Abstract
Enterocytozoon bieneusi is clinically the most significant among the microsporidia infecting humans, causing chronic diarrhea, wasting, and cholangitis in individuals with human immunodeficiency virus/AIDS. The lack of immune reagents is largely due to the absence of methods for laboratory propagation of E. bieneusi. We recently described a procedure for the concentration and purification of spores from diarrheic stool of infected humans. Purified spores were used to immunize mice for production and screening of monoclonal antibodies (MAbs) against E. bieneusi. The eight immunoglobulin M MAbs generated and fully characterized did not cross-react with other human microsporidia or with other microorganisms normally present in stool. One of the MAbs, 2G4, reacted with E. bieneusi spores in stools from monkeys and humans, without background fluorescence, which makes it an ideal diagnostic reagent. It also recognizes intracellular stages of the parasite and will be suitable for determining tissue distribution of E. bieneusi in infected hosts. At least two immunodominant antigens of E. bieneusi of 33,000 and 35,000 Da exist, which were recognized by rabbit and mouse antisera. The availability of MAbs against E. bieneusi will simplify considerably the diagnosis of this infection in humans and will provide tools for epidemiologic investigations regarding the true prevalence of the infection in various human and mammalian populations and the environmental sources of infection.
Enterocytozoon bieneusi, an emerging enteric protozoon, is clinically the most significant among the microsporidia infecting humans and is linked to chronic diarrhea, wasting, and cholangitis in individuals with human immunodeficiency virus/AIDS (5, 6, 23) and those receiving immunosuppressive therapy (13, 28). The infection was first identified two decades ago in individuals with AIDS with severe symptoms of chronic diarrhea and wasting (10). E. bieneusi is rarely symptomatic in immunocompetent individuals, but it may contribute to traveler's diarrhea (11). Like all other microsporidia, E. bieneusi is an intracellular microorganism, which infects the epithelium of the upper small intestine and the hepatobiliary tract (31).
Scientific progress on E. bieneusi has been slow, largely because of lack of in vitro and in vivo models for parasite propagation for laboratory investigations. Also, the identification and purification of large quantities of spores, the environmentally resistant infective form, from feces were technically challenging because of the size and shape of the E. bieneusi spores (1.1 to 1.6 by 0.7 to 1.0 μm). A limited degree of infectivity by E. bieneusi of cultured cells was reported, (30). Natural infections of immunologically competent and immunodeficient macaques have been reported, with distribution of the infection and lesions in the gastrointestinal tract similar to those seen in infected humans (8, 9, 19, 20, 24). E. bieneusi has been successfully transmitted from AIDS patient to simian immunodeficiency virus (SIV)-infected macaques (12, 29) and to immunosuppressed gnotobiotic piglets (18). However, in both models the infection was asymptomatic and very mild and the excretion of spores in the feces was sparse and intermittent. These models provided insufficient spores for laboratory investigations. The lack of sources of E. bieneusi spores also limited the ability to generate immune reagents. Consequently, diagnosis until now depended on PCR-based methods, which is time consuming and requires sophisticated skills and equipment. Two monoclonal antibodies (MAbs) specific for E. bieneusi from Europe have been described (1), but they are unavailable to other investigators. We recently described a method for concentration and purification of E. bieneusi spores from human stool and the production of specific polyclonal antibodies against E. bieneusi (27). Here we describe production and characterization of specific MAbs against E. bieneusi spores of human origin.
MATERIALS AND METHODS
E. bieneusi spores.
Stools from individuals with chronic watery diarrhea were collected in disposable plastic containers at Mulago Hospital, Kampala, Uganda. The samples were screened for the presence of E. bieneusi spores, and spores were purified from positive stools as described elsewhere (27).
MAb production and isotyping.
Female BALB/c mice 6 to 8 weeks old were primed by an intraperitoneal (i.p.) injection of 3 × 107 spores (frozen-thawed five times) emulsified in complete Freund's adjuvant (Difco Laboratories). The same dose emulsified in incomplete Freund's adjuvant (Difco Laboratories) was administered i.p. 14 and 28 days later. A fourth i.p. administration with 3 × 107 spores in phosphate-buffered saline (PBS) was performed 14 days after the third immunization. Mouse spleen cells on fourth day after the last immunization were used for fusion with Ag 8.653 myeloma cells. Hybridoma supernatants were screened by immunofluorescence. Positive hybridomas were cloned three times.
