Abstract
Ferroptosis is a newly discovered iron-dependent programmed cell death characterized by excess lipid peroxidation. It is emerging as a promising target for tumor therapies. In the present study, we first identify Cathepsin S (CTSS) as a novel ferroptosis regulator. CTSS is upregulated in ferroptosis-resistant hepatocellular carcinoma (HCC) cells, and suppression of CTSS sensitizes HCC cells to ferroptosis. Mechanistically, ferroptosis stress induces CTSS maturation and promotes the autophagy-lysosomal degradation of Kelch-like ECH-associated protein 1 (KEAP1). This process blocks KEAP1-dependent, ubiquitination-mediated degradation of nuclear factor E2-related factor 2 (NRF). Consequently, the accumulated NRF2 translocates from the cytoplasm to the nucleus and drives the transcription of anti-ferroptosis genes. In vivo study reveals that CTSS depletion, achieved through either shRNA or the specific inhibitor LY3000328, in combination with a ferroptosis inducer, inhibits HCC tumor growth in orthotopic xenograft mouse models. In conclusion, the above data suggest that CTSS can potentiate ferroptosis in HCC cells and may be a therapeutic target to overcome ferroptosis resistance in HCC patients.
Keywords: CTSS, LY3000328, Kelch-like ECH-associated protein 1, HCC, Nuclear factor E2-related factor 2
Graphical abstract
Highlights
-
•
CTSS is upregulated in ferroptosis-resistant HCC cells.
• Suppressing CTSS sensitizes HCC to ferroptosis both in vitro and in vivo.
• CTSS activates NRF2 by promoting autophagy-lysosomal degradation of KEAP1.
• CTSS may serve as a potential therapeutic target to overcome ferroptosis resistance in HCC.
1. Introduction
Hepatocellular carcinoma (HCC) is a prevalent malignancy globally, ranking as the sixth most common cancer and the third leading cause of cancer-related mortality [1]. Despite advancements in targeted therapies, immunotherapies, and interventional approaches for patients with intermediate to advanced HCC, therapeutic response rates remain suboptimal [2]. This limited efficacy can be attributed to the inherent malignancy and heterogeneity of HCC cells, which frequently exhibit intrinsic or acquired resistance to drug-induced cell death. Consequently, elucidating the molecular mechanisms underlying drug resistance in HCC and identifying novel therapeutic targets remain a critical research priority.
Ferroptosis, a distinct form of programmed cell death characterized in 2012, is defined by iron-dependent accumulation of lipid peroxidation in polyunsaturated fatty acids [3]. Unlike apoptosis, necrosis, and other forms of cell death, ferroptosis exhibits unique morphological characteristics, including compromised plasma membrane integrity, mitochondria shrinkage with condensed membranes, reduced cristae, and chromatin condensation [4,5]. The regulation of ferroptosis primarily involves three fundamental processes: iron homeostasis, cytomembrane lipid peroxidation, and cellular antioxidant capacity [6]. Notably, sorafenib, the first-line targeted therapy approved for intermediate to advanced HCC, has been demonstrated to induce ferroptosis in HCC cells through mechanisms independent of its multi-kinase inhibition [7]. Despite extensive research establishing ferroptosis as a promising target in tumor prevention and treatment, the sensitivity of HCC cells to ferroptosis-inducing agents remains suboptimal. Consequently, strategies to overcome ferroptosis resistance and enhance susceptibility represent an innovative approach to HCC therapy.
Conventional anti-tumor therapies, such as chemotherapy and targeted therapy, can elevate intracellular reactive oxygen species (ROS), inducing oxidative stress to eliminate cancer cells [8]. However, cancer cells possess intrinsic and acquired antioxidant capacities that impede these therapies. Nuclear factor E2-related factor 2 (NRF2) serves as the master regulator of the cellular antioxidant system [9]. Under basal conditions, NRF2 expression is tightly regulated at low levels due to ubiquitination and proteasomal degradation mediated by the E3 ligase Kelch-like ECH-associated protein 1 (KEAP1). During oxidative stress, increased intracellular ROS modify the cysteine residues within KEAP1, leading to conformational changes that disrupt the interaction between KEAP1 and NRF2 [10]. This alteration stabilizes NRF2 in the cytoplasm, facilitating its translocation to the nucleus. Within the nucleus, NRF2 binds to antioxidant response elements (ARE) on DNA, thereby promoting the transcription of downstream cytoprotective target genes [11]. Gain-of-function mutations in NRF2 and loss-of-function mutations in KEAP1 have been identified in HCC, enhancing NRF2 activity [12]. Notably, studies indicate that KEAP1 mutations occur in 7 % of HCC cases, directly affecting KEAP1-mediated NRF2 degradation [13]. Given the central role of the intracellular peroxide-antioxidant system in regulating ferroptosis sensitivity, NRF2 emerges as a key hub for this process. Consequently, targeting the KEAP1-NRF2 signaling axis represents a potential strategy to address ferroptosis resistance and enhance susceptibility to ferroptosis in HCC.
Cathepsin S (CTSS), a member of the cathepsin family, resides within lysosomes and participates in various cellular processes, including protein degradation, lipid metabolism, autophagy, antigen presentation, growth factor receptor recycling, and cellular signaling pathways [14,15]. CTSS is upregulated in the majority of solid tumors and functions as a tumor promoter in various malignancies, such as non-Hodgkin lymphoma, breast cancer, colorectal cancer, gastric cancer, and HCC [[16], [17], [18]]. Initially synthesized as an inactive proteolytic proenzyme (pro-CTSS), CTSS undergoes activation under specific conditions. This activation can occur through cleavage by other proteases or through autocatalysis, yielding the mature active form (mature-CTSS) [19]. Furthermore, CTSS can be secreted extracellularly via exocytosis to degrade the extracellular matrix, thereby promoting tumor invasion and metastasis [20]. Recent investigations have revealed that CTSS orchestrates a pro-tumor microenvironment by cleaving CD74, which regulates MHC-II–dependent antigen presentation [21]. In breast cancer, CTSS binds to BRCA1, facilitating its ubiquitin-mediated degradation through cleavage [18].
Herein, this study uncovered that CTSS upregulation in HCC cells confers ferroptosis resistance through KEAP1 degradation and subsequent NRF2 activation, while CTSS inhibition enhanced the efficacy of ferroptosis inducers in HCC mouse models, establishing CTSS as a potential therapeutic target.
2. Methods
2.1. Reagents
The following chemicals and reagents were used: erastin (HY-15763), tBHQ (HY-100489), Chloroquine (HY-17589A), LY3000328 (HY-15533), and Protein A/G Magnetic Beads (HY–K0202) from MedChem Express; RSL3 (S8155), Ferrostatin-1 (S7243), Liproxstatin-1 (S7699), MG-132 (S2619), and Cycloheximide (S7418) from Selleck; and Ferric citrate (A500061) from Sangon Biotechnology.
2.2. Cell lines and cell culture
PLC/PRF/5, Hep3B, Hepa1-6, and HEK293T cells were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China). All cells were cultured in complete Dulbecco's Modified Eagle's Medium (DMEM; HyClone, USA), supplemented with 10 % fetal bovine serum (Sigma) and 1 % penicillin/streptomycin (Gibco). The cells were maintained in a 37 °C incubator with 5 % CO2.
2.3. Plasmids and lentiviral infection
Human pGMLV-shNT, pGMLV-shCTSS, pGMLV-FLAG-CTSS, pGMLV-FLAG-CTSS-C25A, pGMLV-6xHis-KEAP1, pGMLV-MYC-NRF2, pGMLV-HA-UB, pGMLV-HA-K48-UB, and pGMLV-HA-K63-UB were designed and purchased from Genomeditech Co., Ltd. (Shanghai, China). To establish HCC cells with stable CTSS knockdown, a lentiviral infection system was used. Briefly, 10 μg of the targeted plasmid, 5 μg of psPAX (packaging plasmid), and 7.5 μg of pMD2.G (envelope plasmid) were co-transfected into HEK293T cells using Lipofectamine™ 3000 Reagent (L3000075, Invitrogen, USA) according to the manufacturer's protocols. After 48 h of transfection, the culture supernatants from HEK293T cells were collected and centrifuged. Wild-type PLC/PRF/5 and Hep3B cells, grown to 50 % confluence in 6-well plates, were infected with the collected lentiviral supernatant in the presence of 5 μg/mL polybrene (C0351, Beyotime, China) for 8 h. The media were then replaced with complete DMEM containing 2 μg/mL puromycin (ST551, Beyotime, China) to select for cells with stable CTSS knockdown.
2.4. Small interfering RNA (siRNA) and transfection
Human siRNA targeting KEAP1 and P62 was designed and acquired from Tsingke Biotech Co., Ltd, Beijing, China. To transiently knock down KEAP1 and P62, cells were transfected with these siRNA using Lipofectamine™ RNAiMAX Reagent (13778100, Invitrogen, USA) according to the manufacturer's protocols.
2.5. In vitro establishment of erastin-resistant HCC cells
Erastin-resistant Hepa1-6 cells were established by exposing wild-type HCC cells to progressively increasing concentrations of erastin, ultimately reaching 20 μmol/L. The establishment of erastin-resistant HCC cells was confirmed using CCK-8 assays. To maintain the erastin-resistant phenotype of Hepa1-6 ER cells, these cells were cultured long-term in DMEM medium supplemented with 5 μM erastin. The resistance stability to erastin was routinely assessed using CCK-8 assay at 4-week intervals, ensuring consistent phenotypic retention throughout the study.
