Abstract
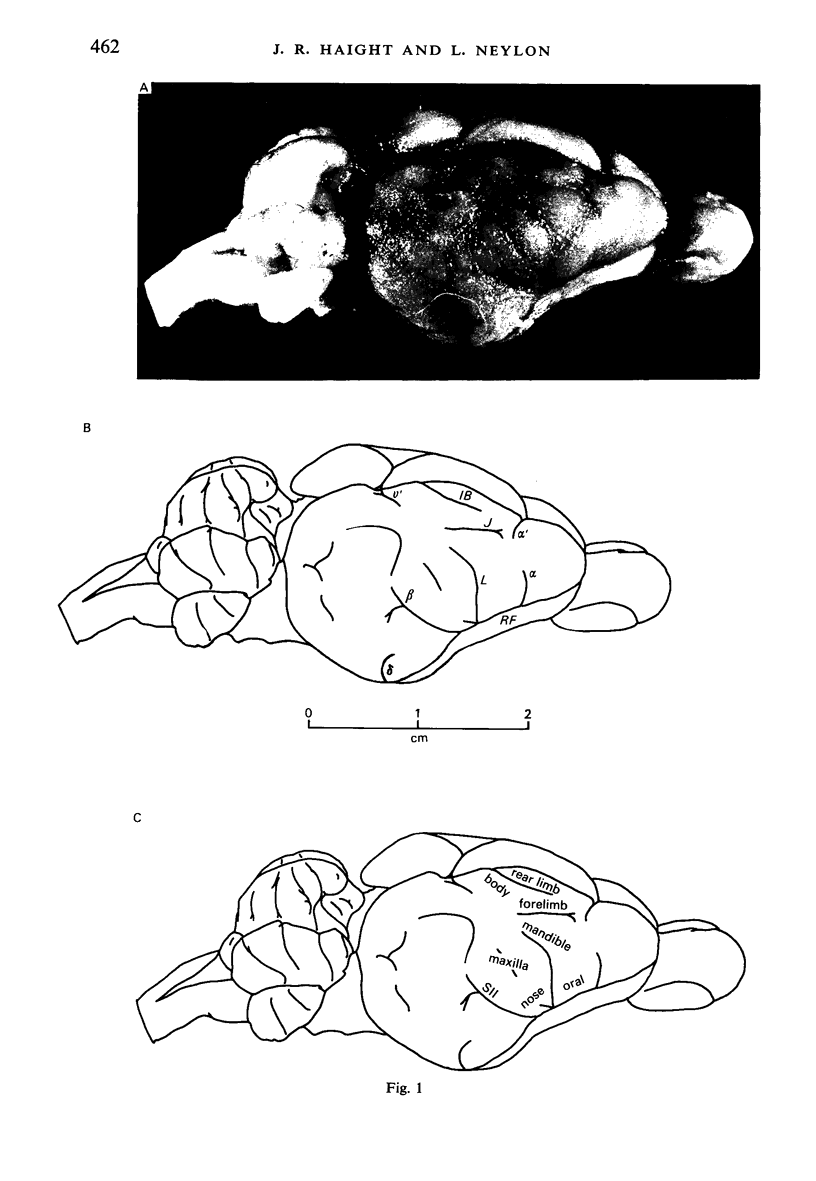
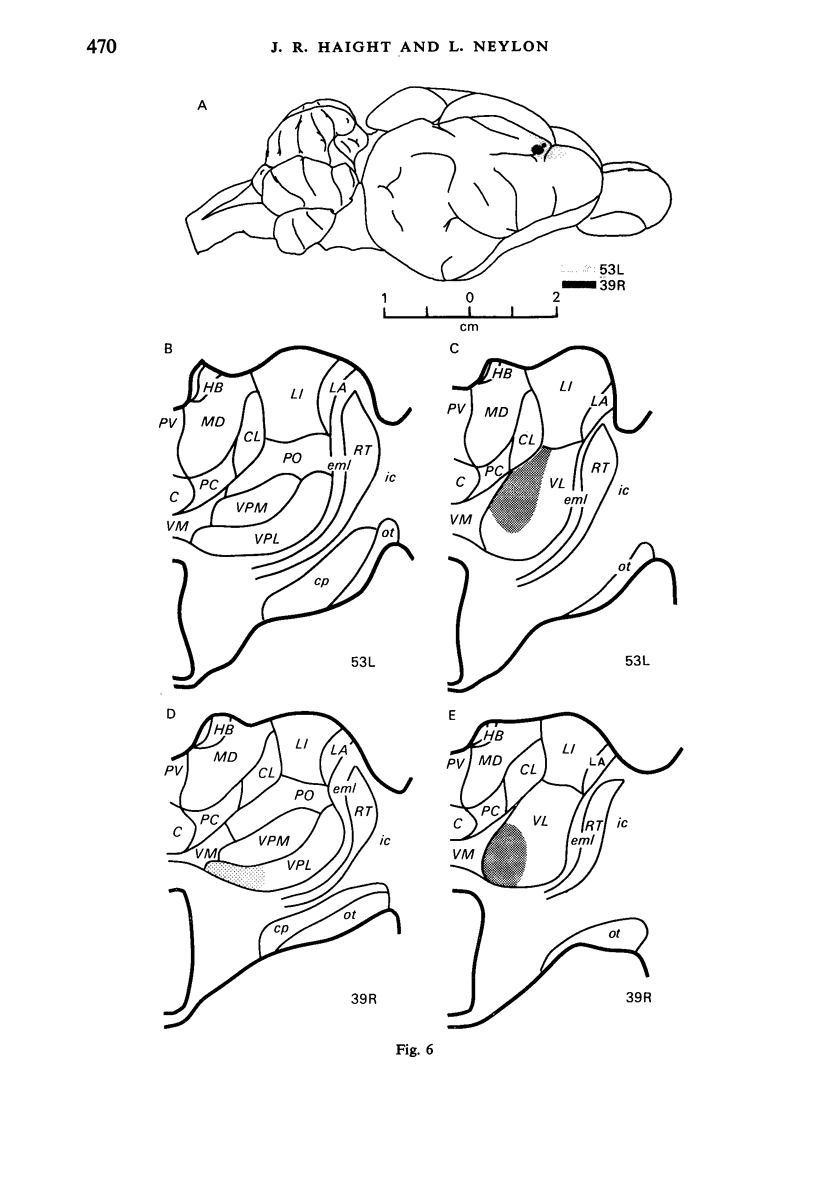
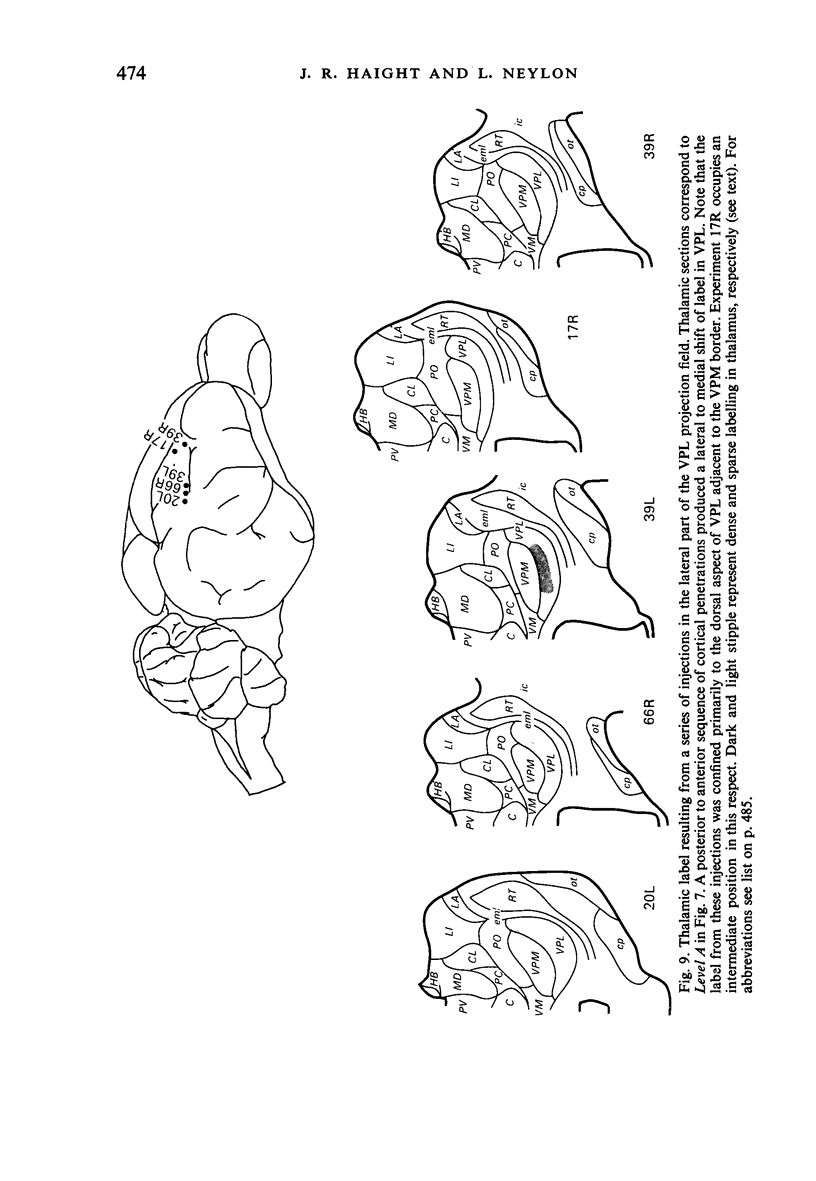
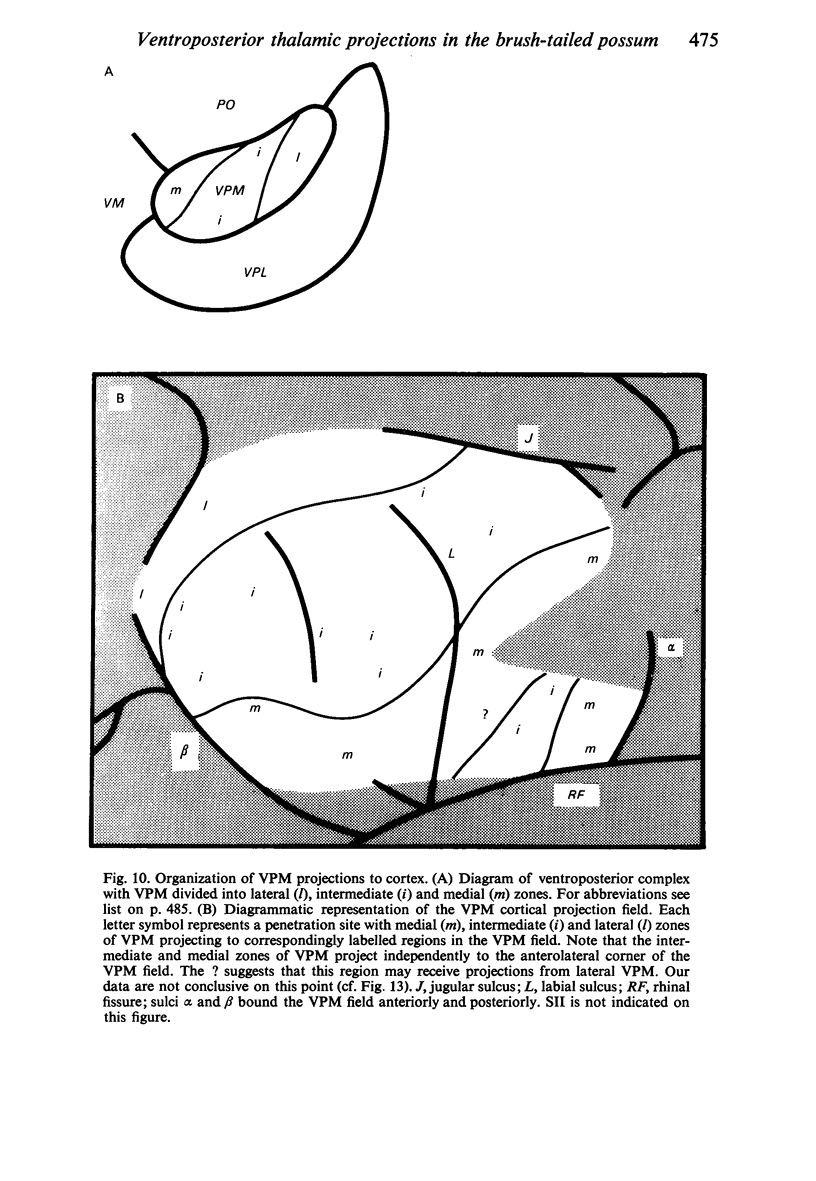
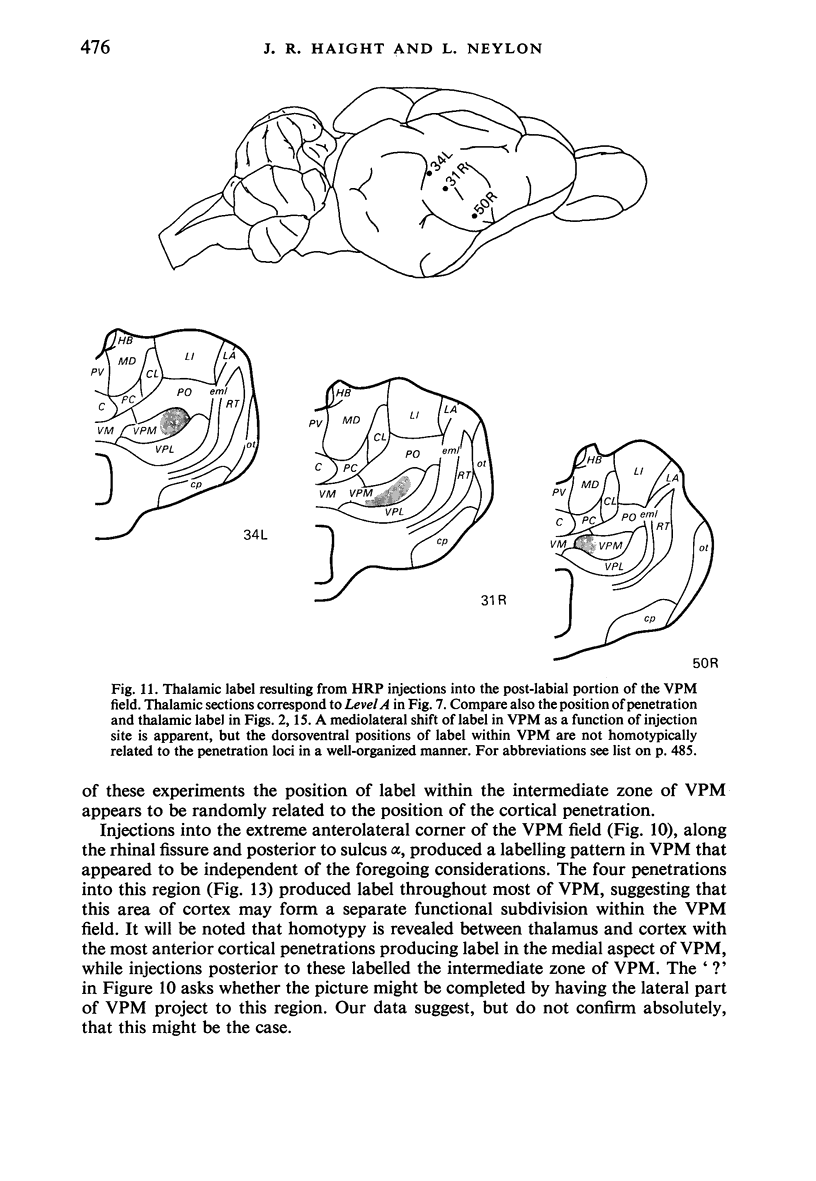
Retrograde transport of horseradish peroxidase (HRP) was used to determine the extent and some of the organizational details of the cortical projection of the ventroposterior thalamic complex (VP) in the marsupial brush-tailed possum, Trichosurus vulpecula. The cortical projection field of VP is coincident with SI as determined by electrophysiological methods, and would appear not to overlap fully the primary motor cortex. Thus, in Trichosurus it appears that the motor and somatic sensory cortical regions are not fully congruent, unlike those of the American opossum, Didelphis, which are. Each division of VP projects discretely, in a non-overlapping manner, to various regions within SI. The ventrolateral subdivision or VPL projects medially and in a strict homotypic manner, though the proportion of VPL cells projecting to cortex is subject to a large amount of variation. The dorsomedial division of VP or VPM projects uniformly to cortex from all areas of that subnucleus, but the strict homotypy characteristic of VPL's projection was not as apparent. VPM also projects to two distinct regions within its cortical field. The posteromedial division of VP or VPP projects to an area of cortex that receives no other VP input but, on the basis of cortical mapping studies, appears to belong to SI. Projections from VPL (and presumably from VPM) to a small area of cortex in the extreme posterolateral part of the VP field correspond to the position expected for, and electrophysiologically confirmed to be, SII.
