Abstract
Background
Tumor necrosis factor-alpha (TNF) is an inflammatory cytokine implicated in the development of many chronic inflammatory diseases and TNF-α inhibitors (TNF-I) are frequently prescribed as treatment. Their malignancy risk is debated, with pro-oncogenic effects of decreased immune surveillance and anti-oncogenic effects of decreasing chronic inflammation. As such, the literature is inconclusive in the malignancy risk of these medications. Here we investigate the malignancy risk in patients with chronic TNF-I exposure.
Methods
This is a single health system, retrospective non-matched cohort study of patients with chronic inflammatory conditions between 1996 and 2023. Patients exposed to TNF-I were identified using the generic medication names and the chronic inflammatory disease indication. The unmatched control cohort consisted of patients with the same chronic inflammatory conditions but without TNF-I exposure. Malignancies in this population were identified using ICD-9 and ICD-10 codes. TNF-I exposure was analyzed as a time-varying covariate prior to model fitting. Hazard ratios (HR) for TNF-I exposure on overall and individual cancer risk were estimated using two-sided Cox proportional hazards regression.
Results
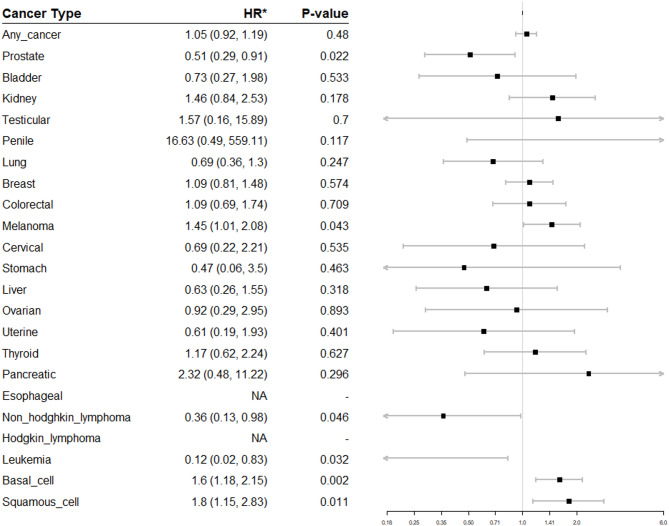
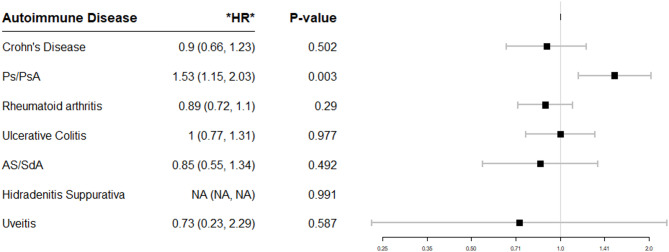
There were 12,941 patients exposed to TNF-I and 37,402 patients unexposed to TNF-I. TNF-I exposure was not associated with overall cancer risk (HR 1.05, 95% CI 0.92–1.19). TNF-I exposure was positively associated with melanoma (HR 1.45, 95% CI 1.01–2.08), basal cell carcinoma (BCC) (HR 1.6, 95% CI 1.18–2.15) and squamous cell carcinoma (SCC) (HR 1.8, 95% CI 1.15–2.83), and negatively associated with prostate cancer (PCa) (HR = 0.51, 95% CI 0.29–0.91), leukemia (HR 0.12, 95% CI 0.02–0.83), and non-Hodgkin lymphoma (NHL) (HR 0.36, 95% CI 0.13–0.98). There was an increased risk of overall malignancy in TNF-I exposed patients with Psoriasis (HR 1.53, 95% CI 1.15–2.03, p = 0.003) but not in other inflammatory conditions.
Conclusions
Patients with TNF-I exposure had higher risk of melanoma, skin SCC and BCC along with lower risk of leukemia, NHL, and PCa. This is consistent with previous melanoma, skin SCC and BCC data and demonstrates novel findings for leukemia, NHL, and PCa. There may need to be differential cancer screening algorithms for patients with different inflammatory conditions and TNF-I exposure.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12950-025-00445-x.
Keywords: Cancer, Neoplasm, Malignancy, Tumor necrosis factor alpha inhibitors, Inflammation, Immunosuppression, Adalimumab, Infliximab, Etanercept
Background
Previous epidemiologic studies have noted an association between chronic inflammation and a variety of malignancies, including lymphoma and skin cancer [1–3]. This association has been linked to both extrinsic and intrinsic causes of inflammation but with the common theme that inflammation alters both local and systemic immune signaling leading to pro-oncogenic conditions for an incipient malignancy such as local angiogenesis and systemic immune evasion [4, 5].
Tumor necrosis factor-alpha (TNF) is an inflammatory cytokine produced by macrophages, T-lymphocytes and other cells in the innate and adaptive immune system. TNF signaling occurs via the NF-kB pathway and dysregulation of this signaling has been implicated in the development of many autoimmune and chronic inflammatory diseases, such as rheumatoid arthritis and ulcerative colitis [6]. As such, a common medication class used to control chronic inflammatory diseases is the TNF Inhibitors (TNF-I), of which there are 5 currently approved for the US market (Adalimumab, Infliximab, Etanercept, Golimumab, and Certolizumab).