2.6. Cell viability assays
HCC cells were plated at a density of 5000 cells per well in a 96-well plate and allowed to attach overnight. The medium in each well was then replaced with complete DMEM containing the indicated concentration of erastin (S7242, Selleck, USA) and RSL3 (S8155, Selleck, USA). After 24 h of incubation, cell viability was measured using the CCK-8 assay kit (40203ES80, Yeasen, China), according to the manufacturer's protocols.
2.7. Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was isolated from cells using RNAiso Plus (9109, Takara, Japan) and successively extracted with chloroform and isopropyl alcohol, according to the manufacturer's protocols. cDNA was subsequently synthesized using the RT reagent Kit (RR047A, Takara, Japan). Quantitative RT-PCR was applied using qPCR SYBR Green Master Mix (11202ES03, Yeasen, China), and the reactions were analyzed with the ABI Prism 7500 Sequence Detection System (Applied Biosystems, USA). The expression levels of genes were normalized to β-actin and quantified using the 2−ΔΔCT method. Primer sequences were listed in the Supplementary Table 2.
2.8. Western blot analysis
Cell and tissue samples were lysed in RIPA buffer (WB6001, WeiaoBio, China), supplemented with PMSF, and Protease and Phosphatase Inhibitors (WB2122, WeiaoBio, China) at 4 °C for 15 min, followed by centrifugation at 12,000g for 15 min at 4 °C. The supernatants were collected for subsequent analysis. To extract cytoplasmic and nuclear proteins, NE-PER Nuclear and Cytoplasmic Extraction Reagents (78833, Thermo Scientific, USA) were used according to the manufacturer's protocols. The extracted proteins were subjected to electrophoresis, transferred to PVDF membranes, and blocked with 5 % non-fat milk at room temperature for 1 h. The membranes were incubated with primary antibodies at 4 °C overnight. On the following day, the membranes were incubated with HRP-conjugated secondary antibodies at room temperature for 1 h, then washed with TBST at least three times. Protein bands were visualized using an enhanced chemiluminescence assay (180–501, Tanon, China).
2.9. Measurement of GSH/GSSG ratio
The GSH and GSSG assay kit was applied to measure the intracellular ratio of GSH/GSSG, according to the manufacturer's protocols. In brief, 1 × 106 cells were lysed, and the lysate was centrifuged at 4 °C. The supernatant was collected for the determination of total glutathione and GSSG levels, respectively. Glutathione reacts with 2-nitrobenzoic acid (DTNB) to produce the yellow 5-thio-2-nitrobenzoic acid (TNB). The concentration of TNB, which reflects the amount of GSH, was quantified spectrophotometrically at 412 nm.
2.10. Lipid peroxidation
The C11-BODIPY (581/591) probe (D3861, Invitrogen, USA) was applied to evaluate intracellular lipid peroxidation. HCC cells grown in a 6-well plate were exposed to DMEM with 5 μM C11-BODIPY (581/591) and incubated at 37 °C, protected from light for 30 min. After incubation, the cells were washed three times with PBS and resuspended for flow cytometric analysis.
2.11. Measurement of malondialdehyde (MDA)
The intracellular MDA levels were quantified using an MDA assay kit, according to the manufacturer's protocols. Briefly, 1 × 106 cells were lysed and centrifuged at 4 °C. The resulting supernatant was treated under high temperature and acidic conditions, allowing MDA to conjugate with thiobarbituric acid (TBA) to form an MDA-TBA adduct. The concentration of the adduct, which reflects MDA levels, was measured spectrophotometrically at 535 nm.
2.12. Co-immunoprecipitation
Cell samples were lysed in IP lysis buffer (WB0105, WeiaoBio, China), supplemented with PMSF, and Protease and Phosphatase Inhibitors (WB2122, WeiaoBio, China) at 4 °C for 15 min, followed by centrifugation at 12,000g for 15 min at 4 °C. The supernatants were collected for subsequent analysis. The supernatant was incubated with the indicated antibodies at 4 °C overnight with gentle rotation. On the second day, Protein A/G Magnetic Beads (HY–K0202, MCE, USA) were added to the lysates to capture the protein-antibody complexes at room temperature for 30 min. The protein-antibody-magnetic beads complexes were then washed five times on ice with IP lysis buffer and resuspended in SDS-PAGE loading buffer. The samples were heated at 100 °C for 10 min to denature the proteins.
2.13. Immunofluorescence assay
HCC cells were seeded onto coverslips and allowed to attach overnight. The following day, cells on the coverslips were fixed with 4 % paraformaldehyde at room temperature for 15 min, permeabilized in 0.2 % Triton X-100 for 10 min and blocked with 5 % BSA at room temperature for 1 h. The samples were then incubated with the specified primary antibodies at 4 °C overnight. On the next day, the samples were incubated with Goat Anti-Rabbit IgG H&L (Alexa Fluor® 594) antibodies (A-11012, Invitrogen, USA) and Goat Anti-Mouse IgG H&L (Alexa Fluor® 488) antibodies (A-10680, Invitrogen, USA) at room temperature for 1 h and washed with PBST three times. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and the slides were visualized under a confocal microscope (Leica, Germany). To evaluate the colocalization between KEAP1 and LAMP1, Mander's coefficient analysis was applied by using ImageJ software.
2.14. Immunohistochemistry assay
HCC tumor tissues were fixed in 4 % paraformaldehyde, embedded in paraffin and sectioned into 4 μm slices. After deparaffinization, hydration, and antigen retrieval, the sections were blocked with goat serum. They were then incubated with the specified primary antibodies at 4 °C overnight. The following day, HRP-conjugated secondary antibodies were applied at room temperature for 1 h, and the sections were subsequently stained with diaminobenzidine (DAB) solution. The slides were independently examined by two pathologists under a light microscope. Immunohistochemical staining was evaluated using a histological scoring system. The positive staining area was scored as follows: 1 (<25 %), 2 (26–50 %), 3 (51–75 %), and 4 (76–100 %). Staining intensity was scored as: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The overall IHC score was calculated by multiplying the scores for positive staining area and staining intensity.
2.15. Spontaneous HCC murine model
Following protocols validated in prior investigations, a plasmid mixture, containing 30 μg MYC + AKT1 co-expressing plasmid, 30 μg pX330 sg-p53 plasmid, and 10 μg SB13 transposase-encoding plasmid prepared in 2 ml of sterile PBS was administered to C57BL/6 mice through tail vein intravenous injection within 3–5 s. Spontaneous HCC developed in mice 3–4 weeks after tail vein injection, as confirmed by IHC staining for HCC-specific markers [22].
2.16. Animal studies
Male 5-week-old BALB/c nude mice and male 5-week-old C57BL/6 mice were purchased from Shanghai Jihui Laboratory Animal Company (Shanghai, China). All mice were housed in a standard pathogen-free (SPF) environment at the Department of Laboratory Animal Science, Fudan University. To establish subcutaneous xenograft models in BALB/c nude mice, 1 × 107 PLC/PRF/5/shCTSS and control PLC/PRF/5/shNT cells were injected into the right flanks of the mice. The mice were randomly divided into four groups: Group 1 consisted of mice bearing PLC/PRF/5/shNT cells and receiving vehicle treatment (0.9 % NaCl intraperitoneally every other day); Group 2 included mice bearing PLC/PRF/5/shNT cells and receiving erastin (15 mg/kg intraperitoneally every other day); Group 3 comprised mice bearing PLC/PRF/5/shCTSS cells and receiving vehicle treatment; and Group 4 included mice bearing PLC/PRF/5/shCTSS cells and receiving erastin. Mice were observed and monitored every other day, and tumor volumes were calculated using the formula: Tumor volume (mm^3) = 0.5 × length × width2. After 28 days, the mice were sacrificed, and subcutaneous tumor weights were measured. For the C57BL/6 mice, 5 × 106 Hepa1-6 cells were injected into the right flanks to establish subcutaneous xenograft models. For the C57BL/6 mice, 5 × 106 Hepa1-6 cells were injected into the right flanks to establish subcutaneous xenograft models. The mice were randomly assigned to four groups: Group 1 received vehicle treatment; Group 2 was treated with erastin; Group 3 was treated with a CTSS inhibitor (LY3000328, 5 mg/kg, intraperitoneally, every other day); and Group 4 received both LY3000328 and erastin. Mice were observed and monitored every other day, and tumor volumes were calculated. After 21 days, the mice were sacrificed, and subcutaneous tumor weights were assessed. Tumor tissues were subsequently analyzed using immunohistochemistry (IHC) assays. Erastin was dissolved in a mixture of 5 % DMSO, 40 % PEG300, 5 % Tween 80, and 50 % water, while LY3000328 was prepared in 20 % DMSO and 80 % PBS, as described previously [23]. All animal care and experimental procedures were conducted in compliance with the guidelines set by the Shanghai Medical Experimental Animal Care Commission.