Full text
PDF



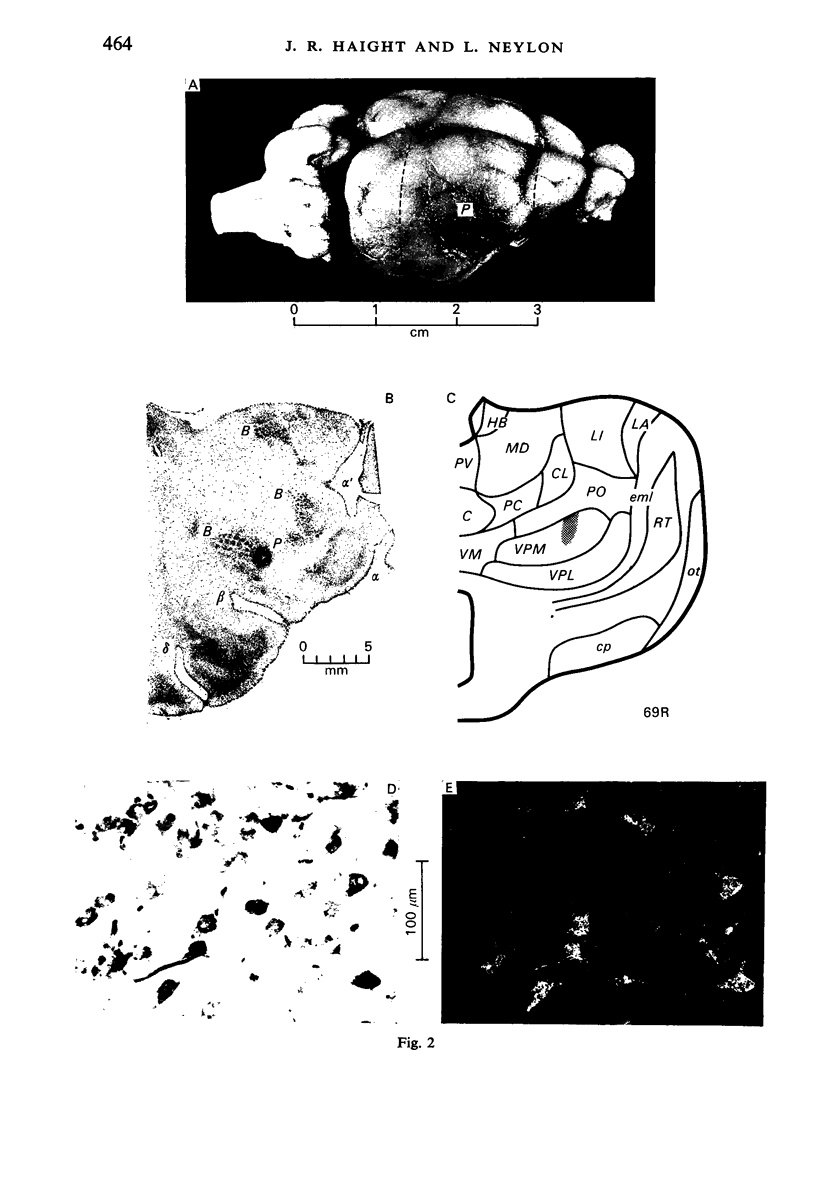

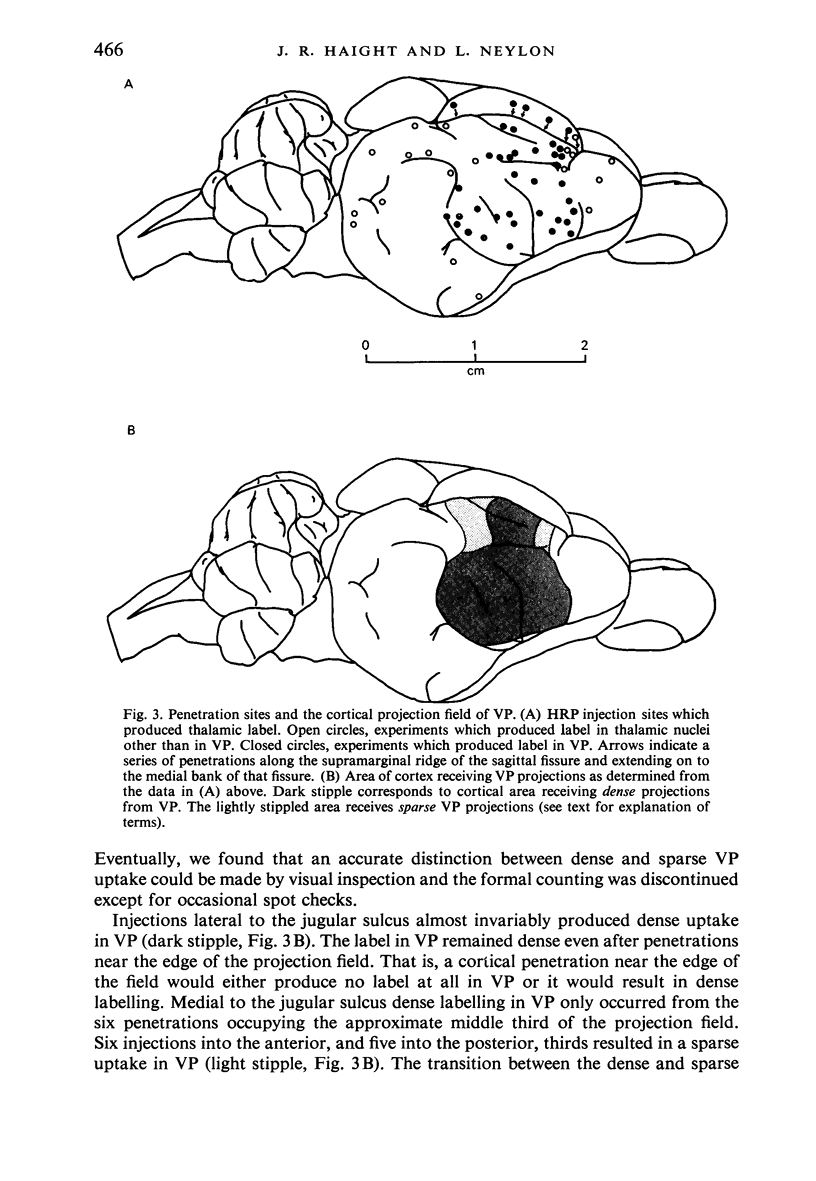
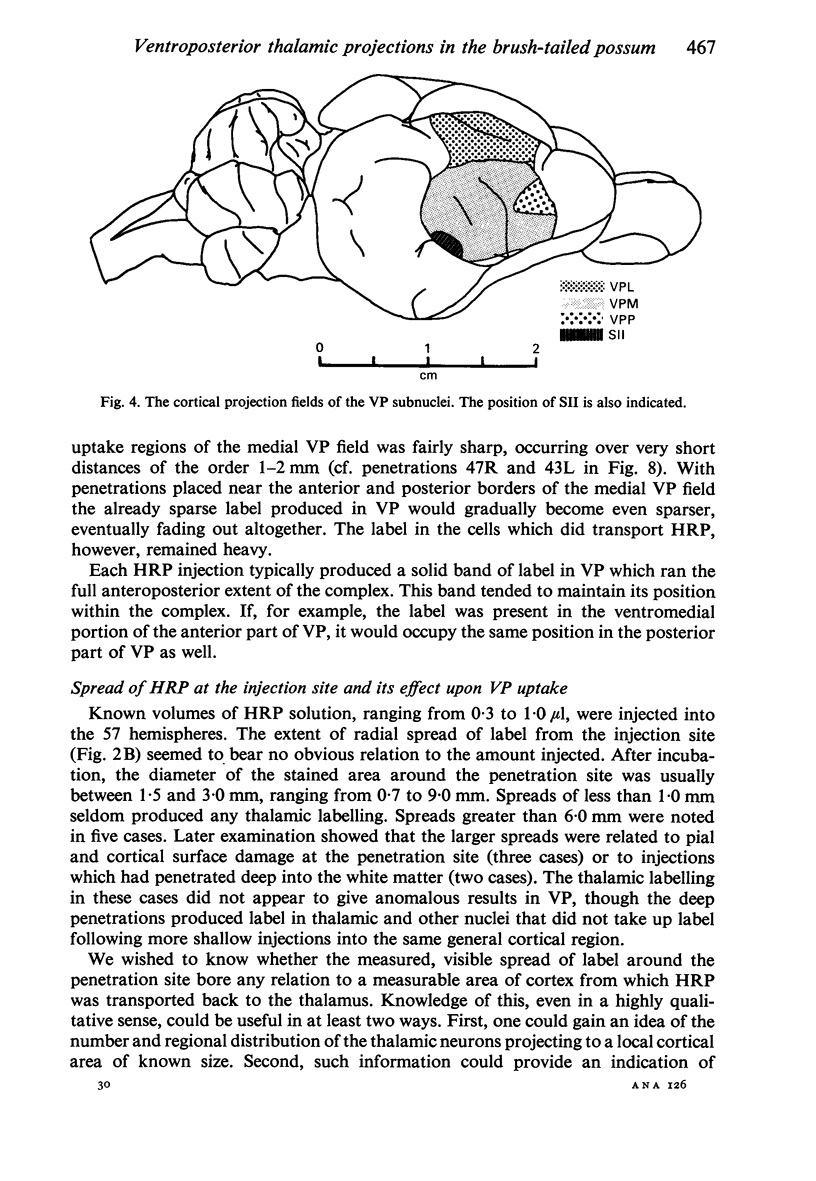
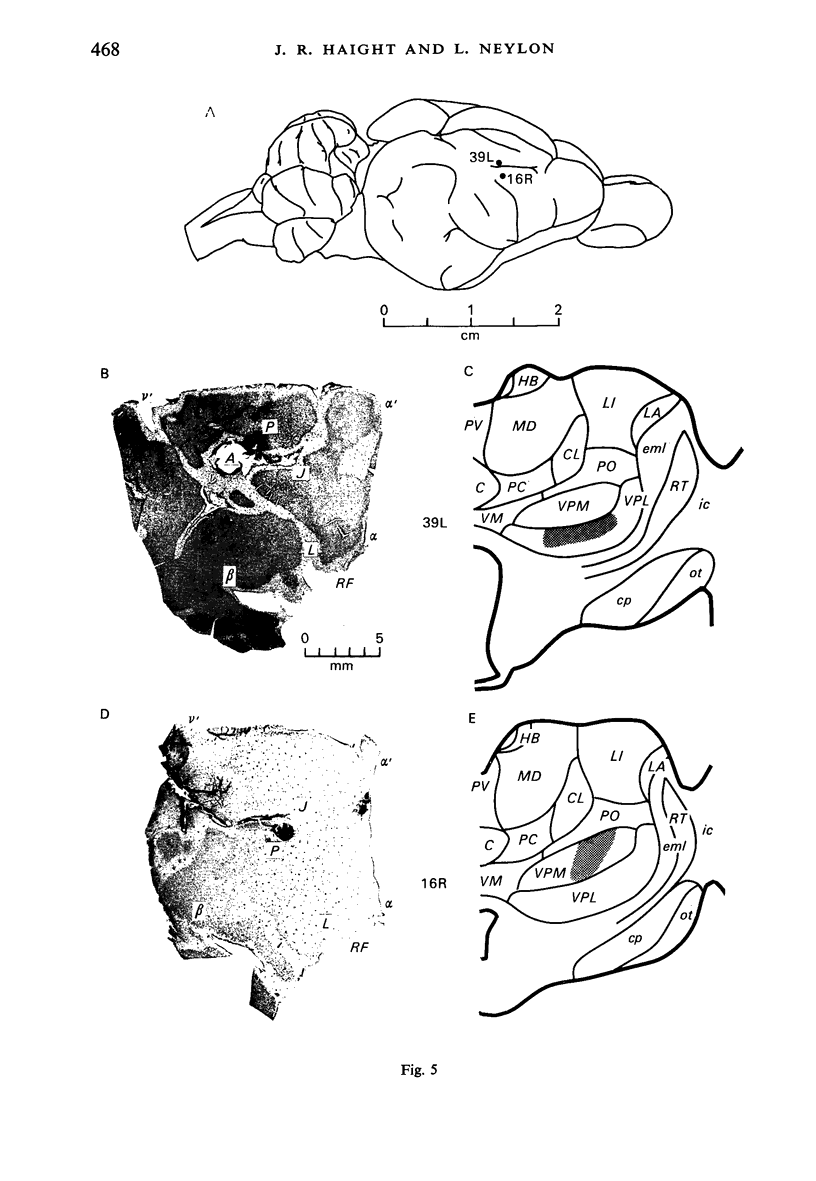









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADEY W. R., KERR D. I. The cerebral representation of deep somatic sensibility in the marsupial phalanger and the rabbit; an evoked potential and histological study. J Comp Neurol. 1954 Jun;100(3):597–624. doi: 10.1002/cne.901000307. [DOI] [PubMed] [Google Scholar]
- Goldby F. An experimental investigation of the motor cortex and its connexions in the phalanger, Trichosurus vulpecula. J Anat. 1939 Oct;74(Pt 1):12–33. [PMC free article] [PubMed] [Google Scholar]
- Goldby F. An experimental study of the thalamus in the phalanger, Trichosurus vulpecula. J Anat. 1943 Apr;77(Pt 3):195–224.4. [PMC free article] [PubMed] [Google Scholar]
- Haight J. R., Weller W. L. Proceedings: Neocortical topography in the brush-tailed possum: variability and functional significance of sulci. J Anat. 1973 Dec;116(Pt 3):473–474. [PubMed] [Google Scholar]
- Jones E. G., Leavitt R. Y. Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol. 1974 Apr 15;154(4):349–377. doi: 10.1002/cne.901540402. [DOI] [PubMed] [Google Scholar]
