Abstract
The shift in cytokine profile during human immunodeficiency virus (HIV) disease progression is influenced by dehydroepiandrosterone sulfate (DHEAS) level. Radioimmunoassay was used to measure plasma DHEAS for 30 treatment-naïve HIV-infected and 30 uninfected individuals. There was a significant negative correlation of viral load with DHEAS level (P < 0.05). Further studies of the use of DHEAS levels for monitoring HIV patients economically are warranted.
Progression of human immunodeficiency virus (HIV) infection is marked by severe immunosuppression, especially during the advanced stages, as a result of selective depletion of CD4 lymphocytes. Primary HIV infection is characterized by detectable humoral and cellular immune responses. During progression to AIDS, there is a shift from a TH1 to a TH2 cytokine profile (3). It has been suggested that this shift in cytokine profile is in part influenced by the increase in the production of cortisol and the reduction of dehydroepiandrosterone (DHEA) (4). The level of change in the cortisol/DHEA ratio could be predictive of progression to AIDS in HIV-infected individuals (2). Studies have shown significant relationships among the CD4 cell count, the development of cachexia, and levels of serum cortisol and DHEA (1). The present study was done on an Indian population to investigate the relationship between the decline in levels of dehydroepiandrosterone sulfate (DHEAS) and HIV infection progression as well as HIV-1 viral load.
Blood samples were collected from 30 treatment-naïve HIV-infected individuals (24 men and 6 women; median age, 33 years; range, 22 to 55 years) and 30 normal healthy age- and sex-matched individuals (24 men and 6 women; median age, 35 years; range, 22 to 58) after obtaining informed consent. Blood samples were drawn between 8 and 10 a.m. on the days of sampling. None of the HIV-infected or the normal healthy individuals had Cushing's syndrome or Addison's disease. The HIV-1 viral load and CD4+ and CD8+ T-cell estimations were done for all the HIV-infected individuals using standard methods.
A commercially available radioimmunoassay (Diagnostic Products Corporation) was used to measure DHEAS concentration. The assay was carried out as per the manufacturer's instructions. Briefly, in polypropylene tubes coated with antibodies to DHEAS, ligands in the patient samples compete with 125I-labeled DHEAS. After incubation and separation of the bound from the free form, the tubes were read in a gamma counter (Wallac; GMI, Inc., Minnesota). The counts were inversely related to the amount of DHEAS in the sample. A calibration curve was then used to quantify the DHEAS in the sample. Viral loads were determined using Amplicor HIV-1 Monitor test v1.5. The CD4+ T-cell counts were measured by flow cytometry for 14 individuals (Becton Dickinson, San Jose, CA) and by the Capcellia assay (Bio-Rad, Paris, France) for the remaining 16 samples. For the correlation analysis between DHEAS and CD4 cell counts, only those 14 patients whose CD4 cell counts were measured by flow cytometry were used.
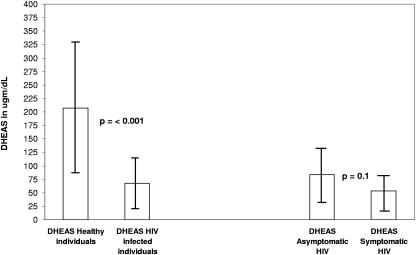
Among the 30 HIV-infected individuals, 16 were asymptomatic, and 13 were symptomatic (one of the individuals' clinical status was not known). The asymptomatic HIV-1-infected individuals had an HIV-1 RNA copy number of 5.2 log10, while the symptomatic individuals had an RNA copy number of 5.5 log10. The mean DHEAS level ± standard deviation in the normal healthy individuals was 207 ± 123 μg/dl (median, 170 μg/dl), and in HIV-infected individuals it was 68 ± 47 μg/dl (median, 61 μg/dl). The mean DHEAS level ± standard deviation among the asymptomatic individuals was 83.5 ± 52 μg/dl (median, 79 μg/dl), and that in the symptomatic individuals was 53 ± 33 μg/dl (median, 46 μg/dl). The difference between the levels of healthy individuals and those of HIV-infected individuals was significant (P < 0.001; Kruskal-Wallis H). The data are shown in Fig. 1. The difference in DHEAS levels between asymptomatic and symptomatic HIV-1-infected individuals was not significant (P = 0.1; Kruskal-Wallis H). There was a trend towards positive correlation between levels of DHEAS and those of CD4+ T cells, with an r value of 0.28, though it was not significant (P > 0.05; regression analysis, F = 1.01).
FIG. 1.
Mean DHEAS levels and standard deviations for healthy and HIV-infected individuals.
The study reported here clearly showed a significantly low level of DHEAS among the Indian HIV-1-infected individuals compared to that of the age- and sex-matched healthy individuals. Symptomatic HIV-1-infected individuals had a lower level of DHEAS than asymptomatic individuals, though the difference was not significant. There was a significant negative correlation between the viral load and the DHEAS level (r = −0.6, P < 0.05) among HIV-infected individuals as a group. Among the asymptomatic group, there was a significant negative correlation between DHEAS and viral load levels (r = −0.55, P < 0.05), whereas for the symptomatic group, it was not significant (r = −0.11, P > 0.05). This may be explained by the DHEAS level dropping below the threshold of any linear relationship to viral loads, with the immune/endocrine system being compromised.
