Abstract
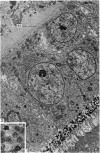
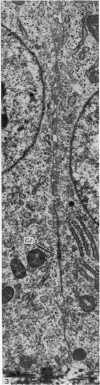
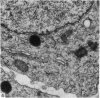
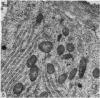
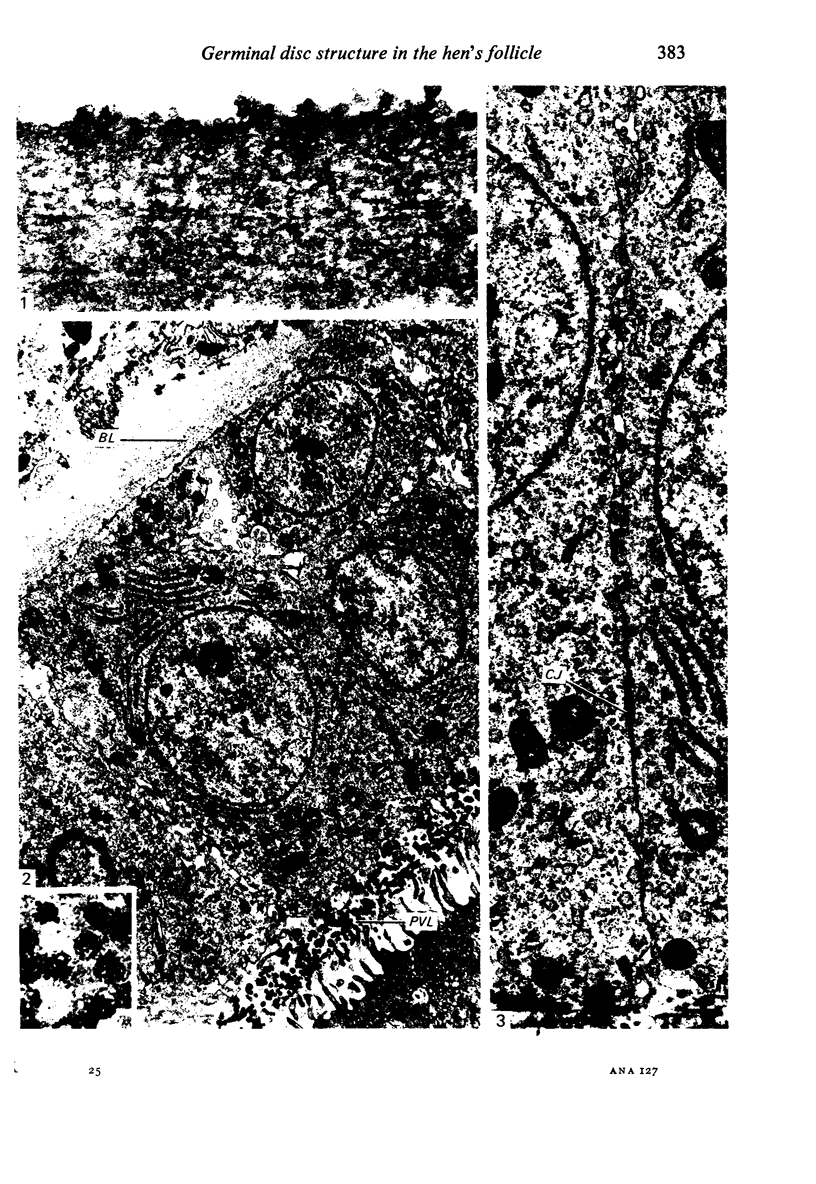
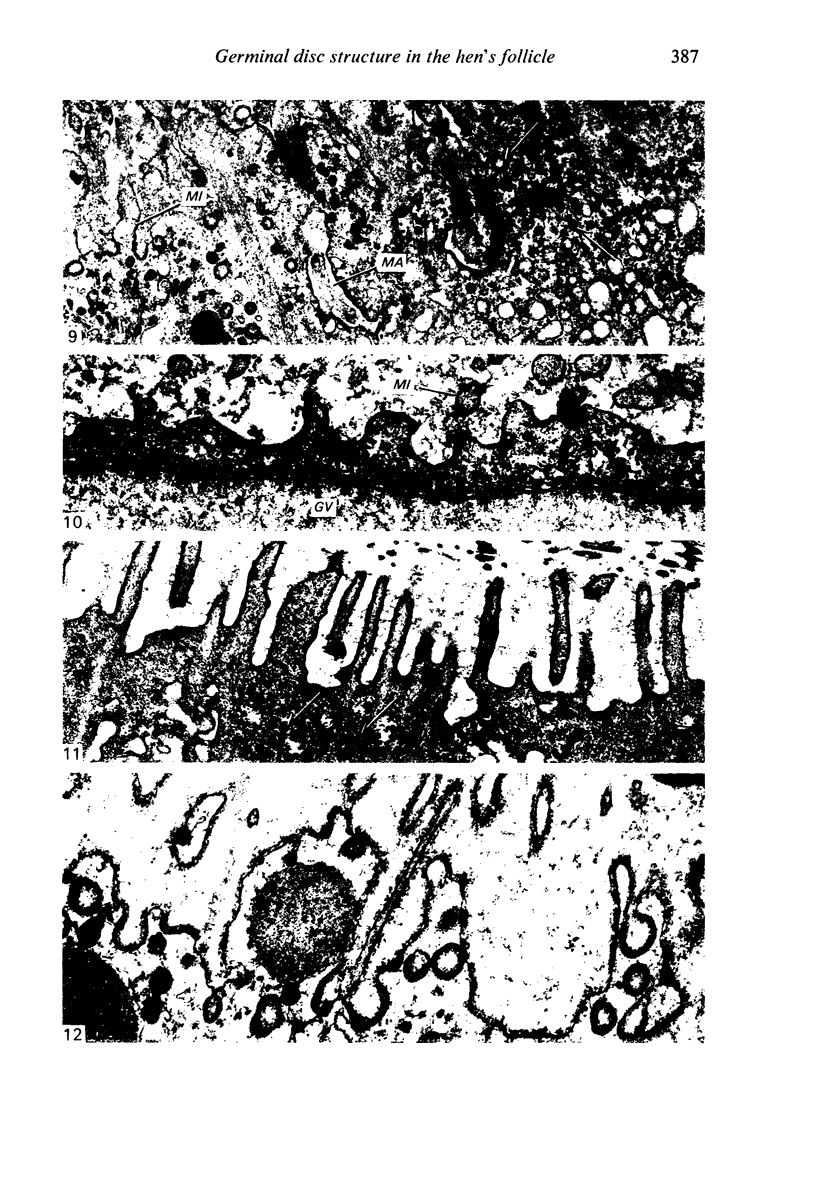
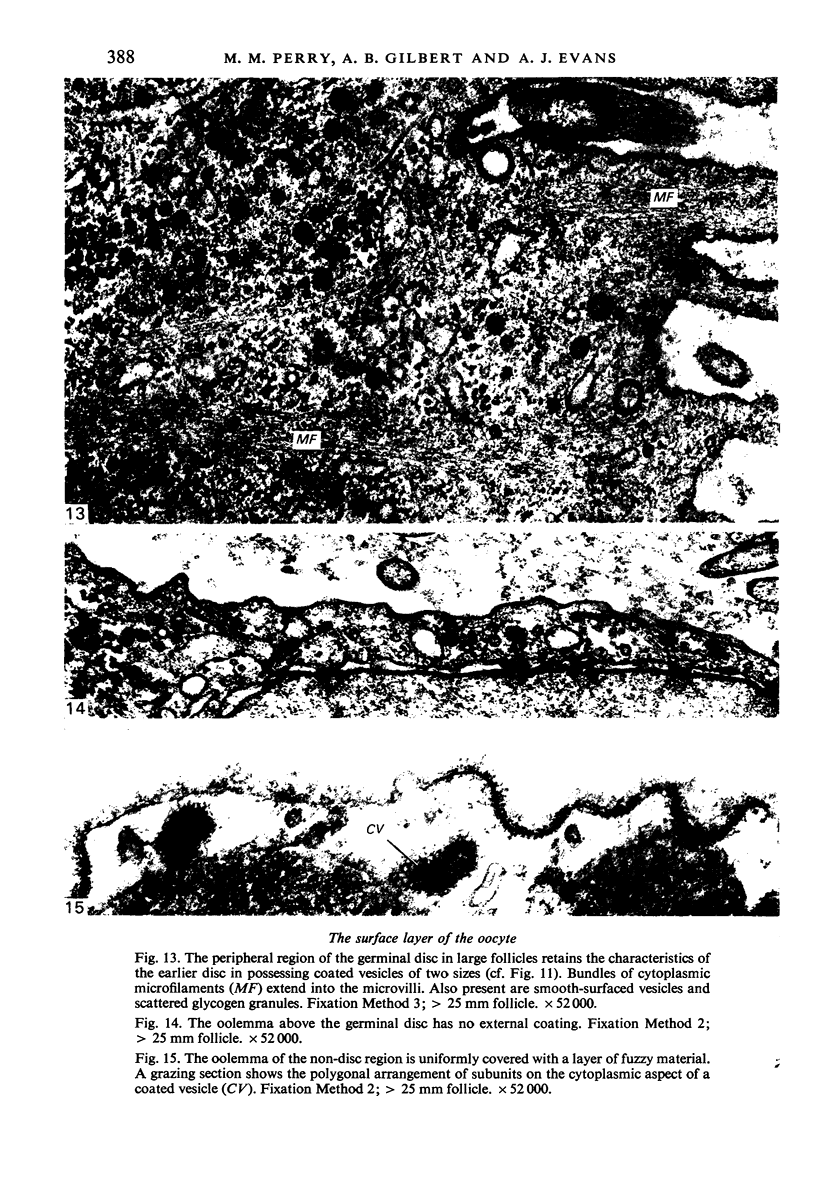
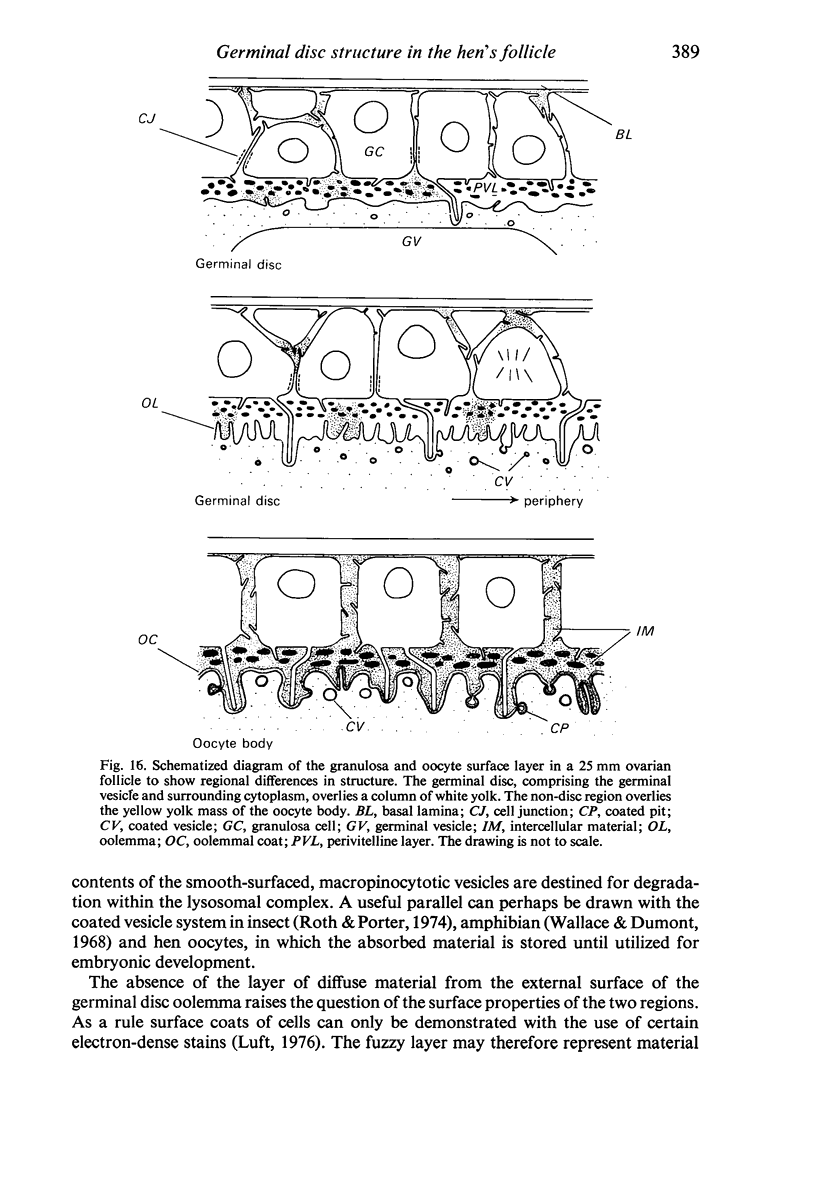
The structure of the ovarian follicle in the region of the germinal disc, which appears as a white plaque at the surface of the oocyte, was examined by electron microscopy and compared with the non-disc region which overlies the yellow yolk mass of the oocyte in the final growth phase. The main differences concerned the granulosa cell layer and the surface layer of the oocyte. In the disc the granulosa cells were less regularly arranged and the spaces between them varied in width. Their mitotic rate was higher than that in the non-disc region, where cell division was seldom observed at maturity. The perivitelline layer was comparatively poorly developed at the periphery of the germinal vesicle in 15 mm follicles, but eventually attained a uniform thickness throughout the follicle. In the intercellular and perivitelline spaces there were smaller amounts of granular material. Marked differences were observed in the ooxyte surface layer. In 15 mm follicles the surface of the germinal disc was thrown into numerous microvilli and some narrow indentations containing macrovilli from the granulosa