Abstract
Since emerging in China in 2010, Duck Tembusu Virus (DTMUV) has caused substantial economic losses to the poultry industry. Guangdong province, a major hub for waterfowl farming and consumption in China, has been severely impacted by DTMUV. Investigating the epidemiology and genetic diversity of DTMUV in Guangdong is essential for developing effective prevention and control strategies. In this study, we collected 427 goose liver samples from five geographically distinct regions in Guangdong province. DTMUV RNA was detected in 69 samples, with positive rates ranging from 12.9 % to 18.6 % in the regions. Eight complete genome sequences were subsequently amplified, and genetic analyses revealed that these strains clustered into three distinct subclades (2.1.1, 2.2, and 3.2), indicating significant genetic heterogeneity among DTMUV circulating in Guangdong’s goose populations. Furthermore, a statistically supported recombination event was identified in one strain, underscoring the role of genetic recombination in driving viral diversity. These findings enhance our understanding of DTMUV’s genetic diversity and evolutionary dynamics in goose populations, thereby providing critical insights into its transmission patterns and evolutionary trends in Guangdong province, China.
Keywords: Duck tembusu virus, Epidemiology, Genetic diversity, Recombination
Introduction
Duck Tembusu virus (DTMUV), a member of the Flaviviridae family and Flavivirus genus, is classified within the Ntaya virus group alongside other medically important flaviviruses including Japanese encephalitis virus (JEV), dengue virus (DENV), West Nile virus (WNV), and Zika virus (Zhang et al., 2017). As a single-stranded positive-sense RNA virus, DTMUV has a spherical structure with a diameter of 45–60 nm and an enveloped capsid (Yang, et al., 2023). Its genome, approximately 11 kb in length, encodes a single open reading frame (ORF) that produces a polyprotein of 3,425 amino acids and further cleavage into three structural proteins (C, prM, and E) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5) (Lei, et al., 2017). Among these, the envelope (E) protein serves critical functions in viral entry, membrane fusion, and immunogenicity, with its hypervariable nature making it a molecular marker for investigating genetic diversity and epidemiological patterns (Cui, et al., 2022). Phylogenetic analyses based on E protein sequences currently delineate DTMUV strains into three distinct clusters (clusters 1–3), reflecting substantial genetic heterogeneity and emphasizing the need for ongoing evolutionary surveillance (Yan, et al., 2022).
First isolated from Culex mosquitoes in Kuala Lumpur, Malaysia in 1955 (Platt, et al., 1975), DTMUV re-emerged in 2010 causing outbreaks characterized by egg production decline and growth retardation in Chinese duck farms (Su, et al., 2011). The virus primarily transmits via mosquito vectors (predominantly Culex spp.), though vertical transmission through eggs and direct parental-to-offspring routes have also been documented (Su, et al., 2011). Notably, a strain of DTMUV was isolated from sparrows near poultry farms, suggesting that wild birds may act as reservoir hosts or amplifying hosts for the virus (Tang, et al., 2013). The expanding host range of DTMUV, which now includes breeder ducks, layer hens, geese, pigeons, mosquitoes, and sparrows, underscores the need for comprehensive surveillance and control measures (Yu, et al., 2018).
Guangdong province is one of the most significant regions for waterfowl farming and consumption in China, with a large-scale poultry industry that contributes substantially to the local economy. However, DTMUV poses a severe threat to this industry, causing significant economic losses due to reduced egg production, stunted growth, and high mortality rates in infected flocks (Su, et al., 2011). Since the first outbreak in 2010, DTMUV has been reported in multiple provinces across China, with Guangdong being one of the most affected regions (Lei, et al., 2017). Therefore, understanding the genetic diversity and molecular epidemiology of DTMUV in Guangdong is essential for developing effective prevention and control strategies. In this study, we collected a total of 427 clinical samples from geese across five cities across Guangdong province to conduct a molecular epidemiological survey of DTMUV. To obtain the highest phylogenetic resolution and to enable recombination analyses, the complete genome sequences were obtained, and phylogenetic analyses were performed to investigate the genetic diversity and potential recombination events of DTMUV.
