Abstract
In the yeast Saccharomyces cerevisiae, the PAH1-encoded phosphatidate (PA) phosphatase plays a major role in the control of diacylglycerol and PA, which are crucial for the synthesis of the storage lipid triacylglycerol and membrane phospholipids as well as for diverse cellular processes. The catalytic core of Pah1 contains the haloacid dehalogenase–like domain, a catalytic domain in diverse phosphatases with the conserved motifs I–IV. In this work, we found the four active site motifs in Pah1 by sequence alignment and AlphaFold modeling and identified Arg-445 as an additional residue conserved in motif II of Pah1 and its orthologs. Mutational analyses of the Pah1 active site motifs showed that the conserved residues (Asp-398 and Asp-400 in motif I, Thr-443 and Arg-445 in motif II, Lys-496 in motif III, and Gly-529, Asn-530, and Asp-534 in motif IV) are essential for PA phosphatase (PAP) activity and the related cellular functions of the enzyme. The limited proteolysis analysis of unphosphorylated Pah1, which mimics the functional dephosphorylated form of Pah1, indicates that its overall structure is not affected by the active site mutations. In the liposome-binding assay, the active site mutations in Pah1 did not affect its association with the membrane. These findings demonstrate that the active site motifs are essential for Pah1 PAP activity and provide a mechanistic basis for lipid-associated diseases caused by mutations in the human lipin PAP.
Keywords: phospholipid, phosphatidate, diacylglycerol, triacylglycerol, PA phosphatase, lipin, lipid metabolism
In the yeast Saccharomyces cerevisiae, Pah1 catalyzes the dephosphorylation of phosphatidate (PA) to diacylglycerol (DAG) (1) (Fig. 1). The Mg2+-dependent PA phosphatase (PAP) plays a crucial role in lipid metabolism by regulating the cellular levels of PA and DAG (1), which are key intermediates in the synthesis of membrane phospholipids and the storage lipid triacylglycerol (TAG), respectively (Fig. 1) (2, 3, 4, 5). The substrate PA is activated with CTP to form CDP–DAG, which is used for the synthesis of major membrane phospholipids phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylglycerol, and cardiolipin (2, 3, 5) (Fig. 1). The product DAG is a direct precursor of TAG whose synthesis is increased in the stationary phase (6, 7) (Fig. 1). DAG can also be channeled into the synthesis of PC and PE in yeast cells supplemented with choline and ethanolamine, respectively, which is essential for the mutants lacking the phospholipid synthesis via CDP–DAG (Fig. 1) (1, 5, 8).
Figure 1.
PAP reaction catalyzed by Pah1 and its role in lipid synthesis and cell physiology. The structures of Pah1 and its substrate (PA) and product (DAG), and their derivatives (CDP–DAG and TAG) are shown. The Pah1 structure predicted by AlphaFold (88, 89, 90) is visualized by the PyMol program. The domains/regions of Pah1 include the amphipathic helix (AH); N-LIP domain; haloacid dehalogenase (HAD)–like domain; RP domain; conserved Trp-637; acidic tail (AT); and intrinsically disordered regions (IDRs). Pah1, which catalyzes the Mg2+-dependent dephosphorylation of PA, plays a key role in the production of DAG for the synthesis of the storage lipid TAG and negatively regulates the synthesis of the PA-derived membrane phospholipids. The DAG produced by Pah1 may also be used for the synthesis of PC and PE when cells are supplemented with choline and ethanolamine, respectively. Additional roles of PA and DAG in cell physiology are indicated. More comprehensive pathways of lipid synthesis may be found in Refs. (2, 3). DAG, diacylglycerol; PA, phosphatidate; PAP, PA phosphatase; PC, phosphatidylcholine; PE, phosphatidylethanolamine; TAG, triacylglycerol.
In addition to their roles in lipid synthesis, PA and DAG participate in various cellular processes, including membrane fission and fusion (9, 10, 11, 12, 13, 14), vesicular trafficking (15, 16, 17, 18, 19), lipid signaling (5, 20, 21, 22, 23, 24, 25), and the regulation of phospholipid synthesis gene expression (26, 27, 28) (Fig. 1). The requirement of Pah1 in controlling the lipid molecules is evident in the enzyme-deficient cells, which exhibit numerous deleterious phenotypes, including aberrant nuclear membrane structure, impaired lipid droplet formation, fatty acid–induced lipotoxicity, defects in vacuole fusion and autophagy, apoptosis, and a reduced chronological lifespan (1, 7, 27, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41). The significance of PAP activity in lipid metabolism and cell physiology extends to higher eukaryotes as well. The dysfunction of Pah1 orthologs in mice and humans, known as lipins (1, 42, 43), is implicated in a range of lipid-related diseases, including lipodystrophy, rhabdomyolysis, inflammation, insulin resistance, peripheral neuropathy, osteomyelitis, congenital dyserythropoietic anemia, and type 2 diabetes (42, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54).
Pah1 is a peripheral membrane enzyme that exerts its PAP activity at the nuclear/ER membrane (55). Its subcellular localization for catalytic function is regulated through phosphorylation and dephosphorylation (56). The phosphorylation of Pah1 by protein kinases stabilizes the enzyme in the cytosol but prevents its interaction with the membrane (32, 57, 58, 59, 60, 61, 62, 63, 64). For its targeted membrane localization, Pah1 is recruited and dephosphorylated by the Nem1–Spo7 protein phosphatase complex in the nuclear/ER membrane (29, 32, 55, 56, 65, 66). The dephosphorylated Pah1, which freely associates with the membrane, recognizes PA, dephosphorylates it, and moves along the membrane for additional rounds of catalysis (67). Unlike the phosphorylated Pah1, its dephosphorylated form is susceptible to degradation by the 20S proteasome (68).
The function of Pah1 and its regulation depend on distinct domains and regions (69) (Fig. 1). The catalytic core of the enzyme, which is required for catalytic function, consists of the N-terminal amphipathic helix for membrane association (55), the N-LIP and haloacid dehalogenase (HAD)–like domains cofolding in the tertiary structure for PAP activity (1, 70), and the WRDPLVDID domain containing a conserved Trp-637 residue for the enzyme function in vivo (71, 72). The intrinsically disordered regions (IDRs) of Pah1 contain most of the phosphorylation sites for the control of the enzyme localization, activity, and stability (56), and the IDR phosphorylation is facilitated by the RP domain (73). The C-terminal acidic tail is responsible for the priming interaction with the Spo7 subunit of the Nem1–Spo7 complex for the recruitment of Pah1 to the ER membrane (65).
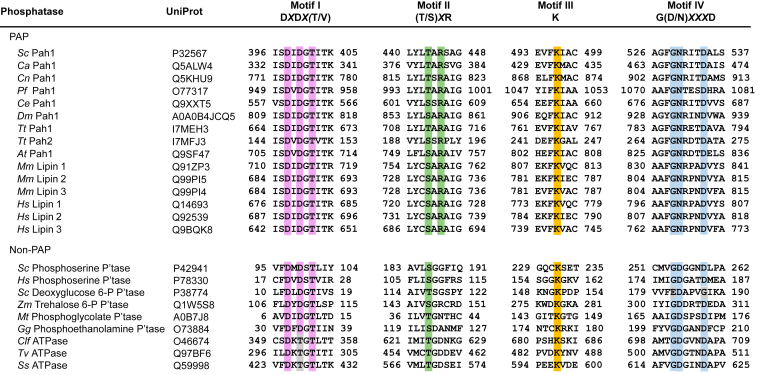
Pah1 belongs to the HAD-like superfamily (1, 74), a group of enzymes that catalyze the phosphoryl transfer reaction on a variety of substrates (75, 76, 77, 78). For the phosphoryl transfer chemistry, the HAD-like enzymes contain the Rossmann-like fold, a conserved catalytic structure that is featured as a central β-sheet composed of five to seven parallel β-strands surrounded by α-helices (75, 79, 80). Studies on the HAD-like enzymes other than Pah1–lipin PAPs have identified four active site motifs (74, 75, 76, 77, 78, 79, 81, 82, 83, 84, 85, 86, 87): motif I, characterized by the sequence DXDX(T/V) at the end of β-strand 1; motif II, which contains the Thr or Ser residue at the end of β-strand 2; motif III, which features a conserved Lys residue within an α-helix surrounding the central β-sheet with its side chain pointing toward the active site; and motif IV at the end of β-strand 4, which is often represented as G(D/N)XXXD but exhibits greater sequence variation (75).
The active site motifs I–IV of Pah1 and its orthologs were shown by sequence alignment (Fig. 2) combined with AlphaFold modeling (88, 89, 90) (Fig. 3) informed by the crystal structure of Tetrahymena thermophila Pah2 (91), which lacks the regulatory domains/regions of Pah1. While the motif I of Pah1 is known for its requirement for catalytic and physiological functions (70), the roles of its motifs II–IV remain unclear. In this study, we demonstrated that all four motifs of Pah1 are essential for catalytic activity and required for its cellular functions. These findings advance the understanding of the catalytic mechanism of Pah1 and provide a mechanistic basis for lipid-associated diseases caused by the mutations of human lipin PAPs in the active site residues.
Figure 2.
Four conserved motifs in the HAD-like domain of PAP and non-PAP enzymes. Clustal Omega alignment of HAD-like domain motifs in PAP and non-PAP enzymes with their UniProt accession numbers; the consensus sequence for each motif is shown above the alignment. Residue numbers are indicated at the start and end of the aligned sequences. The conserved residues of each motif are highlighted in color. At, Arabidopsis thaliana; Ca, Candida albicans; Ce, Caenorhabditis elegans; Clf, Canis lupus familiaris; Cn, Cryptococcus neoformans; Dm, Drosophila melanogaster; Gg, Gallus gallus; Hs, Homo sapiens; Mm, Mus musculus; Mt, Methanothrix thermoacetophila; Pf, Plasmodium falciparum; Sc, Saccharomyces cerevisiae; Ss, Synechocystis sp. PCC 6803; Tt, Tetrahymena thermophila; Tv, Thermoplasma volcanium; Zm, Zea mays. HAD, haloacid dehalogenase; PAP, PA phosphatase.
Figure 3.
Prediction/model of Pah1 active site architecture. The active site of Pah1 predicted by AlphaFold (88, 89, 90) is visualized by the PyMol program. The conserved residues of active site motifs I (Asp-398 and Asp-400), II (Thr-443 and Arg-445), III (Lys-496), and IV (Gly-529, Asn-530, and Asp-534), and β-strains 1 to 6 are indicated. Mg2+ is depicted by the gray circle.