- Killackey H., Ebner F. Convergent projection of three separate thalamic nuclei on to a single cortical area. Science. 1973 Jan 19;179(4070):283–285. doi: 10.1126/science.179.4070.283. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. Retrograde axonal transport of protein. Brain Res. 1971 Jun 18;29(2):363–365. doi: 10.1016/0006-8993(71)90044-8. [DOI] [PubMed] [Google Scholar]
- LENDE R. A. Cerebral cortex: a sensorimotor amalgam in the marsupiala. Science. 1963 Aug 23;141(3582):730–732. doi: 10.1126/science.141.3582.730. [DOI] [PubMed] [Google Scholar]
- LENDE R. A. MOTOR REPRESENTATION IN THE CEREBRAL CORTEX OF THE OPOSSUM (DIDELPHIS VIRGINIANA). J Comp Neurol. 1963 Dec;121:405–415. doi: 10.1002/cne.901210308. [DOI] [PubMed] [Google Scholar]
- LENDE R. A. SENSORY REPRESENTATION IN THE CEREBRAL CORTEX OF THE OPOSSUM (DIDELPHIS VIRGINIANA). J Comp Neurol. 1963 Dec;121:395–403. doi: 10.1002/cne.901210307. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., LaVail M. M. Retrograde axonal transport in the central nervous system. Science. 1972 Jun 30;176(4042):1416–1417. doi: 10.1126/science.176.4042.1416. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., Winston K. R., Tish A. A method based on retrograde intraaxonal transport of protein for identification of cell bodies of origin of axons terminating within the CNS. Brain Res. 1973 Aug 30;58(2):470–477. doi: 10.1016/0006-8993(73)90016-4. [DOI] [PubMed] [Google Scholar]
