Abstract
Glucosinolate hydrolysis in can yield health-promoting isothiocyanates but often results in less bioactive nitriles and epithionitriles. Here, the impact of temperature, light intensity, and photoperiod on glucosinolate metabolism was investigated in red cabbage at two developmental stages: sprouts and mature plants. Controlled simulations of summer and autumn cabbage cultivation revealed that high light and warm temperatures (16-h photoperiod, 23.4 mol m–2 d–1, 25 °C) favored ITC formation, while cold, short-day conditions (8h photoperiod, 7.0 mol m–2 d–1, max 15 °C) enhanced nitriles and epithionitriles. The hydrolysis outcome was associated with the differential expression of specifier and modifier proteins (BoESP1–3, BoESM1). Investigation of individual growing conditions indicated that light is the driving factor regulating specifier proteins, thereby shifting the glucosinolate hydrolysis outcome. Proteome and transcript analyses confirmed the functional link between environmental shifts and glucosinolate metabolic responses. These findings highlight the potential to improve the nutritional quality of crops through targeted cultivation strategies.
Keywords: isothiocyanates, epithiospecifier proteins, epithiospecifier modifier protein, Brassica oleracea, sprouts

Introduction
Vegetables of the group such as broccoli, cabbages, or cauliflower are rich in glucosinolates (GLS). These sulfur-containing plant secondary metabolites accumulate in plant vacuoles, especially in those of specialized S-cells, and are stored either at the intracellular or intercellular level, separately from myrosinases (β-d-thioglucosidases). GLS are valued for their health-promoting effects, which are mainly attributed to isothiocyanates (ITC), electrophilic volatile compounds that are enzymatically released upon plant tissue damage when GLS hydrolysis is initiated. Myrosinase cleaves the glucose moiety, and an unstable intermediate (aglucon) is formed. That aglucon can spontaneously degrade to form an ITC, or at pH values lower than 4, nitrile (cyanideCN) formation can also be favored. ITC are responsible for the pungent taste and flavor of these vegetables and exhibit antimicrobial, anticarcinogenic, and anti-inflammatory effects in humans. , Especially 4-(methylsulfinyl)butyl ITC (sulforaphane) from broccoli or red cabbage is valued for its various health-promoting properties. However, in many vegetables ITC are often not the main GLS hydrolysis products; rather epithionitriles (ETN) and CN are released. − To date, more than 130 distinct GLS have been identified, enabling the formation of a wide and diverse array of specific metabolites as a result of GLS hydrolysis. The formation of CN and ETN is attributed to the presence of additional specifier proteins that can influence the degradation of the unstable aglucon. The epithiospecifier protein (ESP) catalyzes the conversion of aglucons with a terminal double bond to form ETN, while aglucons with a saturated terminal side chain are converted to CN. Therefore, high ESP abundance is considered responsible for the high ETN and CN formation observed for vegetables. , In , apart from ESP, nitrile specifier proteins (NSP) are also involved in GLS hydrolysis and enhance nitrile formation, while the epithiospecifier modifier (ESM) has been reported to enhance ITC release in the presence of ESP. ,
As ESP abundance reduces the potential to profit from ITC-mediated beneficial effects, it is important to find strategies to reduce its abundance or activity in vegetables to enhance ITC formation. While targeted food processing such as mild heating (to 60 °C) or acid addition during salad production, resulting in pH decrease from 6.1 to 4.5, are effective measures to increase ITC levels in foods, , growing vegetables with an increased ITC formation rate is more favorable.
So far, the role of ETN formation as well as ESP in the planta is not well understood. While nitrile formation is involved in indirect plant defense, as it reduces attractiveness for ovipositing insect females and can attract natural enemies of insect larvae, the role of ETN is still unclear. Also, the regulation of ESP by environmental factors has not been well studied. ESP has been reported as a negative regulator of WRKY53-induced leaf senescence. While salicylic acid negatively affects ESP expression, jasmonate has been shown to induce ESP expression, and ESP overexpression in reduced susceptibility toward bacterial and fungal infections. In four ESP isoforms (BoESP1–4) have been reported, of which three are functionally confirmed. , Miao et al. showed that BoESP expression is inducible by abscisic acid and gibberellin, which reduce ITC and induce ETN formation. Moreover, analysis of cis-acting elements in the promotor region also showed that the promotor region of all four BoESP also contained many light-responsive elements.
Preharvest growth temperatures were found to significantly influence ITC formation in rocket salad species, with elevated day/night temperatures of 40/30 °C leading to increased ITC levels. Similarly, Bhat et al. demonstrated that in , increasing growth temperature resulted in higher allyl ITC levels, while the levels of allyl GLS remained unchanged. Additionally, light quality was shown to impact GLS hydrolysis in pak choi ( ssp. chinensis), with reduced UVB exposure promoting CN formation. However, these studies did not investigate the regulatory mechanisms underlying the shifts in GLS hydrolysis outcomes. Recently, our group showed that the harvest date has a strong effect on the ESP abundance and ITC formation in red cabbage. Up to 40-fold higher ITC levels were found in summer red cabbage compared to that harvested in autumn, when CN and ETN increased due to ESP induction, suggesting that abiotic growth factors such as light and temperature regulate ESP activity and GLS hydrolysis outcomes. Therefore, here, we investigated the effect of growth temperature and light regime, individually and combined, on GLS hydrolysis and BoESP abundances as well as BoESP transcript expression in controlled simulations of summer and autumn red cabbage cultivation using mature red cabbage as well as sprouts. Untargeted proteome analysis was conducted to characterize the regulatory mechanisms of GLS hydrolysis. A deeper understanding of the regulatory mechanisms of the formation of health-promoting compounds in cabbage will support efforts to enhance the nutritional benefits of these vegetables for human health.
Materials and Methods
Chemicals
Acetic acid (≥99.5%), allyl ITC (Allyl-ITC; ≥99%), ammonium bicarbonate (≥99.5%), aryl sulfatase, benzonitrile (≥99.9%), 3-butenenitrile (Allyl-CN; ≥98%), DEAE-Sephadex A-25, and (-)-sinigrin hydrate (allyl GLS; Allyl) (≥99%) were obtained from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany). 1,4-Dithiothreitol (≥99%), 4-hydroxybenzyl GLS (≥99%), imidazole (≥99%), and methylene chloride (GC Ultra grade) were purchased from Carl Roth GmbH + Co. KG (Karlsruhe, Germany). DL-Goitrin (OZT; ≥98%) 3-(methylsulfinyl)propyl ITC (3MSOP-ITC; ≥98%), and 4-(methylsulfanyl)butyl-ITC (4MTB-ITC; ≥98%) were purchased from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany). 3-Butenyl GLS (3But; ≥99%), 2-hydroxy-3-butenyl GLS (2OH3But; ≥80%), indol-3-ylmethyl GLS (I3M; ≥99%), 3-(methylsulfinyl)propyl GLS (3MSOP; ≥99%), and 4-(methylsulfinyl)butyl GLS (4MSOB; ≥97%) were obtained from PhytoLab GmbH & Co. KG (Vestenbergsgreuth, Germany). 3-Butenyl ITC (3But-ITC; ≥95%) was purchased from TCI Deutschland GmbH (Eschborn, Germany). 1-Cyano-2,3-epithiopropane (CETP; ≥95%) was synthesized by Sirius Fine Chemicals SiChem GmbH (Bremen, Germany), and 1-cyano-3,4-epithiobutane (CETB) was produced by ASCA GmbH (Berlin, Germany). 4-(Methylsulfinyl)butyl ITC (4MSOB-ITC; ≥97%) was obtained from LKT Laboratories, Inc., MN, USA. Na2SO4 anhydrous (≥99%) was obtained from VWR International GmbH (Darmstadt, Germany). 4-(Methylsulfinyl)butanenitrile (3MSOP-CN), 4-(methylsulfanyl)butanenitrile, 5-(methylsulfinyl)pentanenitrile (4MSOB-CN), and 5-(methylsulfanyl)pentanenitrile (all ≥95% purity) were purchased from Enamine (Riga, Latvia). Acetonitrile (LC-MS grade), methanol (≥99.95%), and trifluoroacetic acid (TFA, LC-MS grade, >99.5%) were purchased from Th. Geyer GmbH & Co. KG (Renningen, Germany). RapiGest SF was obtained from Waters GmbH (Eschborn, Germany). Coomassie protein assay kit was purchased from Thermo Fisher Scientific Inc. (Waltham, USA). Trypsin (MS grade) was obtained from Promega GmbH (Walldorf, Germany). Water used in this study was of ultrapure grade.
