Abstract
Induction of effective immune responses may help prevent cancer progression. Tumor-specific antigens, such as those of human papillomaviruses involved in cervical cancer, are targets with limited intrinsic immunogenicity. Here we show that immunization with low doses (106 infectious units/dose) of a recombinant human adenovirus type 5 encoding a fusion of the E7 oncoprotein of human papillomavirus type 16 to the carboxyl terminus of the surface antigen of hepatitis B virus (HBsAg) induces remarkable E7-specific humoral and cellular immune responses. The HBsAg/E7 fusion protein assembled efficiently into virus-like particles, which stimulated antibody responses against both carrier and foreign antigens, and evoked antigen-specific kill of an indicator cell population in vivo. Antibody and T-cell responses were significantly higher than those induced by a control adenovirus vector expressing wild-type E7. Such responses were not affected by preexisting immunity against either HBsAg or adenovirus. These data demonstrate that the presence of E7 on HBsAg particles does not interfere with particle secretion, as it occurs with bigger proteins fused to the C terminus of HBsAg, and results in enhancement of CD8+-mediated T-cell responses to E7. Thus, fusion to HBsAg is a convenient strategy for developing cervical cancer therapeutic vaccines, since it enhances the immunogenicity of E7 while turning it into an innocuous secreted fusion protein.
Tumor cells of certain types of cancer express proteins, designated as tumor-specific antigens (TSAs), which are not present in nontumor cells. In neoplasias caused by oncoviruses, such as cervical cancers associated with human papillomavirus type 16 (HPV-16) and liver cancers caused by the hepatitis B and C viruses, the viral proteins represent TSAs. A natural mechanism for elimination of chronically infected or transformed cells is activation of cytotoxic T lymphocytes (CTLs) specific for the viral proteins. However, such proteins, are in general weak immunogens and do not induce adequate activation of antigen-specific T cells.
The E6 and E7 products of HPV-16 induce transformation by blocking p53 and retinoblastoma (Rb)-mediated cell cycle control pathways, respectively, and by activating cyclins E and A (44). These proteins are constitutively expressed, albeit at low levels, in preneoplastic as well as cancer tissues and, therefore, represent persistent TSAs. Several lines of evidence suggest that E7 may be an effective immunological target for vaccines against oncogenic HPVs. Cell-mediated immunity to E7 has been demonstrated in HPV-mediated intraepithelial lesions of the uterine cervix (2, 31). Cytolytic T cells to HPV-16 E7 have been found in the blood of women with HPV-16-positive cervical neoplasia (20), and lymphoproliferative responses to E7 were found to inversely correlate with viral load (21). In addition, most cervical intraepithelial lesions caused by HPV regress spontaneously, and the phenomenon is accompanied by macrophage and CD4+ T-cell infiltration (12, 18). Further, preclinical studies have shown that immunization with HPV-16 E7 in various forms elicits CTL responses and protection against tumor cells expressing E7 in mice (10).
At present there is no vaccine against HPV. While prophylactic vaccines using virus-like particles (VLPs) from oncogenic HPVs are under advanced clinical testing (22, 40), formulations intended for the immunotherapy of either incipient or advanced neoplasia showed discrete effects (5, 14, 16, 27, 36). Therefore, methods to develop therapeutic vaccines need to be explored. One way to enhance the immunogenicity of tumor-specific proteins for vaccination purposes may be fusion to an innocuous but highly antigenic protein, such as the small envelope protein of hepatitis B virus (HBV). HBV is unique among animal viruses because infected cells secrete high levels of 22-nm VLPs, which are thought to be used by the virus to sequester circulating antibodies, thus hindering neutralization of infectious virions (15). The small envelop protein [HBV surface antigen, or HBsAg(S)] is the major constituent of HBV VLPs. HBsAg(S) is an integral membrane protein, which has the capacity to self-assemble into empty particles without participation of other viral proteins (11). Because of its intrinsic immunogenic potential, recombinant HBsAg(S) is used worldwide as vaccine against HBV.
