Abstract
Oxygen-vacancy engineering in transition metal oxides enables programmable functionalities by modulating the valence states and local coordination of constituents. Here, we report the selective reduction of cobalt ions in epitaxial SrFe0.5Co0.5O2.5 thin films under reducing gas environments, while iron ions remain unchanged. X-ray absorption spectroscopy reveals an absorption edge shift of 1.65 eV in the Co L-edge upon reduction, and multiplet simulations estimate a decrease in the average Co valence from Co2.91+ to Co2.00+. This site- and element-specific reduction leads to the formation of a structurally distinct oxygen-deficient phase stabilized by oxygen vacancies at tetrahedral sites, as confirmed by density functional theory. Optical spectroscopy reveals an increase in the bandgap from 2.47 eV to 3.04 eV, accompanied by enhanced transparency. Furthermore, simultaneous in situ diffraction and transport measurements confirm fully reversible redox-driven transitions among three phases: reduced defective perovskite, brownmillerite, and oxygen-rich perovskite phases. These findings demonstrate that selective redox control in multi-cation oxides enables the realization of chemically and functionally distinct oxygen-deficient phases.
Subject terms: Synthesis and processing, Materials for energy and catalysis, Fuel cells
Here authors show selective reduction behaviours in a catalytically active iron and cobalt containing multivalent topotactic oxide and demonstrated its low-temperature reversible redox reactions, potentially applicable to energy and electronic devices.
Introduction
Transition metal oxides (TMOs) exhibit diverse emergent properties that have enabled their application in energy storage, catalysis, superconductivity, and electronic devices1–6. Among various tunable parameters, oxygen vacancies have emerged as a powerful handle to modulate the valence state, local structure, and associated functionalities of TMOs. In several systems, oxygen vacancy ordering leads to new structural phases, such as brownmillerite and infinite-layer configurations7–9. These vacancy-engineered phases, governed by local site-preferential vacancy formation, are promising candidates for oxygen-driven programmable matter. The formation and dynamics of oxygen vacancies under controlled environments—such as hydrogen, nitrogen, or argon—have been shown to critically influence the functional properties of perovskite oxides. Prior studies report reversible redox switching in SrCoOy8, oxygen deficiency-induced phase transitions in SrFeOy10, resistive switching behavior and vacancy ordering in brownmillerite films3, and modulation of vacancy behavior through multi-cation substitution1,11.
Recent efforts have focused on stabilizing oxygen-deficient phases in TMOs, particularly in cobalt- and iron-based perovskites, due to their rich redox chemistry and structural flexibility9,12. In cobalt oxides, phase transitions were typically limited between the perovskite and brownmillerite structures. However, a recent report on CaCoO2 demonstrated the formation of an infinitely layered structure stabilized under strong reducing conditions, where the small ionic radius of Ca2+ may introduce chemical pressure and facilitates planar Co−O bonding13. In contrast, SrCoO2 has been demonstrated to form a square spin-tube structure. This indicates that other factors, like chemical pressure from A-site cations, may hamper the formation of an infinitely layered structure in the Sr-based cobalitite4,14. In contrast, iron oxides can adopt a wider range of oxygen stoichiometries without considering the chemical pressure. The associated transitions among perovskite (AFeO3), brownmillerite (AFeO2.5), and infinite-layer (AFeO2) phases are often driven by apical oxygen removal and accompanied by notable structural contraction along the c-axis9,15. However, the redox reactions in mixed B-site systems such as SrFe1−xCoxO2.5 remain underexplored, particularly in terms of whether cooperative or competing interactions between Fe and Co can stabilize unique oxygen-deficient phases. In this study, we demonstrate that the reduction pathway of SrFe0.5Co0.5O2.5 (SFCO) deviates from typical infinite-layer transitions. Instead, it leads to the formation of a defective perovskite phase stabilized by selective Co reduction and oxygen vacancy formation at tetrahedral sites, while Fe remains chemically inert. This behavior is distinct from both SrCoO2.5, which decomposes under reducing conditions, and SrFeO2.5, which retains its structure without substantial reduction. We attribute this structural stability to Fe incorporation, which modifies the local coordination environment and suppresses Co-induced structural collapse16. This behavior highlights the site- and element-specific nature of redox control in multi-cation oxide systems and opens up a new phase space for programmable oxygen-deficient materials.
To systematically investigate the redox behavior of SFCO, we performed annealing experiments under reducing conditions using diluted hydrogen gas. A series of time- and temperature-dependent studies revealed the formation of a distinct structure in SFCO, which is different from the brownmillerite or infinite-layer phases. To assess the compositional dependence, we extended our study to brownmillerite SrFe1−xCoxO2.5 with x = 0, 0.2, and 1.0. Notably, the Fe-rich sample (x = 0) retained its brownmillerite structure, showing only a crystallographic axis rotation consistent with previous studies17. In contrast, the Co-rich sample (x = 1.0) underwent structural decomposition, accompanied by the formation of CoO impurity. The intermediate composition (x = 0.2) exhibited no sign of structural change. These observations highlight the unique redox stability of the x = 0.5 composition, where the incorporation of Fe plays a crucial role in maintaining structural coherence and preventing decomposition11,13,18. The cooperative yet asymmetric redox response of Fe and Co in the SFCO system stabilizes an oxygen-deficient defective perovskite phase that cannot be accessed in single-cation systems.
Results and discussion
Structural transformation via thermal reduction
To reduce the oxygen content in the as-grown brownmillerite (BM) SFCO thin films, we performed post-annealing in a 3% H2/Ar forming gas (FG), which facilitates the removal of lattice oxygen via reaction with hydrogen (Fig. 1a). Films were annealed at various temperatures for 1 hour to investigate temperature-dependent structural evolution. X-ray diffraction (XRD) patterns (Fig. 1b and Supplementary Fig. 1) show that the BM structure is preserved up to 300 °C, with progressive out-of-plane lattice expansion indicating gradual oxygen vacancy formation19. Upon annealing at 400 °C and 500 °C, we observed an abrupt shift in the 008 diffraction peak to lower angles and the disappearance of the 006 diffraction peak, suggesting the collapse of BM ordering and the emergence of a new reduced phase (Fig. 1c). Annealing at 600 °C led to complete loss of diffraction peaks, indicating a breakdown of epitaxial order.