There are conflicting roles of TNF in the tumor microenvironment. It was initially named due to its ability to cause hemorrhagic necrosis of tumors and thus was viewed as a potent anti-oncogenic cytokine, however, its role in oncogenesis is much more complicated [7]. Many malignancies develop and progress in the setting of chronic inflammation, much of which is mediated by TNF via complex signaling cascades involving NF-kB and AP-1 transcription factor complexes [8, 9]. As a result, inhibition TNF and its associated inflammation has become a key molecular target in cancer prevention [10]. On the other hand, TNF blockade decreases immune surveillance, a side effect of immunosuppression which has been found to play a significant pro-oncogenic role in in the literature, especially in cancers that are known to be highly immune responsive, such as melanoma [11–14].
While the majority of the TNF-I literature supports these medications as low-risk for oncogenesis in exposed patients, there are reports of increased malignancy with these medications [15, 16]. Furthermore, the pivotal clinical trials rarely have follow-up data beyond one year of drug treatment, which is a non-conclusive follow-up timeframe to evaluate the risk of solid malignancy [17]. There are a small number of registry studies that have a longer follow-up timeframe, however, these studies largely rely on a spontaneous reporting system for adverse events and are too underpowered to draw conclusions about the long-term risk of de novo solid malignancy [18]. These findings call for an examination and pooled analysis of the risk of overall malignancy in the setting of TNF-I immunosuppression. The current proposal looks to investigate the risk of overall and individual malignancies in patients on long-term TNF-I immunosuppression through a multi-center, single-health system retrospective cohort. Our hypothesis is that long-term TNF-I exposure will increase the risk of de novo malignancy due to decreased immune surveillance.
Materials and methods
Study design
This is a non-interventional observational retrospective cohort study.
Data extraction
Institutional Review Board approval was granted by the Northwestern University Feinberg School of Medicine (IRB# STU00211830) prior to data collection and analysis. The Northwestern Medicine Enterprise Data Warehouse, the central repository containing clinical data of patients treated at all centers within the Northwestern Medicine system, which comprises 11 hospitals in 8 counties in and around Chicago, IL, [19]. was queried for adult (≥ 18 years) patients who presented to any Northwestern Medicine clinic between July 1996 and July 2023, with an associated ICD-9 or ICD-10 code corresponding to a chronic inflammatory condition who were prescribed at least one TNF-I.
Patients exposed to TNF-I were identified using the generic medication names for any of the five TNF-I (adalimumab, infliximab, etanercept, certolizumab, and golimumab) along with the chronic inflammatory condition for which they were prescribed. Chronic inflammatory conditions were grouped based on on-label indications for TNF-I and included Crohn’s Disease (CD), Ulcerative Colitis (UC), Psoriasis/Psoriatic Arthritis (Ps/PsA), Rheumatoid Arthritis (RA), Ankylosing Spondylitis/Spondylarthritis (AS/SpA), Hidradenitis Suppurativa (HS), and Uveitis (UV) (Supplementary Methods 1). All reported malignancies occurring after TNF-I initiation were identified using ICD-9 or ICD-10 codes (Supplementary Methods 2). Demographic data, comorbidities and dates of physician encounters were extracted. Variables including sex, race, and ethnicity were identified via the information entered in the electronic medical record (EMR). When the EHR-reported race of a patient was neither White nor Black, they were extracted as the category “Other”. Patients with missing sex or smoking history were from the analysis. The minimum dosage for inclusion was one month of any TNF-I (40 mg every other week for adalimumab, 5 mg/kg every other week for infliximab, 50 mg twice weekly for etanercept, 400 mg every 2 weeks for certolizumab, or 300 mg across two doses in one month for golimumab). Follow-up time for TNF-I exposed patients was defined as the duration between a patient’s first TNF-I prescription and most recent documented physician encounter.
Control groups
For the control group, we identified patients with the same ICD-9/10 codes for chronic inflammatory diseases for which TNF-I are indicated (CD, UC, Ps/PsA, RA, AS, SpA, HS, US) but did not have any TNF-I prescriptions listed in their charts. These controls were not matched.
Covariates
Medical comorbidities were queried for each patients using the ICD-9 and ICD-10 codes (Supplementary Methods 1). If a cancer was diagnosed less than 6 months after initial exposure to a TNF-I, it was excluded from the analysis.
Outcomes
The primary outcomes were a diagnosis of malignancy stratified by exposure to TNF-I and, if exposed, type of TNF-I medication. Malignancy diagnosis was confirmed by a chart review. Tumor stage at diagnosis, topography, and histologic subtype were determined from biopsy or surgical pathology if available. The incidence of metastatic disease (N + or M+) and stage at diagnosis was also assessed by a chart review.
Statistical analysis
Subjects were divided into two groups based on prior exposure or absence of exposure to a TNF-I. Demographic and clinical variables for each group were presented as medians and interquartile ranges (IQR) for continuous variables and compared between groups using Wilcoxon rank sum test. Categorical variables were summarized as counts and percentages and compared between groups using the chi-squared and Fisher's exact test. Any patient with a malignancy except for non-melanoma skin cancer (NMSC) prior to TNF-I exposure was excluded from the analysis. The proportional assumption was checked prior to the model fitting. TNF-I exposure was considered a time-varying covariate, as TNF-I induction date was not fixed for patients in the TNF-I-exposed group [20]. Models were fit incorporating all patients who developed any malignancy during the study period and individually for each type of cancer. In all models, patients in the TNF-I exposed and unexposed groups who did not develop malignancies were included. For sex-specific malignancies (i.e., prostate, ovarian, cervical, endometrial cancers), we included only subjects of the corresponding sex.
