Abstract
The mechanisms by which probiotic bacteria exert their effects on the immune system are not completely understood, but the epithelium may be a crucial player in the orchestration of the effects induced. In a previous work, we observed that some orally administered strains of lactic acid bacteria (LAB) increased the number of immunoglobulin A (IgA)-producing cells in the small intestine without a concomitant increase in the CD4+ T-cell population, indicating that some LAB strains induce clonal expansion only of B cells triggered to produce IgA. The present work aimed to study the cytokines induced by the interaction of probiotic LAB with murine intestinal epithelial cells (IEC) in healthy animals. We focused our investigation mainly on the secretion of interleukin 6 (IL-6) necessary for the clonal expansion of B cells previously observed with probiotic bacteria. The role of Toll-like receptors (TLRs) in such interaction was also addressed. The cytokines released by primary cultures of IEC in animals fed with Lactobacillus casei CRL 431 or Lactobacillus helveticus R389 were determined. Cytokines were also determined in the supernatants of primary cultures of IEC of unfed animals challenged with different concentrations of viable or nonviable lactobacilli and Escherichia coli, previously blocked or not with anti-TLR2 and anti-TLR4. We concluded that the small intestine is the place where a major distinction would occur between probiotic LAB and pathogens. This distinction comprises the type of cytokines released and the magnitude of the response, cutting across the line that separates IL-6 necessary for B-cell differentiation, which was the case with probiotic lactobacilli, from inflammatory levels of IL-6 for pathogens.
Lactobacillus and Bifidobacterium are normal inhabitants of the human adult gastrointestinal tract. Selected strains from these genera are found in many dairy products, since these microorganisms are regarded as probiotics due to their capacity to improve some biological functions in the host (13, 25). Despite their intensive use, the mechanisms by which probiotic bacteria exert their effects are not completely understood, but the epithelium may be a crucial player in the orchestration of the effects induced (29). Complex interactions occur between probiotic bacteria and the different constituents of the intestinal ecosystem (resident microflora and epithelial and immune cells) (54). These interactions play a major role in the development and maintenance of the activity of the gut-associated lymphoid tissue, including immunoglobulin A (IgA) secretion and CD4+ and CD8+ T-cell activation (16, 42, 45). In previous work, we observed that some orally administered strains of lactic acid bacteria (LAB) were able to increase the number of IgA-producing cells in the small intestines of mice in a dose-dependent manner (4, 42). The increase in the number of IgA+ cells was not always correlated with an increase in the number of CD4+ T cells, indicating that some LAB strains assayed induced the clonal expansion only of B cells triggered to produce IgA (56). It was reported that intestinal epithelial cells (IEC) may be an important source of interleukin 6 (IL-6) to enhance local mucosal IgA+ B-cell responses (18). We also demonstrated that the adherent population of Peyer's patches was responsible for the production of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (44). In our laboratory, we recently showed that the probiotic strain Lactobacillus casei CRL 431 can interact with small-intestine epithelial cells (SIEC) as whole cells, but only their antigenic fragments are able to internalize into SIEC for the subsequent production of cytokines (31). The epithelial cells of the intestinal tract are thought to participate in the onset and regulation of the mucosal immune response to bacteria, especially pathogens, by interacting with the immune cells of the Peyer's patches, lymphoid tissue in the lamina propria of the gut, and the intraepithelial lymphocytes (27). Much of the research on the interactions of lactic acid bacteria with epithelial cells has been conducted on commercial cell lines, such as A-498, J82, T84, HT-29, or CaCo-2. The response of such cloned cells may give valuable information; however, they may not reflect the in vivo situation as clearly as newly isolated cells (3) due to the complexity of the intestinal ecosystem and the high number of microorganisms present in it in healthy animals. The immune system detects microorganisms by discriminating between self and nonself organisms. This discrimination is achieved by a sophisticated system of receptors, the Toll-like receptors (TLRs), which provide considerable specificity for microbial pathogens and discrimination between pathogens and the host while providing an immediate-response system in the setting of danger. TLRs are expressed by myelomonocytic (macrophages and dendritic cells), endothelial, and mucosal epithelial cells, as well as cells from various organ systems (2). TLR4 recognizes lipopolysaccharide (LPS) and gram-negative bacteria, while TLR2 recognizes a variety of microbial components, such as peptidoglycan and lipoteichoic acids, from gram-positive bacteria (1, 50). However, it was reported that when peptidoglycan is purified, it does not stimulate TLR2 (53). Instead, N-acetylmuramyl-l-alanyl-d-isoglutamine (MDP) and peptidoglycan seem to stimulate NOD proteins in the inside of the cell (33). Whereas much has been learned during recent years about the importance of TLRs in the process of immune recognition by myeloid cells, such as macrophages or dendritic cells, the role of TLR expression by epithelial cells is still not completely understood (5). IEC have been shown to secrete a variety of inflammatory cytokines and chemokines after stimulation by pathogenic bacteria. Isolated normal IEC and IEC lines have been shown to produce IL-6 in response to bacterial infection (36). IL-6 is a multifunctional cytokine involved in diverse biological processes, such as host response to enteric pathogens, acute-phase reactions, hematopoiesis, growth factor for normal or neoplastic cells, and terminal differentiation of B lymphocytes (7, 17, 22, 57). IL-6 has traditionally been considered the product of proinflammatory cells (39). However, IL-6 is also known to possess several anti-inflammatory characteristics, such as its ability to down-regulate LPS-induced monocyte IL-1 and TNF-α mRNA expression (36). The aim of this work was to study, by in vitro and ex vivo assays, the cytokines induced by the interaction of two noncommensal (exogenous) lactobacilli, with demonstrated probiotic activity on the immune system, with murine SIEC and large-intestine epithelial cells (LIEC) (primary cell cultures) in healthy animals. We focused our study mainly on the secretion of IL-6 necessary for the clonal expansion of B cells previously observed with probiotic bacteria. The role of TLRs in such interaction was also addressed. This work is the first to report that IEC, stimulated by LAB, generate immune signals to immune cells of the lamina propria, increasing the bidirectional signals from the immune cells to IEC after nonpathogenic-antigen stimulation.
MATERIALS AND METHODS
Animals and bacterial strains.
Six- to 8-week-old BALB/c female mice weighing from 20 to 25 g were obtained from Charles River (Montreal, Canada). Each experimental sample consisted of a group of five mice housed in plastic cages kept in a controlled atmosphere (temperature, 22 ± 2°C; humidity 55% ± 2%) with a 12-h light/dark cycle. The mice were maintained and treated in accordance with the guidelines of the Canadian Council on Animal Care.