Materials and methods
Samples collection and RNA extraction
During 2023, 427 liver tissues samples were aseptically at necropsy from 60- to 180-day-old Magang geese that had died on commercial farms in Foshan, Jiangmen, Zhaoqing, Qingyuan, and Yangjiang—the five cities form the principal goose-producing region in Guangdong Province. None of the flocks had been vaccinated against DTMUV, and antemortem laboratory confirmation of DTMUV infection was unavailable for any case.
Each liver specimen (approximately 30 mg) was homogenized in 500 μL of sterile phosphate-buffered saline (PBS, pH=7.02, GIBCO) using a tissue disruptor. Total RNA was extracted from 200 µL of the clarified supernatant using the OMEGA Total RNA Kit I (R6934, Omega Bio-Tek, Doraville, GA, USA) according to the manufacturer’s protocol. Purified RNA aliquots were cryopreserved at −70°C for subsequent analyses.
Detection and complete genome amplification of DTMUV
All RNA samples were subjected to semi-nested RT-PCR screening targeting the conserved NS5 gene of DTMUV with the following primer set: forward primer AAGCCGACTGAACCATCTGA, reverse outer primer GCTGCGTTRCTRTTYACC TTCT, and reverse inner primer CACATCCATCTTGCCACGAT. All primers were designed in-house. The semi-nested RT-PCR amplification was performed in two stages. In the first stage, the conditions were: reverse transcription at 50°C for 30 min, initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 40 s, annealing at 54°C for 40 s, and extension at 72°C for 1 min, with a final extension at 72°C for 5 min, using the forward primer and reverse outer primer; the reaction was carried out with PrimeScript™ One Step RT-PCR Kit (RR055A). The products from the first stage served as the template for the second stage. In the second stage, the conditions were: initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 40 s, annealing at 52°C for 40 s, and extension at 72°C for 1 min, with a final extension at 72°C for 5 min, using the forward primer and reverse inner primer; the reaction was carried out with TaKaRa PCR Amplification Kit (RR901A).
The complete genomes of DTMUV were recovered from the positive samples through overlapping nested RT-PCR (primers upon request). Amplification products were electrophoresed on 1.0 % agarose gels, visualized using a GelDoc XR+ Imaging System (Bio-Rad, USA), and amplicons of expected sizes were purified for bidirectional Sanger sequencing (Sangon Biotech, Shanghai). To prevent cross-contamination, pre-PCR (reagent preparation) and post-nested PCR (template handling) procedures were performed in spatially separated laboratories, with exclusive use of aerosol-resistant filter tips and calibrated micropipettes.
Sequence comparison and phylogenetic analysis
Raw sequencing reads were assembled and manually curated using SeqMan Pro (DNASTAR Lasergene v7.1, Madison, WI). Nucleotide sequence identities were calculated via the ClustalW algorithm in MegAlign (DNASTAR). Maximum-likelihood (ML) trees were reconstructed in MEGA7 using the General Time Reversible (GTR) nucleotide substitution model and optimized parameters of gamma (Γ)-distribution and proportion of invariable sites (i.e., GTR+Γ+I) selected using jModelTest2. Branch support was assessed through 1,000 bootstrap replicates, with mid-point rooting applied for topological clarity.
Recombination analysis
Putative recombination signals were systematically analyzed using RDP4 with seven embedded methods (RDP, GENECONV, BootScan, MaxChi, Chimera, SiScan, and Distance Plot). Recombination events meeting dual criteria were retained: (1) significant detection (Bonferroni-corrected P < 0.05) by ≥3 independent algorithms, and (2) phylogenetic incongruence confirmation. Breakpoint positions and parental lineages were further validated through SimPlot v3.5.1 similarity scanning
Ethics statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The procedures for sampling and sample processing were approved by the ethics committee of Foshan University. All animals were treated in strict accordance with the Rules for the Implementation of Laboratory Animal Medicine (1998) from the Ministry of Health, China.