HBsAg(S) VLPs have been used as carriers of viral envelop epitopes (8, 29, 30) and as antigens of the malaria parasite (41). The external hydrophilic loop of HBsAg(S) near its major B cell epitope, the “a” determinant, was a preferred site for insertion of foreign antigens. However, antibody rather than T-cell responses was obtained against epitopes inserted at this position, most likely due to suboptimal display of the foreign antigens and restricted CTL induction by this domain. Recently, major histocompatibility complex class I (MHC-I)-restricted CTL responses to HBsAg and HBsAg carrying human immunodeficiency virus epitopes have been primed by DNA vaccines and VLPs (19, 34). Yet the ability of HBsAg to enhance the immunogenicity of tumor antigens has not been explored. In this work we sought to develop an adenovirus (Ad)-based HPV-16 E7 vaccine in which the immunogenicity of E7 was enhanced at the time that its oncogenic capacity was blocked by fusion to an immunogenic integral membrane protein such as HBsAg. Our results show that C-terminal fusion of E7 to HBsAg does not interfere with the ability of this protein to assemble into VLPs and that vaccination with low doses of recombinant Ad encoding HBsAg/E7 fusion proteins induces effective E7-specific antibody and T-cell responses.
MATERIALS AND METHODS
Constructions, plasmid, and virus vectors.
Plasmids pIRES-neo2 (BD Clontech, Heidelberg) containing the HPV-16 E7 (EE7) and EE7Δ1-35 (lacking amino acids 1 to 35) synthetic genes were described elsewhere (4). The HBsAg(S)16EE7 and HBsAg(S)16EE7Δ1-35 fusion genes were generated by PCR cloning as follows. The HBsAg(S) gene was amplified from plasmid pRc/CMV-HBs(S) (obtained through Aldevron Inc. from R. G. Whalen) (6) using the oligonucleotide pair 5′-CTCGAGGATTGGGGA-3′ and 5′-GATATCAATGTATACCCAAAGA-3′. The resulting fragment containing a complete HBsAg(S) gene with no termination codon but an EcoRV site instead was inserted at the EcoRV site into the multiple cloning site of plasmid pIRES-neo2. EE7 and EE7Δ1-35 were amplified using the sense oligonucleotides 5′-GATATCGAGGAGGACGAGATCGA-3′ and 5′-GATATCATGCACGGCGACA-3′, respectively, and the antisense oligonucleotide 5′-GATATCTTACTTGTCGTCGTCGTCCT-3′ for both. The EE7 and EE7Δ1-35 fragments were cloned in frame downstream to the HBsAg(S) sequence at the EcoRV site. Recombinant Ad-HBsAg(S)16EE7 and Ad-HBsAg(S)EE7Δ1-35 were constructed using the Adeno-X expression system (BD Clontech, Heidelberg, Germany), purified, and titrated, as indicated by the manufacturer. In brief, the fusion genes were cloned into the plasmid pShuttle. The resulting expression cassettes were excised with the restriction enzymes I-CeuI and PI-SceI and inserted into the adenovirus genome in the plasmid pAdeno-X, which contains one copy of the recombinant Ad type 5 genome with E1 and E3 deleted. The E1 functions can be complemented in HEK 293T cells. Recombinant Ad-HBsAg(S)16EE7 and Ad-HBsAg(S)EE7Δ1-35 vectors were packaged into infectious, replication-incompetent adenovirus particles by transfecting low-passage HEK 293 cells after linearization of the plasmid with PacI. Isolated viral plaques were amplified on HEK 293T cells, and recombinant adenovirus was harvested and purified. Viral titers in infectious units (IFU)/ml were determined using the BD Adeno-X-Virus Purification and Adeno-X-Rapid Titer kits. The Ad-E7wt vector (where wt is wild-type) (32) was kindly provided by T. Kowalik. Ad-LacZ (serotype 5) was from Q-Biogene. For expression in the yeast Pichia pastoris, the HBsAg(S)16EE7 and HBsAg(S)EE7Δ1-35 fusion genes were amplified devoid of stop codon and fused in frame with a six-His tag in plasmid pPICZ(A) (Invitrogen). All constructs and cloning steps were verified by sequencing using external primers hybridizing with nearby plasmid sequences.
Cell lines and transfections.
