Abstract
Determination of antibody avidity measurements can be difficult in human serum depending on the population evaluated. We evaluated three approaches for the determination of antibody avidity for immunoglobulin G (IgG). These approaches were (i) elution of bound antibody with increasing concentrations of a chaotropic agent using a single serum dilution, (ii) binding interference of multiple serum dilutions by a single concentration of a chaotrope, and (iii) elution of multiple serum dilutions by a single concentration of a chaotrope. Parameters that affect the determination of avidity measurements and their limitations were evaluated with pre- and post-Haemophilus influenzae type b conjugate vaccination sera (n = 89). We determined that elution of low-avidity antibodies present in multiple dilutions of the serum sample by a single concentration of a chaotrope (0.15 M sodium thiocyanate [NaSCN]) was optimal for the determination of avidity measurements throughout a wide range of IgG concentrations (0.94 to 304.6 μg/ml). The percent reduction in concentration as determined by the elution assay with 0.15 M NaSCN correlated highly (r = 0.84) with weighted averages obtained by an elution assay with multiple solutions of NaSCN. The correlation (r = 0.57) between elution and binding interference, when a single concentration of a chaotrope was used, was lower than the correlation between the two elution methods (r = 0.84). We found that the serum dilution, the heterogeneity of the antibody population, and the concentration of the chaotrope were the primary variables affecting avidity determinations. In this study, we present multiple analysis methods depending on the methodology used. We also present the factors that affect the analysis of avidity determinations given the polyclonal nature of human sera. This experimental approach should benefit the evaluation of similar antibodies induced by other bacterial polysaccharide vaccines.
In the United States there has been a dramatic decrease in the incidence of Haemophilus influenzae type b (Hib) disease since the introduction of highly efficacious vaccines in the 1980s (6-8). This success is attributed to the widespread vaccine coverage across the country and to the capacity of polyribosylribitol phosphate (PRP) protein conjugates to elicit long-term protection that can be recalled upon exposure or boosting with a subsequent dose (3, 5, 10, 16). This recall of the established memory has been observed even when a reduced dose of vaccine is used (9). Vaccination elicits memory B- and T-cell clones. With time, the surviving clones tend to be those producing antibodies of higher avidity. Recall of highly avid antibodies by the vaccine antigen would indicate that efficient memory was established. Goldblatt et al. have shown that this is the case with Hib and that antibody avidity can be a marker for the presence of immunological memory (11). However, the long-term benefit of immunological memory as measured by avidity estimates may require additional investigation, especially when only vaccination regimens with three doses are used for young children, as in the case of the meningococcal serogroup C conjugate vaccine (23).
Antibody affinity can be defined as the strength of the binding of a single antibody type (a homogeneous antibody, such as a monoclonal antibody) and a single antigenic target (hapten). In this single-epitope interaction, the affinity constant is the amount of complexed antigen-antibody at equilibrium (13). In human serum the antibody population is heterogeneous (polyclonal in nature), and determination of antibody affinity is not possible. However, adaptations of this affinity concept have been devised, and determinations of the stability of the antigen-antibody interactions in a mixed population of antibodies have been termed antibody avidity determinations (13). Chaotropic agents such as urea or thiocyanate have been preferred for the determination of the average estimate of antibody avidity.
In the laboratory, antibody avidity can be estimated by using a variety of methods. Therefore, antibody avidity estimates will differ according to the methodology used. Each methodology has its own limitations, and it has been difficult to compare the results of one study with those of another given the diversity of approaches to experimentation and analysis. In this investigation, we compare three experimental approaches that represent some of the methodologies used for the determination of anti-PRP antibody avidity (11, 20) and the knowledge gained from other avidity methods used for Streptococcus pneumoniae (2, 24). We present the limitations of each approach and the preferred method of analysis for the data obtained. Similar evaluation of antibody avidities elicited by other bacterial polysaccharide vaccines should benefit from the experimental approach given in this study.
MATERIALS AND METHODS
Serum samples.
A total of 89 sera (46 prevaccination and 43 postvaccination sera) from 51 study participants (age range, 3 to 72 years) who received a single dose of Hib-diphtheria-CRM197 protein conjugate vaccine (HibTITER; Wyeth-Lederle Vaccines and Pediatrics, West Henrietta, N.Y.) were used in this study. Serum samples were collected after informed consent of the participants or their legal guardians before and 8 weeks after vaccination, as part of a colonization intervention study in Alaska in 2001. Adult participants aged 18 to 72 years (n = 18) had no previous history of Hib disease or Hib vaccine receipt, and 16.6% had oropharyngeal swabs that were culture positive for Hib at the time of enrollment. None of the children had a history of confirmed Hib disease. Children (n = 28) aged 3.7 to 11.6 years had previously received a complete Hib vaccination regimen. In this subgroup of children, 35.7% were colonized with Hib at the time of enrollment. Sera were stored frozen at −70°C in 500-μl aliquots until use.
IgG antibody concentrations.