Fig. 1. Structural evolution of SrFe0.5Co0.5O2.5 (SFCO) during thermal reduction.
a Schematic of the reduction process in brownmillerite SFCO thin films on (001) SrTiO3 via annealing in 3% H2/Ar forming gas. Green, red, blue, and grey spheres represent Sr, Fe/Co, Ti, and O atoms, respectively. b X-ray diffraction patterns of as-grown and reduced SFCO films. c Out-of-plane lattice constants as a function of annealing temperature (1 h fixed). d Out-of-plane lattice constants as a function of annealing time at 400 °C. Blue and yellow background regions indicate the brownmillerite phase and the oxygen-deficient perovskite phase, respectively, emphasising the temperature range (300–400 °C) where the structural transition occurs.
To further investigate the time-dependent stability of the reduced phase, we fixed the annealing temperature at 400 °C and varied the annealing time. As shown in Fig. 1d and Supplementary Fig. 2, out-of-plane lattice expansion continued up to 5 hours, reaching ~4.020 Å, but remained unchanged thereafter up to 100 hours, indicating that the reduced phase is thermodynamically stabilized after extended reduction. Importantly, in-plane XRD measurements (Supplementary Fig. 3) confirm that the in-plane lattice parameters are locked by substrate-induced strain from SrTiO3 substrate throughout the reduction process. This anisotropic lattice evolution strongly contrasts with the c-axis contraction, which is typically found in an infinite-layer structure, suggesting that the reduced phase is structurally distinct. Further structural analyses using reciprocal space mapping (RSM, Supplementary Fig. 4) and HAADF-STEM (Supplementary Fig. 5) reveal out-of-plane peak shifts and a Sr−Sr lattice expansion in the vertical direction, while the in-plane periodicity is preserved. The extracted lattice constants from these measurements are summarized in Supplementary Table 1 and show excellent agreement between the XRD and STEM results. Taken together, these observations indicate that the reduced SFCO does not transform into a planar infinite-layer phase, but instead evolves into an oxygen-deficient defective perovskite4,14,16. The lack of long-range superstructure peaks suggests that oxygen vacancies are not ordered.
To understand the formation and stability of the reduced SFCO phase, we performed systematic annealing experiments on BM-SrFe1−xCoxO2.5 films with various compositions (x = 0, 0.2, 0.5, and 1.0), grown under identical conditions and annealed at 400 °C for 5 hours in FG. As summarized in Supplementary Fig. 6, the annealed BM-SrFeO2.5 (x = 0) retained its BM structure, with a minor crystallographic rotation similar to prior observations under vacuum annealing17. In contrast, BM-SrCoO2.5 (x = 1.0) decomposed into a CoO impurity phase, as evidenced by XRD20,21. For BM-SrFe0.8Co0.2O2.5 with x = 0.2, no structural transformation was observed, indicating limited reduction. Notably, only the x = 0.5 composition led to a distinct structural change upon reduction, highlighting the critical role of Fe/Co ratio in determining redox behavior and phase stability.
While Fe remains chemically stable under these reducing conditions, its presence appears to suppress structural collapse by resisting apical oxygen removal, enabling the formation of a stable oxygen-deficient phase. This observation is consistent with prior reports showing that Fe-based perovskites can maintain structural coherence under reducing conditions9, whereas Co-based systems often decompose4,21,22. Note that X-ray reflection revealed an increase in film thickness after reduction (23.40 nm for the as-grown SFCO and 24.30 nm for the reduced SFCO; Supplementary Fig. 7), consistent with the out-of-plane lattice expansion. When converted to unit cell layers, both films show comparable values (19.7 for the as-grown SFCO and 20.1 for the reduced SFCO), confirming that the reduction proceeds without significant structural degradation, while maintaining volumetric integrity. Atomic force microscopy images further revealed that the step-terrace morphology was preserved in both the as-grown and reduced films (Supplementary Fig. 8), indicating that the reduction process does not deteriorate surface integrity. The consistent root-mean-square roughness supports the conclusion that the reduced phase maintains high structural quality, without degradation of surface morphology. These results reinforce that the epitaxial strain is maintained in the reduced phase, while oxygen vacancies are accommodated within the lattice. To evaluate the thermal stability of the reduced phase, we conducted additional annealing at 500 °C. As shown in Supplementary Fig. 9, XRD and X-ray absorption spectroscopy (XAS) measurements after prolonged annealing revealed spectral broadening and structural degradation, indicating that the oxygen-deficient phase becomes unstable at the higher temperatures. This suggests an upper thermal limit for the reversible redox functionality in SFCO films.
Element-specific redox responses of Fe and Co
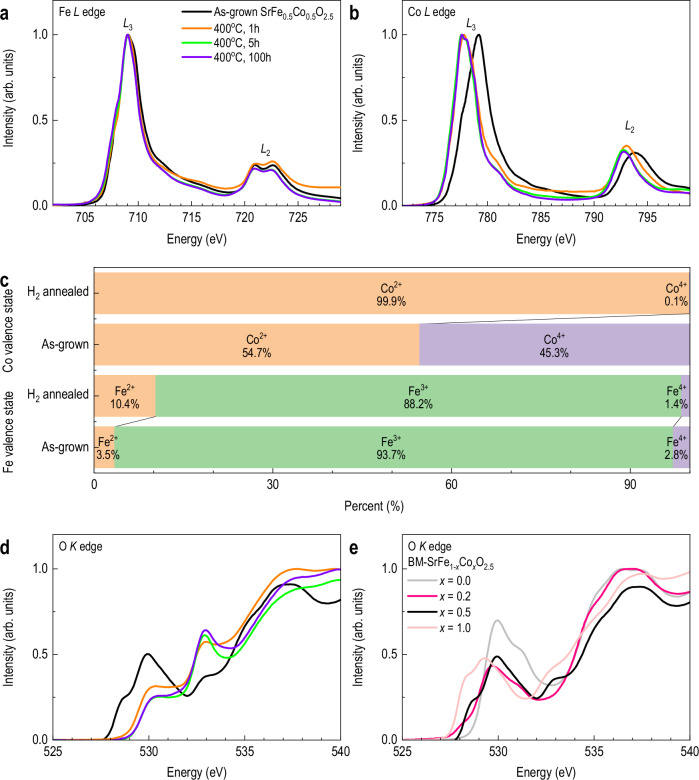
To determine whether the observed structural transformation arises from oxygen vacancy formation, we performed soft XAS at the Fe and Co L-edges. Figure 2a shows the Fe L-edge spectra for the as-grown and reduced SFCO films (annealed for 1, 5, and 100 hours). The spectral shape remains nearly unchanged, closely resembling that of LaFeO3 and SrFe0.8Co0.2O2.5, indicating that the Fe valence state is largely unaffected by the reduction process12,23. In contrast, the Co L-edge spectra exhibit significant changes upon annealing, as shown in Fig. 2b20. An absorption edge shift of 1.65 eV toward lower energies is observed for the Co L3-edges, consistent with a reduction in oxidation state24. To quantify the Co valence states, we performed multiplet simulations using the charge transfer multiplet for X-ray absorption spectroscopy (CTM4XAS)25 code, a ligand-field multiplet framework that enables detailed modeling of transition metal L-edge spectra. The experimental spectrum was fitted by a linear combination of Co4+ and Co2+ configurations, revealing a decrease in the average Co valence from Co2.91+ of the as-grown to Co2.00+ of the reduced (Fig. 2c and Supplementary Figs. 10). These results indicate that the structural transformation is driven by selective Co reduction, while Fe remains chemically inert.