For this work, we used two lactobacillus strains whose immunomodulating capacities had been previously studied in a murine model (34, 43). Overnight cultures (37°C; aerobic conditions) of Lactobacillus casei CRL 431, a human isolate, and Lactobacillus helveticus R389, isolated from Swiss cheese, were obtained in Man-Rogosa-Sharpe (MRS) broth (Difco, Becton Dickson and Company, Sparks, MD). Escherichia coli MM295, a nonpathogenic human isolate, was also used in this study. The strain was isolated, identified, and kindly provided by the Department of Biology of the University of Moncton. Overnight cultures (37°C; aerobic conditions) of E. coli MM295 were obtained in Luria-Bertani broth (Difco, Becton Dickson and Company, Sparks, MD).
Feeding procedures.
Animals received a viable or heat-inactivated (65°C; 30 min) lactobacillus suspension in their drinking water for 2, 5, or 7 consecutive days. Overnight cultures (5 ml) of each strain were suspended in 5 ml sterile 12% nonfat milk and diluted in phosphate-buffered saline (PBS; pH 7.4) solution (Sigma-Aldrich, St. Louis, MO) to a final concentration of 107 cells/ml. The daily suspension intake of lactic acid bacteria was 3.1 ± 0.3 ml/day/mouse. All groups of mice received simultaneously a conventional balanced diet ad libitum. A control group received the same conventional balanced diet but with 12% nonfat milk diluted in PBS (1:30) instead of the LAB suspension.
Primary culture of mouse small- and large-intestine epithelial cells.
Preparation of primary cultures of enterocytes was performed as described previously (48), slightly modified as follows. At the end of each period of the oral administration of lactobacillus suspensions (ex vivo assays) or for the in vitro assays, animals were anesthetized and sacrificed by cervical dislocation. The small and large intestines were removed and placed in Hanks' balanced salt solution (HBSS) (Sigma-Aldrich, St. Louis, MO) containing glucose (2%; Sigma-Aldrich), penicillin (100 U/ml; Sigma-Aldrich), and streptomycin (0.1 mg/ml; Sigma-Aldrich). The intestines were flushed six times with 10 ml of the same buffer, cut into 2- to 3-mm fragments, and collected in HBSS. The large intestine was treated with 5 mM dithiothreitol (Sigma) for 15 min at 37°C to remove the mucus. Then, both the small and large intestines were digested in 20 ml of HBSS containing collagenase (300 U/ml; Sigma-Aldrich C-7657) and dispase (0.1 mg/ml; Gibco, Grand Island, NY) at 25°C and 150-rpm agitation for 45 min and 60 min, respectively. Digestion was stopped by the addition of 20 ml of Dulbecco's modified Eagle medium without phenol red (Gibco) supplemented with heat-inactivated fetal bovine serum (10%; ATCC, Manassas, VA), epidermal growth factor (10 ng/ml; U.S. Biological, Swampscott, Mass.), insulin-transferrin-selenium-A (2.50 μg/ml, 0.55 μg/ml, and 1.68 pg/ml, respectively) from a 100× ready-to-use solution (Gibco), penicillin (100 U/ml; Sigma-Aldrich), and streptomycin (0.1 mg/ml; Sigma-Aldrich). Large fragments were removed using gravity by allowing them to settle (2 min) at the bottom of the flask. The supernatant was transferred to centrifuge tubes and centrifuged for 3 min at 300 rpm. The pellet was washed twice with the culture medium and finally resuspended in the same culture medium at a concentration of 4 × 105 to 6 × 105 organoids (single cells or IEC cluster)/ml. IEC suspensions were then transferred to 96-well cell culture plates (200 μl/well) and incubated for 8 h (37°C; 5% CO2). An in vitro toxicology assay kit, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) based (Sigma-Aldrich), and trypan blue (0.4%) exclusion were used to assess cell viability. Supernatants were recovered for cytokine determination. IL-1α, IFN-γ, IL-6, IL-12, IL-4, and IL-10 were determined using the corresponding mouse IL-X or IFN-γ enzyme-linked immunosorbent assay set (BD OptEIA; BD Biosciences Pharmingen, San Diego, CA).
Resistance of lactobacillus strains to acidic conditions, bile salts, and lysozyme.
Lactobacillus strains were grown overnight in MRS broth (pH 6.8), washed twice with PBS (pH 7.4), and adjusted to 108 cells/ml. The cultures were then resuspended in a 0.3% pepsin (Sigma)-0.5% sodium chloride solution at pH 3 and kept at 37°C for 1 h. The cultures were then centrifuged (4,000 rpm; 15 min; 4°C), washed with PBS, resuspended in a 0.3% bile bovine (Sigma) solution, and kept at 37°C for 1 h. The cultures were finally centrifuged (4,000 rpm; 15 min; 4°C), washed with PBS, resuspended in a 0.1-mg/ml lysozyme (Fluka Sigma) solution, and kept at 37°C for 1 h. Control cultures in PBS were also included. At the beginning of the experiment and after each treatment (pepsin-low pH, bile, or lysozyme), colony counts on MRS agar (37°C; 48 h; AnaeroGen; Oxoid, Basingstoke, England) were carried out to determine cell viability.
In vitro assays for cytokine determination.
In vitro assays for cytokine determination were performed for the two lactobacillus strains and E. coli. The effects of LPS and MDP were also studied. Primary cultures of IEC from untreated animals were used. IEC suspensions were obtained as described above from animals without any treatment. Overnight cultures of L. casei CRL 431, L. helveticus R389, and E. coli MM295 were centrifuged (10,000 rpm; 10 min; 4°C), washed twice with PBS, and resuspended to 109 cells/ml. Viable or heat-inactivated (100°C; 10 min) bacterial suspensions were added to the IEC suspensions to the following final concentrations: 108, 107, 106, and 105 cells/ml. LPS (Sigma-Aldrich) was added to the following final concentrations: 10, 1, 0.1, and 0.01 μg/ml. MDP (Sigma-Aldrich) was added to the following final concentrations: 1,000, 500, 50, and 10 μg/ml. IEC suspensions were incubated for 8 h (37°C; 5% CO2), and supernatants were recovered for cytokine determination as explained above for ex vivo assays.
Blocking of IEC with anti-TLR4 or anti-TLR2 antibodies and challenge with bacterial strains.
Blocking and challenge experiments were performed particularly for IL-6 determination. IEC suspensions from untreated mice were obtained as described above and incubated for 30 min at room temperature in the presence of 40 μg/ml (10 times the blocking concentration suggested by the manufacturer) of functional grade purified anti-mouse TLR4/MD2, anti-mouse TLR2 (eBioscience, San Diego, CA), or Dulbecco's modified Eagle medium (control). After the blocking period with anti-TLRs, viable or heat-inactivated (100°C; 10 min) lactobacillus and E. coli suspensions were added to a final concentration of 107 and 106 cells/ml, respectively. LPS was used as a positive control at a final concentration of 0.1 μg/ml. The IEC suspensions were incubated for 8 h (37°C; 5% CO2), and supernatants were recovered and kept frozen until IL-6 quantification.