Immunoglobulin G (IgG) antibody concentrations were determined by an enzyme-linked immunosorbent assay (ELISA) modification (9) of the method published by Madore et al. (15). Briefly, the standard curve was generated using the reference serum lot 1983 (provided by Carl Frasch, Center for Biological Evaluation and Review; Food and Drug Administration, Bethesda, MD) with a calculated 60.9 μg/ml of IgG antibody. The quality control serum used was PSAB-90 (Claudette Thompson, Dana Farber Cancer Institute, Boston, Mass.). The IgG antibody concentration for this quality control was 60.3 ± 9.45 μg/ml (mean ± standard deviation) (21). IgG determinations were performed either manually or with the assistance of liquid handlers, Packard Multiprobe II (4 tip) and II HT Ex (8 tip) (Perkin-Elmer, Shelton, CT). We estimated the minimum level of detection of the semiautomated ELISA to be 0.11 μg/ml. This limit of detection was similar to that previously reported for the manual assay, 0.12 μg/ml, when the test serum was prediluted 1:50 (20).
Avidity determination using elution with increasing concentrations of a chaotrope.
Since we were interested in avidity determinations for both pre- and postvaccination sera, we followed a method described previously (20). Increasing concentrations (0, 0.005, 0.016, 0.050, 0.150, 0.440, 1.330, and 4.000 M) of sodium thiocyanate (NaSCN) were used as the chaotropic agent (4, 18). The various concentrations of NaSCN were made fresh daily by dilution (10 mM phosphate-buffered saline [PBS] buffer [pH 7.2 to 7.4] with 0.3% Tween 20) of an 8 M NaSCN stock (prepared fresh every week and stored at room temperature in a sealed container). The predetermined serum dilution was loaded into 18 wells and allowed to bind for 1 h at room temperature in a humidified chamber. Four of these wells were not treated with the chaotrope reagent and received only the PBS-Tween 20 buffer described above. The remaining 14 wells were treated with the seven chaotrope concentrations added in duplicate as an elution step following the removal of unbound serum antibodies in the ELISA plate. Solutions were allowed to incubate for 15 min to remove antibodies of low binding capacity to the Hib polysaccharide conjugated to human albumin (HbOHA) antigen (provided by Moon Nahm, www.vaccine.uab.edu). After removal by washing (five times) of the eluted antibodies, detection of bound antibodies with a horseradish peroxidase-conjugated anti-IgG mouse monoclonal antibody (HP 6043; Hybridoma Reagent Laboratories, Baltimore, MD) and tetramethyl benzene as the substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was carried out as previously described (9). A single lot of Immulon 2 microtiter plates (Dynatech Laboratories Inc., Chantilly, VA) was used for both ELISA and all avidity determinations. These avidity assays did not include a standard, because antibody concentrations were not required for data analysis. However, the quality control serum PSAB-90 was included in each assay plate at a serum dilution of 1:400. Avidities using elution with increasing concentrations of the chaotrope were expressed in terms of a weighted average (WA) as previously described (19).
Avidity using binding interference with a single dilution of the chaotrope.
Prior to this study, there was no documentation of a single chaotropic agent concentration for use in Hib following this assay format, as has been described for pneumococcal avidity ELISAs by Antilla et al. (2). Consequently, we analyzed the data generated above to derive a single concentration of the chaotrope (0.15 M NaSCN). This concentration was used to interfere with the binding of serum antibodies that were serially diluted twofold in the microtiter plate. Samples were loaded in quadruplicate at an appropriate dilution ranging from 1:50 to 1:400 (median dilution, 1:100). One set of duplicates was treated with the chaotrope by dilution in PBS-Tween 20 buffer containing 0.15 M NaSCN. The other set of untreated serum duplicates was diluted in PBS-Tween 20 buffer. After incubation of the ELISA plates for 1 h at room temperature in a humidified chamber, the unbound antibodies were removed by washing (five times), and determination of bound antibodies was performed as described in the ELISA protocol. A reference serum, FDA 1983, and the quality control PSAB-90 serum were included in all assay plates at a starting dilution of 1:400. Relative avidities using binding interference with a single dilution of the chaotrope were expressed in terms of the percent reduction in IgG concentration in the presence of the chaotrope compared to the concentration in the absence of the chaotrope (PBS-Tween 20 buffer alone). In both the binding interference and the elution method with a single concentration of NaSCN, avidity was expressed in terms of the percent reduction in the IgG concentration compared to the concentration in the absence of the chaotrope. This methodology differs from the 50% reduction in signal described for mouse sera by van Dam et al. (24).
Avidity using elution with a single dilution of the chaotrope.
This methodology followed the assay format already described for Streptococcus pneumoniae by Antilla et al. (2). We adapted this methodology for determination of Hib antibody avidity using the same reagents and loading pattern as for the binding interference assay, with the exception that the chaotropic concentration (0.15 M NaSCN) was added as a separate elution step after the initial binding of the serially diluted sera. Duplicate wells containing serum dilutions in the absence of the chaotrope were treated with PBS buffer during the elution step after removal of the unbound antibodies. No significant differences (P < 0.01) were found between serum concentrations in the absence of the chaotrope that had this additional PBS treatment and wash step and those that were calculated by the standard ELISA (r = 0.99). As in the binding interference assay, the FDA 1983 reference and PSAB-90 quality control sera were included in each plate. Relative avidities using elution with a single dilution of the chaotrope were expressed in terms of the percent reduction in IgG concentration compared to the concentration in the absence of the chaotrope (2, 11).
Data analysis.