cells. Coated vesicles, 120 nm diameter, appeared to be invaginating from the oolemma, whereas 70 nm coated vesicles were present in the deeper cytoplasm. In follicles of more than 25 nm diameter these structural conformations were evident only at the periphery of the disc; for the most part the 120 nm coated vesicles were absent, and over the germinal vesicle microvilli were of rare occurrence. On the other hand, the bulk of the oocyte surface was highly convoluted throughout this period of growth, numerous granulosa cell macrovilli extended into deep pouches associated with 300 nm coated vesicles, and the oolemma possessed a coating of fuzzy material. These observations suggest that there is a restricted passage of yolk precursors to the surface of the germinal disc, and that the inability to transport yellow yolk into the disc is related to differences in the oolemmal surface coat and the population of coated vesicles. The surface modifications, as well as the proliferation of the granulosa cells, are likely to be influenced by the presence of the germinal vesicle.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLAIRS R. THE RELATIONSHIP BETWEEN OOCYTE AND FOLLICLE IN THE HEN'S OVARY AS SHOWN BY ELECTRON MICROSCOPY. J Embryol Exp Morphol. 1965 Apr;13:215–233. [PubMed] [Google Scholar]
- Beams H. W., Kessel R. G. Synthesis and deposition of oocyte envelopes (vitelline membrane, chorion) and the uptake of yolk in the dragonfly (Odonata:Aeschnidae). J Cell Sci. 1969 Jan;4(1):241–264. doi: 10.1242/jcs.4.1.241. [DOI] [PubMed] [Google Scholar]
