Abstract
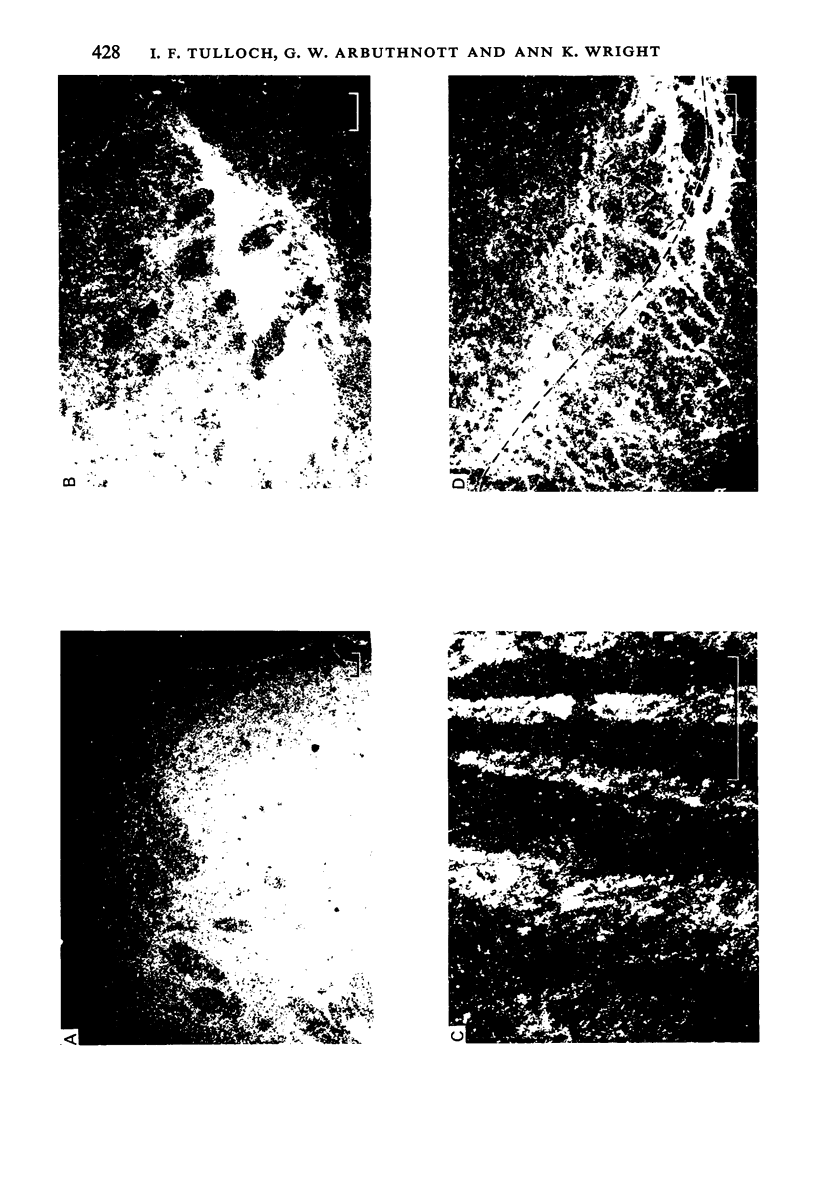
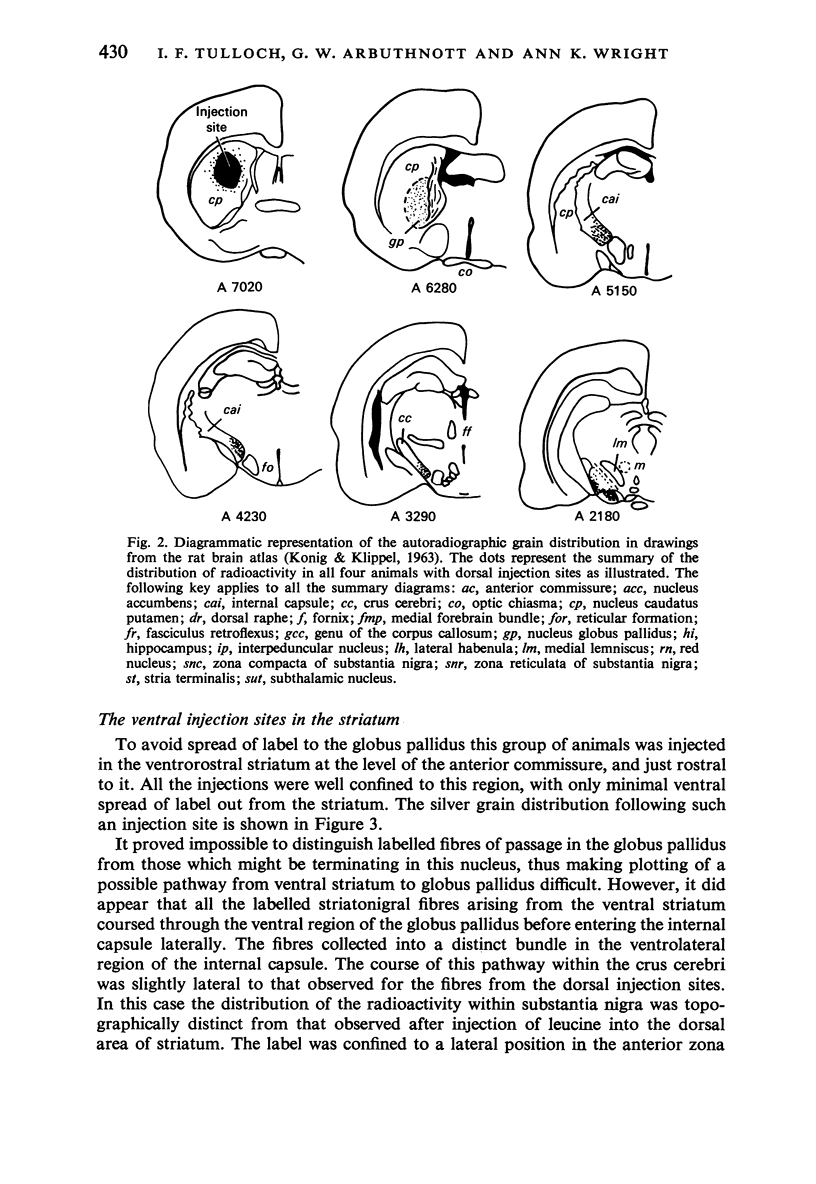
L-[4,5-3H]leucine was injected stereotaxically into various regions of the rat neostriatum. Light microscopic autoradiographic techniques were used to plot the entire efferent pathways of the neostriatum, and in particular, the projections to the substantia nigra (SN). The terminal distribution of the pathways projecting to the ipsilateral SN was predominantly restricted to the zona reticulata region. The dorsal part of the head of the striatum was found to innervate the anterior and medial portions of the zona reticulata, while the ventral area of the head projected to more posterior regions of the SN. A pathway from the tail of the striatum to the lateral and dorsal parts of SN was also demonstrated. Horseradish peroxidase injections, restricted to different areas of the SN, led to retrograde labelling of neurons in the striatum whose distribution confirmed the topographic organization of the striatonigral pathway demonstrated in the autoradiographic studies.
Full text
PDF



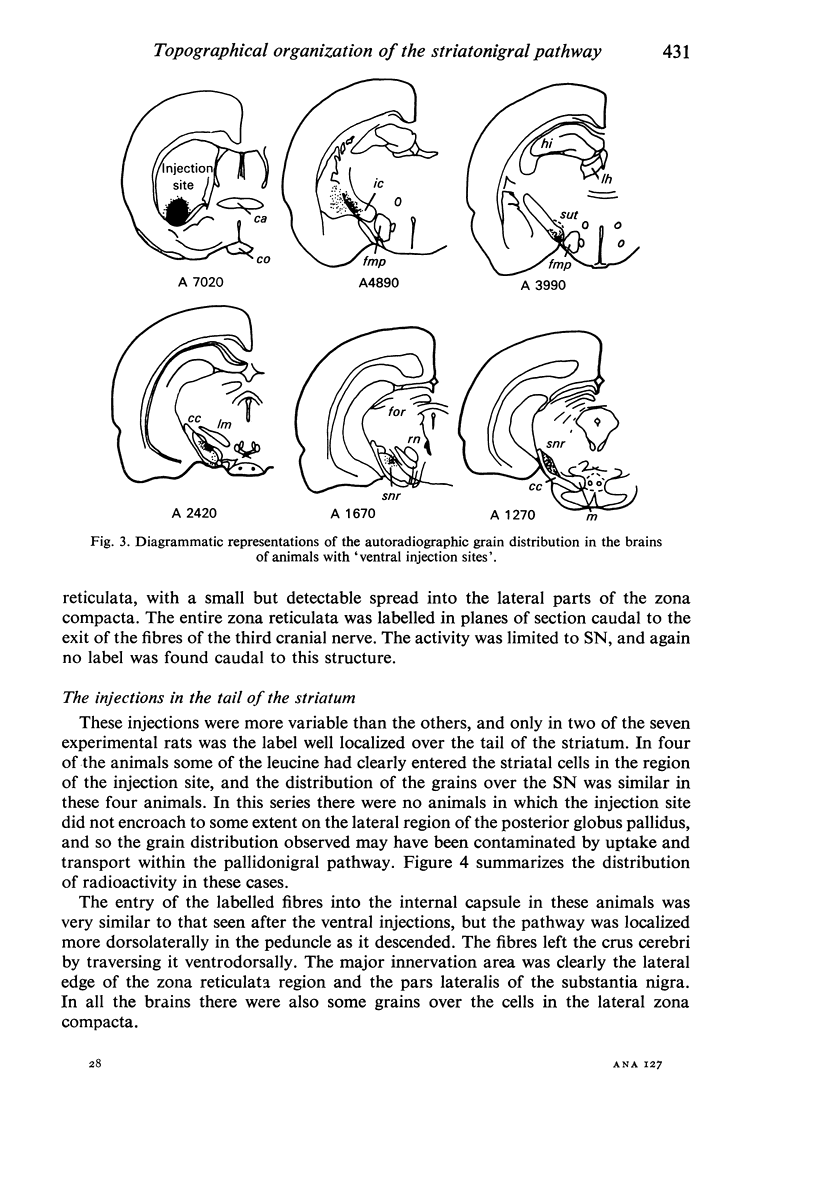
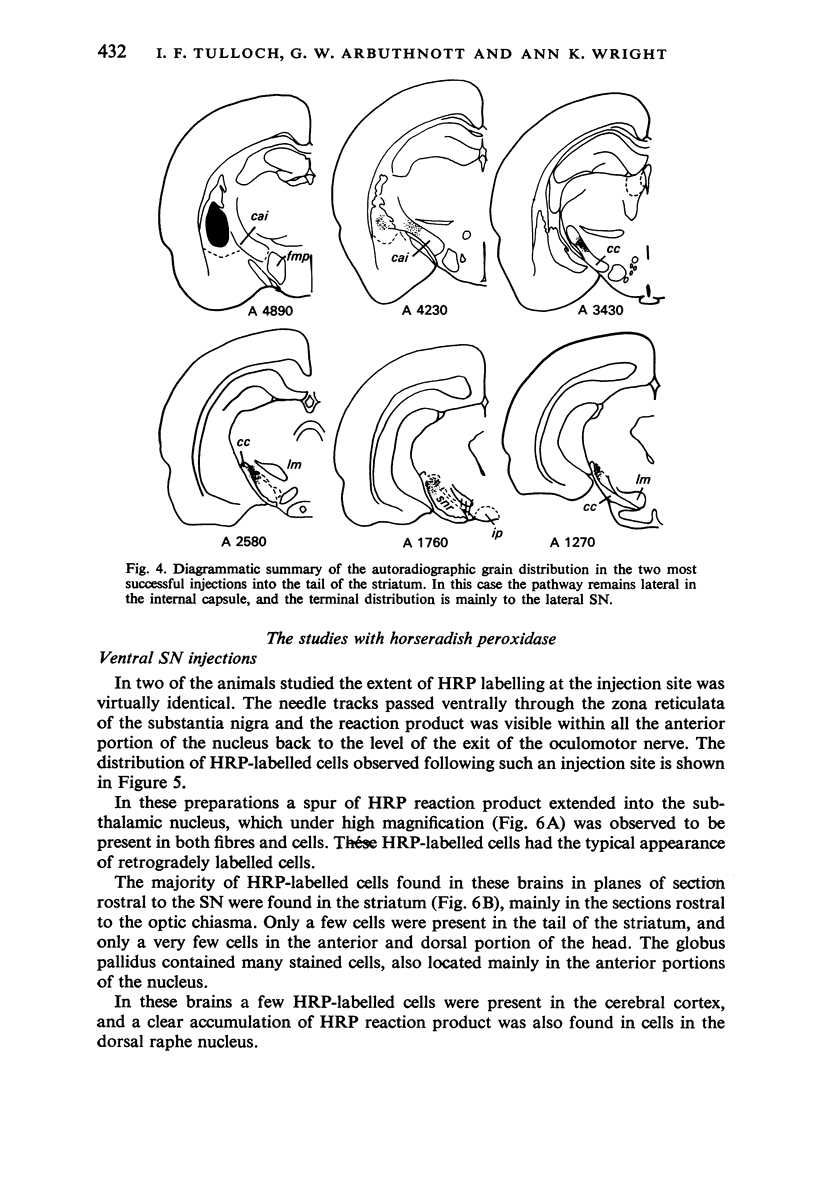

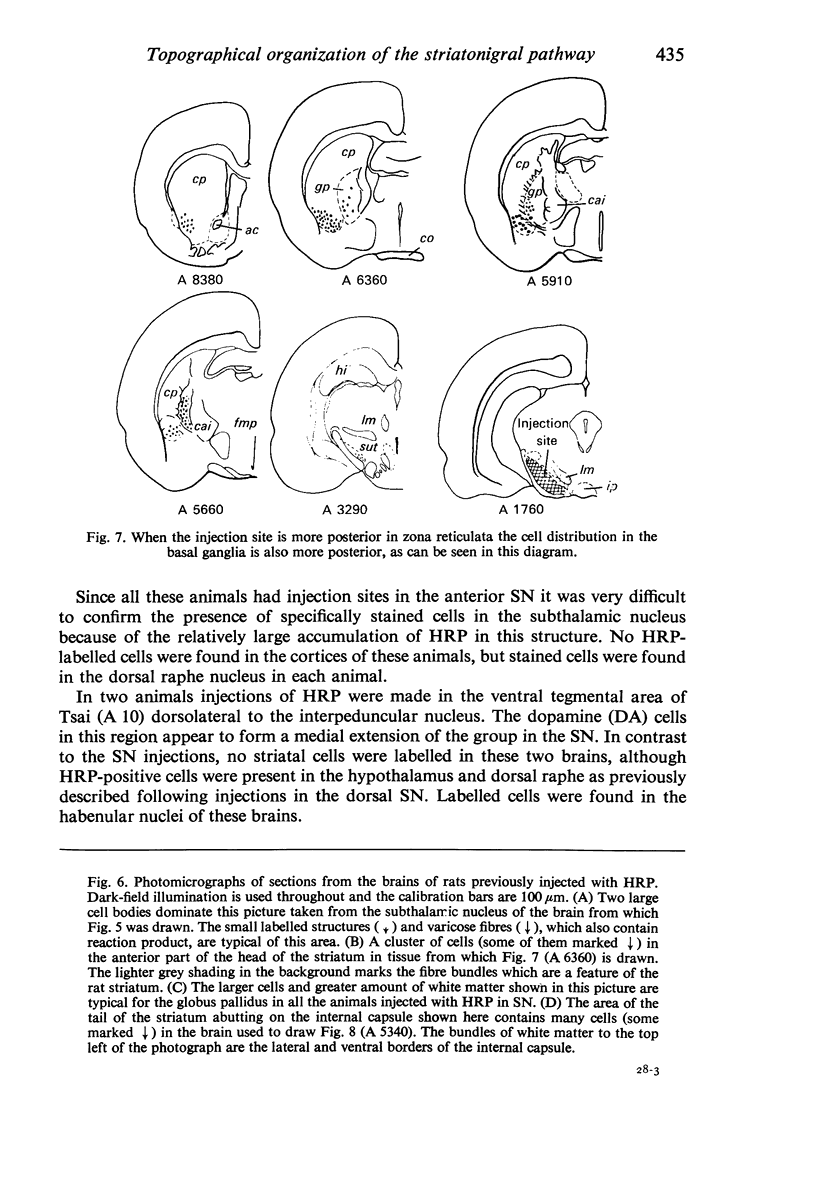






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunney B. S., Achajanian G. K. d-Amphetamine-induced inhibition of central dopaminergic neurons: mediation by a striato-nigral feedback pathway. Science. 1976 Apr 23;192(4237):391–393. doi: 10.1126/science.1257777. [DOI] [PubMed] [Google Scholar]
