Abstract
Breast cancer (BC) is the most prevalent malignancy in women. The emergence of targeted therapies and advancements in comprehensive treatment protocols have significantly improved survival outcomes for patients with early-stage BC. However, individuals with refractory BC, particularly those who have received multiple lines of therapy or presented with distant metastases at diagnosis, continue to face challenges due to the limitations of conventional antibodies and cytotoxic agents in meeting therapeutic needs. Thus, the development of novel and effective treatments for BC, along with strategies to prevent recurrence, remains an urgent priority. Research has shown that nanodrug delivery systems can modify the pharmacokinetic profiles of traditional chemotherapeutic agents, thereby markedly reducing adverse drug reactions. Furthermore, studies have demonstrated that innovative approaches, such as hyaluronic acid-based systems, ultrasound-mediated microbubbles, and antibody-drug conjugates (ADCs), enable targeted and controlled drug release, offering advantages including high drug efficacy, reduced toxicity, and significant antitumor effects. Among these, ADCs have gained increasing attention in BC therapy over recent years. This article provides a comprehensive review of the development and progress of various drug delivery systems in BC treatment, offering an in-depth analysis of their potential applications in clinical practice.
Keywords: Breast cancer, nanomaterials, antibody-drug coupling compounds, drug delivery carriers
Plain Language Summary
Different delivery systems in breast tumors therapy
Breast cancer (BC) represents the most prevalent form of cancer affecting women. The advent of targeted therapies and the enhancement of comprehensive treatment protocols for BC have significantly improved survival outcomes for individuals with early-stage BC. Nevertheless, patients with refractory BC, particularly those who have undergone multiple lines of treatment or initially presented with distant metastases, face challenges as conventional antibodies or cytotoxic drugs fail to meet therapeutic needs. Therefore, the development of novel and effective medications for BC treatment and the prevention of recurrence is an urgent priority. Research indicates that nanodrug delivery systems modify the pharmacokinetic properties of traditional chemotherapeutics, thereby substantially mitigating adverse drug reactions. Additional studies have demonstrated that innovative approaches, such as hyaluronic acid, ultrasound microbubbles, and antibody-drug conjugates (ADCs), enable targeted controlled drug release, offering benefits such as high drug release rates, reduced adverse reactions, and significant antitumor effects. Among these, ADCs have gained increasing prominence in BC therapy in recent years. Now this article presents a comprehensive examination of the present developments and advancements of these different drug delivery carriers in BC therapy.
Introduction
Breast cancer (BC) is not only the most prevalent malignancy in women but also a leading cause of cancer-related mortality. Breast cancer is classified into 4 subtypes based on the expression of estrogen receptor (ER), progesterone receptor (PR), HER-2, and Ki-67: Luminal A (LA), Luminal B (LB), HER-2-overexpressing, and triple-negative breast cancer (TNBC). Standard treatment modalities include endocrine therapy for HR+/ER+ patients, HER-2-targeted therapy for HER2+ patients, chemotherapy and immunotherapy for TNBC patients, and PARP inhibitor therapy for BRCA-mutated TNBC patients. Triple-negative breast cancer is characterized by rapid recurrence, a high incidence of brain metastasis, and a 5-year survival rate below 80%, often exhibiting resistance to broad-spectrum anticancer agents.1,2 Encouragingly, substantial progress has been achieved in the management of localized BC, with a 5-year survival rate approaching 90%. However, despite the use of chemotherapy, radiotherapy, or estrogen blockers, effective treatment strategies for advanced metastatic BC remain limited.3,4 In view of the rising prevalence of long-term diseases and the accelerating process of population aging, there is an urgent need to develop more efficient and less toxic therapies. Consequently, novel drug delivery systems have attracted significant attention and are undergoing vigorous development.
In this context, the research and development of novel drug delivery systems have gained significant attention, with the aim of enhancing drug bioavailability, reducing adverse effects, and achieving precise tumor targeting. 5 Specifically, these systems include various forms such as nanoparticles, hyaluronic acid (HA), ultrasound (US) microbubbles, and antibody-drug conjugates (ADCs), which can protect drugs from degradation in vivo, prolong their circulation time, and deliver them directly to tumor tissues via specific targeting mechanisms, thereby improving therapeutic efficacy while minimizing damage to normal tissues.6 -8 Moreover, novel drug delivery systems can enable intelligent drug release in response to changes in the tumor microenvironment, allowing dynamic adjustment of drug concentrations and further optimizing treatment outcomes. These systems demonstrate promising applications in BC therapy, with the potential to improve patient prognosis. 9 This article systematically reviews the advantages, disadvantages, prospects, and future developments of 4 types of drug delivery systems—nanoparticles, HA, US microbubbles, and ADCs—providing a reference for comprehensive BC treatment strategies (Figure 1).
Figure 1.
Application of various drug delivery systems in breast tumors.
It shows how different formulations (nanometer materials + anticancer drugs, hyaluronic + anticancer drugs, AcidUltrasound Microbubble + Anticancer Drugs, Antibody-drug conjugates) are involved in systemic drug delivery. These delivery systemics aim to achieve targeted therapy by interacting with specific markers and mechanisms in breast cancer.
Nano-Drug Delivery Systems
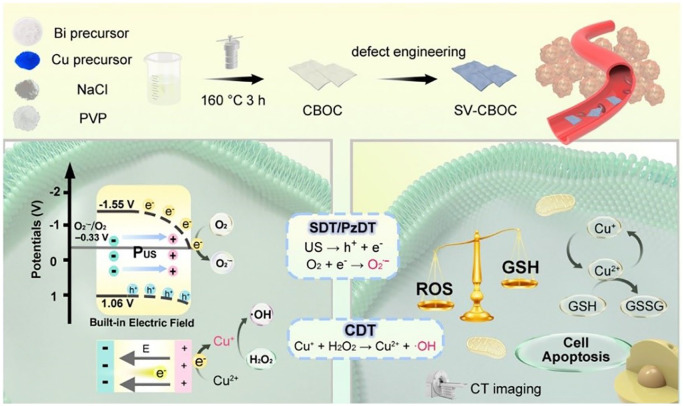
The mechanisms of nanomaterials in targeted therapy can be classified into passive targeting, active targeting, and physicochemical targeting. The advantages of applying nanotechnology in the medical field include reversing drug resistance in tumor chemotherapy, reducing dosing frequency while prolonging drug residence time to achieve sustained-release and controlled-release effects, and enabling surface modification of nanomaterials for the development of multifunctional drug delivery systems, thereby enhancing therapeutic efficacy and minimizing adverse effects. In addition, nanomaterials with unique optical or magnetic properties can be utilized in minimally invasive tumor treatments.10,11 Jiang et al 12 developed a multifunctional nanotherapeutic platform (PEG-PEI-AuAg@HCT, PPAPH) specifically targeting HER2-positive BC. This platform employs gold-silver hollow nanoshells (AuAg HNS) as carriers to achieve synergistic chemotherapy and photothermal therapy. With dual-stimuli-responsive drug release characteristics, it exhibits superior photothermal conversion efficiency under 808 nm laser irradiation, effectively inhibiting tumor growth and reducing tumor size while exerting minimal effects on healthy tissues. The Yang Piaoping team 13 designed Cu-doped BiOCl nanosheets with surface oxygen vacancies (SV-CBOC NSs) for synergistic anticancer therapy (Figure 2). The seamless integration of photodynamic therapy (PDT), sonodynamic therapy (SDT), and chemodynamic therapy (CDT) enhances the cytotoxic effect on tumor cells, offering a novel approach and reference for US-mediated multimodal tumor treatment.
Figure 2.
Schematic diagram of the preparation of SV-CBOC NSs and the proposed synergistic therapeutic mechanism.
Source: Reproduced with permission. 13
SV-CBOC NSs: 2D Cu-doped BOC nanosheets with surface oxygen vacancies.
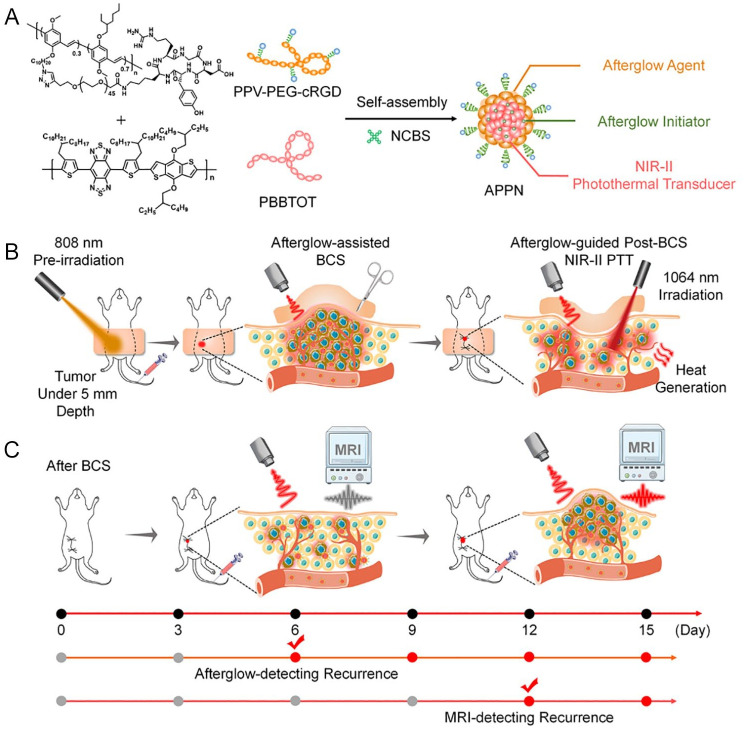
Nanomaterials for targeted BC therapy primarily encompass organic and inorganic nanomaterials. Organic nanomaterials include solid liposomes, liposomes, and polymers.14 -18 Among these, cyclodextrins, cyclic macromolecules approved by the Food and Drug Administration (FDA), have been demonstrated to function as molecular valves that regulate conformational changes in supramolecular systems, thereby facilitating the controlled release of therapeutic payloads. 19 Kaduri et al 20 developed a novel therapeutic strategy targeting tumors via nerve cells. This approach involves the intravenous injection of lipid nanoparticles loaded with the anesthetic bupivacaine. These nanoparticles circulate through the bloodstream to the tumor site, accumulate around nerve cells in BC tissue, and induce paralysis of local nerves as well as disrupt communication between nerve cells and cancer cells. Consequently, this significantly suppresses breast tumor formation and metastasis to the lungs, brain, and bone marrow. Ren et al 21 prepared a liposomal nanomedicine co-delivering doxycycline hydrochloride (Doxy), an antibiotic, and chlorin e6 (Ce6), a photosensitizer. By integrating Ce6-mediated PDT with Doxy-induced autophagy suppression and mitochondrial dysfunction, they achieved efficient photodynamic/immunotherapy for “cold tumor” BC. Zhen et al 22 reported an afterglow/photothermal dual-functional conjugated polymeric nanomaterial (Figure 3), composed of an amphiphilic long afterglow conjugated polymer containing tumor-targeting groups (PPV-PEG-cRGD), a long afterglow initiator (NCBS), and a near-infrared II (NIR-II) photothermal conjugated polymer with high photothermal conversion efficiency (PBBTOT). The afterglow imaging capability of this nanomaterial enables precise localization of minute residual tumor foci following breast-conserving surgery in mice. Subsequently, the NIR-II photothermal imaging function facilitates the precise ablation of localized residual tumor foci, minimizing ionizing radiation side effects while effectively inhibiting the in situ recurrence of BC.