Isotyping was determined by indirect enzyme-linked immunosorbent assay. Briefly, a mixture of goat anti-mouse kappa and lambda light chain antibodies (Southern Biotechnology Associates, Birmingham, AL) was used to coat the enzyme-linked immunosorbent assay plates to capture MAbs in culture supernatants. The isotype of each MAb was detected by horseradish peroxidase-conjugated goat anti-mouse immunoglobulin isotype-specific antibodies. The assay plates were developed as described elsewhere (26).
Immunofluorescence (IF) and confocal microscopy.
Smears from feces and from purified E. bieneusi spores were dried and heat fixed over flame. Smears were blocked with 2% bovine serum albumin in PBS for 20 min at room temperature, washed with PBS, and then incubated with primary antibody (hybridoma supernatants or rabbit anti-E. bieneusi serum at various dilutions in PBS) for 30 min at room temperature. Smears were washed and incubated with either goat antimouse immunoglobulin G (IgG) plus goat antimouse IgM (μ chain specific) or goat antirabbit IgG conjugated with Alexa 488 (Molecular Probes, Eugene, OR) at a dilution of 1/500 in PBS for 30 min at room temperature. Slides were washed and dried, and coverslips were mounted with aqueous mounting medium with antifading compound (1,4-diazobicyclo(2,2,2)-octane; Sigma, St. Louis, MO). Slides were examined by using a fluorescence microscope, and images were captured by confocal microscopy.
The impact of methanol, paraformaldehyde, or acetone fixation on spores.
Smears of purified E. bieneusi spores were dried and fixed with either methanol, ice-cold acetone, or 4% paraformaldehyde for 10 min. Immunofluorescence was then performed with the MAbs and polyclonal antibodies as described above.
Deglycosylation of spores.
Spores were treated with sodium periodate to eliminate carbohydrate epitopes, as described elsewhere (32). Briefly, spores were washed with 50 mM sodium acetate buffer (pH 4.5) and then exposed to 20 mM sodium periodate in 50 mM sodium acetate buffer, pH 4.5, for 1 h at room temperature in the dark. Following a wash with 50 mM sodium acetate buffer, the spores were incubated with 50 mM sodium borohydride in PBS for 30 min at room temperature. Spores were washed with PBS and then used in IF for reactivity with the MAbs. IF was also performed with spores that were deglycosylated enzymatically using Glyko enzymatic deglycosylation kits GK80110 and GK80115 (Prozyme, San Leandro, CA). Glyko enzymatic deglycosylation kit GK80110 removes N-linked and simple O-linked carbohydrates. Glyko enzymatic deglycosylation kit GK80115 removes complex O-linked carbohydrates. To remove N- and O-linked carbohydrates, spores were incubated with enzymes in the kit for 4 h, 24 h, and 5 days. Spores after each time point were washed with PBS and then used in immunofluorescence for reactivity with the MAbs.
The impact of storage on MAbs.
MAbs were dispensed, and aliquots were stored at 4°C and −70°C. Reactivity of these MAbs with E. bieneusi spores was tested by immunofluorescence monthly for 3 months.
Identification of E. bieneusi in infected tissue.
Immunohistochemistry for the localization of E. bieneusi in tissue was performed on formalin-fixed, paraffin-embedded liver tissue of SIV-infected rhesus macaques (Macaca mulatta). These animals were known to be infected with E. bieneusi, as established by examination of gall bladder and small intestine at necropsy and excretion of spores in feces. Tissue sections were cut at 5 μm and immunostained by use of an avidin-biotin-horseradish peroxidase complex technique with diaminobenzidine chromogen, as described elsewhere (18). The tissue sections were also subjected to a microwave pretreatment protocol for antigen retrieval (16). The sections were then stained for E. bieneusi with specific MAbs (1D4 and 2G4). An irrelevant control mouse IgM MAb was included in the assay. Rhesus macaques (Macaca mulatta) were housed at the New England Primate Research Center in a centralized animal biolevel 3 containment facility in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and Harvard Medical School's Animal Care and Use Committee. Animals were tested and found free of simian retrovirus type D, SIV, simian T-lymphotropic virus 1, and herpes B virus before assignment to experimental protocols.
Cross-reactivity studies.
MAbs were analyzed for their cross-reactivity with cell culture-grown microsporidia of the genus Encephalitozoon (E. intestinalis, E. cuniculi, and E. hellem), Vittaforma corneae, Candida albicans, Staphylococcus aureus, and fecal Escherichia coli.