Plant Material
All experiments were conducted at the Leibniz Institute of Vegetable and Ornamental Crops (IGZ) located in Brandenburg, Germany (52°20′56.1″N 13°18′37.2″E). Seeds of red cabbage ( L. var. capitata f. rubra cv. “Integro”) were provided by Bejo Samen GmbH (Sonsbeck, Germany). All harvests were performed in the morning to exclude circadian variation in GLS levels and hydrolysis.
Experiment 1: Simulation of Summer and Autumn Red Cabbage Cultivation
In the first experiment, the effect of growth temperature and light regimes on GLS hydrolysis in mature red cabbage was investigated. Therefore, simulations of summer and autumn red cabbage cultivation were carried out in walk-in phytochambers (ENGIE Deutschland GmbH, Köln, Germany) equipped with mercury-to-metal halide conversion lamps (Clean Ace (Daylight) Mogul Base6500K CCT/90CRI; EYE Lighting International, Mentor, OH, USA). The actual photosynthetic photon flux density (PPFD) was regularly controlled by a LI-250 light meter equipped with a quantum sensor LI-190R (LI-COR GmbH Germany, Bad Homburg, Germany). In experiment 1A, the effects of growth temperature, photoperiod, and light intensity in combination were studied. First, seeds of red cabbage were sown in pots (0.66 L, diameter 12 cm) filled with soil (Pikiererde, Einheitserde Werkverband e.V., Sinntal-Altengronau, Germany), germinated, and grown under moderate conditions in a walk-in phytochamber. The temperature ranged between 15 and 20 °C, and the photoperiod was set to 12 h with a maximum light intensity of 490 μmol m–2 s–1 and a daily light integral (DLI) of 12.4 mol m–2 d–1. Plants were watered with a nutrient solution (EC = 2.0 mS cm–1, pH = 6) during the whole growing phase. After 14 days, the plants were divided into two walk-in phytochambers. In the first chamber, the growing conditions were set up to simulate summer red cabbage cultivation, starting with conditions similar to a Central European Spring, when plants are transferred to the field: temperatures ranging from 7.5 to 12.5 °C, a photoperiod of 12 h with a maximum light intensity of 450 μmol m–2 s–1 and a DLI of 10.5 mol m–2 d–1. After 14 days, the growing conditions were adjusted to early summer field conditions: temperature ranging between 12.5 and 20 °C, 14-h-long photoperiods with a maximum light intensity of 650 μmol m–2 s–1 and a DLI of 18.4 mol m–2 d–1. After another 14 days, summer field conditions were set up for 14 days: temperature increased from 17.5 to 25 °C with 16-h-long photoperiods with a maximum light intensity of 750 μmol m–2 s–1 and a DLI of 23.4 mol m–2 d–1. In the second chamber, simulation of autumn red cabbage cultivation was conducted, starting with early summer field conditions: temperature ranging from 17.5 to 25 °C with 16-h-long photoperiods with maximum light intensity of 650 μmol m–2 s–1 and a DLI of 20.5 mol m–2 d–1. After 14 days, the growth temperature decreased to 15 from 20 °C, photoperiods to 12 h with a maximum light intensity of 550 μmol m–2 s–1 and a DLI of 12.7 mol m–2 d–1. Another 14 days later, autumn field conditions were set up in the walk-in phytochamber with temperatures ranging from 7.5 to 15 °C, 8-h-short photoperiods with a maximum light intensity of 450 μmol m–2 s–1 and a DLI of 7.0 mol m–2 d–1. This experimental setup was based on common red cabbage cultivation practices in Central Europe, incorporating multiple planting dates and reflecting the typical harvest season, which can be from July to December. Additionally, 5 weeks after sowing, plantlets were transplanted into bigger pots (10 L, diameter 29 cm) filled with soil (Topferde, Einheitserde Werkverband e.V., Sinntal-Altengronau, Germany). After 6 weeks of simulations, one-third of the plants in each chamber were harvested, one-third was transferred to the other walk-in phytochamber (swap), and one-third remained at the same growing conditions. Fourteen days later, both the remaining and the swapped plants were harvested.
Furthermore, the effect of growth temperature and light regimes on GLS hydrolysis was also investigated individually, and the experiments were conducted in a similar way as described in the experiment 1A. Briefly, seeds were sown in soil, germinated, and grown at moderate growing conditions for 14 days in a walk-in phytochamber. Then, the growth temperature was set at 17.5–25 °C, photoperiod to 16 h with a maximum light intensity of 550 μmol m–2 s–1 and a DLI of 17.1 mol m–2 d–1. After 6 weeks of cultivation, one-third of the plants was harvested, one-third remained at the same growing conditions, and one-third was transferred to another walk-in phytochamber. In this chamber, for experiment 1B, growth temperature decreased to 7.5–15 °C (autumn temperature), and the light regime remained at 16-h photoperiod with a maximum light intensity of 550 μmol m–2 s–1 and a DLI of 17.1 mol m–2 d–1. In experiment 1C, growth temperature remained at 17.5–25 °C, but the photoperiod decreased to 8 h with a maximum light intensity of 400 μmol m–2 s–1 and a DLI of 6.1 mol m–2 d–1 (autumn light). After 2 weeks, all plants were harvested. An overview of the temperature and light regimes used in the experiment 1 is provided in Figure A.
1.
Experimental setup conditions for simulations of summer and autumn red cabbage cultivation in experiments 1 and 2.
During the harvest, red cabbage heads were cut off, weighed, and halved, and the core stem was removed. One half of the head was immediately shock frozen in liquid nitrogen, freeze-dried, ground to a fine powder (particle size 0.2 mm), and stored at −20 °C until further analyses. The other half was freshly chopped into small pieces of approximately 1 cm in length and mixed well. Then, 10 g of the chopped plant material was filled into glass vials for homogenization, and water was added (1:1, plant material:water). Samples were homogenized using a homogenizer (HO 4, Edmund Bühler GmbH, Bodelhausen, Germany) at 35 000 rpm for 1.5 min and incubated at room temperature for 30 min to achieve enzymatic GLS hydrolysis.
Experiment 2: Effect of Growth Temperature and Photoperiod on GLS Hydrolysis Outcome in Red Cabbage Sprouts
In experiment 2, the effect of growth temperature and photoperiod on GLS hydrolysis was investigated in red cabbage sprouts cultivated under controlled conditions in climate cabinets (Fitotron HGC High Specification Growth Chambers, Weiss Technik UK Limited, Loughborough, United Kingdom) equipped with metal-halide lamps (HQI-BT 400 W/D PRO, OSRAM GmbH, München, Germany). Therefore, two growing conditions, summer and autumn, were simulated (Figure B). During the summer simulation, the temperature was set to 25/15 °C (day/night), the photoperiod was set to 16 h with a light intensity of 300 μmol m–2 s–1 and a DLI of 17.3 mol m–2 d–1, and the air humidity was set to 70%. In the autumn simulation, temperature was set to 15/5 °C (day/night), photoperiod was set to 8 h with a light intensity of 300 μmol m–2 s–1 and a DLI of 8.6 mol m–2 d–1, and the air humidity was set to 70%. Seeds were sown on cellulose sheets in aluminum tray (200 mL) filled with perlite (Perligran Classic, Knauf Performance Materials GmbH, Dortmund, Germany). Seeds and sprouts were watered as required, and no fertilizers were used during the whole growing phase. In the summer simulation, sprouts were sampled after 7 days (day 0) and one-half of the trays was transferred to the autumn simulation. Due to the much slower seed germination and sprout growth in the autumn simulation, the first sampling was carried out 14 days after sowing. The other half of the trays remained in the original simulation as controls. Sprouts were sampled 1, 3, and 7 days after the swap. During the harvest, 20 mL vials were filled with sprouts. The vials were immediately shock frozen in liquid nitrogen and freeze-dried. Afterwards, metal balls were added to the vial, and the samples were homogenized for 1.5 min using an oscillating mill (MM400, Retsch GmbH, Haan, Germany) and stored at −20 °C until further analyses.
