Abstract
The value of West Nile virus immunoglobulin G avidity for distinguishing recent from past infection was investigated using 348 follow-up specimens from 170 viremic blood donors. Low avidity accurately indicated infection within the previous 4 months. However, due to rapid avidity maturation in some individuals, high avidity did not accurately indicate past infection.
New cases of West Nile virus (WNV) infection continue to occur in geographic regions of the United States that first experienced WNV infections in previous years. In these areas of endemicity, a laboratory test is occasionally needed to distinguish current-season from prior-season WNV infection. WNV immunoglobulin M (IgM) detection is not useful for this application, since the antibody may persist for more than a year in some patients (14). WNV IgA follows a persistence pattern similar to that of IgM and is thus not helpful for distinguishing recent from past WNV infection (11).
In other viral infections, IgG avidity has proven to be a valuable tool for distinguishing recent from past infection (1, 2, 8-10, 12, 13, 15). Defined as the strength with which IgG attaches to antigen, IgG avidity matures with time following primary infection (2). When assessing cytomegalovirus (CMV) IgG avidity in pregnant women, for example, a high avidity result for a sample collected during the first trimester indicates that primary infection most likely occurred prior to conception, with little chance of CMV infection of the fetus (1, 10). Within the flavivirus family, dengue virus IgG avidity has proven useful for discriminating primary from secondary infections (6). We thus investigated the utility of WNV IgG for distinguishing recent from past infection. These studies utilized a panel of follow-up specimens collected from WNV-infected blood donors identified by WNV RNA detection (5). The viremic (RNA-positive) period begins within a few days of exposure and typically lasts about 20 days (3); WNV viremia is thus an excellent indicator of infection within the previous 4 weeks.
Specimens.
WNV RNA-positive blood donors were identified by nucleic acid amplification test screening of donations in 2003 and 2004 (4, 5). Plasma from donations confirmed as WNV RNA positive (hereafter referred to as the index donations), as well as plasma or serum specimens collected during follow-up visits, was supplied by the Blood Systems Research Institute and American Red Cross Blood Services. Informed consent was obtained from all donors at the local blood donation site; protocols for nucleic acid amplification test screening and follow-up were approved by local institutional review boards and the Food and Drug Administration.
Methods.
Specimens were tested for WNV IgG using a Food and Drug Administration-cleared enzyme-linked immunosorbent assay kit (Focus Diagnostics, Cypress, CA) (7, 11). IgG avidity was measured using this same enzyme-linked immunosorbent assay kit with a modified procedure (10). IgG-positive serum or plasma specimens were diluted per the package insert and added to duplicate microtiter wells. After an hour at room temperature, the well contents were discarded. Kit wash buffer was then added to one of each pair of duplicate wells, whereas dissociating buffer (kit wash buffer containing 6 M urea [ICN, Aurora, Ohio]) was added to the other well. After 5 min at room temperature, the well contents were discarded and the wash procedure was repeated (including the 5-minute incubation step). All wells were washed once more with kit wash buffer; the assay was then completed as described in the kit insert, and absorbance at 450 nm was measured. For a given specimen, the avidity ratio (AR) was calculated by dividing the absorbance value obtained for the well washed with urea buffer by the absorbance value obtained for the well washed with kit wash buffer.
Findings.
Two samples with markedly different AR values (0.20 and 0.69) in the first avidity assay run were included in all 11 subsequent runs. The mean AR ± standard deviation over these 12 assay runs was 0.17 ± 0.02 for the first sample and 0.68 ± 0.04 for the second sample; the interassay coefficient of variation values were thus 12% and 6%, respectively. Intra-assay coefficient of variation values (eight replicates tested within a single assay run) were 6% and 4%, respectively.