Immunoblotting.
The antigenic profile of E. bieneusi spores was studied by immunoblotting utilizing serum from both mice and rabbit. Reactivity of MAbs with spores was also determined by immunoblotting. Spores (7 × 106/lane) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and electrophoretically transferred to a 0.2-μm-pore-size nitrocellulose membrane (Bio-Rad Laboratories, CA). After transfer, the membrane was blocked with 5% nonfat dried milk powder in PBS containing 0.1% Tween 20 at room temperature for 1 h, washed, and incubated with mouse and rabbit anti-E. bieneusi sera at dilutions of 1/200 and 1/100 in PBS containing 0.1% Tween 20, respectively, again at room temperature for 1 h. The membrane was also incubated with the MAbs. Unrelated mouse IgM MAb and preimmunized mouse and rabbit sera were used as controls. Following washing, strips were incubated with horseradish peroxidase-conjugated goat antirabbit IgG (Sigma) or goat antimouse IgG plus goat antimouse IgM (Southern Biotech) at a dilution of 1/1,000 for 1 h at room temperature and then washed and developed with the TMB peroxidase-substrate system (KPL).
Detection of E. bieneusi spores in fecal samples by PCR and MAbs.
DNA was extracted from feces, and PCR and nested PCR were performed as described elsewhere for amplification of rRNA gene sequence of E. bieneusi (7). MAbs were used on human and monkey fecal smears to detect E. bieneusi spores by immunofluorescence.
Immune electron microscopy.
E. bieneusi spores were incubated at room temperature in 1% bovine serum albumin in PBS for 45 min to block unbound sites and then for 120 min with the MAb 2G4 at concentrations of 1 mg and 0.2 mg/ml. An unrelated IgM MAb was used as an isotype control. The spores were washed with PBS three times and incubated for 60 to 120 min with goat antimouse Ig labeled with 10-μm gold particles (Sigma). The spores were again washed with PBS three times and fixed at room temperature in 4% paraformaldehyde-0.4% glutaraldehyde in 0.15 M Na cacodylate buffer (pH 7.2) for 30 min. They were then rinsed three times at 10-min intervals in 0.1 M ammonium chloride in cacodylate buffer and one time for 10 min in cacodylate buffer alone. After ethanolic dehydration, the material was embedded in LR WHITE resin. The sections were mounted on gold grids, washed, and examined after the electron microscopy.
RESULTS
MAbs and their reactivity with the spore wall.
After six fusions and three cloning procedures, eight hybridoma clones were generated that were reactive against E. bieneusi (Table 1). All MAbs (1D4, 3E9, 2B2, 2G4, 2E9, 4B2, 4C9, and 4C3) were IgM. They were produced in bulk as concentrated cell culture supernatant in Integra Celline 1000 flasks (IBS Integra Biosciences, Baar, Switzerland).
TABLE 1.
Reactivities of monoclonal antibodies with E. bieneusi spores
| MAb | Result with:b
|
|||||
|---|---|---|---|---|---|---|
| Frozen MAba | Spore fixation method
|
Deglycosylation (periodate) | Deglycosylation (enzymatic)c | |||
| Methanol | Paraformaldehyde | Acetone | ||||
| 1D4 | ++ | + | ++++ | ++++ | ++++ | ++++ |
| 3E9 | ++++ | ++ | ++ | ++++ | ++++ | ++++ |
| 2B2 | ++++ | + | ++ | ++++ | ++++ | ++++ |
| 2G4 | ++++ | + | − | ++++ | ++++ | ++++ |
| 2E9 | ++++ | + | ++ | ++++ | ++++ | ++++ |
| 4B2 | ++++ | + | − | ++++ | ++++ | ++++ |
| 4C9 | ++++ | ++ | ++ | ++++ | ++++ | ++++ |
| 4C3 | ++++ | + | ++ | ++++ | ++++ | ++++ |
Reactivities of the MAbs frozen at −70°C were tested after every month for 3 months. Reactivity intensities remained the same during that period.
+, faint fluorescence intensity; ++, weak fluorescence intensity; +++, strong fluorescence intensity; ++++, very strong fluorescent intensity.
Enzymatic deglycosylation removed N-linked, simple O-linked, and complex O-linked carbohydrates but did not alter reactivity of any MAb even after 5 days of deglycosylation.