Glucosinolate Analysis by HPLC-DAD-ToF-MS
Desulfo-GLS were extracted from freeze-dried and ground plant material following the previously reported protocol. Analysis of desulfo-GLS was performed by HPLC-DAD-ToF-MS (1290 Infitiny II LC System with 6230 TOF LC/MS, Agilent Technologies Deutschland GmbH, Waldbronn, Germany), and identification and quantification of desulfo-GLS were carried out as previously reported.
Analysis of Glucosinolate Hydrolysis Products Using GC-MS
In experiment 1, GLS hydrolysis products were extracted from fresh plant material. In experiment 2, GLS hydrolysis products were extracted from 30 mg of freeze-dried plant material after the addition of 270 μL of water and 1 h of incubation at room temperature. For the extraction of GLS hydrolysis products, the protocol described by Wermter et al. was followed. The extracts were analyzed using GC-MS (7890A GC with 5975C Inert XL MSD, Agilent Technologies Deutschland GmbH, Waldbronn, Germany) with an HP-5MS Ultra Inert 30 m column (30 m × 0.25 mm × 0.25 μm) (Agilent Technologies Deutschland GmbH, Waldbronn, Germany). Compound analysis and quantification were performed as outlined in Hanschen using Agilent MassHunter Quantitative Analysis software (Version 10.2). Data from Agilent MSD ChemStation were converted to MassHunter format using MassHunter GC/MS Translator B.07.05.2479.
Protein Extraction and Label-Free Protein Quantification
Proteins from red cabbage heads from experiment 1 were extracted from freeze-dried plant material following the protocol of Friedrich et al. After protein reduction and digestion, the peptides were desalted and analyzed by nanoflow liquid chromatography on a Dionex UltiMate 3000 system (Thermo Scientific) coupled to a Q Exactive Plus mass spectrometer (Thermo Scientific), following the method of Witzel et al., with modifications described by Friedrich et al. The raw proteome data were processed by Proteome Discoverer 2.4 and Sequest HT engine (Thermo Scientific), searching the protein database and the NCBI Annotation Release 100.
cDNA Synthesis and qPCR Analysis
RNA was extracted from 100 mg of freeze-dried red cabbage sprouts from experiment 2 using an RNeasy Plant Mini Kit (QIAGEN, Hilden, Germany), following the manufacturer’s protocol. cDNA synthesis was performed using 2 μg of total RNA with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories GmbH, Munich, Germany) according to the manufacturer’s instructions. BoESP transcript abundance was quantified by qPCR using primer pairs and following the qPCR protocol reported by Witzel et al. BoSANDI and BoTUB6 were chosen as the reference genes. Primer sequences, their annealing temperatures, and the length of the resulting amplicon for BoESP transcript abundances and reference genes are provided in Table S1. Reactions were run on a CFX96 real-time system driven by CFX Manager software 2.1. Each 6 μL reaction comprised 3 μL of SsoAdvanced SYBR Green1314 Supermix (Bio-Rad Laboratories, Hercules, USA), 1 μL of cDNA, and 1 μL of each primer (2 mM), and three technical replicates were included per biological sample. Primer specificity was assessed by inspection of the melting curve. The Cq values of individual well traces were determined using the regression model implemented in the CFX Manager software. Data were analyzed using qbasePLUS software v2.3 (Biogazelle NV, Ghent, Belgium) with normalization based on the two reference genes.
Data Analysis
Experiments 1 and 2 were carried out in three experimental replicates. At each harvest, two biological replicates were sampled and analyzed. Data are shown as means ± standard deviations. Analyses of GLS and their hydrolysis products were performed by using MassHunter version 10.2 (Agilent Technologies Deutschland GmbH, Waldbronn, Germany). To investigate the effect of growth temperature and light regimes on GLS hydrolysis outcomes, data sets were tested for normality (Shapiro-Wilk test) and outliers (z-score). Subsequently, means were compared using one-way ANOVA followed by Tukey’s post hoc test using TIBCO Statistica 13 (StatSoft Europe GmbH, Hamburg, Germany). A significance level of p ≤ 0.05 was considered statistically significant. Principal component analysis (PCA) including normalized abundances of all identified proteins was carried out in experiment 1A after centering the data and dividing by standard deviation. Furthermore, grouped abundances of all identified proteins were used for hierarchical clustering with the Euclidean distance function and complete linkage method. Both analyses of the proteome data set were carried out by Proteome Discoverer 2.4.
Results
Photoperiod and Light Intensity Affect GLS Biosynthesis and Hydrolysis in Mature Red Cabbage
The effect of growth temperature and light regime, individually and in combination, on GLS biosynthesis and hydrolysis was tested in simulations of summer and autumn red cabbages cultivated under controlled conditions.
In the first experiment, when temperature and light regimes were tested in combination, GLS amounts ranged from 2.2 to 2.5 μmol g–1 dry matter (DM) after 6 weeks of summer or autumn simulations, and levels did not change after 2 more weeks of autumn simulation (A 2) or after the shift of the autumn cabbage plants to the summer conditions (AS 2) (Figure A and Table S2). In the summer simulation, a strong enhancement in GLS abundance was observed between the first (S 1) and the second harvest (S 2), and GLS levels increased by 6-fold. Also, GLS levels were enhanced in plants shifted from summer to autumn cultivation (SA 2), as the levels of 2OH3But, Allyl, and 4MSOB were significantly increased compared to those at the first harvest time point (S 1) (Figure A). In the simulation where only the temperature regime changed, an enhancement in GLS accumulation was observed between the first (S 1) and the second harvest time points (S 2 and SA 2), but there was no difference between plants grown exclusively in the summer simulation (S 2) and plants shifted to the autumn temperature settings (SA 2) (Figure B). When the effect of light regime was tested individually, a significant increment in 3MSOP, 4MSOB, and 3But was found between the first (S 1) and second (S 2) harvest time point. Autumn light treatment significantly reduced GLS levels (Figure C)
2.
Effect of growth temperature and light regime, tested in combination (A, D, G) and individually (growth temperature: B, E, H; light regime: C, F, I), on glucosinolate (GLS) levels and hydrolysis in mature red cabbage. Amounts of single GLS [μmol g–1 dry matter (DM)] depending on the temperature and light regime tested in combination (A) and individually (B, C). Amounts of GLS hydrolysis products grouped into isothiocyanates (ITC), nitriles (CN; cyanides), and epithionitriles (ETN) [μmol g–1 DM] depending on the temperature and light regime tested in combination (D) and individually (E, F). Proportion of ITC, CN, and ETN among total GLS hydrolysis products [%] depending on the temperature and light regime tested in combination (G) and individually (H, I). S 1, A 1 = plants harvested after 6 weeks of summer (S) or autumn (A) simulation. S 2, A 2 = plants harvested after 8 weeks of S and A simulation. S-A 2 = plants grown in the S simulation for 6 weeks and then transferred to the A simulation for 2 weeks. A-S 2 = plants grown in the A simulation for 6 weeks and then transferred to the S simulation for 2 weeks. 3-(Methylsulfinyl)propyl GLS (3MSOP), 2-hydroxy-3-butenyl GLS (2OH3But), allyl GLS (Allyl), 4-(methylsulfinyl)butyl GLS (4MSOB), 3-butenyl GLS (3But), 3-(methylsulfanyl)propyl GLS (3MTP), indol-3-ylmethyl-GLS (I3M). Values show means and standard deviations of three experimental replicates (n = 3). Different small letters indicate significant differences in the amounts of single GLS and their hydrolysis products, while different capital letters indicate significant differences in the total GLS amounts (D, E, F) (ANOVA, Tukey HSD test, p ≤ 0.05).
Subsequently, the outcome of GLS hydrolysis was evaluated using GC-MS. The amounts of single GLS hydrolysis products are displayed in Figure S1 and Tables S2–S4 and reflect the GLS levels. Total amounts (Figure D–F) as well as relative formation (Figure G–I) of ITC, CN, and ETN were calculated. In general, GLS hydrolysis resulted in all experiments and at all harvest time points predominantly in the formation of CN and ETN, representing on average 82 ± 4% of all GLS hydrolysis products.