- Bunney B. S., Aghajanian G. K. The precise localization of nigral afferents in the rat as determined by a retrograde tracing technique. Brain Res. 1976 Dec 3;117(3):423–435. doi: 10.1016/0006-8993(76)90751-4. [DOI] [PubMed] [Google Scholar]
- Bédard P., Larochelle L., Parent A., Poirier L. J. The nigrostriatal pathway: a correlative study based on neuroanatomical and neurochemical criteria in the cat and the monkey. Exp Neurol. 1969 Nov;25(3):365–377. doi: 10.1016/0014-4886(69)90131-9. [DOI] [PubMed] [Google Scholar]
- Carpenter M. B., Peter P. Nigrostriatal and nigrothalamic fibers in the rhesus monkey. J Comp Neurol. 1972 Jan;144(1):93–115. doi: 10.1002/cne.901440105. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Gottlieb D. I., Hendrickson A. E., Price J. L., Woolsey T. A. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res. 1972 Feb 11;37(1):21–51. doi: 10.1016/0006-8993(72)90344-7. [DOI] [PubMed] [Google Scholar]
- Deniau J. M., Feger J., Le Guyader C. Striatal evoked inhibition of identified nigro-thalamic neurons. Brain Res. 1976 Mar 5;104(1):152–156. doi: 10.1016/0006-8993(76)90656-9. [DOI] [PubMed] [Google Scholar]
- DiFiglia M., Pasik P., Pasik T. A Golgi study of neuronal types in the neostriatum of monkeys. Brain Res. 1976 Sep 17;114(2):245–256. doi: 10.1016/0006-8993(76)90669-7. [DOI] [PubMed] [Google Scholar]
- Dray A., Gonye T. J., Oakley N. R. Caudate stimulation and substantia nigra activity in the rat. J Physiol. 1976 Aug;259(3):825–849. doi: 10.1113/jphysiol.1976.sp011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faull R. L., Carman J. B. Ascending projections of the substantia nigra in the rat. J Comp Neurol. 1968 Jan;132(1):73–92. doi: 10.1002/cne.901320104. [DOI] [PubMed] [Google Scholar]