- Bell W. L., Barth R. H., Jr Initiation of yolk deposition by juvenile hormone. Nat New Biol. 1971 Apr 14;230(15):220–222. doi: 10.1038/newbio230220a0. [DOI] [PubMed] [Google Scholar]
- Follett B. K., Nicholls T. J., Redshaw M. R. The vitellogenic response in the South African clawed toad (Xenopus laevis Daudin). J Cell Physiol. 1968 Oct;72(2 Suppl):91+–91+. doi: 10.1002/jcp.1040720408. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Farquhar M. G. Functions of coated vesicles during protein absorption in the rat vas deferens. J Cell Biol. 1967 Nov;35(2):357–376. doi: 10.1083/jcb.35.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A. B., Evans A. J., Perry M. M., Davidson M. H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J Reprod Fertil. 1977 May;50(1):179–181. doi: 10.1530/jrf.0.0500179. [DOI] [PubMed] [Google Scholar]
- Gipson I. Electron microscopy of early cleavage furrows in the chick blastodisc. J Ultrastruct Res. 1974 Dec;49(3):331–347. doi: 10.1016/s0022-5320(74)90049-5. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Birdwell C. R. Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro. I. Characterization of the response of granulosa cells to FGF and EGF. Endocrinology. 1977 Apr;100(4):1108–1120. doi: 10.1210/endo-100-4-1108. [DOI] [PubMed] [Google Scholar]