2.17. Bioinformatics
RNA sequencing for whole transcriptome analysis was applied using Illumina NovaSeq 6000 platform, following the manufacturer's protocol. Total RNA was extracted from both wild-type Hepa1-6 and erastin-resistant Hepa1-6 cells using RNAiso Plus (9109, Takara, Japan). Three biological replicates underwent sequencing analysis after quality assessment with total read outputs of 5–8 Gb and base call accuracy exceeding 90 % at Q20 threshold. Differentially expressed gene analysis and volcano plots were generated using R software (www.r-project.org). To examine CTSS mRNA expression levels in HCC tumor tissues and normal tissues, mRNA sequence data from the LIHC dataset of TCGA database were downloaded through Cbioportal. Pearson correlation tests were used to evaluate the relationship between CTSS expression and NRF2 target genes. For functional enrichment analysis, a total of 372 HCC patients were divided into high and low CTSS expression groups based on the median CTSS expression level. Gene Set Enrichment Analysis (GSEA) was conducted with the GSEA software (http://www.gsea-msigdb.org/gsea/index.jsp, version 4.0.0). To investigate the relationship between CTSS expression and responses to anti-cancer drugs, the Cancer Therapeutics Response Portal (CTRP) was utilized as previously described [24]. The transcriptome sequencing data generated in this study have been deposited in NCBI Sequence Read Archive (SRA) and are accessible through SRA number PRJNA1251835.
2.18. Statistics
Statistical analyses were performed using SPSS 23.0 (IBM, Armonk, NY, USA), GraphPad Prism 8.0 (GraphPad Inc.), and R software (version 4.2.1). Pearson's correlation test was applied to analyze linear relationships. The student's t‐test was performed to analyze the differences between the two groups with a normal distribution. Analysis of variance (ANOVA) was used to compare differences among more than two groups. All data were acquired from at least three independent biological replicates and were presented as mean ± standard deviation (SD). p < 0.05 (two-tailed) was considered statistically significant.
3. Results
3.1. CTSS is overexpressed in ferroptosis-resistant HCC cells
To investigate the underlying mechanisms of ferroptosis resistance in HCC, we established an erastin-resistant HCC cell line, HepaER. Erastin, a direct inhibitor of the cystine/glutamate antiporter (xCT), induces ferroptosis by depleting intracellular cysteine [25]. We exposed wild-type murine-derived Hepa1-6 cells to gradually increasing concentrations of erastin over three months (Fig. 1A). The established HepaER cells demonstrated reduced cell death and increased viability compared to parental cells following erastin treatment, as verified by CCK8 assay (Fig. 1B). RNA sequencing and differential gene analysis were conducted on HepaER-cells and their parental wild-type cells. The volcano plots highlighted CTSS among the top five upregulated genes in erastin-resistant HCC cells (Fig. 1C). Among the eleven members of the cathepsin protease family, CTSS ranked as the most significantly overexpressed gene (Fig. 1D). Western blot analysis demonstrated a significant upregulation of CTSS protein expression in HepaER cells compared to wild-type Hepa1-6 cells (Fig. 1E). Further analysis using dataset GSE173905 from the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database revealed that CTSS was also overexpressed in RSL3-resistant breast cancer cells (Fig. 1F) [26]. To assess the correlation between CTSS expression and anti-cancer drug response, we analyzed data from the Cancer Therapeutics Response Portal (CTRP) (http://portals.broadinstitute.org/ctrp.v2.1/), which integrates gene expression data with responses to 481 compounds across cancer cell lines. We identified a positive association between CTSS expression levels and the sensitivity of HCC cell lines to ferroptosis inducers, erastin and sorafenib (Fig. 1G and S1A).
Fig. 1.
CTSS is upregulated in erastin-resistant HCC cells, and ferroptosis promotes the maturation of CTSS. (A) A schematic diagram of the construction of erastin-resistant HCC cells. (B) Erastin-resistant Hepa1-6 cells were validated by CCK-8 assay. (C) Volcano plot of differential gene analysis between HepaER cells and wild-type Hepa1-6 cells from RNA sequencing data. (D) Cathepsin family genes mRNA expression in HepaER cells and wild-type Hepa1-6 cells. (E) Western blot analysis of CTSS protein expression in the indicated cells. (F) CTSS mRNA expression in RSL3-resistant cells and parental control cells. (G) Correlation between CTSS expression and erastin sensitivity based on the HCC cell lines from Cancer Therapeutics Response Portal (CTRP) database. (H) CTSS mRNA expression in tumor and adjacent normal tissue samples from LIHC data set in TCGA database. (I) qRT-PCR analysis of CTSS mRNA expression in matched tumor and adjacent normal tissues from spontaneous HCC murine model. (J) Western blot analysis of CTSS protein expression in randomly selected 12-paired HCC tumors (T) and adjacent normal tissues (N). (K) Pro-CTSS and mature-CTSS protein expression levels from the indicated cells were assessed using Western blot. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. For FAC treatment, PLC/PRF/5 cells were pre-treated with 1000 μM FAC for 24 h before collection, and Hep3B cells were pre-treated with 500 μM FAC for 24 h before collection. (L) Western blot analysis of pro-CTSS and mature-CTSS protein expression from the indicated cells. For erastin treatment, PLC/PRF/5 cells were pre-treated with 5 μM and 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 1 μM and 2 μM erastin for 24 h before collection. For Fer-1 and Lx-1 treatment, PLC/PRF/5 cells and Hep3B cells were pre-treated with 10 μM Fer-1 and Lx-1 for 24 h before collection. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Furthermore, analysis of RNA-sequencing data from the LIHC dataset in The Cancer Gene Atlas (TCGA) database and mRNA expression in matched tumor and adjacent normal tissues from a spontaneous HCC murine model revealed significant upregulation of CTSS mRNA expression in HCC (p < 0.0001; Fig. 1H–I). In randomly selected 12 pairs of HCC and adjacent non-tumor samples, the protein expression of CTSS was markedly higher in HCC tissues compared to adjacent non-tumor tissues (Fig. 1J). CTSS is initially synthesized as an inactive proenzyme (pro-CTSS), consisting of 331 amino acids. Under specific conditions, pro-CTSS is converted into its active form (mature-CTSS) through proteolytic cleavage [14]. To identify the potential role of CTSS in HCC cell lines under ferroptosis stress, PLC/PRF/5 and Hep3B cells were treated with two different ferroptosis inducers, erastin and ferric ammonium citrate (FAC). A remarkable increase was observed in the ratio of mature-CTSS (24 kDa) to pro-CTSS (35 kDa) under ferroptosis stress conditions (Fig. 1K), and this phenotype could be reversed by addition of ferroptosis inhibitors Ferrostatin-1 (Fer-1) and Liprostatin-1 (Lx-1; Fig. 1L). Together, these results suggest that ferroptosis stress facilitates the maturation of CTSS.
3.2. Knockdown of CTSS sensitizes HCC cells to ferroptosis
Following the observation that CTSS was upregulated in ferroptosis-resistant HCC cells and its expression negatively correlated with ferroptosis sensitivity, we investigated whether CTSS knockdown could sensitize HCC cells to ferroptosis inducers. Under erastin treatment, CTSS knockdown contributed to reduced cell viability in PLC/PRF/5 cells compared to control shNT cells, indicating enhanced susceptibility to erastin in CTSS-suppressed cells. A similar effect was observed in Hep3B and Hepa1-6 cells, suggesting that CTSS knockdown-induced hypersensitivity to erastin was not cell line-specific (Fig. 2A, Figure S1B-D and S2A-B). Notably, CTSS depletion also led to increased cell death under RSL3 treatment, another ferroptosis inducer that inhibits GPX4 (Fig. 2B). These results implied that CTSS may regulate downstream sensitivity of HCC cells to ferroptosis. Morphological changes, primarily characterized by cell shrinkage, were observed under an optical microscope in erastin-treated CTSS knockdown cells (Fig. 2C, Figs. S1E and S2C).
Fig. 2.
CTSS knockdown sensitizes HCC cells to ferroptosis. (A–B) The indicated cells were treated with increasing concentrations of erastin and RSL3 for 24 h, and the cell viability was measured by CCK-8 assay. (C) The morphological changes in the indicated cells untreated or treated with erastin for 24 h by an optical microscope. Red arrow indicated cell shrinkage. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before observation, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before observation. Scale bar, 50 μm. (D) The ratio of GSH/GSSH was measured in the indicated cells pre-treated with erastin for 24 h. (E) The levels of intracellular lipid peroxidation were detected by the C11-BODIPY probe. PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. (F–G) The contents of MDA and 4-HNE were measured in the indicated cells. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
As aberrant lipid peroxidation is a hallmark of ferroptosis and glutathione (GSH) functions as an endogenous ferroptosis inhibitor through conversion of toxic lipid peroxidation products into non-toxic substances, we first assessed the ratio of reduced to oxidized glutathione (GSH/GSSG) in CTSS knockdown cells and shNT cells [27]. Analysis revealed decreased GSH/GSSG ratios in PLC/PRF/5/shCTSS and Hep3B/shCTSS cells (Fig. 2D, Figs. S1F and S2D). We next measured intracellular lipid peroxidation levels using C11-BODIPY probe. Both erastin treatment and CTSS inhibition accelerated lipid peroxidation accumulation in HCC cells, whereby CTSS knockdown exacerbated lipid peroxidation under erastin exposure in PLC/PRF/5 and Hep3B cells (Fig. 2E). 4-Hydroxynonenal (4-HNE) and malondialdehyde (MDA), lipid peroxidation products, are established markers of ferroptosis [28]. Further evaluation of 4-HNE and MDA content in HCC cells demonstrated that both erastin treatment and CTSS suppression individually elevated intracellular 4-HNE and MDA levels, with a more pronounced effect observed in erastin-treated CTSS knockdown HCC cells (Fig. 2F–G, Figure S1G-H and S2E-F). These results indicate that CTSS knockdown sensitizes HCC to ferroptosis.