Figure 3.
Schematic diagram of APPN for postoperative imaging-guided adjuvant therapy and early recurrence diagnosis and treatment after BCS. APPN, afterglow/photothermal polymeric nanoparticles; BCS, breast-conserving surgery. A. APPN is composed of an amphiphilic long-persistent luminescence conjugated polymer (PPV-PEG-cRGD) containing tumor-targeting groups, a long-persistent luminescence initiator (NCBS), and a near-infrared material with high photothermal conversion efficienciency. B. The afterglow imaging function of APPN enables precise localization of small residual tumor foci in mice after surgery, reducing the side effects of ionizing radiation while effectively inhibiting the recurrence of breast cancer in situ. C. APPN’s long-lasting luminescence imaging function can sensitively detect recurrent tumor cells that have not yet formed microscopic solid tumors in vivo, with a detection sensitivity superior to that of traditional magnetic resonance imaging.
Source: Reproduced with permission. 22
Inorganic nanomaterials encompass quantum dots (QDs), 23 silica-based nanomaterials (MSNs), 24 metallic nanomaterials, 25 and carbon-based nanomaterials. 26 Liang et al 27 reported a mitochondrial oxidative stress amplifier, MitoCAT-g, consisting of atomically dispersed gold particles (CAT-g) supported on carbon dots and further surface-modified with triphenylphosphine and cinnamaldehyde. Their study demonstrated that MitoCAT-g particles specifically target mitochondria, consuming mitochondrial glutathione in an atom-economical manner to amplify reactive oxygen species (ROS) damage induced by cinnamaldehyde, ultimately leading to cancer cell apoptosis [Figure 4].
Figure 4.
Fabrication of HM-NPs@G. Schematic of MitoCAT-g nanoparticle structure (A) and its mechanism: targeting mitochondria, reacting with GSH via AU-S bonds to deplete GSH, trigger ROS production, and induce apoptosis (B). HM-NPs@G: hybrid membrane-camouflaged reactive oxygen species (ROS)-responsive nanoparticles loaded with Gboxin. 27
Source: Reproduced with permission. 27
Shi et al 28 developed cancer cell-mitochondrial hybrid membrane-camouflaged, ROS-responsive nanoparticles loaded with Gboxin, enabling noninvasive targeted delivery of Gboxin to glioblastoma multiforme (GBM) mitochondria. The hybrid membrane-encapsulated biomimetic nanoparticles significantly prolonged the circulation half-life by evading immune clearance, thereby overcoming the limitations of Gboxin therapy. This innovative nanoplatform can effectively penetrate the blood-brain barrier and selectively deliver Gboxin to GBM mitochondrial targets, thus inhibiting GBM tumor cell proliferation (Table 1).
Table 1.
Nano drug delivery system in breast tumor.
| Therapeutic agents | Nanocarrier system | Effect | Reference |
|---|---|---|---|
| Dual tyrosine kinase inhibitor ZD6474 | ZD6474-AuNP | Inhibit tumor cell proliferation, migration, invasion and induce apoptosis; tumor volume reduction | Sarkar et al 29 |
| 5-Fu | 5-Fu-AuNP | Increase the expression of mitogen-activated protein kinase phosphatase 1 and histone H3, reduce the expression of thymidylate synthase; increase the sensitivity of tumor cells to 5-Fu, induce tumor cell death. | Liszbinski et al 30 |
| DOX | DOX-LDGI-NP | Enhance the penetration ability of drugs in the tumor area, promote Tumor cell apoptosis; synergistic therapy is better than single drug therapy. | Xu et al 31 |
| Cisplatin, docetaxel | NACLAT1-AuNP | Photothermal ablation effect kills tumor cells; reduces recurrence and metastasis. | Alhussan et al 32 |
| miR708 | miR708-AuNP | Target miR708 low-expression tumor cell clones, inhibit tumor cell metastasis; reduce lung metastasis of TNBC. | Ramchandani et al 33 |
| miR34a | miR34a-LBL-AuNP | Inhibit the expression of tumor proliferation genes Sirt1 and Bcl-2, effectively inhibit tumor cell proliferation; tumor cell proliferation decreased by 33%. | Goyal et al 34 |
| Pd[DMBil1]-PEG750 | Photosensitizer-Silica Core—NP | Generates potent reactive oxygen species, oxidatively damages tumor cells; noninvasively induces TNBC cell death. | Riley et al 35 |
| Mitoxantrone Hydrochloride | Liposome | Capable of slowing the growth of advanced recurrent or metastatic breast cancer. | Wang et al 36 |
| PTX | Liposome | DLTs of paclitaxel liposome injection were evaluated. | Koudelka and Turánek 37 |
| ThermoDox in combination with Microwave Hyperthermia | Lyso-thermosensitive liposomal DOX | Bioequivalence of ThermoDox and improved efficacy (3.7 times) in combination with Hyperthermia. | Fraguas-Sánchez et al 38 |
| Veldoreotid | CdS/ZnS core-shell type quantum Dots | Anticancer activity was confirmed by SRB assay and scintigraphy. | Jin et al 39 |
| Fluorescent cRGDY-PEG-Cy5.5 C dots | Silica NPs | Explored as an imaging tool to detect cancer before surgery. Feasibility of conducting preoperative sentinel lymph node mapping | Chopra 40 |
| PTX & Phenelzine Sulfate | Albumin-bound NPs | Dose-limiting toxicities were observed in patients. | Jain et al 41 |
| PTX & carboplatin | Albumin-bound NPs | The effective cytotoxic drug in neoadjuvant treatment. Significantly improved progression-free survival, and overall survival. | Ghosh et al 42 |
| PTX carboplatin, trastuzumab/bevacizumab | Albumin-bound NPs | Clinical complete response in the neoadjuvant setting, Toxicity of the combinations in HER2 positive and HER2 negative breast cancer assessed. | Omidi et al 43 |
| Pembrolizumab (Pbr)/PTX | Albumin-bound NPs | Pembrolizumab targets immune cells within the tumor which improved therapeutic response. | Shokooh et al 44 |
| PTX, Gemcitabine & Bevacizumab PTX, & Ixabepilone | Albumin-stabilized NPs | 6-month progression-free survival (PFS) Rate, PFS increased 20% up to 5 years. | Jain et al 41 |
| Carboplatin, PTX, & Bevacizumab | Albumin-stabilized NPs | Overall survival was improved from the start of treatment to 80 months. | Jain et al 41 |
| Carboplatin & PTX | Albumin-stabilized NPs | Fewer patients experienced serious adverse events. | Oneda et al 45 |
| PTX, Epirubicin & Cyclophosphamide | Albumin-bound NPs | Adverse reactions are reduced and toxicity is reduced. | Shimada et al 46 |
| PTX&Cyclophosphamide | Albumin-bound NPs | Disease-free survival (18 months) improved. | Sabatelle et al 47 |
| Resveratrol (RES) | PLGA NP | Vercome DOX resistance therapy, Depict potential in the treatment of DOX-resistant BC. | Zhao et al 48 |
| RES | AuNPs | Innovative green nanotechnology approach using RES and gum arabic,3 fold higher cell cytotoxicity of RES coated NPs compared to RES. | Thipe et al 49 |
| RES | SLNs | RES loaded SLNs for effective BC treatment, RES loaded SLNs exhibited a significant inhibitory effect on BC proliferation by the induced apoptotic mechanism. | Wang et al 50 |
| Morin | AuNPs | To increase bioavailability and cytotoxicity,MCF-7 cells undergo apoptosis, cell necrosis, and cell cycle arrest at the G2/M phase. | Kondath et al 51 |
| Baicalin | AuNPs | A functionalized AuNPs for delivery of flavonoid derivative to evaluate anticancer activity, Cyclo-dextrin conjugated AuNPs showed antiproliferation, cytotoxic, and apoptotic activity against MCF-7 cells. | Lee et al 52 |
| Baicalin | Magnetic NPs | Controlled synthesis of PEG-coated iron oxide NPs for delivery of baicalein against TNBC, Demonstrated cell cycle arrest at various phases, apoptotic cell death, DNA damage confirmed by TUNEL assay. | Kavithaa et al 53 |
| Fisetin | α-Tocopherol-poly (lactic acid) based | Polymeric micelles for enhancing the anticancer efficacy, Enhanced, Dose-dependent cytotoxicity of Fisetin loaded nanocarriers in-vitro, reduced tumor burden, higher cell apoptosis in MCF-7 bearing xenograft mice model. | Wang et al 54 |
| Fisetin | PLA NPs | Increase solubility and therapeutic efficacy, Reduction in the dose and systemic adverse events. | Feng et al 55 |
| Luteolin | Superparamagnetic iron oxide NPs | Determine the cytotoxic efficacy of luteolin and FA conjugated Superparamagnetic iron oxide NPs, Depicted higher cytotoxic, apoptotic, and necrotic effects. Folic acid conjunction with NPs facilitated lutein release in acidic conditions. | Alpsoy et al 56 |
| Tamoxifen and Quercet | PLGA NPs | Improve pharmacokinetic parameters and efficacy, Higher cellular uptake, and cytotoxicity, controlled angiogenesis, no measurable hepatotoxicity, and oxidative stress. Improved pharmacokinetics. | Jain et al 57 |
| Quercetin | MSNs | pH-responsive MSNs- for dual drug targeting of quercetin and topotecan, Enhanced intracellular uptake, enhanced apoptosis induction, and improved tumor growth inhibition. | Zang et al 58 |
| Quercetin | PLA NPs | Biodegradable polymeric nanoparticles for the delivery of quercetin, improved cytotoxicity. | Kumari et al 59 |
| Lycopene | Whey protein isolate NPs | Improved bioavailability, and anticancer activity by fabricated protein NPs, NPs depicted higher tumor growth inhibition with improved bioavailability in Wistar rats. | Jain et al 60 |
| Lycopene | NLCs | Improved oral absorption, solubility, and cytotoxicity of lycopene, Drug loaded NLCs depicted enhanced cytotoxicity, oral bioavailability, and better absorption. | Jain et al 61 |
Abbreviations: DOX, doxorubicin; NLCs, nanostructured lipid carriers; NP, nanoparticle; PLA, polylactic acid; PTX, paclitaxel; RES, resveratrol.
