Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most prevalent bacterial pathogens leading to various kinds of infections, but the characteristics of this superbug with both strong biofilm-producing and intracellular invasive capabilities is rarely reported. This study aimed to investigate the genotypic and phenotypic features of this superbug with above two properties.
Methods
Phenotypic resistance profiling of MRSA clinical isolates was performed via the VITEK 2 AST-GP67 Test Kit. Biofilm production was assessed via crystal violet staining and the Congo red agar (CRA) method. The biofilm-degrading activity was tested using Proteinase K, Dispersin B, and DNase I. The intracellular invasive capability was evaluated via dilution plate count and immunofluorescence assay. Genotyping was performed using multilocus sequence typing and staphylococcal protein A typing methods, and virulence genes were detected via polymerase chain reaction. Flow cytometry was performed to assess the cytotoxicity of the dominant MRSA clones.
Results
A high prevalence (21.6%) of MRSA isolates exhibiting strong biofilm-forming capability was observed in this study, including 70 strains with the highest level of biofilm production (optical density > 0.4). DNase I exhibited the most effective biofilm-degrading activity, with the biofilm-degrading percentage of 78.6% of the strains exceeding 50%. Simultaneously, 71.4% of the isolates exhibited strong invasive capability into A549 cells. ST5-t2460 (48.6%), ST59-t437 (20%), and ST239-t030 (11.4%) were identified as the predominant clones. In particular, ST5-t2460 and ST239-t030 clones exhibited broader antibiotic resistance to gentamicin, ciprofloxacin, levofloxacin, moxifloxacin, and tetracycline compared with ST59-t437 clone. In addition, a higher percentage of the isolates belonging to ST5-t2460 (91.2%) and ST239-t030 (100%) clones demonstrated stronger intracellular invasive capability relative to those belonging to ST59-t437 clone (14.3%). Furthermore, ST5-t2460 and ST239-t030 clones displayed stronger cytotoxicity and carried higher proportions of adhesion-related genes (fnbA, sdrD, sasC) and other virulence genes (sea, seb, sec, isdB, lukE-D, tsst-1).
Conclusions
This is the first report of the phenotypic–genotypic characteristics of MRSA with both strong biofilm-producing and virulence potential, with ST5-t2460, ST59-t437, and ST239-t030 clones accounting for the major genotypes. Further exploration of specific virulence genes correlating to the pathogenesis of this superbug is deemed essential for developing targeted infection control and treatment strategies in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-025-04258-z.
Keywords: MRSA, Biofilm, Virulence gene, Molecular characterization, Epidemiology, Infection control
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is a notorious multidrug-resistant (MDR) bacterium responsible for a range of infectious diseases, including septic shock, endocarditis, and severe pneumonia [1]. Although the total prevalence rate of MRSA is relatively lower globally, predominant clones persistently prevail and evolve in a temporally and geographically dependent manner [2]. Since 2000, ST239-t030-SCCmecIII has rapidly emerged as the predominant S. aureus clone in tertiary hospitals in China, replacing ST239-t037-SCCmecIII [3]. Subsequently, an obvious shift in the dominant clones was observed from 2013 to 2016, when ST239-t030 was replaced by ST59-t437 [4]. Another study suggested that the dominance of ST239 gradually declined between 2008 and 2019, with ST5 replacing ST239 as the predominant epidemic lineage [5]. Numerous studies have demonstrated that the S. aureus genotype affects infection complications, severity, and mortality. In particular, infections caused by ST121 clone are associated with longer hospital stays and extended antimicrobial therapy, whereas clonal complex (CC) 398 MSSA infections are linked to higher mortality rates [6, 7]. The less commonly reported MRSA strains—ST22 and ST630—have shown significantly higher virulence in both in vitro and in vivo models compared with the more prevalent clones [8, 9]. In a previous study, MRSA with strong biofilm-producing capability was associated with strains from Spa-CC064 and MLST CC5 and CC8 [10]. Overall, these findings underscore the strong correlation between S. aureus pathogenicity and its molecular genotype.
Multiple extracellular and cell-surface factors are correlated with the pathogenesis of S. aureus, participating in the processes of adhesion and invasion into host cells, resistance to phagocytosis, and biofilm formation [11]. Biofilm formation is widely recognized as the key mechanism underlying the persistence and chronicity of S. aureus infections [12]. Biofilms are multicellular microbial communities that adhere to substrates, interfaces, or each other and are embedded in an extracellular polymeric substance (EPS) matrix, exhibiting distinct phenotypic changes in growth, gene expression, and protein synthesis [13]. EPS, the hallmark of biofilms, primarily comprises polysaccharides, proteins, and extracellular DNA (eDNA) [14, 15]. Polysaccharides facilitate water retention, promoting bacterial colonization and survival [16], whereas surface proteins such as Protein A, fibronectin-binding proteins (FnBPA and FnBPB), S. aureus surface proteins (SasG), biofilm-associated proteins (Bap), and clumping factor B (ClfB) mediate adhesion [17–21]. eDNA, released via autolysis, further stabilizes a biofilm and reinforces its structural integrity [22]. S. aureus forms biofilms on the host tissues (such as the heart valves, joints, and urinary tract) and external devices (such as catheters and prosthetic joints) to generate a “community-like” environment that enhances physical and chemical stability [23]. This structure provides stronger protection for bacteria from immune defenses compared with its planktonic form [24, 25]. In addition, as a facultative intracellular pathogen, S. aureus manipulates host autophagy pathways to invade both professional and nonprofessional phagocytic cells, thereby establishing intracellular niches that facilitate immune evasion [26]. The resultant intracellular persistence allows bacteria to survive within host cells and, upon cell death or immune activation, they re-enter the bloodstream or other tissues, resulting in hematogenous dissemination and distant-site infections [27]. This mechanism is a major contributor to the persistence and recurrence of S. aureus bacteremia and infections [28]. However, little is known about this superbug that possesses strong biofilm-producing and intracellular invasive capability.
Therefore, this study aimed to investigate the molecular epidemiology, phenotypic resistance characteristics, and in vitro pathogenic potential of MRSA strains with strong biofilm-producing and intracellular invasive capability, specifically focusing on clinical isolates from Hohhot—a region in China with limited data regarding this issue. Existing studies on MRSA in China have predominantly concentrated on economically developed regions such as East and South China. As a representative city in the northwestern region with lower density of population, the molecular characteristics of MRSA clinical isolates in Hohhot was not thoroughly explored as yet. This study holds substantial significance for regional public health and offers region-specific insights that may contribute to a more comprehensive understanding of the epidemiology and pathogenicity of MRSA clinical isolates in this region.
Methods
Identification of the MRSA clinical isolates
Between 2010 and 2023, 1,072 nonrepetitive MRSA clinical isolates were collected from the Affiliated Hospital of Inner Mongolia Medical University (details in Additional file 1). Species identification was conducted using Matrix-Assisted Laser Desorption/n Time of Flight Mass Spectrometry (MALDI-TOF MS) equipment (microTyper MS, Tianrui, China). mecA was amplified via polymerase chain reaction (PCR) with the MRSA N315 strain as the positive control strain. The primers used in the study are provided in Additional file 2. The isolates positive for mecA were confirmed as MRSA [29, 30].
Biofilm formation assay
MRSA isolates were prepared as bacterial suspensions standardized to 0.5 McFarland and diluted 100-fold in TSB. These suspensions were then added into a 96-well plate and incubated statically at 37 °C for 24 h. After incubation, the nonadherent bacteria were removed, and the biofilms were fixed with methanol for 15 min and then stained with crystal violet for 20 min. To quantify biofilm formation, acetic acid was added to destain the crystal violet, followed by gentle mixing for 10 min. The absorbance (optical density [OD]) of the destained solution was measured at 620 nm. All measurements were performed in triplicate, with TSB without bacteria serving as the negative control (ODc). The biofilm-producing capability was categorized based on OD values: OD > 4× ODc indicated strong biofilm production; 2× ODc ≤ OD ≤ 4× ODc indicated moderate biofilm production; OD ≤ 2× ODc indicated weak biofilm production, and OD ≤ ODc indicated no biofilm production [31].