We compared cancer rates between subjects in the TNF-I exposed and unexposed groups using Cox proportional hazards regression analysis, adjusting for age, race, sex, smoking status and underlying inflammatory disease type. Exposure time for all patients was the duration of time between a patient’s first TNF-α-I prescription and most recent TNF-I prescription. Follow-up time for all patients was the duration between a patient’s first TNF-I prescription and last physician encounter [21–24]. We conducted a random 5% chart review of the entire cohort to ensure accuracy.
Results of regression models were presented as forest plots with hazard ratios and 95% confidence intervals. As a sensitivity analysis, we added patients with missing sex, race, and smoking status, by including a new group “unknown” for these categorical variables and repeated the multivariate Cox regression analyses [25]. P values < 0.05 were considered statistically significant throughout the study and determined in a two-sided manner.
Results
For the health system cohort, there were a total of 50,343 patients with chronic inflammatory disorders, 12,941 of whom had TNF-I exposure and 37,402 of whom did not have TNF-I exposure. Overall, these patients developed 5,576 primary malignancies (11%) during the study period with a median follow-up of 88 months (IQR 40–147 months). Of 12,941 patients exposed to a TNF-I, there was a median post-TNF-I exposure follow-up of 44 months (IQR 20–77 months) (Table 1). Rates of individual malignancy diagnosis are available in Supplementary Table 1.
Table 1.
System wide patient demographics and disease states by TNF-I exposure
| TNF-I Exposed (N = 12,941) | TNF-I Unexposed (N = 37,402) | Total (N = 50,343) | P-value | |
|---|---|---|---|---|
| Any Cancer | < 0.001* | |||
| Yes | 827 (6%) | 4,749 (13%) | 5,576 (11%) | |
| No | 12,114 (94%) | 32,653 (87%) | 44,767 (89%) | |
| Age (years) | 0.002* | |||
| Median (Q1, Q3) | 47 (34, 58) | 47 (32, 60) | 47 (33, 60) | |
| Range | 18–97 | 18–99 | 18–99 | |
| Race | < 0.001* | |||
| Black | 977 (7.7%) | 4,108 (11%) | 5,085 (10%) | |
| White | 9,662 (75%) | 27,255 (73%) | 36,917 (73%) | |
| Other | 447 (3.5%) | 1,302 (3.5%) | 1,749 (3.5%) | |
| Unknown | 1,855 (14%) | 4,737 (13%) | 6,592 (13%) | |
| Sex | < 0.001* | |||
| Female | 7,980 (62%) | 24,780 (66%) | 32,760 (65%) | |
| Male | 4,961 (38%) | 12,622 (34%) | 17,583 (35%) | |
| Smoking Status | < 0.001* | |||
| Current | 1,295 (10%) | 4,347 (12%) | 5,642 (11%) | |
| Former | 2,920 (23%) | 9,553 (26%) | 12,473 (25%) | |
| Never | 8,726 (67%) | 23,502 (63%) | 32,228 (64%) | |
| Follow-up (months, median) | < 0.001* | |||
| Overall (Q1, Q3) | 99 (52, 163) | 84 (35, 142) | 88 (40, 147) | |
| Post-Exposure (Q1, Q3) | 44 (20, 77) | |||
| Range | 0 − 367 | 0–279 | 0–367 | |
| Rheumatoid Arthritis | 3,882 (30%) | 11,894 (32%) | 15,776 (31%) | < 0.001* |
| Crohn’s Disease | 2,215 (17%) | 5,964 (16%) | 8,179 (16%) | 0.002* |
| Ulcerative Colitis | 2,873 (22%) | 11,799 (32%) | 14,672 (29%) | < 0.001* |
| Psoriasis/Psoriatic Arthritis | 2,951 (23%) | 742 (2.0%) | 3,693 (7.3%) | < 0.001* |
| Ankylosing Spondylitis/Spondyloarthritis | 1,177 (9.1%) | 6,253 (17%) | 7,430 (15%) | < 0.001* |
| Hidradenitis Suppurativa | 225 (1.7%) | 3,755 (10%) | 3,980 (7.9%) | < 0.001* |
| Uveitis | 196 (1.5%) | 2,061 (5.5%) | 2,257 (4.5%) | < 0.001* |
*P < 0.05 denotes significance
In the adjusted model (Fig. 1), there was no increased risk of overall malignancy (HR 1.05, p = 0.48). TNF-I exposure was associated with an increased risk of three classes of malignancy: melanoma (HR 1.45, p = 0.043), cutaneous squamous cell carcinoma (SCC) (HR 1.80, p = 0.011), and basal cell carcinoma (BCC) (HR 1.6, p = 0.002). TNF-I exposure was associated with a decreased risk of three classes of malignancy: non-Hodgkin lymphoma (NHL) (HR 0.36, p = 0.046), leukemia (HR 0.12, p = 0.032), and prostate cancer (PCa) (HR 0.51, p = 0.022). There were no statistically significant differences in the risk of any other malignancy.
Fig. 1.
Hazard ratios (HR) for TNF-I exposure and malignancy diagnosis
Further analyses were performed by individual inflammatory conditions (Fig. 2) and by individual TNF-I drug exposure (Fig. 3). There was a statistically significant increased risk of overall malignancy in TNF-I exposed patients with Ps/PsA (HR 1.53, p = 0.003). There were no statistically significant differences in the risk of malignancy for other inflammatory disease or by individual medication (adalimumab, certolizumab, etanercept or infliximab). There were not enough patients exposed to golimumab to enable a risk calculation.