Nanodelivery systems not only demonstrate remarkable potential in BC vaccine development but also leverage unique physical and chemical properties. These properties include enhancing drug bioavailability, improving targeting specificity, minimizing side effects, and protecting drugs from degradation within the body. 62 In BC vaccine research, nanodelivery systems can augment immune responses via multiple mechanisms, such as promoting antigen uptake by antigen-presenting cells, stabilizing antigens and prolonging their retention in the body, as well as activating specific immune signaling pathways. 63 Nanodelivery systems can enhance vaccine targeting through both passive and active strategies. Passive targeting primarily depends on nanoparticle size and surface modification to facilitate accumulation in tumor tissues. Active targeting involves modifying nanoparticles with specific ligands, such as antibodies or peptides, to recognize and bind to receptors on tumor cell surfaces. Notably, nanodelivery systems can shield vaccine antigens from enzymatic and other forms of degradation, extending their circulation time, which is critical for improving vaccine stability and efficacy. 64 Furthermore, nanoparticles can function as adjuvants, enhancing immune responses by activating specific receptors on immune cells, such as Toll-like receptors (TLRs). 65
Of course, during the circulation of BC nanomedicines in the body, they inevitably interact with the immune system. These interactions significantly influence the efficacy and safety of nanomedicines. For example, BC nanomedicines can activate macrophages, promoting their polarization toward the antitumor M1 phenotype, thereby enhancing their antitumor activity. 66 In addition, macrophages can phagocytose nanomedicines, affecting their distribution and metabolism in the body. Furthermore, BC nanomedicines can modulate the maturation and function of dendritic cells (DCs), promoting their capture and presentation of tumor antigens and thus activating T-cell-mediated immune responses. Therefore, to improve the efficacy and safety of BC nanomedicines, it is essential to rationally regulate their interactions with the immune system. 67 By modifying the surface of nanomedicines with highly biocompatible polymers, antibodies, peptides, and so on, nonspecific interactions with immune cells can be minimized, thereby improving their targeting and biocompatibility. Combining BC nanomedicines with immunotherapeutic agents (such as immune checkpoint inhibitors, cytokines, etc.) can further enhance immune responses and improve therapeutic efficacy.68,69 The interaction between BC nanomedicines and the immune system is a complex process involving multiple immune cells and signaling pathways. In-depth research into these interactions and their underlying mechanisms can aid in the development of safer and more effective BC nanomedicines, offering new treatment options for BC patients.
Hyaluronic acid Delivery Carriers
Hyaluronic acid is a naturally occurring linear polysaccharide with excellent biocompatibility and biodegradability. It targets tumors via the cell surface receptor cluster of differentiation 44 (CD44).70,71 CD44, a receptor exhibiting high affinity for HA, serves as a tumor marker or targeted receptor due to its overexpression and specificity in tumor cells. When conjugated with nanomedicine carriers, CD44 ligands can enhance the affinity of nanocarriers for tumor cells. 72 Hyaluronic acid is recognized for its efficient tumor-targeting capability, which is attributed to the presence of CD44 ligands within its structure.
Moreover, the presence of various functional groups within the HA structure enables further structural modification and functionalization, making it a promising carrier material for tumor-targeted delivery. 73 Hyaluronic acid serves as an ideal polymeric carrier for systemic drug administration and has been extensively applied in the delivery of diverse therapeutic agents. 74 Zhang et al 75 co-encapsulated the chemotherapeutic drug cisplatin (Cis) and chloroquine (CQ) into hyaluronan-based nanogels, markedly enhancing the intracellular delivery efficiency of Cis and achieving efficient, targeted therapy for TNBC. Compared with the free drug combination (Cis + CQ), this HA/Cis/CQ nanogel formulation maintains robust antitumor efficacy while significantly reducing nephrotoxicity-associated adverse effects (Table 2).
Table 2.
HA-anticancer drug conjugates.
| Carrier | Drug | Connection method | Reference |
|---|---|---|---|
| Low molecular weight HA |
Paclitaxel | Glucuronic acid hydrazide | Luo et al 76 |
| High molecular weight HA |
Butyric acid | N-Acetylglucosamine Hydroxyesterification | Piskin 77 |
| High molecular weight HA |
Carborane | Glucose carboxyl esterification | Meo et al 78 |
| High molecular weight HA |
Paclitaxel | Carboxyl esterification of glucoaldehyde | Wang 79 |
Characterisation of HA
Hyaluronic acid enhances drug uptake in cells with high expression of its receptors, thereby potentiating therapeutic efficacy. Cluster of differentiation 44, the primary receptor for HA, is a transmembrane glycoprotein that exhibits elevated expression in various tumors. The CD44 receptor undergoes alternative exon splicing and posttranscriptional modifications in gene expression, resulting in multiple isoforms. 80 Anttila et al 81 demonstrated that stromal HA accumulation correlates with tumor progression by evaluating HA levels and CD44 expression; however, they argued that HA accumulation is not associated with CD44 expression on the surface of human ovarian cancer cells. Zhao et al 82 found that CD44 promotes cell proliferation in lung cancer cells through activation of mitogen-activated protein kinase (MAPK) signaling, whereas deletion of the CD44 gene inhibits Kras-mediated lung adenocarcinoma proliferation in mice. Furthermore, the interaction between HA and CD44 facilitates ankyrin binding to MDR-1, leading to the efflux of chemotherapeutic drugs and chemotherapy resistance in tumor cells. 83
Targeted therapy with HA
Hyaluronic acid demonstrates excellent biocompatibility and exhibits no immunogenicity. 84 HA can bind to the overexpressed cluster of differentiation protein 44 (CD44) receptors on cancer cell membranes, thereby conferring tumor-targeting properties. Notably, CD44 receptors are upregulated in various BC subtypes, with significant overexpression observed in TNBC. Li et al 85 synthesized targeted micelles with an average diameter of 120 nm using an HA-deoxycholic acid (DOCA) copolymer loaded with paclitaxel (Taxol), achieving a drug loading content of 34%. MTT assays were performed to analyze and compare the cytotoxicity of this formulation with clinically used paclitaxel in MDA-MB-231 BC cells and normal human embryonic lung fibroblasts (HELF). The results indicated that paclitaxel-loaded HA micelles exhibited enhanced cytotoxicity against CD44-positive tumor cells compared with paclitaxel alone, while demonstrating reduced toxicity toward HELF cells at equivalent concentrations. Studies have highlighted that breast tumor cells contain high levels of hyaluronidase (HAase), which can rapidly degrade HA-based drug carriers, releasing the drug and targeting tumor cells.86,87 Stenzel 88 developed a pH-responsive HA micellar system by conjugating thiolated HA (HA-SH) with the lateral unsaturated bonds of phosphatidylcholine via click chemistry methodology. The resultant copolymer spontaneously formed doxorubicin-encapsulated micelles, achieving a 10% drug loading efficiency. The release kinetics were evaluated at pH 7.4 and 5.0, revealing approximately 2-fold higher doxorubicin release in an acidic environment (pH 5.0) compared with physiological conditions (pH 7.4) over a 48-h period. Wang et al 89 developed HA-modified liposomes loaded with cisplatin (CDDP) and hesperidin (Hes), enhancing the efficacy of chemotherapy in TNBC. Hyaluronic acid modification promotes the uptake and retention of nanoparticles in BC cell lines. These nanoparticles mitigate inflammation by inhibiting the PI3K/Akt/mTOR pathway and improve chemosensitivity by inhibiting epithelial-mesenchymal transition, effectively suppressing the invasion and metastasis of TNBC while exhibiting reduced side effects on normal tissues.
Hydrogen sulfide (H2S) has been recognized as the third gasotransmitter implicated in various physiological and pathological processes. Endogenous H2S or moderate exogenous H2S levels promote tumor cell survival, whereas increased expression of H2S-generating enzymes or exposure to high concentrations of H2S-releasing compounds may exhibit antitumor effects.90,91 ADT-OH, a commonly used H2S-releasing agent, contains the ~3H-1,2-dithiol-3-thione moiety within its molecular structure. 92 The synthesis of HA-ADT is achieved through the chemical conjugation of HA with ADT-OH. Dong et al 93 investigated the effects of HA-ADT on human BC cell progression. The findings revealed that HA-ADT released higher levels of H2S compared with NaHS and GYY4137. Hyaluronic acid-ADT exhibited superior inhibitory effects on the proliferation and viability of human BC cells relative to NaHS and GYY4137, while also reducing their migratory and invasive capabilities. These results suggest that HA-ADT may play a pivotal role in suppressing the growth, migration, and invasion of human BC cells. The integration of these in vitro findings supports the development of a promising formulation with enhanced tumor-targeting efficacy and improved safety for normal cells, highlighting the potential benefits of active tumor targeting. 94
As evident from the above, both in vitro and in vivo studies robustly support the concept that conjugating HA to the outer surface of micelles enhances the targeting of tumor cells. When combined with passive targeting, this active targeting strategy offers a promising approach for delivering chemotherapeutics to tumors that overexpress CD44. 95 As depicted in Figure 5, HA-based nanomicelles exhibit excellent biological safety and possess notable capabilities for drug release, blood compatibility, and systemic tumor targeting, thus presenting a favorable prospect for clinical oncology treatment.96,97
Figure 5.
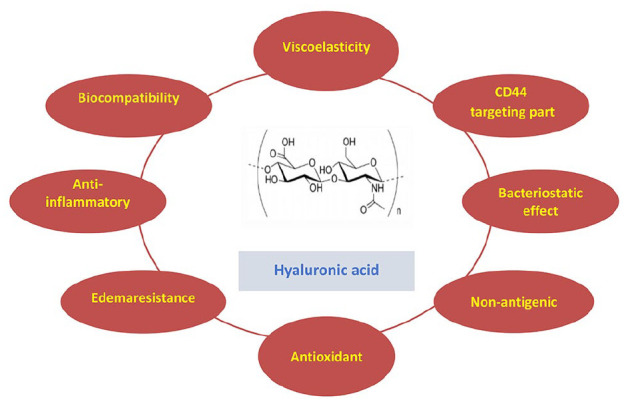
Structure and function of hyaluronic acid (HA).
These include viscoelasticity, CD44-targeting part, bacteriostatic effect, nonantigenic nature, antioxidant, edemaresistance, anti-inflammatory action and biocompatibility, highlighting its multifaceted biomedical potential.
Ultrasound Microbubble Delivery Vehicle
Ultrasound imaging technology is a real-time imaging modality in which contrast agent microbubbles preferentially accumulate within the rich microvasculature of tumors for tumor visualization.98,99 Traditional intravenous drug administration methods are limited by low tumor-targeting concentrations, short drug circulation times, and significant adverse reactions, such as nephrotoxicity and bone marrow suppression. In recent years, studies have confirmed that microbubbles can serve as carriers for chemotherapeutic agents (e.g., paclitaxel). Ultrasound-targeted microbubble destruction (UTMD), as a drug and gene delivery system, enables targeted and controlled drug release, offering advantages such as high drug release efficiency, uniform distribution, prolonged circulation time, reduced adverse effects, and enhanced antitumor efficacy, thereby demonstrating substantial potential.