In the combined temperature–light simulation, elevated ITC levels were formed in the summer simulation at the second harvest time point (S 2) compared to the first harvest (S 1), as well as compared to autumn conditions (A 1, A 2; AS 2) (Figure D). The swap from summer to autumn settings decreased absolute ITC formation by 4.6-fold (Figure D) while relative formation declined from 26% to 11% (SA 2). Correspondingly, the relative level of CN formation was increased (Figure G). Absolute GLS hydrolysis outcome did not change between the first (A 1) and second harvest (A 2) of autumn plants, and plants shifted to the summer simulation (AS 2) (Figure D). However, the shift of autumn red cabbage to the summer growing conditions (AS 2) increased relative ITC formation by 50% (compared to A 2), reaching ITC formation rate of summer red cabbage (S 1 & S 2) (Figure G). In the simulation where only the temperature regime changed, total ITC, CN, and ETN levels increased between the first (S 1) and the second harvest (S 2 & SA 2) (Figure E). Relative CN formation increased between the first (S 1) and second harvest (S 2 & SA 2), while ETN formation decreased (Figure H). Autumn temperature regime (S 2 & SA 2) had no effect on absolute (Figure E) and relative ITC, ETN, and CN formation (Figure H). When the effect of light regime was tested individually, the amounts of ITC, CN, and ETN increased from the first harvest (S 1) to the second harvest (S 2), when compared to the first harvest (Figure F). After swap to autumn light treatment (SA 2), absolute ITC as well as CN levels decreased (Figure F), and relative ETN formation was slightly but significantly enhanced by 1.36-fold (Figure I).
Abundance of Two ESP Isoforms Correlates with GLS Hydrolysis Formation
NanoLC-MS/MS analysis was performed to gain insight into the molecular background of GLS formation and hydrolysis. A total of 3257 proteins were identified in the analysis. Principal component analysis (PCA) revealed a grouping of technical replicates and showed that the shift from summer to autumn conditions provoked only minor changes in the proteome, while the plant’s adaptation from autumn to summer conditions caused stronger alterations in the proteome (Figure ). PCA of all four treatments, displayed in Figure S3, revealed a high degree of variation in the proteome of summer and autumn red cabbage. Plants adapting to the shift, from summer to autumn or vice versa, show greater similarity based on the principal component. Furthermore, the dendrogram of a hierarchical clustering analysis (Figure S4) carried out with these data sets depicts a balanced tree in which the two experiments are clearly separated.
3.
Assessment of technical and biological variation in protein expression profiles of red cabbage adapting either from summer to autumn conditions (A) or from autumn to summer conditions (B) using principal component analysis. Normalized abundances of all identified proteins in the data set were used for the calculation. Each dot represents one nanoLC-MS/MS experiment.
In the adaptation from summer to autumn conditions, which is the natural developmental process for biennial red cabbage, 341 proteins were differentially abundant (Table S5). Proteins that increased in their abundance (n = 101 proteins) included several translation initiation factors (XP_013620479.1, XP_013590151.1, XP_013633847.1, XP_013585856.1, XP_013603128.1) and RNA-binding proteins (XP_013605607.1, XP_013631475.1, XP_013615245.1, XP_013600155.1, XP_013630288.1), reflecting the targeted transition from the vegetative to the dormant stage. Proteins with decreased abundance (n = 240 proteins) included ribosomal proteins (XP_013622637.1, XP_013618113.1, XP_013583730.1, XP_013637304.1, XP_013625244.1), proteins involved in photosynthesis (XP_013628336.1, XP_013585372.1, XP_013613241.1, XP_013593518.1, XP_013616079.1, XP_013600114.1, XP_013608420.1), and transport processes (XP_013636024.1, XP_013587671.1, XP_013591857.1, XP_013620383.1, XP_013612489.1, XP_013620698.1, XP_013630717.1, XP_013621417.1, XP_013607161.1, XP_013587651.1, XP_013630573.1, XP_013614074.1), indicating a general decline in physiological processes to prepare the plant for overwintering.
In the adaptation from autumn to summer conditions, 578 proteins were differentially abundant, and the identified proteins reflect an imposition of stress on the plant due to this climatic shift (Table S6). Increased in their abundance (n = 192) are proteins involved in cellular organization and the cell cycle (XP_013620468.1, XP_013598882.1, XP_013611140.1, XP_013587011.1, XP_013605530.1, Bo2g040080.1, Bo1g022580.1, Bo3g107240.1, XP_013597875.1, XP_013605018.1, XP_013598863.1, XP_013622303.1, XP_013625531.1, XP_013637710.1, XP_013611737.1, XP_013621011.1), heat shock proteins (XP_013625547.1, XP_013625891.1, XP_013638033.1, XP_013629357.1, XP_013621322.1), and other stress-related proteins (XP_013620214.1, XP_013630140.1, XP_013606747.1, XP_013629246.1, XP_013609020.1, XP_013599573.1, XP_013618190.1, XP_013633579.1, XP_013621339.1, XP_013633437.1, XP_013603994.1, XP_013601492.1). Additionally, BoESM1 (Bo5g131590.1) as well as BoESP2 (XP_013599019.1) were identified in this group. Proteins with decreased abundance (n = 386 proteins) included those involved in RNA metabolism or translation (Bo6g004630.1, XP_013618188.1, XP_013587164.1, XP_013597071.1, XP_013611149.1, XP_013586592.1, Bo5g106250.1, Bo1g128470.1, XP_013593395.1, XP_013636109.1, XP_013586459.1, Bo9g179000.1, Bo4g134520.1, XP_013629411.1, Bo5g109800.1, XP_013603087.1, Bo3g139990.1) and transport processes (XP_013622744.1, XP_013628815.1, XP_013600842.1, Bo3g149330.1, XP_013604798.1, XP_013611643.1, XP_013612790.1, XP_013605478.1, XP_013605526.1, XP_013631705.1, XP_013620571.1, XP_013629346.1, XP_013590379.1, XP_013611646.1), among others.
In 2019, Witzel et al. identified and characterized three BoESP isoforms in . The proteome analysis identified two of these isoforms, BoESP1 (XP_013587912.1) and BoESP2 (XP_013599019.1), as well as one ESM isoform candidate (BoESM1, Bo5g131590.1) (Figure ). BoESP1 isoform was more abundant than BoESP2 at all growing conditions and at any harvest time points. Significantly higher abundancies of both ESP isoforms in cabbages grown under summer conditions at the second harvest time point (S 2), or cabbages shifted to autumn cultivation (SA 2), were found when temperature and light were investigated in combination (Figure A). In the autumn simulation, both ESP isoforms were significantly most abundant in the second harvest (A 2). Their abundancies did not change between the first harvest (A 1) and the cabbages shifted to the summer simulation (AS 2). BoESM1 candidate abundance was not affected when temperature and light regimes were tested in combination.
4.
Effect of growth temperature and light regime, tested in combination (A) and individually (growth temperature: B; light regime: C) on the protein expression of epithiospecifier proteins BoESP1 (XP_013587912.1) and BoESP2 (XP_013599019.1), as well as putative epithiospecifier modifier protein BoESM1 (Bo5g131590.1) in mature red cabbage. S 1, A 1 = plants harvested after 6 weeks of summer (S) or autumn (A) simulation. S 2, A 2 = plants harvested after 8 weeks of S and A simulation. S-A 2 = plants grown in the S simulation for 6 weeks and then transferred to the A simulation for 2 weeks. A-S 2 = plants grown in the A simulation for 6 weeks and then transferred to the S simulation for 2 weeks. Values show means and standard deviations of three experimental replicates (n = 3). Different letters indicate significant differences in the protein expression (ANOVA, Tukey HSD test, p ≤ 0.05).
When the effect of temperature was investigated individually, BoESP1 showed the lowest abundance in cabbages transferred from summer to autumn cultivation (SA 2) and the abundances of BoESP2 as well as the BoESM1 candidate were not affected (Figure B). Significant enhancement of BoESP1 and BoESM1 candidate abundance could be observed in the individual investigation of the light regime at the second harvest time point (S 2) compared to the first one (S 1) (Figure C). Moreover, a shift of the summer cabbage plants into cultivation with autumn light conditions (SA 2) caused further increments in the abundance of BoESP1 as well as BoESP2 (Figure C).