Vero and HeLa cells were grown in Dulbecco's modified Eagle's medium, and RMA cells were grown in RPMI 1640 medium, with both media containing glutamax and 1 g/liter of glucose (Invitrogen) supplemented with 10% fetal bovine serum (FBS). HEK 293T cells (BD Clontech) were propagated in Dulbecco's modified Eagle's medium containing 4.5 g/liter of glucose supplemented with various amounts of FBS (2 to 10%) in order to modulate the speed of cell growth. All cells were maintained at 37°C under 5% CO2 atmosphere. Cells were transfected at 50 to 70% confluence using FuGENE (Roche, Mannheim, Germany) as suggested by the manufacturer.
Immunofluorescence.
Cells growing on glass coverslips in separate wells of 24-multiwell plates were transfected with the indicated plasmids. Nearly 48 h after transfection, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature and washed once with phosphate-buffered saline (PBS). Background fluorescence was reduced by quenching free aldehyde groups in 50 mM NH4Cl-PBS for 10 min. The cells were permeabilized by immersing the coverslips in PBS-0.2% Triton X-100 for 5 min at room temperature, washed again with PBS, and blocked in blocking solution (2% fetal calf serum, 2% bovine serum albumin, 0,2% gelatin, in PBS) for 30 min. Antibodies were diluted in blocking solution diluted 1:10 in PBS. The cells were incubated for 1 h with each antibody and were washed six times in PBS between the first and second antibody. The nuclei were stained with propidium iodide (Molecular Probes) as indicated by the manufacturer. Antibodies used were anti-flag M2 mouse monoclonal (Sigma), anti-HBsAg (Dako), and Cy2-conjugated anti-mouse antibodies (Jackson ImmunoResearch). Fluorescence was analyzed with a Leica TCS-SP confocal microscope equipped with a ×63 objective, with the pinhole set at 1 Airy disk unit.
Deglycosylation by endoglycosydase H.
HeLa cells (106) were infected with Ad-HBsAg(S)EE7Δ1-35 at a multiplicity of infection (MOI) of 50 for 1 h at 37°C and 5% CO2 and incubated for a further 48 h. Then the cells were lysed with buffer containing 1% NP-40, 0.1% SDS, 50 mM sodium acetate (pH 5.5) 50 mM β-mercaptoethanol, and protease inhibitors (Complete; Roche Diagnostics) for 1 h at 4°C. After cold spinning at 15,000 × g, aliquots of 100 μl of the supernatant were incubated for 1 h at 4°C in the presence or absence of 100 mU/ml of endoglycosydase H (Roche Diagnostics).
Expression and purification of E7 and HBsAg/E7 proteins.
The complete HPV-16 E7 protein carrying a carboxyl-terminal six-His tag was expressed in Escherichia coli using a T7 expression plasmid [pET28a(+)] (Novagen). Expression of HBsAg(S)/E7 fusion proteins in yeast was performed with the P. pastoris system (Invitrogen) using the X-33 strain and selection of recombinant clones on plates containing 100 μg/ml of zeocin. The His-tagged E7 and fusion proteins were purified on nickel-agarose columns (Novagen) as indicated by the manufacturer. The purified proteins were quantified using the Bradford assay (Bio-Rad).
Western blotting.
HEK 293T ells growing on 6-cm plates were transfected with 2 μg of plasmid as indicated and incubated for 48 h. The cells were then washed twice with PBS and lysed in sodium dodecyl sulfate (SDS) loading buffer containing 1 mM dithiothreitol. The cellular proteins were separated on 15% or 4 to 20% gradient (NuPAGE; Invitrogen) polyacrylamide gels by SDS-polyacrylamide gel electrophoresis, blotted onto polyvinylidene difluoride membranes, blocked with 5% milk in PBS containing 0.1% Tween 20, and incubated at room temperature with horseradish peroxidase-conjugated anti-FLAG M2 antibodies (Sigma). Antibody binding was visualized with enhanced chemiluminescence reagent (Renaissance; NEN-Perkin Elmer).
Electron microscopy.