Results and discussion
In this study, 427 goose liver tissues samples were screened for DTMUV through semi-nested RT-PCR targeting the NS5 gene, followed by Sanger sequencing and BLAST-based sequence confirmation. DTMUV RNA was detected in 69 samples, yielding an overall prevalence of 16.2 % (69/427; 95 % CI: 12.8–20.0 %). Geographic stratification revealed that Foshan exhibiting the highest prevalence at 18.6 % (18/97; 95 % CI: 12.1–27.4 %), followed by Zhaoqing (17.4 %, 16/92; 95 % CI: 10.3–26.7 %), Jiangmen (16.0 %, 13/81; 95 % CI: 8.8–25.8 %), Yangjiang (15.3 %, 11/72; 95 % CI: 7.9–25.7 %), and Qingyuan (12.9 %, 11/85; 95 % CI: 6.6–21.9 %). These findings demonstrate active DTMUV transmission within Guangdong’s major waterfowl production zones. Although the positive rate varied slightly among different sampling sites (ranging from 12.9 % to 18.6 %), the differences were not statistically significant (χ² = 1.206, p > 0.05). Therefore, the spatial distribution of DTMUV can be considered relatively uniform across these zones. However, areas with relatively higher prevalence rates, particularly Foshan and Zhaoqing, still warrant prioritized surveillance and targeted biosecurity interventions to mitigate outbreak risks. This study provides a comprehensive molecular epidemiological profile of DTMUV in Guangdong’s geese, providing critical baseline data for optimizing regional prevention strategies and early warning systems.
Based on similarity and phylogenetic analyses of screening-derived sequences, 8 out of the 69 positive samples were selected for complete genome amplification and sequencing to better characterize the genetic diversity of DTMUV strains detected in this study. The complete genome sequences generated in this study have been deposited in GenBank under accession numbers PQ758739–PQ758746. Sequence comparison analyses showed that the complete genome sequences generated in this study shared 89.0 % to 99.2 % genome-wide pairwise identity with each other. Meanwhile, the polyprotein sequences derived from the eight newly obtained complete genome sequences displayed 95.5–99.3 % amino acid identity with each other. When compared to reference sequences retrieved from GenBank, the newly sequenced genomes showed 80.7–99.2 % nucleotide identity at the genome-wide level, and 78.1–99.3 % amino acid identity at the polyprotein level.
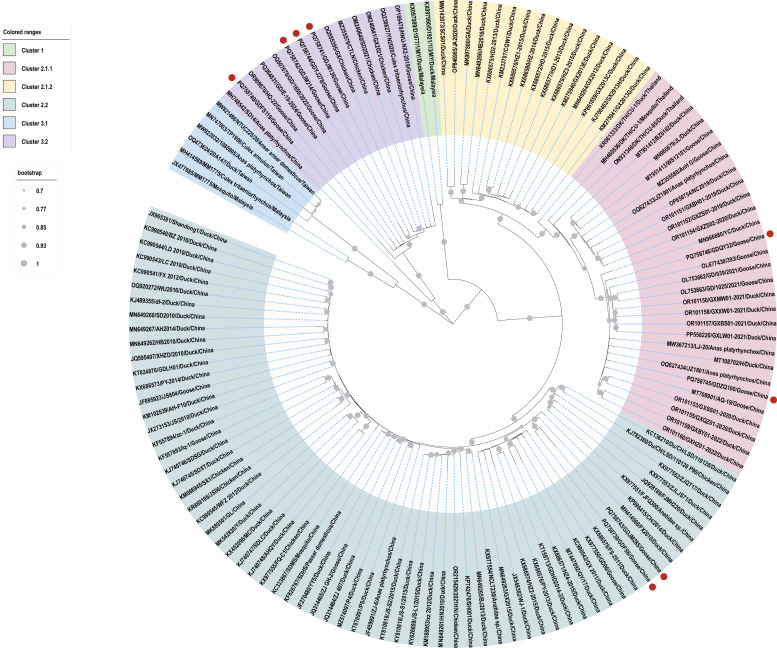
The maximum-likelihood phylogenetic tree reconstructed from all currently available complete genome sequences in GenBank demonstrates that DTMUV can be phylogenetically categorized into three major clusters (Cluster 1-3), with Cluster 2 and Cluster 3 further subdivide into three (subclusters 2.1.1, 2.1.2, and 2.2) and two (subclusters 3.1 and 3.2) distinct sublineages, respectively (Fig. 1). Our study expands this genomic landscape by contributing eight newly sequenced complete genomes from Guangdong province, China, which were distributed across three subclusters: four strains (GDFS-119, GDJM-214, GDJM-136, GDYJ-278) clustered within subcluster 3.2; two strains (GDQY-32, GDZQ-100) aligned with subcluster 2.1.1; and the remaining two (GDJM-285, GDFS-6) grouped into subcluster 2.2 (Fig. 1). Epidemiologically, subclade 2.1.1 was responsible for the 2010–2013 nationwide epizootic in ducks and geese and continues to circulate at moderate levels; it remains fully susceptible to the live-attenuated vaccine FX2010-180P (Li, et al., 2014). Subclade 2.2, first detected in 2012, shows the highest cross-species transmission rate among poultry and has been linked to sporadic neurologic outbreaks in breeder ducks (Zhou, et al., 2023). Sub-clade 3.2 emerged around 2018, rapidly spread to chickens, ducks, geese and mosquitoes, and is now dominant in southern China (Lei, et al., 2017; Yan, et al., 2022). These findings confirm extensive genetic heterogeneity of DTMUV in China and emphasize the need for continuous, subclade-specific surveillance and periodic vaccine seed updates.