In addition, the biofilm-producing ability of MRSA strains was evaluated by inoculating them onto Congo red agar (CRA) plates. The CRA medium was prepared using blood agar (Solarbio, Beijing China) as the base, supplemented with 18 g of sucrose (Macklin, Shanghai China) per 500 mL of blood agar medium. After autoclaving at 121 °C for 15 min, 0.4 g of Congo red dye (Solarbio, Beijing China) was added. Well-isolated MRSA colonies, grown on standard blood agar plates, were selected and inoculated onto the prepared CRA plates. The plates were incubated at 37 °C for 24 h, followed by an additional 24-hour incubation at room temperature. Strains forming black colonies were considered strong biofilm producers; those forming black colonies with reddish hues were classified as weak biofilm producers; and strains forming entirely red colonies were identified as non-biofilm producers [32].
Biofilm degradation assay
This study evaluated the biofilm-degrading activity of three extracellular matrix–degrading agents on MRSA biofilms: protein-degrading enzyme (Proteinase K), polysaccharide poly-N-acetylglucosamine (PNAG)–degrading enzyme (dispersin B), and eDNA-degrading enzyme (DNase I). Proteinase K (RT403, 20 mg/mL) was purchased from TIANGEN Biotech (Beijing) Co., Ltd. β-N-acetylglucosaminidase (S10237, 32.5 U/mL) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. DNase I (D8071, 5 mg/mL) was purchased from Beijing Solarbio Science & Technology Co., Ltd. Biofilm formation was assessed as described previously. After 24 h of incubation, the supernatant was removed, and 200 µL of each degradation agent (20 µg/mL) was added to the wells containing the biofilms. The plates were incubated at 37 °C for 2 h and stained with crystal violet. To assess biofilm degradation, the experimental groups included a control group (no degradation agents), single-agent groups (Proteinase K, dispersin B, or DNase I), and combination groups (Proteinase K + dispersin B, Proteinase K + DNase I, or dispersin B + DNase I). All tests were performed in triplicate [33, 34].
Antimicrobial susceptibility testin
Antimicrobial susceptibility was assessed using the VITEK2 Compact 60 and the VITEK 2 AST-GP67 Test Kit (bioMerieux, Durham, NC, USA). Sixteen antibiotics were tested, including penicillin (PEN), gentamicin (GEN), rifampicin (RIF), oxacillin (OXA), ciprofloxacin (CIP), levofloxacin (LVX), moxifloxacin (MFX), clindamycin (CLI), erythromycin (ERY), linezolid (LNZ), vancomycin (VAN), quinupristin/dalfopristin (QDA), tetracycline (TCY), tigecycline (TGC), cefoxitin screening (OXSF), and clindamycin induction (DTST). The results were interpreted with reference to the Clinical and Laboratory Standards Institute (CLSI M100-S34) guidelines [35]. The antimicrobial susceptibility results for tigecycline (TGC) were interpreted according to the breakpoints established in version 15.0 of the guidelines published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in 2025 [36].
Cell invasion assay
A549 cells were cultured in DMEM (Giboc, Suzhou China) supplemented with 10% fetal bovine serum (VivaCell, Shanghai China) and seeded at a density of 1.5 × 105 cells per well in a 24-well plate. After 12-h incubation at 37 °C under 5% CO2 atmosphere, MRSA was added at a multiplicity of infection (MOI) of 100:1 and coincubated with the cells for 2 h. Following incubation, the supernatant was removed, and the cells were treated with 200 µg/mL gentamicin (MeilunBio) for 1 h to eliminate any extracellular bacteria (antimicrobial susceptibility testing confirmed that all MRSA strains were sensitive to this concentration of gentamicin). The cells were then lysed with sterile water to release the intracellular MRSA, and bacterial colony-forming units were quantified using the plate count method. Each invasion assay was performed in triplicate [8].
Immunofluorescence staining
A549 cells were seeded into a 24-well plate at the density of 1.5 × 105 cells per well and incubated at 37 °C for 12 h under 5% CO2. Next, the cells were infected with MRSA at an MOI of 100:1 and coincubated at 37 °C for 2 h. Subsequently, the culture supernatant was discarded, and the cells were fixed with 4% paraformaldehyde (Solarbio, Beijing China) for 20 min. Then, 200 µL of rabbit anti-EsxA polyclonal antibody (Huada Protein, Beijing China) was added, and the mixture was incubated at room temperature for 20 min. Following the removal of the primary antibody, the cells were incubated with 200 µL of fluorescently labeled secondary antibody (AF 488 Goat Anti-Rabbit IgG, Lablead, Beijing China) for 20 min at room temperature. Permeabilization was then achieved by incubating the cells with 0.1% Triton X-100 (Solarbio, Beijing China) for 20 min, after which the cytoskeleton was stained with SF954 Phalloidin (Solarbio, Beijing China). The slides were mounted with a DAPI-containing medium (Solarbio, Beijing China), and MRSA invasion into A549 cells was observed and imaged using a laser-scanning confocal microscope (Nikon AX, Shanghai China) [37].
Multilocus sequence typing (MLST)
Genomic DNA from the MRSA strains was extracted using a bacterial genomic DNA extraction kit (TianGen, Beijing China) and stored at − 20 °C for subsequent staphylococcal protein a (Spa) typing and virulence gene detection. PCR amplification was performed on seven housekeeping genes (arc, aro, glp, gmk, pta, tpi, and yqiL) from the MRSA isolates using the primers listed in Additional file 2. The same primers were then used for Sanger sequencing of the PCR products, which were analyzed on an ABI 3730XL DNA Analyzer (Applied Biosystems, USA). Sequence data were processed using Seqman software, wherein chromatograms were inspected and sequences were assembled. The assembled sequences were then compared with known alleles in the MLST database (http://saureus.mlst.net) to determine the sequence type (ST) of the MRSA strains [38].
Spa typing
In this study, the DNA of 70 MRSA strains was PCR-amplified for the spa gene and subsequently sequenced. The spa gene sequence includes a stable forward primer (5′-TAAAGACGATCCTTCGGTGAGC-3′) and a reverse primer (5′-CAGCAGTAGTGCCGTTTGCTT-3′) (Additional file 2). The base immediately following the forward primer is defined as the starting point, and the base immediately before the reverse primer marks the termination. The sequence was analyzed in 24-bp repeat units and categorized into 2–15 polymorphic repeat sequences. Sequences shorter than 24 bp were excluded from the analysis. Spa types were assigned by submitting the sequences to the Ridom Web Server (http://spaserver.ridom.de), and genotypes were determined by comparing the order of repeat units with those in the database [39].
Detection of virulence genes
Conventional PCR was performed to detect 41 virulence-associated genes specific for S. aureus, including adhesion-related genes (icaA, icaD, icaB, altA, clfA, clfB, fnbA, fnbB, fib, bap, bbp, cna, eno, ebpS, efb, sdrC, sdrD, sdrE, sasA, sasC, sasG, and sasX), toxic shock syndrome toxin gene (tsst-1), exfoliative toxin genes (eta and etb), leukocidin genes (pvl, lukDE, and lukM), hemolysin genes (hla, hlb, hld, hlgA, hlgB, and hlgC), enterotoxins (sea, seb, sec, sed, and see), and cation transport genes (isdA and isdB). The PCR reaction was performed in a total volume of 20 µL, comprising template DNA, gene-specific primers, 2× Taq PCR Mix (TianGen, Beijing China), and ddH₂O. The thermal cycling conditions were as follows: initial denaturation at 95 °C for 5 min; followed by 30–35 cycles of denaturation at 95 °C for 30 s, annealing at 50–60 °C for 30 s (depending on primer Tm), and extension at 72 °C for 30 s to 1 min; with a final extension at 72 °C for 10 min. PCR products were separated by electrophoresis on a 1.5% agarose gel and visualized under UV illumination. The detailed information of the primers is provided in Additional file 2 [40].