Immunofluorescence study of TLR2 expression after lactobacillus feeding.
An immunohistochemistry assay previously described (8), slightly modified this time, was used to study the expression of TLR2 in IEC after lactobacillus feeding. After 2, 5, and 7 consecutive days of administration of viable or heat-inactivated lactobacilli, IEC suspensions were prepared as described above. Twenty microliters of each cell suspension was placed in 10-well immunofluorescence slides. The cells were allowed to dry (37°C; 45 min) and fixed with formalin (ICC fixation buffer; BD Biosciences PharMingen, San Diego, CA). Fixed IEC were stained with 25 μg/ml of fluorescein isothiocyanate-conjugated anti-mouse TLR2 (eBiosciences) (37°C; 30 min), followed by two washes with PBS. Fixed cells of the small intestine were mounted and examined by three independent observers using a fluorescent-light microscope.
Statistical analysis.
The results for each experimental point are the mean of three independent experiments. Data were analyzed using the one-way analysis of variance procedure of SPSS software. The differences among means were detected by Duncan's multiple-range test (SPSS software). Data were considered significantly different when the P value was <0.05.
RESULTS
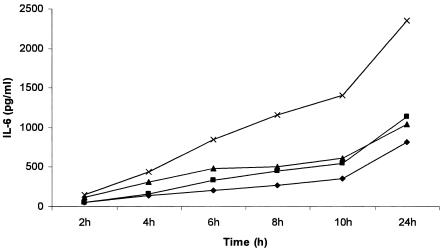
The collagenase-dispase digestion of the small and large intestines yielded intestinal cells mostly in large clumps, but also as single cells or pairs of cells. The viability of fresh isolated cells was higher than 90%, based on trypan blue exclusion. Figure 1 shows the course in time of IL-6 production by SIEC and LIEC in the presence of LPS. From 6 to 24 h of culture, the levels of IL-6 production by control and challenged (LPS) cells were significantly different (P < 0.05). After culture for 8 h, 32 to 36% of the cells remained viable as revealed by the MTT toxicology assay kit. This level of viability was maintained even after culture for 48 h. An 8-hour incubation period was chosen as the culture time for all subsequent experiments.
FIG. 1.
Time course of LPS-induced IL-6 response by IEC. IL-6 production by small (▪) and large (×) IEC challenged with 0.1 μg/ml LPS compared to unchallenged controls (⧫ for SIEC and ▴ for LIEC).
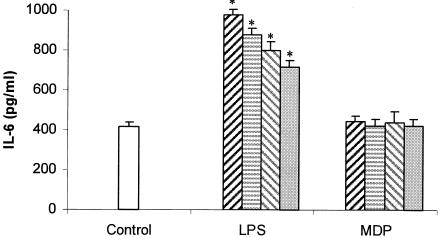
IL-6 production when SIEC from untreated mice were challenged with different concentrations of LPS or MDP is shown in Fig. 2. SIEC showed unresponsiveness toward increasing concentrations of MDP (from 10 to 500 μg/ml), whereas LPS steadily enhanced the IL-6 secretion as its concentration increased from 0.01 to 10 μg/ml.
FIG. 2.
Effects of the concentration of LPS and MDP on IL-6 production. In vitro effects of 10 (dark hatched bar), 1 (striped bar), 0.1 (light hatched bar), or 0.01 (shaded bar) μg/ml of LPS and 500 (dark hatched bar), 200 (striped bar), 100 (light hatched bar), or 10 (shaded bar) μg/ml of MDP on the production of IL-6 by SIEC. *, significantly different from control (P < 0.05). The error bars indicate standard deviations for 3 determinations per mouse.
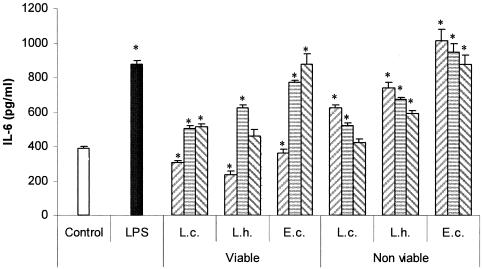
Figure 3 shows the profiles of IL-6 production by SIEC from untreated animals that were cocultured for 8 h with different concentrations (108, 107, or 106 cells/ml) of viable or heat-inactivated cultures of lactobacilli or E. coli and LPS. For the highest concentration of viable lactobacilli and E. coli assessed (108 cells/ml), a significant diminution of IL-6 production by SIEC was observed. The viability of these cells was similar to that of control cells (according to the MTT test); however, the pH of the culture medium dropped from 7.2 to approximately 5.9 in all cases due to the metabolic activity of viable cultures during the incubation period. In order to determine whether this diminution on IL-6 production could also be due to the proteolytic activity of lactobacilli (especially the highly proteolytic L. helveticus R389) on IEC, we performed the same experiment, this time comparing L. helveticus R389 with its nonproteolytic mutant L. helveticus L89 (34). A profile of IL-6 production similar to the one already observed for L. helveticus R389 was observed for its nonproteolytic mutant (data not shown). Viable cultures showed an IL-6 production profile different from the one found with nonviable cultures. For the former, a peak in IL-6 production was observed at 107 cells/ml for Lactobacillus and at 106 cells/ml for E. coli, whereas for nonviable cultures, the highest concentration of lactobacilli assayed (108 cells/ml) was the most effective for IL-6 production by SIEC. Viable and nonviable E. coli cells showed high levels of IL-6 production (Fig. 3).
FIG. 3.
Effects of the concentration of bacteria on IL-6 production by SIEC. In vitro effects of viable or heat-inactivated L. casei CRL 431 (L.c.), L. helveticus R389 (L.h.), and E. coli (E.c.) at different concentrations, 108 (left hatched bars), 107 (striped bars), or 106 (right hatched bars) cells/ml, on the production of IL-6 by SIEC. LPS was used as a positive control (0.1 μg/ml). *, significantly different from control (P < 0.05). The error bars indicate standard deviations.
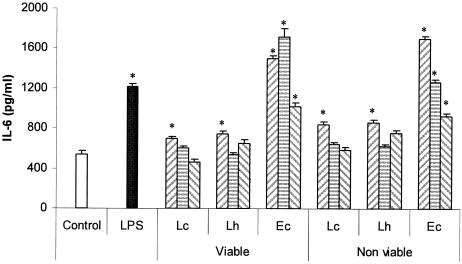
Figure 4 shows the pattern of IL-6 production by LIEC from untreated mice that were cocultured for 8 h with different concentrations (107, 106, or 105 cells/ml) of viable or heat-inactivated cultures of lactobacilli or E. coli and LPS. Due to the fact that 108 cells/ml of bacteria diminished IL-6 production by inhibiting IEC, that concentration was no longer used. As for the small intestine, maximum IL-6 production was observed at a bacterial concentration of 107 cells/ml for Lactobacillus and at 106 cells/ml for E. coli, whereas for nonviable cultures, the highest concentration of lactobacilli induced the highest IL-6 production by SIEC. The values obtained for E. coli were significantly different from the control value for both viable and nonviable cultures for all the bacterial concentrations assayed.