ELISA antibody concentrations were calculated by using a 4-parameter logistic curve-fitting analysis as described by Plikyatis et al. (17) against the standard curve generated by the reference serum FDA 1983. Avidities using elution with increasing concentrations of the chaotrope (eight threefold dilutions ranging from 0 to 4 M NaSCN) and a single serum dilution were expressed in terms of a WA as previously described (19). The following formula was used for determination of WAs: WA = antilog[∑(percent reduction in OD450 × log10 NASCN concentration)/∑ percent reduction in OD450], where OD450 is optical density at 450 nm. This WA is equivalent to the NaSCN concentration at which there is a reduction in the majority of the ELISA IgG absorbance of each serum sample. When the dilution factor used in this assay is factored in, avidity is expressed as an avidity index (AI), calculated by multiplying WA by the serum dilution factor (20). Avidities using binding interference or elution with a single dilution of the chaotrope and serial dilutions of the serum sample were expressed in terms of the percent reduction in IgG concentration in the presence of the chaotrope compared to the IgG concentration in the absence of the chaotrope (2, 11). In this study, and only for purposes of comparison, the percent reduction in concentration was transformed to WA using the formula y = a + bx, calculated as y = 0.79 + (−0.00963 × percent reduction in concentration), where y is the calculated weighted average, a is the y intercept calculated as 0.79, b is the slope calculated as −0.00963, and x is the known variable, percent reduction in antibody concentration. When the weighted averages were >0.79 M NaSCN, the relationship between WA and the percent reduction at a single concentration was no longer linear, and a hillslope adjustment of the data was required to maintain linearity. For hillslope adjustment, the following formula was used: y = min + (max − min)/[1 + (X/EC50)∧hillslope], calculated as y = 0.0363 + (0.9666 − 0.0363)/[1 + (X/0.5358) exp(−1.3511)], where min is the bottom of the curve, max is the top of the curve, X is the percent reduction in OD450, and EC50 is the median effective concentration of NaSCN. Comparisons among data sets were performed with Pearson's product moment correlation coefficient. Significant levels of correlation were set at a P value of <0.05. All data were analyzed with Excel (Microsoft Windows 2000) and Sigma Plot version 8.0 (SPSS, Plover, Wis.).
RESULTS
Determination of a single concentration of chaotrope.
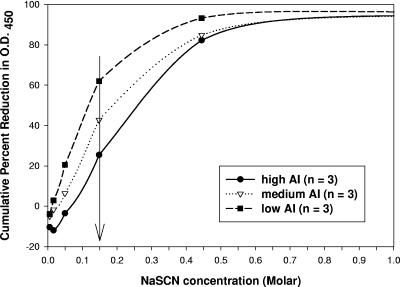
We analyzed the WA obtained from 89 sera tested for avidity determinations by elution of a single serum dilution with multiple chaotrope concentrations to validate the selection of the 0.15 M NaSCN concentration. For this purpose, we selected 9 out of 89 sera that had high, medium, and low AIs. These sera yielded OD450 values between 0.5 and 1.0 in the absence of NaSCN. Their cumulative percent reduction in OD450 was calculated, and the average was plotted against the NaSCN concentration yielding the corresponding reduction in OD450 (Fig. 1). A concentration of 0.15 M NaSCN allowed for discrimination between the high, medium, and low antibody avidities in this panel of sera.
FIG. 1.
Cumulative percent reduction in OD450 caused by the addition of increasing concentrations of NaSCN to three groups of sera exhibiting high, medium, and low AIs after elution with multiple chaotrope concentrations. The mean percent reduction of three sera per category is plotted against the NaSCN concentration (Table 1 gives individual percent reductions). Arrow indicates 0.15 M NaSCN as the optimal concentration.
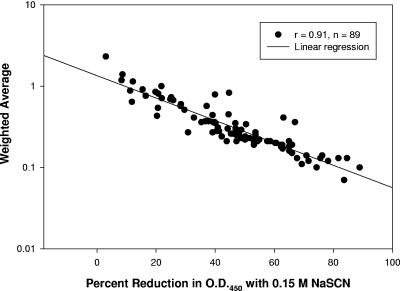
We then calculated the percent reduction in OD at two flanking concentrations (0.05 M and 0.44 M) used in the elution method with multiple chaotrope concentrations. Each serum's individual percent reduction in OD450 at 0.05, 0.15, and 0.44 M was correlated to the corresponding WA to confirm that a 0.15 M NaSCN concentration was robust across all 89 sera tested. As shown in Fig. 2, there was a high level of correlation (r = 0.91) when the percent reduction at 0.15 M NaSCN was compared to the overall WA. In addition, the data were evenly distributed along the line of linear regression. A similar analysis using the percent reduction in OD450 at 0.05 M NaSCN for the same 89 sera yielded a lower correlation (r = 0.75), and the majority of the data shifted to a reduction in OD450 of ≤40%. When the percent reduction in OD450 at 0.44 M NaSCN was used instead, the correlation (r) between the WA and the percent reduction in OD450 was 0.80 and ∼50% of the data shifted toward a greater (80 to 100%) reduction in OD450. Therefore, a concentration of 0.15 M NaSCN was used for the comparison of binding interference and elution avidity assays that used a single chaotrope concentration.
FIG. 2.
