and the tracheal diverticulum was found. The left upper lobe and the basal segment of the lower lobe had airway occlusion, and the corresponding lung tissue was atelectasis. Bronchoscopy showed that the carina was fine and soft as a line. The main bronchus and the left and right main bronchus were enlarged, the membrane was thin, multiple diverticula could be seen, and the cartilage ring was slender and collapsed dynamically. | Anti-infection, cough, phlegm, asthma, anti-inflammation and other treatments. | Get better |
Discussion
Diagnosis and differential diagnosis
MKS is a rare congenital condition with controversial etiology. It is primarily associated with abnormal development of elastic fibers and smooth muscle in the trachea and main bronchial wall. The core pathological changes include atrophy or loss of elastic fibers in the trachea and main bronchi, along with thinning of the smooth-muscle layer [9, 10]. This structural abnormality leads to reduced airway wall tension, resulting in abnormal dilatation during inspiration due to negative pressure and collapse during expiration due to lack of support, thereby causing dynamic airway obstruction and impairing normal ventilatory function [10]. Additionally, weakness in the airway walls may lead to diverticulum formation, further impeding mucus clearance and increasing the risk of infection [11, 12]. Typical symptoms include chronic respiratory infections, cough, expectoration, dyspnea, spontaneous pneumothorax, and signs of tracheal dilatation. Pneumothorax may be the first manifestation in some patients [12]. In severe cases, signs such as “bellows” breath sounds or stridor may be present [9]. Patients often experience recurrent lower respiratory tract infections, pneumothorax, bullae, postoperative respiratory complications, pulmonary fibrosis, and deterioration of pulmonary function. The key to diagnosis lies in chest computed tomography (CT), which demonstrates marked dilatation of the trachea and main bronchi (tracheal transverse diameter > 34 mm and main bronchi > 24 mm), along with diverticula or dynamic collapse [10, 12]. Bronchoscopy allows direct visualization of airway dilatation, diverticula, secretion retention, and exclusion of other obstructive lesions [11]. Pulmonary function tests typically reveal mixed ventilatory dysfunction (predominantly obstructive but sometimes combined with restriction), with markedly decreased expiratory flow [10, 11].
The patient was a middle-aged woman who had experienced intermittent cough and expectoration without obvious inducement for over 10 years. The sputum was white and viscous, accompanied by chest tightness and shortness of breath. Symptoms worsened with activity and recurred frequently, consistent with the general manifestations of chronic obstructive pulmonary disease (COPD). Due to an acute exacerbation, a chest CT performed at a local hospital showed consolidation and atelectasis in the left upper lobe, corresponding bronchial stenosis, mediastinal and interlobar fissure displacement. Pulmonary function tests revealed extremely severe mixed ventilatory dysfunction, and the bronchodilation test was negative (-). She was subsequently referred to our hospital for further treatment. However, her chest CT and pulmonary function test results did not meet the diagnostic criteria for COPD. The chest CT demonstrated dilation and irregularity of the main tracheal cavity, with tracheal diverticula observed. Airway occlusion was noted in the left upper lobe and basal segment of the lower lobe, with corresponding atelectasis of lung tissue. Inflammation was present in the left lung and right middle lobe; the main pulmonary artery and right pulmonary artery trunk were widened, and mild left-sided pleural effusion was detected. Fiberoptic bronchoscopy revealed a thin and soft carina, enlargement of the main bronchus and bilateral main bronchi, a thin membrane, multiple diverticula, thin cartilage rings, and dynamic collapse, confirming MKS.
It should be differentiated from the following diseases: (1) Acquired tracheal dilatation, such as chronic infections (e.g., tuberculosis, aspergillosis), relapsing polychondritis, etc [11].; (2) Tracheomalacia: characterized by airway collapse during expiration but without congenital structural abnormalities [13]; (3) Pulmonary fibrosis-related airway changes: attention should be paid to whether it is secondary to interstitial lung disease, such as idiopathic pulmonary fibrosis; (4) Other causes of recurrent pneumothorax, such as pulmonary bullous disease, Marfan syndrome, etc [12]. A case of an elderly female patient with pulmonary fibrosis was reported in the literature, which is currently the oldest diagnosed female case, suggesting that MKS should be included in the differential diagnosis of recurrent infections in the elderly [14]. Some patients exhibit an insidious course with no significant clinical or imaging changes for a long time, leading to delayed diagnosis [15, 16].