Reactivity of MAbs.
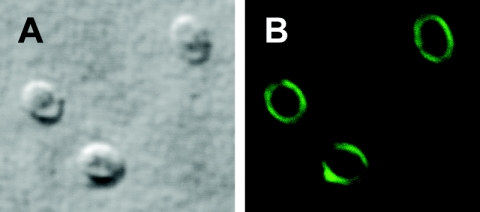
All MAbs reacted strongly with the E. bieneusi spores. Reactivity of a representative MAb, 2G4, with an exospore is shown in Fig. 1 and 2. Storage of the MAbs at −70°C did not affect reactivities of any of the MAbs except the MAb 1D4 (Table 1). The reactivity of 1D4 was reduced from a strong to a weak reaction after the first testing following 1 month of freezing, without further deterioration. Fixation of spores with acetone had no effect on reactivity with the MAbs, but it was severely affected by fixation with methanol and paraformaldehyde (Table 1). Whereas methanol fixation did not entirely abolish reactivity of any of the MAbs, paraformaldehyde fixation eliminated reactivities of two MAbs (2G4 and 4B2). Periodate and enzymatic deglycosylation did not impact the extent of reactivity of any of the eight MAbs (Table 1).
FIG. 1.
Confocal image of immunofluorescence staining of E. bieneusi spore wall with the MAb 2G4 (B). Image A is a differential interference contrast image of image B.
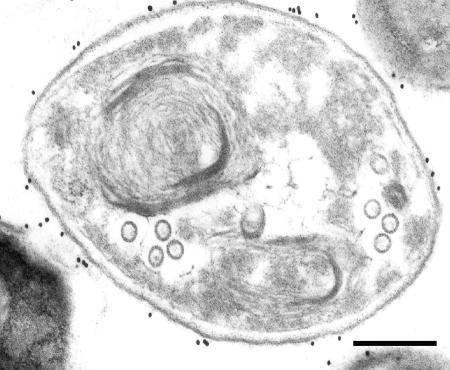
FIG. 2.
Immunogold electron micrograph of an E. bieneusi spore stained with the MAb 2G4. The exospore layer of the spore is detected by the MAb. Bar = 200 nM.
Cross-reactivity.
None of the MAbs reacted with E. intestinalis, E. hellem, E. cuniculi, Staphylococcus aureus, or Escherichia coli by indirect immunofluorescence.
Immunohistochemistry.
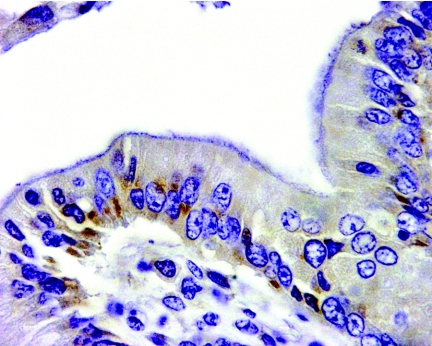
MAbs 1D4 and 2G4, reacted by immunohistochemistry with macaque tissue infected with E. bieneusi, identified intracellular parasites at the basal perinuclear location in gall bladder epithelial cells (Fig. 3).
FIG. 3.
Immunohistochemistry with the MAb 1D4. The antibody identified E. bieneusi in a perinuclear location of biliary epithelial cells of a rhesus macaque spontaneously infected with E. bieneusi (rhesus macaque, gall bladder; original magnification, ×400). MAb 2G4 gives the same staining profile (not shown). Nuclei are stained blue. The isotype control did not stain anything (not shown).
Immunoblotting.
None of the MAbs reacted in immunoblots. However, rabbit and mouse polyclonal specific antibodies (27) reacted with two antigens of 33,000 and 35,000 Da (Fig. 4).
FIG. 4.