Fig. 2.
Forest plot of Hazard ratios (HR) of overall malignancy after TNF-I exposure by autoimmune disease type
Fig. 3.
Forest plot of Hazard ratios (HR) of overall malignancy by individual TNF-I exposure
A sensitivity analysis was performed which included patients with missing sex and smoking demographic info, which added a total of 5,866 new patients. Upon time-dependent analysis, TNF-I exposure remained associated with an increased risk of cutaneous SCC (HR 1.76, p = 0.014) and BCC (HR 1.58, p = 0.003). TNF-I exposure also remained associated with a decreased risk of NHL (HR 0.36, p = 0.047), leukemia (HR 0.11, p = 0.03), and PCa (HR 0.50, p = 0.02). Melanoma was no longer significant (HR 1.43, p = 0.052) (Supplementary Fig. 1). Ps/PsA patients remained at elevated risk of cancer after TNF-I exposure (HR 1.52, p = 0.004) (Supplementary Fig. 2). There were no differences by individual TNF-I (Supplementary Fig. 3).
Discussion
Our aggregate multi-hospital system-wide data were extracted from 50,343 subjects followed for 76,564 person-years, defining a sample size similar to previous large registry-based cohort studies [26, 27]. Our findings are largely consistent with prior studies that have demonstrated increased risk of basal cell carcinomas [28–32] and melanomas [33–35], however, our findings of decreased risks of PCa, leukemia, and NHL are novel. This is the first example of a malignancy for which TNF-I use appears protective, which is theoretically consistent with the proposed association between chronic inflammation and the development of PCa [1]. To our knowledge, this is also the first institutional cohort to demonstrate increased risk of overall malignancy in Ps/PsA patients exposed to TNF-I in the literature.
It merits discussion why our institutional analysis demonstrated novel findings of risk of individual cancers. The validity our findings is supported by the large size of the study and its contemporary nature as we incorporated patients in the current era of widespread TNF-I use as opposed to prior negative studies in the past [36–38]. Data from our system wide repository were collected in a standardized fashion and thus likely more consistent and detailed than those from national registries drawing on heterogeneous sources [26, 27]. This is the fundamental improvement of our study over previous studies, all of which used insurance databases, national registries, post-marketing surveillance studies, or had a much smaller sample size. Our study was able to directly query the charts of over 50,000 patients within a single health system and directly compare long term cancer diagnoses with a median follow-up of over seven years. As a result of our stratified analysis, our cohort study is similar limited by follow-up time as previous studies. There is also a concern that patients on TNF-I had more frequent healthcare interactions and would thus be more likely to have cancers identified. However, our analysis did not identify any increased risk of any screen-detected cancer (prostate, colorectal, lung, breast, cervical) while patients on a TNF-I were less likely to be diagnosed with prostate cancer, arguing against increased rates of cancer diagnosis as an artifact of receiving more frequent medical care. Our sensitivity analysis (Supplementary Fig. 1) included 6,000 more patients with missing sex and smoking status demographic information and confirmed all findings except for increased melanoma risk.
For our novel finding of an overall lower risk of developing PCa in patients exposed to TNF-I, there is a plausible mechanism. There are multiple studies evaluating inflammation and the PCa microenvironment that demonstrate a causal link between chronic inflammation and the development of PCa, ranging from malignant conversion of precursor lesions to invasion and metastasis [39–41]. This agrees with Hellgren et al., who found that there was an identical 50% reduction of relative risk of prostate cancer in Ankylosing Spondylitis patients exposed to TNF-I [42]. That paper, however, was only designed to look at cancers on a large scale, nationwide cohort level and was limited to about 9,000 Ankylosing Spondylitis patients. We have validated that same finding in a much more diverse and larger inflammatory disease cohort. The other literature citations that investigate cancer as an outcome perform a similarly superficial analysis, with many of them identifying a single disease entity, such as psoriasis, but not differentiating by drug exposure, grouping all malignancies together, or do not separate out the individual biologic drugs and instead group them all together as a single clinical entity [31, 33–47]. This provides a possible link between TNF mediated inflammation and PCa development with a profound protective effect of TNF-I administration. This both warrants further prospective investigation into TNF-I therapeutics and their potential ability to alter PCa disease course and further investigation to upstream and downstream signaling in the TNF cascade to identify an intervenable molecular target. This also has implications for PCa screening in patients exposed to TNF-I that should be further studied, including the possibility that their screening could be de-intensified.