Fig. 2. Element-specific electronic structure evolution upon reduction.
X-ray absorption spectra (XAS) of (a) Fe L-edge and (b) Co L-edge for as-grown and reduced SrFe0.5Co0.5O2.5 (SFCO) films. c Estimated Fe and Co valence states from multiplet fitting. Lists relative proportions of Fe4+, Fe3+, Fe2+ and Co4+, Co2+ in as-grown and 5 h reduced films. d O K-edge XAS spectra for SFCO films before and after reduction, showing changes in hybridization. e O K-edge XAS spectra for SrFe1−xCoxO2.5 with different x values.
To support this estimation, we performed multiplet simulations using CTM4XAS to fit the Fe and Co L-edge spectra. To refine the CTM4XAS simulations, we included multiple coordination environments for each oxidation state. For Fe, four components were considered: Fe4+ (octahedral), Fe3+ (octahedral and tetrahedral), and Fe2+ (tetrahedral), based on prior reports identifying Fe3+ species in both sites under strain or oxygen-deficient conditions26,27. Co spectra were simulated using Co4+ (octahedral) and Co2+ (tetrahedral), consistent with known valence distributions in cobaltite24,28. The Fe L-edge spectrum showed a dominant Fe3+ character (Fe4+: Fe3+: Fe2+ = 2.80: 93.74: 3.46), corresponding to an estimated oxygen stoichiometry of SrFe0.5Co0.5O2.497. The Co L-edge fitting yielded a Co4+: Co2+ ratio of 45.34: 54.66, corresponding to SrFe0.5Co0.5O2.453. The combined analysis supports an overall oxygen content of SrFe0.5Co0.5O2.475 ± 0.022 for the as-grown film, consistent with the BM phase. The simulated spectral weights and corresponding oxygen stoichiometry estimates for both Fe and Co L-edges are summarized in Fig. 2c. These results stand in contrast to previous reports on mixed B-site perovskites, such as SrFe1−xCuxO3−δ and SrFe1−xCoxO3−δ, where both B-site cations exhibited simultaneous redox behavior, yet no structurally distinct oxygen-deficient phase was stabilized under comparable reducing conditions29,30.
We further investigated the element-specific magnetic response using X-ray magnetic circular dichroism (XMCD) at the Fe and Co L-edges at 130 K. As shown in Supplementary Fig. 11, only the O2-annealed (O2, 400 °C, 5 h anneal) sample exhibited discernible XMCD signals, confirming the presence of finite magnetic moments in the oxidized phase (SrFe0.5Co0.5O2.5+δ). In contrast, no XMCD signal was detected in either the as-grown or reduced samples, suggesting the absence of net magnetic moments. These valence changes correlate with the anisotropic lattice evolution observed in RSM and STEM analyses, supporting the formation of an oxygen-deficient defective perovskite phase.
To further verify the element-specific valence changes associated with reduction, we examined the O K-edge XAS of SFCO films before and after annealing (Fig. 2d). In the reduced film, the feature within the 527.8–531.1 eV range, typically associated with Co 3 d − O 2p hybridization31, is significantly suppressed, consistent with the removal of oxygen atoms near Co sites. In contrast, a pronounced intensity enhancement is observed near 533 eV. This spectral feature, measured in total electron yield (TEY) mode, has previously been attributed to the σ* resonance of surface-stabilized O2− superoxide species in oxygen-deficient cobalt oxides32. The formation of these species is facilitated by oxygen vacancies and the reduced transition metal states, particularly near the film surface33. To further elucidate the element-specific hybridization, we measured the O K-edge spectra of BM-SrFe1−xCoxO2.5 with varying x values (x = 0.0, 0.2, 0.5, and 1.0) (Fig. 2e). The spectral region from 530 to 535 eV reflects hybridization between transition metal 3 d and O 2p orbitals, with contributions from both Fe 3 d − O 2p (near 529.5–530.5 eV) and Co 3 d − O 2p (within 527.8–531.1 eV) interactions. These compositional spectra serve as fingerprints to distinguish local oxygen environments in mixed B-site perovskites. Quantitative multiplet fitting, summarized in Fig. 2c, confirms that the selective reduction of Co is accompanied by oxygen deficiency, further validating the proposed redox mechanism. Additionally, electron energy loss spectroscopy (EELS) measurements (Supplementary Fig. 12) show a discernible chemical shift in the Co L3-edge toward lower energy after annealing, confirming the reduction of cobalt ions. This local electronic structure change is consistent with the observed O K-edge spectral evolution and supports our interpretation of Co-selective reduction. In contrast, no significant energy shift was observed for the Fe L-edge in the EELS spectra, further supporting the conclusion that iron remains largely chemically inert during the reduction process.
Role of the apical oxygen sites and Co-selective reduction pathway
To gain atomistic insight into the site preference of oxygen vacancies and the mechanism underlying Co-selective reduction, we performed density functional theory (DFT) calculations (Fig. 3). We evaluated the oxygen vacancy formation enthalpy for three distinct oxygen sites in the BM-SFCO structure34–39: equatorial oxygen within the octahedral layers, tetrahedral oxygen within the tetrahedral layers, and apical oxygen connecting tetrahedral and octahedral layers, as illustrated in Fig. 3a.
Fig. 3. DFT calculations of oxygen vacancy formation.