3.3. CTSS promotes NRF2 activation and suppresses K48-linked ubiquitination of NRF2
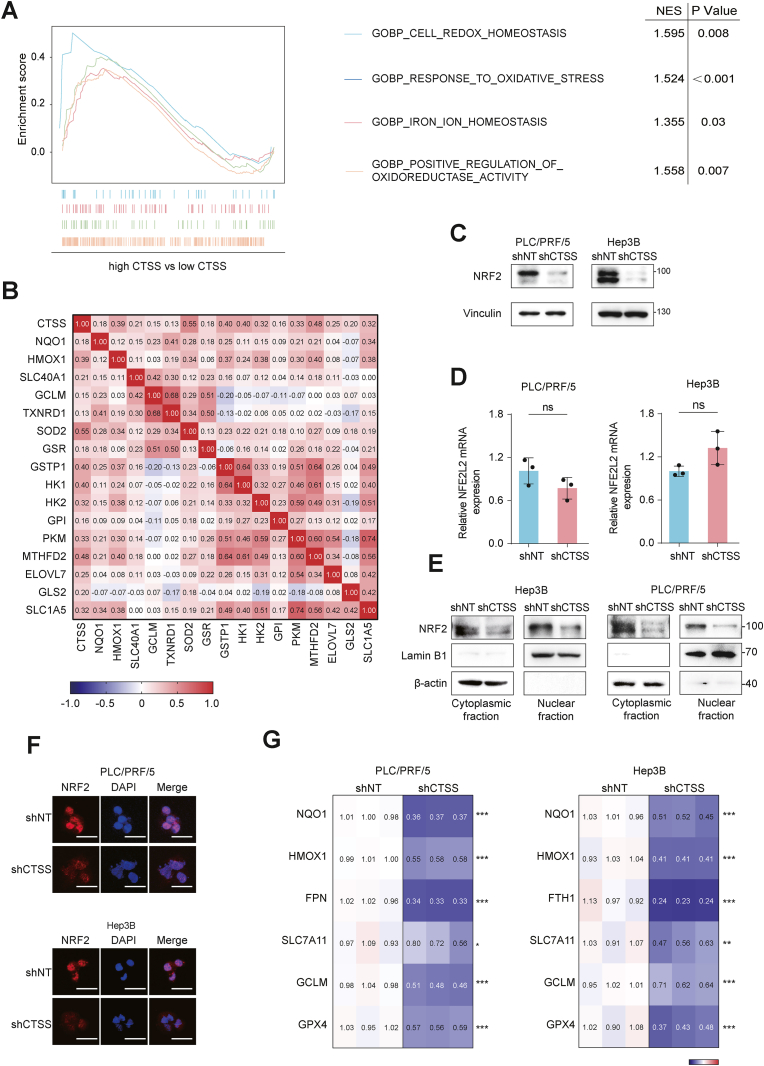
To elucidate the molecular mechanisms underlying CTSS-mediated regulation of ferroptosis sensitivity in HCC, we analyzed mRNA sequencing data from the LIHC data set and stratified HCC patients into low and high CTSS expression groups. Gene Set Enrichment Analysis (GSEA) revealed that CTSS expression was significantly associated with pathways related to cell redox homeostasis, response to oxidative stress, iron ion homeostasis, and positive regulation of the oxidoreductase activity pathway (Fig. 3A). Given that NRF2 serves as the principal regulator of the cellular antioxidant responses protecting cells from endogenous and exogenous oxidative stress, we examined whether CTSS affected NRF2 expression to regulate ferroptosis in HCC [29]. Given NRF2's role as a transcription factor, we further explored the relationship between CTSS expression and NRF2 target genes. Pearson correlation analysis suggested that CTSS exhibited significant positive correlations with NRF2 target genes, particularly those involved in antioxidant defense, such as NQO1, HMOX1, TXNRD1, and SOD2 (Fig. 3B and Fig. S3A–F). In vitro study revealed that NRF2 protein expression was significantly reduced in shCTSS cells compared to parental shNT cells, while NFE2L2 mRNA levels remained unchanged (Fig. 3C–D). This suggested that CTSS may modulate NRF2 expression post-transcriptionally.
Fig. 3.
CTSS promotes NRF2 translocation into the nucleus and activation. (A) Gene Set Enrichment Analysis (GSEA) between high CTSS expression group and low CTSS expression group from LIHC data set with normalized enrichment score (NES) and nominal P value. (B) Correlation between CTSS expression and NRF2 downstream target genes from LIHC data set in TCGA database. (C) Western blot analysis of NRF2 protein expression in the indicated cells. (D) qRT-PCR analysis of NFE2L2 mRNA expression in the indicated cells. (E) Western blot analysis of NRF2 protein expression in cytoplasmic fraction and nuclear fraction from the indicated cells. (F) The intracellular NRF2 localization in the indicated cells was observed using fluorescent microscopy. Red: NRF2; Blue: DAPI. Scale bar, 50 μm. (G) qRT-PCR analysis of NRF2-targeted anti-ferroptosis genes mRNA expression in the indicated cells. The mRNA expression of targeted genes in CTSS knockdown cells was normalized to parental control cells as shown in the heatmap. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
We extracted nucleus and cytoplasm proteins from shNT and shCTSS HCC cells to assess NRF2 nuclear translocation. Western blot analysis revealed a significant decrease in both cytosolic and nuclear NRF2 protein levels in CTSS-knockdown cells (Fig. 3E). This finding was further confirmed by immunofluorescence staining, which demonstrated weaker nuclear NRF2 staining in shCTSS cells compared to shNT cells (Fig. 3F). Consistently, several NRF2 target genes associated with anti-ferroptosis, including NQO1, HMOX1, GCLM, SLC7A11, GPX4, FPN and FTH1 were downregulated in PLC/PRF/5/shCTSS and Hep3B/shCTSS (Fig. 3G–and Figure S3G-H). These results suggest that CTSS promotes NRF2 nuclear translocation and activation, thereby enhancing the cellular response to ferroptosis.
After establishing CTSS-mediated regulation of NRF2 at the post-transcription level, we assessed NRF2 protein stability by treating HCC cells with 20 μg/mL cycloheximide (CHX) for various durations. CHX, a protein synthesis inhibitor, is widely employed to examine protein half-life. The half-life of NRF2 was remarkably shortened in PLC/PRF/5/shCTSS and Hep3B/shCTSS cells, suggesting that CTSS inhibition promotes NRF2 degradation (Fig. 4A). To thoroughly elucidate the mechanism underlying CTSS-mediated NRF2 degradation, we treated shCTSS and shNT cells with 10 μM MG-132, a proteasome inhibitor (Fig. 4B). MG-132 treatment reversed the NRF2 protein downregulation resulting from CTSS suppression, indicating that CTSS modulates NRF2 degradation through the ubiquitin-proteasome pathway.
Fig. 4.
CTSS knockdown facilitates degradation of NRF2 mediated by the K48-linked ubiquitination. (A) The half-life of NRF2 protein expression in CTSS knockdown cells and parental control cells was measured by western blot assay. HCC cells were treated with 20 μg/mL cycloheximide (CHX) for the indicated time before collection. (B) Western blot analysis of NRF2 protein expression in the indicated cells untreated or treated with MG-132. For MG-132 treatment, PLC/PRF/5 and Hep3B cells were pre-treated with 20 μM MG-132 for 8 h before collection. (C) The endogenous ubiquitination levels of NRF2 in the indicated cells were measured by western blot assay. HCC cells were pre-treated with 20 μM MG-132 for 8 h before being lysed and anti-NRF2 primary antibodies were added, followed by immunoblotting with anti-ubiquitin antibody. (D) The exogenous ubiquitination levels of NRF2 in HEK-293T cells were measured by western blot assay. HEK-293T cells were co-transfected with the indicated plasmids. After 48 h of transfection, 20 μM MG-132 was added for 8 h before being lysed and immunoprecipitated with anti-MYC primary antibodies, followed by immunoblotting with anti-HA antibody. (E–F) The endogenous K48-linked and K63-linked ubiquitination levels of NRF2 in the indicated cells were measured by western blot assay. HCC cells were pre-treated with 20 μM MG-132 for 8 h before being lysed, and anti-NRF2 primary antibodies were added, followed by immunoblotting with anti-K48-ubiquitin and K63-ubiquitin antibodies. (G) The exogenous K48-linked and K63-linked ubiquitination levels of NRF2 in HEK-293T cells were measured by western blot assay. HEK-293T cells were co-transfected with the indicated plasmids (HA-tagged K48-Ub and K64-Ub plasmids referred to other lysines were mutated into arginin except K48 or K63 residue). After 48 h of transfection, 20 μM MG-132 was added for 8 h before being lysed and immunoprecipitated with anti-MYC primary antibodies, followed by immunoblotting with anti-HA antibody. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Ubiquitination analysis revealed that endogenous NRF2 exhibited increased ubiquitination in PLC/PRF/5/shCTSS and Hep3B/shCTSS cells than shNT cells (Fig. 4C). To validate these findings, we co-transfected MYC-tagged NRF2, HA-tagged ubiquitin (Ub), and either shNT or shCTSS plasmids into HEK-293T cells and performed an exogenous ubiquitination assay. Consistent with the endogenous ubiquitination results, exogenous NRF2 ubiquitination was increased in the presence of the shCTSS plasmid (Fig. 4D).