Results
Active site motifs I–IV of Pah1 are required for PAP activity
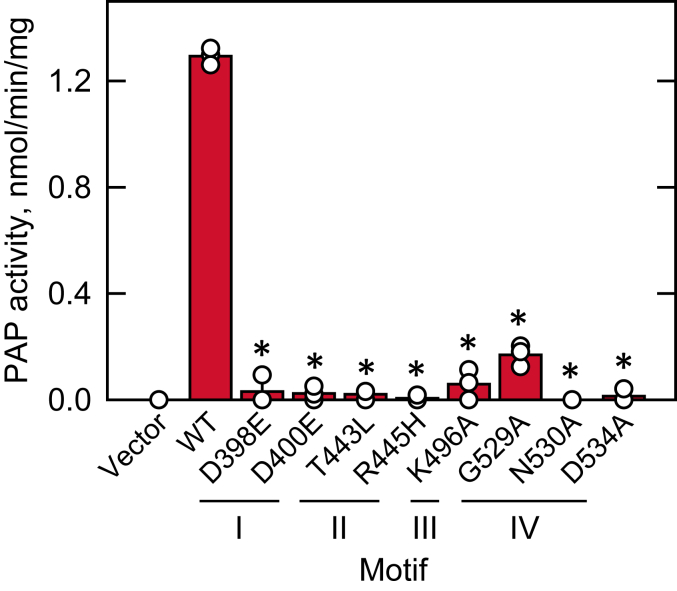
Sequence alignment showed that the HAD-like domain of Pah1 contains four motifs (Fig. 2), which are located within the active site that is predicted by the AlphaFold modeling (88, 89, 90) (Fig. 3). The active site motifs of Pah1 are characterized by the conserved residues, which are Asp-398, Asp-400, and Thr-402 in motif I, Thr-443 and Arg-445 in motif II, Lys-496 in motif III, and Gly-529, Asn-530, and Asp-534 in motif IV (Figures 2 and 3). Arg-445, which is not conserved in non-PAP proteins, was identified in our analysis as a conserved residue of the active site in Pah1 and its orthologs (Figs. 2 and 3). To examine the importance of the conserved residues for PAP activity, we examined their mutational effects. By site-directed mutagenesis, we generated the plasmid-borne PAH1 alleles containing point mutations for the conserved residues (Table 1). The D398E and D400E mutations conserve the negative charge; the T443L mutation replaces the polar residue with a nonpolar hydrophobic residue; the R445H mutation conserves the positive charge; K496A mutation replaces the positive charge with a nonpolar residue; G529A mutation introduces steric hindrance; the N530A mutation replaces the polar uncharged residue to a nonpolar residue; and D534A mutation replaces the negative charge to the nonpolar residue. In particular, the T443L and R445H mutations were made to mirror human lipin–related disease mutations. The mutant alleles were expressed in the pah1Δ app1Δ dpp1Δ lpp1Δ strain (8), which lacks all the PAP-encoding genes, PAH1 (1), APP1 (8), DPP1 (92), and LPP1 (93). The use of the quadruple mutant ensured that cellular PAP activity was solely attributed to the plasmid-borne PAH1 allele. The exponential phase cell extracts were prepared from the PAP-deficient strain expressing Pah1 active site mutants and analyzed for PAP activity by measuring the release of 32Pi from 32P-labeled PA (94). In the PAP assay, the substrate PA was delivered to the assay mixture as a uniform Triton X-100/PA-mixed micelle to mimic the membrane surface for catalysis (95). As expected, the PAP-deficient strain exhibited PAP activity (1.2 nmol/min/mg) by the expression of WT Pah1 (Fig. 4). Compared with the WT enzyme, active site mutants in the motifs I, II, and III showed almost complete lack of PAP activity. Of the mutants in the motif IV, N530A and D534A were inactive, whereas G529A showed a very weak activity (0.17 nmol/min/mg), which is sevenfold lower than that of the WT enzyme (Fig. 4). These results indicate that the conserved residues in the active site motifs I–IV are crucial for PAP activity.
Table 1.
Plasmids and strains used in this study
| Plasmid or strain | Genotype or relevant characteristics | Source or reference |
|---|---|---|
| Plasmid | ||
| pRS415 | Single-copy E. coli/S. cerevisiae shuttle vector with LEU2 | (139) |
| Derivative | ||
| pGH315 | PAH1 with native promoter in pRS415 | (59) |
| pGH315-D398E | pGH315 with the D398E mutation in PAH1 | (98) |
| pGH315-D400E | pGH315 with the D400E mutation in PAH1 | (98) |
| pGH315-T443L | pGH315 with the T443L mutation in PAH1 | This study |
| pGH315-R445H | pGH315 with the R445H mutation in PAH1 | This study |
| pGH315-K496A | pGH315 with the K496A mutation in PAH1 | This study |
| pGH315-G529A | pGH315 with the G529A mutation in PAH1 | This study |
| pGH315-N530A | pGH315with the N530A mutation in PAH1 | This study |
| pGH315-D534A | pGH315 with the D534A mutation in PAH1 | This study |
| YCplac33 | Single-copy E. coli/S. cerevisiae shuttle vector with URA3 | (140) |
| Derivative | ||
| YCplac33-SEC63-GFP | YCplac33 with SEC63-GFP fusion | (33) |
| pET-15b | E. coli IPTG-inducible expression vector with N-terminal His6-tag fusion | Novagen |
| Derivative | ||
| pGH313 | pET-15b with PAH1 coding sequence | (1) |
| pGH313-D398E | pGH313 with the D398E mutation in PAH1 | (70) |
| pGH313-T443L | pGH313 with the T443L mutation in PAH1 | This study |
| pGH313-K496A | pGH313 with the K496A mutation in PAH1 | This study |
| pGH313-N530A | pGH313 with the N530A mutation in PAH1 | This study |
| pGH313-ΔAH | pGH313 with the ΔAH (Δ2-18) mutation in PAH1 | This study |
| Strain | ||
| S. cerevisiae | ||
| RS453 | MATaade2-1 his3-11,15 leu2-3112 trp1-1 ura3-52 | (141) |
| Derivative | ||
| SS1026 | pah1Δ::TRP1 | (29) |
| W303-1A | MATaade2-1 can1-100 his3-11,15 leu2-3112 trp1-1 ura3-1 | (142) |
| Derivative | ||
| GHY66 | pah1Δ::URA3 app1Δ::natMX4 dpp1Δ::TRP1/Kanrlpp1Δ::HIS3/Kanr | (8) |
| E. coli | ||
| DH5⍺ | F- Φ80 lacZΔM15Δ (lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rk- mk+) phoA supE44 λ−thi-1 gyrA96 relA1 | (124) |
| NiCo21 (DE3)pLysSRARE2 | can::CBD fhuA2 [Ion] ompT gal (λ DE3) [dcm] amA::CBD sly::CBD glmS6Ala ΔhsdS λ DE3 = λ sBamHIo ΔEcoRI-B int::(lacI::PlacUV5::T7 gene1) i21 Δnin5 pLysSRARE2 | New England Biolabs |
Figure 4.
Effect of Pah1 active site motif mutations on PAP activity. The pah1Δ app1Δ dpp1Δ lpp1Δ (GHY66) cells were transformed with pRS415 (vector), pGH315 (PAH1), and pGH315 derivatives containing active site motif mutations. The yeast transformants were grown at 30 °C in SC-Leu medium to the exponential phase. Cell extracts were prepared and measured (30 μg) for PAP activity by following the release of 32Pi from [32P]PA. The data are means ± SD (error bars) from triplicate determinations; the individual data points for each experiment are shown. ∗p < 0.05 versus WT. PAP, PA phosphatase.
Pah1 active site mutants exhibit reduced stability and phosphorylation
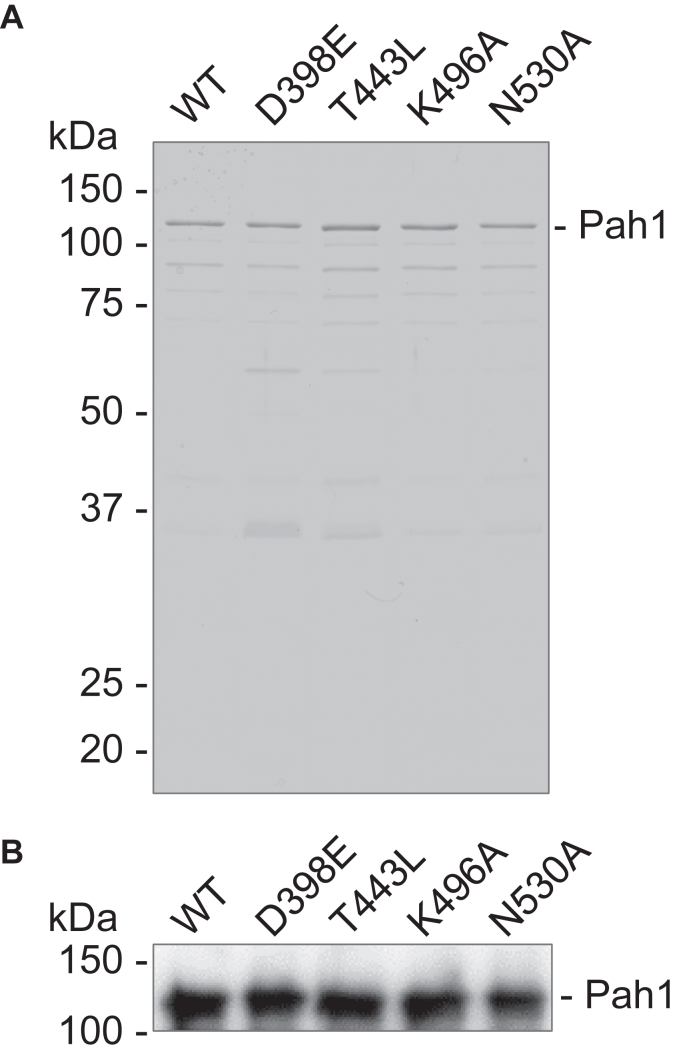
To confirm that activity deficiency of the Pah1 mutants was not because of the lack of the enzymes, we examined their expression by immunoblot analysis with anti-Pah1 antibody. As described previously (96), WT Pah1 was detected from the extracts of the exponential-phase cells used for PAP assay (Fig. 5). Likewise, its active site mutants were all detected but at a lower level (Fig. 5A). Since WT and mutant forms of Pah1 are expressed from the same gene promoter, the different enzyme levels are most likely because of a difference in their stability. In addition to their reduced levels, the active site mutants showed a faster electrophoretic mobility, which was readily noticeable in a low-percentage (6%) polyacrylamide gel (Fig. 5B). The electrophoretic mobility of Pah1 reflects its phosphorylation state (66, 73), and the faster mobility of the active mutants suggests that they are less phosphorylated than the WT enzyme. The phosphorylation state of Pah1 is intimately related to its stability, and its phosphorylation-deficient form is unstable in the cell (96). Accordingly, these results indicate the active site mutants of Pah1, which lack PAP activity, are less phosphorylated, and unstable.
Figure 5.
Cellular level and phosphorylation state of Pah1 active site motif mutants. The pah1Δ app1Δ dpp1Δ lpp1Δ (GHY66) cells were transformed with pRS415 (vector), pGH315 (PAH1), and pGH315 derivatives containing the active site motif mutations. The yeast transformants were grown at 30 °C in SC-Leu medium to the exponential phase. Samples (30 μg) of the cell extracts were separated by SDS-PAGE using the 12% (A) and 6% (B) polyacrylamide gels, transferred to a PVDF membrane, and probed with anti-Pah1 (A and B) and anti-Pgk1 (A) antibodies. The relative amounts of Pah1 (A) were determined by image analysis using iBright 1500 Imager and iBright Analysis software. The positions of Pah1, Pgk1, and molecular mass standards are indicated. The immunoblots shown are representative of three independent experiments. The data are means ± SD (error bars) from triplicate determinations; the individual data points for each experiment are shown. ∗p < 0.05 versus WT.