Upon admission, the patient’s inflammatory markers were slightly elevated, but there were no obvious abnormalities in fungal or tuberculosis-related pathogenic examinations. Tuberculosis and fungal infections were therefore not considered at this stage. Chest CT revealed dilatation of the main tracheal cavity and formation of tracheal diverticula without evident interstitial changes. Fiberoptic bronchoscopy demonstrated MKS. A liquid-based smear of bronchial lavage (from the left upper lobe) showed a few neutrophils, scattered macrophages, and ciliated columnar epithelial cells. Interstitial lung disease was not considered. Relapsing polychondritis (RP) is a rare autoimmune disease characterized by recurrent inflammation of cartilage-rich tissues and proteoglycan-rich structures, commonly involving the ear, nose, and respiratory tract, and associated with various systemic manifestations [17, 18]. Although specific biomarkers are lacking, elevations in inflammatory markers (e.g., CRP and ESR) can be detected in some patients [19]. FDG PET/CT may reveal increased metabolic activity in the oto-naso-tracheobronchial triad, aiding early diagnosis [18, 20, 21]. Dynamic high-resolution CT can assess airway stenosis and cartilage destruction [22]. Pathological examination of cartilage tissue biopsies shows inflammatory cell infiltration and cartilage destruction, though negative results do not exclude the diagnosis [19, 20]. The patient lacked typical otorhinolaryngological manifestations of RP, and chest CT and fiberoptic bronchoscopy findings did not support the diagnosis of RP. Tracheomalacia is a condition characterized by weakening of the tracheal wall structure, leading to dynamic airway collapse, particularly during expiration. Studies suggest possible associations with abnormal development or dysfunction of tracheal smooth muscle. For example, genetic defects in the T-type calcium channel CACNA1H affect smooth-muscle contraction and cytoskeletal organization, resulting in congenital tracheal stenosis or malacia [2]. Preterm infants, especially those with bronchopulmonary dysplasia, have a higher incidence [23], and RP is often associated with tracheomalacia (e.g., 100% of type I RP patients exhibit tracheomalacia and subglottic stenosis) [23]. Bronchoscopy, which allows direct visualization of tracheal collapse, remains the gold standard for clinical diagnosis [7, 8]. Fiberoptic bronchoscopy revealed a thin and soft carina, enlargement of the main bronchus and bilateral bronchi, a thin membrane, multiple diverticula, slender cartilage rings, and dynamic collapse, suggesting MKS. There was insufficient evidence to diagnose tracheomalacia. This patient did not present with recurrent pneumothorax as the initial symptom, and chest CT and other examinations did not support pulmonary bullous disease or Marfan syndrome at present. However, the diagnosis of Marfan syndrome primarily relies on Ghent criteria and genetic testing. Since the patient did not undergo relevant genetic testing, there is insufficient evidence to rule out Marfan syndrome.
Treatment and prognosis
The treatment of MKS primarily focuses on symptomatic support, infection control, and surgical intervention. Physical therapies, such as chest percussion and postural drainage, can facilitate mucus clearance and reduce the risk of infection due to retention in patients with impaired expectoration [24]. Commonly used medications include mucolytic agents (e.g., acetylcysteine) and bronchodilators (e.g., β₂ receptor agonists), which improve mucus fluidity and alleviate airway collapse [16, 24]. Bronchoscopy may be employed to assist in secretion removal, particularly in cases of severe sputum retention or atelectasis [16]. In patients with respiratory muscle weakness or significant airway collapse, nocturnal noninvasive ventilation can enhance ventilation and oxygenation [16, 24]. For dynamic airway collapse, continuous positive airway pressure (CPAP) or positive end-expiratory pressure (PEEP) can maintain airway patency, reduce the work of breathing, and improve symptoms [7, 25]. This is especially beneficial for children or adults unable to generate sufficient PEEP independently [26]. In cases of acute respiratory failure, invasive ventilation may be required; however, careful selection of the endotracheal tube type and cuff pressure is essential to avoid airway injury due to tracheal dilatation and softening [27]. Airway stenting can be considered when necessary, with temporary stents providing rapid relief of dyspnea in severe tracheobronchomalacia (TBM). However, complications such as mucus plugging, granulation tissue proliferation, and stent migration must be monitored [28]. Long-term stenting requires a rigorous risk-benefit assessment, and newer flexible porous stents (e.g., four-helix designs) may reduce complications [28, 29]. Biodegradable scaffolds or those coated with epithelial cells represent promising future options to minimize the risk of mucus plugs and infections [28].