To characterize the specific type of polyubiquitination modification involved in CTSS-mediated NRF2 ubiquitination, we examined various ubiquitin linkages. Ubiquitination occurs at seven lysine residues and one methionine site (K6, K11, K27, K29, K33, K48, K63, and M1), each mediating distinct biological functions [30]. Among these eight types of ubiquitin chain linkages, K48- and K63-linked ubiquitin chains are the most extensively characterized. K48-linked ubiquitination targets proteins for proteasomal degradation, while K63-linked ubiquitination mediates cell signaling pathways [31].
To identify the specific ubiquitin linkage, we performed immunoprecipitation (IP) assays using anti-K48 and anti-K63 ubiquitin antibodies in CTSS knockdown and control PLC/PRF/5 and Hep3B cells. CTSS depletion increased K48-linked ubiquitination of NRF2 without affecting K63-linked ubiquitination (Fig. 4E–F). To further validate these results, we co-transfected HA-tagged K48-Ub and K63-Ub plasmids (containing lysine-to-arginine mutations except at K48 or K63) with MYC-tagged NRF2 and either shNT or shCTSS plasmids into HEK-293T cells. Consistent with our endogenous ubiquitination results, CTSS suppression specifically enhanced K48-linked ubiquitination of NRF2 without affecting K63-linked ubiquitination (Fig. 4G). These findings indicate that CTSS regulates NRF2 degradation through K48-linked ubiquitination.
3.4. CTSS regulates ferroptosis sensitivity in HCC cells in an NRF2-dependent manner
To further elucidate whether CTSS-mediated resistance to ferroptosis in HCC was NRF2-dependent, we overexpressed NRF2 in PLC/PRF/5/shCTSS and Hep3B/shCTSS cells by transfecting them with a MYC-tagged NRF2 plasmid and treated the CTSS-knockdown cells with the NRF2 activator tert-Butylhydroquinone (tBHQ). The increased sensitivity to ferroptosis inducers, erastin and RSL3, observed in CTSS-knockdown HCC cells was restored to levels comparable to those in parental control cells following NRF2 overexpression and activation (Fig. 5A–C and Fig. S4A–B). Additionally, in PLC/PRF/5/shCTSS and Hep3B/shCTSS cells, NRF2 overexpression or tBHQ treatment mitigated both the CTSS depletion-induced increase in MDA levels and decline in the GSH/GSSG ratio (Fig. 5D–E and Fig. S4C–D). Overexpression of CTSS significantly reduced cellular sensitivity to erastin, and knockdown of NRF2 increased the sensitivity to ferroptosis inducers. The inability of CTSS overexpression to rescue ferroptosis resistance in shNRF2 cells confirms that CTSS acts upstream of NRF2 (Fig. 5F). Based on these results, we verify that NRF2 is required for CTSS-mediated regulation of ferroptosis sensitivity.
Fig. 5.
CTSS regulates the sensitivity to ferroptosis in HCC cells dependent on NRF2. (A–B) The indicated cells were pre-treated with 20 μM tert-Butylhydroquinone (tBHQ) for 24h, followed by treatment with increasing concentrations of erastin and RSL3 for 24 h, and the cell viability was measured by CCK-8 assay. (C) The morphological changes in the indicated cells by an optical microscope. PLC/PRF/5 and Hep 3B cells were pre-treated with erastin, and untreated or treated with tBHQ for 24 h. Red arrow indicated cell shrinkage. Scale bar, 50 μm. (D) The ratio of GSH/GSSH was measured in the indicated cells pre-treated with erastin. For tBHQ treatment, cells were pre-treated with 20 μM tBHQ for 24h before collection. (E) The contents of MDA were measured in the indicated cells pre-treated with 20 μM tBHQ for 24h. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. (F) The indicated cells were treated with increasing concentrations of erastin for 24 h and the cell viability was measured by CCK-8 assay. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.5. CTSS activates NRF2 by disrupting KEAP1-mediated NRF2 degradation
E3 ligase adaptor KEAP1 serves as the principal negative regulator of NRF2. Under basal conditions, NRF2 is rapidly ubiquitinated and degraded through the ubiquitin-proteasome pathway mediated by KEAP1. In response to oxidative stress, accumulated intracellular ROS modified cysteine residues on KEAP1, thereby blocking KEAP1-mediated ubiquitination of NRF2 [10]. We hypothesized that CTSS might influence NRF2 protein expression in a KEAP1-dependent manner. To test this hypothesis, we first examined the protein levels of NRF2 and KEAP1 in CTSS-knockdown HCC cells. A concurrent decrease in NRF2 and an increase in KEAP1 protein levels were observed in PLC/PRF/5/shCTSS and Hep3B/shCTSS cells (Fig. 6A). Subsequently, we transfected siKEAP1 into shNT and shCTSS cells to knock down KEAP1. Our data demonstrated that KEAP1 depletion significantly increases the protein levels of NRF2 in both shNT and shCTSS cells. The NRF2 downregulation induced by CTSS depletion was partially abolished in CTSS and KEAP1 double-knockdown HCC cells compared to parental cells (Fig. 6B). Furthermore, endogenous ubiquitin assays were performed in CTSS and KEAP1 double-knockdown cells. The increased ubiquitination of NRF2 observed in CTSS knockdown cells was reversed in CTSS and KEAP1 double knockdown cells. This phenomenon was particularly evident for K48-linked ubiquitination of NRF2 (Fig. 6C–D). These results indicated that CTSS regulated NRF2 ubiquitination and degradation, at least partly, through KEAP1, and also suggested that CTSS may also contribute to NRF2 protein stability via KEAP1-independent mechanisms.
Fig. 6.
CTSS binds to KEAP1 and disrupts the interaction between KEAP1 and NRF2. (A) Western blot analysis of KEAP1 protein expression in the indicated cells. (B) Western blot analysis of NRF2 and KEAP1 protein expression in the indicated cells. For construction of KEAP1 knockdown HCC cells, KEAP1-siRNA was transfected into HCC cells for 48 h before collection. (C) The endogenous ubiquitination levels of NRF2 in the indicated cells were measured in the indicated cells transfected with KEAP1-siRNA for 48 h by western blot assay. HCC cells were pre-treated with 20 μM MG-132 for 8 h before being lysed, and anti-NRF2 primary antibodies were added, followed by immunoblotting with anti-ubiquitin antibody. (D) The endogenous K48-linked ubiquitination levels of NRF2 in the indicated cells transfected with KEAP1-siRNA for 48 h were measured by western blot assay. HCC cells were pre-treated with 20 μM MG-132 for 8 h before being lysed, and anti-NRF2 primary antibodies were added, followed by immunoblotting with anti-K48-ubiquitin antibody. (E) Interaction between endogenous CTSS and KEAP1 was detected by Co-IP. Cells were untreated with erastin, lysed and immunoprecipitated with anti-KEAP1 antibody, followed by immunoblotting with anti-CTSS antibody. Reversely, cells were lysed and immunoprecipitated with anti-CTSS antibody, followed by immunoblotting with anti-KEAP1 antibody. (F) The colocalization between CTSS and KEAP1 was detected by immunofluorescence staining. Red: CTSS. Green: KEAP1. Blue: DAPI. Scale bar, 20 μm. (G) Interaction between endogenous KEAP1 and NRF2 in the indicated cells untreated or treated with erastin was detected by Co-IP. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. Cells were lysed and immunoprecipitated with anti-KEAP1, followed by immunoblotting with anti-NRF2 antibody. Reversely, cells were lysed and immunoprecipitated with anti-NRF2, followed by immunoblotting with anti-KEAP1 antibody. (H) Interaction between exogenous KEAP1 and NRF2 in HEK-293T cells untreated or treated with erastin was detected by Co-IP. HEK-293T cells were co-transfected with the indicated plasmids. After 48 h of transfection, 2 μM erastin was added for 24 h before being lysed and immunoprecipitated with anti-MYC primary antibodies, followed by immunoblotting with anti-His antibody. Reversely, cells were lysed and immunoprecipitated with anti-His, followed by immunoblotting with anti-MYC antibody. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
We then conducted co-immunoprecipitation (co-IP) assays in wild-type PLC/PRF/5 and Hep3B cells and found a direct interaction between KEAP1 and CTSS (Fig. 6E). Immunofluorescence staining revealed that CTSS and KEAP1 were colocalized in the cytoplasm of these cells (Fig. 6F). Given the observed interaction between CTSS and KEAP1, along with the opposing changes in protein levels of KEAP1 and NRF2 upon CTSS alteration, we hypothesized that CTSS might interfere with the KEAP1-NRF2 interaction, thereby restraining KEAP1-mediated NRF2 degradation. Data from the co-IP assay suggested a rapid, robust increase in the interaction between endogenous KEAP1 and NRF2 in CTSS-knockdown cells compared to parental control cells. We then treated cells with erastin and found that erastin treatment blocked the KEAP1-NRF2 interaction regardless of CTSS levels (Fig. 6G). This effect may be attributed to erastin-induced oxidative stress, which elevated intracellular ROS levels to promote a conformational change in KEAP1, thus disrupting the formation of the KEAP1-NRF2 complex. We then co-transfected MYC-tagged NRF2, His-tagged KEAP1, and either shNT or shCTSS plasmids into HEK-293T cells, followed by treatment with erastin or DMSO. Consistent with these findings, the exogenous co-IP assay observed the same phenomenon (Fig. 6H). Collectively, these results demonstrate that CTSS disrupts the KEAP1-NRF2 interaction.