Cytotoxicity assay
The cytotoxicity of the MRSA isolates was evaluated using flow cytometry. Adherent A549 cells were coincubated with MRSA strains (MOI = 100) at 37 °C for 2 h under 5% CO2. After discarding the supernatant, 1 mL of DMEM containing 200 µg/mL gentamicin was added for 1 h to eliminate the residual free bacteria. The cells were then trypsinized, centrifuged at 1000 ×g for 5 min, and washed twice with PBS. The pellet was resuspended in 1× Annexin V Binding Buffer and stained with FITC-labeled Annexin V and propidium iodide for 15 min (FITC Annexin V Apoptosis Detection Kit, BD Pharmingen, Beijing, China). The samples were immediately analyzed using a flow cytometer (BD Canto II, USA) [41].
Statistical analysis
All analyses were performed using GraphPad Prism 10 (GraphPad Software Inc., San Diego, CA, USA). The results derived from samples between the two groups were subjected to unpaired two-tailed Student’s t-test and χ2 test. P-values of < 0.05 were considered to indicate statistical significance.
Results
Screening of MRSA strains with strong and strong biofilm-producing capability
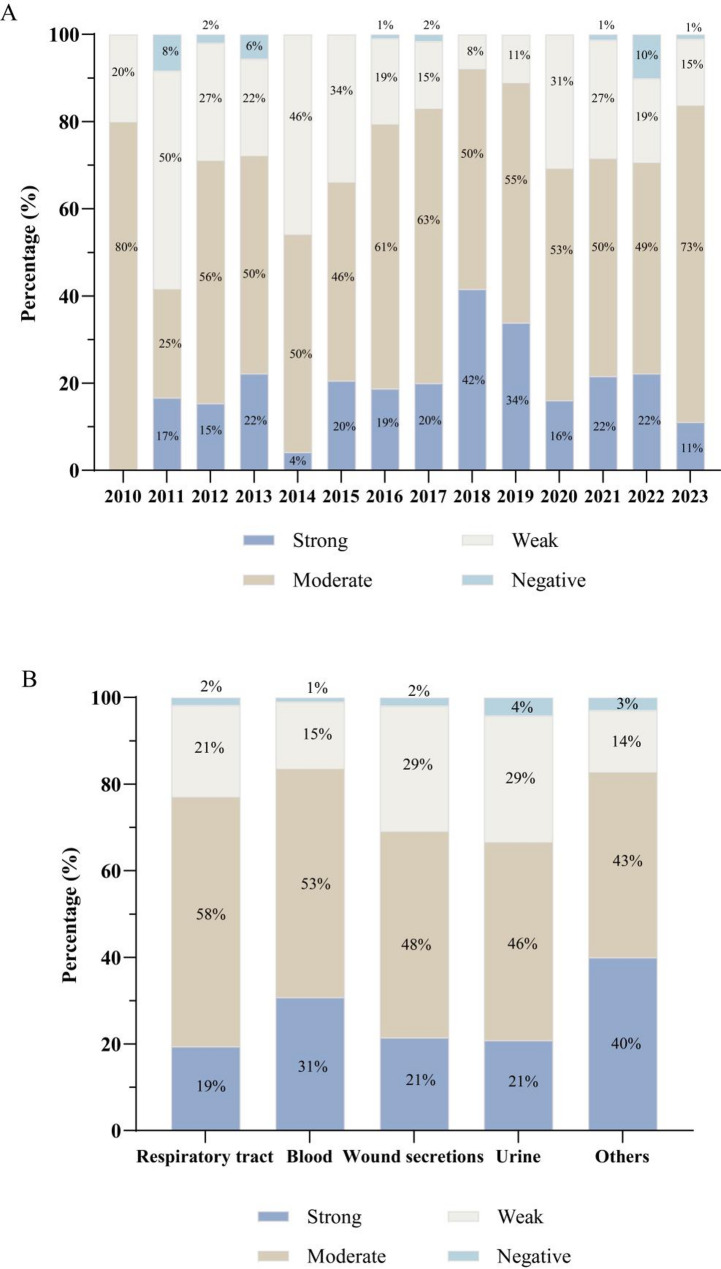
A total of 1,072 MRSA isolates were collected from clinical infection samples during 2010–2023. The biofilm-producing abilities of these isolates were evaluated using both the crystal violet staining assay and the Congo red agar (CRA) method. 1.7% isolates (19/1072) were identified to be non-biofilm producers, while 21.6% (232/1072), 54.4% (584/1072) and 22.1% (237/1072) isolates were classified as strong, moderate and weak biofilm producers, respectively. Over time, the proportion of moderate-to-strong biofilm-producing MRSA strains increased from 42% in 2011 to 92% in 2018, stabilizing at 84% in 2023 (Fig. 1A). Moderate-to-strong biofilm producers were most frequently detected in blood samples (84%), followed by respiratory samples (77%) and wound secretion samples (69%) (Fig. 1B). Based on three independent crystal violet staining assays, we selected 70 MRSA strains exhibiting the highest biofilm-producing capability (optical density > 0.4) for further analysis (Additional file 3).
Fig. 1.
Evaluation of biofilm formation capability of 1,072 clinical MRSA isolates. (A) The proportion of 1072 MRSA strains was categorized by the collection year, covering clinical samples from 2010 to 2023. (B) The proportion of biofilm-producing MRSA strains categorized by the sample source, including respiratory (throat swab, sputum, bronchoalveolar lavage fluid), blood, wound secretion, urine, and other sample types such as ascitic fluid, rectal swabs, and synovial fluid
EPS component analysis of MRSA strains with strong biofilm-producing capability
We performed biofilm degradation experiments on 70 strong biofilm-producing MRSA isolates and noted a consistent degradation trend across both single-agent and dual-agent treatments. DNase I exhibited the highest degradation capability, with 78.6% (55/70) of the strains showing a degradation rate of ≥ 50%, followed by Proteinase K (60%, 42/70) and dispersin B (21.4%, 15/70) (Fig. 2A). Furthermore, the average degradation rates of the three degradative agents on the 70 biofilm-producing MRSA strains confirmed the same trend: DNase I (61.3%) > Proteinase K (47.8%) > dispersin B (38.3%) (Fig. 2B). Therefore, we proposed that the EPS of moderate-to-strong biofilm-producing MRSA strains is primarily composed of eDNA, followed by proteins, with polysaccharides being the least abundant component.
Fig. 2.
Biofilm degradation experiment of MRSA strains with strong biofilm-producing capability. (A) The proportion of strains with a biofilm degradation rate of ≥ 50% using three degrading agents among 70 MRSA strains. (B) The average degradation rates of 70 strong biofilm-producing MRSA isolates treated with single and dual degradation agents
Antimicrobial susceptibility test
Antimicrobial susceptibility of 70 clinical MRSA isolates with strong biofilm-producing capability was evaluated (Fig. 3A). All isolates were susceptible to vancomycin, linezolid, quinupristin/dalfopristin, and tigecycline (100%). However, over 50% exhibited resistance to multiple antibiotics, including penicillin (100%), gentamicin (52.9%), oxacillin (95.7%), ciprofloxacin (67.1%), levofloxacin (64.3%), moxifloxacin (61.4%), clindamycin (81.4%), erythromycin (85.7%), tetracycline (71.4%), and cefoxitin (94.3%). Notably, the highest co-resistance rate (78.6%) was observed for a combination of five antibiotics: penicillin, oxacillin, clindamycin, erythromycin, and cefoxitin. Additionally, three isolates (Nos. 16, 310, and 1025) displayed phenotypic susceptibility to both oxacillin and cefoxitin. However, since they were confirmed to carry the mecA gene, they were classified as oxacillin-susceptible mecA-positive S. aureus (OS-MRSA). Source-based analysis revealed that among the 70 clinical MRSA isolates with strong biofilm-producing capability, the majority were derived from respiratory (60%) and secretion samples (22.9%), while their detection rate in blood specimens was significantly lower (10%) (Fig. 3B). This trend was particularly evident in the resistance rate to gentamicin (Fig. 3C).