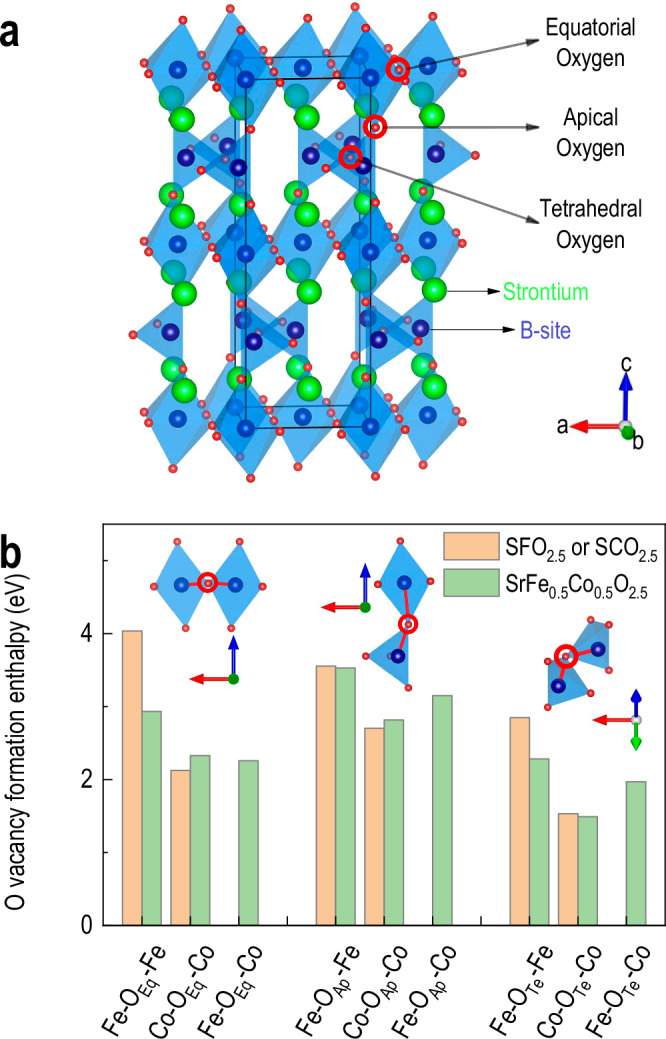
a Atomic structure of brownmillerite SFCO indicates three inequivalent oxygen sites. In the schematic, green, blue, and red spheres represent Sr, Fe/Co, and O atoms, respectively. b Computed oxygen vacancy formation enthalpies at different oxygen sites and B-site configurations.
Since the experimental results indicate a selective reduction of Co ions while Fe remains largely unchanged, we considered different cationic environments surrounding each oxygen site, including Fe−Fe, Fe−Co, and Co−Co configurations. As shown in Fig. 3b, the formation enthalpy for oxygen vacancies is consistently lower when the oxygen is coordinated by Co-containing pairs. For instance, while Fe−O−Fe configurations at equatorial sites yield vacancy formation enthalpies close to 3 eV, Co−O−Co and Co−O−Fe configurations result in significantly lower values around 2 eV. This suggests that cobalt incorporation into the local environment promotes easier vacancy formation. Notably, even in Fe−O−Fe geometries, the enthalpy decreases as more Co atoms are introduced into the simulation cell, implying that the second-nearest neighbour also affects vacancy energetics.
When comparing different geometric sites for the same Co−Fe composition (x = 0.5), the tetrahedral oxygen sites exhibit the lowest average vacancy formation enthalpy (1.97 eV), followed by equatorial sites (2.25 eV), while apical sites show the highest average value (>3 eV). These trends confirm that oxygen vacancies preferentially form at tetrahedral sites in SFCO. This pathway contrasts sharply with the infinite-layer phase formation, which requires removal of apical oxygen atoms to induce square-planar coordination and c-axis contraction. Our DFT calculations demonstrate that, in general, the removal of oxygen ions from apical sites is relatively difficult when compared to equatorial sites. Combined with the random distribution of Co and Fe ions, it is expected that there will be randomized oxygen coordination in the reduced SFCO. In addition, XRD and STEM do not show superlattice peaks or electron diffraction patterns associated with long-range vacancy ordering but indicate the expansion of the lattice along the out-of-plane direction. This suggests that the oxygen vacancies, while site-selective, are likely disordered at the macroscopic scale, resulting in a defective perovskite phase without long-range structural modulation.
Therefore, we interpret the reduced phase as an oxygen vacancy-stabilized defective perovskite with local site selectivity, rather than a vacancy-ordered phase. These findings have important implications for the reduction mechanism in SFCO. Unlike infinite-layer phases, where they are developed via removal of apical oxygen and lattice contraction, our data suggest that the reduction proceeds mainly via tetrahedral oxygen removal, leading to a structurally stable, anisotropic, and defective perovskite phase. This interpretation aligns with our experimental observations of c-axis expansion and preserved in-plane coherence during reduction (Supplementary Fig. 3). Furthermore, our results support a pathway distinct from both SrFeOy and SrCoOy, where SrFeO2.5 typically maintains its BM structure under mild reducing conditions and SrCoO2.5 decomposes into CoO-like phases9,15,40,41. In contrast, our SFCO films exhibit a cooperative but asymmetric redox behavior, selective reduction of Co accompanied by retention of the Fe valence state, leading to the emergence of a stable oxygen-deficient phase (Supplementary Fig. 6). This site-specific redox pathway has not been observed in conventional single-cation perovskites and underscores the potential for achieving programmable oxygen-vacancy configurations in multi-cation systems9,15,40,42. Selective Co reduction in SFCO, particularly at tetrahedral sites, enables a structural and electronic landscape that is chemically and functionally distinct from previously reported perovskite and infinite-layer oxides.
Suppression of inter-band transition and bandgap tuning via selective reduction
To further investigate the electronic consequences of selective cobalt reduction and oxygen vacancy formation, we examined the optical properties of SrFe1−xCoxO2.5 films using spectroscopic ellipsometry. Figure 4a shows the optical conductivity spectra of BM-SrFe1−xCoxO2.5 films with varying Fe/Co compositions. For BM-SrCoO2.5 (x = 1.0), two distinct absorption peaks appear near 1.5 eV and 2.5 eV, corresponding to d − d and p − d transitions, respectively, consistent with prior reports43,44. In contrast, BM-SrFeO2.5 (x = 0.0) displays peaks near 2.7 eV and 4.0 eV, attributed to p − d transitions involving Fe−O hybridization10,45. The SrFe1−xCoxO2.5 films with x = 0.2 and 0.5 exhibit intermediate spectral features, reflecting a combination of Fe- and Co-related optical transitions. Notably, incorporation of Co leads to suppression of the 4 eV peak and enhanced spectral weight below 3 eV.
Fig. 4. Optical properties of brownmillerite and reduced SFCO films.