Pah1 active site mutants fail to complement the pah1Δ phenotypes
Loss of Pah1 function in S. cerevisiae results in a variety of cellular changes (reviewed in Ref. (5)). Prominent phenotypes of the pah1Δ mutant include a decrease in the TAG level and a reciprocal increase in the level of membrane phospholipids (1, 7, 37). The reduced TAG synthesis caused by a defect in DAG production correlates with a significantly reduced number of cytoplasmic lipid droplets (97, 98). In contrast, the increased level of membrane phospholipids, which is caused by the accumulation of PA that serves as a phospholipid precursor and as a transcriptional regulator for the derepression of phospholipid biosynthetic genes (2, 5, 26), is associated with the aberrant expansion of the nuclear/ER membrane (29, 70). Moreover, yeast cells lacking Pah1 function manifest diverse physiological defects that include a shortened chronological lifespan, apoptotic cell death in stationary phase, and an inability to grow at high temperature and on nonfermentable carbon sources (1, 29, 30, 36, 98). To examine whether the active site mutants of Pah1 complement the pah1Δ phenotypes, they were expressed on a single-copy plasmid in the pah1Δ or pah1Δ app1Δ dpp1Δ lpp1Δ cells (Table 1).
To assess the effect of the Pah1 mutants on lipid synthesis, the pah1Δ transformants were labeled to steady state with [2-14C]acetate and harvested in the stationary phase, then their lipids were extracted and analyzed by TLC (Fig. 6). Stationary-phase cells were examined for lipid analysis as the effect of Pah1 is pronounced when the TAG level is highest (1, 7). Consistent with previous findings (1, 7), pah1Δ cells harboring an empty vector showed a very low level of TAG (3%) but a significantly high level of phospholipids (66%). The expression of WT Pah1 in pah1Δ cells resulted in a substantial increase in the TAG level (44%) with a reciprocal decrease in the level of phospholipids (33%). In contrast to the WT enzyme, its active site mutants, except for G529A (9%) and D534A (6%), did not significantly increase the TAG level of pah1Δ cells.
Figure 6.
Effect of Pah1 active site motif mutations on the synthesis of TAG and phospholipids. The pah1Δ (SS1026) cells were transformed with pRS415 (vector), pGH315 (PAH1), and pGH315 derivatives containing the active site motif mutations. The transformants were grown at 30 °C to the stationary phase in SC-Leu medium containing 1 μCi/ml [2-14C]acetate. Radiolabeled lipids were extracted, separated by one-dimensional TLC, and quantified by phosphorimaging coupled with an image analysis by ImageQuant. The levels of TAG and phospholipids in each strain were normalized to its total 14C-labeled chloroform-soluble fraction. The data are means ± SD (error bars) from biological triplicates. The individual data points are also shown. ∗p < 0.05 versus WT. TAG, triacylglycerol.
The pah1Δ mutant, which contains the elevated level of PA (1, 28, 37, 99), exhibits the derepression of phospholipid biosynthetic genes (e.g., INO1, CHO1, OPI3) containing the UASINO cis-acting element in the promoter (27, 28, 29) through the Opi1/Ino2–Ino4 (Henry) regulatory circuit (2, 3, 5, 26, 100). In this regulation, the Opi1 repressor is sequestered at the nuclear/ER membrane through its binding to PA and does not inhibit the activator function of the Ino2–Ino4 complex in the transcription of the UASINO-containing genes (101). To determine the Pah1-mediated regulation of phospholipid biosynthetic gene expression, we examined the level of the CHO1-encoded PS synthase. Consistent with the previous finding (27), the elevated level of Cho1 in pah1Δ cells was reduced (∼1.6-fold) by the expression of WT Pah1 (Fig. 7A). However, little change was observed in the Cho1 level by the active site mutants (Fig. 7A).
Figure 7.
Effect of Pah1 active site motif mutations on the complementation of the pah1Δ physiological defects.A, pah1Δ app1Δ dpp1Δ lpp1Δ (GHY66) cells transformed with pRS415 (vector), pGH315 (PAH1), or pGH315 derivatives were grown in SC-Leu medium to the exponential phase. The expression of Cho1 was detected from cell extracts (10 μg) by immunoblot analysis with anti-Cho1 antibody, quantified by ImageQuant analysis, and normalized to the level of WT Pah1. B, pah1Δ cells harboring YCplac33-SEC63-GFP were transformed with pRS415 (vector), pGH315 (PAH1), or a pGH315 derivative. The yeast transformants were grown in SC-Leu-Ura medium to the exponential phase, examined by fluorescence microscopy for the signal of the ER marker Sec63-GFP, and scored for normal nuclear morphology from ≥200 cells. C, pah1Δ cells transformed with pRS415 (vector), pGH315 (PAH1), or a pGH315 derivative were grown to the stationary phase and stained with BODIPY 493/503. Lipid droplets were visualized by fluorescence microscopy from ≥300 cells. The black and white lines in the box plot are the median and mean values, respectively, and the white circles are the outlier data points of the 5th and 95th percentile. D, pah1Δ cells transformed with pRS415 (vector), pGH315 (PAH1), or a pGH315 derivative were grown to saturation and adjusted to an absorbance of 0.67 at 600 nm, followed by 10-fold serial dilution. About 5 μl of the diluted cultures were spotted on SC-Leu plates with 2% glucose (left and center) or 2% glycerol (right) and incubated at 30 °C (left and right) or 37 °C (center). The cell growth on glucose and glycerol plates was scored after incubation for 3 and 4 days, respectively. The data in A, B, and C are means ± SD (error bars) from triplicate determinations. ∗p < 0.05 versus the WT. ER, endoplasmic reticulum.
The increased synthesis of membrane phospholipids in the pah1Δ mutant is responsible for the nuclear/ER membrane expansion leading to an aberrant nuclear morphology (27, 29, 70). The nuclear morphology of pah1Δ cells harboring WT Pah1 or its active site mutant was monitored by the coexpression of the nuclear/ER marker Sec63-GFP. While the expression of WT Pah1 in pah1Δ cells restored normal, round nuclear morphology, the complementation effect was not clearly shown by the active site mutants (Fig. 7B). As previously reported (72, 102, 103), the expression of WT Pah1 in pah1Δ cells restored the formation of cytoplasmic lipid droplets (∼7 lipid droplets/cell). However, the expression of the active site mutants in motifs I–IV, which lack PAP activity, did not significantly increase the lipid droplets (2–3 lipid droplets/cell). The G529A mutant exhibiting weak PAP activity showed a partial effect on lipid droplet formation (∼5 lipid droplets/cell) (Fig. 7C). Moreover, the active site mutants, except for G529A and D534A, did not restore the growth defect of pah1Δ cells at 37 °C or on growth medium containing glycerol, a nonfermentable carbon source (Fig. 7D).
Effect of Pah1 active site mutations on the kinetics of PAP activity
We further investigated Pah1 active site mutants, which do not exhibit the cellular function, for PAP activity in a more defined system. The His6-tagged Pah1 and its D398E (motif I), T443L (motif II), K496A (motif III), and N530A (motif IV) mutants were heterologously expressed in Escherichia coli and purified by immobilized metal affinity chromatography and ion exchange chromatography. The E. coli-expressed Pah1 lacks phosphorylation (59), mimicking the dephosphorylated form of the enzyme, which is functional on the nuclear/ER membrane of S. cerevisiae (56, 104). Moreover, the unphosphorylated form of Pah1 can associate with the membrane in the absence of Nem1–Spo7, the protein phosphatase that catalyzes the dephosphorylation of Pah1 (67). The purified WT and the active site mutant forms of Pah1 were confirmed by SDS-PAGE (Fig. 8A) and immunoblot analysis with anti-Pah1 antibody (Fig. 8B).
Figure 8.
Purification of Pah1 and its active site motif mutants. The His6-tagged Pah1 and its catalytic motif mutants were expressed in Escherichia coli and purified by immobilized metal affinity chromatography. A, samples of the purified enzymes were subjected to SDS-PAGE (12% polyacrylamide gel) and stained with Coomassie blue. B, the purified enzyme preparations were subjected to immunoblot analysis with anti-Pah1 antibody. The positions of Pah1 and molecular mass standards are indicated. The data shown are representative of two experimental replicates.
The purified WT and active site mutant enzymes were examined for their kinetic parameters of PAP activity. In the PAP assay, Triton X-100 is used to create mixed micelles with PA, providing a surface for catalysis (95, 105, 106). Therefore, PA concentration is expressed as a surface concentration (mol %) rather than a molar concentration (95). This system ensures that the measured PAP activity is dependent on the surface concentration of PA and independent of its molar concentration (95). Consistent with previous findings (1, 70), WT Pah1 displayed positive cooperative kinetics with respect to the PA surface concentration, with a Vmax of 12 μmol/min/mg, a Km for PA of 3 mol%, and a Hill number of 3 (Fig. 9). However, the mutant enzymes did not show PAP activity at all the concentrations examined, and their kinetic parameters could not be determined (Fig. 9).
Figure 9.
Effect of Pah1 active site motif mutations on the kinetics of PAP activity. The PAP activity of WT Pah1 and its catalytic motif mutants was measured as a function of the PA surface concentration (mol %); the enzyme activity was measured by following the release of 32Pi from [32P]PA. The surface concentration of PA (mol %) was adjusted by maintaining the molar concentration of PA at 0.2 mM and varying the molar concentration of Triton X-100 (106). The data are means ± SD (error bars) from triplicate assays. Some error bars are contained within the data symbols. PAP, PA phosphatase.