- Manson J. The somatosensory cortical projection of single nerve cells in the thalamus of the cat. Brain Res. 1969 Feb;12(2):489–492. doi: 10.1016/0006-8993(69)90021-3. [DOI] [PubMed] [Google Scholar]
- Megirian D., Johnson J. I., Haight J. R. Le rapport entre les circonvolutions du cortex cérébral et les projections sensorielles chez le wombat Wombatus ursinus. J Physiol (Paris) 1972;65(Suppl):448A–448A. [PubMed] [Google Scholar]
- Pubols B. H., Jr, Pubols L. M., DiPette D. J., Sheely J. C. Opossum somatic sensory cortex: a microelectrode mapping study. J Comp Neurol. 1976 Jan 15;165(2):229–245. doi: 10.1002/cne.901650208. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Jr, Pubols L. M. Somatic sensory representation in the thalamic ventrobasal complex of the Virginia opossum. J Comp Neurol. 1966 May;127(1):19–34. doi: 10.1002/cne.901270103. [DOI] [PubMed] [Google Scholar]
- Rees S., Hore J. The motor cortex of the brush-tailed possum (Trichosurus vulpecula): motor representation, motor function and the pyramidal tract. Brain Res. 1970 Jun 15;20(3):439–451. doi: 10.1016/0006-8993(70)90173-3. [DOI] [PubMed] [Google Scholar]