Fig. 3.
Antimicrobial susceptibility test results of 70 MRSA strains with biofilm-producing capability. (A) Abbreviations: PEN, penicillin; GEN, gentamicin; RIF, rifampicin; OXA, oxacillin; CIP, ciprofloxacin; LVX, levofloxacin; MFX, moxifloxacin; CLI, clindamycin; ERY, erythromycin; LNZ, linezolid; VAN, vancomycin; QDA, quinupristin/dalfopristin; TCY, tetracycline; TGC, tigecycline; OXSF, cefoxitin screening; DTST, clindamycin induction. (B) Distribution of 70 MRSA isolates across different specimen sources, including respiratory (throat swab, sputum, bronchoalveolar lavage fluid), blood, wound secretion, urine, and other sample types such as ascitic fluid, rectal swabs, and synovial fluid. (C) Distribution of 37 gentamicin-resistant MRSA isolates across different specimen sources
Cell invasion assay of strong biofilm-producing MRSA isolates
To identify high-virulence MRSA isolates, we employed the dilution plate count method to quantify the MRSA strains internalized by A549 cells. Given the current lack of a universally accepted classification system for the cell invasion capability of S. aureus, we selected the widely used reference strain N315 as an internal control in our experimental system. Its invasion rate (5.03%) was adopted as a practical threshold to distinguish between high and low invasiveness under standardized assay conditions. Among the 70 strong biofilm-producing MRSA isolates, 50 exhibited strong invasiveness (invasion rate > 5.03%, 71.4%), whereas 20 displayed weak invasiveness (invasion rate < 5.03%, 28.6%) (Fig. 4). Immunofluorescence staining was performed to visualize the cell invasion effect of the MRSA isolates (Fig. 5). Notably, the majority (71.4%) of strong biofilm-producing MRSA isolates demonstrated strong invasion capability, suggesting a correlation between various virulence factors in MRSA strains, either through mechanistic or epidemiological links.
Fig. 4.
Cell invasion assay of strong biofilm-producing MRSA isolates. The invasion rates of 70 MRSA isolates were determined using the dilution plate count method. The cell invasion rate of the reference strain N315 (5.03%) was used as the threshold to classify the isolates (as shown by the dashed line). Strains with an invasion rate greater than 5.03% were defined as highly invasive MRSA, while those with an invasion rate below 5.03% were classified as weakly invasive MRSA
Fig. 5.
Immunofluorescence staining results. Visualization of MRSA cell invasion using immunofluorescence staining, with MRSA-760 representing a strong invasion strain (cell invasion rate = 26.47%) and MRSA-547 a weak invasion strain (cell invasion rate = 1.97%)
Molecular typing of MRSA isolates with strong biofilm-producing capability
MLST analysis of 70 strong biofilm-producing MRSA isolates identified 12 distinct STs within 8 CCs. The most prevalent ST was ST5 (50%, 35/70), followed by ST59 (20%, 14/70) and ST239 (11.4%, 8/70). Spa typing revealed 12 types, with t2460 being the most common one (48.6%, 34/70), followed by t437 (20%, 14/70) and t030 (11.4%, 8/70). The dominant ST-Spa combinations were ST5-t2460 (48.6%, 34/70), ST59-t437 (20%, 14/70), and ST239-t030 (11.4%, 8/70), demonstrating a strong correlation between the ST and Spa types (Table 1). In addition, this study also identified other highly virulent MRSA clones, including ST188 (2.9%, 2/70), ST45 (2.9%, 2/70), ST22 (1.4%, 1/70), ST121 (1.4%, 1/70), and ST630 (1.4%, 1/70). Furthermore, less common STs, including ST223, ST139, and ST2592 (1.4%, 1/70), were also observed in this study.
Table 1.
Molecular typing results of MRSA isolates with strong biofilm-producing capability
| CC (no.) | MLST (no.) | Spa (no.) | Strong invasion | Weak invasion |
|---|---|---|---|---|
| CC1 (3) | ST188 (2) | t189 (2) | 2 | 0 |
| ST2592 (1) | t114 (1) | 0 | 1 | |
| CC5 (35) | ST5 (35) | t2460 (34) | 31 | 3 |
| t1084 (1) | 1 | 0 | ||
| CC7 (1) | ST7 (1) | t091 (1) | 1 | 0 |
| CC8 (9) | ST239 (8) | t030 (8) | 8 | 0 |
| ST630 (1) | t377 (1) | 0 | 1 | |
| CC22 (1) | ST22 (1) | t1977 (1) | 0 | 1 |
| CC45 (2) | ST45 (2) | t1081 (2) | 2 | 0 |
| CC59 (14) | ST59 (14) | t437 (14) | 2 | 12 |
| CC121 (1) | ST121 (1) | t159 (1) | 1 | 0 |
| Singletons (4) | ST223 (2) | t3622 (2) | 1 | 1 |
| ST39 (2) | t034 (2) | 1 | 1 |
Distribution of virulence genes among predominant clones of strong biofilm-producing MRSA.
This study employed conventional PCR to analyze the frequency of virulence genes among MRSA isolates, with the aim of elucidating the relationship between virulence gene distribution, genetic background, and pathogenic potential. A total of 41 virulence genes were identified among 70 strong biofilm-producing MRSA isolates. Genes associated with adhesion and biofilm formation—including icaA, icaD, eno, fib, sdrC, and efb—were universally detected across all isolates (100% prevalence). In addition, altA, clfA, clfB, fnbB, ebpS, and sdrE were found in the majority of isolates (prevalence > 80%), indicating a widespread presence of adhesion-related genes in strains with strong biofilm-producing capability. Similarly, hemolysin-related genes (hla, hlb, hld, hlgA, hlgB, and hlgC) were commonly detected (Additional file 4).
Molecular typing analysis further revealed that ST5-t2460 and ST239-t030 clones harbored a significantly higher proportion of adhesion-related genes (fnbA, sdrD, and sasC) and other virulence factors (isdB, lukE-D, and tsst-1) compared with ST59-t437. Moreover, ST5-t2460 isolates predominantly carried the enterotoxin genes seb and sec, while sea was more commonly associated with ST239-t030. In contrast, the pvl gene was primarily detected in ST59-t437 isolates, with a significantly higher prevalence than in other clonal types (P < 0.01), suggesting that highly virulent strains possess an expanded repertoire of virulence genes that contribute to their pathogenicity. (Table 2).
Table 2.