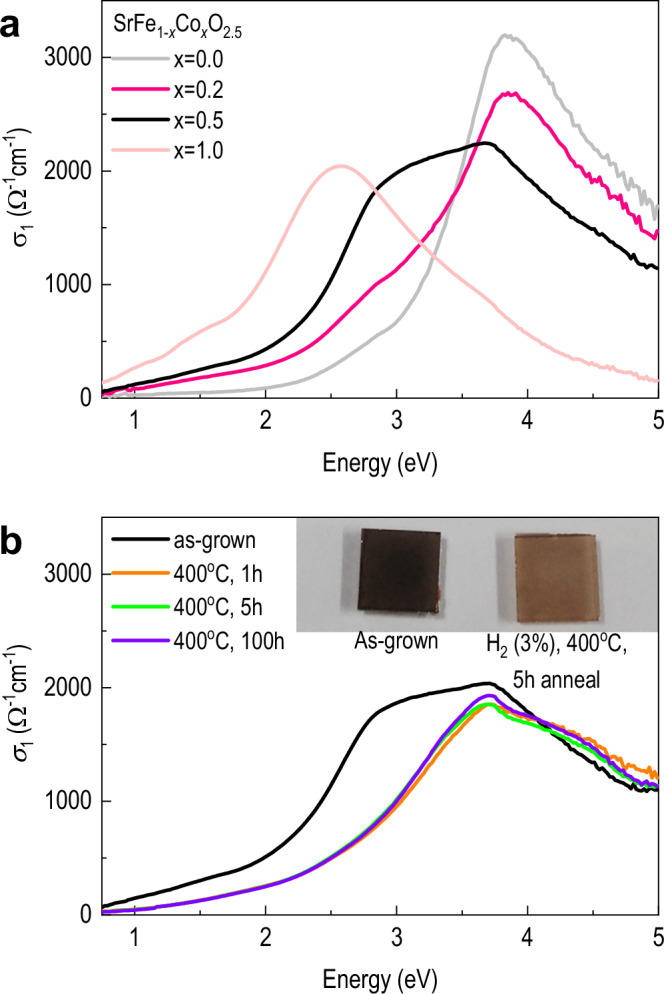
a Optical conductivity spectra of SrFe1−xCoxO2.5 films (x = 0, 0.2, 0.5, and 1.0), showing composition-dependent absorption features. b Optical conductivity spectra of SrFe0.5Co0.5O2.5 before and after reduction, highlighting spectral weight suppression and bandgap widening from 2.47 eV to 3.04 eV. Inset: photographs of as-grown (dark and opaque) and reduced (lighter and partially transparent) films on SrTiO3, visually confirming enhanced transparency upon reduction.
To track the evolution of optical properties upon reduction, we measured the optical conductivity spectra of the x = 0.5 films annealed for various durations. A significant decrease in optical conductivity between 2 eV and 3.7 eV was observed after reduction, while the high-energy peak near 4 eV remained largely unchanged (Fig. 4b). This behavior suggests that the suppressed spectral weight is primarily associated with Co−O transitions, consistent with the selective Co reduction observed by XAS. Additionally, the optical bandgap increased from 2.47 eV (as-grown SFCO) to 3.04 eV (reduced SFCO), indicative of enhanced transparency8,46. This transition is visually confirmed in the inset of Fig. 4, where reduced films appear significantly lighter and more transparent than the as-grown samples. Such tunable optical responses, achieved via chemical reduction, highlight the potential of oxygen-deficient SFCO phases for optoelectronic/electrochromic applications requiring bandgap control and visible transparency.
Reversible redox transitions and formation of multiple distinct states in SFCO thin films
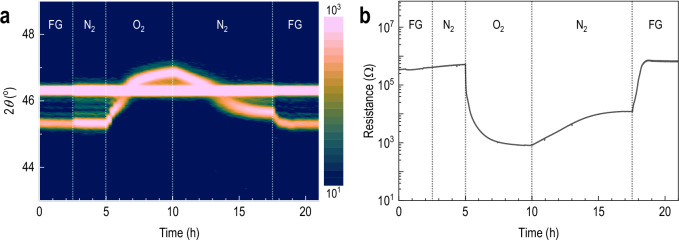
To explore the reversibility of phase transitions in SFCO, we performed simultaneous in situ experiments under controlled gas environments. These experiments revealed the presence of three structurally and electronically distinct states: the BM phase, the oxygen-deficient reduced phase, and the oxygen-rich perovskite-like phase. These states were identified through simultaneous monitoring of lattice parameters and resistance (Fig. 5) and further corroborated by the appearance or disappearance of BM superlattice reflections (Supplementary Fig. 13).
Fig. 5. In situ monitoring of phase reversibility.
a In situ x-ray diffraction and b resistance measurements during sequential 3% H2/Ar forming gas switching at 400 °C, showing structural reversibility and electronic modulation among three distinct phases.
Initially, the SFCO film was annealed at 400 °C under FG for ~2.5 hours, leading to the formation of the reduced phase. When the atmosphere was switched to high-purity N2 at the same temperature, no noticeable change in either lattice constant or electrical resistance was observed, indicating that the reduced phase is structurally and electronically stable under inert conditions. Subsequent annealing in pure O2 gas at 400 °C induced a rapid decrease in both lattice constant (Fig. 5a) and electrical resistance (Fig. 5b), suggesting re-oxidation toward an oxygen-rich SFCO phase. Notably, this transition occurred without abrupt changes in transport or diffraction signatures, indicating a continuous, diffusion-driven oxidation process. However, full oxidation to an ideal SrFe0.5Co0.5O3 phase was not achieved under these conditions, likely due to limited oxygen partial pressure. Previous studies have shown that such full oxidation requires high-pressure oxygen or ozone treatments40,41,47.
The resistance evolution mirrors the redox-induced structural changes: reduction led to charge localization and increased resistivity, while oxidation enhances carrier delocalization and reduces resistivity. This interplay reflects an intrinsic trade-off between optical transparency and electrical conductivity. While reduction enhances transparency and widens the bandgap, it simultaneously drives the material toward an insulating state. The ability to modulate both optical transparency and electrical conductivity via controlled redox tuning suggests that SFCO thin films could be promising candidates for multifunctional devices, including resistive switching memory and optoelectronic modulators48–54.
Upon switching the atmosphere to high-purity N2 at the same temperature, we observed a slight increase in the lattice constant and a corresponding increase in resistance. Expanded-range in situ XRD patterns revealed the reappearance of superlattice peaks such as 006BM and 0010BM, indicating partial reversion to the BM structure. This transformation is attributed to oxygen loss from the oxygen-rich perovskite-like state under inert conditions. To rule out the potential effect of residual oxygen during N2 annealing, we performed control experiments under high-purity N255. The absence of structural degradation or phase decomposition under N2 annealing is confirmed by XRD in Supplementary Fig. 14. As shown in Supplementary Fig. 15, no structural degradation was observed even after multiple redox cycles (up to six cycles of alternating FG and O2 annealing), confirming that the reduced phase is a structurally stable defective perovskite. The transient appearance of BM peaks upon switching from O2 to N2 (Supplementary Fig. 13) further supports an oxygen-mediated transformation pathway. After prolonged annealing in N2, we observed a saturation in both resistance and lattice contraction. These values, however, did not fully return to the initial BM levels observed in the reduced state12. Switching back to FG restored the reduced phase lattice constant and electrical resistance, confirming the repeatability of the redox transition. Additional redox cycling experiments (Supplementary Fig. 15) further demonstrated stable and reversible switching between the three phases without structural degradation, underscoring the robustness of the oxygen-deficient phase under cyclic operation. Note that it is important to clarify that while we identify three structurally distinct states, BM, reduced defective perovskite, and oxygen-rich SFCO, these classifications are based primarily on their transport and diffraction signatures.