- Fonnum F., Grofová I., Rinvik E., Storm-Mathisen J., Walberg F. Origin and distribution of glutamate decarboxylase in substantia nigra of the cat. Brain Res. 1974 May 10;71(1):77–92. doi: 10.1016/0006-8993(74)90192-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Munoz M., Nicolaou M. N., Tulloch I. F., Wright A. K., Arguthnott G. W. Feedback loop or output pathway in striato-nigral fibres? Nature. 1977 Jan 27;265(5592):363–365. doi: 10.1038/265363a0. [DOI] [PubMed] [Google Scholar]
- Grofová I., Rinvik E. An experimental electron microscopic study on the striatonigral projection in the cat. Exp Brain Res. 1970;11(3):249–262. doi: 10.1007/BF01474385. [DOI] [PubMed] [Google Scholar]
- Grofová I. The identification of striatal and pallidal neurons projecting to substantia nigra. An experimental study by means of retrograde axonal transport of horseradish peroxidase. Brain Res. 1975 Jun 27;91(2):286–291. doi: 10.1016/0006-8993(75)90550-8. [DOI] [PubMed] [Google Scholar]
- Hattori T., Fibiger H. C., McGeer P. L. Demonstration of a pallido-nigral projection innervating dopaminergic neurons. J Comp Neurol. 1975 Aug 15;162(4):487–504. doi: 10.1002/cne.901620406. [DOI] [PubMed] [Google Scholar]
- Hattori T., McGeer P. L., Fibiger H. C., McGeer E. G. On the source of GABA-containing terminals in the substantia nigra. Electron microscopic autoradiographic and biochemical studies. Brain Res. 1973 May 17;54:103–114. doi: 10.1016/0006-8993(73)90037-1. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Yang H. Y., Racagni G., Costa E. Projections of substance P containing neurons from neostriatum to substantia nigra. Brain Res. 1977 Feb 25;122(3):541–544. doi: 10.1016/0006-8993(77)90464-4. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L. Inhibition of substance P release from the isolated rat substantia nigra by GABA [proceedings]. Br J Pharmacol. 1977 Mar;59(3):486P–486P. [PMC free article] [PubMed] [Google Scholar]
- Kanazawa I., Emson P. C., Cuello A. C. Evidence for the existence of substance P-containing fibres in striato-nigral and pallido-nigral pathways in rat brain. Brain Res. 1977 Jan 7;119(2):447–453. doi: 10.1016/0006-8993(77)90323-7. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Marshall G. R., Kelly J. S. Afferents to the rat substantia nigra studied with horseradish peroxidase, with special reference to fibres from the subthalamic nucleus. Brain Res. 1976 Oct 22;115(3):485–491. doi: 10.1016/0006-8993(76)90364-4. [DOI] [PubMed] [Google Scholar]
- Kemp J. M., Powell T. P. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971 Sep 30;262(845):383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Koslow S. H., Racagni G., Costa E. Mass fragmentographic measurement of norepinephrine dopamine, serotonin and acetylcholine in seven discrete nuclei of the rat tel-diencephalon. Neuropharmacology. 1974 Dec;13(12):1123–1130. doi: 10.1016/0028-3908(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- McNair J. L., Sutin J., Tsubokawa T. Suppression of cell firing in the substantia nigra by caudate nucleus stimulation. Exp Neurol. 1972 Nov;37(2):395–411. doi: 10.1016/0014-4886(72)90083-0. [DOI] [PubMed] [Google Scholar]