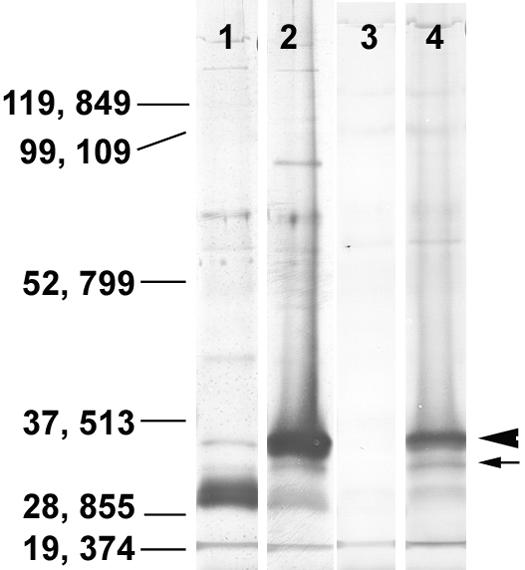
Immunoblot reactivity of preimmunized rabbit serum (lane 1), postimmunized rabbit antiserum (lane 2), preimmunized mouse serum (lane 3), and postimmunized mouse antiserum (lane 4) with E. bieneusi antigens. Both rabbit and mouse antisera against E. bieneusi specifically reacted with two antigens of 33,000 and 35,000 Da.
Detection of spores in feces by MAbs.
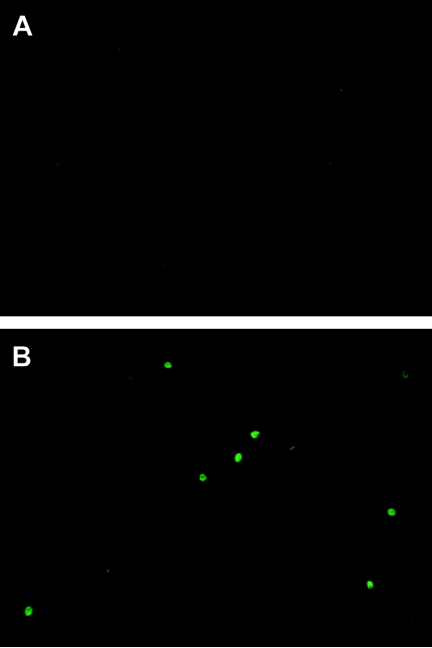
All MAbs reacted by indirect IF with E. bieneusi spores in human and monkey fecal smears. Some MAbs gave background fluorescence; however, reactive spores were still very strongly and clearly identifiable. The MAb 2G4 did not give any background fluorescence (Fig. 5) and was selected for the detection of spores in fecal samples of infected humans and macaques. The rabbit antisera also did not give background fluorescence. A comparative evaluation of the 2G4 and rabbit antisera with PCR was performed as shown in Table 2, indicating equal sensitivities of antibodies with primary but not with nested PCR. MAb 2G4 was also analyzed for E. bieneusi detection with human stool samples. A total of 35 human stool samples were analyzed, and 5 were found positive by the first PCR and also by immunofluorescence with the MAb 2G4 (Table 3).
FIG. 5.
MAb 2G4 specifically detects E. bieneusi spores by immunofluorescence in a fecal smear (image B). Image A is a fecal smear stained with an unrelated mouse IgM control MAb.
TABLE 2.
Detection of E. bieneusi spores in stool of rhesus macaque
| Detection by PCR (first and nested PCR) | No. of samples with result
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All samples (n = 38) | Detection by MAb 2G4a,b
|
Detection by rabbit antibodya,b
|
|||||||
| + | ++ | +++ | ++++ | + | ++ | +++ | ++++ | ||
| Positive by both PCR | 22 | 3 | 4 | 7 | 8 | 2 | 4 | 5 | 11 |
| Negative by first PCR but positive by nested PCR | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Negative by both PCR | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Spores were counted in 10 microscopic fields at a magnification of ×400.
+, 40 to 100 spores; ++, 101 to 400 spores; +++, 401 to 1,000 spores; ++++, >1,000 spores.
TABLE 3.
Detection of E. bieneusi spores in human stool
| Detection by first PCR | No. of samples with result
|
||||
|---|---|---|---|---|---|
| All samples (n = 35) | Detection by MAb 2G4a,b
|
||||
| + | ++ | +++ | ++++ | ||
| Positive by first PCR | 5 | 1 | 1 | 1 | 2 |
| Negative by PCR | 30 | 0 | 0 | 0 | 0 |
Spores were counted in 10 microscopic fields at a magnification of ×400.
+, 40 to 100 spores; ++, 101 to 400 spores; +++, 401 to 1,000 spores; ++++, >1,000 spores.