Shift to Low Temperature and Short Photoperiods Enhances ETN Formation in Red Cabbage Sprouts
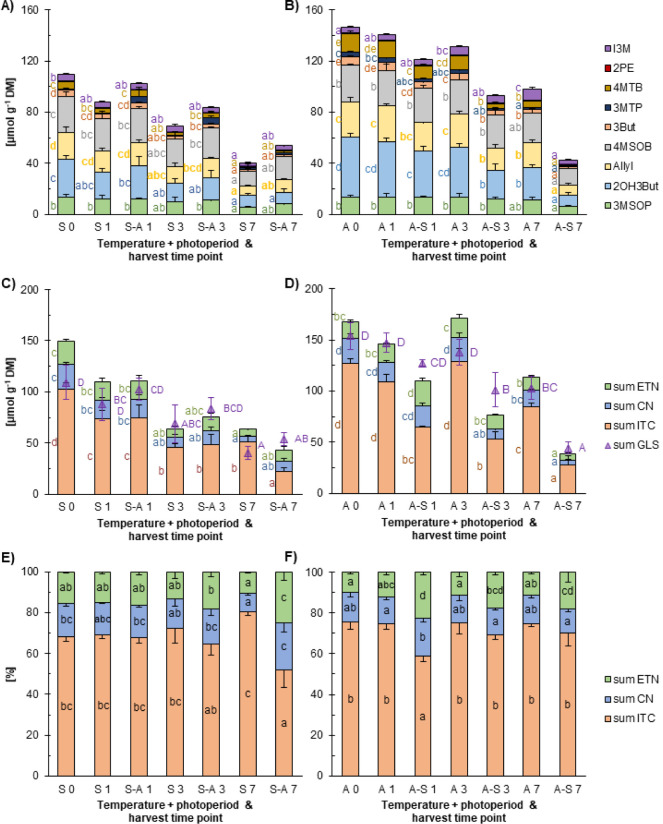
To investigate how fast GLS hydrolysis shifts in response to different growing conditions, the effects of temperature and light regimes on GLS biosynthesis and hydrolysis were tested in red cabbage sprouts under simulated summer and autumn conditions. Fully developed sprouts were transferred to the opposite growing conditions and sampled after 1, 3, and 7 days. In both simulations, the highest GLS levels were found at the sampling time point 0 (110 μmol g–1 DM in summer (S 0) or 147 μmol g–1 DM in autumn conditions (A 0) (Figure A,B). In sprouts that germinated and remained in the summer simulation (S 1–7) or were transferred to the autumn simulation (S-A 1–7), a continuous decrease in GLS levels down to 40 μmol g–1 DM (S 7) or 54 μmol g–1 DM (S-A 7) was observed. Similarly, in sprouts germinated in the autumn simulation and then transferred to the summer growing conditions (A-S 1–7), a strong and continuous decrease in GLS levels was shown. Sprouts that grew only in the autumn simulation (A 1–7) showed significantly lower GLS levels only at day 7 (A 7) when compared to the earlier harvests, and overall levels remained relatively high.
5.
Effect of growth temperature and photoperiod on glucosinolate (GLS) levels and hydrolysis in red cabbage sprouts. Amounts of single GLS [μmol g–1 dry matter (DM)] (A and B), amounts of GLS hydrolysis products grouped into isothiocyanates (ITC), nitriles (CN; cyanides), and epithionitriles (ETN) [μmol g–1 DM] (C and D), and proportion of ITC, CN, and ETN among total GLS hydrolysis products [%] (E and F) depending on the temperature and light regime. S 0, S 1, S 3, and S 7 = sprouts germinated and grown in summer growing conditions, and harvested at time points 0, 1, 3, and 7 days. A 0, A 1, A 3, A 7 = sprouts germinated and grown in autumn growing conditions, harvested at time points 0, 1, 3, and 7 days. S-A 1, S-A 3, and S-A 7 = sprouts germinated in summer growing conditions, transferred to autumn conditions, and harvested at time points 1, 3, and 7 days after the transfer. A-S 1, A-S 3, A-S 7 = sprouts germinated in autumn growing conditions, transferred to summer conditions, and harvested at time points 1, 3, and 7 days after the transfer. 3-(Methylsulfinyl)propyl GLS (3MSOP), 2-hydroxy-3-butenyl GLS (2OH3But), allyl GLS (Allyl), 4-(methylsulfinyl)butyl GLS (4MSOB), 3-butenyl GLS (3But), 3-(methylsulfanyl)propyl GLS (3MTP), 4-(methylsulfanyl)butyl GLS (4MTB), indol-3-ylmethyl-GLS (I3M). Values show means and standard deviations of three experimental replicates (n = 3). Different small letters indicate significant differences in the amounts of single GLS and their hydrolysis products, while different capital letters indicate significant differences in the total GLS amounts (C, D) (ANOVA, Tukey HSD test, p ≤ 0.05).
Analysis of GLS hydrolysis revealed that ITC were the most prominent GLS hydrolysis products in red cabbage sprouts regardless of the treatment and at all sampling time points reaching 128 μmol g–1 DM (Figure C,D). A continuous and significant decrease in the levels of all GLS hydrolysis products across the experiment and later sampling time points reflected the continuous decrease in GLS amounts (Figure C,D). Sprouts grown exclusively in autumn conditions showed a significant decline in the ITC, CN, and ETN levels only at the last harvest time point (Figure D). Relative formation of ITC was significantly lower, while CN and ETN formation was significantly higher in sprouts 7 days after the shift from summer to autumn conditions (S-A 7) compared to sprouts grown for the same time exclusively in summer conditions (S 7) (Figure E). No differences were found in the relative formation of GLS hydrolysis products between the different sampling time points in sprouts grown exclusively in the autumn conditions (A 0–7) (Figure F). Notably, relative ETN formation was significantly higher in the sprouts shifted from autumn to summer growing conditions at all sampling time points (A-S 1–7).
As the profiling of protein abundance was successful for only two main isoforms, the transcript abundance of four known ESP isoforms was analyzed via RT-qPCR. BoESP1 (LOC106296341) abundance was significantly induced in sprouts transferred from summer to autumn simulation as early as 1 day after the shift, and transcript levels peaked at 7 days after the shift (S-A 7) (Figure ). BoESP2 (LOC106306810) transcript abundance was induced only 1 day after the shift from summer to autumn growing conditions (S-A 1).
6.
Expression of BoESP1–4 transcripts in red cabbage sprouts germinated and grown in summer (S) growing conditions, harvested at time points 0, 1, 3, and 7 days after the transfer (S 0, S 1, S 3, S 7), as well as sprouts germinated in S growing conditions, transferred to A conditions, and harvested at time points 1, 3, and 7 days after the transfer (S-A 1, S-A 3, S-A 7). Values show means and standard deviations of three experimental replicates (n = 3). Different letters indicate significant differences in the transcript expression (ANOVA, Tukey HSD test, p ≤ 0.05).
Overall, GLS levels in red cabbage sprouts decreased throughout the experiment and under different treatments. A shift from the simulation with warm growth temperatures and long photoperiods to a cold environment and short days caused a significant shift in GLS hydrolysis outcomes, leading to less ITC and more pronounced CN and ETN formation after 7 days. Likewise, transcripts of BoESP1 and BoESP2 were induced as early as 1 day after the shift.
Discussion
GLS-derived ITC found in vegetables are known for their numerous health benefits, including antimicrobial, antioxidant, anti-inflammatory, chemoprotective, and neuroprotective properties. − However, GLS hydrolysis in mature varieties often results in the formation of less bioactive CN and ETN, and recent studies provide evidence that not only genetic factors but also abiotic growth factors affect GLS biosynthesis as well as the outcome of GLS hydrolysis. ,,, Previously, we showed that the time point of harvesting during the growth season has a strong effect on GLS hydrolysis in different varieties, suggesting that growth temperature and light regimes regulate ESP induction which, in turn, modifies GLS hydrolysis. Therefore, here the individual and combined effects of temperature, light intensity, and photoperiod on GLS metabolism were investigated in mature red cabbage as well as in sprouts, as the developmental stage also affects plant secondary metabolite profiles and levels.