(continued)
| Functional categories | Virulence genes | MRSA(n = 70) n (%) |
Genotypes | ||
|---|---|---|---|---|---|
| ST5-t2460 (n = 34) n (%) |
ST59-t437 (n = 14) n (%) | ST239-t030 (n = 8) n (%) | |||
| Cation transport | isdA | 67 (95.7) | 34 (100) | 13 (92.9) | 7 (87.5) |
| isdB | 67 (95.7) | 34 (100) | 12 (85.7) | 8 (100) | |
| Enterotoxins | sea | 12 (17.1) | 1 (2.9) | 3 (21.4) | 8 (100) |
| seb | 32 (45.7) | 31 (91.2) | 0 | 0 | |
| sec | 32 (45.7) | 31 (91.2) | 0 | 0 | |
| sed | 0 | 0 | 0 | 0 | |
| see | 0 | 0 | 0 | 0 | |
| Toxic shock syndrome toxin | tsst-1 | 32 (45.7) | 31 (91.2) | 0 | 0 |
| Leukocidin | pvl | 7 (10) | 1 (2.9) | 5 (35.7) | 0 |
| lukM | 0 | 0 | 0 | 0 | |
| lukD-E | 58 (82.9) | 34 (100) | 5 (35.7) | 8 (100) | |
| Exfoliative toxin | eta | 1 (1.4) | 0 | 0 | 0 |
| etb | 1 (1.4) | 0 | 0 | 0 | |
| Hemolysin | hla | 68 (97.1) | 34 (100) | 13 (92.9) | 8 (100) |
| hlb | 70 (100) | 34 (100) | 14 (100) | 8 (100) | |
| hld | 70 (100) | 34 (100) | 14 (100) | 8 (100) | |
| hlgA | 68 (97.1) | 34 (100) | 13 (92.9) | 8 (100) | |
| hlgB | 68 (97.1) | 34 (100) | 13 (92.9) | 8 (100) | |
| hlgC | 65 (92.9) | 34 (100) | 12 (85.7) | 8 (100) | |
Table 2.
Distribution of virulence genes among dominant STs of MRSA strains with strong biofilm-producing capability
| Functional categories | Virulence genes | MRSA(n = 70) n (%) |
Genotypes | ||
|---|---|---|---|---|---|
| ST5-t2460 (n = 34) n (%) |
ST59-t437 (n = 14) n (%) | ST239-t030 (n = 8) n (%) | |||
| Adhesion factors | icaA | 70 (100) | 34 (100) | 14 (100) | 8 (100) |
| icaD | 70 (100) | 34 (100) | 14 (100) | 8 (100) | |
| icaB | 25 (35.7) | 11 (32.4) | 5 (20) | 2 (8) | |
| altA | 60 (85.7) | 32 (94.1) | 9 (15) | 6 (10) | |
| clfA | 64 (91.4) | 34 (100) | 12 (85.7) | 8 (100) | |
| clfB | 69 (98.6) | 34 (100) | 14 (100) | 8 (100) | |
| fnbA | 55 (78.6) | 33 (97.1) | 6 (42.9) | 8 (100%) | |
| fnbB | 68 (97.1) | 34 (100) | 14 (100) | 8 (100) | |
| fib | 70 (100) | 34 (100) | 14 (100) | 8 (100) | |
| bap | 1 (1.4) | 1 (2.9) | 0 | 0 | |
| bbp | 2 (2.9) | 0 | 0 | 0 | |
| cna | 17 (24.3) | 0 | 0 | 8 (100) | |
| eno | 70 (100) | 34 (100) | 14 (100) | 8 (100) | |
| epbs | 69 (98.6) | 34 (100) | 14 (100) | 8 (100) | |
| sdrC | 70 (100) | 34 (100) | 14 (100) | 8 (100) | |
| sdrD | 51 (72.9) | 34 (100) | 1 (7.1) | 8 (100) | |
| sdrE | 58 (82.9) | 34 (100) | 8 (57.1) | 7 (87.5) | |
| efb | 70 (100) | 34 (100) | 14 (100) | 8 (100) | |
| sasA | 25 (35.7) | 10 (29.4) | 6 (42.9) | 2 (25) | |
| sasC | 53 (75.7) | 32 (94.1) | 5 (35.7) | 7 (87.5) | |
| sasG | 0 | 0 | 0 | 0 | |
| sasX | 0 | 0 | 0 | 0 |
Characteristics of the major clones ST5-t2460, ST59-t437, and ST239-t030
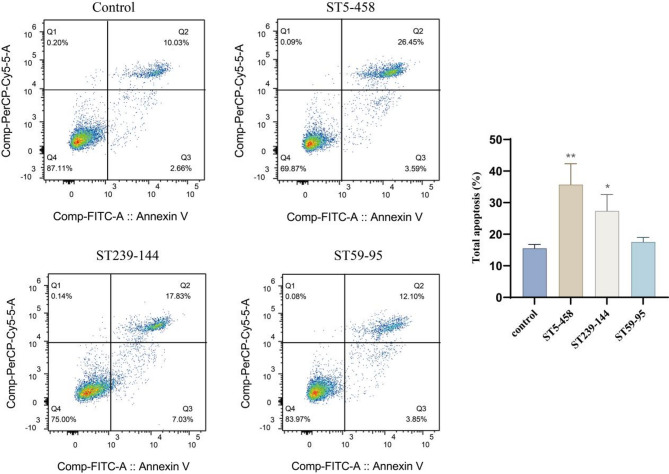
In addition to the distribution patterns of virulence genes, this study also compared the molecular typing results of MRSA isolates with their antimicrobial susceptibility profiles and cytotoxic potential. Among the ST5-t2460 and ST239-t030 clones, the majority of isolates were resistant to gentamicin (82.9%; 87.5%), ciprofloxacin (94.3%; 100%), levofloxacin (94.3%; 100%), moxifloxacin (91.4%; 87.5%), and tetracycline (97.1%; 100%). In contrast, the majority of ST59-t437 isolates were susceptible to the following antibiotics: gentamicin (0%), ciprofloxacin (14.3%), levofloxacin (7.1%), moxifloxacin (7.1%), and tetracycline (35.7%) (Fig. 6A). Regarding cellular invasiveness, 94.1% (32/34) and 100% (8/8) of the ST5-t2460 and ST239-t030 isolates, respectively, were classified as highly invasive, whereas only 14.3% (2/14) of the ST59-t437 isolates exhibited similar invasiveness (Fig. 6B). Flow cytometry confirmed that ST5-t2460 and ST239-t030 MRSA isolates exhibited significantly higher virulence (apoptosis ratio = 35.7%; 27.4%) than ST59-t437 isolates (apoptosis ratio = 17.6%) (Fig. 7).
Fig. 6.
The characteristics of phenotypic resistance and cellular invasiveness of the major MRSA clonal lineages identified in this study. (A) Among the isolates resistant to five antibiotics (GEN, gentamicin; CIP, ciprofloxacin; LVX, levofloxacin; MFX, moxifloxacin; TCY, tetracycline), the predominant sequence types were ST5, ST239, and ST59. (B) The correlation between MLST types and cell invasion ability is shown, with the y-axis indicating invasion rate (%) and the x-axis representing MLST types, where each data point reflects the invasion result of a single MRSA isolate
Fig. 7.
Flow cytometry analysis of cellular virulence in three major clonal types of MRSA. Control group: living cells without bacterial treatment; ST5-458 group: strong invasive MRSA strains, cell invasion rate = 20%; ST239-144 group: strong invasive MRSA strains, cell invasion rate = 16.9%; ST59-95 group: weak invasive MRSA strains, cell invasion rate = 1.03%. Statistical significance was determined by single factor analysis of variance: * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001
Discussion
The pathogenicity of S. aureus largely relies on its ability to adhere to host cells or the extracellular matrix, which is crucial for its further invasion into host cells or biofilm formation [11]. EPS is an essential component of the biofilm matrix, as it plays a key role in the persistence of cells in complex environments, providing significant resistance to external stress and damage [25]. Therefore, disrupting and degrading EPS is a critical challenge in the medical field [42]. In this study, all MRSA isolates were biofilm producers, with 76.1% (816/1072) exhibiting moderate-to-strong biofilm-producing capability. The biofilm-degrading efficiency follows the following pattern: DNase I > Proteinase K > dispersin B, which is consistent with the finding reported previously [43]. Furthermore, Izano reported that mature S. aureus biofilms resist dispersin B degradation, whereas DNase I significantly disrupts or dissolves aged biofilms in vitro, possibly due to PNAG’s predominant role in early biofilm development [33]. Besides eDNA, S. aureus utilizes covalently linked peptidoglycan-associated surface proteins (CWA proteins) to promote adhesion and biofilm formation [11]. Concurrently, these proteins interact directly or indirectly with integrins, facilitating bacterial invasion of nonphagocytic host cells [44]. Notably, 71.4% of the strong biofilm-producing MRSA isolates also exhibited high invasiveness, suggesting a significant correlation between biofilm formation and bacterial invasiveness. Moreover, the highly virulent MRSA strains with strong biofilm-producing capability have not been studied in detail to date, which makes identifying and monitoring this superbug even more important in the present scenario.