Surgical interventions are also effective. Tracheobronchoplasty, which reinforces collapsed airway walls using suture or suspension techniques, is indicated for both congenital and acquired TBM [30]. Tracheal resection may be effective for localized stenosis or tumor-related cases, though repair of long tracheal defects (> 5 cm) remains challenging [31, 32]. Emerging technologies, such as tissue-engineered tracheal transplantation using decellularized tracheal scaffolds, promote epithelialization and cartilage regeneration for repairing long-segment defects [31, 33]. Dynamic bronchoscopy remains the gold standard for diagnosis, and adjunctive therapies such as balloon dilation or laser ablation can be performed during the procedure [34]. Additionally, stem cell-based therapies targeting the Wnt/BMP/SHH signaling pathway may promote tracheal cartilage regeneration [35, 36]. Electrochemical therapy has been reported to reduce airway compression caused by capillary malformations in cases involving vascular abnormalities [37].
Lung transplantation may be considered as a potential treatment option for end-stage patients who progress to respiratory failure, although strict evaluation of airway conditions is essential. Due to the significant dilatation and softening of the trachea and main bronchi, anastomosis during transplantation poses a considerable challenge, and some patients may be excluded from transplantation due to extensive airway involvement [38]. Early pulmonary rehabilitation is recommended to enhance the quality of life in affected individuals. The prognosis of this disease varies depending on individual differences and disease severity, with outcomes primarily influenced by the prevention and management of complications such as recurrent infections and respiratory failure. In this case, the patient’s symptoms were alleviated following general treatments including oxygen therapy, anti-infection measures, and mechanical expectoration. It is emphasized that early diagnosis, active infection control, and personalized treatment strategies are critical to improving long-term outcomes.
Limitations
The majority of cases were derived from previously published journals, leading to an emphasis on symptoms, imaging findings, and treatment approaches, while detailed descriptions of patient signs were often lacking. Additionally, the diagnosis of Marfan syndrome primarily depends on the Ghent criteria and genetic testing. Since this patient did not undergo relevant genetic testing, there is insufficient evidence to definitively exclude Marfan syndrome.
Conclusion
MKS is a rare and frequently overlooked disease that requires early identification through the integration of imaging findings and clinical manifestations. Bronchoscopy remains the gold standard for diagnosing this condition, and it is recommended to perform fiberoptic bronchoscopy as soon as possible to confirm the diagnosis. Surgical intervention may be considered when appropriate, and lung transplantation is a feasible option for patients who progress to end-stage respiratory failure. Early diagnosis and individualized treatment strategies can significantly improve prognosis. Based on literature analysis, the misdiagnosis rate of MKS remains high, often accompanied by significant diagnostic delays. Additionally, the proportion of secondary MKS cases has increased, challenging the traditional perception that MKS is exclusively congenital. Despite clear diagnostic criteria, the nonspecific clinical manifestations of MKS frequently lead to misdiagnosis. This highlights the importance for clinicians to consider rare diseases, particularly MKS, in patients presenting with refractory chronic obstructive pulmonary disease (COPD)-like symptoms, emphasizing the need for careful differentiation.
Acknowledgements
Not Applicable.
Abbreviations
- MKS
Mounier Kuhn syndrome
- COPD
Chronic obstructive pulmonary disease
- PEEP
Positive End-Expiratory Pressure
- TBM
Tracheobronchomalacia
Authors’ contributions
All authors contributed equally to this work.
Funding
This research is supported by the key clinical specialty project of Qinghai Province.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable. This is not a clinical trial, and therefore no registration details are available.
Consent for publication
The patient and her family in this case have given written informed consent for her personal or clinical details and for any identifying images to be released in this case.
Competing interests
The authors declare no competing interests.
Informed consent statement
Written informed consent has been obtained from the patient to publish this case report and any accompanying images.
Conflict of interest
The author declares that there is no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sai Yuan and Weiran Li contributed equally to this work.