- Greenfield M. L. The oocyte of the domestic chicken shortly after hatching, studied by electron microscopy. J Embryol Exp Morphol. 1966 Jun;15(3):297–316. [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. The structure and properties of the cell surface coat. Int Rev Cytol. 1976;45:291–382. doi: 10.1016/s0074-7696(08)60081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur S. K., Holtzman E., Schwartz I. L., Walter R. Correlation between pinocytosis and hydroosmosis induced by neurohypophyseal hormones and mediated by adenosine 3',5'-cyclic monophosphate. J Cell Biol. 1971 Jun;49(3):582–594. doi: 10.1083/jcb.49.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon L. A., Wild A. E. Localisation of proteins in coated micropinocytotic vesicles during transport across rabbit yolk sac endoderm. Cell Tissue Res. 1976 Aug 20;171(2):175–193. doi: 10.1007/BF00219405. [DOI] [PubMed] [Google Scholar]
- PRESS N. AN UNUSUAL ORGANELLE IN AVIAN OVARIES. J Ultrastruct Res. 1964 Jun;10:528–546. doi: 10.1016/s0022-5320(64)80027-7. [DOI] [PubMed] [Google Scholar]
- Paulson J., Rosenberg M. D. The function and transposition of lining bodies in developing avian oocytes. J Ultrastruct Res. 1972 Jul;40(1):25–43. doi: 10.1016/s0022-5320(72)80020-0. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. M., Gilbert A. B., Evans A. J. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J Anat. 1978 Mar;125(Pt 3):481–497. [PMC free article] [PubMed] [Google Scholar]