FIG. 4.
Effects of the concentration of bacteria on IL-6 production by LIEC. In vitro effects of viable or heat-inactivated L. casei CRL 431 (Lc), L. helveticus R389 (Lh), and E. coli (Ec) at different concentrations, 107 (left hatched bars), 106 (striped bars), or 105 (right striped bars) cells/ml, on the production of IL-6 by LIEC. LPS was used as a positive control (0.1 μg/ml). *, significantly different from control (P < 0.05). The error bars indicate standard deviations.
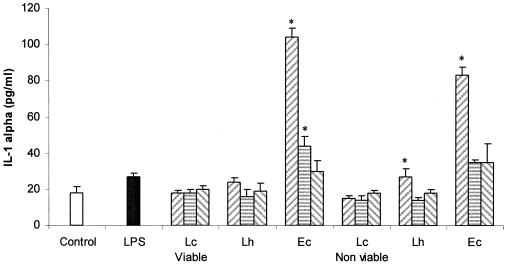
Figure 5 shows the IL-1α production by SIEC from untreated animals challenged with different concentrations of viable or heat-inactivated lactobacilli or E. coli. Only E. coli and nonviable L. helveticus were able to induce the secretion of IL-1α at the highest concentration assayed. For LIEC, no IL-1α was detected.
FIG. 5.
Effects of the concentration of bacteria on IL-1α production by SIEC. In vitro effects of viable or heat-inactivated L. casei CRL 431 (Lc), L. helveticus R389 (Lh), and E. coli (Ec) at different concentrations, 107 (left hatched bars), 106 (striped bars), or 105 (left hatched bars) cells/ml, on the production of IL-1α by SIEC. LPS was used as a positive control (0.1 μg/ml). *, significantly different from control (P < 0.05). The error bars indicate standard deviations.
No IL-4, IL-10, IFN-γ, or IL-12 was observed when small or large IEC explants were challenged with viable or heat-inactivated lactobacilli or E. coli.
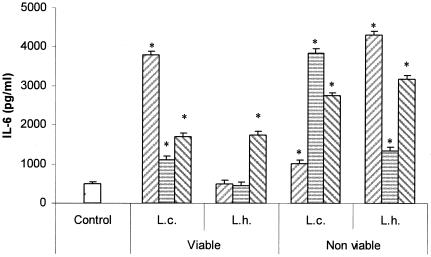
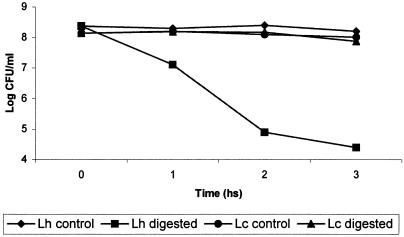
Figure 6 shows the IL-6 production by cultured (8 h) IEC from mice that received viable or nonviable suspensions of L. casei CRL 431 or L. helveticus R389 for 2, 5, or 7 consecutive days. The profiles observed for the strains were different, and so were the profiles determined for each strain when administered as viable or nonviable cultures. In general, larger amounts of IL-6 were detected in the supernatants of the IEC of mice that received nonviable cultures than in the supernatants of animals that were given viable bacteria. A remarkable fact is that the ex vivo production of IL-6 was much higher than the values observed in vitro, but IL-10 was detected neither in in vitro nor in ex vivo cultures of SIEC. When we compared levels of IL-6 production after the administration of viable cultures of lactobacilli, L. casei induced a higher IL-6 production than L. helveticus. In order to determine whether this difference could be related to the different resistances of the strains to the biological barriers (low pH of stomach, pepsin, bile, and lyzosyme) found during gastrointestinal transit, we performed a simulated in vitro digestion of the strains. Figure 7 shows the results obtained: a 4-log-unit loss in cell viability was observed for L. helveticus R389, whereas a negligible loss in cell viability (less than 1 log unit) was determined for L. casei CRL 431.
FIG. 6.
Effects of the oral administration of lactobacilli on IL-6 production by SIEC. Ex vivo effects of the administration of viable and nonviable cultures of Lactobacillus casei CRL 431 (L.c.) and Lactobacillus helveticus R389 (L.h.) for 2 (left hatched bars), 5 (striped bars), or 7 (right striped bars) consecutive days on the production of IL-6 by SIEC. *, significantly different from control (P < 0.05). The error bars indicate standard deviations.
FIG. 7.
Resistance of lactobacilli to simulated gastrointestinal digestion. Effects of acidic conditions, bile salts, and lysozyme exposure on the cell viability of L. casei CRL 431 (Lc) and L. helveticus R389 (Lh). Viable cells were exposed for 1 h to a solution of pepsin-NaCl, pH 3, followed by 1 h of incubation in the presence of bile, and finally, the cells were treated with lysozyme for 1 h.
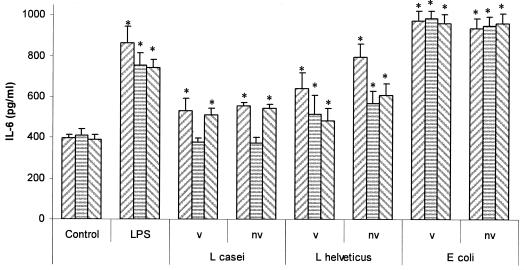
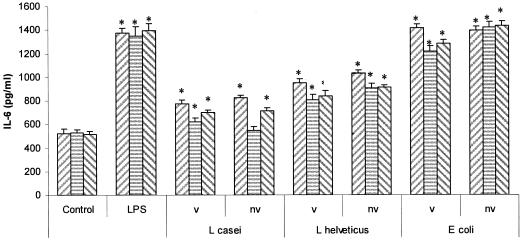
Figure 8 shows the results of the culture of viable or nonviable lactobacilli or E. coli with SIEC that had been previously treated with anti-TLR2 or anti-TLR4. The basal production of IL-6 by IEC was not affected by the incubation with 40 μg/ml of each anti-TLR prior to coculture with bacteria. For viable or nonviable L. casei CRL 431, anti-TLR2 completely inhibited IL-6 production by SIEC, while anti-TLR4 had no effect on IL-6 secretion compared to the untreated control. For viable or heat-inactivated L. helveticus R389, both anti-TLRs only partially inhibited IL-6 production by SIEC compared to the untreated control. Moreover, the partial inhibition was also statistically significant (P < 0.05) compared to the untreated sample (SIEC treated only with viable or nonviable L. helveticus R389), except for SIEC treated with anti-TLR2 and viable L. helveticus R389. On the other hand, IL-6 production by SIEC challenged with E. coli was not affected when the cells were preincubated with anti-TLRs. Finally, Fig. 9 shows the IL-6 production by LIEC cocultured with viable or nonviable lactobacilli or E. coli after preincubation with anti-TLR2 or anti-TLR4. Only anti-TLR2 was able to block IL-6 secretion when IEC were challenged with nonviable L. casei. On the other hand, nonviable E. coli, as well as LPS, induced the same levels of IL-6 in anti-TLR-treated cells than when the bacterium was added directly to freshly isolated cells. For all the other cases (viable and nonviable L. helveticus), only a partial inhibition of IL-6 was observed for anti-TLR2 or anti-TLR4. Moreover, this partial inhibition was also statistically significant compared to the untreated sample (SIEC treated only with viable or nonviable L. helveticus R389).