References
- 1.Ferrada M, Rimland CA, Quinn K, et al. Defining clinical subgroups in relapsing polychondritis: A prospective observational cohort study. Arthritis Rheumatol Hoboken NJ. 2020;72(8):1396–402. 10.1002/art.41270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Lu C, Ma L, et al. The T-Type calcium channel CACNA1H is required for smooth muscle cytoskeletal organization during tracheal tubulogenesis. Adv Sci Weinh Baden-Wurtt Ger. 2024;11(44):e2308622. 10.1002/advs.202308622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verleden SE, Hendriks JMH, Snoeckx A, et al. Small airway disease in Pre-Chronic obstructive pulmonary disease with emphysema: A Cross-Sectional study. Am J Respir Crit Care Med. 2024;209(6):683–92. 10.1164/rccm.202301-0132OC. [DOI] [PubMed] [Google Scholar]
- 4.Cazier P, Chassagnon G, Dhote T, et al. Reversal of cylindrical bronchial dilatations in a subset of adults with cystic fibrosis treated with elexacaftor/tezacaftor/ivacaftor. Eur Respir J. 2024;63(3):2301794. 10.1183/13993003.01794-2023. [DOI] [PubMed] [Google Scholar]
- 5.Debban CL, Ambalavanan A, Ghosh A, et al. Dysanapsis genetic risk predicts lung function across the lifespan. Am J Respir Crit Care Med. 2024;210(12):1421–31. 10.1164/rccm.202401-0011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith BM, Kirby M, Hoffman EA, et al. Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA. 2020;323(22):2268–80. 10.1001/jama.2020.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.W PZWY. COPD-Associated expiratory central airway collapse: current concepts and new perspectives. Chest. 2025;167(4):1024–43. 10.1016/j.chest.2024.11.015. [DOI] [PubMed]
- 8.Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29–50. 10.1164/rccm.201907-1292SO. [DOI] [PubMed] [Google Scholar]
- 9.Piazza C, Bolzoni A, Giudice M, Peretti G. [Laryngeal carcinoma associated with congenital tracheobronchomegaly (Mounier-Kuhn syndrome): a case report]. Acta Otorhinolaryngol Ital. 2002;22(1):34–8. [PubMed] [Google Scholar]
- 10.Sun B, Dai H. [Tracheobronchomegaly: a report of 3 cases and literature review]. Chin J Tuberc Respir Dis. 2011;34(8):600–3. [PubMed] [Google Scholar]
- 11.Celik B, Bilgin S, Yuksel C. Mounier-Kuhn syndrome: a rare cause of bronchial dilation. Tex Heart Inst J. 2011;38(2):194–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Unlu EN, Annakkaya AN, Balbay EG, et al. An unusual cause of recurrent spontaneous pneumothorax: the Mounier-Kuhn syndrome. Am J Emerg Med. 2016;34(1):e1221–2. 10.1016/j.ajem.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Santiago-Burruchaga M, Zalacain-Jorge R, Vazquez-Cordero C. Are airways structural abnormalities more frequent in children with recurrent lower respiratory tract infections? Respir Med. 2014;108(5):800–5. 10.1016/j.rmed.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Boglou P, Papanas N, Oikonomou A, Bakali S, Steiropoulos P. Mounier-Kuhn syndrome in an elderly female with pulmonary fibrosis. Case Rep Med. 2016;2016:8708251. 10.1155/2016/8708251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharitonov VA, Fakhrutdinov AM. [A rare case of tracheobronchomegaly]. Lik Sprava. 1993;(2–3):149–50. [PubMed]
- 16.Gouder C, Bilocca D, Fsadni P, Montefort S. A delayed diagnosis of Mounier-Kuhn syndrome. BMJ Case Rep. 2014;2014:bcr2014203674. 10.1136/bcr-2014-203674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertz P, Costedoat-Chalumeau N, Ferrada MA, et al. Relapsing polychondritis: clinical updates and new differential diagnoses. Nat Rev Rheumatol. 2024;20(6):347–60. 10.1038/s41584-024-01113-9. [DOI] [PubMed] [Google Scholar]
- 18.Purohit P, Preet K, Mittal BR, et al. FDG PET/CT in an interesting case of paraneoplastic relapsing polychondritis associated with adenocarcinoma of the lung. Clin Nucl Med. 2024;49(9):e482–3. 10.1097/RLU.0000000000005345. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Cheng L, Zhao M, et al. Development and validation of diagnostic and activity-assessing models for relapsing polychondritis based on laboratory parameters. Front Immunol. 2023;14:1274677. 10.3389/fimmu.2023.1274677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwok SH, Choi F. Relapsing polychondritis: the Oto-Rhino-Tracheobronchial triad on 18 F-FDG PET/CT. Clin Nucl Med. 2023;48(8):699–701. 10.1097/RLU.0000000000004698. [DOI] [PubMed] [Google Scholar]