Active site mutations of Pah1 do not affect its overall structure and interaction with liposomes
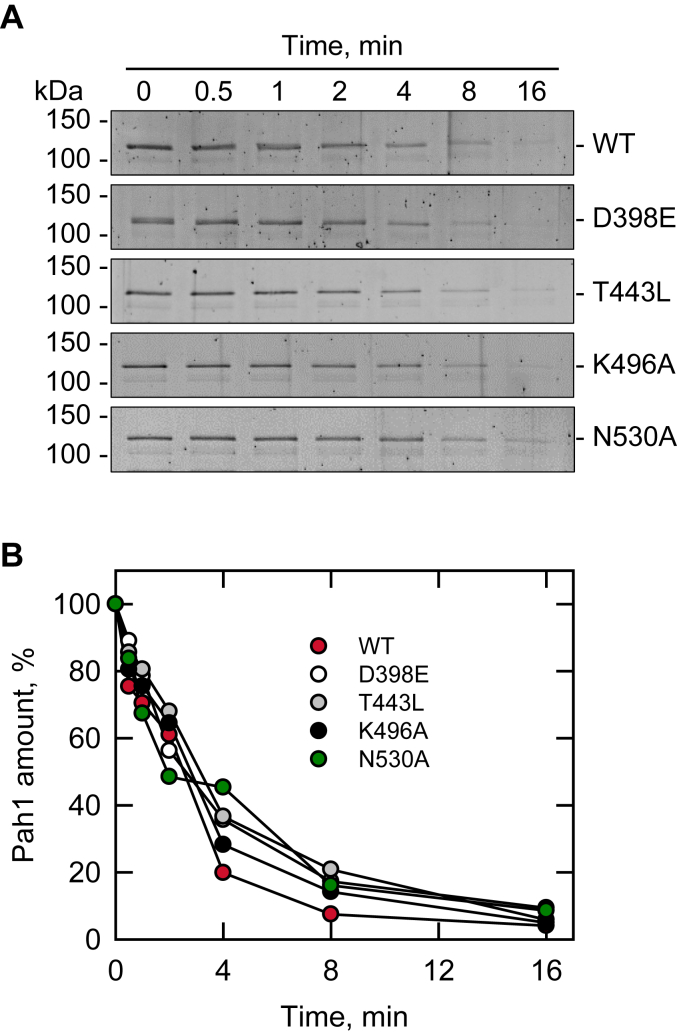
AlphaFold modeling (88, 89, 90) shows that the overall structure of Pah1 is not significantly affected by the active site mutations D398E, T443L, K496A, and N530A. To determine whether these mutations cause an overall structural change, we performed a limited proteolysis assay (107, 108). The WT and mutant forms of the E. coli-expressed, unphosphorylated Pah1 were incubated with chymotrypsin for different periods, and the protein digests were separated by SDS-PAGE (Fig. 10A). The analysis of the full-length Pah1 showed that WT and its active mutants were digested in a very similar extent (Fig. 10B). This result suggests that the overall structure of dephosphorylated Pah1, which functions on the nuclear/ER membrane, is not affected by the active site mutations D398E, T443L, K496A, and N530A.
Figure 10.
Limited chymotrypsin digestion of Pah1 and its active site motif mutants.A, samples (0.1 μg) of purified Pah1 and its active site motif mutants were incubated at 23 °C for the indicated periods with 25 ng of chymotrypsin in phosphate-buffered saline (pH 7.4). The digestion mixtures were separated by SDS-PAGE (12% polyacrylamide gel) and stained with SYPRO Ruby. The full-length position of Pah1 and its mutant forms and molecular mass standards are indicated. The data are representative of duplicate experiments. B, the amounts of full-length Pah1 and its mutant forms remaining after chymotrypsin treatment were determined by image analysis using iBright 1500 Imager and iBright Analysis software. The data are the average of duplicate experiments.
We next examined the interaction of unphosphorylated Pah1 with liposomes whose phospholipid contents PC/PE/PI/PS/PA (33.75:22.5:22.5:11.25:10 mol%) reflect the composition of the nuclear/ER membrane and the subcellular location where dephosphorylated Pah1 associates for catalytic activity (67, 109). To assess only its membrane association, Pah1 was incubated with liposomes in the absence of the cofactor Mg2+ required for catalysis (1, 105). Liposomes were collected by centrifugation and examined for the presence of Pah1 by immunoblot analysis with anti-Pah1 antibody. As described previously (67), WT Pah1 was found exclusively in the pellet of liposomes (Fig. 11). Similarly, the active site mutants were also found almost exclusively in the pellet fraction. As expected, Pah1 mutant (ΔAH), which is defective in membrane association because of the lack of the amphipathic helix (55), showed a great reduction in the liposomal pellet (Fig. 11). These results indicate that the active site mutations of Pah1 do not affect its ability to interact with the membrane.
Figure 11.
Liposome interaction of Pah1 and its active site motif mutants. WT and mutant forms of Pah1 (0.1 μg) were incubated for 15 min at 30 °C with (10 mM phospholipid) PC/PE/PI/PS/PA liposomes. Following the incubation, liposomes were precipitated by centrifugation at 100,000g for 1 h at 4 °C. The pellet (P) fraction was suspended in buffer to the same volume as that of the supernatant (S) fraction, and equal volumes of the fractions were subjected to SDS-PAGE, followed by immunoblot analysis with anti-Pah1 antibody. A, the immunoblot shown is representative of two experiments. The positions of Pah1 and molecular mass standards are indicated. B, the amounts of Pah1 associated with the liposome were quantified by image analysis using iBright 1500 Imager and iBright Analysis software. The bars are means of the experimental replicates; the individual data points are shown. PA, phosphatidate; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine.
Discussion
Pah1, which controls the levels of PA and DAG at the nuclear/ER membrane, plays a major role not only in lipid synthesis but also in diverse cellular processes (29, 32, 55, 56, 65, 66, 102, 110, 111). Pah1 belongs to the Mg2+-dependent HAD-like phosphatase superfamily, characterized by the DXDX(T/V) motif (1, 70, 74). This superfamily encompasses a broad range of phosphatases with diverse substrate specificities (74). Research on other HAD-like family members suggests a four-motif active site, with the DXDX(T/V) sequence designated as motif I (74). Studies on phosphoserine phosphatase, a non-Pah–lipin PAP enzyme, have demonstrated the importance of all four motifs for its activity (82). In motif I, the first Asp acts as a nucleophile, forming a phosphoryl–aspartate intermediate, whereas the second Asp deprotonates a water molecule to facilitate hydrolysis of this intermediate (76, 85). Motifs II and III are thought to stabilize the reaction intermediate (75), and motif IV coordinates the essential Mg2+ ion (75, 82). Sequence alignments show that Pah1, like its mammalian lipin homologs, contains all four motifs within its HAD-like domain (Fig. 2). In this work, we identified the active site motifs I–IV of Pah1 and its orthologs (Fig. 2) and examined Pah1 as a model for the Mg2+-dependent PAPs to show that the conserved residues of the motifs are crucial for PAP activity and the cellular functions of the enzyme.
Our findings demonstrated that the Pah1 mutations in the active site motifs I–IV abolish PAP activity without affecting the enzyme interaction with the liposome membrane. The catalytically inactive Pah1 failed to rescue the pah1Δ phenotypes, including reduced levels of TAG and lipid droplets, aberrant nuclear morphology, increased expression of Cho1 PS synthase, and growth defects at high temperature and on glycerol medium. Thus, the conserved residues of Pah1 in the motifs I–IV are essential for PAP activity and thereby for the control of lipid synthesis, gene regulation, and other cellular processes. The proteolysis analysis suggests that the overall structure of Pah1 is not significantly affected by its active site mutations. Interestingly, however, the Pah1 mutants were less abundant in the cell and exhibited a reduced phosphorylation state. The lower cellular level of the mutant protein is likely to result from an increased susceptibility to proteolysis under the reduced state of phosphorylation (68, 96). It is yet unclear why the active site mutants are in a lower phosphorylation state. Pah1 mutations that do not directly affect PAP activity also affect the phosphorylation state and stability of the enzyme (71, 72). Thus, we cannot conclude that PAP activity per se regulates Pah1 phosphorylation. Considering that the cells lacking Pah1 PAP activity exhibit diverse physiological changes, the reduced phosphorylation of mutant Pah1 may result from a decreased protein kinase activity or by an increased protein phosphatase activity in the PAP-deficient cell, which is not complemented by the expression of the nonfunctional enzyme.
We identified Arg-445 as a motif II residue conserved in Pah1 and its orthologs but not in other HAD-like enzymes (Fig. 2). The conservation of the arginine residue in Pah1–lipin PAPs could stem from a difference in enzyme structure requirements and/or its substrate specificity. Pah1–lipin PAPs contains the N-LIP domain (42), which is not present in other members of the HAD-like enzymes such as phosphoserine phosphatase (82). The N-LIP domain and the HAD-like domain cofold to form a two-domain structure as the catalytic core of PAP (42, 69, 70, 91). Unlike the HAD-like domain, which contains the active site motifs, the role of the N-LIP domain in PAP activity is unclear. In kinetic analysis, a Pah1 variant containing the G80R mutation in the N-LIP domain exhibits a greater reduction in Vmax when compared with a change in Km (70), suggesting the contribution of the N-LIP domain to catalysis more than to substrate binding. While containing the N-LIP domain, Pah1–lipin PAPs lack a cap domain (69, 91), a mobile element influencing substrate specificity in many HAD-like phosphatases (75, 112). Protein modeling (113) indicates that the active site of Pah1 is smaller than that of non-PAP HAD-like enzymes (114), suggesting that it can accommodate only the phosphate group of PA. Indeed, the acyl chain composition of PA has no effect on the catalytic activity of Pah1 and lipin 1 (67, 115). Unlike the non-PAP enzymes acting on water-soluble substrates (74, 75, 76, 77, 78), Pah1–lipin PAPs utilize water-insoluble PA as a substrate (1, 67, 115). Further research is needed to determine whether structural differences are associated with the role of the conserved arginine residue of Pah1–lipin PAP enzymes.
Given that Lys-496 in motif III is crucial for the catalytic activity of Pah1, any modification of the active site is likely to abolish the cellular function of the enzyme. As reported here, other studies have shown that Pah1 mutated at Lys-496 is nonfunctional and does not complement the pah1Δ phenotypes, such as irregular nuclear morphology and reduced amounts of TAG and lipid droplets (116, 117). While the functional deficiency of the K496A mutant is caused by the lack of PAP activity, Laframboise et al. (117) attributed the mutational effect to the defect of the enzyme in localizing to the inner nuclear membrane in association with the lack of the lysine acetylation. The acetylation of Lys-496 was indicated by the analysis of Pah1 expressed heterologously in human embryonic kidney 293T cells overexpressing the Esa1 acetyltransferase (116). Despite this finding, the acetylation of Pah1 at Lys-496 is yet unclear in a native physiological condition as proteomic analysis of S. cerevisiae does not show this lysine to be acetylated (118). In addition, Li et al. (116) erroneously described that Lys-496 in the HAD-like domain of Pah1 corresponds to Lys-425 in the IDR of lipin 1. Lys-425 is one of the two residues (Lys-425 and Lys-595) that are acetylated by TIP60 acetyltransferase in response to fatty acid stimulation (116). The acetylation of Lys-425 and Lys-595 in lipin 1 does not affect its PAP activity but facilitates the translocation of the enzyme to the ER membrane for catalytic function, contributing to TAG synthesis and lipid droplet formation (116). In the optimal sequence alignment (42), the human lipin 1 residue corresponding to the Pah1 Lys-496 in the HAD-like domain is Lys-776 (Fig. 2), which is not subject to acetylation (116). Pah1 contributes to TAG synthesis and lipid droplet formation through its catalytic function mainly at the nuclear/ER membrane, not at the inner nuclear membrane (29, 32, 55, 56, 65, 66, 67). Accordingly, the role of Lys-496 as an acetylation site for the localization control of Pah1 is intriguing but remains uncertain.
The motif IV residues (Gly-529, Asn-530, and Asp-534) were also shown to be important for PAP activity and the physiological functions of the enzyme. Of the three residues, Asn-530 showed the strongest mutational effect. Like Asp-179 (motif IV) of phosphoserine phosphatase (82), Asn-530 is predicted to play a role in the coordination of Mg2+ in the active site (Fig. 3). Interestingly, the G529A mutant, which retained limited PAP activity in the cell extract, and the D534A mutant, which showed essentially no enzymatic activity in the extract, mostly complemented growth of pah1Δ cells at 37 °C and on glycerol-containing medium. The expression of both mutants slightly increased the TAG level of pah1Δ cells when compared with the vector control. Since TAG production requires Pah1 PAP activity, this indicated that the G529A and D534A mutant enzymes retain a very low level of PAP activity that is sufficient to complement these particular pah1Δ mutant phenotypes.