For our finding of decreased risk of NHL and leukemia, this is a surprising finding and contradicts much of the previous literature [48, 49]. There have been multiple case reports of patients on TNF-I such as adalimumab developing acute myelogenic leukemia, leading some to draw conclusions that the hematopoietic malignancy risk is elevated in patients on TNF-I [50–52]. These reports have to led to TNF-I receiving an FDA black box warning for increased NHL risk [53]. As such, we would have expected to see a signal of increased risk of these malignancies, if there were any signal at all. However, our study found the opposite. Of note, Calip et al. was one of the largest studies to find an association between TNF-I and NHL but was based on a commercial insurance database as opposed to directly querying patient charts [49]. There is basic science literature looking at TNF and TNF-receptor expression in leukemias that support our finding. Yamashita and Passegué found that inflammation-induced TNF signaling prevents hematopoietic stem cell necroptosis and initiates emergency myelopoiesis through NF-κB-dependent mechanisms, hence promoting hematopoietic stem cell survival and persistent hematopoietic regeneration that can lead to malignant hematopoiesis [54]. Similarly, basic science studies of chronic lymphocytic lymphomas (CLL) found aberrantly high TNF-receptor expression in the most actively proliferating lymph nodes as well as TNF-receptor stimulation leading to increased viability of these CLL cells [55]. Given these basic science findings, there is a plausible microbiologic mechanism for blockade of TNF or its receptor to downregulate pro-oncogenic signals in the bone marrow that may otherwise lead to NHL or leukemia.
Our findings of increased risk of melanoma, BCC, and SCC agree with the established literature. Melanoma is an exquisitely immune-sensitive malignancy [56]. and, as such, the mainstay of advanced melanoma treatment is immunotherapy with agents such as pembrolizumab and atezolizumab [57]. Even for rarer melanomas, such as uveal melanoma which occur in immune-privileged spaces, there is a possible pro-oncogenic effect of TNF-I [13]. and there seems to be a role for immunotherapy as treatment [14]. This further adds to the strong evidence in the literature that TNF-I can lead to skin cancer development via the inhibition of immune surveillance and suggests that patients on these medications should be screened much more intensively with Total Body Skin Exams for the development of these cutaneous malignancies.
Our finding of increased risk of malignancy in Ps/PsA is also novel in the TNF-I exposed patient cohort. Previously in the literature, Ps/PsA has been associated with a wide range of malignancies, both cutaneous (NMSC) and non-cutaneous (lymphoma, lung, bladder, colorectal) [58]. The hypothesis is generally that the chronic low-level inflammation seen in Ps/PsA in combination with its treatments accounts for this elevated cancer risk but the ability to draw a causal link is confounded by the fact that the main risk factors for developing Ps/PsA, such as smoking, alcohol consumption, and obesity, are all also risk factors for developing cancer [59, 60]. In our specific population of interest, Jung et al. found that TNF-I exposure in Ps/PsA patients led to an overall increased risk of malignancy and increased risk of NHL whereas patients exposed to other non-TNF-I biologic therapies did not demonstrate the same increased risk [61]. Our study evaluated patient charts and calculated long-term follow up after TNF-I exposure, thereby improving on the evidence from Jung et al., which was based on a national insurance database and did not quantify follow-up after TNF-I exposure. This emphasizes the concern of increased malignancy risk in Ps/PsA and warrants further investigation into the need for intensified screening in this population.
There are some limitations of the present study based on its retrospective design. First, this retrospective analysis can provide associations but is not equipped to provide causal relationships between exposures and outcomes. This is partially mitigated by our study being the largest study to date in the literature, aggregating data from over 50,000 patients, and the length of our median follow-up. Second, there is the possibility of confounding by disease severity or screening intensity in which TNF-I were prescribed preferentially to individuals with worse disease and higher cancer risk or that they undergo more contact with the medical system. However, in our cohort, the number of medical encounters was not different, nor was there a consistent trend amongst screen-detected cancers. There was also some missing sex and smoking history data, which was excluded in the main analysis to select for the highest quality data. We then included those patients with missing data in a sensitivity analysis that did not substantively change the findings. Finally, date of exposure was defined only as it was entered in the Northwestern system which can be inaccurate and does not consistently measure medication compliance.
Conclusions
There is a higher risk of melanoma, skin SCC and BCC along with lower risk of leukemia, NHL, and PCa in patients exposed to TNF-I. There may need to be differential cancer screening algorithms for patients with TNF-I exposure and more aggressive screening for Ps/PsA patients with TNF-I exposure given these findings.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- TNF
Tumor necrosis factor-alpha
- TNF-I
Tumor necrosis factor-alpha inhibitors
- CD
Crohn’s Disease
- UC
Ulcerative Colitis
- Ps/PsA
Psoriasis/Psoriatic Arthritis
- RA
Rheumatoid Arthritis
- AS/SpA
Ankylosing Spondylitis/Spondylarthritis
- HS
Hidradenitis Suppurativa
- UV
Uveitis
- EMR
Electronic medical record
- IQR
Interquartile range
- NMSC
Non-melanoma skin cancer
- RR
Relative Risk
- HR
Hazard Ratio
- SCC
Squamous cell carcinoma
- BCC
Basal cell carcinoma
- NHL
Non-Hodgkin lymphoma
- PCa
Prostate cancer
- CLL
Chronic lymphocytic lymphoma
Authors’ contributions
(CRediT author statement): CD: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing – Original Draft, Writing – Review & Editing; JMR: Investigation, Data Curation, Writing – Review & Editing; CY: Investigation, Data Curation; JN: Investigation, Data Curation; DI: Validation, Investigation, Data Curation, Writing – Review & Editing; PS: Methodology, Investigation; XM: Methodology, Validation, Formal analysis, Visualization;, Formal analysis, Visualization; SK: Methodology, Validation, Formal analysis, Visualization; HZ: Methodology, Validation, Formal analysis, Writing – Review & Editing; SB: Methodology, Investigation; WT: Methodology, Validation; EMS: Conceptualization, Supervision, Project Administration, Funding Acquisition; SK: Conceptualization, Methodology, Validation, Resources, Supervision, Project Administration, Funding Acquisition. All authors read and approved the final manuscript.