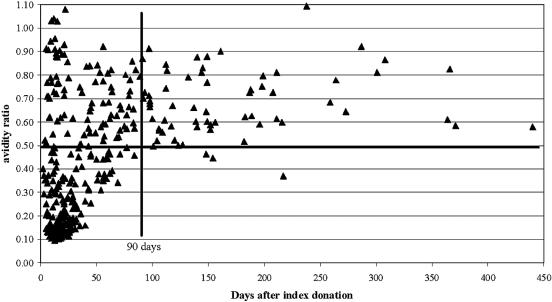
Figure 1 shows the distribution of WNV IgG avidity values for 348 follow-up specimens from 170 viremic blood donors, plotted as a function of days postindex. Two major observations were apparent from visual examination of Fig. 1: (i) nearly all samples collected >90 days postindex exhibited AR values of ≥0.50, and (ii) nearly all samples with AR values of <0.50 were collected ≤90 days postindex. Based on these findings, an AR of ≥0.50 was defined as high avidity and an AR of <0.50 was defined as low avidity. The somewhat unexpected finding evident in Fig. 1 was that many samples collected ≤90 days postindex exhibited high avidity. The exact numbers of samples in the four quadrants evident in Fig. 1 were as follows: 95 high-avidity samples collected ≤90 days postindex, 61 high-avidity samples collected >90 days postindex, 3 low-avidity samples collected >90 days postindex, and 189 low-avidity samples collected ≤90 days postindex. Thus, of 64 samples collected >90 days postindex, 61 (95%) exhibited high WNV IgG avidity. Of 192 samples with low WNV IgG avidity, 189 (98%) were collected ≤90 days postindex. These 189 low-avidity specimens represented 67% of the 284 specimens collected ≤90 days postindex; i.e., 33% (95/284) of samples collected during this time frame exhibited high WNV IgG avidity.
FIG. 1.
WNV IgG AR values for follow-up specimens plotted as a function of days postindex. The dark solid vertical line indicates 90 days postindex, and the dark solid horizontal line indicates an AR value of 0.50.
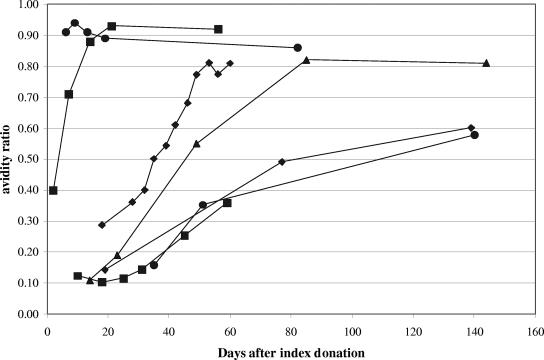
Figure 2 presents AR results for serial follow-up specimens from seven donors. In donors whose initial follow-up sample exhibited low avidity, AR values increased steadily over time; there was no apparent fluctuation around the AR value used to define low versus high avidity (0.50). Once high avidity was attained, there was no decrease to low avidity.
FIG. 2.
WNV IgG AR values in serial samples from selected donors. Each line series represents a separate donor.
The index (WNV RNA-positive) specimens from 36 of the 170 donors included in the study were positive for WNV IgG. Avidity testing of these samples showed that 28 exhibited low avidity (AR range, 0.10 to 0.45) and 8 exhibited high avidity (AR range, 0.54 to 0.65).
A cluster of 41 specimens with high WNV IgG avidity collected during the first 25 days of follow-up is evident in Fig. 1. The WNV IgG status of the index donations from the 20 donors contributing these 41 follow-up specimens was investigated. Ten index donations were WNV IgG negative, five were IgG positive with low avidity, and five were IgG positive with high avidity; thus, donors contributing early follow-up samples with high IgG avidity were heterogeneous with respect to the WNV IgG result of the index specimen.