The dependence of Pah1 function on the active site motifs provides an insight into the mechanistic basis for the lipin-related diseases in humans. Specifically, three conserved residues are of interest: Thr-443 and Arg-445 in motif II of Pah1 correspond to Ser-734 of lipin 2 (UniProt no.: Q92539) and Arg-725 of lipin 1 (UniProt no.: Q14693), respectively, whereas Gly-529 in motif IV of Pah1 corresponds to Gly-799 of lipin 1 (UniProt no.: Q14693) (Fig. 2). The S734L mutation in lipin 2 (motif II), which eliminates PAP activity (49), causes Majeed syndrome, an inflammatory disorder characterized by recurrent osteomyelitis, fever, dyserythropoietic anemia, and skin inflammation (53, 119). Mutations R725H (motif II) and G799R (motif IV) in lipin 1 cause rhabdomyolysis (120), marked by myoglobinuria (121). Notably, the motif II mutations in lipins 1 and 2 result in distinct lipin-related diseases, which may be based on their differential tissue expression (43) and thus different levels of PAP activity. In T. thermophila Pah2 (UniProt no.: I7MFJ3), mutations S191L (motif II) and G267R (motif IV) abolish PAP activity (91), further highlighting the importance of the conserved active site residues whose mutation in human lipins leads to a disease condition.
Experimental procedures
Reagents
All chemicals were reagent grade. Growth medium was sourced from Difco Laboratories. Enzymes and reagents for DNA manipulations came from New England Biolabs. Carrier DNA for yeast transformations was purchased from Clontech. Qiagen was the source of nickel–nitrilotriacetic acid agarose resin and kits for plasmid and DNA gel extractions. Roche was the source for EDTA-free cOmplete ULTRA protease inhibitor tablets. Ampicillin, nucleotides, silica gel TLC plates, ATP, Triton X-100, and bovine serum albumin were acquired from Millipore–Sigma. Bio-Rad supplied the DNA size ladders, molecular mass protein standards, and reagents used for electrophoresis and immunoblotting. InstantBlue Coomassie protein stain was purchased from Expedeon. Lipids were purchased from Avanti Polar Lipids. Radiochemicals and scintillation counting supplies were acquired from Revvity and National Diagnostics, respectively. Q-Sepharose, Protein A-Sepharose CL-4B, polyvinylidene difluoride membrane, and enhanced chemifluorescence substrate were acquired from GE Healthcare. Thermo Fisher Scientific supplied Malachite green, BODIPY 493/503, and SYPRO Ruby.
Antibodies
Rabbit polyclonal antibodies were generated against the peptide TSIDKEFKKLSVSKAGA (residues 778–794) of Pah1 (59) and MVESDEDFAPQEFPH (residues 1–15) of Cho1 (122). The IgG fraction of the antibodies was purified by affinity chromatography (123) with Protein A-Sepharose CL-4B and used for immunoblot analyses. Mouse anti-Pgk1 antibody was from Abcam (product number: ab113687; lot number: 2101050637). Goat anti-rabbit IgG antibody conjugated with alkaline phosphatase was from Thermo Fisher Scientific (product number: 31340; lot number: NJ178812). Goat anti-mouse IgG antibody conjugated with alkaline phosphatase was from Millipore–Sigma (product number: A3562; lot number: SLBG1482V).
Plasmids, strains, and growth conditions
The plasmids and strains used in this study are listed in Table 1. The isolation of plasmid DNA, PCR amplification, restriction enzyme digestion, and DNA ligation were performed by standard methods (124, 125, 126). Plasmids pGH315 and pGH313 were used for the expression of Pah1 and its mutants in S. cerevisiae and E. coli, respectively. The derivatives of pGH315 and pGH313 containing the PAH1 mutations in the active site motifs I–IV and amphipathic helix were constructed by the PCR-mediated site-directed mutagenesis (102), and the mutations were confirmed by DNA sequencing. E. coli strain DH5α was used for plasmid propagation (124), and the strain NiCo21(DE3)pLysS RARE2 (New England Biolabs) was used for heterologous expression of Pah1. E. coli was grown at 37 °C in lysogeny broth media (1% tryptone, 0.5% yeast extract, 1% NaCl, pH 7.0), and its plasmid transformant was selected with appropriate antibiotics. The S. cerevisiae strains pah1Δ (SS1026) and pah1Δ app1Δ dpp1Δ lpp1Δ (GHY66) were used for the expression of plasmid-borne PAH1 and its mutant alleles. S. cerevisiae transformants were grown at 30 °C in synthetic complete media lacking an appropriate nutrient for plasmid maintenance. Plasmid transformations of E. coli (124) and S. cerevisiae (127) were performed by standard methods. The growth of E. coli and S. cerevisiae in liquid media was monitored by measuring the absorbance at 600 nm. Solid growth media contained 1.5% and 2% agar for E. coli and S. cerevisiae, respectively.
Preparation of yeast cell extracts
All steps were conducted at 4 °C. The exponential-phase cultures (absorbance at 600 nm = 0.5) of S. cerevisiae pah1Δ app1Δ dpp1Δ lpp1Δ expressing PAH1 and its mutant alleles were lysed by mechanical disruption with glass beads (0.5-mm diameter) in the buffer containing 50 mM Tris–HCl (pH 7.5), 10 mM 2-mercaptoethanol, 100 mM sucrose, and the EDTA-free protease inhibitor cocktail (94). The lysate was centrifuged at 1500g for 10 min to remove the unbroken cells and glass beads, and the supernatant was used as cell extract.
Purification of Pah1
E. coli NiCo21(DE3)pLysS RARE2 harboring the pGH313 or its derivatives was grown in lysogeny broth containing chloramphenicol (34 μg/ml) and ampicillin (100 μg/ml) until absorbance at 600 nm reaches 1.0 and was added with 1 mM isopropyl-β-D-thiogalactoside to express the His6-tagged Pah1 (1) and its mutants. The epitope-tagged proteins were isolated from the induced culture by immobilized metal affinity chromatography with nickel–nitrilotriacetic acid agarose. The affinity-purified enzymes were further purified by anion exchange chromatography with Q-Sepharose (1, 66) and stored at −80 °C until use.
Preparation of Triton X-100/PA-mixed micelles
PA dissolved in chloroform was dried in vacuo, followed by its suspension in Triton X-100 to prepare Triton X-100/PA-mixed micelles (94). The molar percent of PA in the Triton X-100/PA-mixed micelle was calculated by the formula: mol %PA = 100 × [PA (molar)]/([PA (molar)] + [Triton X-100 (molar)]). The PA concentration was kept below 15 mol% to ensure that the structure of the Triton X-100/PA-mixed micelles was similar to that of pure Triton X-100 micelles (128, 129).
Preparation of liposomes
Unilamellar liposomes, which are composed of PC/PE/PI/PS/PA (33.75:22.5:22.5:11.25:10 mol%), were prepared by the extrusion method of MacDonald et al. (130). Briefly, the phospholipid mixture (10 mM) dissolved in chloroform was dried under a stream of nitrogen to form a thin film, and the residual solvent was removed in vacuo for 30 min. Dried phospholipids were resuspended in 50 mM Tris–HCl (pH 7.5) buffer containing 150 mM NaCl. After five cycles of freezing (−80 °C) and thawing (37 °C), the phospholipid suspension was repeatedly extruded through a polycarbonate filter with the pore size of 100 nm (67). The resulting liposomes were stored at 4 °C and used within 1 week.
PAP assay
PAP activity was measured at 30 °C for 20 min by following the release of water-soluble 32Pi from chloroform-soluble [32P]PA (1, 94). [32P]PA was enzymatically synthesized from 1, 2-dioleoyl DAG and [γ-32P]ATP with E. coli DAG kinase (94). The PAP reaction mixture contained 50 mM Tris–HCl (pH 7.5), 0.2 mM [32P]PA (5000–10,000 cpm/nmol), 2 mM Triton X-100, 1 mM MgCl2, 10 mM 2-mercaptoethanol, and the indicated amount of enzyme protein.
Pah1–liposome interaction assay
Pah1 (0.1 μg) was incubated for 15 min at 30 °C with liposomes (10 mM phospholipid) in 50 mM Tris–HCl (pH 7.5) buffer containing 150 mM NaCl in a total volume of 20 μl. Following the incubation, the reaction mixture was centrifuged at 100,000g for 1 h at 4 °C, and the pellet was resuspended in the supernatant volume of the same buffer. The equal volumes of the supernatant and pellet fractions were subjected to SDS-PAGE (131), and the presence of Pah1 in the fraction was detected by immunoblotting (132, 133, 134) with rabbit anti-Pah1 antibody (59).
Limited proteolysis
The purified preparation (0.1 μg) of Pah1 and its mutant derivatives was incubated at 23 °C with 25 ng of chymotrypsin in phosphate-buffered saline (pH 7.4) for various periods (0–16 min). The protein digests were separated by SDS-PAGE using 12% polyacrylamide gels and stained with SYPRO Ruby.
Lipid analysis
[2-14C]acetate labeling of lipids (135), extraction (136), and separation by one-dimensional TLC (hexane/diethyl ether/glacial acetic acid [40:10:1 v/v]) on silica gel plates (137) were performed as described previously. After resolution, the 14C-labeled lipids were visualized by phosphorimaging with the Storm 860 Molecular Imager (GE Healthcare) and quantified by ImageQuant software (GE Healthcare) using a standard curve of [2-14C]acetate. To confirm the identity of the radiolabeled lipids, their migration on the silica gel was compared with authentic standards visualized by iodine vapor staining.
Analyses of lipid droplets and nuclear/ER morphology
A Nikon Eclipse NiU microscope with an EGFP/FITC/Cy2/AlexaFluor 488 filter was used for the analysis of lipid droplets and nuclear/ER membrane morphology. Fields of view from one z-plane were captured with a DS-Qi2 camera, and images were analyzed with NIS-Elements BR software or FIJI software. Lipid droplets were imaged from stationary phase cells (absorbance at 600 nm ∼3.5) after staining with 2 mM BODIPY 493/503 in phosphate-buffered saline (pH 7.4) (27). Nuclear/ER membrane morphology was imaged from yeast cells expressing Sec63-GFP (33). The average number of lipid droplets per cell and percentage of cells with a round nucleus were scored from a total of ≥300 cells.
SDS-PAGE and immunoblot analysis
SDS-PAGE (131) and immunoblotting (133, 134) were performed by standard procedures. Normalized gel loading was confirmed by protein concentration determination according to the method of Bradford (138) with bovine serum albumin as the standard. Protein transfer from polyacrylamide gels to polyvinylidene difluoride membranes was monitored by Ponceau S staining. The protein blots were probed with rabbit anti-Pah1 (2 μg/ml), rabbit anti-Cho1 (0.25 μg/ml), or mouse anti-Pgk1 (2 μg/ml) antibody. The antibody-treated membrane was incubated with alkaline phosphatase–conjugated goat anti-rabbit IgG antibody or goat anti-mouse IgG antibody at a dilution of 1:5,000, and the immune complexes were detected with an enhanced chemifluorescence substrate for alkaline phosphatase. The fluorescence signal from the immunoblot was detected by Invitrogen iBright 1500 imager, and the signal intensity was analyzed using the iBright Analysis Software.