- ROTH T. F., PORTER K. R. YOLK PROTEIN UPTAKE IN THE OOCYTE OF THE MOSQUITO AEDES AEGYPTI. L. J Cell Biol. 1964 Feb;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. Intestinal transport of antibodies in the newborn rat. J Cell Biol. 1973 Jul;58(1):189–211. doi: 10.1083/jcb.58.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjeide O. A., Galey F., Grellert E. A., I-San Lin R., De Vellis J., Mead J. F. Macromolecules in oocyte maturation. Biol Reprod. 1970 Jun;2(Suppl):14–43. doi: 10.1095/biolreprod2.supplement_2.14. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Dumont J. N. The induced synthesis and transport of yolk proteins and their accumulation by the oocyte in Xenopus laevis. J Cell Physiol. 1968 Oct;72(2 Suppl):73–89. doi: 10.1002/jcp.1040720407. [DOI] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. II. Kinetics of protein uptake and digestion by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):123–134. doi: 10.1083/jcb.64.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyburn G. M., Aitken R. N., Johnston H. S. The ultrastructure of the zona radiata of the ovarian follicle of the domestic fowl. J Anat. 1965 Jul;99(Pt 3):469–484. [PMC free article] [PubMed] [Google Scholar]
- Wyburn G. M., Johnston H. S., Aitken R. N. Specialised plasma membranes in the preovulatory follicle of the fowl. Z Zellforsch Mikrosk Anat. 1965 Sep 24;68(1):70–79. doi: 10.1007/BF00332346. [DOI] [PubMed] [Google Scholar]