The observed GLS profiles of mature red cabbage, predominantly aliphatic GLS, such as 4MSOB, 3MSOP, and 2OH3But, were in line with previous studies. ,, Higher total GLS amounts were found in cabbages exposed to the summer temperature and light regime at the second harvest time point. This indicates a strong ontogenetic effect between 8- and 10-week-old plants, which was also observed by Heinze et al. in pak choi (, ssp. chinensis). Higher GLS levels were also found in the summer red cabbage compared to the later harvests in two consecutive years of a field experiment. By investigating the effect of temperature and light regimes individually, we found that light regime was the major factor influencing GLS levels, as only low light and short days but not lower temperature reduced GLS levels. Similarly, the induction of aliphatic GLS under high light growing conditions was recently described by Ishihara et al. in purple-pigmented kale genotypes, but sinigrin induction was also observed in a green kale genotype in the past. This may be due to the fact that GLS biosynthesis depends on sulfate assimilation, which is regulated by light. Huseby et al. demonstrated that light regulation of GLS biosynthesis genes, as well as many genes of primary sulfate assimilation, is controlled by the HY5 transcription factor. Also, different responses of the transcription factors MYB28 and MYB29 that are involved in aliphatic GLS biosynthesis in , under different light environments contributed to the induction of GLS biosynthesis under a high light regime. Liu et al. also observed an increase in sinigrin with increasing photoperiod in kale sprouts. However, in our study, higher total GLS amounts were found in sprouts when germinated and grown under cold temperatures with short days at all harvest time points, indicating that in sprouts, the response to photoperiod may be genotype-specific.
With regard to GLS hydrolysis outcome, the simulation of summer and autumn red cabbage cultivation under controlled conditions partially reflected the seasonal effects observed in red cabbage grown in the field. Relatively more ITC were formed upon GLS hydrolysis in mature plants grown under warm temperatures and long photoperiods with high light intensities. Conversely, short photoperiods, low light conditions, and low growth temperatures favored ETN formation, as also observed in our recent field study. However, shifts in the GLS hydrolysis outcome were less pronounced under controlled conditions, possibly due to lower light intensity compared to the natural field conditions. Nevertheless, reduced light intensity and shorter photoperiods still led to increased ETN formation, suggesting that the light regime may be the driving factor for seasonal changes in GLS hydrolysis outcome.
While the reduction in light alone induced both BoESP1 and BoESP2, temperature reduction reduced BoESP1, and the combined simulation (summer to autumn) showed no effect, although the hydrolysis was shifted toward ETN formation. However, as GLS levels declined during the autumn simulation, it can be expected that the same level of ESP will result in higher ETN and CN formation, as under such conditions, likely lower aglucon levels would be available to be either converted to ETN or CN, which would be favored with the same ESP level, instead of being spontaneously degraded to ITC. Correspondingly, in summer cabbages harvested at the second harvest time point, BoESP abundances were enhanced to correspond with the strong induction of GLS levels between the first and the second harvest time point. In contrast, the induction of BoESP1 and BoESP2 in the autumn growing conditions at the second harvest time point likely resulted from the interaction of ontogeny and growth conditions. The BoESM1 candidate, which likely redirects the formation of CN and ETN to ITC, was induced under summer light conditions at the second harvest time point. This corresponds with the observed increase in GLS levels between the first and second harvest, although it did not lead to an induced ITC formation. It should also be noted that the activity of specifier proteins is influenced by pH and the presence of Fe2+ ions and may not always directly correlate with their abundance. , Higher BoESM abundance was also observed in summer red cabbage in our previous field study. Furthermore, Mbudu et al. found higher formation of ITC in tissues with relatively higher BoESM1 abundance along with a higher ratio of myrosinase to ESP activity. Increased myrosinase activity likely leads to greater production of unstable GLS aglucon, thereby raising the probability of spontaneous breakdown into ITC, assuming ESP abundance remains unchanged. ,
The proteome profiles also confirmed the effectiveness of the applied treatments as they induced the anticipated physiological responses. On the one hand is the decrease in physiological processes due to the adaptation of summer plants to autumnal conditions, and on the other hand is stress-related regulation in autumn plants transferred to summer conditions.
The results of the red cabbage sprout experiment confirmed that the shift from simulated summer (25/15 °C day/night, 16 h photoperiod) to autumn (15/5 °C day/night, 8-h photoperiod) growing conditions significantly impacts GLS hydrolysis. While overall GLS levels declined over time in all treatments, likely reflecting natural developmental changes observed in previous studies, sprouts exposed to lower temperatures and shorter photoperiods showed a notable shift in GLS hydrolysis outcomes, with decreased ITC and enhanced CN and ETN formation. This change was most pronounced 7 days after the shift, suggesting a time-dependent physiological adaptation. The observed increase in ETN and CN formation corresponded with the induced expression of BoESP1, BoESP2, and BoESP3 transcripts shortly after the environmental shift, indicating a functional link between ESP expression and the observed changes in GLS hydrolysis. Particularly, BoESP1 transcript abundance peaked 7 days after the shift, matching the strong decline in ITC formation and the enhanced production of CN and ETN (Figures A and ). This aligns with observations showing that BoESP transcription responds strongly to abscisic acid or gibberellin, which have been implicated in controlling many processes during plant development as well as stress responses, resulting in increased ETN levels under these treatments. These findings support the hypothesis that environmental factors not only affect GLS biosynthesis but also modulate the hydrolysis pathway by regulating specific ESP isoforms. Notably, sprouts continuously grown in autumn conditions maintained relatively high ITC formation over the experiment, indicating that the autumn environment alone does not necessarily reduce ITC formation in sprouts, and that the regulation of GLS hydrolysis changes during plant development.
The findings support the hypothesis, as derived from a previous field study that temperature, light intensity, and photoperiod significantly influence GLS metabolism during the harvest season in red cabbage. Simulated field cultivation of red cabbage in phytochambers under controlled light and temperature conditions and a subsequent analysis of GLS hydrolysis products, specifier proteins, and their transcript expression revealed that intense light and warm conditions promoted ITC formation, while autumn-like conditions enhanced CN and ETN through regulation of specifier proteins. Possibly, the enhanced ITC formation during the warmer, longer summer days, when pest and pathogen pressure is also higher, serves as an efficient defense mechanism, as ITC act as natural deterrents. Furthermore, as allyl-ITC was shown to induce stomatal closure in leading to suppression of water loss, , enhancement of ITC formation during summer may be a strategy to manage expected drought stress during warm days. Conversely, lower ITC formation in autumn may allow cabbage plants to be less apparent to specialist herbivores that often use ITC as recognition cues.
Taken together, our findings deepen our understanding of how abiotic growth factors influence health-relevant phytochemical profiles in vegetables and highlight the potential to optimize cultivation strategies for improved nutritional quality.
Supplementary Material
Acknowledgments
Bejo Samen GmbH is gratefully thanked for providing the seeds. Maria Skoruppa, Jessica Eichhorn, Andrea Maikath, Elke Büsch, and Gundula Aust are thanked for their excellent technical assistance.
Glossary
Abbreviations
- GLS
glucosinolate
- ITC
isothiocyanate
- CN
nitrile (cyanide)
- ETN
epithionitrile
- ESP
epithiospecifier protein
- ESM
epithiospecifier modifier protein
- DM
dry matter
- A
autumn
- S
summer
- 3MSOP
3-(methylsulfinyl)propyl GLS
- 2OH3But
2-hydroxy-3-butenyl GLS
- Allyl
allyl GLS
- 4MSOB
4-(methylsulfinyl)butyl GLS
- 3But
3-butenyl GLS
- 3MTP
3-(methylsulfanyl)propyl GLS
- 4MTB
4-(methylsulfanyl)butyl GLS
- 2PE
2-phenylethyl GLS
- I3M
indol-3-ylmethyl GLS
- 3But-ITC
3-butenyl ITC
- 3MTP-ITC
3-(methylsulfanyl)propyl ITC
- 4MTB-ITC
4-(methylsulfanyl)butyl ITC
- 2PE-ITC
2-phenylethyl ITC
- OZT
5-vinyl-1,3-oxazolidine-2-thione
- 3MSOP-ITC
3-(methylsulfinyl)propyl ITC
- 4MSOB-ITC
4-(methylsulfinyl)butyl ITC
- Allyl-CN
3-butenenitrile
- 3But-CN
4-pentenenitrile
- 2OH3But-CN
3-hydroxypentenenitrile
- 3MTP-CN
4-(methylsulfanyl)butanenitrile
- 4MTB-CN
5-(methylsulfanyl)pentanenitrile
- 3MSOP-CN
4-(methylsulfinyl)butanenitrile
- 4MSOB-CN
5-(methylsulfinyl)pentanenitrile
- IAN
indole-3-acetonitrile
- CETP
1-cyano-2,3-epithiopropane
- CETB
1-cyano-3,4-epithiobutane
- CHETB
1-cyano-2-hydroxy-3,4-epithiobutane
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.5c06284.