Data analysis
Kinetic parameters were determined by the enzyme kinetics module of the SigmaPlot software (Grafiti LLC). Microsoft Excel software was utilized for statistical analysis; p values <0.5 were taken to be statistically significant.
Data availability
All data are contained within the article.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We acknowledge Shoily Khondker and Emily Bostrom for the helpful discussions during the course of this work. This work was supported, in whole or in part, by National Institutes of Health grant GM136128 from the United States Public Health Service.
Author contributions
G. J. S., G.-S. H., and G. M. C. conceptualization; G. J. S., J. M. K., and G.-S. H. methodology; G. J. S. validation; G. J. S., P. K. S., R. J., J. M. K., G.-S. H., and G. M. C. formal analysis; G. J. S., P. K. S., R. J., J. M. K., and G.-S. H. investigation; G. J. S. and G.-S. H. resources; G. J. S., P. K. S., R. J., and G. M. C. data curation; G. J. S. writing–original draft; G. J. S., P. K. S., R. J., J. M. K., G.-S. H., and G. M. C. writing–review & editing; G. J. S. visualization; G.-S. H. supervision; G. M. C. project administration; G. M. C. funding acquisition.
Funding and additional information
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Todd R. Graham
References
- 1.Han G.-S., Wu W.-I., Carman G.M. The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 2006;281:9210–9218. doi: 10.1074/jbc.M600425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carman G.M., Han G.-S. Regulation of phospholipid synthesis in the yeast Saccharomyces cerevisiae. Ann. Rev. Biochem. 2011;80:859–883. doi: 10.1146/annurev-biochem-060409-092229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry S.A., Kohlwein S., Carman G.M. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics. 2012;190:317–349. doi: 10.1534/genetics.111.130286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carman G.M., Han G.-S. Fat-regulating phosphatidic acid phosphatase: a review of its roles and regulation in lipid homeostasis. J. Lipid Res. 2019;60:2–6. doi: 10.1194/jlr.S087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatek J.M., Han G.-S., Carman G.M. Phosphatidate-mediated regulation of lipid synthesis at the nuclear/endoplasmic reticulum membrane. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865 doi: 10.1016/j.bbalip.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor F.R., Parks L.W. Triacylglycerol metabolism in Saccharomyces cerevisiae relation to phospholipid synthesis. Biochim. Biophys. Acta. 1979;575:204–214. doi: 10.1016/0005-2760(79)90022-5. [DOI] [PubMed] [Google Scholar]
- 7.Pascual F., Soto-Cardalda A., Carman G.M. PAH1-encoded phosphatidate phosphatase plays a role in the growth phase- and inositol-mediated regulation of lipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2013;288:35781–35792. doi: 10.1074/jbc.M113.525766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chae M., Han G.-S., Carman G.M. The Saccharomyces cerevisiae actin patch protein App1p is a phosphatidate phosphatase enzyme. J. Biol. Chem. 2012;287:40186–40196. doi: 10.1074/jbc.M112.421776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao M.J., Prestegard J.H. Fusion of phosphatidic acid-phosphatidylcholine mixed lipid vesicles. Biochim. Biophys. Acta. 1979;550:157–173. doi: 10.1016/0005-2736(79)90204-9. [DOI] [PubMed] [Google Scholar]
- 10.Koter M., de K.B., van Deenen L.L. Calcium-induced aggregation and fusion of mixed phosphatidylcholine-phosphatidic acid vesicles as studied by 31P NMR. Biochim. Biophys. Acta. 1978;514:255–263. doi: 10.1016/0005-2736(78)90296-1. [DOI] [PubMed] [Google Scholar]
- 11.Blackwood R.A., Smolen J.E., Transue A., Hessler R.J., Harsh D.M., Brower R.C., et al. Phospholipase D activity facilitates Ca2+-induced aggregation and fusion of complex liposomes. Am. J. Physiol. 1997;272:C1279–C1285. doi: 10.1152/ajpcell.1997.272.4.C1279. [DOI] [PubMed] [Google Scholar]
- 12.Weigert R., Silletta M.G., Spano S., Turacchio G., Cericola C., Colanzi A., et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 13.Goni F.M., Alonso A. Structure and functional properties of diacylglycerols in membranes. Prog. Lipid Res. 1999;38:1–48. doi: 10.1016/s0163-7827(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 14.Chernomordik L., Kozlov M.M., Zimmerberg J. Lipids in biological membrane fusion. J. Membr. Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- 15.Roth M.G. Molecular mechanisms of PLD function in membrane traffic. Traffic. 2008;9:1233–1239. doi: 10.1111/j.1600-0854.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 16.Morris A.J. Regulation of phospholipase D activity, membrane targeting and intracellular trafficking by phosphoinositides. Biochem. Soc. Symp. 2007:247–257. doi: 10.1042/BSS0740247. [DOI] [PubMed] [Google Scholar]
- 17.Maissel A., Marom M., Shtutman M., Shahaf G., Livneh E. PKCeta is localized in the Golgi, ER and nuclear envelope and translocates to the nuclear envelope upon PMA activation and serum-starvation: C1b domain and the pseudosubstrate containing fragment target PKCeta to the Golgi and the nuclear envelope. Cell Signal. 2006;18:1127–1139. doi: 10.1016/j.cellsig.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Lehel C., Olah Z., Jakab G., Szallasi Z., Petrovics G., Harta G., et al. Protein kinase C epsilon subcellular localization domains and proteolytic degradation sites. A model for protein kinase C conformational changes. J. Biol. Chem. 1995;270:19651–19658. doi: 10.1074/jbc.270.33.19651. [DOI] [PubMed] [Google Scholar]
- 19.Baron C.L., Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 20.Exton J.H. Signaling through phosphatidylcholine breakdown. J. Biol. Chem. 1990;265:1–4. [PubMed] [Google Scholar]
- 21.Exton J.H. Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 22.Testerink C., Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005;10:368–375. doi: 10.1016/j.tplants.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Waggoner D.W., Xu J., Singh I., Jasinska R., Zhang Q.X., Brindley D.N. Structural organization of mammalian lipid phosphate phosphatases: implications for signal transduction. Biochim. Biophys. Acta. 1999;1439:299–316. doi: 10.1016/s1388-1981(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 24.Kudo S., Shiino H., Furuta S., Tamura Y. Yeast transformation stress, together with loss of Pah1, phosphatidic acid phosphatase, leads to Ty1 retrotransposon insertion into the INO4 gene. FASEB J. 2020;34:4749–4763. doi: 10.1096/fj.201901811RR. [DOI] [PubMed] [Google Scholar]
- 25.Dey P., Su W.M., Han G.-S., Carman G.M. Phosphorylation of lipid metabolic enzymes by yeast Pkc1 protein kinase C requires phosphatidylserine and diacylglycerol. J. Lipid Res. 2017;58:742–751. doi: 10.1194/jlr.M075036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carman G.M., Henry S.A. Phosphatidic acid plays a central role in the transcriptional regulation of glycerophospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:37293–37297. doi: 10.1074/jbc.R700038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han G.-S., Carman G.M. Yeast PAH1-encoded phosphatidate phosphatase controls the expression of CHO1-encoded phosphatidylserine synthase for membrane phospholipid synthesis. J. Biol. Chem. 2017;292:13230–13242. doi: 10.1074/jbc.M117.801720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaspar M.L., Aregullin M.A., Chang Y.F., Jesch S.A., Henry S.A. Phosphatidic acid species 34:1 mediates expression of the myo-inositol 3-phosphate synthase gene INO1 for lipid synthesis in yeast. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos-Rosa H., Leung J., Grimsey N., Peak-Chew S., Siniossoglou S. The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 2005;24:1931–1941. doi: 10.1038/sj.emboj.7600672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irie K., Takase M., Araki H., Oshima Y. A gene, SMP2, involved in plasmid maintenance and respiration in Saccharomyces cerevisiae encodes a highly charged protein. Mol. Gen. Genet. 1993;236:283–288. doi: 10.1007/BF00277124. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz C., Cid V.J., Lussier M., Molina M., Nombela C. A large-scale sonication assay for cell wall mutant analysis in yeast. Yeast. 1999;15:1001–1008. doi: 10.1002/(SICI)1097-0061(199907)15:10B<1001::AID-YEA400>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.O'Hara L., Han G.-S., Peak-Chew S., Grimsey N., Carman G.M., Siniossoglou S. Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J. Biol. Chem. 2006;281:34537–34548. doi: 10.1074/jbc.M606654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han G.-S., O'Hara L., Carman G.M., Siniossoglou S. An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J. Biol. Chem. 2008;283:20433–20442. doi: 10.1074/jbc.M802903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasser T., Qiu Q.S., Karunakaran S., Padolina M., Reyes A., Flood B., et al. The yeast lipin 1 orthologue Pah1p regulates vacuole homeostasis and membrane fusion. J. Biol. Chem. 2012;287:2221–2236. doi: 10.1074/jbc.M111.317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lussier M., White A.M., Sheraton J., di P.T., Treadwell J., Southard S.B., et al. Large scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics. 1997;147:435–450. doi: 10.1093/genetics/147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park Y., Han G.-S., Mileykovskaya E., Garrett T.A., Carman G.M. Altered lipid synthesis by lack of yeast Pah1 phosphatidate phosphatase reduces chronological life span. J. Biol. Chem. 2015;290:25382–25394. doi: 10.1074/jbc.M115.680314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassaninasab A., Han G.-S., Carman G.M. Tips on the analysis of phosphatidic acid by the fluorometric coupled enzyme assay. Anal. Biochem. 2017;526:69–70. doi: 10.1016/j.ab.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherr G.L., LaMassa N., Li E., Phillips G., Shen C.H. Pah1p negatively regulates the expression of V-ATPase genes as well as vacuolar acidification. Biochem. Biophys. Res. Commun. 2017;491:693–700. doi: 10.1016/j.bbrc.2017.07.127. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z., He G., Han G.-S., Zhang J., Catanzaro N., Diaz A., et al. Host Pah1p phosphatidate phosphatase limits viral replication by regulating phospholipid synthesis. PLoS. Pathog. 2018;14 doi: 10.1371/journal.ppat.1006988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman M.A., Mostofa M.G., Ushimaru T. The Nem1/Spo7-Pah1/lipin axis is required for autophagy induction after TORC1 inactivation. FEBS J. 2018;285:1840–1860. doi: 10.1111/febs.14448. [DOI] [PubMed] [Google Scholar]