Correlation of the calculated percent reduction in OD450 at 0.15 M NaSCN with the WA as a measurement of avidity. Both the x and y data sets are derived from avidity determinations using an elution assay with increasing concentrations of the chaotrope. There is a high level of correlation (r = 0.91; P < 0.01) at 0.15 M NaSCN. Similar analyses using adjacent concentrations, 0.05 and 0.44 M NaSCN, demonstrated lower levels of correlation (r = 0.75 and 0.8, respectively). In addition, the data points were not evenly distributed along the line of linear regression (data not shown).
Comparison of IgG and avidity measurements.
Table 1 gives the ELISA IgG and corresponding avidity measurements (percent reduction in IgG concentration) for the panel of 16 sera (7 pre- and 9 postvaccination sera; 9 were from individuals <18 years old, and 7 were from individuals >18 years old; levels of anti-PRP IgG, 0.94 to 304.6 μg/ml) randomly selected from the initial 89 sera. These 16 sera were analyzed in parallel by elution or binding interference with 0.15 M NaSCN of anti-PRP antibodies. Table 2 gives the weighted averages, avidity indexes, and percentages of reduction in OD450 (at 0.15 M NaSCN, one of seven dilutions used) for the same panel of sera after elution with increasing concentrations of the chaotrope (0 M to 4 M). Antibody concentrations could not be determined by the elution method with increasing concentrations of NaSCN, since there was no standard curve and a single serum dilution was used for avidity determinations.
TABLE 1.
ELISA IgG concentrations and corresponding avidity measurements for a panel of 16 sera after elution or binding interference with 0.15 M NaSCN
| Seruma | Elution with 0.15 M NaSCN
|
Binding interference with 0.15 M NaSCN
|
||||
|---|---|---|---|---|---|---|
| IgG concn (μg/ml) at:
|
% Reduction in IgG concnb | IgG concn (μg/ml) at:
|
% Reduction in IgG concnb | |||
| 0 M NaSCN | 0.15 M NaSCN | 0 M NaSCN | 0.15 M NaSCN | |||
| 1 | 13.99 | 7.87 | 43.7 | 12.84 | 7.53 | 41.4 |
| 2 | 16.89 | 10.89 | 35.5 | 14.92 | 13.24 | 11.3 |
| 3 | 2.05 | 0.77 | 62.4 | 2.01 | 0.57 | 71.6 |
| 4 | 3.48 | 1.66 | 52.3 | 2.84 | 1.44 | 49.3 |
| 5 | 1.04 | 0.53 | 49.0 | 0.78 | 0.43 | 44.9 |
| 6 | 6.21 | 4.59 | 26.1 | 5.49 | 0.84 | 84.7 |
| 7 | 4.66 | 2.36 | 49.4 | 4.38 | 2.03 | 53.7 |
| 8 | 569.98 | 132.99 | 76.7 | 555.8 | 127.5 | 77.1 |
| 9 | 177.92 | 98.31 | 44.7 | 169.74 | 175.89 | −3.6 |
| 10 | 9.79 | 5.58 | 43.0 | 8.96 | 5.64 | 37.1 |
| 11 | 9.26 | 5.7 | 38.4 | 7.47 | 4.28 | 42.7 |
| 12 | 32.13 | 12.3 | 61.7 | 29.69 | 10.32 | 65.2 |
| 13 | 10.26 | 4.15 | 59.6 | 8.18 | 3.69 | 54.9 |
| 14 | 7.78 | 1.95 | 74.9 | 7.93 | 1.66 | 79.1 |
| 15 | 40.61 | 21.07 | 48.1 | 37.18 | 15.24 | 59.0 |
| 16 | 12.44 | 8.9 | 28.5 | 9.15 | 13.35 | −45.9 |
| PSAB-90 | 70.7 | 35.7 | 49.5 | 66.0 | 30.9 | 53.1 |
Sera 1 to 7 were prevaccination and sera 9 to 16 were postvaccination sera. PSAB-90 results represent the mean of four independent assays. Values for sera with high CVs (>20%) are boldfaced.
Percent reduction in IgG antibody concentration (μg/ml) in the presence of the chaotrope NaSCN (0.15 M).
TABLE 2.
Avidity measurements for a panel of 16 sera after elution with increasing concentrationsa of NaSCN
| Serumb | WAc | AId | Serum dilution factor | % Reduction in OD450e |
|---|---|---|---|---|
| 1 | 0.35 | 87.5 | 250 | 40.1 |
| 2 | 0.41 | 100 | 250 | 32.8 |
| 3 | 0.15 | 15.4 | 100 | 67.7 |
| 4 | 0.25 | 37.5 | 150 | 47.1 |
| 5 | 0.41 | 20.5 | 50 | 63.1 |
| 6 | 0.67 | 100.5 | 150 | 75.8 |
| 7 | 0.21 | 30 | 150 | 58.6 |
| 8 | 0.12 | 960 | 8,000 | 78.2 |
| 9 | 0.25 | 1,000 | 4,000 | 53.6 |
| 10 | 0.37 | 74 | 200 | 37.7 |
| 11 | 0.28 | 58 | 200 | 41.3 |
| 12 | 0.30 | 45 | 150 | 44.2 |
| 13 | 0.17 | 42.5 | 250 | 62.8 |
| 14 | 0.13 | 26 | 200 | 81.7 |
| 15 | 0.44 | 22 | 50 | 39.1 |
| 16 | 0.54 | 106 | 200 | 20.6 |
| PSAB-90 | 0.32 | 128.8 | 400 | NA |
Concentrations were 0 to 4 M.