DISCUSSION
In a previous publication we described the development of methods for concentration and purification of E. bieneusi spores from human with watery diarrheic stool, which led to the production of rabbit polyclonal specific antibody (27). In this communication we describe the generation and characterization of eight MAbs against E. bieneusi using the same concentration and purification procedures. Despite four immunizations and six fusion attempts, we were unsuccessful in our attempts to immortalize IgG-producing hybridoma. Carbohydrate antigens are known to induce strong IgM responses (4), but despite treatment with periodate and enzymatic deglycosylation, no loss of reactivity of any of the eight MAbs was observed. Since lipids are also known to induce strong IgM responses (17), the MAbs will be evaluated accordingly. It is important to point out that fixation of spores with methanol for 10 min, which is a known lipid solubilizer (21), almost entirely eliminated reactivity of six MAbs and reduced the reactivity of two MAbs to half. But methanol is also known to cause alteration in the structure of proteins due to disruption of hydrophobic bonds which contribute to the maintenance of the tertiary structure of proteins (2, 3), and therefore, the loss of MAb reactivity could also be due to the denaturation of the protein epitopes. In contrast to methanol fixation, the fixation of spores with another lipid dissolver, acetone, which dissolves simple lipids and glycolipids but not phospholipids (14, 15), did not affect the reactivity of any of the MAbs. If the antibodies are against lipid antigens, then either spore fixation time with acetone (10 min) was insufficient to dissolve lipids or the MAbs are against phospholipid antigens.
Of the eight, MAb 2G4 reacted with E. bieneusi spores in stools from monkeys and humans without background fluorescence, which makes it an ideal diagnostic reagent. The MAb was comparable to the polyclonal rabbit antisera in its efficacy. The use of MAbs should replace the labor-intensive and highly specialized PCR procedures that are currently used for quantitative detection of E. bieneusi in stool (22, 25). Since 2G4 did not detect spores in those macaque stool samples that were positive by nested PCR, only first PCR was performed with human stool samples. Also, the nested PCR is not particularly useful for clinical laboratories because of the possibility of contamination. Therefore, the sensitivity of the MAb 2G4 is equivalent to that of the first PCR, which is the most likely clinical lab test that can be performed today. The sensitivity and specificity of MAb-based spore detection will be compared with detection by real-time PCR in future. Labeling of 2G4 with fluorescent dyes will make it possible to apply the reagent to direct immunofluorescence testing. Since MAb 2G4 recognizes intracellular stages of the parasite, it will also be a suitable reagent for determining tissue distribution of E. bieneusi in infected hosts.
Information on antigens of E. bieneusi does not exist. Though MAbs did not react with E. bieneusi antigens in immunoblots, the polyclonal antibodies demonstrated the existence of at least two antigens of E. bieneusi of 33,000 and 35,000 Da. These antigens seem to be important immunodominant antigens, since only they were recognized by antisera generated in two different animal species (rabbit and mouse). The nonreactivity of MAbs in blots could be due to insufficient antigen in the blot or the destruction of the epitopes by the SDS-PAGE procedure.
In conclusion, the availability of MAbs against E. bieneusi will facilitate and simplify considerably the future diagnosis of this infection in humans and should make it possible to conduct comprehensive and systematic epidemiologic investigations regarding the true prevalence of this infection not only in the human immunodeficiency virus/AIDS population but also in the general populations in developing and in developed countries. It will be possible to trace the source of the infection, whether from contact or water or both, and the occurrence and distribution in other mammalian species. The MAbs will also be useful tools for studying immunodominant antigens and will help in isolating and characterizing surface proteins that may play a role in parasite attachment and invasion of the host cell.
Acknowledgments
This study was supported by grants R01AI43196, R21AI52792, and P01DK55510 from the National Institutes of Health and EPASTAR Program R828043.
We thank Najuka Florence for technical assistance. We also thank Karen Boisvert, New England Primate Research Center, for the confocal microscopy.