3.6. CTSS participates in lysosomal degradation of KEAP1 during ferroptosis
NRF2 binds to the DGR motif of KEAP1 through its ETGE motif. Some proteins with the ETGE-like motif can also bind to KEAP1, interfering with the formation of the KEAP1-NRF2 complex and KEAP1-mediated NRF2 degradation [31]. While we demonstrated that CTSS directly interacted with KEAP1, CTSS does not possess an ETGE-like motif. Given that CTSS is localized in lysosomes involved in protein degradation and is activated during ferroptosis, we hypothesized that CTSS might participate in the lysosomal degradation of KEAP1 under ferroptosis stress. To test this hypothesis, we treated HCC cells with erastin and found that erastin-induced ferroptosis significantly decreased KEAP1 protein levels in PLC/PRF/5/shNT and Hep3B/shNT cells (Fig. S5A). We also measured mRNA expression of KEAP1 in these cells and found no significant difference in KEAP1 mRNA expression between CTSS-knockdown and parental cells, regardless of erastin treatment (Fig. 7A). These results excluded the possibility that CTSS regulated KEAP1 at the transcriptional level and suggested that CTSS suppressed KEAP1 at the protein level.
Fig. 7.
CTSS promotes lysosomal degradation of KEAP1. (A) qRT-PCR analysis of KEAP1 mRNA expression in the indicated cells untreated or treated with erastin. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. (B) Western blot analysis of KEAP1 protein expression in the indicated cells untreated or treated with erastin. PLC/PRF/5 cells were pre-treated with 10 μM, and Hep3B cells were pre-treated with 2 μM erastin for 24 h, followed by treatment with 20 μM chloroquine (CQ) for 24 h before collection. (C) Interaction between endogenous KEAP1 and LAMP1 in the indicated cells untreated or treated with erastin was detected by Co-IP. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. Cells were lysed and immunoprecipitated with anti-KEAP1, followed by immunoblotting with anti-LAMP1 antibody. Reversely, cells were lysed and immunoprecipitated with anti-LAMP1, followed by immunoblotting with anti-KEAP1 antibody. (D–E) The colocalization between KEAP1 and LAMP1 in the indicated cells untreated or treated with erastin was detected by immunofluorescence staining. Red: LAMP1. Green: KEAP1. Blue: DAPI. Scale bar, 20 μm. The co-localization of KEAP1 and CTSS was quantified using Mander's coefficient. (F) Interaction between endogenous KEAP1 and mature-CTSS in the indicated cells untreated or treated with erastin and FAC was detected by Co-IP. For erastin treatment, PLC/PRF/5 cells were pre-treated with 10 μM erastin for 24 h before collection, and Hep3B cells were pre-treated with 2 μM erastin for 24 h before collection. For FAC treatment, PLC/PRF/5 cells were pre-treated with 1000 μM FAC for 24 h before collection, and Hep3B cells were pre-treated with 500 μM FAC for 24 h before collection. (G) The indicated cells were treated with increasing concentrations of erastin for 24 h, and the cell viability was measured by CCK-8 assay. (H) Western blot analysis of exogenous CTSS and KEAP1 protein expression in the indicated cells. HEK-293T cells were co-transfected with the indicated plasmids. After 48 h of transfection, 2 μM erastin was added for 24 h before being lysed. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
We then treated PLC/PRF/5 and Hep3B cells with the lysosome inhibitor chloroquine (CQ) and found that CQ treatment restored the erastin-induced KEAP1 downregulation. Conversely, CQ caused no alteration in the reduction of KEAP1 in CTSS knockdown HCC cells following erastin treatment (Fig. 7B and Fig. S5B). Both the co-IP assay and immunofluorescence staining revealed that upon erastin treatment, the direct interaction between KEAP1 and LAMP1, a lysosome marker, was diminished in CTSS-knockdown cells compared to control cells (Fig. 7C–E and Fig. S5C–F). These results showed that CTSS regulated erastin-mediated KEAP1 degradation through the lysosomal pathway. Both erastin and FAC accelerated CTSS maturation and enhanced the interaction between mature-CTSS and KEAP1 (Fig. 7F). These results indicate that ferroptosis stress facilitates the cleavage of KEAP1 mediated by mature-CTSS, highlighting a novel role for CTSS in the lysosomal degradation of KEAP1.
To further identify the role of CTSS in the degradation of KEAP1, we generated a catalytically inactive CTSS mutant (CTSS-C25A) by mutating the cysteine residue at amino acid 25 [18]. Overexpression of wild-type CTSS contributed to increased cell viability compared to control vector cells, indicating enhanced resistance to erastin and RSL3 in CTSS-WT HCC cells (Fig. 7G and Fig. S5G). Consistently, an increase in GSH/GSSG ratios and a decrease in MDA were observed in CTSS-WT HCC cells, indicating that overexpression of CTSS confers ferroptosis resistance in HCC cells. Intriguingly, ectopic overexpression of CTSS-C25A produced no effects on cellular sensitivity to ferroptosis inducers or intracellular levels of the GSH/GSSG ratio and MDA (Fig. S5G–I). These findings underscore that the protease activity of CTSS is indispensable for modulating the sensitivity to ferroptosis inducers. To further validate that CTSS degrades KEAP1 through its protease activity, we transfected HEK293T cells with a His-KEAP1 plasmid alongside an equal amount of either CTSS-WT or CTSS-C25A plasmids. Notably, the protein levels of exogenous KEAP1 protein decreased in cells transfected with CTSS-WT, whereas CTSS-C25A transfection did not affect KEAP1 abundance (Fig. 7H). This demonstrated that CTSS-mediated KEAP1 degradation strictly depended on its proteolytic activity.
To investigate the biological role of CTSS-mediated KEAP1 degradation under basal conditions, we performed cell proliferation and colony formation assays in CTSS-knockdown HCC cells with additional KEAP1 knockdown or NRF2 overexpression. CTSS depletion significantly impaired cell proliferation and reduced colony-forming capacity. KEAP1 knockdown or NRF2 overexpression in CTSS-deficient cells restored cell proliferation and colony formation, confirming that CTSS regulates the proliferative capacity of HCC through KEAP1 degradation and subsequent NRF2 activation (Fig. S6).
3.7. CTSS inhibition augments the anti-tumor activity of erastin via enhanced ferroptosis sensitivity
To evaluate whether CTSS suppression could improve the anti-tumor efficacy of erastin in vivo, we established subcutaneous xenograft models in BALB/c nude mice and C57BL/6 mice. In BALB/c nude mice, we implanted PLC/PRF/5/shCTSS cells and control cells subcutaneously and administered either erastin or normal saline (Fig. 8A). Tumor volume and weight were notably reduced in both the erastin-treated shNT group and the normal saline-treated shCTSS group compared to the vector control group. The combination of CTSS depletion and erastin treatment resulted in a further substantial reduction in tumor volume and weight (Fig. 8B–D). Immunohistochemical (IHC) staining assays revealed that erastin treatment led to increased levels of 4-HNE and CD71 in tumors derived from PLC/PRF/5/shCTSS cells compared to those from parental cells (Fig. 8E–F). Tumors from PLC/PRF/5/shCTSS cells exhibited lower nuclear NRF2 levels, which were augmented by erastin treatment. Conversely, CTSS knockdown led to increased KEAP1 expression levels, while erastin treatment reduced KEAP1 expression (Fig. 8G–H). These reciprocal changes in NRF2 and KEAP1 observed in the mouse study were consistent with our in vitro findings.
Fig. 8.
CTSS inhibition augments the antitumor activity of erastin in vivo. (A–B) BALB/c nude mice were transplanted with PLC/PRF/5/shNT or PLC/PRF/5/shCTSS cells. At the 14th day after implantation, mice were intraperitoneally injected with 15 mg/kg erastin or normal saline every other day. On the 28th day after implantation, they were sacrificed, and tumors were separated. (C–D) Tumor volumes and weights were measured. (E–H) Immunohistochemistry analysis of 4HNE, CD71, NRF2, and KEAP1 of tumor xenografts. Scale bar, 20 μm. All data are acquired from at least three independent experiments and presented as the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
In C57BL/6 mice, we further assessed the anti-tumor effects of the CTSS inhibitor LY3000328 combined with erastin and found similar results (Figure S7A and Figure S8). Additionally, neither erastin nor LY3000328 caused any detectable toxicity in the liver, lungs, kidneys, or heart (Fig. S7B–C). These results suggest that CTSS depletion potentiates the anti-tumor activity of erastin by suppression of the KEAP-NRF2 signaling pathway and enhancement of ferroptosis sensitivity in vivo.