Studies have shown low DHEAS levels to be of negative prognostic value during the course of HIV infection (5). Moreover, it has also been shown that the initiation of antiretroviral therapy can induce an increase in circulating DHEAS (5). The increased DHEAS level may be directly inhibitory to HIV viral replication, as it has been shown that DHEA protects against acute lethal viral infection and can inhibit the HIV-1 latency reactivation (6). There are some recent reports on lower DHEAS levels in HIV- and hepatitis C virus-coinfected patients than in HIV-monoinfected individuals (7). Schifitto et al. have shown that in HIV-1-infected individuals with low plasma levels of DHEAS, there is a trend towards a TH2 cytokine profile (10).
Previous studies have been done in areas where subtype B is predominant. The present study was conducted in an area where subtype C is the predominant strain (9). It is now recognized that subtype C will be the globally predominant HIV-1 type (8). From our study, it appears that the changes in DHEAS levels are more a consequence of immune/endocrine function decline and that they are unrelated to the infecting virus subtype. Further studies are required to explore the use of plasma DHEAS levels with CD4+ T-cell estimation (U.S. $14) for monitoring HIV patients, especially those on therapy. The DHEAS assay (U.S. $9) is 1/10 the cost of viral load estimation (U.S. $89) and may be a useful surrogate marker for economical management of patients. Presently, commercial enzyme-linked immunosorbent assay reagents have become available, making DHEAS measurements possible in laboratories attached to secondary-level hospitals. Most secondary-level hospitals in countries like India currently have facilities for enzyme-linked immunosorbent assay to screen for blood-borne viruses. This also obviates the need for expensive equipment for radioimmunoassay applications. In countries like India, physicians prescribing antiretroviral therapy find three monthly viral load measurements more expensive than therapy for a year. The argument in favor of using DHEAS measurement for monitoring successful antiretroviral therapy is its low cost. Further cohort-based studies need to be undertaken.
REFERENCES
- 1.Christeff, N., E. A. Nunez, and M. L. Gougeon. 2000. Changes in cortisol/DHEA ratio in HIV-infected men are related to immunological and metabolic perturbations leading to malnutrition and lipodystrophy. Ann. N. Y. Acad. Sci. 917:962-970. [DOI] [PubMed] [Google Scholar]
- 2.Christeff, N., N. Gherbi, O. Mammes, M. T. Dalle, S. Gharakhanian, O. Lortholary, J. C. Mechior, and E. A. Nunez. 1997. Serum cortisol and DHEA concentrations during HIV infection. Psychoneuroendocrinology 22(Suppl. 1):S11-S18. [DOI] [PubMed] [Google Scholar]
- 3.Clerici, M., and G. M. Shearer. 1993. A TH1 to TH2 switch is a critical step in the etiology of HIV infection. Immunol. Today 14:107-111. [DOI] [PubMed] [Google Scholar]
- 4.Clerici, M., M. Bevilacqua, T. Vago, M. L. Villa, G. M. Shearer, and G. Norbiato. 1994. An immunoendocrinological hypothesis of HIV infection. Lancet 343:1552-1553. [DOI] [PubMed] [Google Scholar]
- 5.Ferrando, S. J., J. G. Rabkin, and L. Poretsky. 1999. Dehydroepiandrosterone sulfate (DHEAS) and testosterone: relation to HIV illness stage and progression over one year. J. Acquir. Immune Defic. Syndr. 22:146-154. [DOI] [PubMed] [Google Scholar]
- 6.Loria, R. M., T. H. Inge, S. S. Cook, A. K. Szakal, and W. Regelson. 1988. Protection against acute lethal viral infections with the native steroid dehydroepiandrosterone (DHEA). J. Med. Virol. 26:301-314. [DOI] [PubMed] [Google Scholar]
- 7.Mauboussin, J. M., A. Mahamat, H. Peyriere, I. Rouanet, P. Fabbro-Peray, J. P. Daures, and D. Vincent. 2004. Low plasma levels of dehydroepiandrosterone sulphate in HIV-positive patients coinfected with hepatitis C virus. HIV Med. 5:151-157. [DOI] [PubMed] [Google Scholar]
- 8.Osmanov, S., C. Pattou, N. Walker, B. Schwardlander, J. Esparza and the WHO-UNAIDS Network for HIV Isolation and Characterization. 2002. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J. Acquir. Immune Defic. Syndr. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 9.Ramalingam, S., R. Kannangai, T. S. Vijayakumar, D. Mathai, O. C. Abraham, S. Subramanian, P. Rupali, M. V. Jesudason, and G. Sridharan. 2005. Subtype & cytokine profiles of HIV infected individuals from south India. Indian J. Med. Res. 121:226-234. [PubMed] [Google Scholar]
- 10.Schifitto, G., M. P. McDermott, T. Evans, T. Fitzgerald, J. Schwimmer, L. Demeter, and K. Kieburtz. 2000. Autonomic performance and dehydroepiandrosterone sulfate levels in HIV-1-infected individuals: relationship to TH1 and TH2 cytokine profile. Arch. Neurol. 57:1027-1032. [DOI] [PubMed] [Google Scholar]