- Nauta H. J., Pritz M. B., Lasek R. J. Afferents to the rat caudoputamen studied with horseradish peroxidase. An evaluation of a retrograde neuroanatomical research method. Brain Res. 1974 Feb 22;67(2):219–238. doi: 10.1016/0006-8993(74)90274-1. [DOI] [PubMed] [Google Scholar]
- Nauta W. J., Mehler W. R. Projections of the lentiform nucleus in the monkey. Brain Res. 1966 Jan;1(1):3–42. doi: 10.1016/0006-8993(66)90103-x. [DOI] [PubMed] [Google Scholar]
- Niimi K., Ikeda T., Kawamura S., Inoshita H. Efferent projections of the head of the caudate nucleus in the cat. Brain Res. 1970 Jul 29;21(3):327–343. doi: 10.1016/0006-8993(70)90415-4. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Hökfelt T., Pernow B. Distribution of substance P-like immuno-reactivity in the rat central nervous system as revealed by immunohistochemistry. Med Biol. 1974 Dec;52(6):424–427. [PubMed] [Google Scholar]
- Precht W., Yoshida M. Blockage of caudate-evoked inhibition of neurons in the substantia nigra by picrotoxin. Brain Res. 1971 Sep 10;32(1):229–233. doi: 10.1016/0006-8993(71)90171-5. [DOI] [PubMed] [Google Scholar]
- Rinvik E. Demonstration of nigrothalamic connections in the cat by retrograde axonal transport of horseradish peroxidase. Brain Res. 1975 Jun 13;90(2):313–318. doi: 10.1016/0006-8993(75)90312-1. [DOI] [PubMed] [Google Scholar]