In this study, we demonstrated the selective reduction of Co ions in epitaxial SFCO thin films while maintaining the chemical inertness of Fe ions. Through a combination of soft X-ray absorption spectroscopy, density functional theory calculations, and spectroscopic ellipsometry, we revealed that oxygen vacancies preferentially form near Co sites, leading to the formation of an oxygen-deficient defective perovskite phase. This phase exhibits a widened optical bandgap and enhanced transparency compared to the BM phase, without significant structural degradation. Comparative experiments on SrFe1−xCoxO2.5 with varying x compositions confirmed that the presence of Fe plays a crucial role in stabilizing the reduced phases. While Co-rich samples (x = 1.0) decomposed into impurity phases, Fe-rich and intermediate compositions (x = 0.0, 0.2, and 0.5) preserved structural coherence upon reduction, with x = 0.5 showing distinct defective perovskite formation. The incorporation of Fe modifies the local coordination environment, suppressing Co-induced collapse and promoting the stability of oxygen-deficient phases under reducing conditions. Our findings highlight the cooperative yet asymmetric redox behavior in multi-cation oxide systems, offering a new avenue for programmable oxygen-vacancy engineering and multifunctional oxide design. The selective control of cation-specific reduction not only enables tunable optical and electronic properties but also offers insights into the design of robust, multifunctional perovskite oxides for future device applications.
Methods
Material synthesis
Epitaxial thin films of SrFe1−xCoxO2.5, with compositions x = 0.0, 0.2, 0.5, and 1.0, were grown on single-crystalline SrTiO3 (001) substrates by pulsed laser deposition (PLD). The deposition was carried out at 600 °C under an oxygen partial pressure of 100 mTorr. A pulsed Nd:YAG laser (energy density: 0.75 J cm−2, repetition rate: 4 Hz) was used as the ablation source. All substrates were pre-treated via standard thermal annealing to ensure atomically flat step-and-terrace morphology (Supplementary Fig. 8)56. After deposition, the films were cooled to room temperature under the same oxygen partial pressure. Film thicknesses were measured using X-ray reflectivity, and structural characterization was conducted with a high-resolution X-ray diffractometer (D8 Discover, Bruker). Reciprocal space mapping confirmed that all films remained fully strained to the SrTiO3 substrate. Post-deposition annealing was performed in a tube furnace under a reducing atmosphere of FG. The system was initially purged with FG at a flow rate of 60 sccm for one hour, followed by a reduction to 20 sccm prior to temperature ramping. The samples were heated at a rate of 200 °C hr−1 to the target annealing temperature, followed by isothermal annealing for durations of up to 100 hours.
Materials characterizations
XAS measurements were carried out at the 2 A beamline of the Pohang Accelerator Laboratory using TEY mode at 300 K57. For accurate comparison, the Fe and Co L-edge spectra were normalized to the intensity of their respective L3-edges, while the O K-edge spectra were normalized to the background and the absorption edge near 565 eV. Multiplet fitting of the Fe and Co L-edge XAS spectra was performed using CTM4XAS, a ligand-field multiplet simulation code for TMOs, to estimate oxidation states from linear combinations of reference spectra. Optical conductivity spectra were acquired by spectroscopic ellipsometry in the photon energy range of 0.75–6.0 eV near the Brewster angle at room temperature58. To examine the feasibility of redox-driven structural reversibility in SrFe0.5Co0.5Ox films, in situ X-ray diffraction and resistance measurements were performed simultaneously using a dome stage (Nextron XRDMPS-CH). To evaluate the redox reversibility, in situ cycling experiments were conducted by alternately switching the gas environment between FG and O2 at 400 °C. Each gas cycle was maintained for several hours, and both structural and electrical responses were continuously monitored. These redox cycling experiments were repeated for up to six full cycles, confirming the phase reversibility and stability of the reduced and oxidized states (Fig. 5 and Supplementary Fig. 15). The dome chamber was interfaced with a high-resolution diffractometer and equipped with mass flow controllers to precisely regulate gas atmosphere and a PID controller for temperature stability. HAADF-STEM images and EDS element maps were obtained using a Cs-corrected STEM (Thermo Fisher Themis Z) operated at 300 kV. The convergence semi-angle and inner collection angle were set to 30 mrad and 50 mrad, respectively. Interatomic distances were quantified using custom python scripts. EEL spectral imaging was performed using a monochromated STEM (Thermo Fisher G2 Cube) operated at 80 kV. The convergence semi-angle was 13.5 mrad, and the full width at half maximum of the zero-loss peak was ~250 meV during spectral imaging.
Theoretical analysis
All DFT calculations of SrBOx (B = Co, Fe) were carried out using the plane-wave-based Vienna Ab initio Simulation Package VASP59,60 version 5.4.4, within the generalized gradient approximation (GGA) using the Perdew−Burke−Ernzerhof for solids (PBEsol) exchange–correlation functional61. The energy cutoff for the plane-wave basis set was 600 eV, employing projected augmented wave potentials62,63. A 6 × 6 × 2 k-point mesh was utilized for sampling the Brillouin zone for a 36-atom unit cell and scaled linearly with the supercell size. We used a 2 × 2 × 1 supercell for all calculations to accommodate Fe-Co solid solution on B-site and decrease periodic effects. The bulk geometry was optimised with a force convergence criterion of 1 meV/Å, and the individual components of the stress tensor were converged to ≤ 0.1 kB. Magnetism of B-site cations, Co, and Fe was treated with the PBEsol collinear spin density approximation in the GGA with an onsite Hubbard U (GGA + U) scheme64. An onsite Coulomb parameter U = 4 eV was applied for all B-site cations (Co and Fe) to account for the increased Coulomb repulsion between the semi-filled 3 d states65. The magnetic moments of B-site cations were initiated in the G–type antiferromagnetic state spins antiferromagnetically aligned within (001) planes between adjacent (001) planes66.