FIG. 8.
Involvement of TLRs in the interactions of bacteria with SIEC. In vitro effects of viable (v) or nonviable (nv) L. casei CRL 431 (107 cells/ml), L. helveticus R389 (107 cells/ml), and E. coli (106 cells/ml) on the production of IL-6 by SIEC previously treated or not (left hatched bars) with anti-TLR2 (striped bars) or anti-TLR4 (right hatched bars). *, significantly different from control (P < 0.05). The error bars indicate standard deviations.
FIG. 9.
Involvement of TLRs in the interactions of bacteria with LIEC. In vitro effects of viable (v) or nonviable (nv) L. casei CRL 431 (107 cells/ml), L. helveticus R389 (107 cells/ml), and E. coli (106 cells/ml) on the production of IL-6 by LIEC previously treated or not (left hatched bars) with anti-TLR2 (striped bars) or anti-TLR4 (right hatched bars). *, significantly different from control (P < 0.05). The error bars indicate standard deviations.
The ex vivo immunofluorescence assays carried out to evaluate the effects of L. casei CRL 431 or L. helveticus R389 administration showed no differences with respect to the controls in the expression of TLR2. No differences in fluorescence (three independent observers) were detected in IEC isolated from the small intestines of mice that received viable or nonviable lactobacillus cultures for 2, 5, or 7 consecutive days compared to controls.
DISCUSSION
The gut microflora is undoubtedly important in supporting a functional yet balanced immune system; the processes that lead to this balance can be emulated by transiently colonizing the gastrointestinal tract with appropriate strains of microbes that are delivered orally as probiotics and that influence the host microflora and immune functions of the immune cells associated with the gut (9). In spite of the amount of knowledge about cytokine release already obtained using cancerous cell lines, their main limitation is the fact that they are probably not subject to the same control mechanisms as normal cells are. Even though enzymatically dissociated normal cells fail to attach and survive, they are useful in suspension for short-term experiments (28). Additionally, it has been shown that intestinal murine cells may respond very rapidly (even within 1 h) to a number of stimuli in vitro (6, 46). On this basis, we decided to use a primary culture of normal small- and large-intestine epithelial cells for a better understanding of how probiotic lactobacilli influence their activities. This mouse model of intestinal cells had been previously characterized, and it has been reported to maintain all the key features of the normal intact intestinal mucosa. In this work, the characteristics of cell isolates and cell viability of primary cultures were in accordance with previous reports (28, 30, 48).
We first studied the effects on IL-6 production of the antigenic interaction of the principal component of gram-positive bacterial cell walls (10), the muramyl dipeptide (MDP), with IEC compared with LPS (Fig. 1 and 2), a potent inducer of IL-6 (23) and a component of gram-negative bacterial cell walls. We demonstrated that only a high dose of MDP (1,000 μg/ml) was able to induce a small but significant increase in IL-6 production (data not shown). This result agrees with a previous report that established that up to a 1,000-fold-higher concentration of peptidoglycan than of LPS may be required to induce cytokine secretion by activated macrophages (14). The tolerance of IEC for MDP stimulation might be related to an adaptive tolerance developed in the small intestine for concomitant gram-positive bacteria to prevent inflammatory reactions.
We then turned to the study of the in vitro interaction of the two noncommensal lactobacillus strains with the IEC compared to a nonpathogenic human isolate of E. coli (Fig. 3 and 4). We observed the importance of the dose of bacteria in their viable form, as well as nonviable cultures. In our model, maximum IL-6 production was observed for both LAB strains when they were added to a concentration of 107 cells/ml. For E. coli, this maximum was observed with 106 cells/ml. It should be noted that E. coli induced a higher IL-6 response even when it was added at a 10-fold-lower concentration than that of lactobacilli. The diminution of IL-6 production when cocultured with 108 cells/ml of lactobacilli or 108 and 107 cells/ml of E. coli might be due to a diminution in the pH of the culture medium by metabolically active bacteria. For heat-inactivated cultures, IL-6 production increased as the bacterial concentration increased. Heat treatment probably affected the number of cell wall epitopes involved in cell-to-cell communication. There are controversial reports on the production of IL-6 after the contact of LAB with Caco-2 cells. A gram-positive nonpathogenic bacterium (Bacillus subtilis) was reported to increase IL-6 both in its viable form and as a chemically inactivated culture (24). On the other hand, another study reported no IL-6 found after the contact of several lactobacillus strains with Caco-2 cells cocultured with leukocytes (37). Our results using normal murine IEC showed the ability of these cells to produce IL-6 after their contact with nonpathogenic bacteria. These findings underline the importance of the use of primary culture models for a better approach to the understanding of the interaction of LAB with IEC, which is the first step in the activation of the immune cells associated with the gut.
No IL-4, IL-10, IFN-γ, or IL-12 was detected in the supernatants of IEC challenged with lactobacilli or E. coli. Our results agree with previous findings (29) in which a lactobacillus strain used at different bacterial concentrations did not by itself up-regulate mRNA for IL-10 in the human colon epithelial cell line T84, HT-29, or Caco-2. IL-10 production by IEC after stimulation with LAB was reported only in cocultures (19). In our opinion, IL-10 is not released from IEC so as not to down-regulate the immune cells associated with the gut. IL-1α, but not IL-1β, was reported to be constitutively expressed in the rat intestinal epithelial cell line IEC-6 (49). In this ex vivo assay, IL-1α (Fig. 5) was detected only for viable or heat-inactivated cultures of E. coli or with nonviable L. helveticus. The absence of IFN-γ or IL-12 ex vivo production by SIEC reveals that the interaction of nonpathogenic bacteria with IEC is unable to induce the mechanisms of an inflammatory response as pathogens do.