Funding
The Polsky Urologic Institute, Northwestern University.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was granted approval by Northwestern University Institutional Review Board (IRB# STU00211830).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shah SC, Itzkowitz SH. Colorectal Cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. 2022;162(3):715–e7303. 10.1053/j.gastro.2021.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morand S, Staats H, Creeden JF, Iqbal A, Kahaleh B, Stanbery L, Dworkin L, Nemunaitis J. Molecular mechanisms underlying rheumatoid arthritis and cancer development and treatment. Future Oncol. 2020;16(9):483–95. 10.2217/fon-2019-0722. [DOI] [PubMed] [Google Scholar]
- 3.Vaengebjerg S, Skov L, Egeberg A, Loft ND. Prevalence, incidence, and risk of Cancer in patients with psoriasis and psoriatic arthritis: A systematic review and Meta-analysis. JAMA Dermatol. 2020;156(4):421–9. 10.1001/jamadermatol.2020.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50(3):184–95. 10.1002/1097-0029(20000801)50:3%3C;184::AID-JEMT2%3E;3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr). 2020;43(1):1–18. Epub 2020 Jan 3. PMID: 31900901. 10.1007/s13402-019-00489-1. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25(3):409–16. PMID: 16951987. 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 9.Qi D, Lu M, Xu P, Yao X, Chen Y, Gan L, Li Y, Cui Y, Tong X, Liu S, Zhao J, Liu N, Ye X. Transcription factor ETV4 promotes the development of hepatocellular carcinoma by driving hepatic TNF-α signaling. Cancer Commun (Lond). 2023;43(12):1354–72. Epub 2023 Sep 5. PMID: 37670477; PMCID: PMC10693303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozooni Z, Ghadyani R, Soleimani S, Ahangar ER, Sheikhpour M, Haghighi M, Motallebi M, Movafagh A, Aghaei-Zarch SM. TNF-α, and TNFRs in Gastrointestinal cancers. Pathol Res Pract. 2024;263:155665. 10.1016/j.prp.2024.155665. [DOI] [PubMed] [Google Scholar]
- 11.DePry JL, Reed KB, Cook-Norris RH, Brewer JD. Latrogenic immunosuppression and cutaneous malignancy. Clin Dermatol. 2011;29(6):602–13. 10.1016/j.clindermatol.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Kearney CJ, Vervoort SJ, Hogg SJ, Ramsbottom KM, Freeman AJ, Lalaoui N, Pijpers L, Michie J, Brown KK, Knight DA, Sutton V, Beavis PA, Voskoboinik I, Darcy PK, Silke J, Trapani JA, Johnstone RW, Oliaro J. Tumor immune evasion arises through loss of TNF sensitivity. Sci Immunol. 2018;3(23):eaar3451.PMID: 29776993. 10.1126/sciimmunol.aar3451. [DOI] [PubMed] [Google Scholar]
- 13.Damento G, Kavoussi SC, Materin MA, Salomão DR, Quiram PA, Balasubramaniam S, Pulido JS. Clinical and histologic findings in patients with uveal melanomas after taking tumor necrosis factor-α inhibitors. Mayo Clin Proc. 2014;89(11):1481-6. doi: 10.1016/j.mayocp.2014.08.012. Epub 2014 Nov 3. Erratum in: Mayo Clin Proc. 2014 Dec;89(12):1739. Balasubramaniam, Soranya [Corrected to Balasubramaniam, Saranya]. PMID: 25444484. 10.1016/j.mayocp. [DOI] [PubMed] [Google Scholar]
- 14.Rossi E, Schinzari G, Zizzari IG, Maiorano BA, Pagliara MM, Sammarco MG, Fiorentino V, Petrone G, Cassano A, Rindi G, Bria E, Blasi MA, Nuti M, Tortora G. Immunological backbone of uveal melanoma: is there a rationale for immunotherapy? Cancers (Basel). 2019;11(8):1055. PMID: 31357439; PMCID: PMC6721347. 10.3390/cancers11081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer LK, Galloway JB, Lunt M, Davies R, Low AL, Dixon WG, Watson KD, BSRBR Control Centre Consortium, Symmons DP, Hyrich KL. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British society for rheumatology biologics register for rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):497–503. 10.1136/annrheumdis-2016-209389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frew JW, Jiang CS, Singh N, Grand D, Navrazhina K, Vaughan R, Krueger JG. Malignancy and infection risk during adalimumab therapy in hidradenitis suppurativa. Clin Exp Dermatol. 2020;45(7):859–65. 10.1111/ced.14264. [DOI] [PubMed] [Google Scholar]
- 17.Williams CJM, Peyrin-Biroulet L, Ford AC. Systematic review with meta‐analysis: malignancies with anti‐tumour necrosis factor‐α therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:447–58. 10.1111/apt.12624. [DOI] [PubMed] [Google Scholar]
- 18.Zhdanava M, Kachroo S, Manceur AM, Ding Z, Holiday C, Zhao R, Godwin B, Pilon D. Persistence among patients with Crohn disease previously treated with an Anti-tumor necrosis factor inhibitor and switching or cycling to another biologic agent. Clin Ther. 2023;45(8):770–7. 10.1016/j.clinthera.2023.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Starren JB, Winter AQ, Lloyd-Jones DM. Enabling a learning health system through a unified enterprise data warehouse: the experience of the Northwestern university clinical and translational sciences (NUCATS) Institute. Clin Transl Sci. 2015;8:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie D, Yang W, Jepson C, Roy J, Hsu JY, Shou H, Anderson AH, Landis JR, Feldman HI. Chronic renal insufficiency cohort (CRIC) study investigators. Statistical methods for modeling Time-Updated exposures in cohort studies of chronic kidney disease. Clin J Am Soc Nephrol. 2017;12(11):1892–9. 10.2215/CJN.00650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical report. Berlin:Springer Verlag;1966. [Google Scholar]