- 21.Kamada H, Takanami K, Toyama Y, Saito M, Takase K. 18F-FDG PET/CT imaging of vasculitis complicated with relapsing polychondritis. Clin Nucl Med. 2020;45(7):e327–8. 10.1097/RLU.0000000000003060. [DOI] [PubMed] [Google Scholar]
- 22.Sangle SR, Hughes CD, Barry L, et al. Relapsing polychondritis - A single centre study in the united Kingdom. Autoimmun Rev. 2023;22(8):103352. 10.1016/j.autrev.2023.103352. [DOI] [PubMed] [Google Scholar]
- 23.Gunatilaka CC, Higano NS, Hysinger EB, et al. Increased work of breathing due to tracheomalacia in neonates. Ann Am Thorac Soc. 2020;17(10):1247–56. 10.1513/AnnalsATS.202002-162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodring JH, Howard RS, Rehm SR. Congenital tracheobronchomegaly (Mounier-Kuhn syndrome): a report of 10 cases and review of the literature. J Thorac Imaging. 1991;6(2):1–10. [PubMed] [Google Scholar]
- 25.Clinical grade manufacture of 3D printed patient specific biodegradable devices for pediatric airway support - PubMed. Accessed May 22. 2025. https://pubmed.ncbi.nlm.nih.gov/36041362/ [DOI] [PubMed]
- 26.Gunatilaka CC, Hysinger EB, Schuh A, et al. Neonates with tracheomalacia generate Auto-Positive End-Expiratory pressure via glottis closure. Chest. 2021;160(6):2168–77. 10.1016/j.chest.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SN, Wu AS, Miao JB, Chen S, Jiang J. Airway management for a patient with tracheobronchomegaly undergoing lobectomy: a case report. BMC Anesthesiol. 2023;23(1):357. 10.1186/s12871-023-02324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Du T, Zhang H, Zhang Y, Qiao A. Advances in studies on tracheal stent design addressing the related complications. Mater Today Bio. 2024;29:101263. 10.1016/j.mtbio.2024.101263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Yao X, Wang Z, et al. A flexible porous chiral auxetic tracheal stent with ciliated epithelium. Acta Biomater. 2021;124:153–65. 10.1016/j.actbio.2021.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Gikandi A, Chiu P, Crilley N, et al. Outcomes of patients undergoing surgery for complete vascular rings. J Am Coll Cardiol. 2024;84(14):1279–92. 10.1016/j.jacc.2024.05.078. [DOI] [PubMed] [Google Scholar]
- 31.Soriano L, Khalid T, Whelan D, et al. Development and clinical translation of tubular constructs for tracheal tissue engineering: a review. Eur Respir Rev Off J Eur Respir Soc. 2021;30(162):210154. 10.1183/16000617.0154-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei S, Zhang Y, Luo F, Duan K, Li M, Lv G. Tissue-engineered tracheal implants: advancements, challenges, and clinical considerations. Bioeng Transl Med. 2024;9(4):e10671. 10.1002/btm2.10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Dharmadhikari S, Shontz KM, et al. Regeneration of partially decellularized tracheal scaffolds in a mouse model of orthotopic tracheal replacement. J Tissue Eng. 2021;12:20417314211017417. 10.1177/20417314211017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin M, Yarmus L, Rendina EA, et al. Multi-institutional retrospective analysis of adverse events following rigid tracheobronchoscopy. Respirol Carlton Vic. 2021;26(1):87–91. 10.1111/resp.13873. [DOI] [PubMed] [Google Scholar]
- 35.Kishimoto K, Furukawa KT, Luz-Madrigal A, et al. Bidirectional Wnt signaling between endoderm and mesoderm confers tracheal identity in mouse and human cells. Nat Commun. 2020;11(1):4159. 10.1038/s41467-020-17969-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Liu X, Wang Y, et al. Enhancing long-segmental tracheal restoration: A self-repairing hydrogel loaded with chondrocytokines for sutureless anastomosis and cartilage regeneration. Mater Today Bio. 2024;28:101208. 10.1016/j.mtbio.2024.101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalmády S, Csoma Z, Besenyi Z, et al. New treatment option for capillary lymphangioma: Bleomycin-Based electrochemotherapy of an infant. Pediatrics. 2020;146(6):e20200566. 10.1542/peds.2020-0566. [DOI] [PubMed] [Google Scholar]
- 38.Mitterbauer A, Hoetzenecker K, Birner P, et al. Clinical-radiological, histological and genetic analyses in a lung transplant recipient with Mounier-Kuhn syndrome and end-stage chronic obstructive pulmonary disease. Clin Respir J. 2015;9(3):375–9. 10.1111/crj.12139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.