Characteristics of US microbubble for drug delivery
The efficacy of chemotherapy for tumors depends on the effective concentration and duration of chemotherapeutic drugs at the tumor site. Chemotherapeutic agents can prolong survival in BC patients; however, adverse reactions remain the primary issue limiting their clinical application. Paclitaxel, a lipophilic drug, is widely used as a chemotherapeutic agent for BC but often induces numerous adverse reactions during chemotherapy. Encapsulating paclitaxel within microbubbles can reduce drug toxicity and side effects while enhancing local drug concentration within the tumor. Tang et al 100 investigated the effects of UTMD-mediated suppressor of cytokine signaling 3 (SOCS3) gene therapy on the biological characteristics and epithelial-mesenchymal transition of BC stem cells by establishing an in vivo BC model and culturing BC stem cells in vitro. The results indicated that UTMD-mediated SOCS3 gene therapy exhibited superior therapeutic effects. Takabatake et al 101 studied the inhibitory impact of gefitinib and taxane microbubble complexes on BC cell lines MDA-MB-231 (EGFR-positive) and MCF7/ADR (EGFR and HER2-positive). These findings suggested that gefitinib and taxane microbubble complexes could overcome drug resistance in these cancer cells, thereby enhancing the effect of taxanes on MCF7/ADR cells.
Researchers have also prepared dual-modified lipid CLR8LP (DOX + VER) microbubbles co-loaded with doxorubicin (DOX) and verapamil (VER) to investigate their inhibitory effects on the proliferation of human BC MCF7 cells. The results showed that co-encapsulating DOX and VER within co-modified liposomes significantly increased the cytotoxic efficacy against MCF7 cells. Overexpression of P-glycoprotein (Pgp) on tumor cell membranes is one of the primary mechanisms of drug resistance.102 -104 A commonly used strategy is to combine Pgp inhibitors with chemotherapeutic drugs. Verapamil, as a Pgp inhibitor, and dual-modified liposomes (CLR8LP) with excellent tumor-targeting delivery capabilities were used as carriers for chemotherapeutic drugs and Pgp inhibitors to create microbubble complexes. This enables chemotherapeutic drugs, with the synergistic effect of Pgp inhibitors, to effectively accumulate within cells, inhibiting and killing tumor cells. Ultrasound microbubbles carrying drugs not only reduce drug toxicity and side effects but also increase local drug concentration within tumors, enhancing the cytotoxic effect on tumor tissues.
Targeting of US microbubble in therapy
Ultrasonic microbubbles, as carriers, can not only reduce drug side effects in systemic circulation while enhancing drug concentration in pathological regions but also protect drugs and gene fragments from degradation by nucleases and clearance by the reticuloendothelial system. Gene therapy involves the use of gene engineering techniques to introduce exogenous genes into target cells for correcting functional defects or providing new functions. 105 Ultrasonic targeted microbubble destruction technology represents a targeted and efficient gene delivery method that minimizes injury, as supported by the excellent cavitation effect of microbubbles. 106 Some researchers 107 combined targeted ultrasonic microbubbles with CD/TK bicistronic suicide gene plasmids and prodrugs to transfect BC MCF7 cells expressing the KDR promoter, followed by US irradiation. The results demonstrated that the KDR promoter could drive the targeted expression of CD/TK bicistronic suicide genes in MCF7 cells, and the KDRP-CD/TK gene exhibited a targeted cytotoxic effect on MCF7 cells in vitro. Survivin, a member of the inhibitor of apoptosis protein family, promotes tumor proliferation. By inhibiting survivin gene expression and interfering with survivin Thr34 phosphorylation, cancer cells undergoing mitosis can be induced to undergo apoptosis, thereby enhancing the apoptosis-inducing effects of chemotherapeutic drugs on cancer cells. 108 Researchers have constructed an siRNA eukaryotic expression vector to silence the human survivin gene 109 and prepared lipid microbubbles loaded with this vector using the reverse-phase evaporation method with mechanical agitation. In vitro experiments showed that gene-loaded targeted microbubbles could specifically bind to BC cells with high HER-2 expression and induce apoptosis. The safety, efficacy, and stability of microbubbles as gene carriers have been confirmed in numerous experiments conducted by scholars worldwide; however, their clinical application still faces many challenges. Some scholars 110 noted as early as the 2015 Annual Meeting of the American Association for Cancer Research (AACR) that MPDL3280A exhibited durable efficacy in advanced TNBC. Programmed death 1/PDL1 immunotherapy is currently a hot topic in antitumor therapy, but no studies have been reported on conjugating anti-PD1 monoclonal antibodies to microbubbles for BC treatment.
This indicates that ultrasonic microbubbles can enhance the permeability of tumor cell membranes, induce mechanical damage to vascular endothelium and adjacent tumor tissues, and enlarge endothelial gaps. Ultrasonic microbubbles are capable of delivering chemotherapeutic agents such as paclitaxel in a targeted manner, thereby reducing drug toxicity and side effects, increasing local drug concentration in tumors, and potentiating the cytotoxic effects of chemotherapeutics on tumor cells. Ultrasonic microbubbles can facilitate the introduction of exogenous genes into BC cells to correct functional defects in target cells and promote apoptosis. Furthermore, they can deliver monoclonal antibody carriers for targeted immunotherapy, activating the immune system, enhancing immune responses, and inhibiting tumor initiation and progression. Currently, there are limited reports on microbubbles carrying drugs and genes targeting tumor parenchymal cells. Therefore, future research should focus on identifying safe and efficient targets for tumor parenchymal cells to improve the precision of ultrasonic microbubble-mediated targeted therapy.
Application of ADCs
Antibody-drug conjugates represent a novel strategy in cancer therapy. By exploiting the high specificity of monoclonal antibodies for binding to antigens on the surface of cancer cells, ADCs deliver potent cytotoxic agents directly to tumor cells. This approach integrates the targeted precision of antibodies with the robust antitumor efficacy of cytotoxic drugs, achieving high potency and reduced systemic toxicity. 111 To date, T-DM1 remains the sole ADC approved for the treatment of advanced HER-2-positive BC, and its success has catalyzed the development of next-generation ADCs.
The mechanism of action of ADCs
The design of ADCs comprises 3 critical components: the antibody, the cytotoxic drug, and the linker. 112 Antibody-drug conjugates combine the highly specific targeting capability of antibodies with potent small-molecule cytotoxic agents, maintaining stability in the circulatory system. As depicted in Figure 6, upon binding to antigens expressed on the surface of tumor cells, ADCs undergo intracellular degradation and release their payload within the cell, achieving a high local drug concentration that effectively and precisely eliminates tumor cells. By integrating the targeting properties of monoclonal antibodies with the cytotoxicity of chemotherapeutic agents, ADCs represent a significant advancement over traditional chemotherapy and targeted therapy. 113 When specific antigens on the surface of tumor cells are kinase receptors or molecules involved in signaling pathways associated with tumor growth, differentiation, and proliferation, the antibody component of ADCs binds to these antigens, thereby blocking downstream signaling pathways and inhibiting tumor cell proliferation. 114 In addition, ADCs can induce apoptosis via antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). 115
Figure 6.
Hyaluronic acid-decorated nanomicelle-mediated active and passive targeting.
Source: Reproduced with permission. 96
Micelle formation via HA-polymer conjugation, tumor accumulation by EPR effect after intravenous injection and CD44 receptor mediated endocytosis for intracellular drug release.
Trastuzumab emtansine (T-DM1) is a novel ADC that demonstrates biological activity analogous to trastuzumab. It selectively targets and delivers the potent antimicrotubule agent DM-1 to HER-2-positive tumor cells, effectively and specifically eliminating them. The initial success of T-DM1 in BC treatment has expedited advancements in this field, with over 60 new ADCs currently in development.116 -118 Nevertheless, ADC development continues to encounter substantial challenges, including complex drug structures, targeting efficiency, and adverse effects, which have contributed to suboptimal outcomes in clinical trials. 119 Of particular significance is the balance between lower-than-expected efficacy and higher-than-expected toxicity, highlighting the necessity for further innovation in designing next-generation ADCs. 120 In summary, ADCs provide novel opportunities to enhance the prognosis of BC patients. Through ongoing optimization of drug molecule design and target selection, improved treatment options for BC can be anticipated in the future. 121
Clinical application of ADCs
Antibody-drug conjugates selectively deliver cytotoxic agents or radioisotopes to malignant cells through targeted monoclonal antibodies. 122 In the TNBC field, ADCs that have entered clinical trials include DS-8201a, sacituzumab govitecan, ladiratuzumab vedotin (SGN-LIV1A), CAB-ROR2-ADC, anti-CA6-DM4 immunoconjugate (SAR566658), AVID100, and U3-1402. 123 Although atezolizumab and albumin-bound paclitaxel have been approved, their combination is currently limited to PD-L1-positive TNBC patients, highlighting ADCs as a promising targeted therapeutic option for the remaining 60% of PD-L1-negative patients.
DS-8201, also known as T-DXd, is an ADC comprising trastuzumab, a peptide-based cleavable linker, and a topoisomerase I inhibitor payload. 124 The amino acid sequence of its antibody component is identical to that of trastuzumab, and it is conjugated to the cytotoxic agent DXd via a protease-cleavable tetrapeptide linker, achieving a drug-to-antibody ratio (DAR) of 8. The phase III DESTINY-Breast03 trial demonstrated that T-DXd significantly reduced the risk of mortality by 36% in patients with HER2-positive BC, with a median progression-free survival (PFS) more than 4 times longer compared with the T-DM1 arm (28.8 months vs. 6.8 months, HR = 0.33, P < 0.0001). In addition, 79% of participants achieved an objective response, including 21% who attained a complete response. 125 These findings indicate that, compared with T-DM1, T-DXd provides clinically and statistically significant improvements in survival and sustained PFS benefits for patients with pretreated HER2-positive metastatic BC. Furthermore, T-DXd has shown manageable and tolerable safety profiles even with prolonged treatment exposure. Recently, results from a phase I dose-escalation study (NCT02564900) were published to evaluate the safety and tolerability of DS-8201 in HER2-positive BC patients who progressed after prior T-DM1 therapy. 126 The dosing regimens were 5.4 mg/kg or 6.4 mg/kg every 3 weeks, and common grade 3 or higher adverse events included anemia (17%), neutropenia (14%), leukopenia (9%), and thrombocytopenia (8%). The trial confirmed controllable safety for DS-8201. Moreover, an ongoing phase III clinical trial (NCT03529110) is enrolling 500 patients with HER2-positive, unresectable, and/or metastatic BC who have received prior trastuzumab or taxane-based chemotherapy. This trial compares DS-8201 to T-DM1, with PFS as the primary endpoint and overall survival (OS) and objective response rate (ORR) as secondary endpoints.