To further elucidate the distribution of the clonal types of highly virulent MRSA strains with strong biofilm-producing and intracellular invasive capability, we conducted molecular typing detection on strong biofilm-producing MRSA isolates from Inner Mongolia (2011–2023). The analysis revealed that ST5-t2460, ST59-t437, and ST239-t030 were the most prevalent genotypes, which conformed to the results of a 2022 study [45]. Invasive capability and cytotoxicity assessments further revealed that ST5-t2460 and ST239-t030 were the dominant high-virulence MRSA clones, exhibiting strong biofilm formation, invasiveness, and cytotoxicity, whereas ST59-t437 clones primarily displayed strong biofilm formation, but weak invasive potential. In parallel, we observed that most MRSA isolates exhibited multidrug resistance, with ST5-t2460 and ST239-t030 clones displaying a broader resistance profile, including penicillin, methicillin, cefoxitin, gentamicin, quinolones, and tetracyclines. In contrast, ST59-t437 clones were mostly sensitive to antibiotics. Furthermore, all ST239-t030-MRSA isolates were resistant to rifampin, tetracycline, and fluoroquinolones, which is consistent with the findings of several previous studies [4, 46]. Currently, in China, the resistance of MRSA isolates to quinolones, aminoglycosides, and rifamycin has sharply decreased. In fact, a study by Wang et al. suggested that this shift in the resistance phenotypes was primarily due to the clonal displacement from CC8-ST239 to CC59-ST59 [45]. The ST239-MRSA clone exhibited significant adaptation to enhanced antibiotic resistance. However, Hamid suggested a negative correlation between antibiotic resistance and the expression of toxin genes in MRSA isolates belonging to the ST239 clone, with toxin-producing clones exhibiting non-MDR characteristics. It is proposed that MDR ST239-MRSA strains acquire toxin genes via mobile elements, thereby compromising long-term antibiotic resistance [47]. In contrast, our findings revealed that ST239 and ST5 MRSA strains in our study displayed a high prevalence of virulence genes in addition to broad-spectrum antibiotic resistance. This finding suggests that, over time, dominant MRSA clones bypass genetic compensation mechanisms, acquiring both virulence and resistance following a clone-specific phenomenon.
As another important key determinant of pathogenicity in S. aureus, the distribution of specific virulence genes varied greatly among different clonal subgroups [48]. For instance, Fan reported that ST5 strains exhibited higher vancomycin heteroresistance, biofilm expression, and prevalence of adhesion/virulence genes (fnbB, tst, and sec) compared with non-ST5 strains [49]. Similarly, Bingjie Wang et al. found that CC5-MRSA strains frequently harbor tsst-1; moreover, compared with CC59 and CC8, they often carry adhesion-related genes such as fnbA and sdr [45]. In our study, the highly virulent MRSA clones ST5-t2460 and ST239-t030 carried a higher number of key microbial surface components (MSCRAMMs) and exotoxins, compared with ST59-t437 and other clonal strains. Consistent with our findings, Pereyra previously showed that S. aureus internalized into bovine mammary epithelial cells significantly overexpressed adhesion- and biofilm-related genes, particularly those encoding FnBPs and IcaD [50]. Similarly, Jenkins reported that S. aureus can transition from asymptomatic colonization to an invasive pathogen through the sustained upregulation of sdrC, fnbA, and hla [51]. Besides adhesion, sdrD enhances S. aureus virulence and survival in the bloodstream by promoting neutrophil-mediated bacterial killing [52]. The FnBP-Fn-α5β1 integrin pathway is known to be a major bacterial internalization mechanism, and IsdB directly binds to β3-containing integrins to promote bacterial adhesion and internalization. This finding suggests that IsdB synergizes with FnBPs to facilitate invasion through integrin interactions [18, 53]. In addition to MSCRAMMs, exotoxins secreted by highly virulent MRSA strains contribute to host tissue damage and enhance bacterial virulence. Staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin-1 (TSST-1) act as potent superantigens that non-specifically activate large populations of T cells through interaction with major histocompatibility complex class II (MHC-II) and specific Vβ regions of T cell receptors, particularly Vβ2 in the case of TSST-1. This results in massive release of proinflammatory cytokines, such as TNF-α, IL-2, and IFN-γ, triggering a cytokine storm and systemic immune dysregulation. In contrast, the bicomponent leukotoxin LukE-D (LukED) targets specific chemokine receptors on host immune cells, such as CCR5, and forms oligomeric pores on the cell membrane, leading to cell lysis and contributing to immune evasion and tissue damage [48, 54, 55].
Moreover, Chen reported that ST59-MRSA has relatively low adhesion and invasion capacities [56], whereas another study indicated that 88.7% of ST59-SCCmec IV-t437 isolates exhibited strong biofilm formation [57]. Consistent with these findings, the present study results confirmed that ST59-t437 clones exhibited robust biofilm formation but weaker cell invasion. Among the 1072 biofilm-producing MRSA isolates, the strongest biofilm producers − MRSA-4 (OD = 2.5) and MRSA-689 (OD = 1.801)—belonged to this clonal lineage. However, ST59-t437 clones exhibited substantial variability in their biofilm-producing capability, with some strains exhibiting weaker formation. Similarly, the cytotoxicity of ST59 clones varied between subtypes. In our study, ST59 MRSA clones exhibited significantly lower cytotoxicity than ST5 and ST239, whereas Liao et al. found that the ST59-t437 SCCmec IVa subtype showed higher pathogenicity, including stronger cytotoxicity, faster growth, and higher toxin secretion [58]. These discrepancies highlight the need for further investigation into the virulence and pathogenicity of ST59 clones under different conditions and between different subtypes. Furthermore, although a previous study suggested that pvl-positive ST59 clones have superior adhesion compared with other MRSA clones [59], we did not observe this trend, likely because most ST59 strains in our study lacked pvl, thus warranting further research with a larger sample size to clarify this finding.
Conclusions
This study reported a high prevalence of MRSA clinical isolates with strong biofilm-producing capability in a tertiary teaching hospital in Hohhot, China. Besides, this study revealed that the local strains possessed notable biofilm-producing and intracellular invasive abilities simultaneously, along with distinct phenotypic and genotypic profiles. Although the epidemiology and virulence potential of MRSA clinical isolates have been extensively investigated in other regions of China, data from Hohhot remains scarce. By addressing this gap, the present study highlights the regional significance of MRSA dissemination and its contribution to the broader national surveillance landscape. The predominant clones—ST5-t2460, ST59-t437, and ST239-t030—exhibited clear clone-dependent traits, including variable antibiotic resistance profiles, virulence potential, and expression of specific virulence genes. Collectively, these findings offer key molecular insights into the pathogenicity of hypervirulent MRSA strains and emphasize the need for continued regional monitoring and targeted investigation of virulence-associated genetic markers.