- 22.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 23.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual percent change in trend analysis. Stat Med. 2009;28:3670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon DH, Mercer E, Kavanaugh A. Observational studies on the risk of cancer associated with tumor necrosis factor inhibitors in rheumatoid arthritis: a review of their methodologies and results. Arthritis Rheum. 2012;64(1):21–32. 10.1002/art.30653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):121. 10.21037/atm.2018.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyboe Andersen N, Pasternak B, Basit S, Andersson M, Svanström H, Caspersen S, Munkholm P, Hviid A, Jess T. Association between tumor necrosis factor-α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. 2014;311(23):2406–13. 10.1001/jama.2014.5613. [DOI] [PubMed] [Google Scholar]
- 27.Askling J, Baecklund E, Granath F, Geborek P, Fored M, Backlin C, Bertilsson L, Cöster L, Jacobsson LT, Lindblad S, Lysholm J, Rantapää-Dahlqvist S, Saxne T, van Vollenhoven R, Klareskog L, Feltelius N. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish biologics register. Ann Rheum Dis. 2009;68(5):648–53. 10.1136/ard.2007.085852. [DOI] [PubMed] [Google Scholar]
- 28.D’Arcy ME, Beachler DC, Pfeiffer RM, Curtis JR, Mariette X, Seror R, Mahale P, Rivera DR, Yanik EL, Engels EA. Tumor necrosis factor inhibitors and the risk of Cancer among older Americans with rheumatoid arthritis. Cancer Epidemiol Biomarkers Prev. 2021;30(11):2059–67. 10.1158/1055-9965.EPI-21-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott FI, Mamtani R, Brensinger CM, Haynes K, Chiesa-Fuxench ZC, Zhang J, Chen L, Xie F, Yun H, Osterman MT, Beukelman T, Margolis DJ, Curtis JR, Lewis JD. Risk of nonmelanoma skin Cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin Cancer. JAMA Dermatol. 2016;152(2):164–72. 10.1001/jamadermatol.2015.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amari W, Zeringue AL, McDonald JR, Caplan L, Eisen SA, Ranganathan P. Risk of non-melanoma skin cancer in a National cohort of veterans with rheumatoid arthritis. Rheumatology (Oxford). 2011;50(8):1431–9. 10.1093/rheumatology/ker113. [DOI] [PubMed] [Google Scholar]
- 31.Peleva E, Exton LS, Kelley K, Kleyn CE, Mason KJ, Smith CH. Risk of cancer in patients with psoriasis on biological therapies: a systematic review. Br J Dermatol. 2018;178(1):103–13. 10.1111/bjd.15830. [DOI] [PubMed] [Google Scholar]
- 32.Wang JL, Yin WJ, Zhou LY, Zhou G, Liu K, Hu C, Zuo XC, Wang YF. Risk of non-melanoma skin cancer for rheumatoid arthritis patients receiving TNF antagonist: a systematic review and meta-analysis. Clin Rheumatol. 2020;39(3):769–78. 10.1007/s10067-019-04865-y. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886–95. [DOI] [PubMed] [Google Scholar]
- 34.Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, Walsh C, Lawson R, Reynolds A, Emery P. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70(11):1895–904. 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 35.Wetzman A, Lukas C, Gaujoux-Viala C, Mamtani R, Barnetche T, Combe B, Morel J, Szafors P. Risk of Cancer after initiation of targeted therapies in patients with rheumatoid arthritis and a prior cancer: systematic review with Meta-Analysis. Arthritis Care Res (Hoboken). 2023;75(2):260–71. 10.1002/acr.24784. [DOI] [PubMed] [Google Scholar]
- 36.Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, Etanercept, and Infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20(2):119–30. 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 37.Moulis G, Sommet A, Béné J, Montastruc F, Sailler L, Montastruc JL, Lapeyre-Mestre M. Cancer risk of anti-TNF-α at recommended doses in adult rheumatoid arthritis: a meta-analysis with intention to treat and per protocol analyses. PLoS ONE. 2012;7(11):e48991. 10.1371/journal.pone.0048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64(6):1035–50. 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tewari AK, Stockert JA, Yadav SS, Yadav KK, Khan I. Inflammation and prostate Cancer. Adv Exp Med Biol. 2018;1095:41–65. 10.1007/978-3-319-95693-0_3. [DOI] [PubMed] [Google Scholar]
- 40.Porter CM, Shrestha E, Peiffer LB, Sfanos KS. The Microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018;21(3):345–54. 10.1038/s41391-018-0041-1. [DOI] [PubMed] [Google Scholar]