- Rinvik E., Grofová I., Ottersen O. P. Demonstration of nigrotectal and nigroreticular projections in the cat by axonal transport of proteins. Brain Res. 1976 Aug 13;112(2):388–394. doi: 10.1016/0006-8993(76)90293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo J. Projections from the body of the caudate nucleus in the rhesus monkey. Exp Neurol. 1970 Apr;27(1):1–15. doi: 10.1016/0014-4886(70)90196-2. [DOI] [PubMed] [Google Scholar]
- Szabo J. The course and distribution of efferents from the tail of the caudate nucleus in the monkey. Exp Neurol. 1972 Dec;37(3):562–572. doi: 10.1016/0014-4886(72)90099-4. [DOI] [PubMed] [Google Scholar]
- Szabo J. The efferent projections of the putamen in the monkey. Exp Neurol. 1967 Dec;19(4):463–476. doi: 10.1016/0014-4886(67)90166-5. [DOI] [PubMed] [Google Scholar]
- Tassin J. P., Cheramy A., Blanc G., Thierry A. M., Glowinski J. Topographical distribution of dopaminergic innervation and of dopaminergic receptors in the rat striatum. I. Mictoestimation of [3H] dopamine uptake and dopamine content in microdiscs. Brain Res. 1976 May 7;107(2):291–301. doi: 10.1016/0006-8993(76)90227-4. [DOI] [PubMed] [Google Scholar]
- Ternaux J. P., Héry F., Bourgoin S., Adrien J., Glowinski J., Hamon M. The topographical distribution of serotoninergic terminals in the neostriatum of the rat and the caudate nucleus of the cat. Brain Res. 1977 Feb;121(2):311–326. doi: 10.1016/0006-8993(77)90154-8. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- VONEIDA T. J. An experimental study of the course and destination of fibers arising in the head of the caudate nucleus in the cat and monkey. J Comp Neurol. 1960 Aug;115:75–87. doi: 10.1002/cne.901150107. [DOI] [PubMed] [Google Scholar]