Sera 1 to 7 were prevaccination and sera 9 to 16 were postvaccination sera. PSAB-90 results represent the means of four independent assays.
Determined as given under “Data analysis” in Materials and Methods.
Calculated by multiplying the WA by the dilution factor.
Since this avidity assay does not include a standard in the assay plate, we could not determine the percent reduction in concentration, but rather determined the percent reduction in OD450. NA, not applicable.
Agreement between elution methods at 0.15 M NaSCN.
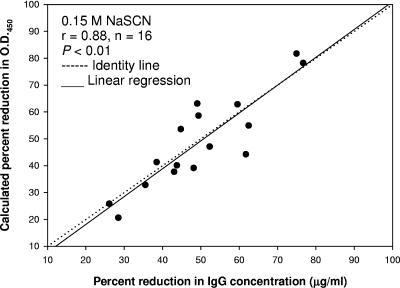
The percent reduction in OD450 at 0.15 M was derived by a mathematical conversion of the data obtained from the elution with increasing concentrations of chaotrope for a panel of 16 sera. The correlation between the mathematically calculated percent reduction in OD450 and the experimental (in the laboratory) percent reduction in IgG concentration using 0.15 M NaSCN was significant (P < 0.01; r = 0.88), as shown in Fig. 3. When only the percent reduction in OD450 was compared for both methods (both axes), there was a slight trend for the percent reduction in OD450 obtained from the experimental elution ELISA with 0.15 M NaSCN to be below the line of identity, although the correlation was still highly significant (P < 0.01; r = 0.86).
FIG. 3.
Correlation between the calculated percent reduction in OD450 (y axis) and the experimental percent reduction in the IgG concentration (x axis) of sera (n = 16) tested with 0.15 M NaSCN by two elution methods. The calculated percent reduction was derived from duplicate wells treated with 0.15 M NaSCN during the elution assay with increasing concentrations of the chaotrope (0 to 4 M NaSCN). In this type of elution assay, data were normally expressed as WAs. The experimental percent reduction in concentration was derived from the elution of serially diluted sera with a single NaSCN concentration (0.15 M).
Correlation between elution methods with single or increasing concentrations of chaotrope.
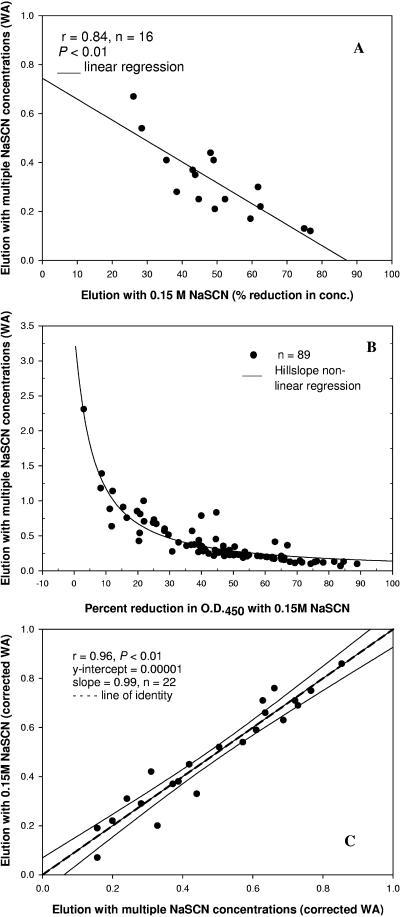
We initially compared the WA obtained by the elution method of a single serum dilution with increasing concentrations of NaSCN with the percent reduction in IgG concentration obtained by elution of serially diluted sera (n = 16) with 0.15 M NaSCN. There was an inverse correlation between these data sets (P < 0.01; r = 0.84), as shown in Fig. 4A. However, it became apparent that in sera with higher WAs (>0.7 M NaSCN; y intercept in Fig. 4A), the relationship between the WA and the percent reduction in OD was affected by a lack of linearity (the y axis has a logarithmic scale in Fig. 2). The hillslope nonlinear regression analysis of the data given in Fig. 2 shows the marked effect due to a lack of linearity (Fig. 4B). To coalesce the experimental results by the two methodologies, six additional sera with WAs of >0.7 M were tested by elution with 0.15 M for a total of 22 sera. The WA obtained by elution of a single serum dilution with increasing concentrations of NaSCN was adjusted using the hillslope formula and compared for linearity with the calculated WA using a linear equation conversion of the percent reduction in IgG concentration as determined by the elution method with a single concentration of chaotrope and serial serum dilutions. These conversions of the data allowed for the reporting of either data set in the same measuring unit (weighted molar concentration of NaSCN) and allowed for the comparison of data obtained by either method (Fig. 4C).
FIG. 4.
Correlation between the percent reduction in IgG concentration (μg/ml) as calculated by the single-concentration elution assay (0.15 M NaSCN), and the WA of the NaSCN molar concentrations as a measurement of the serum avidity in a panel of 16 sera. (A) There is an inverse correlation (r = 0.835) between the data obtained by these two elution methods. (B) The relationship between the calculated percent reduction in OD450 at 0.15 M NaSCN and the overall WA of the NaSCN molar concentrations is not linear and follows a hillslope regression line. (C) A linear regression (r = 0.96; slope, 0.99) can be obtained by correction of the overall WA using the hillslope formula and the calculated WA (using a linear equation conversion of the percent reduction in OD450) based on elution with 0.15 M NaSCN. These transformations allow for the conversion of elution data obtained from either a single concentration or increasing concentrations of the chaotrope into the same measuring unit (WA of NaSCN molar concentrations). When a single concentration of the chaotrope is used, the sera are serially diluted. When increasing concentrations of the chaotrope are used, the sera are loaded at a single dilution.