Trophoblast cell surface antigen 2 (Trop-2), a glycoprotein widely expressed in various tumor types, is also referred to as tumor-associated calcium signal transducer 2 (TACSTD2), membrane component 1 surface marker 1 (M1 S1), gastrointestinal antigen 733-1 (GA733-1), and epithelial glycoprotein 1 (EGP-1). First identified in a human choriocarcinoma cell line in 1981, it belongs to the category of cell surface receptors. Trop-2 serves as a critical signaling molecule regulating tumor growth. 127 It is overexpressed in malignant tumors such as BC, gastric cancer, colorectal cancer, and nonsmall cell lung cancer. Through mediation of multiple signaling pathways, Trop-2 regulates cell proliferation and apoptosis, thereby participating in tumor development. 128 Particularly in TNBC cells, this characteristic makes Trop-2 an ideal target for ADCs. 129 Sacituzumab govitecan (SG), a Trop-2-targeted ADC, exerts antitumor activity by conjugating an anti-Trop-2 antibody with the cytotoxic agent SN-38 (7-ethyl-10-hydroxycamptothecin). 130 The final results of the phase III ASCENT study demonstrated that SG significantly prolonged median PFS (5.6 months vs. 1.7 months, HR = 0.39, P < 0.001) and overall survival (OS) (12.1 months vs. 6.7 months, HR = 0.48, P < 0.001) compared with single-agent chemotherapy in patients with unresectable, recurrent, or refractory locally advanced/metastatic TNBC who had received ⩾ 2 prior lines of standard chemotherapy. The most common grade 3/4 treatment-related adverse events were neutropenia and diarrhea, which could be managed with symptomatic support. Furthermore, the final analysis of the phase III TROPiCS-02 study indicated that SG was superior to physician’s choice of chemotherapy in patients with endocrine therapy-resistant metastatic hormone receptor (HR)-positive/HER2-negative BC who had previously received taxanes, endocrine therapy, CDK4/6 inhibitors, and 2-4 lines of chemotherapy, with median OS of 14.4 months versus 11.2 months, respectively (HR = 0.79, P = 0.02). 131 A single-arm, multicenter, phase II clinical trial evaluated the efficacy and safety of sacituzumab govitecan in patients with recurrent/refractory metastatic TNBC. 132 Results showed an objective response rate (ORR) of 30%, including 2 complete responses and 19 partial responses, with a median PFS of 6 months and median OS of 16.6 months. Immunohistochemical analysis revealed moderate to strong Trop-2 expression in 88% of patients. The most common grade ⩾ 3 adverse events included neutropenia (39%), leukopenia (16%), anemia (14%), and diarrhea (13%), with a febrile neutropenia incidence rate of approximately 7%. The trial confirmed the favorable efficacy and tolerability of sacituzumab govitecan in metastatic TNBC. Another ongoing multicenter, randomized, open-label, phase III clinical trial (NCT02574455) is enrolling patients with refractory/metastatic TNBC or recurrence after at least 2 prior lines of chemotherapy. This trial compares sacituzumab govitecan to the physician’s choice of chemotherapy (capecitabine, eribulin, gemcitabine, and vinorelbine), with PFS as the primary endpoint and OS, ORR, and other indicators as secondary endpoints.
SGN-LIV1A represents an additional ADC therapeutic, comprising a monoclonal antibody targeting LIV1 conjugated to the microtubule inhibitor monomethyl auristatin E (MMAE). 133 LIV1 is a transmembrane protein with zinc transporter and metalloproteinase activities, exhibiting high expression in over 90% of breast tumors but limited expression in normal tissues. 134 An ongoing open-label, dose-escalation Phase I clinical trial (NCT01969643) is evaluating the safety and tolerability of SGN-LIV1A in patients with metastatic BC. The interventional strategies in this study include the administration of SGN-LIV1A as a single agent or in combination with trastuzumab. The primary endpoint is the incidence of adverse events, while secondary endpoints include blood concentrations of SGN-LIV1A and its metabolites, overall response rate (ORR), and other indicators. Currently, multiple clinical studies for this drug are underway, with results anticipated within the next 2 years. Other ADCs under investigation include CAB-ROR2-ADC, SAR566658, AVID100, and U3-1402. CAB-ROR2-ADC integrates conditionally active biologics (utilizing BioAtla’s proprietary CAB technology) with an antibody targeting ROR2, and its tolerability, safety, and antitumor activity are being assessed in patients with solid tumors. The anti-CA6-DM4 immunoconjugate (SAR566658) is an ADC consisting of a ds6 antibody targeting carbonic anhydrase 6 (CA6) coupled with the microtubule inhibitor DM4. 135 AVID100 is the first epidermal growth factor receptor (EGFR)-targeted ADC to achieve clinical progress. AVID100 was evaluated in a multicenter, dose-escalation Phase IIa clinical trial in TNBC patients, initially demonstrating promising results, 136 but recent updates indicate the trial was terminated due to insufficient efficacy. U3-1402 is an ADC where the HER3 monoclonal antibody patritumab is conjugated to the topoisomerase I inhibitor deruxtecan (Dxd) via a tetrapeptide linker. 137 Previous data indicate that HER3 is expressed in approximately 50% to 70% of BC patients, yet few therapies specifically target HER3. 138 At the 2022 ASCO meeting, the novel HER3-targeted ADC patritumab deruxtecan demonstrated efficacy in treating HER3-expressing metastatic BC. The U31402-A-J101 study enrolled 182 patients with HER3-expressing metastatic BC, including 113 HR +/HER2-, 53 TNBC, and 14 HER2+ patients. Following treatment with patritumab deruxtecan, overall survival (OS) was 14.6, 14.6, and 19.5 months, respectively, while PFS was 5.5, 7.4, and 11.0 months across the 3 subtypes. The study demonstrated the efficacy of patritumab deruxtecan irrespective of HER3 expression levels, with favorable safety, as only 10% of patients discontinued treatment due to intolerance. Researchers believe that HER3 will emerge as a critical target for treating diseases such as BC.
In summary, the application of ADCs in BC treatment has demonstrated significant progress and evolution in recent years. By leveraging the targeting specificity of antibodies to deliver cytotoxic agents to designated lesions, ADCs exert enhanced antitumor efficacy. Despite over 2 decades of investigation in oncology, ADC development has been relatively slow due to challenges in target selection, conjugation technology, antibody optimization, and drug release. However, advancements in fourth-generation ADC technology have established a promising contemporary landscape. In TNBC, SGN-LIV1A underwent a series of clinical trials, leading to the full FDA approval of the targeted anticancer agent Trodelvy, expanding its indication to adult patients who have received at least 2 prior systemic therapies, including 1 for metastatic or unresectable locally advanced TNBC. In addition, as depicted in Figure 7, ADCs such as ladiratuzumab vedotin, SAR566658, CAB-ROR2-ADC, and U3-1402 are currently under clinical evaluation (Figures 8, 9). 139
Figure 7.
Schematic of the microbubble-based, ultrasound-assisted photodynamic therapy (PDT) strategy. 90 PDT, photodynamic therapy; PGL, porphyrin-grafted lipid; MB, microbubbles; LFUS, low-frequency ultrasound; CEUS, contrast-enhanced ultrasound.
Figure 8.
Schematic representation of ADC drug structure and its mechanism of action.
ADCs consist of 3 parts: an antibody, a linker, and a cytotoxic drug. The antibody recognizes antigens on the surface of breast cancer cells, and the ADC-antigen complex is then endocytosed into the cell. Subsequently, the linker is cleaved in the lysosome, releasing the cytotoxic payload. The payload disrupts DNA or microtubules, inducing apoptosis in cancer cells.
Figure 9.
Timeline of current and future ADC development in BC.
The evolution of antibody-drug conjugates in the field of breast cancer is well-defined: from HER2 targeting to multitarget coverage including TROP2, from later-line treatments to front-line/new adjuvant therapies, and gradually moving toward personalized, combination treatments and resistance resolution.
Conclusions
In brief, within the current treatment landscape, BC patients face significant challenges related to drug resistance or nonresponsiveness, highlighting the necessity for developing novel anticancer agents as a promising strategy to further improve patient outcomes. The 4 aforementioned technologies each exhibit distinct advantages. Given the diverse mechanisms of action employed by these drugs, it is crucial to select or customize therapies based on individual patient characteristics. For example, antiangiogenic compounds may achieve optimal efficacy when targeted to the interstitial space rather than intracellularly, whereas chemotherapeutic agents function most effectively upon intracellular delivery. Furthermore, the heterogeneity of the BC cell population emphasizes the importance of targeting multiple cell types expressing varied surface markers to effectively reduce tumor burden and eliminate cancer cells. An effective multitargeted approach may also require the incorporation of several distinct targeting ligands, representing a current and future research focus in anticancer drug development. In conclusion, the optimal pharmacotherapeutic strategy aims to maximize therapeutic efficacy, overcome drug resistance, and minimize adverse effects. Advancing research on these drug delivery systems and developing safer, more effective targeted therapies for BC may offer new treatment opportunities for patients.
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Dongmin Yu  https://orcid.org/0000-0002-8455-7425
https://orcid.org/0000-0002-8455-7425
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Author Contributions: Cunjun Mai: Conceptualization; Formal analysis; Methodology; Writing—original draft.
Huiyang Tang: Data curation; Investigation.
Guie Lai: Data curation; Formal analysis; Investigation.
Lulin Liu: Methodology; Resources; Writing—review & editing.
Wenzhen Huang: Data curation; Formal analysis; Project administration.
Kang He: Formal analysis; Project administration.
Qingyang Liu: Data curation; Investigation; Resources.
You Sun: Resources; Writing—review & editing.
Dongmin Yu: Funding acquisition; Methodology; Writing—review & editing.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the National Natural Science Foundation of China (grant no. 82460495), Jiangxi Provincial Natural Science Foundation (grant nos. 20242BAB216064 and 20242BAB25353), Jiangxi Provincial Health Commission Science and Technology Plan Project (grant no. SKJP220225453) and Ganzhou City, Jiangxi Province “Science and Technology + health care” Joint Program Project (grant no. 2023LNS36669).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials: Not applicable.