These in vitro findings allowed us to analyze the in vivo IL-6 levels induced in IEC after the oral administration of lactobacillus strains. The ex vivo culture of SIEC from mice fed with viable suspensions of L. casei or L. helveticus showed the higher capacity of the former to induce IL-6 production (Fig. 6). The increase in IL-6 production would allow us to understand the increase in the number of IgA+ cells in the intestinal lamina propria observed previously (34, 43), even when the CD4+ population was not affected after the oral administration of these bacteria.
The higher capacity of intestinal isolates than of LAB isolated from fermented foods to overcome in vitro simulated biological barriers has already been reported (20, 55). In this study, higher resistance to a simulated gastrointestinal transit was observed in L. casei than in L. helveticus (Fig. 7), in agreement with a previous finding (26). The intestinal origin of the L. casei strain used, compared to the dairy origin of L. helveticus, might be partly responsible for the difference observed. Cell wall structures might have been more affected by the gastric transit in L. helveticus than in L. casei. It was recently demonstrated by transmission electron microscopy (31) that the probiotic strain L. casei CRL 431 can interact with SIEC as whole cells, but only their antigenic fragments are able to internalize into SIEC for the subsequent production of cytokines by these IEC and the immune cells associated with the gut when this strain is orally administered to mice (43). The profile of IL-6 production when mice received heat-inactivated lactobacillus suspensions differed from the one observed when viable cultures were administered. For heat-inactivated cultures, L. helveticus, in general, induced a higher production of IL-6 by IEC than L. casei in a dose-dependent manner. A wide variety of stressful conditions, including heat shock, induce the synthesis of heat shock proteins. The ability of these proteins to activate immune cells to secrete proinflammatory cytokines is well documented (40) and could be a reason for the higher IL-6 production by heat-inactivated than by viable cultures.
It is known that deregulated inflammation leads to massive production of proinflammatory cytokines, such as TNF-α, IL-1, and IL-6. IL-6 has been traditionally considered as the product of proinflammatory cells (39); however, it is also known to possess several anti-inflammatory characteristics (36). It is within this context that we believe that probiotic viable bacteria (L. casei CRL 431 and L. helveticus R389) are able to deliver immune signals to IEC for the up-regulation of IL-6 production to an extent that would help B-cell differentiation into plasmocytes without reaching inflammatory levels that could cause intestinal damage. When compared to the gram-negative E. coli, the strains of lactobacilli used in this study induced lower levels of IL-6, probably just those necessary for B-cell differentiation into plasma cells in the absence of inflammation.
Toll-like receptors are a family of transmembrane receptors that recognize repetitive patterns, i.e., pathogen-associated molecular patterns, present in diverse microbes, including gram-positive and gram-negative bacteria. TLR4 is required for the recognition of LPS, whereas TLR2 is required for the recognition of gram-positive microorganisms, among others (35). Therefore, we aimed at determining whether the nonpathogenic bacterium-IEC interactions observed were taking place through TLR2 and/or TLR4. In the small intestine, we observed that IL-6 production was inhibited when viable or nonviable cultures of L. casei CRL 431 interacted with IEC previously treated with anti-mouse TLR2 antibody (Fig. 8). This fact would suggest that L. casei CRL 431-IEC interaction is achieved completely through TLR2. However, for L. helveticus R389, only a partial inhibition of IL-6 production was observed in anti-TLR2-coated IEC. This fact suggests that this strain has other epitopes involved in the interaction with IEC (29). As expected, the interaction between E. coli and SIEC was not affected by anti-TLR2. Anti-TLR2-coated IECs were still sensitive to LPS. This could be due to the fact that TLR2 also recognizes several atypical types of LPS whose chemical structures are close to traditional LPS from enterobacteria (50). We wanted, then, to determine the participation of TLR4 in LAB-IEC interaction in the small intestine. As expected, we observed that anti-TLR4 did not interfere with the interaction between L. casei CRL 431 and IEC. However, the effect of L. helveticus R389 (viable or nonviable) on IL-6 production was partially blocked by anti-TLR4. This fact suggests that this strain displays cell wall structures partially resembling ligands that bind TLR4. The partial inhibition of IL-6 production in anti-TLR4-treated IEC challenged with LPS could be due to the TLR4-independent recognition of LPS (52). In the large intestine (Fig. 9), only the interaction between L. casei and IEC was blocked by anti-TLR2, similar to what was observed for SIEC. In all other cases, LIEC were responsive to the presence of LAB or E. coli even when coated with anti-TLR2 or anti-TLR4. It was recently reported (32) that the gram-negative Helicobacter pylori activates innate immunity via TLR2 but not via TLR4, as expected for gram-negative pathogens. Moreover, receptors other than TLRs could sense the presence of bacteria in this highly populated ecological niche or there could be less exclusivity between TLR2 and TLR4 and some gram-positive and gram-negative bacterial strains, respectively.
The intestinal epithelium is continually confronted by a variety of commensal bacteria and pathogen-associated molecular patterns. However, in the absence of pathogenic bacteria, the lamina propria maintains a controlled state of inflammation through the development of a careful system of controlled TLR expression (35). The expression of TLRs has been reported to increase during the course of infections (15, 47, 51) or inflammatory bowel disease (8). Although TLR2 is normally present in epithelial cells, it plays a limited role in inflammation. It may be activated under conditions in which bacterial cell wall concentrations within the intestine are pathologically high (38, 41).
We demonstrated that probiotic LAB, even when they are gram-positive microorganisms, can use receptors other than TLR2 to send immune signals to IEC (such as TLR4, as for L. helveticus R389), and the immune signals released would be closer to those of inflammation. However, the in vitro findings do not always correlate well with the in vivo results, since both lactobacillus strains are able to increase the number of IgA+ cells in the small-intestine lamina propria without evoking an inflammatory response, as was reported in previous works (34, 56). The fact that L. helveticus R389 binds to TLR4 might be involved in better protection against enteric infections by competition with pathogens for adhesion sites. The intensity of the TLR-epitope interaction was seen to depend on the antigenic concentration and the viability status of the specimen at the strain-IEC level. This work is the first to report, by in vitro and ex vivo assays with primary cultures of IEC from healthy animals, that probiotic strains with already demonstrated immunomodulating capacities can activate IEC using the same TLRs as gram-positive or gram-negative pathogens but eliciting different immune responses. Most works performed with cocultures of cell lines of IEC and LAB report that immune cells are responsible for the immune signals to IEC (21). We believe that IEC are the first line to generate immune signals to the underlying immune cells in the lamina propria. This probiotic-bacteria-IEC interaction, which releases signals from the IEC, could play a major role in the innate immune response induced by LAB. Even though the E. coli strain used was nonpathogenic, it shares many structural and surface features with the pathogenic strains of E. coli (11, 12). We conclude that the epithelial cells from the small and large intestines make a major distinction between probiotic LAB and pathogens. This distinction comprises the type of cytokines released and the magnitude of the response: for example, cutting across the line that separates “IL-6 necessary for B-cell differentiation,” which is the case for probiotic lactobacilli, from “inflammatory levels of IL-6” for pathogens.