We also found that the serum dilution affected the calculation of the WA as well as the determination of the percent reduction in IgG concentrations in the presence of 0.15 M NaSCN. Table 3 gives an example of the effect that serum dilution can have on avidity assay determinations of a single serum sample (serum 306B). The overall coefficient of variance (CV) in the calculated avidity (percent reduction in IgG concentration) for this serum sample across dilutions was 39.5%.
TABLE 3.
Example of the effect of serum dilution on avidity determinations
| IgG concn (μg/ml) at:
|
% Reduction in IgG concna | Calculated WAb | Serum dilution testedc | |
|---|---|---|---|---|
| 0 M NaSCN | 0.15 M NaSCN | |||
| 13.88 | 11.98 | 13.7 | 0.66 | 1/100 |
| 13.92 | 9.79 | 29.7 | 0.50 | 1/200 |
| 11.97 | 8.06 | 32.7 | 0.48 | 1/400 |
| 10.00 | 5.86 | 41.5 | 0.39 | 1/800 |
Calculated as (IgG concentration at 0 M NaSCN − IgG concentration at 0.15 M NaSCN)/(IgG concentration at 0 M NaSCN) × 100. The elution method was used to calculate the IgG concentration (expressed in μg/ml) in the presence of 0.15 M NaSCN.
Calculated as 0.79 + (−0.00963 × percent reduction in IgG concentration [μg/ml]).
A single serum sample (serum 306B) is given as an example of the effect that serum dilution can have on avidity assay determinations. The overall CV in the calculated WA for this serum across dilutions was 39.5%.
Agreement between binding interference and elution with a single concentration of chaotrope.
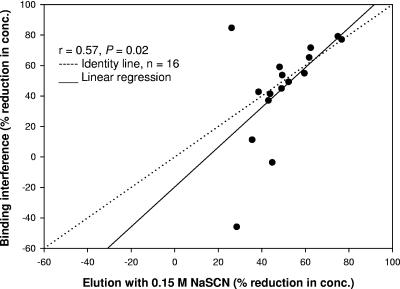
The correlation (P = 0.02; r = 0.57) between the percent reduction in IgG concentration obtained experimentally by the binding interference and the elution methods using 0.15 M NaSCN is shown in Fig. 5. Of the 16 sera tested in parallel and using serial serum dilutions for both methods, 4 sera were in marked disagreement (>20% difference from the line of identity) and 2 of these 4 sera showed an increase rather than a reduction in IgG concentration when tested by the binding interference method. Coefficients of variance for these four sera were higher (range, 23.5 to 39.5%) by the elution method than by the standard ELISA, indicating that nonparallelism was observed in these serum samples (17) and that a different antibody population was being measured in these sera than in the standard reference serum. All other sera had CVs of <20%. The CVs for three of these sera in the binding interference assay were also high (range, 23.6 to 459.3%). The overall correlation when OD was used for the comparison of these methods was slightly improved (P < 0.02; r = 0.61). However, the three sera with high CVs in the binding interference assay were still in disagreement.
FIG. 5.
Correlation between binding interference and elution with a single concentration of NaSCN for a panel of 16 sera (serially diluted). Of the four outliers, two were prevaccination and two were postvaccination sera. These outlier sera were nonparallel with the standard curve, and they had a CV of ≥20%.
DISCUSSION
Estimates of serum antibody avidity are dependent on the homogeneity of the antibodies present in the serum sample. The more heterogeneous the antibody population, the more difficult it is to establish a true measurement of antibody avidity (13, 24). The ideal antibody population for measurement of avidity would be a homogeneous monoclonal antibody population. In this situation, an accurate estimate of the true affinity constant of that single antigen-antibody reaction is determined. However, human serum is far from this ideal antibody population, and in many cases the populations of antibodies are so variable in their binding to the target antigen that the reaction is nonparallel. Chaotropic agents, such as ammonium or sodium thiocyanate, have been used widely for dissociation (elution) or binding interference of the antigen-antibody reaction, allowing only those antibodies with higher avidities to bind to the target antigen (2, 4, 11, 18, 19, 20). The basis of this interaction is dependent on the concentration of thiocyanate compound to interfere with the electrostatic interactions that keep the antigen-antibody complex formed. Researchers have used concentrations ranging from 0 to 5 M of the thiocyanate compound. The higher the avidity of the antibodies in the serum sample, the stronger the antigen-antibody complex that is formed, and therefore a higher concentration of thiocyanate is required to break or prevent the formation of such antigen-antibody complexes. In this study, we gave examples of different concentrations of chaotrope that are required to elute antibodies ranging from strong to weak antigen-antibody interactions. Antibody avidity determinations that use a single concentration of thiocyanate are available for other bacterial polysaccharide antigens, such as pneumococcus (2). An elution with a higher concentration of thiocyanate (0.5 M NaSCN) is used in the elution assay for anti-pneumococcal polysaccharide antibodies. This study defines a single concentration of chaotrope (0.15 M NaSCN) for Hib PRP antigen-antibody avidity determinations (as given in Fig. 2). Gray and Shaw reported artifacts during elution with lower concentrations of thiocyanate in a pneumococccal polysaccharide method (12). We also observed an increase in OD450 at lower concentrations of thiocyanate in occasional adult serum samples tested for Hib avidity using the multiple NaSCN elution method (data not shown). In addition, 4 out of 16 sera showed discrepant percent reductions in IgG concentration when the binding interference method was used with 0.15 M NaSCN. However, use of a single chaotrope concentration in an elution format eliminates these potential artifacts, which bias the final WA calculation.