4. Discussion
Ferroptosis, a regulated form of cell death characterized by iron-dependent lipid peroxidation, holds promise for treating various diseases, including HCC, stroke, and ischemia-reperfusion injury [32]. However, current therapies face challenges in effectively inducing ferroptosis in these disease contexts. Overcoming resistance to ferroptosis is, therefore, a crucial step towards developing novel therapeutic strategies. In the current study, we demonstrated that CTSS depletion sensitized erastin-induced ferroptosis and inhibited HCC tumorigenesis in vitro. Consistent results were observed in both immunodeficient nude mice and immunocompetent C57B/6 mice, indicating the tumor-suppressive role of CTSS in ferroptosis. Further research is needed to elucidate whether CTSS modulates the tumor immune environment.
Since the term ferroptosis was first coined in 2012, our understanding of its impact on tumorigenesis and tumor therapy has become more comprehensive. Ferroptosis is now acknowledged for its dual effects on cancer [33]. On one hand, ferroptosis can act as a tumor suppressor. Several studies have revealed that certain oncogenes can inhibit ferroptosis, whereas tumor suppressor genes can promote it. TP53, a prominent tumor suppressor, has been reported to downregulate SLC7A11 expression, thereby enhancing ferroptosis [34]. Additionally, the oncogenic RAS and PI3K signaling pathways, which are often activated in cancer, are involved in suppressing ferroptosis [35]. On the other hand, ferroptosis can also assist tumor cells in escaping from immune surveillance. Overexpression of CD36 on tumor-infiltrating CD8+ T cells accelerates fatty acid uptake, resulting in excessive lipid peroxidation and ferroptosis, which impairs the anti-tumor function of CD8+ T cells [36]. Furthermore, damage-associated molecular patterns released from ferroptotic cancer cells can alter the tumor immune environment. For instance, KRASG12D and HMGB1 released from ferroptotic cancer cells can polarize macrophages towards a pro-inflammatory phenotype, thereby promoting tumor growth [37,38].
NRF2 plays a crucial role in defending against oxidative stress, and its activation can confer resistance to tumor therapies [39,40]. In this study, we elucidated that CTSS promoted NRF2 accumulation and activation, which in turn led to resistance to ferroptosis. Conversely, the depletion of CTSS sensitized HCC cells to ferroptosis. Analysis of data in the TCGA database indicated a positive correlation between CTSS expression and NRF2 in gastric carcinoma, colorectal carcinoma, breast cancer, and HCC. Additionally, the LIHC dataset confirmed a positive association between CTSS expression and NRF2 target genes. IHC analysis from animal studies also corroborated the positive relationship between CTSS and NRF2 expression.
NRF2 is negatively regulated by KEAP1-mediated ubiquitination and degradation. In the canonical pathway, intracellular ROS modify cysteine residues on KEAP1, resulting in a conformational change that disrupts the interaction between KEAP1 and NRF2 [10]. In the non-canonical pathway, the autophagy-related protein P62 mimics the ETGE binding motif on NRF2. P62 competes with NRF2 for binding to KEAP1, and the KEAP1-p62 complex is subsequently sequestered in the autophagosome, thereby protecting NRF2 from degradation [41]. Besides, NRF2 can be regulated through KEAP1-independent mechanisms. NRF2 can be recognized and bound by β-TrCP via a distinct motif, leading to NRF2 ubiquitination and degradation [42]. The interaction affinity between β-TrCP and NRF2 is enhanced by GSK3β and repressed by the PI3K/AKT signaling pathway [43,44]. In this study, we identified that CTSS regulated NRF2 protein expression post-transcriptionally in a KEAP1-dependent manner. We confirmed a physical interaction between CTSS and KEAP1, and found that CTSS knockdown increased the formation of the KEAP1-NRF2 complex. Notably, CTSS does not contain DLG or ETGE motifs, indicating that its effect on blocking the KEAP1-mediated NRF2 degradation did not rely on competitively binding. Considering that CTSS is a lysosomal protease and that KEAP1 protein levels are elevated in CTSS knockdown cells, we hypothesized that CTSS may cleave and degrade KEAP1. Subsequent studies suggested a proteolytic role of CTSS in KEAP1's autophagy-lysosomal degradation. Since p62 is a scaffold protein involved in autophagosome formation, we then attempted to explore the relationship between CTSS and p62. We found no significant change in p62 protein levels following CTSS knockdown. Conversely, p62 depletion led to a decrease in CTSS protein levels (Fig. S9). These results suggested that CTSS functioned downstream of p62, and its regulatory effect on NRF2 may depend on p62 or be part of the p62-KEAP1-NRF2 pathway, which required further investigation.
Previous studies have reported that CTSS is overexpressed in malignant tissues and cells and is involved in tumorigenesis [45]. Mature CTSS is activated from pro-CTSS either through proteolytic cleavage by other proteases or autocatalysis under specific conditions. Our study is the first to demonstrate that ferroptosis stress promotes the maturation of CTSS, which can be reversed by ferroptosis inhibitors. Both pro-CTSS and mature-CTSS can bind to KEAP1, and treatment with ferroptosis inducers such as erastin and FAC increased the binding affinity between mature-CTSS and KEAP1. Specifically, erastin treatment increased the interaction between KEAP1 and LAMP1 and decreased KEAP1 protein levels; these effects were diminished in CTSS-knockdown cells. The erastin-induced downregulation of KEAP1 exacerbates the accumulation and activation of NRF2, accelerating NRF2-mediated anti-ferroptosis response and establishing a negative feedback loop that suppresses ferroptosis. We propose that this mechanism represents an intrinsic or acquired cellular protective system against ferroptosis stress, contributing to ferroptosis resistance. Inhibition of CTSS by small molecules or antibodies impairs the cellular protective system, consequently sensitizing cancer cells to ferroptosis. Our studies in mice have shown that CTSS inhibition can cooperate with erastin to inhibit HCC progression through the promotion of ferroptosis.
Emerging studies have revealed that follicular lymphoma exhibits frequent CTSS overexpression and gain-of-function mutations. Hyperactive CTSS promotes CD74 cleavage, thereby enhancing MHC-II antigen presentation and increasing the infiltration of CD4+ T cells in the tumor microenvironment, ultimately establishing an immunosuppressive microenvironment. Conversely, CTSS inhibition disrupts crosstalk between lymphoma cells and CD4+ follicular helper T cells, activating CD8+ cytotoxic T lymphocytes, which suppress tumor cell growth [21]. In colorectal cancer, CTSS regulates autophagy in tumor-associated macrophages, driving their polarization toward the pro-tumorigenic M2 phenotype [46]. Additionally, CTSS impairs the antigen presentation capacity of cancer-associated fibroblasts, thereby inhibiting CD8+ T cell-mediated tumor cytotoxicity [47]. Given that the tumor immune microenvironment constitutes a complex and dynamic network of cellular and molecular interactions, the role of CTSS cannot be simplistically categorized as uniformly pro-tumorigenic or anti-tumorigenic. Its immunomodulatory effects appear context-dependent, varying across tumor types, immune cell subsets, and spatial-temporal conditions. In subsequent studies, we aim to systematically investigate the roles of CTSS in reshaping the TME.
Outside of HCC, accumulating evidence indicates that CTSS is overexpressed in multiple solid tumors, including stomach, breast, and colorectal cancers, which exhibit intrinsic or acquired resistance to ferroptosis [[48], [49], [50]]. Furthermore, conventional and emerging therapeutic modalities, such as chemotherapy, radiotherapy, and immunotherapy, have been shown to exert anti-tumor effects by promoting ferroptosis. Notably, targeting CTSS to enhance tumor susceptibility to ferroptosis has emerged as a novel therapeutic strategy to improve the therapeutic efficacy. CTSS has been reported to be involved in the pathogenesis of diverse non-neoplastic diseases, including psoriasis, acute respiratory distress syndrome, pulmonary fibrosis, and hepatic fibrosis [[51], [52], [53]]. Mechanistically, CTSS contributes to disease progression by modulating inflammatory cascades, extracellular matrix remodeling, and cellular stress responses. Therapeutic targeting of CTSS represents a promising therapeutic strategy to recalcitrant inflammatory and fibrotic disorders.
In conclusion, our study underscores a critical relationship between CTSS expression and ferroptosis sensitivity. The results indicate that CTSS enhances the anti-ferroptosis capacity of HCC cells through activating NRF2. CTSS interacts with KEAP1 to disrupt KEAP1-mediated NRF2 ubiquitination and degradation. Ferroptosis stress accelerates the maturation of CTSS, leading to increased autophagy-lysosomal degradation of KEAP1 and subsequently promoting the expression of NRF2-dependent anti-ferroptosis genes. CTSS inhibition can potentiate erastin-induced ferroptosis in HCC cells, positioning it as a candidate therapeutic target for overcoming ferroptosis resistance in HCC patients.