REFERENCES
- 1.Accoceberry, I., M. Thellier, I. Desportes-Livage, A. Achbarou, S. Biligui, M. Danis, and A. Datry. 1999. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi. J. Clin. Microbiol. 37:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu, K. R., and D. J. Douglas. 2000. Methanol-induced conformations of myoglobin at pH 4.0. Biochemistry 39:14702-14710. [DOI] [PubMed] [Google Scholar]
- 3.Babu, K. R., A. Moradian, and D. J. Douglas. 2001. The methanol-induced conformational transitions of beta-lactoglobulin, cytochrome c, and ubiquitin at low pH: a study by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 12:317-328. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., and J. M. Davie. 1975. Clonal dominance. I. Restricted nature of the IgM antibody response to group A streptococcal carbohydrate in mice. J. Exp. Med. 141:1291-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan, R. T., A. Cali, R. L. Owen, and H. C. Spencer. 1991. Microsporidia: opportunistic pathogens in patients with AIDS. Prog. Clin. Parasitol. 2:1-26. [PubMed] [Google Scholar]
- 6.Bryan, R. T., and R. Weber. 1993. Microsporidia. Emerging pathogens in immunodeficient persons. Arch. Pathol. Lab. Med. 117:1243-1245. [PubMed] [Google Scholar]
- 7.Buckholt, M. A., J. H. Lee, and S. Tzipori. 2002. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 68:2595-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalifoux, L. V., A. Carville, D. Pauley, B. Thompson, A. A. Lackner, and K. G. Mansfield. 2000. Enterocytozoon bieneusi as a cause of proliferative serositis in simian immunodeficiency virus-infected immunodeficient macaques (Macaca mulatta). Arch. Pathol. Lab. Med. 124:1480-1484. [DOI] [PubMed] [Google Scholar]
- 9.Chalifoux, L. V., J. MacKey, A. Carville, D. Shvetz, K. C. Lin, A. Lackner, and K. G. Mansfield. 1998. Ultrastructural morphology of Enterocytozoon bieneusi in biliary epithelium of rhesus macaques (Macaca mulatta). Vet. Pathol. 35:292-296. [DOI] [PubMed] [Google Scholar]
- 10.Desportes, I., Y. Le Charpentier, A. Galian, F. Bernard, B. Cochand-Priollet, A. Lavergne, P. Ravisse, and R. Modigliani. 1985. Occurrence of a new microsporidan: Enterocytozoon bieneusi n.g., n. sp., in the enterocytes of a human patient with AIDS. J. Protozool. 32:250-254. [DOI] [PubMed] [Google Scholar]
- 11.Fournier, S., O. Liguory, V. Garrait, J. P. Gangneux, C. Sarfati, F. Derouin, and J. M. Molina. 1998. Microsporidiosis due to Enterocytozoon bieneusi infection as a possible cause of traveller's diarrhea. Eur. J. Clin. Microbiol. Infect. Dis. 17:743-744. [DOI] [PubMed] [Google Scholar]
- 12.Green, L. C., P. J. Didier, L. C. Bowers, and E. S. Didier. 2004. Natural and experimental infection of immunocompromised rhesus macaques (Macaca mulatta) with the microsporidian Enterocytozoon bieneusi genotype D. Microbes. Infect. 6:996-1002. [DOI] [PubMed] [Google Scholar]
- 13.Guerard, A., M. Rabodonirina, L. Cotte, O. Liguory, M. A. Piens, S. Daoud, S. Picot, and J. L. Touraine. 1999. Intestinal microsporidiosis occurring in two renal transplant recipients treated with mycophenolate mofetil. Transplantation 68:699-707. [DOI] [PubMed] [Google Scholar]
- 14.Gunston, K. D., and D. A. Davey. 1980. The effect of cold acetone fractionation on the total phospholipid value and the lecithin/sphingomyelin ratio of amniotic fluid. S. Afr. Med. J. 57:916-917. [PubMed] [Google Scholar]
- 15.Hayes, E. P., P. Scala, and G. Witz. 1986. Lipid composition of liver microsomes from rats treated with acetone. Toxicol. Lett. 31:139-145. [DOI] [PubMed] [Google Scholar]
- 16.Hendricks, E. E., E. Ludlage, S. Bussell, K. George, F. H. Wegner, and K. G. Mansfield. 2004. Wasting syndrome and disruption of the somatotropic axis in simian immunodeficiency virus-infected macaques with Mycobacterium avium complex infection. J. Infect. Dis. 190:2187-2194. [DOI] [PubMed] [Google Scholar]
- 17.Ide, T., M. Sata, H. Nakano, H. Suzuki, and K. Tanikawa. 1997. Increased serum IgM class anti-lipid A antibody and therapeutic effect of ursodeoxycholic acid in primary biliary cirrhosis. Hepatogastroenterology 44:1569-1573. [PubMed] [Google Scholar]