We have judiciously analyzed the data obtained from the elution of a single dilution of serum samples with increasing concentrations of NaSCN to define the appropriate concentration needed to distinguish the avidity profiles of mixed populations of anti-PRP antibodies present in human sera. This type of research became a necessity during our investigation of the antibody avidity of Alaska Natives participating in a Hib colonization study conducted by the Arctic Investigations Program, Centers for Disease Control and Prevention (CDC), Anchorage, AK. Our preliminary observations indicated that the elution of a single serum dilution with multiple thiocyanate concentrations was limited by the serum dilution itself. Although this was a known factor, we had previously corrected for this effect by taking the serum dilution into consideration as a variable and calculating avidity indices (WA times dilution factor) as previously published (20). However, some of the serum samples that we encountered during the Hib colonization study had very high IgG antibody concentrations (>50 μg/ml) that required serum dilutions of >1:1,000 in order to obtain an initial OD of 0.5 to 1.0 as defined by this methodology (as given in Table 2). These high dilutions were in many cases 2 or 3 times higher than the dilution of the standard used in the ELISA. High dilutions of the serum sample bring three major consequences. (i) Serum dilution can bias the population of antibodies that are allowed to bind to the antigen. (ii) Targeting a serum dilution to a given OD is experimentally inaccurate despite individual analysis of the binding curve and careful predilution of serum samples. (iii) As the avidity index accounts for serum dilution, an avidity index may be falsely elevated due to dilution factors that exceed the dilution factor of the standard reference serum. Another observation that prompted this research was the fact that avidity in serum samples with low OD450 values (≤0.2) could not be accurately evaluated by this methodology.
Since human serum is polyclonal in nature, the major limitation of diluting a given serum sample to a predetermined OD is that a single cross section of the antibody population is analyzed. Under this methodology, there is no capacity to accurately calculate concentrations or the level of parallelism (17) that a given sample has in the presence or absence of chaotrope. We had been limited to this type of methodology in the past, due to the lack of information regarding a single concentration of chaotrope. The 0.15 M concentration defined here allows for the use of a binding interference method or an elution method using this single concentration and serially diluted sera.
Based on our experimental and analytical results, we favor the elution over the binding interference method for Hib PRP antigen. Elution with 0.15 M NaSCN allows for determination of antibody concentrations as well as information about parallelism, since multiple serum dilutions are used. Most serum samples were prediluted at 1:75, regardless of the antibody concentrations, and then serially diluted to obtain accurate avidity calculations (data not shown). Since Hib IgG antibody concentrations in the absence of chaotrope were not affected by the additional incubation step with PBS-Tween 20 buffer (r = 0.99), this methodology can be used to determine not only the antibody concentration but also the relative serum antibody avidity. We also define a conversion mechanism that enables comparison of data collected by either elution method (single or multiple chaotrope concentrations), therefore facilitating comparisons between previous and current studies. There is a need for simple and rapid avidity assays that are ELISA based for assessment of antibody avidity. In the past, comparative studies of Hib vaccine formulations and follow-up investigations of vaccine failures have benefited from avidity determinations (1, 4, 14, 22). In addition, there is new interest in the evaluation of memory induced after conjugate vaccination using antibody avidity as a potential marker (11). This study provides a simple methodology that should facilitate the evaluation of antibody avidity elicited by Hib conjugate vaccines.
Acknowledgments
P. Gomez de Leon was the recipient of a sabbatical grant from the Universidad Nacional Autónoma de México for a six-month internship at the CDC. Willie Spear was a participant in the Oak Ridge Institute of Technology training fellowship at the CDC.
We thank Henry Baggett and Lisa Bulkow of the Arctic Investigations Program for surveillance data and serum samples.