References
- 1. Li X, Guo Q, Chen Q, et al. Reconciling the cooperative-competitive patterns among tumor and immune cells for triple-negative breast cancer treatment using multimodule nanocomplexes. Adv Mater. 2024;36:e2312219. doi: 10.1002/adma.202312219 [DOI] [PubMed] [Google Scholar]
- 2. Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple—negative breast cancer—expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19:91-113. doi: 10.1038/s41571-021-00565-2 [DOI] [PubMed] [Google Scholar]
- 3. Huppert LA, Gumusay O, Idossa D, Rugo HS. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J Clin. 2023;73:480-515. doi: 10.3322/caac.21777 [DOI] [PubMed] [Google Scholar]
- 4. Chumsri S. Advancing outcomes of metastatic HER2-positive breast cancer. Lancet. 2023;401(10390):1746-1747. doi: 10.1016/s0140-6736(23)00805-x [DOI] [PubMed] [Google Scholar]
- 5. Bai J, Gao Y, Zhang G. The treatment of breast cancer in the era of precision medicine. Cancer Biol Med. 2025;22:322-347. doi: 10.20892/j.issn.2095-3941.2024.0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajput MU, Ali SW, Kainat W, Madani S, Ding W. Enhanced transdermal drug delivery and breast cancer spheroid penetration using hyaluronic acid-coated ethosomes. Colloids Surf B Biointerfaces. 2025;254:114817. doi:0.1016/j.colsurfb.2025.114817 [DOI] [PubMed] [Google Scholar]
- 7. Davis AA, Hesse J, Pereira PMR, Ma CX. Novel treatment approaches utilizing antibody-drug conjugates in breast cancer. NPJ Breast Cancer. 2025;11:42. doi: 10.1038/s41523-025-00743-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kunte R, Sabale P, Waghmare S, Jiwankar MN, Sabale V. Advancements in nanoparticle-based targeted drug delivery systems for breast cancer. Pharm Nanotechnol. Published online March 11, 2025. doi: 10.2174/0122117385339882241206095441 [DOI] [PubMed] [Google Scholar]
- 9. Singhai H, Raikwar S, Rathee S, Jain SK. Emerging combinatorial drug delivery strategies for breast cancer: a comprehensive review. Curr Drug Targets. 2025;26:331-349. doi: 10.2174/0113894501352081241211090911 [DOI] [PubMed] [Google Scholar]
- 10. Qiao K, Pan Y, Zhang S, et al. Cold exposure therapy sensitizes nanodrug-mediated radioimmunotherapy of breast cancer. ACS Nano. 2024;18:29689-29703. doi: 10.1021/acsnano.4c09021 [DOI] [PubMed] [Google Scholar]
- 11. Shang R, Yang F, Gao G, Luo Y, You H, Dong L. Bioimaging and prospects of night pearls-based persistence phosphors in cancer diagnostics. Exploration (Beijing). 2024;4:20230124. doi: 10.1002/exp.20230124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao L, Chang F, Tong Y, et al. A multifunctional bimetallic nanoplatform for synergic local hyperthermia and chemotherapy targeting HER2-positive breast cancer. Adv Sci (Weinh). 2024;11:e2308316. doi: 10.1002/advs.202308316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu C, Dong Y, Zhu X, et al. Oxygen vacancy piezoelectric nanosheets constructed by a photoetching strategy for ultrasound “unlocked” tumor synergistic therapy. Nano Lett. 2024;24:8008-8016. doi: 10.1021/acs.nanolett.4c01656 [DOI] [PubMed] [Google Scholar]
- 14. Wong KY, Nie Z, Wong MS, Wang Y, Liu J. Metal-drug coordination nanoparticles and hydrogels for enhanced delivery. Adv Mater. 2024;36:e2404053. doi: 10.1002/adma.202404053 [DOI] [PubMed] [Google Scholar]
- 15. Deng B, Liu S, Wang Y, et al. Oral nanomedicine: challenges and opportunities. Adv Mater. 2024;36:e2306081. doi: 10.1002/adma.202306081 [DOI] [PubMed] [Google Scholar]
- 16. Hu M, Li X, You Z, Cai R, Chen C. Physiological barriers and strategies of lipid-based nanoparticles for nucleic acid drug delivery. Adv Mater. 2024;36:e2303266. doi: 10.1002/adma.202303266 [DOI] [PubMed] [Google Scholar]
- 17. Zhao Y, Zhang Z, Pan Z, Liu Y. Advanced bioactive nanomaterials for biomedical applications. Exploration (Beijing). 2021;1:20210089. doi: 10.1002/exp.20210089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng C, Tan P, Nie G, Zhu M. Biomimetic and bioinspired nano-platforms for cancer vaccine development. Exploration (Beijing). 2023;3:20210263. doi: 10.1002/exp.20210263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miao T, Wang J, Zeng Y, Liu G, Chen X. Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: from bench to bedside. Adv Sci (Weinh). 2018;5:1700513. doi: 10.1002/advs.201700513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaduri M, Sela M, Kagan S, et al. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases. Sci Adv. 2021;7:eabj5435. doi: 10.1126/sciadv.abj5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao H, Li X, Li B, et al. Nanodrug inducing autophagy inhibition and mitochondria dysfunction for potentiating tumor photo-immunotherapy. Small. 2023;19:e2300280. doi: 10.1002/smll.202300280 [DOI] [PubMed] [Google Scholar]
- 22. Qu R, He D, Wu M, et al. Afterglow/photothermal bifunctional polymeric nanoparticles for precise postbreast-conserving surgery adjuvant therapy and early recurrence theranostic. Nano Lett. 2023;23:4216-4225. doi: 10.1021/acs.nanolett.3c00191 [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Wang B, Zhang X, et al. Activatable graphene quantum-dot-based nanotransformers for long-period tumor imaging and repeated photodynamic therapy. Adv Mater. 2023;35:e2211337. doi: 10.1002/adma.202211337 [DOI] [PubMed] [Google Scholar]
- 24. Ma L, Song X, Yu Y, Chen Y. Two-dimensional silicene/silicon nanosheets: an emerging silicon-composed nanostructure in biomedicine. Adv Mater. 2021;33:e2008226. doi: 10.1002/adma.202008226 [DOI] [PubMed] [Google Scholar]
- 25. Zheng D, Pisano F, Collard L, et al. Toward plasmonic neural probes: SERS detection of neurotransmitters through gold-nanoislands-decorated tapered optical fibers with sub-10 nm gaps. Adv Mater. 2023;35:e2200902. doi: 10.1002/adma.202200902 [DOI] [PubMed] [Google Scholar]
- 26. Urgel JI, Sánchez-Grande A, Vicent DJ, Jelínek P, Martín N, Écija D. On-surface covalent synthesis of carbon nanomaterials by harnessing carbon gem-polyhalides. Adv Mater. 2024;36:e2402467. doi: 10.1002/adma.202402467 [DOI] [PubMed] [Google Scholar]
- 27. Gong N, Ma X, Ye X, et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat Nanotechnol. 2019;14:379-387. doi: 10.1038/s41565-019-0373-6 [DOI] [PubMed] [Google Scholar]
- 28. Zou Y, Sun Y, Wang Y, et al. Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment. Nat Commun. 2023;14:4557. doi: 10.1038/s41467-023-40280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarkar S, Konar S, Prasad PN, et al. Micellear gold nanoparticles as delivery vehicles for dual tyrosine kinase inhibitor ZD6474 for metastatic breast cancer treatment. Langmuir. 2017;33:7649-7659. doi: 10.1021/acs.langmuir.7b01072 [DOI] [PubMed] [Google Scholar]
- 30. Liszbinski RB, Romagnoli GG, Gorgulho CM, Basso CR, Pedrosa VA, Kaneno R. Anti-EGFR-coated gold nanoparticles in vitro carry 5-fluorouracil to colorectal cancer cells. Materials (Basel). 2020;13:375. doi: 10.3390/ma13020375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu C, Feng Q, Yang H, et al. A light-triggered mesenchymal stem cell delivery system for photoacoustic imaging and chemo-photothermal therapy of triple negative breast cancer. Adv Sci (Weinh). 2018;5:1800382. doi: 10.1002/advs.201800382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alhussan A, Bozdoğan EPD, Chithrani DB. Combining gold nanoparticles with other radiosensitizing agents for unlocking the full potential of cancer radiotherapy. Pharmaceutics. 2021;13:442. doi: 10.3390/pharmaceutics13040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramchandani D, Lee SK, Yomtoubian S, Han MS, Tung CH, Mittal V. Nanoparticle delivery of miR-708 mimetic impairs breast cancer metastasis. Mol Cancer Ther. 2019;18:579-591. doi: 10.1158/1535-7163.Mct-18-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goyal R, Kapadia CH, Melamed JR, Riley RS, Day ES. Layer-by-layer assembled gold nanoshells for the intracellular delivery of miR-34a. Cell Mol Bioeng. 2018;11:383-396. doi: 10.1007/s12195-018-0535-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riley RS, O’Sullivan RK, Potocny AM, Rosenthal J, Day ES. Evaluating nanoshells and a potent biladiene photosensitizer for dual photothermal and photodynamic therapy of triple negative breast cancer cells. Nanomaterials (Basel). 2018;8:658. doi: 10.3390/nano8090658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang L, Cao J, Li C, et al. Efficacy and safety of mitoxantrone hydrochloride liposome injection in Chinese patients with advanced breast cancer: a randomized, open-label, active-controlled, single-center, phase II clinical trial. Invest New Drugs. 2022;40:330-339. doi: 10.1007/s10637-021-01182-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koudelka S, Turánek J. Liposomal paclitaxel formulations. J Control Release. 2012;163:322-334. doi: 10.1016/j.jconrel.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 38. Fraguas-Sánchez AI, Martín-Sabroso C, Fernández-Carballido A, Torres-Suárez AI. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother Pharmacol. 2019;84:689-706. doi: 10.1007/s00280-019-03910-6 [DOI] [PubMed] [Google Scholar]
- 39. Jin N, Sun Y, Shi W, et al. Type-I CdS/ZnS core/shell quantum dot-gold heterostructural nanocrystals for enhanced photocatalytic hydrogen generation. J Am Chem Soc. 2023;145:21886-21896. doi: 10.1021/jacs.3c06065 [DOI] [PubMed] [Google Scholar]
- 40. Chopra A. Pegylated bis(4-(N-(2-naphthyl)phenylamino)phenyl)-fumaronitrile (NPAPF) nanoparticles. In: Molecular Imaging and Contrast Agent Database [Internet]. National Center for Biotechnology Information; 2004. [PubMed] [Google Scholar]
- 41. Jain V, Kumar H, Anod HV, et al. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J Control Release. 2020;326:628-647. doi: 10.1016/j.jconrel.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 42. Ghosh S, Javia A, Shetty S, et al. Triple negative breast cancer and non-small cell lung cancer: clinical challenges and nano-formulation approaches. J Control Release. 2021;337:27-58. doi: 10.1016/j.jconrel.2021.07.014 [DOI] [PubMed] [Google Scholar]
- 43. Omidi Y, Mobasher M, Castejon AM, Mahmoudi M. Recent advances in nanoscale targeted therapy of HER2-positive breast cancer. J Drug Target. 2022;30:687-708. doi: 10.1080/1061186x.2022.2055045 [DOI] [PubMed] [Google Scholar]
- 44. Shokooh MK, Emami F, Duwa R, Jeong J-H, Yook S. Triple-negative breast cancer treatment meets nanoparticles: current status and future direction. J Drug Deliv Sci Technol. 2022;71:103274. [Google Scholar]