- 41.Göbel A, Dell’Endice S, Jaschke N, Pählig S, Shahid A, Hofbauer LC, Rachner TD. The role of inflammation in breast and prostate Cancer metastasis to bone. Int J Mol Sci. 2021;22(10):5078. 10.3390/ijms22105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellgren K, Dreyer L, Arkema EV, Glintborg B, Jacobsson LT, Kristensen LE, Feltelius N, Hetland ML, Askling J, ARTIS Study Group, For the DANBIO Study Group. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis. 2017;76(1):105–11. 10.1136/annrheumdis-2016-209270. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto K, Ikeya K, Kato M, Matsuura A, Watanabe F, Takano R, Tamura S, Tani S, Osawa S, Hanai H. Assessment of Long-Term efficacy and safety of adalimumab in patients with ulcerative colitis: results from a 6-Year Real-World clinical practice. Dig Dis. 2019;37:11–20. 10.1159/000493121. [DOI] [PubMed] [Google Scholar]
- 44.Sartini A, Scaioli E, Liverani E, Bellanova M, Ricciardiello L, Bazzoli F, Belluzzi A. Retention rate, persistence and safety of adalimumab in inflammatory bowel disease: A Real-Life, 9-Year, Single-Center experience in Italy. Dig Dis Sci. 2019;64(3):863–74. 10.1007/s10620-018-5329-4. [DOI] [PubMed] [Google Scholar]
- 45.Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, Gelfand JM. The risk of Cancer in patients with psoriasis: A Population-Based cohort study in the health improvement network. JAMA Dermatol. 2016;152(3):282–90. 10.1001/jamadermatol.2015.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517–24. 10.1136/annrheumdis-2011-201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, Danese S. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: A systematic review and network Meta-analysis. Clin Gastroenterol Hepatol. 2016;14(10):1385–e139710. 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 48.Deepak P, Sifuentes H, Sherid M, Stobaugh D, Sadozai Y, Ehrenpreis ED. T-cell non-Hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-α) inhibitors: results of the REFURBISH study. Am J Gastroenterol. 2013;108(1):99–105. 10.1038/ajg.2012.334. [DOI] [PubMed] [Google Scholar]
- 49.Calip GS, Patel PR, Adimadhyam S, Xing S, Wu Z, Sweiss K, Schumock GT, Lee TA, Chiu BC. Tumor necrosis factor-alpha inhibitors and risk of non-Hodgkin lymphoma in a cohort of adults with rheumatologic conditions. Int J Cancer. 2018;143(5):1062–71. 10.1002/ijc.31407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saba NS, Kosseifi SG, Charaf EA, Hammad AN. Adalimumab-induced acute myelogenic leukemia. South Med J. 2008;101(12):1261–2. 10.1097/SMJ.0b013e318188950a. [DOI] [PubMed] [Google Scholar]
- 51.Bittencourt AL, Oliveira PD, Bittencourt VG, Carvalho EM, Farre L. Adult T-cell leukemia/lymphoma triggered by adalimumab. J Clin Virol. 2013;58(2):494–6. 10.1016/j.jcv.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Devarkonda V, Balmuri S, Akabane MACC, Akabane H. Adalimumab-associated Philadelphia chromosome positive acute lymphoblastic leukaemia in a patient with Crohn’s disease. BMJ Case Rep. 2023;16(10):e255604. 10.1136/bcr-2023-255604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.U.S. Food Drug Administration. Follow-up to the June 4, 2008 early communication about the ongoing safety review of tumor necrosis factor (TNF) blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi) 2009;2017.
- 54.Yamashita M, Passegué E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell. 2019;25(3):357–e3727. 10.1016/j.stem.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dürr C, Hanna BS, Schulz A, Lucas F, Zucknick M, Benner A, Clear A, Ohl S, Öztürk S, Zenz T, Stilgenbauer S, Li-Weber M, Krammer PH, Gribben JG, Lichter P, Seiffert M. Tumor necrosis factor receptor signaling is a driver of chronic lymphocytic leukemia that can be therapeutically targeted by the flavonoid Wogonin. Haematologica. 2018;103(4):688–97. 10.3324/haematol.2017.177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marzagalli M, Ebelt ND, Manuel ER. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol. 2019;59:236–50. 10.1016/j.semcancer.2019.08.002. Epub 2019 Aug 9. PMID: 31404607. [DOI] [PubMed] [Google Scholar]
- 57.Ralli M, Botticelli A, Visconti IC, Angeletti D, Fiore M, Marchetti P, Lambiase A, de Vincentiis M, Greco A. Immunotherapy in the treatment of metastatic melanoma: current knowledge and future directions. J Immunol Res. 2020;2020:9235638. 10.1155/2020/9235638. PMID: 32671117; PMCID: PMC7338969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loft ND, Vaengebjerg S, Skov L. Cancer risk in patients with psoriasis: should we be paying more attention? Expert Rev Clin Immunol. 2020;16(5):479–92. Epub 2020 Apr 24. PMID: 32279582. [DOI] [PubMed] [Google Scholar]
- 59.Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232(6):633–9. 10.1159/000455840. Epub 2017 Feb 23. PMID. [DOI] [PubMed] [Google Scholar]
- 60.Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol. 2014;170(2):304–14. PMID: 24117435. 10.1111/bjd.12670. [DOI] [PubMed] [Google Scholar]
- 61.Jung JM, Kim YJ, Chang SE, Lee MW, Won CH, Lee WJ. Cancer risks in patients with psoriasis administered biologics therapy: a nationwide population-based study. J Cancer Res Clin Oncol. 2023;149(19):17093–102. 10.1007/s00432-023-05387-6. Epub 2023 Sep 27. PMID: 37755577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.