Given these findings, two measures should be taken into account to better control the spread and infection of the high-risk MRSA clones. Firstly, it is imperative to establish an epidemiological surveillance system in Hohhot and its surrounding regions. Surveillance should prioritize the real-time monitoring of dominant clones and the dynamic evolution of their virulence determinants. Systematic data collection will enable early warning of emergence of high-risk clone and guide timely public health interventions. Secondly, to curb the transmission of dominant multidrug-resistant MRSA clones, stricter and more precise antibiotic stewardship programs must be implemented, based on the specific resistance profiles of local strains. These regionally adapted policies will enhance the rational use of antimicrobials and mitigate selective pressure driving antimicrobial resistance. Collectively, these measures will help to control the regional spread of hypervirulent MRSA clones and reduce the overall burden of antimicrobial resistance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all Lab Medicine Department staff for assisting with isolates collection, identification and storage.
Abbreviations
- MRSA
Methicillin-resistant Staphylococcus aureus
- MLST
Multilocus sequence typing
- PCR
Polymerase chain reaction
- MDR
Multi-drug resistant
- EPS
Exopolysaccharides
- eDNA
Extracellular DNA
- PNAG
Polysaccharide poly-N-acetylglucosamine
- PEN
Penicillin
- GEN
Gentamicin
- RIF
Rifampicin
- OXA
Oxacillin
- CIP
Ciprofloxacin
- LVX
Levofloxacin
- MFX
Moxifloxacin CLI: clindamycin
- ERY
Erythromycin
- LNZ
Linezolid
- VAN
Vancomycin
- QDA
Quinupristin/dalfopristin
- TCY
Tetracycline
- TGC
Tigecycline
- OXSF
Cefoxitin screening
- DTST
Clindamycin induction
- MOI
Multiplicity of infection
- Spa
Staphylococcal protein a
- STs
Sequence types
- CCs
Clonal complexes
Author contributions
JRW conceived the idea and designed the experiment. MHH is responsible for all experimental content and manuscript writing. JRW and MHH participated in data analysis and manuscript revision. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 82260416), Joint funds of public hospital in Inner Mongolian Autonomous Region (2024GLLH0302), and the Zhixue Project-Zhiyuan Funding of Inner Mongolia Medical University (No, ZY20241209).
Data availability
The datasets generated and/or analyzed during the current study are available in the Genome Sequence Archive (GSA) repository, under accession number CRA028060 (https://ngdc.cncb.ac.cn/gsa/browse/CRA028060). All other data supporting the findings of this study are available in the additional files of this article.
Declarations
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki. The bacterial strains used in this study were isolated from the routine biological specimens, which were obtained during the clinical diagnosis and management of the patients. Also, rights and health of the subjects were not under threat, and no personal identifying information was used during this study. The mutated isolates were destroyed using autoclaving device and finally processed as medical wastes, according to the regulation of Affiliated hospital of Inner Mongolian Medical University. According to the national regulation on ethical review (No. 2016–11, 12/01/2016), the requirement for the informed consent was waived by the Ethical Committee of Inner Mongolian Medical University, which belongs to the Office of Scientific Research of Inner Mongolian Medical University. Meanwhile, this study was approved by the Ethical Committee of the Inner Mongolian Medical University (Reference No. YKD202201166).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakhundi S, Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31:e00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Liu Y, Jiang X, Chen M, Wang H. Rapid change of Methicillin-Resistant Staphylococcus aureus clones in a Chinese tertiary care hospital over a 15-Year period. Antimicrob Agents Chemother. 2010;54:1842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Sun S, Yang C, Chen H, Yin Y, Li H, et al. The changing pattern of population structure of Staphylococcus aureus from bacteremia in China from 2013 to 2016: ST239-030-MRSA replaced by ST59-t437. Front Microbiol. 2018;9:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Zhao L, Liu Y, Wang Y, Jian Y, Zhao N, et al. Mechanisms of high-level fosfomycin resistance in Staphylococcus aureus epidemic lineage ST5. J Antimicrob Chemother. 2022;77:2816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouiller K, Gbaguidi-Haore H, Hocquet D, Cholley P, Bertrand X, Chirouze C. Clonal complex 398 methicillin-susceptible Staphylococcus aureus bloodstream infections are associated with high mortality. Clin Microbiol Infect. 2016;22:451–5. [DOI] [PubMed] [Google Scholar]
- 7.Rao Q, Shang W, Hu X, Rao X. Staphylococcus aureus ST121: a globally disseminated hypervirulent clone. J Med Microbiol. 2015;64:1462–73. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Xiao Y, Shen L, Wan C, Wang B, Zhou P et al. Phenotypic and genomic analysis of the hypervirulent methicillin-resistant Staphylococcus aureus ST630 clone in China. mSystems. 2024; 9:e00664–24. [DOI] [PMC free article] [PubMed]
- 9.Zhao H, Wu X, Wang B, Shen L, Rao L, Wang X, et al. Phenotypic and genomic analysis of the hypervirulent ST22 methicillin-resistant Staphylococcus aureus in China. mSystems. 2023;8:e0124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naicker PR, Karayem K, Hoek KGP, Harvey J, Wasserman E. Biofilm formation in invasive Staphylococcus aureus isolates is associated with the clonal lineage. Microb Pathog. 2016;90:41–9. [DOI] [PubMed] [Google Scholar]
- 11.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schilcher K, Horswill AR. Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol Mol Biol Rev. 2020;84:e00026–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aboelnaga N, Elsayed SW, Abdelsalam NA, Salem S, Saif NA, Elsayed M, et al. Deciphering the dynamics of methicillin-resistant Staphylococcus aureus biofilm formation: from molecular signaling to nanotherapeutic advances. Cell Commun Signal. 2024;22:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flemming H-C, Van Hullebusch ED, Neu TR, Nielsen PH, Seviour T, Stoodley P, et al. The biofilm matrix: multitasking in a shared space. Nat Rev Microbiol. 2023;21:70–86. [DOI] [PubMed] [Google Scholar]
- 16.Marincola G, Jaschkowitz G, Kieninger A-K, Wencker FDR, Feßler AT, Schwarz S, et al. Plasmid-Chromosome crosstalk in Staphylococcus aureus: a horizontally acquired transcription regulator controls polysaccharide intercellular Adhesin-mediated biofilm formation. Front Cell Infect Microbiol. 2021;11:660702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue T, You Y, Shang F, Sun B. Rot and Agr system modulate fibrinogen-binding ability mainly by regulating ClfB expression in Staphylococcus aureus NCTC8325. Med Microbiol Immunol. 2012;201:81–92. [DOI] [PubMed] [Google Scholar]
- 18.Josse J, Laurent F, Diot A. Staphylococcal adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front Microbiol. 2017;8:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, et al. A novel Staphylococcus aureus biofilm phenotype mediated by the Fibronectin-Binding proteins, FnBPA and FnBPB. J Bacteriol. 2008;190:3835–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills KB, Maciag JJ, Wang C, Crawford JA, Enroth TJ, Keim KC, et al. Staphylococcus aureus skin colonization is mediated by SasG lectin variation. Cell Rep. 2024;43:114022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penadés JR. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol. 2001;183:2888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFrancesco AS, Masloboeva N, Syed AK, DeLoughery A, Bradshaw N, Li G-W, et al. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc Natl Acad Sci U S A. 2017;114:E5969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueiredo AMS, Ferreira FA, Beltrame CO, Côrtes MF. The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit Rev Microbiol. 2017;43:602–20. [DOI] [PubMed] [Google Scholar]
- 24.Manasherob R, Mooney JA, Lowenberg DW, Bollyky PL, Amanatullah DF. Tolerant small-colony variants form prior to resistance within a Staphylococcus aureus biofilm based on antibiotic selective pressure. Clin Orthop Relat Res. 2021;479:1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin W, Wang Y, Liu L, He J. Biofilms: the microbial protective clothing in extreme environments. Int J Mol Sci. 2019;20:3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, et al. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One. 2008;3:e1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulcahy ME, O’Brien EC, O’Keeffe KM, Vozza EG, Leddy N, McLoughlin RM. Manipulation of autophagy and apoptosis facilitates intracellular survival of Staphylococcus aureus in human neutrophils. Front Immunol. 2020;11:565545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vozza EG, Mulcahy ME, McLoughlin RM. Making the most of the host; targeting the autophagy pathway facilitates Staphylococcus aureus intracellular survival in neutrophils. Front Immunol. 2021;12:667387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiramatsu K, Kihara H, Yokota T. Analysis of borderline-resistant strains of methicillin-resistant Staphylococcus aureus using polymerase chain reaction. Microbiol Immunol. 1992;36:445–53. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe S, Nsofor CA, Thitiananpakorn K, Tan X-E, Aiba Y, Takenouchi R et al. Metabolic remodeling by RNA polymerase gene mutations is associated with reduced β-lactam susceptibility in oxacillin-susceptible MRSA. mBio. 2024; 15:e00339–24. [DOI] [PMC free article] [PubMed]
- 31.Brahma U, Sharma P, Murthy S, Sharma S, Chakraborty S, Appalaraju SN, et al. Decreased expression of FemXAB genes and Fnbp mediated biofilm pathways in OS-MRSA clinical isolates. Sci Rep. 2019;9:16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva-Santana G, Baêta Júnior ES, Silva Conceição GM, Aguiar-Alves F, Lima Brandão ML, Lopes-Torres EJ, et al. Intervention of Corynebacterium striatum in the sessile lifestyle of Staphylococcus aureus wild-type and mutants for Ica genes in polymicrobial biofilms. Microb Pathog. 2025;204:107577. [DOI] [PubMed] [Google Scholar]
- 33.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of Poly-N-Acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74:470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank KL, Patel R. Poly-N-Acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-Positive Staphylococcus lugdunensis isolates. Infect Immun. 2007;75:4728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Huang T, Xu K, Li C, Li Y. Molecular characteristics and virulence gene profiles of Staphylococcus aureus isolates in Hainan, China. BMC Infect Dis. 2019;19:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Committee on. Antimicrobial Susceptibility Testing. 2025.