- 18.Kondova, I., K. Mansfield, M. A. Buckholt, B. Stein, G. Widmer, A. Carville, A. Lackner, and S. Tzipori. 1998. Transmission and serial propagation of Enterocytozoon bieneusi from humans and Rhesus macaques in gnotobiotic piglets. Infect. Immun. 66:5515-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansfield, K. G., A. Carville, D. Hebert, L. Chalifoux, D. Shvetz, K. C. Lin, S. Tzipori, and A. A. Lackner. 1998. Localization of persistent Enterocytozoon bieneusi infection in normal rhesus macaques (Macaca mulatta) to the hepatobiliary tree. J. Clin. Microbiol. 36:2336-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansfield, K. G., A. Carville, D. Shvetz, J. MacKey, S. Tzipori, and A. A. Lackner. 1997. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian-immunodeficiency-virus-inoculated macaques with hepatobiliary disease. Am. J. Pathol. 150:1395-1405. [PMC free article] [PubMed] [Google Scholar]
- 21.Mastro, R., and M. Hall. 1999. Protein delipidation and precipitation by tri-n-butylphosphate, acetone, and methanol treatment for isoelectric focusing and two-dimensional gel electrophoresis. Anal. Biochem. 273:313-315. [DOI] [PubMed] [Google Scholar]
- 22.Menotti, J., B. Cassinat, R. Porcher, C. Sarfati, F. Derouin, and J. M. Molina. 2003. Development of a real-time polymerase-chain-reaction assay for quantitative detection of Enterocytozoon bieneusi DNA in stool specimens from immunocompromised patients with intestinal microsporidiosis. J. Infect. Dis. 187:1469-1474. [DOI] [PubMed] [Google Scholar]
- 23.Molina, J. M., C. Sarfati, B. Beauvais, M. Lemann, A. Lesourd, F. Ferchal, I. Casin, P. Lagrange, R. Modigliani, F. Derouin, et al. 1993. Intestinal microsporidiosis in human immunodeficiency virus-infected patients with chronic unexplained diarrhea: prevalence and clinical and biologic features. J. Infect. Dis. 167:217-221. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz, D. A., D. C. Anderson, S. A. Klumpp, and H. M. McClure. 1998. Ultrastructure of atypical (teratoid) sporogonial stages of Enterocytozoon bieneusi (Microsporidia) in naturally infected rhesus monkeys (Macacca mulatta). Arch. Pathol. Lab. Med. 122:423-429. [PubMed] [Google Scholar]
- 25.Sestak, K., P. P. Aye, M. Buckholt, K. G. Mansfield, A. A. Lackner, and S. Tzipori. 2003. Quantitative evaluation of Enterocytozoon bieneusi infection in simian immunodeficiency virus-infected rhesus monkeys. J. Med. Primatol. 32:74-81. [DOI] [PubMed] [Google Scholar]
- 26.Sheoran, A. S., S. Chapman, P. Singh, A. Donohue-Rolfe, and S. Tzipori. 2003. Stx2-specific human monoclonal antibodies protect mice against lethal infection with Escherichia coli expressing Stx2 variants. Infect. Immun. 71:3125-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheoran, A. S., X. Feng, S. Kitaka, L. Green, C. Pearson, E. S. Didier, S. Chapman, J. K. Tumwine, and S. Tzipori. 2005. Purification of Enterocytozoon bieneusi from stools and production of specific antibodies. J. Clin. Microbiol. 43:387-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sing, A., K. Tybus, J. Heesemann, and A. Mathis. 2001. Molecular diagnosis of an Enterocytozoon bieneusi human genotype C infection in a moderately immunosuppressed human immunodeficiency virus seronegative liver-transplant recipient with severe chronic diarrhea. J. Clin. Microbiol. 39:2371-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzipori, S., A. Carville, G. Widmer, D. Kotler, K. Mansfield, and A. Lackner. 1997. Transmission and establishment of a persistent infection of Enterocytozoon bieneusi, derived from a human with AIDS, in simian immunodeficiency virus-infected rhesus monkeys. J. Infect. Dis. 175:1016-1020. [DOI] [PubMed] [Google Scholar]
- 30.Visvesvara, G. S., G. J. Leitch, S. Wallace, C. Seaba, D. Erdman, and E. P. Ewing, Jr. 1996. Adenovirus masquerading as microsporidia. J. Parasitol. 82:316-319. [PubMed] [Google Scholar]
- 31.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodward, M. P., W. W. Young, Jr., and R. A. Bloodgood. 1985. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J. Immunol. Methods 78:143-153. [DOI] [PubMed] [Google Scholar]