Fig. 1.
Phylogenetic analysis based on the complete genome sequence of DTMUV strains. Phylogenetic tree constructed using the maximum likelihood (ML) method in MEGA7, under the GTR+Γ+I model. The analysis includes 1,000 replicates of the alignment, with bootstrap values indicated at nodes (>70 % shown). The red dot denotes the sequence determined in this study.
In addition, although Cluster 2 strains currently predominate in global genomic databases, our findings align with emerging evidence of Cluster 3′s increasing epidemiological prevalence. Specifically, four of our eight sequences clustered within Cluster 3, corroborating recent reports of its rising prevalence (Yang, et al., 2023) and expanded host range, which includes chickens, ducks, geese, and mosquitoes (Lei, et al., 2017; Yan, et al., 2022). The phylogeographic distribution of Guangdong strains across multiple subclusters suggests either independent introduction events or ongoing local diversification. Collectively, these findings highlight three critical insights: (1) China remains a hotspot for DTMUV genetic diversity; (2) Cluster 3 may be undergoing selective expansion, potentially replacing the historical dominance of Cluster 2; and (3) the Guangdong waterfowl production system serves as an important interface for DTMUV evolution. These insights underscore the imperative for sustained genomic surveillance across avian species and geographic regions to monitor potential host-adaptation mutations and zoonotic threats.
Recombination, a key driver of viral diversity and a critical mechanism for viral evolution and emergence (Simon-Loriere and Holmes, 2011), remains understudied in DTMUV, with only limited report of potential event (Dai et al., 2015). To address this knowledge gap, we conducted a comprehensive recombination analysis incorporating all publicly available and eight DTMUV complete genomes newly sequenced from this study. Using multiple algorithms in the RDP4 suite (RDP, GENECONV, BootScan, MaxChi, Chimera, SiScan, and Distance Plot), we identified a statistically supported recombination event (P < 0.05) in strain GDJM-285. Bootscan analysis via SimPlot further delineated two recombination breakpoints at nucleotide positions 3,506 and 6,002, partitioning the genome into three phylogenetically distinct regions: Region A (1–3,505 nt) and Region C (6,003–10,999 nt) showed closest homology to the Chinese duck strain FX2010 (subcluster 2.2), whereas Region B (3,506–6,002 nt) exhibited the highest similarity to strain GDZQ-100 (subcluster 2.1.1) from this study (Fig. 2A). Maximum-likelihood phylogenies of individual regions revealed significant topological discordance, confirming GDJM-285 as a recombinant strain (Fig. 2B). Notably, both the recombinant (GDJM-285) and its putative minor parental strain (GDZQ-100) originated from Guangdong province, China, suggesting that this region's intensive waterfowl farming systems may facilitate viral co-circulation and genetic exchange. This finding emphasizes the urgency for longitudinal surveillance integrating whole-genome sequencing and recombination screening, particularly in regions with high poultry density, to preempt the emergence of novel pathogenic variants.
Fig. 2.
Recombination within the genome of DTMUV strain GDJM-285. (A) Bootscan analysis of the complete genome sequence of DTMUV strain GDJM-285 was conducted using SimPlot program. Parameters: 200 bp window size, 20 bp step size, GapStrip: On, 100 replicates, Kimura (2-parameter), and a T/t ratio of 2.0 in Neighbor-Joining analysis. (B) The phylogenetic trees reconstructed based on three regions of the genome (1–3,505, 3,506–6,002, and 6,003–10,999) using the maximum likelihood (ML) method in MEGA7 under the best-fit substitution model.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by Guangdong Basic and Applied Basic Research Foundation (2024A1515012840).