Acknowledgments
This project was supported by the Atlantic Innovation Fund (AIF), the Atlantic Canada Opportunities Agency (ACOA), and the Natural Sciences and Engineering Research Council (NSERC) of Canada. G. Vinderola is a postdoctoral fellow of CONICET-Argentina (Consejo Nacional de Investigaciones Científicas y Técnicas).
We are grateful to Jairo Duarte for his technical assistance.
REFERENCES
- 1.Abreu, M. T., L. S. Thomas, E. T. Arnold, K. Lukasek, K. S. Michelsen, and M. Arditi. 2003. TLR signaling at the intestinal epithelial interface. J. Endotoxin Res. 9:322-330. [DOI] [PubMed] [Google Scholar]
- 2.Abreu, M. T., and M. Arditi. 2004. Innate immunity and toll-like receptors: clinical implication of basic science research. J. Pediatr. 144:421-429. [DOI] [PubMed] [Google Scholar]
- 3.Aldhous, M. C., A. N. Shmakov, J. Bode, and S. Ghosh. 2001. Characterization of conditions for the primary culture of human small intestinal epithelial cells. Clin. Exp. Immunol. 125:32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez, S., N. Gobbato, E. Bru, A. P. de Ruiz Holgado, and G. Perdigón. 1998. Specific immunity induction at mucosal level by viable Lactobacillus casei. Perspective for oral vaccine development. Food Agric. Immunol. 10:79-87. [Google Scholar]
- 5.Backhed, F., and M. Hornef. 2003. Toll-like receptor 4-mediated signaling by epithelial surfaces: necessity or threat? Microbes Infect. 5:951-959. [DOI] [PubMed] [Google Scholar]
- 6.Bai, A.-P., Q. Ouyang, W. Zhang, C.-H. Wang. and S.-F. Li. 2004. Probiotics inhibit TNF-α-induced interleukin-8 secretion of HT29 cells. World J. Gastroenterol. 10:455-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beagley, K. W., J. H. Eldridge, W. K. Aicher, J. Mestecky, S. Difabio, H. Kiyono, and J. R. McGhee. 1991. Payer's patch B cells with memory cell characteristics undergo terminal differentiation within 24 hours in response to interleukin-6. Cytokine 3:107-116. [DOI] [PubMed] [Google Scholar]
- 8.Cario, E., and D. K. Podolsky. 2000. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68:7010-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross, M. L. 2002. Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol. Med. Microbiol. 34:245-253. [DOI] [PubMed] [Google Scholar]
- 10.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Leeuwenhoek 76:159-184. [PubMed] [Google Scholar]
- 11.Dougan, G., A. Haque, D. Pickard, G. Frankel, P. O'Goara, and J. Wain. 2001. The Escherichia coli gene pool. Curr. Opin. Microbiol. 4:90-94. [DOI] [PubMed] [Google Scholar]
- 12.Dozois, C. M., and R. Curtis. 1999. Pathogenicity diversity of Escherichia coli and the emergence of ‘exotic’ islands in the gene stream. Vet. Res. 30:157-179. [PubMed] [Google Scholar]
- 13.Dunne, C., L. Murphy, S. Flynn, L. O'Mahony, S. O'Halloran, M. Feeney, D. Morrissey, G. Thornton, G. Fitzgerald, C. Daly, B. Kiely, E. M. M. Quigley, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 1999. Probiotics: from myth to reality. Demonstration of functionality in animal model of disease and in human clinical trials. Antonie Leeuwenhoek 76:279-292. [PubMed] [Google Scholar]
- 14.Erickson, K. L., and N. E. Hubbard. 2000. Probiotic immunomodulation in health and disease. J. Nutr. 130(Suppl. 25):403S-409S. [DOI] [PubMed] [Google Scholar]
- 15.Faure, E., L. Thomas, H. Xu, A. E. Medvedev, O. Equils, and M. Arditi. 2001. Bacterial lipopolysaccharide and IFN-γ induce toll-like receptor 2 and toll-like receptor 4 expression in human endothelial cells: role of NF-κB activation. J. Immunol. 166:2018-2024. [DOI] [PubMed] [Google Scholar]
- 16.Gleeson, M., and A. W. Cripps. 2004. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunol. Med. Microbiol. 42:21-23. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich, M. E., and D. W. McGee. 1998. Regulation of mucosal B cell immunoglobulin secretion by intestinal epithelial cell-derived cytokines. Cytokine 10:948-955. [DOI] [PubMed] [Google Scholar]
- 18.Goodrich, M. E., and D. W. McGee. 1999. Effect of intestinal epithelial cell cytokines on mucosal B-cell IgA secretion: enhancing effect of epithelial-derived IL-6 but not TGF-β on IgA+ B cells. Immunol. Lett. 67:11-14. [DOI] [PubMed] [Google Scholar]
- 19.Haller, D., C. Bode, W. P. Hammes, A. M. A. Pfeifer, E. J. Schiffrin, and S. Blum. 2000. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 47:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haller, D., H. Colbus, M. G. Ganzle, P. Scherenbacher, C. Bode, and W. P. Hammes. 2001. Metabolic and functional properties of lactic acid bacteria in the gastro-intestinal ecosystem: a comparative in vitro study between bacteria of intestinal and fermented food origin. Syst. Appl. Microbiol. 24:218-226. [DOI] [PubMed] [Google Scholar]
- 21.Haller, D., P. Serrant, G. Peruisseau, C. Bode, W. P. Hammes, E. Schiffrin, and S. Blum. 2002. IL-10 producing CD14low monocytes inhibit lymphocyte-dependent activation of intestinal epithelial cells by commensal bacteria. Microbiol. Immunol. 46:195-205. [DOI] [PubMed] [Google Scholar]
- 22.Hedger, S., M. Svensson, and C. Svanborg. 1992. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect. Immun. 60:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosoi, T., R. Hirose., S. Saegusa., A. Ametani., K. Kiuchi, and S. Kaminogawa. 2003. Cytokine responses of human intestinal epithelial-like Caco-2 cells to the non-pathogenic bacterium Bacillus subtilis (natto). Int. J. Food Microbiol. 82:255-264. [DOI] [PubMed] [Google Scholar]
- 25.Isolauri, E., S. Salminen, and A. C. Ouwehand. 2004. Microbial-gut interactions in health and disease. Probiotics. Best Pract. Res. Clin. Gastroenterol. 18:299-313. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen, C. N., V. R. Nielsen, A. E. Hayford, P. L. Møller, K. F. Michaelsen, A. Paerregaard, B. Sandström, M. Tvede, and M. Jakobsen. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedinger, M., K. Haffen, and P. Simon-Assmann. 1987. Intestinal tissue and cell cultures. Differentiation 36:71-85. [DOI] [PubMed] [Google Scholar]