Supplementary information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2025-00558200). Also, this work was partially supported under the framework of the international cooperation program managed by the NRF (NRF-2022K2A9A1A01098180). First-principles calculations (V.R.C. and K.P.) were supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Science and Engineering Division. We acknowledge computational resources provided by the National Energy Research Scientific Computing Centre (NERSC), which is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231 using NERSC award BES-ERCAP-m1057. H.O. is supported by Grants-in-Aid for Scientific Research A (22H00253) from the Japan Society for the Promotion of Science (JSPS). Part of this work was supported by the Crossover Alliance to Create the Future with People, Intelligence and Materials, and by the Network Joint Research Centre for Materials and Devices. H.J. acknowledges the support of the Korea Basic Science Institute (National Research Facilities and Equipment Centre) grant funded by the Ministry of Education (grant No. RS-2024-00435344). S.P. and S.Y. acknowledge support from the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (RS-2024-00460372). We thank Nextron Corporation for the generous technical support.
Author contributions
J.L. synthesized the samples and conducted in situ XRD experiments with H.C., S.R., and J.C. Optical measurements and analysis were performed by J.L. and Y.S. XAS experiments and their interpretation were carried out by J.L. and Y.K. K.C.P. and V.R.C. performed first-principles calculations. In-plane XRD patterns were measured by G.K. and H.O. S.P. and S.Y. conducted STEM, EELS, and EDS experiments and analyses. H.J. initiated and supervised the project. All authors contributed to the discussion of results and writing of the manuscript.
Peer review
Peer review information
Nature Communications thanks Chonglin Chen, Woo Seok Choi, Felix Gunkel, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the main figures generated in this study are available at Figshare: 10.6084/m9.figshare.28944977.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62612-1.
References
- 1.Myung, J. H., Neagu, D., Miller, D. N. & Irvine, J. T. Switching on electrocatalytic activity in solid oxide cells. Nature537, 528–531 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Wan, L. F., Incorvati, J. T., Poeppelmeier, K. R. & Prendergast, D. Building a fast lane for Mg diffusion in α-MoO3 by fluorine doping. Chem. Mater.28, 6900–6908 (2016). [Google Scholar]
- 3.Acharya, S. K. et al. Epitaxial brownmillerite oxide thin films for reliable switching memory. ACS Appl Mater. Interfaces8, 7902–7911 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Yang, Q. et al. Solid-state electrochemical thermal transistors. Adv. Funct. Mater.33, 2214939 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Li, D. et al. Superconductivity in an infinite-layer nickelate. Nature572, 624–627 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Goodenough, J. B. Electronic and ionic transport properties and other physical aspects of perovskites. Rep. Prog. Phys.67, 1915–1993 (2004). [Google Scholar]
- 7.Bertaut, E. F., Blum, P. & Sagnieres, A. Structure du ferrite bicalcique et de la brownmillerite. Acta Cryst.12, 149–159 (1959). [Google Scholar]
- 8.Jeen, H. et al. Reversible redox reactions in an epitaxially stabilized SrCoOx oxygen sponge. Nat. Mater.12, 1057–1063 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Tsujimoto, Y. et al. Infinite-layer iron oxide with a square-planar coordination. Nature450, 1062–1065 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Khare, A. et al. Topotactic metal-insulator transition in epitaxial SrFeOx thin films. Adv. Mater.29, 1606566 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Tikhonovich, V., Naumovich, E., Logvinovich, D., Kharton, V. & Vecher, A. Oxygen deficiency and phase transitions in SrCo1–x–yFexCryO3–δ (x= 0.10–0.40, y= 0–0.05). J. Solid State Electrochem.7, 77–82 (2003). [Google Scholar]
- 12.Lee, J. et al. Redox-driven nanoscale topotactic transformations in epitaxial SrFe0.8Co0.2O3-x under atmospheric pressure. Phys. Rev. Appl10, 054035 (2018). [Google Scholar]
- 13.Kim, W. J. et al. Geometric frustration of Jahn–Teller order in the infinite-layer lattice. Nature615, 237–243 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Li, H.-B. et al. Dehydration of electrochemically protonated oxide: SrCoO2 with square spin tubes. J. Am. Chem. Soc.143, 17517–17525 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Hayward, M. A. & Rosseinsky, M. J. Materials chemistry: cool conditions for mobile ions. Nature450, 960 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Bian, Z. et al. Solid-state electrochemical thermal transistors with strontium cobaltite–strontium ferrite solid solutions as the active layers. ACS Appl. Mater. Interfaces15, 23512–23517 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Khare, A. et al. Directing oxygen vacancy channels in SrFeO2.5 epitaxial thin films. ACS Appl Mater. Interfaces10, 4831 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Li, Q. et al. Structural stability of Lanthanum-based oxygen-deficient perovskites in redox catalysis: a density functional theory study. Catal. Today347, 142–149 (2020). [Google Scholar]
- 19.He, S. et al. La0.6Sr0.4CoO3-δ films under deoxygenation: Magnetic and electronic transitions are apart from the structural phase transition. Adv. Funct. Mater.34, 2313208 (2024). [Google Scholar]
- 20.Jeen, H. et al. Topotactic phase transformation of the brownmillerite SrCoO2.5 to the perovskite SrCoO3–δ. Adv. Mater.25, 3651 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa, N. et al. Reduction and oxidation of SrCoO2.5 thin films at low temperatures. Dalton Trans.41, 10507–10510 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Jin, L. et al. Understanding structural incorporation of oxygen vacancies in perovskite cobaltite films and potential consequences for electrocatalysis. Chem. Mater.34, 10373–10381 (2022). [Google Scholar]
- 23.Abbate, M. et al. Controlled-valence properties of La1−xSrxFeO3 and La1−xSrxMnO3 studied by soft-x-ray absorption spectroscopy. Phys. Rev. B46, 4511–4519 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Hu, Z. et al. Difference in spin state and covalence between La1-xSrxCoO3 and La2-xSrxLi0.5Co0.5O4. J. Alloy Compd.343, 5–13 (2002). [Google Scholar]