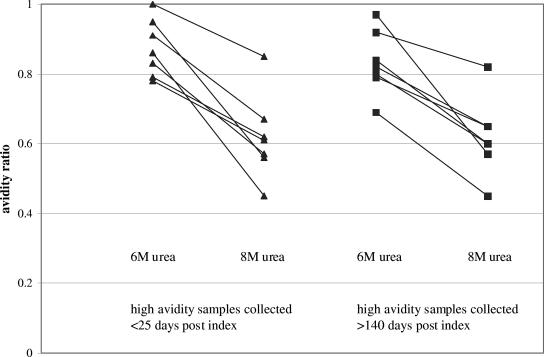
We next asked if using dissociating buffer containing more urea would distinguish early-follow-up high-avidity samples from late-follow-up high-avidity samples. Seven samples with AR values of >0.70 collected <25 days postindex and seven samples with similar AR values collected >140 days postindex were retested in parallel using routine dissociating buffer (6 M urea) and 8 M urea as dissociating buffer. As expected, the distributions of AR values obtained using 6 M urea were similar in the early-follow-up and late-follow-up groups (Fig. 3); likewise, the distributions of AR values obtained using 8 M urea were similar in the two groups. When the group means obtained using a given urea concentration were compared, no significant differences were observed (data not shown). Thus, more stringent dissociating conditions did not distinguish early-follow-up high-avidity samples from late-follow-up high-avidity samples.
FIG. 3.
Influence of an increased urea concentration on high AR values in relation to time of sample collection (days postindex). A solid line connects the value obtained using 6 M urea and the value obtained using 8 M urea for a given specimen.
Comments.
Our findings show that a low WNV IgG avidity index is a potentially useful adjunct to existing serologic assays for identifying WNV infection within the previous 4 months; 98% of follow-up samples with low avidity were collected from viremic blood donors within 90 days (3 months) of the index specimen, and the index specimen was collected within a month of infection (3).
Surprisingly, 33% of follow-up samples collected within 90 days of the index specimen exhibited high IgG avidity. Particularly noteworthy was a cluster of high-avidity specimens collected ≤25 days postindex. We hypothesized that these specimens were collected from the small percentage of viremic blood donors whose index specimens already contained WNV IgG; however, only half the donors contributing early-follow-up samples with high avidity were IgG positive at index. Next we hypothesized that early-follow-up and late-follow-up samples with similar high avidities when using 6 M urea would become distinguishable (i.e., early-follow-up sample avidities lower than late-follow-up sample avidities) using 8 M urea as dissociating buffer. However, the experimental results did not support this hypothesis; samples with similar AR distributions using 6 M urea also showed similar AR distributions using 8 M urea. Taken together, these findings indicate that WNV IgG avidity matures very rapidly in some donors following exposure to the virus. The explanation for this phenomenon remains unknown; one possibility is that these individuals were exposed to another flavivirus in the past and produce high-avidity WNV IgG as part of an anamnestic response to common flavivirus antigens. Further studies measuring neutralizing antibodies to a battery of flaviviruses in index and follow-up specimens are required to test this hypothesis; insufficient sample volumes precluded our conducting such studies using the existing specimen panel.
Measurement of WNV IgG avidity could be a useful tool for determining if WNV infection should be considered in the differential diagnosis for selected patients from areas of endemicity. For example, an elderly South Dakota resident presents in September with recent onset of fever and altered mental status; serologic tests detect both WNV IgM and WNV IgG, but the WNV IgM index of 1.93 (reference range of <0.90) is lower than typically observed in patients with recent WNV infection. A low WNV IgG avidity result would provide strong support for recent infection, indicating that WNV neuroinvasive disease should be included in the differential diagnosis. However, a high WNV IgG avidity value would not be useful for determining the time since WNV infection. The clinical utility of WNV IgG avidity data thus differs from the clinical utility of avidity results for other viral infections, where high avidity essentially excludes the possibility of recent infection. It will be important to ensure that clinicians and laboratorians understand this difference so that WNV IgG avidity results are appropriately interpreted.