- 29.Ma, D., P. Forsythe, and J. Bienenstock. 2004. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect. Immun. 72:5308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macartney, K. K., D. C. Baumgart, S. R. Carding, J. O. Brubaker, and P. A. Offit. 2000. Primary murine small intestinal epithelial cells, maintained in long-term culture, are susceptible to rotavirus infection. J. Virol. 74:5597-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maldonado Galdeano, C., and G. Perdigón. 2004. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 97:673-681. [DOI] [PubMed] [Google Scholar]
- 32.Mandel, L., A. P. Moran, A. Cocchiarella, J. M. Houghton, N. Taylor, J. G. Fox, T. C. Wang, and E. A. Kurt-Jones. 2004. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via Toll-like receptor 2 but not Toll-like receptor 4. Infect. Immun. 72:6446-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuguchi, T., A. Takagi, T. Matsuzaki, M. Nagaoka, K. Ishikawa, T. Yokohura, and Y. Yoshikai. 2003. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 10:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matar, C., J. C. Valdez, M. Medina, M. Rachid, and G. Perdigón. 2001. Immunomodulating effect of milks fermented by Lactobacillus helveticus and its non-proteolytic variant. J. Dairy Res. 68:601-609. [DOI] [PubMed] [Google Scholar]
- 35.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implication for host-microbial interaction in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 36.Miller, T. L., and D. W. McGee. 2002. Epithelial cells respond to proteolytic and non-proteolytic detachment by enhancing interleukin-6 responses. Immunology 105:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita, H., F. He, T. Fuse, A. C. Ouwehand, H. Hashimoto, M. Hosoda, K Mizumachi, and J.-I. Kurisaki. 2002. Adhesion of lactic acid bacteria to Caco-2 cells and their effect on cytokine secretion. Microbiol. Immunol. 46:293-297. [DOI] [PubMed] [Google Scholar]
- 38.Naik, S., E. J. Kelly, L. Meijer, S. Pettersson, and I. R. Sanderson. 2001. Absence of toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J. Pediatr. Gastroenterol. Nutr. 32:449-453. [DOI] [PubMed] [Google Scholar]
- 39.Ng, E. K., N. Panesar, W. E. Longo, M. J. Shapiro, D. L. Kaminski, K. C. Tolman, and J. E. Mazuski. 2003. Human intestinal epithelial and smooth muscle cells are potent producers of IL-6. Mediators Inflamm. 12:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is putative endogenous ligand of the toll-like receptor-4 complex. J. Biol. Chem. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 41.Otte, J. M., E. Cario, and D. K. Podolsky. 2004. Mechanisms of cross hyporesponsiveness to toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126:1054-1070. [DOI] [PubMed] [Google Scholar]
- 42.Perdigón, G., R. Fuller, and R. Raya. 2001. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2:27. [PubMed] [Google Scholar]
- 43.Perdigón G., S. Alvarez, and A. Pesce de Ruiz Holgado. 1991. Immunoadjuvant activity of oral Lactobacillus casei: influence of dose on the secretory immune response and protective capacity in intestinal infections. J. Dairy Res. 58:485. [DOI] [PubMed] [Google Scholar]
- 44.Perdigón, G., C. Maldonado Galdeano, J. C. Valdez, and M. Medici. 2002. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 56:S21-S26. [DOI] [PubMed] [Google Scholar]
- 45.Rhee, K. J., P. Sethupathi, A. Driks, D. K. Lanning, and K. L. Knight. 2004. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 172:1118-1124. [DOI] [PubMed] [Google Scholar]
- 46.Sennikov, S. V., V. Temchura. V. A. Kozlov, and V. A. Trufakin. 2002. The influence of conditioned medium from mouse intestinal epithelial cells on the proliferative activity of crypt cells: role of granulocyte-macrophage colony-stimulating factor. J. Gastroenterol. 37:1048-1051. [DOI] [PubMed] [Google Scholar]
- 47.Shuto, T., A. Imasato, H. Jono, A. Sakai, H. Xu, T. Watanabe, D. D. Rixter, H. Kai, A. Andalibi, F. Linthicum, et al. 2002. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced toll-like receptor 2 expression via a negative cross-talk with p38 MPA kinase. J. Biol. Chem. 277:17263. [DOI] [PubMed] [Google Scholar]
- 48.Slorach E. M., F. C. Campbell, and J. R. Dorin. 1999. A mouse model of intestinal stem cell function and regeneration. J. Cell Sci. 112:3029-3038. [DOI] [PubMed] [Google Scholar]
- 49.Stadnyk, A., G. R. Sisson, and C. C. M. Waterhouse. 1995. IL-1α is constitutively expressed in the rat intestinal epithelial cell line IEC-6. Exp. Cell Res. 220:298-303. [DOI] [PubMed] [Google Scholar]
- 50.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 51.Tötemeyer, S., N. Foster, P. Kaiser, D. J. Maskell, and C. E. Bryant. 2003. Toll-like receptor expression in C3H/HeN and C3H/HeJ mice during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 71:6653-6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Triantafilou, K., M. Triantafilou, and R. L. Dedrick. 2001. A CD14-independent LPS receptor cluster. Nat. Immunol. 2:338-359. [DOI] [PubMed] [Google Scholar]
- 53.Uehori, J., M. Matsumoto, S. Tsuji, T. Akazawa, O. Takeuchi, S. Akira, T. Kawata, I. Azuma, K. Tuyoshima, and T. Seya. 2003. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis Calmette-Guerin peptidoglycan. Infect. Immun. 71:4238-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umesaki, Y., and H. Setoyama. 2000. Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes Infect. 2:1343-1351. [DOI] [PubMed] [Google Scholar]
- 55.Vinderola, C. G., and J. A. Reinheimer. 2003. Lactic acid starter and probiotic bacteria: a comparative in vitro study of probiotic characteristics and biological barrier resistance. Food Res. Intern. 36:895-904. [Google Scholar]
- 56.Vintiñi, E., S. Alvarez, M. Medina, M. Medici, M. V. de Budeguer, and G. Perdigón. 2000. Gut mucosal immunostimulation by lactic acid bacteria. Biocell 23:223. [PubMed] [Google Scholar]
- 57.Weinstein D. L., B. L. O'Neill, and E. S. Metcalf. 1997. Salmonella typhi stimulation of human intestinal epithelial cell induces secretion of epithelial cell-derived interleukin-6. Infect. Immun. 65:395-404. [DOI] [PMC free article] [PubMed] [Google Scholar]