Table S1: qPCR primer sequences, their annealing temperatures, and the length of the resulting amplicon for BoESP and reference genes’ transcript abundance. Table S2: Amounts of glucosinolates and their hydrolysis products depending on temperature and light regime in mature red cabbage. Table S3: Amounts of glucosinolates and their hydrolysis products depending on growth temperature in mature red cabbage. Table S4: Amounts of glucosinolates and their hydrolysis products depending on light regime in mature red cabbage. Table S5: List of proteins with significantly differential expression patterns in response to the shift from summer to autumn conditions. Table S6: List of proteins with significantly differential expression patterns in response to the shift from autumn to summer conditions. Table S7: Amounts of glucosinolates and their hydrolysis products depending on temperature and light regime during cultivation of red cabbage sprouts (XLSX)
Figure S1: Amounts of single glucosinolate hydrolysis products depending on temperature and light regime in mature red cabbage. Figure S2: Amounts of single glucosinolate hydrolysis products depending on temperature and light regime in red cabbage sprouts. Figure S3: Assessment of technical and biological variation in protein expression profiles in red cabbage harvested after 8 weeks of summer and autumn simulation or red cabbage adapting to the shift either from summer to autumn conditions or from autumn to summer conditions using principal component analysis. Normalized abundances of all identified proteins in the data set were used for the calculation. Each dot represents one nanoLC-MS/MS experiment. Figure S4: Hierarchical clustering analysis of the grouped abundances of all identified proteins, represented by colors from white to dark blue as shown in the color key, in red cabbage harvested after 8 weeks of summer and autumn simulation or red cabbage adapting to the shift either from summer to autumn conditions or from autumn to summer conditions (PDF)
This work is part of the project OPTIGLUP, which was financially supported by the Leibniz Association (Leibniz-Junior Research Group: J16/2017). F.S.H is financially supported by the Leibniz Association (Leibniz Programme for Women Professors: P126/2021).
The authors declare no competing financial interest.
References
- Blažević I., Montaut S., Burcul F., Olsen C. E., Burow M., Rollin P., Agerbirk N.. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry. 2020;169:112100. doi: 10.1016/j.phytochem.2019.112100. [DOI] [PubMed] [Google Scholar]
- Koroleva O. A., Gibson T. M., Cramer R., Stain C.. Glucosinolate-accumulating S-cells in Arabidopsis leaves and flower stalks undergo programmed cell death at early stages of differentiation. Plant J. 2010;64(3):456–469. doi: 10.1111/j.1365-313X.2010.04339.x. [DOI] [PubMed] [Google Scholar]
- Uda Y., Kurata T., Arakawa N.. Effects of pH and ferrous ion on the degradation of glucosinolates by myrosinase. Agric. Biol. Chem. 1986;50(11):2735–2740. doi: 10.1080/00021369.1986.10867832. [DOI] [Google Scholar]
- Bell L., Oloyede O. O., Lignou S., Wagstaff C., Methven L.. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018;62(18):e1700990. doi: 10.1002/mnfr.201700990. [DOI] [PubMed] [Google Scholar]
- Palliyaguru D. L., Yuan J. M., Kensler T. W., Fahey J. W.. Isothiocyanates: translating the power of plants to people. Mol. Nutr. Food Res. 2018;62(18):e1700965. doi: 10.1002/mnfr.201700965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. Y., Zhang J. K., Zheng L., Chen Y.. The functional role of sulforaphane in intestinal inflammation: a review. Food Funct. 2022;13(2):514–529. doi: 10.1039/D1FO03398K. [DOI] [PubMed] [Google Scholar]
- Yagishita Y., Fahey J. W., Dinkova-Kostova A. T., Kensler T. W.. Broccoli or sulforaphane: is it the source or dose that matters? Molecules. 2019;24(19):3593. doi: 10.3390/molecules24193593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Púčiková V., Rohn S., Hanschen F. S.. Glucosinolate accumulation and hydrolysis in leafy vegetables are influenced by leaf age. J. Agric. Food Chem. 2023;71(30):11466–11475. doi: 10.1021/acs.jafc.3c01997. [DOI] [PubMed] [Google Scholar]
- Hanschen F. S., Schreiner M.. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017;8:1095. doi: 10.3389/fpls.2017.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopsch R., Witzel K., Artemyeva A., Ruppel S., Hanschen F. S.. Genotypic variation of glucosinolates and their breakdown products in leaves of Brassica rapa . J. Agric. Food Chem. 2018;66(22):5481–5490. doi: 10.1021/acs.jafc.8b01038. [DOI] [PubMed] [Google Scholar]
- Matusheski N. V., Swarup R., Juvik J. A., Mithen R., Bennett M., Jeffery E. H.. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 2006;54(6):2069–2076. doi: 10.1021/jf0525277. [DOI] [PubMed] [Google Scholar]
- Hanschen F. S., Kühn C., Nickel M., Rohn S., Dekker M.. Leaching and degradation kinetics of glucosinolates during boiling of Brassica oleracea vegetables and the formation of their breakdown products. Food Chem. 2018;263:240–250. doi: 10.1016/j.foodchem.2018.04.069. [DOI] [PubMed] [Google Scholar]
- Wittstock U., Kurzbach E., Herfurth A. M., Stauber E. J.. Glucosinolate breakdown. Adv. Bot. Res. 2016;80:125–169. doi: 10.1016/bs.abr.2016.06.006. [DOI] [Google Scholar]
- Wittstock U., Meier K., Dörr F., Ravindran B. M.. NSP-dependent simple nitrile formation dominates upon breakdown of major aliphatic glucosinolates in roots, seeds, and seedlings of Arabidopsis thaliana Columbia-0. Front. Plant Sci. 2016;7:1821. doi: 10.3389/fpls.2016.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusheski N. V., Jeffery E. H.. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem. 2001;49(12):5743–5749. doi: 10.1021/jf010809a. [DOI] [PubMed] [Google Scholar]
- Hanschen F. S.. Acidification and tissue disruption affect glucosinolate and S-methyl-l-cysteine sulfoxide hydrolysis and formation of amines, isothiocyanates and other organosulfur compounds in red cabbage (Brassica oleracea var. capitata f. rubra) Food Res. Int. 2024;178:114004. doi: 10.1016/j.foodres.2024.114004. [DOI] [PubMed] [Google Scholar]
- Jeschke V., Gershenzon J., Vassao D. G.. Insect detoxification of glucosinolates and their hydrolysis products. Adv. Bot. Res. 2016;80:199–245. doi: 10.1016/bs.abr.2016.06.003. [DOI] [Google Scholar]
- Miao Y., Zentgraf U.. The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell. 2007;19(3):819–830. doi: 10.1105/tpc.106.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel K., Abu Risha M., Albers P., Börnke F., Hanschen F. S.. Identification and characterization of three epithiospecifier protein isoforms in Brassica oleracea . Front. Plant Sci. 2019;10:1552. doi: 10.3389/fpls.2019.01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Xia C., Yu S., Wang J., Zhao Y., Wang Q.. Enhancing health-promoting isothiocyanates in Chinese kale sprouts via manipulating BoESP. Hortic. Res. 2023;10(4):uhad029. doi: 10.1093/hr/uhad029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper J., Wagstaff C., Bell L.. Growth temperature influences postharvest glucosinolate concentrations and hydrolysis product formation in first and second cuts of rocket salad. Postharvest Biol. Biotechnol. 2020;163:111157. doi: 10.1016/j.postharvbio.2020.111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R., Faiz S., Ali V., Khajuria M., Mukherjee D., Vyas D.. Effect of temperature and insect herbivory on the regulation of glucosinolate-myrosinase system in Lepidium latifolium . Physiol. Plant. 2021;172(1):53–63. doi: 10.1111/ppl.13289. [DOI] [PubMed] [Google Scholar]
- Heinze M., Hanschen F. S., Wiesner-Reinhold M., Baldermann S., Grafe J., Schreiner M., Neugart S.. Effects of developmental stages and reduced UVB and low UV conditions on plant secondary metabolite profiles in pak choi (Brassica rapasubspchinensis) J. Agric. Food Chem. 2018;66(7):1678–1692. doi: 10.1021/acs.jafc.7b03996. [DOI] [PubMed] [Google Scholar]