REFERENCES
- 1.Bodeus, M., S. Feyder, and P. Goubau. 1998. Avidity of IgG antibodies distinguishes primary from nonprimary cytomegalovirus infection in pregnant women. Clin. Diagn. Lab. Immunol. 9:9-16. [DOI] [PubMed] [Google Scholar]
- 2.Bottinger, B., and I. P. Jensen. 1997. Maturation of rubella IgG avidity over time after acute rubella infection. Clin. Diagn. Virol. 8:105-111. [DOI] [PubMed] [Google Scholar]
- 3.Busch, M. P., and S. L. Stramer. 2005. Viremia and antibody studies in WNV infected blood donors. Centers for Disease Control and Prevention West Nile virus website. [Online.] www.cdc.gov/ncidod/dvbid/westnile/conf/February_2005.htm. Accessed 8 March 2005.
- 4.Centers for Disease Control and Prevention. 2004. Update: West Nile virus screening of blood donations and transfusion-associated transmission—United States, 2003. Morb. Mortal. Wkly. Rep. 53:281-284. [PubMed] [Google Scholar]
- 5.Custer, B., P. A. Tomasulo, E. L. Murphy, S. Caglioti, D. Harpool, P. McEvoy, and M. P. Busch. 2004. Triggers for switching from minipool testing by nucleic acid technology to individual-donation nucleic acid testing for West Nile virus: analysis of 2003 data to inform 2004 decision making. Transfusion 44:1547-1554. [DOI] [PubMed] [Google Scholar]
- 6.de Souza, V. A, S. Fernandes, E. S. Araujo, A. F. Tateno, O. M. Oliveira, R. R. Oliveira, and S. C. Pannuti. 2004. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J. Clin. Microbiol. 42:1782-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogrefe, W. R., R. Moore, M. Lape-Nixon, M. Wagner, and H. E. Prince. 2004. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J. Clin. Microbiol. 42:4641-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanno, A., and Y. Kazuyama. 2002. Immunoglobulin G antibody avidity assay for serodiagnosis of hepatitis C virus infection. J. Med. Virol. 68:229-233. [DOI] [PubMed] [Google Scholar]
- 9.Morrow, R. A., D. Friedrich, E. Krantz, and A. Wald. 2004. Development and use of a type-specific antibody avidity test based on herpes simplex virus type 2 glycoprotein G. Sex. Transm. Dis. 31:503-515. [DOI] [PubMed] [Google Scholar]
- 10.Prince, H. E., and A. L. Leber. 2002. Validation of an in-house assay for cytomegalovirus immunoglobulin G (CMV IgG) avidity and relationship of avidity to CMV IgM levels. Clin. Diagn. Lab. Immunol. 9:824-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince, H. E., L. H. Tobler, M. Lape-Nixon, G. A. Foster, S. L. Stramer, and M. P. Busch. 2005. Development and persistence of West Nile virus-specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J. Clin. Microbiol. 43:4316-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puchhammer-Stockl, E., B. Schmied, A. Rieger, M. Sarcletti, M. Geit, R. Zangerle, and H. Hofmann. 2005. Low proportion of recent human immunodeficiency virus (HIV) infections among newly diagnosed cases of HIV infection as shown by the presence of HIV-specific antibodies of low avidity. J. Clin. Microbiol. 43:497-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson, P., S. Beynon, R. Whybin, C. Brennan, U. Vollmer-Conna, I. Hickie, and A. Lloyd. 2003. Measurement of EBV-IgG anti-VCA avidity aids the early and reliable diagnosis of primary EBV infection. J. Med. Virol. 70:617-623. [DOI] [PubMed] [Google Scholar]
- 14.Roehrig, J. T., D. Nash, B. Maldin, A. Labowitz, D. A. Martin, R. S. Lanciotti, and G. L. Campbell. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg. Infect. Dis. 9:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roque-Afonso, A. M., L. Grangeot-Keros, B. Roquebert, D. Desbois, J. D. Poveda, V. Mackiewicz, and E. Dussaix. 2004. Diagnostic relevance of immunoglobulin G avidity for hepatitis A virus. J. Clin. Microbiol. 42:5121-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]