REFERENCES
- 1.Amir, J., X. Liang, and D. M. Granoff. 1990. Variability in the functional activity of vaccines induced antibody to Haemophilus influenzae type b. Pediatr. Res. 27:358-364. [DOI] [PubMed] [Google Scholar]
- 2.Antilla, M., J. Eskola, H. Åhman, and H. Käyhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 3.Black, S. B., H. R. Shinefield, P. Ray, E. M. Lewis, B. Fireman, R. Hiatt, D. C. Madore, C. L. Johnson, J. G. Hackell, et al. 1993. Safety of combined oligosaccharide conjugate Haemophilus influenzae type b (HbOC) and whole cell diphtheria-tetanus toxoids-pertussis vaccine in infancy. Pediatr. Infect. Dis. J. 12:981-985. [DOI] [PubMed] [Google Scholar]
- 4.Breukels, M. A., E. M. Jol-van der Zijde, M. J. D. vanTol, and G. T. Rijkers. 2002. Concentration and avidity of anti-Haemophilus influenzae type b (Hib) antibodies in serum samples obtained from patients for whom Hib vaccination failed. Clin. Infect. Dis. 34:191-197. [DOI] [PubMed] [Google Scholar]
- 5.Bulkow, L. R., R. B. Wainwright, G. W. Letson, S. J. Chang, and J. I. Ward. 1993. Comparative immunogenicity of four Haemophilus influenzae type b conjugate vaccines in Alaska Native infants. Pediatr. Infect. Dis. J. 12:484-492. [DOI] [PubMed] [Google Scholar]
- 6.Carlone, G. M., B. A. Perkins, T. Popovic, N. Rosenstein, and S. Romero-Steiner. 2002. Haemophilus influenzae type B, Neisseria meningitidis, Streptococcus pneumoniae, and Corynebacterium diphtheriae vaccines, p. 418-431. In N. R. Rose, R. G. Hamilton, and B. Detrick (ed.), Manual of clinical laboratory immunology, 6th ed. American Society for Microbiology, Washington, D.C.
- 7.Centers for Disease Control and Prevention. 1996. Progress toward elimination of Haemophilus influenzae type B disease among infants and children—United States, 1987-1995. Morb. Mortal. Wkly. Rep. 45:901-906. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2002. Summary of notifiable diseases—United States, 2000. Morb. Mortal. Wkly. Rep. 49:1-102. [PubMed] [Google Scholar]
- 9.Fernandez, J., S. Balter, J. Feris, E. Gómez, Z. Garib, P. L. Castellanos, J. Sánchez, S. Romero-Steiner, and O. S. Levine. 2000. Randomized trial of the immunogenicity of fractional-dose regimens of PRP-T Haemophilus influenzae type b conjugate vaccine. Am. J. Trop. Med. Hyg. 62:485-490. [DOI] [PubMed] [Google Scholar]
- 10.Frasch, C. E. 1995. Haemophilus influenzae type b conjugate and combination vaccines. Clin. Immunother. 4:376-386. [Google Scholar]
- 11.Goldblatt, D., A. R. J. P. M. Pinto Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming to Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 177:1112-1115. [DOI] [PubMed] [Google Scholar]
- 12.Gray, B. M., and D. R. Shaw. 1993. Artifacts with the thiocyanate elution method for estimating relative antibody avidity. J. Immunol. Methods 157:269-271. [DOI] [PubMed] [Google Scholar]
- 13.Harlow, E., and D. Lane. 1998. Using antibodies: a laboratory manual, 1st ed., p. 23-37. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 14.Hetherington, S. V., and A. F. Rutkowski. 1990. Antibody affinity in infants after immunization with conjugated capsular polysaccharide from Haemophilus influenzae type b. J. Infect. Dis. 162:1185-1188. [DOI] [PubMed] [Google Scholar]
- 15.Madore, D., P. Anderson, B. D. Baxter, G. M. Carlone, K. M. Edwards, R. G. Hamilton, P. Holder, H. Käyhty, D. C. Phipps, C. C. A. Peeters, R. Schneerson, G. R. Siber, J. I. Ward, and C. E. Frasch. 1996. Interlaboratory study evaluating quantitation of antibodies to Haemophilus influenzae type b polysaccharide by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 3:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madore, D.V., C.L. Johnson-Kraines, E. P. Rothstein, D. H. Smith, et al. 1999. Kinetics of antibody response to Haemophilus influenzae type b vaccines. Curr. Med. Res. Opin. 15:105-112. [DOI] [PubMed] [Google Scholar]
- 17.Plikaytis, B., P. F. Holder, L. B. Pais, S. E. Maslanka, L. L. Gheesling, and G. M. Carlone. 1994. Determination of parallelism and non-parallelism in bioassay dilution curves. J. Clin. Microbiol. 32:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Steiner, S., D. M. Musher, M. S. Cetron, L. B. Pais, J. E. Groover, A. E. Fiore, B. D. Plikaytis, and G. M. Carlone. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Steiner, S., J. Fernandez, C. Biltoft, M. E. Wohl, J. Feris, S. Balter, O. S. Levine, and G. M. Carlone. 2001. Functional antibody activity elicited by fractional doses of Haemophilus influenzae type b conjugate vaccine (polyribosylribitol phosphate-tetanus conjugate). Clin. Diagn. Lab. Immunol. 8:1115-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Steiner, S., W. Spear, N. Brown, P. Holder, T. Hennessy, P. Gomez de Leon, and G. M. Carlone. 2004. Measurement of serum bactericidal activity specific for Haemophilus influenzae type b by using a chromogenic and fluorescent metabolic indicator. Clin. Diagn. Lab. Immunol. 11:89-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlesinger, Y., D. M. Granoff, and the Vaccine Study Group. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 267:1489-1494. [PubMed] [Google Scholar]
- 23.Trotter, C., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 24.van Dam, G. J., A. F. M. Verheul, G. J. W. J. Zigterman, M. J. de Reuver, and H. Snippe. 1989. Estimation of the avidity of antibodies in polyclonal antisera against Streptococcus pneumoniae type 3 by inhibition ELISA. Mol. Immunol. 26:269-274. [DOI] [PubMed] [Google Scholar]