- 25.Stavitski, E. & De Groot, F. M. The CTM4XAS program for EELS and XAS spectral shape analysis of transition metal L edges. Micron41, 687–694 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Hirai, K. et al. Strain-induced significant increase in metal-insulator transition temperature in oxygen-deficient Fe oxide epitaxial thin films. Sci. Rep.5, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tassel, C. & Kageyama, H. Square planar coordinate iron oxides. Chem. Soc. Rev.41, 2025–2035 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury, S. et al. High-temperature insulating ferromagnetic state in charge-disproportionated and spin-state-disproportionated strained SrCoO2. 5 thin film. APL Mater.12, 051129 (2024). [Google Scholar]
- 29.Vieten, J. et al. Redox behavior of solid solutions in the SrFe1-xCuxO3-δ system for application in thermochemical oxygen storage and air separation. Energy Technol.7, 131–139 (2019). [Google Scholar]
- 30.Fujishiro, F., Oshima, N., Sakuragi, T. & Oishi, M. Oxygen desorption properties of perovskite-type SrFe1−xCoxO3−δ: B-site mixing effect on the reduction properties of Fe and Co ions. J. Solid State Chem.312, 123254 (2022). [Google Scholar]
- 31.Karvonen, L. et al. and Co-L XANES study on oxygen intercalation in perovskite SrCoO3-δ. Chem. Mater.22, 70–76 (2010). [Google Scholar]
- 32.Che, Q. et al. Operando soft X-ray absorption of LaMn1–xCoxO3 perovskites for CO oxidation. ACS Catal.14, 11243–11251 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem.3, 546–550 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Sayle, T. X. T., Parker, S. C. & Catlow, C. R. A. The role of oxygen vacancies on ceria surfaces in the oxidation of carbon monoxide. Surf. Sci.316, 329 (1994). [Google Scholar]
- 35.Lu, G., Linsebigler, A. & Yates, J. T. Jr Photooxidation of CH3Cl on TiO2 (110): a mechanism not involving H2O. J. Phys. Chem.99, 7626–7631 (1995). [Google Scholar]
- 36.Henderson, M. A., Epling, W. S., Perkins, C. L., Peden, C. H. F. & Diebold, U. Interaction of molecular oxygen with the vacuum-annealed TiO2(110) surface: molecular and dissociative channels. J. Phys. Chem. B103, 5328 (1999). [Google Scholar]
- 37.Campbell, C. T. & Peden, C. H. Oxygen vacancies and catalysis on ceria surfaces. Science309, 713–714 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Yang, C. W. et al. Chemical activity of oxygen vacancies on ceria: a combined experimental and theoretical study on CeO2(111). Phys. Chem. Chem. Phys.16, 24165–24168 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Schaub, R. et al. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110). Phys. Rev. Lett.87, 266104 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Takeda, Y. et al. Phase relation and oxygen-non-stoichiometry of perovskite-like compound SrCoOx (2.29 <x <2.80). Z. Anorg. Allg. Chem.540, 259 (1986). [Google Scholar]
- 41.Long, Y. W., Kaneko, Y., Ishiwata, S., Taguchi, Y. & Tokura, Y. Synthesis of cubic SrCoO3 single crystal and its anisotropic magnetic and transport properties. J. Phys. Condes. Matter23, 245601 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Radaelli, P. G. & Cheong, S.-W. Structural phenomena associated with the spin-state transition in LaCoO3. Phys. Rev. B66, 094408 (2002). [Google Scholar]
- 43.Choi, W. S. et al. Reversal of the lattice structure in SrCoOx epitaxial thin films studied by real-time optical spectroscopy and first-principles calculations. Phys. Rev. Lett.111, 097401 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Yang, Q. et al. Unusually large thermopower change from +330 to −185 μV K−1 of brownmillerite SrCoO2.5. ACS Appl Electron Mater.2, 2250–2256 (2020). [Google Scholar]
- 45.Lee, J. H. et al. Strongly coupled magnetic and electronic transitions in multivalent strontium cobaltites. Sci. Rep.7, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posadas, A. B., Lin, C., Demkov, A. A. & Zollner, S. Bandgap engineering in perovskite oxides: Al-doped SrTiO3. Appl Phys. Lett.103, 142906 (2013). [Google Scholar]
- 47.Takeda, Y. et al. Phase relation in the oxygen nonstoichiometric system, SrFeOx (2.5 ≤ x ≤ 3.0). J. Solid State Chem.63, 237 (1986). [Google Scholar]
- 48.Jia, T. et al. The influence of oxygen vacancy on the electronic and optical properties of ABO3−δ (A= La, Sr, B= Fe, Co) perovskites. Phys. Chem. Chem. Phys.21, 20454–20462 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Fazl-Ur-Rahman, K. & Periyasamy, G. Role of oxygen vacancy ordering on structure and reactivity of iron-doped Sr-based perovskites: a computational study. J. Solid State Chem.317, 123734 (2023). [Google Scholar]
- 50.Darwish, M. & Pohl, L. Insulator metal transition-based selector in crossbar memory arrays. Electron Mater.5, 17–29 (2024). [Google Scholar]
- 51.Hu, G. et al. Cu/MgO-based resistive random access memory for neuromorphic applications. Appl. Phys. Lett.124, 142109 (2024). [Google Scholar]
- 52.Steele, B. C. & Heinzel, A. Materials for fuel-cell technologies. Nature414, 345–352 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Ohta, H. et al. Giant thermoelectric Seebeck coefficient of a two-dimensional electron gas in SrTiO3. Nat. Mater.6, 129–134 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Choi, H. et al. Organometal halide perovskite-based photoelectrochemical module systems for scalable unassisted solar water splitting. Adv. Sci.10, 2303106 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu, N. P. et al. Electric-field control of tri-state phase transformation with a selective dual-ion switch. Nature546, 124–128 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Kareev, M. et al. Atomic control and characterization of surface defect states of TiO2 terminated SrTiO3 single crystals. Appl Phys. Lett.93, 061909 (2008). [Google Scholar]
- 57.Abbate, M. et al. Probing depth of soft x-ray absorption spectroscopy measured in total-electron-yield mode. Surf. Interface Anal.18, 65–69 (1992). [Google Scholar]
- 58.Aspnes, D. E. Spectroscopic ellipsometry - past, present, and future. Thin Solid Films571, 334–344 (2014). [Google Scholar]
- 59.Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater. Sci.6, 15–50 (1996). [DOI] [PubMed] [Google Scholar]
- 60.Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 61.Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett.100, 039902 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Blochl, P. E. Projector augmented-wave method. Phys. Rev. B50, 17953 (1994). [DOI] [PubMed] [Google Scholar]
- 63.Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B59, 1758–1775 (1999). [Google Scholar]
- 64.Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B57, 1505–1509 (1998). [Google Scholar]
- 65.Nakayama, K. et al. Transition-metal distribution in brownmillerite Ca2FeCoO5. Inorg. Chem.58, 10209–10216 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Battle, P. D., Gibb, T. C. & Lightfoot, P. The crystal and magnetic-structures of Sr2CoFeO5. J. Solid State Chem.76, 334–339 (1988). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the main figures generated in this study are available at Figshare: 10.6084/m9.figshare.28944977.