References
- Cui Y., Pan Y., Guo J., Wang D., Tong X., Wang Y., Li J., Zhao J., Ji Y., Wu Z., Zeng P., Zhou J., Feng X., Hou L., Liu J. The evolution, genomic eepidemiology, and transmission dynamics of Tembusu virus. Viruses. 2022;14:1236. doi: 10.3390/v14061236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Li Z., Tao P. Evolutionary analysis of Tembusu virus: evidence for the emergence of a dominant genotype. Infect. Genet. Evol. 2015;32:124–129. doi: 10.1016/j.meegid.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Lei W., Guo X., Fu S., Feng Y., Tao X., Gao X., Song J., Yang Z., Zhou H., Liang G. The genetic characteristics and evolution of Tembusu virus. Vet. Microbiol. 2017;201:32–41. doi: 10.1016/j.vetmic.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Li G., Gao X., Xiao Y., Liu S., Peng S., Li X., Shi Y., Zhang Y., Yu L., Wu X., Yan P., Yan L., Teng Q., Tong G., Li Z. Development of a live attenuated vaccine candidate against duck Tembusu viral disease. Virology. 2014;450-451:233–242. doi: 10.1016/j.virol.2013.12.028. [DOI] [PubMed] [Google Scholar]
- Platt G.S., Way H..J., Bowen E.T., Simpson D.I., Hill M.N., Kamath S., Bendell P.J., Heathcote O.H. Arbovirus infections in Sarawak, October 1968–February 1970 Tembusu and Sindbis virus isolations from mosquitoes. Ann. Trop. Med. Parasitol. 1975;69:65–71. doi: 10.1080/00034983.1975.11686984. [DOI] [PubMed] [Google Scholar]
- Simon-Loriere E., Holmes E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011;9:617–626. doi: 10.1038/nrmicro2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Li S., Hu X., Yu X., Wang Y., Liu P., Lu X., Zhang G., Hu X., Liu D., Li X., Su W., Lu H., Mok N.S., Wang P., Wang M., Tian K., Gao G.F. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS. One. 2011;6 doi: 10.1371/journal.pone.0018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Diao Y., Yu C., Gao X., Ju X., Xue C., Liu X., Ge P., Qu J., Zhang D. Characterization of a Tembusu virus isolated from naturally iinfected house sparrows (passer domesticus) in Northern China. Transbound. Emerg. Dis. 2013;60:152–158. doi: 10.1111/j.1865-1682.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- Yan D., Li X., Wang Z., Liu X., Dong X., Fu R., Su X., Xu B., Teng Q., Yuan C., Zhang Z., Liu Q., Li Z. The emergence of a disease caused by a mosquito origin cluster 3.2 Tembusu virus in chickens in China. Vet. Microbiol. 2022;272 doi: 10.1016/j.vetmic.2022.109500. [DOI] [PubMed] [Google Scholar]
- Yang Q., Ding Y., Yao W., Chen S., Jiang Y., Yang L., Bao G., Yang K., Fan S., Du Q., Wang Q., Wang G. Pathogenicity and interspecies transmission of cluster 3 tembusu vvirus strain TMUV HQ-22 isolated from geese. Viruses. 2023;15:2449. doi: 10.3390/v15122449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Lin Y., Tang Y., Diao Y. Evolution of Tembusu virus in ducks, chickens, geese, sparrows, and mosquitoes in northern China. Viruses. 2018;10:485. doi: 10.3390/v10090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Chen S., Mahalingam S., Wang M., Cheng A. An updated review of avian-origin Tembusu virus: a newly emerging avian flavivirus. J. Gen. Virol. 2017;98:2413–2420. doi: 10.1099/jgv.0.000908. [DOI] [PubMed] [Google Scholar]
- Zhou P., Ma B., Gao Y., Xu Y., Li Z., Jin H., Luo R. Epidemiology, genetic diversity, and evolutionary dynamics of Tembusu virus. Arch. Virol. 2023;168:262. doi: 10.1007/s00705-023-05885-5. [DOI] [PubMed] [Google Scholar]