- 45. Oneda E, Meriggi F, Zanotti L, et al. Innovative approach for the prevention of chemotherapy-induced peripheral neuropathy in cancer patients: a pilot study with the hilotherm device, the poliambulanza hospital experience. Integr Cancer Ther. 2020;19:1534735420943287. doi: 10.1177/1534735420943287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimada H, Ueda S, Saeki T, et al. Neoadjuvant triweekly nanoparticle albumin-bound paclitaxel followed by epirubicin and cyclophosphamide for stage II/III HER2-negative breast cancer: evaluation of efficacy and safety. Jpn J Clin Oncol. 2015;45:642-649. doi: 10.1093/jjco/hyv055 [DOI] [PubMed] [Google Scholar]
- 47. Sabatelle RC, Chu NQ, Blessing W, et al. Decreased lung metastasis in triple negative breast cancer following locally delivered supratherapeutic paclitaxel-loaded polyglycerol carbonate nanoparticle therapy. Biomacromolecules. 2024;25:1800-1809. doi: 10.1021/acs.biomac.3c01258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao Y, Huan ML, Liu M, et al. Doxorubicin and resveratrol co-delivery nanoparticle to overcome doxorubicin resistance. Sci Rep. 2016;6:35267. doi: 10.1038/srep35267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thipe VC, Panjtan Amiri K, Bloebaum P, et al. Development of resveratrol-conjugated gold nanoparticles: interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int J Nanomedicine. 2019;14:4413-4428. doi: 10.2147/ijn.S204443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang W, Zhang L, Chen T, et al. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules. 2017;22:1814. doi: 10.3390/molecules22111814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kondath S, Srinivas Raghavan B, Anantanarayanan R, Rajaram R. Synthesis and characterisation of morin reduced gold nanoparticles and its cytotoxicity in MCF-7 cells. Chem Biol Interact. 2014;224:78-88. doi: 10.1016/j.cbi.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 52. Lee D, Ko WK, Hwang DS, et al. Use of baicalin-conjugated gold nanoparticles for apoptotic induction of breast cancer cells. Nanoscale Res Lett. 2016;11:381. doi: 10.1186/s11671-016-1586-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kavithaa K, Paulpandi M, Padma PR, Sumathi SJRA. Induction of intrinsic apoptotic pathway and cell cycle arrest via baicalein loaded iron oxide nanoparticles as a competent nano-mediated system for triple negative breast cancer therapy. 2016;6:64531-64543. [Google Scholar]
- 54. Wang L, Zhang DZ, Wang YX. Bioflavonoid fisetin loaded α-tocopherol-poly(lactic acid)-based polymeric micelles for enhanced anticancer efficacy in breast cancers. Pharm Res. 2017;34:453-461. doi: 10.1007/s11095-016-2077-z [DOI] [PubMed] [Google Scholar]
- 55. Feng C, Yuan X, Chu K, Zhang H, Ji W, Rui M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int J Biol Macromol. 2019;125:700-710. doi: 10.1016/j.ijbiomac.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 56. Alpsoy L, Baykal A, Akal ZJJOS, Magnetism N. Luteolin-loaded spion as a drug carrier for cancer cell in vitro. 2018;31:467-474. [Google Scholar]
- 57. Jain AK, Thanki K, Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol Pharm. 2013;10:3459-3474. doi: 10.1021/mp400311j [DOI] [PubMed] [Google Scholar]
- 58. Zang X, Cheng M, Zhang X, Chen X. Quercetin nanoformulations: a promising strategy for tumor therapy. Food Funct. 2021;12:6664-6681. doi: 10.1039/d1fo00851j [DOI] [PubMed] [Google Scholar]
- 59. Kumari A, Kumar V, Yadav SK. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: a green approach. PLoS ONE. 2012;7:e41230. doi: 10.1371/journal.pone.0041230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jain A, Sharma G, Ghoshal G, et al. Lycopene loaded whey protein isolate nanoparticles: an innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int J Pharm. 2018;546:97-105. doi: 10.1016/j.ijpharm.2018.04.061 [DOI] [PubMed] [Google Scholar]
- 61. Jain A, Sharma G, Kushwah V, et al. Fabrication and functional attributes of lipidic nanoconstructs of lycopene: an innovative endeavour for enhanced cytotoxicity in MCF-7 breast cancer cells. Colloids Surf B Biointerfaces. 2017;152:482-491. doi: 10.1016/j.colsurfb.2017.01.050 [DOI] [PubMed] [Google Scholar]
- 62. Maleki H, Aiyelabegan HT, Javadi P, et al. Nanotechnology-mediated precision drug delivery strategies for breast cancer treatment. Biomed Pharmacother. 2025;188:118224. doi: 10.1016/j.biopha.2025.118224 [DOI] [PubMed] [Google Scholar]
- 63. Lotter C, Stierli MA, Puligilla RD, Huwyler J. Dual targeted lipid nanoparticles for enhanced DNA delivery and transfection of breast cancer cells. Eur J Pharm Biopharm. 2025;209:114674. doi: 10.1016/j.ejpb.2025.114674 [DOI] [PubMed] [Google Scholar]
- 64. Alaei E, Hashemi F, Farahani N, et al. Peptides in breast cancer therapy: from mechanisms to emerging drug delivery and immunotherapy strategies. Pathol Res Pract. 2025;269:155946. doi: 10.1016/j.prp.2025.155946 [DOI] [PubMed] [Google Scholar]
- 65. Hadri SH, Riaz A, Abid J, et al. Emerging nanostructure-based strategies for breast cancer therapy: innovations, challenges, and future directions. Med Oncol. 2025;42:188. doi: 10.1007/s12032-025-02743-z [DOI] [PubMed] [Google Scholar]
- 66. Jin H, Meng X, Feng J. Mechanisms of tumor-associated macrophages in breast cancer and treatment strategy. Front Immunol. 2025;16:1560393. doi: 10.3389/fimmu.2025.1560393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jiang R, Yang L, Liu X, et al. Genetically engineered macrophages reverse the immunosuppressive tumor microenvironment and improve immunotherapeutic efficacy in TNBC. Mol Ther. 2025;33:3339-3359. doi: 10.1016/j.ymthe.2025.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rahdari T, Mahdavimehr M, Ghafouri H, Ramezanpour S, Ehtesham S, Asghari SM. Advancing triple-negative breast cancer treatment through peptide decorated solid lipid nanoparticles for paclitaxel delivery. Sci Rep. 2025;15:6043. doi: 10.1038/s41598-025-90107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yapar EA, Ozdemir MN, Cavalu S, Dagıstan ÖA, Ozsoy Y, Kartal M. Phytoactive molecules and nanodelivery approaches for breast cancer treatment: current and future perspectives. Curr Pharm Biotechnol. 2025;26:795-812. doi: 10.2174/0113892010299183240529094844 [DOI] [PubMed] [Google Scholar]
- 70. LeSavage BL, Zhang D, Huerta-López C, et al. Engineered matrices reveal stiffness-mediated chemoresistance in patient-derived pancreatic cancer organoids. Nat Mater. 2024;23:1138-1149. doi: 10.1038/s41563-024-01908-x [DOI] [PubMed] [Google Scholar]
- 71. Han X, Wang F, Shen J, et al. Ultrasound nanobubble coupling agent for effective noninvasive deep-layer drug delivery. Adv Mater. 2024;36:e2306993. doi: 10.1002/adma.202306993 [DOI] [PubMed] [Google Scholar]
- 72. Cao L, Yang Y, Zheng Y, et al. X-ray-triggered CO-release from gold nanocluster: all-in-one nanoplatforms for cancer targeted gas and radio synergistic therapy. Adv Mater. 2024;36:e2401017. doi: 10.1002/adma.202401017 [DOI] [PubMed] [Google Scholar]
- 73. Lee Y, Shinn J, Xu C, Dobson HE, Neamati N, Moon JJ. Hyaluronic acid-bilirubin nanomedicine-based combination chemoimmunotherapy. Nat Commun. 2023;14:4771. doi: 10.1038/s41467-023-40270-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guo Y, Wang SZ, Zhang X, et al. In situ generation of micrometer-sized tumor cell-derived vesicles as autologous cancer vaccines for boosting systemic immune responses. Nat Commun. 2022;13:6534. doi: 10.1038/s41467-022-33831-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gao M, Deng H, Zhang Y, et al. Hyaluronan nanogel co-loaded with chloroquine to enhance intracellular cisplatin delivery through lysosomal permeabilization and lysophagy inhibition. Carbohydr Polym. 2024;323:121415. doi: 10.1016/j.carbpol.2023.121415 [DOI] [PubMed] [Google Scholar]
- 76. Luo Y, Ziebell MR, Prestwich GD. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules. 2000;1:208-218. doi: 10.1021/bm000283n [DOI] [PubMed] [Google Scholar]
- 77. Piskin E. Biodegradable polymers in medicine. In: Scott G, ed. Degradable Polymers. Springer; 2002;321-379. [Google Scholar]
- 78. Meo CD, Panza L, Capitani D, et al. Hyaluronan as carrier of carboranes for tumor targeting in boron neutron capture therapy. Biomacromolecules. 2007;8:552-559. doi: 10.1021/bm0607426 [DOI] [PubMed] [Google Scholar]
- 79. Wang JF. Paclitaxel-loaded microspheres incorporated into chitosan and hyaluronic acid films for prevention of post-surgical adhesions. [Thesis, The University of British Columbia]. 2001. [Google Scholar]
- 80. Zhang L, Yang P, Chen J, et al. CD44 connects autophagy decline and ageing in the vascular endothelium. Nat Commun. 2023;14:5524. doi: 10.1038/s41467-023-41346-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Anttila MA, Tammi RH, Tammi MI, Syrjänen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150-155. [PubMed] [Google Scholar]
- 82. Zhao P, Damerow MS, Stern P, et al. CD44 promotes Kras-dependent lung adenocarcinoma. Oncogene. 2013;32:5186-5190. doi: 10.1038/onc.2012.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang C, Sheng Y, Shi X, et al. CD44/HA signaling mediates acquired resistance to a PI3Kα inhibitor. Cell Death Dis. 2020;11:831. doi: 10.1038/s41419-020-03037-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mohammed AI, Celentano A, Paolini R, et al. High molecular weight hyaluronic acid drastically reduces chemotherapy-induced mucositis and apoptotic cell death. Cell Death Dis. 2023;14:453. doi: 10.1038/s41419-023-05934-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li J, Yin T, Wang L. et al. Biological evaluation of redox-sensitive micelles based on hyaluronic acid-deoxycholic acid conjugates for tumor-specific delivery of paclitaxel. Int J Pharm. 2015;483(1-2):38-48. doi:10.1016/j.ijpharm.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 86. McGuire J, Taguchi T, Tombline G, et al. Hyaluronidase inhibitor delphinidin inhibits cancer metastasis. Sci Rep. 2024;14:14958. doi: 10.1038/s41598-024-64924-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu Z, Hou P, Fang J, et al. Mesenchymal stromal cells confer breast cancer doxorubicin resistance by producing hyaluronan. Oncogene. 2023;42:3221-3235. doi: 10.1038/s41388-023-02837-w [DOI] [PubMed] [Google Scholar]
- 88. Stenzel MH. Bioconjugation using thiols: old chemistry rediscovered to connect polymers with nature’s building blocks. ACS Macro Lett. 2013;2:14-18. doi: 10.1021/mz3005814 [DOI] [PubMed] [Google Scholar]
- 89. Wang X, Song Y, Yu L, et al. Co-delivery of hesperetin and cisplatin via hyaluronic acid-modified liposome for targeted inhibition of aggression and metastasis of triple-negative breast cancer. ACS Appl Mater Interfaces. 2023;15:34360-34377. doi: 10.1021/acsami.3c03233 [DOI] [PubMed] [Google Scholar]