- 37.Bin L, Kim BE, Brauweiler A, Goleva E, Streib J, Ji Y, et al. Staphylococcus aureus α-toxin modulates skin host response to viral infection. J Allergy Clin Immunol. 2012;130:683–e6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Fang F, Zhao J, Lou N, Li C, Huang T, et al. Molecular characteristics and virulence gene profiles of Staphylococcus aureus causing bloodstream infection. Braz J Infect Dis. 2018;22:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frénay HM, Bunschoten AE, Schouls LM, van Leeuwen WJ, Vandenbroucke-Grauls CM, Verhoef J, et al. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. Eur J Clin Microbiol Infect Dis. 1996;15:60–4. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Lin D, Huang Z, Zhang J, Xie W, Liu P, et al. Clonality, virulence genes, and antibiotic resistance of Staphylococcus aureus isolated from blood in Shandong, China. BMC Microbiol. 2021;21:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suardana IW, Swacita IBN, Pinatih KJP, Suharsono H, Widiasih DA. Assessment of cell cycle and induction of apoptosis by Shiga-like toxin produced by Escherichia coli O157:H7 in T47D breast cancer cells using flow cytometry. Asian Pac J Cancer Prev. 2022;23:3247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakshmi SA, Alexpandi R, Shafreen RMB, Tamilmuhilan K, Srivathsan A, Kasthuri T, et al. Evaluation of antibiofilm potential of four-domain α-amylase from Streptomyces griseus against exopolysaccharides (EPS) of bacterial pathogens using Danio rerio. Arch Microbiol. 2022;204:243. [DOI] [PubMed] [Google Scholar]
- 43.Bowden LC, Finlinson J, Jones B, Berges BK. Beyond the double helix: the multifaceted landscape of extracellular DNA in Staphylococcus aureus biofilms. Front Cell Infect Microbiol. 2024;14:1400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster TJ. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2016;35:1923–31. [DOI] [PubMed] [Google Scholar]
- 45.Wang B, Xu Y, Zhao H, Wang X, Rao L, Guo Y, et al. Methicillin-resistant Staphylococcus aureus in china: a multicentre longitudinal study and whole-genome sequencing. Emerg Microbes Infections. 2022;11:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Z, Gu F-F, Guo X-K, Ni Y-X, He P, Han L-Z. Antimicrobial resistance and molecular characterization of Staphylococcus aureus causing childhood pneumonia in Shanghai. Front Microbiol. 2017;8:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abd El-Hamid MI, Sewid AH, Samir M, Hegazy WAH, Bahnass MM, Mosbah RA, et al. Clonal diversity and epidemiological characteristics of ST239-MRSA strains. Front Cell Infect Microbiol. 2022;12:782045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12:547–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan Y-X, Chen M-T, Li N-Y, Liu X-F, Yang M-J, Chen Y-C et al. Sequence type 5 (ST5) as a possible predictor of bacterial persistence in adult patients with Methicillin-Resistant Staphylococcus aureus pneumonia treated with Vancomycin. Microbiol Spectr. 2022; 10:e01348–22. [DOI] [PMC free article] [PubMed]
- 50.Pereyra EAL, Picech F, Renna MS, Baravalle C, Andreotti CS, Russi R, et al. Detection of Staphylococcus aureus adhesion and biofilm-producing genes and their expression during internalization in bovine mammary epithelial cells. Vet Microbiol. 2016;183:69–77. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, et al. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio. 2015;6:e02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Askarian F, Uchiyama S, Valderrama JA, Ajayi C, Sollid JUE, van Sorge NM, et al. Serine-aspartate repeat protein D increases Staphylococcus aureus virulence and survival in blood. Infect Immun. 2017;85:e00559-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zapotoczna M, Jevnikar Z, Miajlovic H, Kos J, Foster TJ. Iron-regulated surface determinant B (IsdB) promotes Taphylococcus aureus adherence to and internalization by non-phagocytic human cells. Cell Microbiol. 2013;15:1026–41. [DOI] [PubMed] [Google Scholar]
- 54.Schlievert PM, Davis CC. Device-associated menstrual toxic shock syndrome. Clin Microbiol Rev. 2020;33:e00032-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ono HK, Hirose S, Narita K, Sugiyama M, Asano K, Hu D-L, et al. Histamine release from intestinal mast cells induced by Staphylococcal enterotoxin A (SEA) evokes vomiting reflex in common marmoset. PLoS Pathog. 2019;15:e1007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen F, Yin Y, Chen H, Jin L, Li S, Wang R et al. Phenotypic and Genomic Comparison of Staphylococcus aureus Highlight Virulence and Host Adaptation Favoring the Success of Epidemic Clones. mSystems. 7:e00831-22. [DOI] [PMC free article] [PubMed]
- 57.Yang X, Qian S, Yao K, Wang L, Liu Y, Dong F, et al. Multiresistant ST59-SCCmec IV-t437 clone with strong biofilm-forming capacity was identified predominantly in MRSA isolated from Chinese children. BMC Infect Dis. 2017;17:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao F, Gu W, Fu X, Yuan B, Zhang Y. Comparison of virulence-related determinants between the ST59-t437 and ST239-t030 genotypes of methicillin-resistant Staphylococcus aureus. BMC Microbiol. 2021;21:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X, Sun K, Luo Q, Duan Y, Chen F. Emergence and spread of pvl-positive genotypic CA-MRSA ST59 with increased adhesion capacity from wounds in hospitals. J Infect. 2019;79:612–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Genome Sequence Archive (GSA) repository, under accession number CRA028060 (https://ngdc.cncb.ac.cn/gsa/browse/CRA028060). All other data supporting the findings of this study are available in the additional files of this article.