- Púčiková V., Witzel K., Rohn S., Hanschen F. S.. Season-dependent variation in the contents of glucosinolates and S-methyl-l-cysteine sulfoxide and their hydrolysis in Brassica oleracea . Food Chem. 2025;465(Pt 2):142100. doi: 10.1016/j.foodchem.2024.142100. [DOI] [PubMed] [Google Scholar]
- Renz M., Dekker M., Rohn S., Hanschen F. S.. Plant matrix concentration and redox status influence thermal glucosinolate stability and formation of nitriles in selected Brassica vegetable broths. Food Chem. 2023;404(Pt A):134594. doi: 10.1016/j.foodchem.2022.134594. [DOI] [PubMed] [Google Scholar]
- Wermter N. S., Rohn S., Hanschen F. S.. Seasonal variation of glucosinolate hydrolysis products in commercial white and red cabbages (Brassica Oleracea Varcapitata) . Foods. 2020;9(11):1682. doi: 10.3390/foods9111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschen F. S.. Domestic boiling and salad preparation habits affect glucosinolate degradation in red cabbage (Brassica oleracea var. capitata f. rubra) Food Chem. 2020;321:126694. doi: 10.1016/j.foodchem.2020.126694. [DOI] [PubMed] [Google Scholar]
- Friedrich K., Wermter N. S., Andernach L., Witzel K., Hanschen F. S.. Formation of volatile sulfur compounds and S-methyl-l-cysteine sulfoxide in Brassica oleracea vegetables. Food Chem. 2022;383:132544. doi: 10.1016/j.foodchem.2022.132544. [DOI] [PubMed] [Google Scholar]
- Palliyaguru D. L., Yang L., Chartoumpekis D. V., Wendell S. G., Fazzari M., Skoko J. J., Liao Y., Oesterreich S., Michalopoulos G. K., Kensler T. W.. Sulforaphane diminishes the formation of mammary tumors in rats exposed to 17beta-estradiol. Nutrients. 2020;12(8):2282. doi: 10.3390/nu12082282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm C., Wagner A. E.. Brassica-derived plant bioactives as modulators of chemopreventive and inflammatory signaling pathways. Int. J. Mol. Sci. 2017;18(9):1890. doi: 10.3390/ijms18091890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo L., Iori R., Rollin P., Bramanti P., Mazzon E.. Isothiocyanates: an overview of their antimicrobial activity against human infections. Molecules. 2018;23(3):624. doi: 10.3390/molecules23030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan M., Fahey J. W., Zimmerman A. W., Zhou X., Panjwani A. A.. The role of isothiocyanate-rich plants and supplements in neuropsychiatric disorders: a review and update. Front. Nutr. 2024;11:1448130. doi: 10.3389/fnut.2024.1448130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Alegre S., Pascual J., Trotta A., Yang W., Yang B., Seyednasrollah F., Burow M., Kangasjarvi S.. Growth conditions trigger genotype-specific metabolic responses that affect the nutritional quality of kale cultivars. J. Exp. Bot. 2025;76(5):1427–1445. doi: 10.1093/jxb/erae169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justen V. L., Fritz V. A., Cohen J. D.. Seasonal variation in glucosinolate accumulation in turnips grown under photoselective nettings. Hortic., Environ. Biotechnol. 2012;53(2):108–115. doi: 10.1007/s13580-012-0106-3. [DOI] [Google Scholar]
- Renz M., Andernach L., Kaufmann M., Rohn S., Hanschen F. S.. Degradation of glucosinolates and formation of isothiocyanates, nitriles, amines, and N,N’-dialk(en)yl thioureas during domestic boiling of red cabbage. Food Chem. 2024;435:137550. doi: 10.1016/j.foodchem.2023.137550. [DOI] [PubMed] [Google Scholar]
- Lefsrud M. G., Kopsell D. A., Sams C. E.. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. Hortscience. 2008;43(7):2243–2244. doi: 10.21273/HORTSCI.43.7.2243. [DOI] [Google Scholar]
- Huseby S., Koprivova A., Lee B. R., Saha S., Mithen R., Wold A. B., Bengtsson G. B., Kopriva S.. Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in. J. Exp. Bot. 2013;64(4):1039–1048. doi: 10.1093/jxb/ers378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Chen H. C., Cao B., Lei J. J., Chen G. J.. Molecular Characterization of MYB28 Involved in Aliphatic Glucosinolate Biosynthesis in Chinese Kale (Brassica oleracea var. alboglabra Bailey) Front. Plant Sci. 2017;8:1083. doi: 10.3389/fpls.2017.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neequaye M., Stavnstrup S., Harwood W., Lawrenson T., Hundleby P., Irwin J., Troncoso-Rey P., Saha S., Traka M. H., Mithen R., Ostergaard L.. CRISPR-Cas9-mediated gene editing of MYB28 genes impair glucoraphanin accumulation of Brassica oleracea in the field. CRISPR J. 2021;4(3):416–426. doi: 10.1089/crispr.2021.0007. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Chun J. H., Kim H. R., Kim S. J., Lim Y. P., Park S. U.. Variation of glucosinolate accumulation and gene expression of transcription factors at different stages of Chinese cabbage seedlings under light and dark conditions. Nat. Prod. Commun. 2014;9(4):533–537. doi: 10.1177/1934578X1400900428. [DOI] [PubMed] [Google Scholar]
- Liu K. Z., Gao M. F., Jiang H. Z., Ou S. Y., Li X. P., He R., Li Y. M., Liu H. C.. Light Intensity and photoperiod affect growth and nutritional quality of Brassica microgreens. Molecules. 2022;27(3):883. doi: 10.3390/molecules27030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchernig J. C., Burow M., Wittstock U.. Evolution of specifier proteins in glucosinolate-containing plants. BMC Evol. Biol. 2012;12:127. doi: 10.1186/1471-2148-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschen F. S., Klopsch R., Oliviero T., Schreiner M., Verkerk R., Dekker M.. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017;7:40807. doi: 10.1038/srep40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. J., Critchley C., Pun S., Chaliha M., O’Hare T. J.. Key role of Fe (2+) in epithiospecifier protein activity. J. Agric. Food Chem. 2010;58(15):8512–8521. doi: 10.1021/jf904532n. [DOI] [PubMed] [Google Scholar]
- Mbudu K. G., Witzel K., Bornke F., Hanschen F. S.. Glucosinolate profile and specifier protein activity determine the glucosinolate hydrolysis product formation in kohlrabi (Brassica oleracea var. gongylodes) in a tissue-specific way. Food Chem. 2025;465(Pt 2):142032. doi: 10.1016/j.foodchem.2024.142032. [DOI] [PubMed] [Google Scholar]
- Mocniak L. E., Elkin K., Bollinger J. M.. Lifetimes of the aglycone substrates of specifier proteins, the autonomous iron enzymes that dictate the products of the glucosinolate-myrosinase defense system in Brassica plants. Biochemistry. 2020;59(26):2432–2441. doi: 10.1021/acs.biochem.0c00358. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Sun J. Y., Hu Z. W., Cheng C. X., Lin S. E., Zou H. X., Yan X. F.. Variation in glucosinolate accumulation among different sprout and seedling stages of broccoli(Brassica oleracea varitalica) Plants. 2022;11(12):1563. doi: 10.3390/plants11121563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K., Zhou W. G., Chen F., Luo X. F., Yang W. Y.. Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front. Plant Sci. 2018;9:416. doi: 10.3389/fpls.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon M. A. R., Jahan M. S., Rahman T., Hossain M. A., Muroyama D., Minami I., Munemasa S., Mori I. C., Nakamura Y., Murata Y.. Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ. 2011;34(11):1900–1906. doi: 10.1111/j.1365-3040.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- Salehin M., Li B. H., Tang M., Katz E., Song L., Ecker J. R., Kliebenstein D. J., Estelle M.. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019;10(1):4021. doi: 10.1038/s41467-019-12002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrix V., Reichelt M., Mitchell-Olds T., Kliebenstein D. J., Gershenzon J.. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13(12):2793–2807. doi: 10.1105/tpc.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.