- 90. Gao W, Liu YF, Zhang YX, et al. The potential role of hydrogen sulfide in cancer cell apoptosis. Cell Death Discov. 2024;10:114. doi: 10.1038/s41420-024-01868-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Opoku-Damoah Y, Zhang R, Ta HT, Xu ZP. Therapeutic gas-releasing nanomedicines with controlled release: advances and perspectives. Exploration (Beijing). 2022;2:20210181. doi: 10.1002/exp.20210181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cai F, Xu H, Cao N, et al. ADT-OH, a hydrogen sulfide-releasing donor, induces apoptosis and inhibits the development of melanoma in vivo by upregulating FADD. Cell Death Dis. 2020;11:33. doi: 10.1038/s41419-020-2222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dong Q, Yang B, Han JG, et al. A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Lett. 2019;455:60-72. doi: 10.1016/j.canlet.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 94. Yu S, Cao Z, Cai F, et al. ADT-OH exhibits anti-metastatic activity on triple-negative breast cancer by combinatorial targeting of autophagy and mitochondrial fission. Cell Death Dis. 2024;15:463. doi: 10.1038/s41419-024-06829-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Müller S, Sindikubwabo F, Cañeque T, et al. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020;12:929-938. doi: 10.1038/s41557-020-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wickens JM, Alsaab HO, Kesharwani P, et al. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov Today. 2017;22:665-680. doi: 10.1016/j.drudis.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhou YQ, Liu DQ, Chen SP, et al. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol Sin. 2020;41:1041-1048. doi: 10.1038/s41401-020-0394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ling B, Gungoren B, Yao Y, et al. Truly tiny acoustic biomolecules for ultrasound imaging and therapy. Adv Mater. 2024;36:e2307106. doi: 10.1002/adma.202307106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xia L, Chen M, Dong C, et al. Spatiotemporal Ultrasound-Driven Bioorthogonal Catalytic Therapy. Adv Mater. 2023;35:e2209179. doi: 10.1002/adma.202209179 [DOI] [PubMed] [Google Scholar]
- 100. Tang X, Hao N, Zhou Y, Liu Y. Ultrasound targeted microbubble destruction-mediated SOCS3 attenuates biological characteristics and epithelial-mesenchymal transition (EMT) of breast cancer stem cells. Bioengineered. 2022;13:3896-3910. doi: 10.1080/21655979.2022.2031384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Takabatake D, Fujita T, Shien T, et al. Tumor inhibitory effect of gefitinib (ZD1839, Iressa) and taxane combination therapy in EGFR-overexpressing breast cancer cell lines (MCF7/ADR, MDA-MB-231). Int J Cancer. 2007;120:181-188. doi: 10.1002/ijc.22187 [DOI] [PubMed] [Google Scholar]
- 102. Thomas JR, Frye WJE, Robey RW, Gottesman MM. Progress in characterizing ABC multidrug transporters in zebrafish. Drug Resist Updat. 2024;72:101035. doi: 10.1016/j.drup.2023.101035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Thumar M, Liu M. Applications in molecular ultrasound imaging: present and future. Adv Ultrasound Diagn Ther. 2019;3:62-75. [Google Scholar]
- 104. Liu L, Zhang Q, Wang C, et al. Single-cell diagnosis of cancer drug resistance through the differential endocytosis of nanoparticles between drug-resistant and drug-sensitive cancer cells. ACS Nano. 2023;17:19372-19386. doi: 10.1021/acsnano.3c07030 [DOI] [PubMed] [Google Scholar]
- 105. Roth TL, Marson A. Genetic disease and therapy. Annu Rev Pathol. 2021;16:145-166. doi: 10.1146/annurev-pathmechdis-012419-032626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huang S, Shang M, Guo L, et al. Hydralazine loaded nanodroplets combined with ultrasound-targeted microbubble destruction to induce pyroptosis for tumor treatment. J Nanobiotechnology. 2024;22:193. doi: 10.1186/s12951-024-02453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li XH, Zhou P, Wang LH, et al. The targeted gene (KDRP-CD/TK) therapy of breast cancer mediated by SonoVue and ultrasound irradiation in vitro. Ultrasonics. 2012;52:186-191. doi: 10.1016/j.ultras.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 108. Cao Y, Tang H, Wang G, et al. Targeting survivin with tanshinone IIA inhibits tumor growth and overcomes chemoresistance in colorectal cancer. Cell Death Discov. 2023;9:351. doi: 10.1038/s41420-023-01622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen ZY, Liang K, Sheng XJ, et al. Optimization and apoptosis induction by RNAi with UTMD technology in vitro. Oncol Lett. 2012;3:1030-1036. doi: 10.3892/ol.2012.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology (Williston Park). 2015;29:375-385. [PubMed] [Google Scholar]
- 111. Fatima SW, Khare SK. Benefits and challenges of antibody drug conjugates as novel form of chemotherapy. J Control Release. 2022;341:555-565. doi: 10.1016/j.jconrel.2021.12.013 [DOI] [PubMed] [Google Scholar]
- 112. Wei Q, Li P, Yang T, et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J Hematol Oncol. 2024;17:1. doi: 10.1186/s13045-023-01509-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jabbour E, Paul S, Kantarjian H. The clinical development of antibody—drug conjugates—lessons from leukaemia. Nat Rev Clin Oncol. 2021;18:418-433. doi: 10.1038/s41571-021-00484-2 [DOI] [PubMed] [Google Scholar]
- 114. Tarantino P, Tolaney SM. The dawn of the antibody-drug conjugates era: how T-DM1 reinvented the future of chemotherapy for solid tumors. Cancer Res. 2022;82:3659-3661. doi: 10.1158/0008-5472.Can-22-2324 [DOI] [PubMed] [Google Scholar]
- 115. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327-344. doi: 10.1038/s41571-021-00470-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fang J, Guo L, Zhang Y, Guo Q, Wang M, Wang X. The target atlas for antibody-drug conjugates across solid cancers. Cancer Gene Ther. 2024;31:273-284. doi: 10.1038/s41417-023-00701-3 [DOI] [PubMed] [Google Scholar]
- 117. Jin Y, Schladetsch MA, Huang X, Balunas MJ, Wiemer AJ. Stepping forward in antibody-drug conjugate development. Pharmacol Ther. 2022;229:107917. doi: 10.1016/j.pharmthera.2021.107917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126:4278-4288. doi: 10.1002/cncr.33102 [DOI] [PubMed] [Google Scholar]
- 119. Passaro A, Jänne PA, Peters S. Antibody-drug conjugates in lung cancer: recent advances and implementing strategies. J Clin Oncol. 2023;41:3747-3761. doi: 10.1200/jco.23.00013 [DOI] [PubMed] [Google Scholar]
- 120. Foldi J, Geyer CE, Jr. Precision medicine for metastatic TNBC: the FUTURE is now. Cell Res. 2023;33:491-492. doi: 10.1038/s41422-023-00815-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tarantino P, Ricciuti B, Pradhan SM, Tolaney SM. Optimizing the safety of antibody-drug conjugates for patients with solid tumours. Nat Rev Clin Oncol. 2023;20:558-576. doi: 10.1038/s41571-023-00783-w [DOI] [PubMed] [Google Scholar]
- 122. Malli Cetinbas N, Monnell T, Soomer-James J, et al. Tumor cell-directed STING agonist antibody-drug conjugates induce type III interferons and anti-tumor innate immune responses. Nat Commun. 2024;15:5842. doi: 10.1038/s41467-024-49932-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ning WJ, Liu X, Zeng HY, An ZQ, Luo WX, Xia NS. Recent progress in antibody-based therapeutics for triple-negative breast cancer. Expert Opin Drug Deliv. 2022;19:815-832. doi: 10.1080/17425247.2022.2093853 [DOI] [PubMed] [Google Scholar]
- 124. Cortés J, Hurvitz SA, Im SA, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: long-term survival analysis of the DESTINY-Breast03 trial. Nat Med. 2024;30:2208-2215. doi: 10.1038/s41591-024-03021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105-117. doi: 10.1016/s0140-6736(22)02420-5 [DOI] [PubMed] [Google Scholar]
- 126. Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:816-826. doi: 10.1016/s1470-2045(19)30097-x [DOI] [PubMed] [Google Scholar]
- 127. Kuo P, Elboudwarej E, Zavodovskaya M, et al. Trop-2 expression in non-small cell lung cancer. PLoS ONE. 2025;20:e0321555. doi: 10.1371/journal.pone.0321555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. LeVee A, Wong M, Ruel N, et al. Trop-2 expression as a biomarker of response to sacituzumab govitecan in patients with HER2-negative metastatic breast cancer: a pilot study. Cancer Treat Res Commun. 2025;44:100954. doi: 10.1016/j.ctarc.2025.100954 [DOI] [PubMed] [Google Scholar]
- 129. Sakach E, Sacks R, Kalinsky K. Trop-2 as a therapeutic target in breast cancer. Cancers (Basel). 2022;14:5936. doi: 10.3390/cancers14235936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529-1541. doi: 10.1056/NEJMoa2028485 [DOI] [PubMed] [Google Scholar]
- 131. Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1423-1433. doi: 10.1016/s0140-6736(23)01245-x [DOI] [PubMed] [Google Scholar]
- 132. Bardia A, Mayer IA, Diamond JR, et al. Efficacy and safety of anti-trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol. 2017;35:2141-2148. doi: 10.1200/jco.2016.70.8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Al Jarroudi O, El Bairi K, Curigliano G, Afqir S. Antibody-drug conjugates: a new therapeutic approach for triple-negative breast cancer. Cancer Treat Res. 2023;188:1-27. doi: 10.1007/978-3-031-33602-7_1 [DOI] [PubMed] [Google Scholar]
- 134. Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. NPJ Breast Cancer. 2022;8:95. doi: 10.1038/s41523-022-00468-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Paul S, Konig MF, Pardoll DM, et al. Cancer therapy with antibodies. Nat Rev Cancer. 2024;24:399-426. doi: 10.1038/s41568-024-00690-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Melear J, Lakhani N, O’Shaughnessy JA, et al. Abstract A088: novel anti-EGFR antibody-drug conjugate AVID100: a phase 2a trial in patients with EGFR-overexpressing advanced solid tumors. 2019;18:A088-0A88. [Google Scholar]
- 137. Yu HA, Goto Y, Hayashi H, et al. HERTHENA-Lung01, a phase II trial of patritumab deruxtecan (HER3-DXd) in epidermal growth factor receptor-mutated non-small-cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum-based chemotherapy. J Clin Oncol. 2023;41:5363-5375. doi: 10.1200/jco.23.01476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Papa F, Grinda T, Rassy E, et al. Long road towards effective HER3 targeting in breast cancer. Cancer Treat Rev. 2024;129:102786. doi: 10.1016/j.ctrv.2024.102786 [DOI] [PubMed] [Google Scholar]
- 139. Xiao T, Ali S, Mata D, Lohmann AE, Blanchette PS. Antibody-drug conjugates in breast cancer: ascent to destiny and beyond-a 2023 review. Curr Oncol. 2023;30:6447-6461. doi: 10.3390/curroncol30070474 [DOI] [PMC free article] [PubMed] [Google Scholar]