- 41.Desfougeres Y., Vavassori S., Rompf M., Gerasimaite R., Mayer A. Organelle acidification negatively regulates vacuole membrane fusion in vivo. Sci. Rep. 2016;6 doi: 10.1038/srep29045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Péterfy M., Phan J., Xu P., Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein. Lipin. Nat. Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 43.Donkor J., Sariahmetoglu M., Dewald J., Brindley D.N., Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 2007;282:3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 44.Csaki L.S., Dwyer J.R., Fong L.G., Tontonoz P., Young S.G., Reue K. Lipins, lipinopathies, and the modulation of cellular lipid storage and signaling. Prog. Lipid Res. 2013;52:305–316. doi: 10.1016/j.plipres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reue K., Donkor J. Genetic factors in type 2 diabetes: all in the (lipin) family. Diabetes. 2007;56:2842–2843. doi: 10.2337/db07-1288. [DOI] [PubMed] [Google Scholar]
- 46.Reue K., Brindley D.N. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 2008;49:2493–2503. doi: 10.1194/jlr.R800019-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reue K. The lipin family: mutations and metabolism. Curr. Opin. Lipidol. 2009;20:165–170. doi: 10.1097/MOL.0b013e32832adee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reue K., Dwyer J.R. Lipin proteins and metabolic homeostasis. J. Lipid Res. 2009;50:S109–S114. doi: 10.1194/jlr.R800052-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donkor J., Zhang P., Wong S., O'Loughlin L., Dewald J., Kok B.P., et al. A conserved serine residue is required for the phosphatidate phosphatase activity but not transcriptional coactivator functions of lipin-1 and lipin-2. J. Biol. Chem. 2009;284:29968–29978. doi: 10.1074/jbc.M109.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J., Lee W.N., Phan J., Saad M.F., Reue K., Kurland I.J. Lipin deficiency impairs diurnal metabolic fuel switching. Diabetes. 2006;55:3429–3438. doi: 10.2337/db06-0260. [DOI] [PubMed] [Google Scholar]
- 51.Zhang P., Verity M.A., Reue K. Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab. 2014;20:267–279. doi: 10.1016/j.cmet.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Mosawi Z.S., Al-Saad K.K., Ijadi-Maghsoodi R., El-Shanti H.I., Ferguson P.J. A splice site mutation confirms the role of LPIN2 in Majeed syndrome. Arthritis Rheum. 2007;56:960–964. doi: 10.1002/art.22431. [DOI] [PubMed] [Google Scholar]
- 53.Ferguson P.J., Chen S., Tayeh M.K., Ochoa L., Leal S.M., Pelet A., et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J. Med. Genet. 2005;42:551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang P., Reue K. Lipin proteins and glycerolipid metabolism: roles at the ER membrane and beyond. Biochim. Biophys. Acta. 2017;1859:1583–1595. doi: 10.1016/j.bbamem.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karanasios E., Han G.-S., Xu Z., Carman G.M., Siniossoglou S. A phosphorylation-regulated amphipathic helix controls the membrane translocation and function of the yeast phosphatidate phosphatase. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17539–17544. doi: 10.1073/pnas.1007974107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khondker S., Han G.-S., Carman G.M. Phosphorylation-mediated regulation of the Nem1-Spo7/Pah1 phosphatase cascade in yeast lipid synthesis. Adv. Biol. Regul. 2022;84 doi: 10.1016/j.jbior.2022.100889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi H.-S., Su W.-M., Han G.-S., Plote D., Xu Z., Carman G.M. Pho85p-Pho80p phosphorylation of yeast Pah1p phosphatidate phosphatase regulates its activity, location, abundance, and function in lipid metabolism. J. Biol. Chem. 2012;287:11290–11301. doi: 10.1074/jbc.M112.346023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khondker S., Kwiatek J.M., Han G.S., Carman G.M. Glycogen synthase kinase homolog Rim11 regulates lipid synthesis through the phosphorylation of Pah1 phosphatidate phosphatase in yeast. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi H.-S., Su W.-M., Morgan J.M., Han G.-S., Xu Z., Karanasios E., et al. Phosphorylation of phosphatidate phosphatase regulates its membrane association and physiological functions in Saccharomyces cerevisiae: identification of Ser602, Thr723, and Ser744 as the sites phosphorylated by CDC28 (CDK1)-encoded cyclin-dependent kinase. J. Biol. Chem. 2011;286:1486–1498. doi: 10.1074/jbc.M110.155598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su W.-M., Han G.-S., Casciano J., Carman G.M. Protein kinase A-mediated phosphorylation of Pah1p phosphatidate phosphatase functions in conjunction with the Pho85p-Pho80p and Cdc28p-cyclin B kinases to regulate lipid synthesis in yeast. J. Biol. Chem. 2012;287:33364–33376. doi: 10.1074/jbc.M112.402339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su W.-M., Han G.-S., Carman G.M. Cross-talk phosphorylations by protein kinase C and Pho85p-Pho80p protein kinase regulate Pah1p phosphatidate phosphatase abundance in Saccharomyces cerevisiae. J. Biol. Chem. 2014;289:18818–18830. doi: 10.1074/jbc.M114.581462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsieh L.-S., Su W.-M., Han G.-S., Carman G.M. Phosphorylation of yeast Pah1 phosphatidate phosphatase by casein kinase II regulates its function in lipid metabolism. J. Biol. Chem. 2016;291:9974–9990. doi: 10.1074/jbc.M116.726588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassaninasab A., Hsieh L.S., Su W.M., Han G.-S., Carman G.M. Yck1 casein kinase I regulates the activity and phosphorylation of Pah1 phosphatidate phosphatase from Saccharomyces cerevisiae. J. Biol. Chem. 2019;294:18256–18268. doi: 10.1074/jbc.RA119.011314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khondker S., Han G.-S., Carman G.M. Protein kinase Hsl1 phosphorylates Pah1 to inhibit phosphatidate phosphatase activity and regulate lipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2024;300 doi: 10.1016/j.jbc.2024.107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karanasios E., Barbosa A.D., Sembongi H., Mari M., Han G.-S., Reggiori F., et al. Regulation of lipid droplet and membrane biogenesis by the acidic tail of the phosphatidate phosphatase Pah1p. Mol. Biol. Cell. 2013;24:2124–2133. doi: 10.1091/mbc.E13-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su W.-M., Han G.-S., Carman G.M. Yeast Nem1-Spo7 protein phosphatase activity on Pah1 phosphatidate phosphatase is specific for the Pho85-Pho80 protein kinase phosphorylation sites. J. Biol. Chem. 2014;289:34699–34708. doi: 10.1074/jbc.M114.614883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwiatek J.M., Carman G.M. Yeast phosphatidic acid phosphatase Pah1 hops and scoots along the membrane phospholipid bilayer. J. Lipid Res. 2020;61:1232–1243. doi: 10.1194/jlr.RA120000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsieh L.-S., Su W.-M., Han G.-S., Carman G.M. Phosphorylation regulates the ubiquitin-independent degradation of yeast Pah1 phosphatidate phosphatase by the 20S proteasome. J. Biol. Chem. 2015;290:11467–11478. doi: 10.1074/jbc.M115.648659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stukey G.J., Han G.S., Carman G.M. Architecture and function of yeast phosphatidate phosphatase Pah1 domains/regions. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2024;1869 doi: 10.1016/j.bbalip.2024.159547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han G.-S., Siniossoglou S., Carman G.M. The cellular functions of the yeast lipin homolog Pah1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 2007;282:37026–37035. doi: 10.1074/jbc.M705777200. [DOI] [PubMed] [Google Scholar]
- 71.Park Y., Han G.-S., Carman G.M. A conserved tryptophan within the WRDPLVDID domain of yeast Pah1 phosphatidate phosphatase is required for its in vivo function in lipid metabolism. J. Biol. Chem. 2017;292:19580–19589. doi: 10.1074/jbc.M117.819375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park Y., Stukey G.J., Jog R., Kwiatek J.M., Han G.-S., Carman G.M. Mutant phosphatidate phosphatase Pah1-W637A exhibits altered phosphorylation, membrane association, and enzyme function in yeast. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stukey G.J., Han G.-S., Carman G.M. Phosphatidate phosphatase Pah1 contains a novel RP domain that regulates its phosphorylation and function in yeast lipid synthesis. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.105025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuznetsova E., Nocek B., Brown G., Makarova K.S., Flick R., Wolf Y.I., et al. Functional diversity of haloacid dehalogenase superfamily phosphatases from Saccharomyces cerevisiae: biochemical, structural, and evolutionary insights. J. Biol. Chem. 2015;290:18678–18698. doi: 10.1074/jbc.M115.657916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burroughs A.M., Allen K.N., Dunaway-Mariano D., Aravind L. Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J. Mol. Biol. 2006;361:1003–1034. doi: 10.1016/j.jmb.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 76.Allen K.N., Dunaway-Mariano D. Phosphoryl group transfer: evolution of a catalytic scaffold. Trends Biochem. Sci. 2004;29:495–503. doi: 10.1016/j.tibs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 77.Lu Z., Dunaway-Mariano D., Allen K.N. HAD superfamily phosphotransferase substrate diversification: structure and function analysis of HAD subclass IIB sugar phosphatase BT4131. Biochemistry. 2005;44:8684–8696. doi: 10.1021/bi050009j. [DOI] [PubMed] [Google Scholar]
- 78.Huang H., Pandya C., Liu C., Al-Obaidi N.F., Wang M., Zheng L., et al. Panoramic view of a superfamily of phosphatases through substrate profiling. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E1974–E1983. doi: 10.1073/pnas.1423570112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medvedev K.E., Kinch L.N., Dustin S.R., Pei J., Grishin N.V. A fifth of the protein world: rossmann-like proteins as an evolutionarily successful structural unit. J. Mol. Biol. 2021;433 doi: 10.1016/j.jmb.2020.166788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rao S.T., Rossmann M.G. Comparison of super-secondary structures in proteins. J. Mol. Biol. 1973;76:241–256. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- 81.Collet J.F., Stroobant V., Pirard M., Delpierre G., Van Schaftingen E. A new class of phosphotransferases phosphorylated on an aspartate residue in an amino-terminal DXDX(T/V) motif. J. Biol. Chem. 1998;273:14107–14112. doi: 10.1074/jbc.273.23.14107. [DOI] [PubMed] [Google Scholar]
- 82.Collet J.F., Stroobant V., Van Schaftingen E. Mechanistic studies of phosphoserine phosphatase, an enzyme related to P-type ATPases. J. Biol. Chem. 1999;274:33985–33990. doi: 10.1074/jbc.274.48.33985. [DOI] [PubMed] [Google Scholar]
- 83.Li Y.F., Hata Y., Fujii T., Hisano T., Nishihara M., Kurihara T., et al. Crystal structures of reaction intermediates of L-2-haloacid dehalogenase and implications for the reaction mechanism. J. Biol. Chem. 1998;273:15035–15044. doi: 10.1074/jbc.273.24.15035. [DOI] [PubMed] [Google Scholar]
- 84.Morais M.C., Zhang W., Baker A.S., Zhang G., Dunaway-Mariano D., Allen K.N. The crystal structure of bacillus cereus phosphonoacetaldehyde hydrolase: insight into catalysis of phosphorus bond cleavage and catalytic diversification within the HAD enzyme superfamily. Biochemistry. 2000;39:10385–10396. doi: 10.1021/bi001171j. [DOI] [PubMed] [Google Scholar]