CRediT authorship contribution statement
Ru-Chen Xu: Writing – original draft, Methodology, Formal analysis. Jia-Lei Sun: Methodology, Formal analysis. Fu Wang: Visualization, Methodology, Investigation. Hua-Hua Liu: Methodology, Formal analysis. Zhuo-Ran Qi: Formal analysis, Data curation. Xuan Shi: Methodology, Data curation. Xiang-Nan Yu: Resources, Formal analysis. Tao-Tao Liu: Methodology, Data curation. Shu-Qiang Weng: Visualization, Data curation. Ling Dong: Writing – review & editing. Xi-Zhong Shen: Supervision, Project administration, Funding acquisition. Ji-Min Zhu: Writing – review & editing, Supervision, Project administration, Conceptualization.
Funding information
This study was supported by the National Natural Science Foundation of China (81970505, 82173122, and 82303161), the Shanghai Science and Technology Commission (19ZR1434700, 22ZR1411800, and 23YF1405900), and the China Postdoctoral Science Foundation (2023M740679).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the staff of the Department of Laboratory Animal Science, Fudan University, for their assistance in animal study and Shen Cai at the Laboratory of Medical Molecular Virology, Fudan University, for providing them with a guide to operate the confocal microscope.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2025.103815.
Contributor Information
Xi-Zhong Shen, Email: shen.xizhong@zs-hospital.sh.cn.
Ji-Min Zhu, Email: zhu.jimin@zs-hospital.sh.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Bray F., Laversanne M., Sung H., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N. Engl. J. Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagoda N., von Rechenberg M., Zaganjor E., et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedmann Angeli J.P., Schneider M., Proneth B., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockwell B.R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X., Niu X., Chen R., et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64(2):488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung E.C., Vousden K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer. 2022;22(5):280–297. doi: 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- 9.Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative stress in cancer. Cancer Cell. 2020;38(2):167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98(3):1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S., Pi J., Zhang Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway. Redox Biol. 2022;54 doi: 10.1016/j.redox.2022.102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34(1):21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Q., Zhu H., Dong L., et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell. 2019;179(5):1240. doi: 10.1016/j.cell.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Olson O.C., Joyce J.A. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat. Rev. Cancer. 2015;15(12):712–729. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- 15.Fonović M., Turk B. Cysteine cathepsins and extracellular matrix degradation. Biochim. Biophys. Acta. 2014;1840(8):2560–2570. doi: 10.1016/j.bbagen.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Dheilly E., Battistello E., Katanayeva N., et al. Cathepsin S regulates antigen processing and T cell activity in non-hodgkin lymphoma. Cancer Cell. 2020;37(5):674–689.e12. doi: 10.1016/j.ccell.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Xu J., Li D., Ke Z., et al. Cathepsin S is aberrantly overexpressed in human hepatocellular carcinoma. Mol. Med. Rep. 2009;2(5):713–718. doi: 10.3892/mmr_00000161. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Jin H., Seo H.R., et al. Regulating BRCA1 protein stability by cathepsin S-mediated ubiquitin degradation. Cell Death Differ. 2019;26(5):812–825. doi: 10.1038/s41418-018-0153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turk V., Stoka V., Vasiljeva O., et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim. Biophys. Acta. 2012;1824(1):68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small D.M., Burden R.E., Jaworski J., et al. Cathepsin S from both tumor and tumor-associated cells promote cancer growth and neovascularization. Int. J. Cancer. 2013;133(9):2102–2112. doi: 10.1002/ijc.28238. [DOI] [PubMed] [Google Scholar]
- 21.Bararia D., Hildebrand J.A., Stolz S., et al. Cathepsin S alterations induce a tumor-promoting immune microenvironment in follicular lymphoma. Cell Rep. 2020;31(5) doi: 10.1016/j.celrep.2020.107522. [DOI] [PubMed] [Google Scholar]
- 22.Xu R.C., Wang F., Sun J.L., et al. A novel murine model of combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Transl. Med. 2022;20(1):579. doi: 10.1186/s12967-022-03791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo S.U., Min K.J., Woo S.M., et al. Z-FL-COCHO, a cathepsin S inhibitor, enhances oxaliplatin-mediated apoptosis through the induction of endoplasmic reticulum stress. Exp. Mol. Med. 2018;50(8):1–11. doi: 10.1038/s12276-018-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao F., Deng Y., Zhao Y., et al. A targetable LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat. Commun. 2021;12(1):7333. doi: 10.1038/s41467-021-27452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan R., Xie E., Li Y., et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022;32(7):687–690. doi: 10.1038/s41422-022-00642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P., Lin Q., Sun S., et al. Inhibition of cannabinoid receptor type 1 sensitizes triple-negative breast cancer cells to ferroptosis via regulating fatty acid metabolism. Cell Death Dis. 2022;13(9):808. doi: 10.1038/s41419-022-05242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassannia B., Vandenabeele P., Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q., Meng Y., Li D., et al. Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct. Targeted Ther. 2024;9(1):55. doi: 10.1038/s41392-024-01769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht F., Zocchi M., Alimohammadi F., et al. Regulation of antioxidants in cancer. Mol. Cell. 2024;84(1):23–33. doi: 10.1016/j.molcel.2023.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dikic I. Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson A.D., Zhang N.Y., Xu P., et al. The lysine 48 and lysine 63 ubiquitin conjugates are processed differently by the 26 s proteasome. J. Biol. Chem. 2009;284(51):35485–35494. doi: 10.1074/jbc.M109.052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockwell B.R., Friedmann Angeli J.P., Bayir H., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Kang R., Kroemer G., et al. Broadening Horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18(5):280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 34.Liu D.S., Duong C.P., Haupt S., et al. Inhibiting the system x(C)(-)/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat. Commun. 2017;8 doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y.N., Lou S.Y., Lu J., et al. Selective PI3Kδ inhibitor TYM-3-98 suppresses AKT/mTOR/SREBP1-mediated lipogenesis and promotes ferroptosis in KRAS-Mutant colorectal cancer. Cell Death Dis. 2024;15(7):474. doi: 10.1038/s41419-024-06848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X., Xiao L., Liu L., et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33(5):1001–1012.e5. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai E., Han L., Liu J., et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G., Liao C., Chen J., et al. Targeting the MCP-GPX4/HMGB1 axis for effectively triggering immunogenic ferroptosis in pancreatic ductal adenocarcinoma. Adv. Sci. (Weinh.) 2024;11(21) doi: 10.1002/advs.202308208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cloer E.W., Goldfarb D., Schrank T.P., et al. NRF2 activation in cancer: from DNA to protein. Cancer Res. 2019;79(5):889–898. doi: 10.1158/0008-5472.Can-18-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Homma S., Ishii Y., Morishima Y., et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 2009;15(10):3423–3432. doi: 10.1158/1078-0432.Ccr-08-2822. [DOI] [PubMed] [Google Scholar]
- 41.Komatsu M., Kurokawa H., Waguri S., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12(3):213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 42.Chowdhry S., Zhang Y., McMahon M., et al. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32(32):3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patibandla C., van Aalten L., Dinkova-Kostova A.T., et al. Inhibition of glycogen synthase kinase-3 enhances NRF2 protein stability, nuclear localisation and target gene transcription in pancreatic beta cells. Redox Biol. 2024;71 doi: 10.1016/j.redox.2024.103117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rada P., Rojo A.I., Evrard-Todeschi N., et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell Biol. 2012;32(17):3486–3499. doi: 10.1128/mcb.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sevenich L., Bowman R.L., Mason S.D., et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 2014;16(9):876–888. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang M., Liu J., Shao J., et al. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol. Cancer. 2014;13:43. doi: 10.1186/1476-4598-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harryvan T.J., Visser M., de Bruin L., et al. Enhanced antigen cross-presentation in human colorectal cancer-associated fibroblasts through upregulation of the lysosomal protease cathepsin S. J. Immunother. Cancer. 2022;10(3) doi: 10.1136/jitc-2021-003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H., Li Q., Chen X., et al. Targeting SOX13 inhibits assembly of respiratory chain supercomplexes to overcome ferroptosis resistance in gastric cancer. Nat. Commun. 2024;15(1):4296. doi: 10.1038/s41467-024-48307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y., Liu Z., Liu G., et al. Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1. Cell Metab. 2023;35(10):1688–1703.e10. doi: 10.1016/j.cmet.2023.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H., Yu K., Hu H., et al. METTL17 coordinates ferroptosis and tumorigenesis by regulating mitochondrial translation in colorectal cancer. Redox Biol. 2024;71 doi: 10.1016/j.redox.2024.103087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma F., Plazyo O., Billi A.C., et al. Single cell and spatial sequencing define processes by which keratinocytes and fibroblasts amplify inflammatory responses in psoriasis. Nat. Commun. 2023;14(1):3455. doi: 10.1038/s41467-023-39020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ainscough J.S., Macleod T., McGonagle D., et al. Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36γ. Proc. Natl. Acad. Sci. U. S. A. 2017;114(13):E2748–e2757. doi: 10.1073/pnas.1620954114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuo T., Xie Q., Liu J., et al. Macrophage-derived cathepsin S remodels the extracellular matrix to promote liver fibrogenesis. Gastroenterology. 2023;165(3):746–761.e16. doi: 10.1053/j.gastro.2023.05.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.