- Rockel A. J., Heath C. J., Jones E. G. Afferent connections to the diencephalon in the marsupial phalanger and question of sensory convergence in the "posterior group" of the thalamus. J Comp Neurol. 1972 May;145(1):105–129. doi: 10.1002/cne.901450107. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Sessle B. J. Somatic afferent input to posterior thalamic neurones and their axon projection to the cerebral cortex in the cat. J Physiol. 1968 May;196(1):19–35. doi: 10.1113/jphysiol.1968.sp008491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L. A., Watson C. R. Proceedings: An experimental study of the ventrolateral thalamic nucleus of the brush-tailed possum. J Anat. 1973 Dec;116(Pt 3):472–472. [PubMed] [Google Scholar]
- Welker W. I. Principles of organization of the ventrobasal complex in mammals. Brain Behav Evol. 1973;7(4):253–336. doi: 10.1159/000124417. [DOI] [PubMed] [Google Scholar]
- Weller W. L. Barrels in somatic sensory neocortex of the marsupial Trichosurus vulpecula (brush-tailed possum). Brain Res. 1972 Aug 11;43(1):11–24. doi: 10.1016/0006-8993(72)90271-5. [DOI] [PubMed] [Google Scholar]
- Weller W. L., Haight J. R. Proceedings: Barrels and somatotopy in S I neocortex of the brush-tailed possum. J Anat. 1973 Dec;116(Pt 3):474–474. [PubMed] [Google Scholar]