- 85.Lahiri S.D., Zhang G., Dunaway-Mariano D., Allen K.N. Caught in the act: the structure of phosphorylated beta-phosphoglucomutase from Lactococcus lactis. Biochemistry. 2002;41:8351–8359. doi: 10.1021/bi0202373. [DOI] [PubMed] [Google Scholar]
- 86.Wang W., Kim R., Jancarik J., Yokota H., Kim S.H. Crystal structure of phosphoserine phosphatase from Methanococcus jannaschii, a hyperthermophile, at 1.8 A resolution. Structure. 2001;9:65–71. doi: 10.1016/s0969-2126(00)00558-x. [DOI] [PubMed] [Google Scholar]
- 87.Calderone V., Forleo C., Benvenuti M., Cristina T.M., Rossolini G.M., Mangani S. The first structure of a bacterial class B Acid phosphatase reveals further structural heterogeneity among phosphatases of the haloacid dehalogenase fold. J. Mol. Biol. 2004;335:761–773. doi: 10.1016/j.jmb.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 88.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abramson J., Adler J., Dunger J., Evans R., Green T., Pritzel A., et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500. doi: 10.1038/s41586-024-07487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khayyo V.I., Hoffmann R.M., Wang H., Bell J.A., Burke J.E., Reue K., et al. Crystal structure of a lipin/Pah phosphatidic acid phosphatase. Nat. Commun. 2020;11:1309. doi: 10.1038/s41467-020-15124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toke D.A., Bennett W.L., Dillon D.A., Wu W.-I., Chen X., Ostrander D.B., et al. Isolation and characterization of the Saccharomyces cerevisiae DPP1 gene encoding for diacylglycerol pyrophosphate phosphatase. J. Biol. Chem. 1998;273:3278–3284. doi: 10.1074/jbc.273.6.3278. [DOI] [PubMed] [Google Scholar]
- 93.Toke D.A., Bennett W.L., Oshiro J., Wu W.-I., Voelker D.R., Carman G.M. Isolation and characterization of the Saccharomyces cerevisiae LPP1 gene encoding a Mg2+-independent phosphatidate phosphatase. J. Biol. Chem. 1999;273:14331–14338. doi: 10.1074/jbc.273.23.14331. [DOI] [PubMed] [Google Scholar]
- 94.Carman G.M., Lin Y.-P. Phosphatidate phosphatase from yeast. Methods Enzymol. 1991;197:548–553. doi: 10.1016/0076-6879(91)97182-x. [DOI] [PubMed] [Google Scholar]
- 95.Carman G.M., Deems R.A., Dennis E.A. Lipid signaling enzymes and surface dilution kinetics. J. Biol. Chem. 1995;270:18711–18714. doi: 10.1074/jbc.270.32.18711. [DOI] [PubMed] [Google Scholar]
- 96.Pascual F., Hsieh L.-S., Soto-Cardalda A., Carman G.M. Yeast Pah1p phosphatidate phosphatase is regulated by proteasome-mediated degradation. J. Biol. Chem. 2014;289:9811–9822. doi: 10.1074/jbc.M114.550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adeyo O., Horn P.J., Lee S., Binns D.D., Chandrahas A., Chapman K.D., et al. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 2011;192:1043–1055. doi: 10.1083/jcb.201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fakas S., Qiu Y., Dixon J.L., Han G.-S., Ruggles K.V., Garbarino J., et al. Phosphatidate phosphatase activity plays a key role in protection against fatty acid-induced toxicity in yeast. J. Biol. Chem. 2011;286:29074–29085. doi: 10.1074/jbc.M111.258798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glotzer M., Murray A.W., Kirschner M.W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 100.Carman G.M., Henry S.A. Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog. Lipid Res. 1999;38:361–399. doi: 10.1016/s0163-7827(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 101.Loewen C.J.R., Gaspar M.L., Jesch S.A., Delon C., Ktistakis N.T., Henry S.A., et al. Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science. 2004;304:1644–1647. doi: 10.1126/science.1096083. [DOI] [PubMed] [Google Scholar]
- 102.Mirheydari M., Dey P., Stukey G.J., Park Y., Han G.-S., Carman G.M. The Spo7 sequence LLI is required for Nem1-Spo7/Pah1 phosphatase cascade function in yeast lipid metabolism. J. Biol. Chem. 2020;295:11473–11485. doi: 10.1074/jbc.RA120.014129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han G.-S., Kwiatek J.M., Hu K.S., Carman G.M. Catalytic core function of yeast Pah1 phosphatidate phosphatase reveals structural insight into its membrane localization and activity control. J. Biol. Chem. 2024;300 doi: 10.1016/j.jbc.2023.105560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carman G.M., Han G.-S. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 2009;284:2593–2597. doi: 10.1074/jbc.R800059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin Y.-P., Carman G.M. Purification and characterization of phosphatidate phosphatase from Saccharomyces cerevisiae. J. Biol. Chem. 1989;264:8641–8645. [PubMed] [Google Scholar]
- 106.Lin Y.-P., Carman G.M. Kinetic analysis of yeast phosphatidate phosphatase toward Triton X-100/phosphatidate mixed micelles. J. Biol. Chem. 1990;265:166–170. [PubMed] [Google Scholar]
- 107.Carey J. A systematic and general proteolytic method for defining structural and functional domains of proteins. Methods Enzymol. 2000;328:499–514. doi: 10.1016/s0076-6879(00)28415-2. [DOI] [PubMed] [Google Scholar]
- 108.Fontana A., de Laureto P.P., Spolaore B., Frare E., Picotti P., Zambonin M. Probing protein structure by limited proteolysis. Acta Biochim. Pol. 2004;51:299–321. [PubMed] [Google Scholar]
- 109.Reinhard J., Starke L., Klose C., Haberkant P., Hammaren H., Stein F., et al. MemPrep, a new technology for isolating organellar membranes provides fingerprints of lipid bilayer stress. EMBO J. 2024;43:1653–1685. doi: 10.1038/s44318-024-00063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siniossoglou S., Santos-Rosa H., Rappsilber J., Mann M., Hurt E. A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J. 1998;17:6449–6464. doi: 10.1093/emboj/17.22.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jog R., Han G.-S., Carman G.M. Conserved regions of the regulatory subunit Spo7 are required for Nem1-Spo7/Pah1 phosphatase cascade function in yeast lipid synthesis. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.104683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allen K.N., Dunaway-Mariano D. Markers of fitness in a successful enzyme superfamily. Curr. Opin. Struct. Biol. 2009;19:658–665. doi: 10.1016/j.sbi.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ye B., Tian W., Wang B., Liang J. CASTpFold: Computed atlas of surface topography of the universe of protein folds. Nucleic Acids Res. 2024;52:W194–W199. doi: 10.1093/nar/gkae415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rao K.N., Kumaran D., Seetharaman J., Bonanno J.B., Burley S.K., Swaminathan S. Crystal structure of trehalose-6-phosphate phosphatase-related protein: biochemical and biological implications. Protein Sci. 2006;15:1735–1744. doi: 10.1110/ps.062096606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han G.-S., Carman G.M. Characterization of the human LPIN1-encoded phosphatidate phosphatase isoforms. J. Biol. Chem. 2010;285:14628–14638. doi: 10.1074/jbc.M110.117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li T.Y., Song L., Sun Y., Li J., Yi C., Lam S.M., et al. Tip60-mediated lipin 1 acetylation and ER translocation determine triacylglycerol synthesis rate. Nat. Commun. 2018;9:1916. doi: 10.1038/s41467-018-04363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laframboise S.J., Deneault L.F., Denoncourt A., Downey M., Baetz K. Uncovering the role of the yeast lysine acetyltransferase NuA4 in the regulation of nuclear shape and lipid metabolism. Mol. Cell Biol. 2024;44:237–288. doi: 10.1080/10985549.2024.2366206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaluarachchi D.S., Friesen H., Baryshnikova A., Lambert J.P., Chong Y.T., Figeys D., et al. Exploring the yeast acetylome using functional genomics. Cell. 2012;149:936–948. doi: 10.1016/j.cell.2012.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferguson P.J., El-Shanti H.I. Autoinflammatory bone disorders. Curr. Opin. Rheumatol. 2007;19:492–498. doi: 10.1097/BOR.0b013e32825f5492. [DOI] [PubMed] [Google Scholar]
- 120.Jaradat S.A., Amayreh W., Al-Qa'qa' K., Krayyem J. Molecular analysis of LPIN1 in Jordanian patients with rhabdomyolysis. Meta Gene. 2016;7:90–94. doi: 10.1016/j.mgene.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.David W.S. Myoglobinuria. Neurol. Clin. 2000;18:215–243. doi: 10.1016/s0733-8619(05)70187-0. [DOI] [PubMed] [Google Scholar]
- 122.Choi H.-S., Han G.-S., Carman G.M. Phosphorylation of yeast phosphatidylserine synthase by protein kinase A: identification of Ser46 and Ser47 as major sites of phosphorylation. J. Biol. Chem. 2010;285:11526–11536. doi: 10.1074/jbc.M110.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harlow E., Lane D. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. Using Antibodies: A Laboratory Manual. [Google Scholar]
- 124.Sambrook J., Fritsch E.F., Maniatis T. 2nd Ed. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1989. Molecular Cloning, A Laboratory Manual. [Google Scholar]
- 125.Rose M.D., Winston F., Heiter P. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 1990. Methods in Yeast Genetics: A Laboratory Course Manual. [Google Scholar]
- 126.Innis M.A., Gelfand D.H. In: PCR Protocols. A Guide to Methods and Applications. Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. Academic Press, Inc.; San Diego: 1990. pp. 3–12. [Google Scholar]
- 127.Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robson R.J., Dennis E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton X-100. J. Phys. Chem. 1977;81:1075–1078. [Google Scholar]
- 129.Lichtenberg D., Robson R.J., Dennis E.A. Solubilization of phospholipids by detergents: structural and kinetic aspects. Biochim. Biophys. Acta. 1983;737:285–304. doi: 10.1016/0304-4157(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 130.MacDonald R.C., MacDonald R.I., Menco B.P., Takeshita K., Subbarao N.K., Hu L.R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- 131.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 132.Guengerich F.P., Wang P., Davidson N.K. Estimation of isozymes of microsomal cytochrome P-450 in rats, rabbits, and humans using immunochemical staining coupled with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1982;21:1698–1706. doi: 10.1021/bi00536a035. [DOI] [PubMed] [Google Scholar]
- 133.Burnette W. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 134.Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- 135.Morlock K.R., Lin Y.-P., Carman G.M. Regulation of phosphatidate phosphatase activity by inositol in Saccharomyces cerevisiae. J. Bacteriol. 1988;170:3561–3566. doi: 10.1128/jb.170.8.3561-3566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 137.Henderson R.J., Tocher D.R. In: Lipid Analysis. Hamilton R.J., Hamilton S., editors. IRL Press; New York: 1992. pp. 65–111. [Google Scholar]
- 138.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 139.Sikorski R.S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gietz R.D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 141.Wimmer C., Doye V., Grandi P., Nehrbass U., Hurt E.C. A new subclass of nucleoporins that functionally interact with nuclear pore protein NSP1. EMBO J. 1992;11:5051–5061. doi: 10.1002/j.1460-2075.1992.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thomas B., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.