Cell monolayers were fixed in 2% glutaraldehyde-2 mM CaCl2-0.08 M sodium cacodylate buffer (pH 7.2), postfixed in 1% OsO4, block stained with aqueous uranylacetate, dehydrated in a graded series of ethanol, and embedded in Epon. Ultrathin sections (70 nm) were contrasted with uranylacetate and lead and examined with a Tecnai 12 transmission electron microscope (Fei, Hillsboro, Oregon) at 80 KV. For the ultrastructural analysis of HBsAg VLPs, negative staining of purified fractions was performed. Briefly, 10-μl drops of each fraction were put on Parafilm. Upside-down electron microscopy grids were laid on top of the drops, incubated for 5 min at room temperature, washed in PBS, and subjected to negative staining with uranylacetate (10 μl/grid; 1.5 min at room temperature). Samples were then rinsed in water, air dried, and visualized and photographed with a Zeiss EM-10 microscope.
Immunizations.
Female 5- to 8-week-old BALB/c (H2d) and C57BL/6 (H2b) mice (WiGa-Charles River, Hamburg, Germany) were kept under conventional pathogen-free housing and caring conditions at the animal facility of the Deutsches Krebsforschungszentrum (DKFZ; German Cancer Research Center). All animal treatments were approved by the Animal Care Commission of the Government of Baden-Württemberg. Immunizations were performed with a 50-μl Hamilton syringe and a 30-gauge needle to inject a volume of 30 to 40 μl per dose. Mice were inoculated with 106 IFU/dose of either Ad-HBsAg(S)16EE7, Ad-HBsAg(S)EE7Δ1-35, or Ad-LacZ or with 107 IFU/dose of Ad-E7wt. All doses were administered 50% subcutaneously (dorsal skin at the level of the cervical vertebrae) and 50% intramuscularly (i.m.; muscle tibialis anterior). Protein immunizations were administered i.m. using 2 to 4 μg/dose of HBV vaccine (Engerix-B [20 μg/ml]; Glaxo Smith-Kline Beecham) or recombinant HBsAg(S)E7Δ1-35 VLPs.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with 1 μg/ml of either commercially available recombinant HBsAg(S) (Engerix-B), His-tagged E7 protein, β-galactosidase (Sigma), or adenovirus hexon protein, as indicated, in 0.1 M carbonate buffer, pH 9.3, at 4°C and blocked in 5% fetal calf serum (FCS) in PBS for 2 h at room temperature. Plates were subsequently washed, and 100 μl of 5% serum (diluted in 0.5% milk-0.1% Tween 20 in PBS) was added to each well and incubated for 1 h at 37°C. Bound antibody was detected using goat anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Molecular Probes) for 1 h at 37°C. Plates were developed by adding 100 μl of O-phenylenediamine (Sigma). After 30 min at room temperature in a darkened area, the reaction was stopped by adding 50 μl of 1 M sulfuric acid per well. Plates were read at 450 nm. Positive controls with anti-HPV-16 E7 antibodies (Oncogene) and negative controls (without serum or antibody) were run in parallel. The cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the normal sera plus three standard deviations. Titers of anti-HBsAg in serum were determined by comparison with World Health Organization-defined standards and multiplying the serum dilution with the measured antibody level (mIU/ml). Detection of HBsAg particles was performed with Monolisa (Bio-Rad).
T-cell cultures.
T cells were isolated from the spleens of immunized mice 7 days after the booster immunization. Spleen cell suspensions were filtered through 70-μm-pore-size cell strainers (Beckton Dickinson) and pretreated for 5 min at room temperature with 0.8% NH4Cl-0.1% KHCO3 to deplete erythrocytes. After centrifugation, pellets of splenocytes were resuspended in RPMI 1640 medium supplemented with 10% FCS, 50 μM 2-mercaptoethanol, 10 IU of penicillin/ml, 20 μg of streptomycin/ml, and 10 IU/ml of recombinant interleukin-2 (rIL-2; Roche, Mannheim, Germany) and incubated for 2 h at 37°C with 5% CO2. Nonadherent cells were then collected for enzyme-linked immunospot assay (ELISPOT) analysis (see below) or were in vitro restimulated before the intracellular gamma interferon (IFN-γ) staining was performed. T cells were restimulated in vitro with RMA cells loaded with E7(49-57) peptide (E7 peptide with residues 49 to 57) by addition to the culture medium (0.1 μM of peptide). Loaded cells were subsequently γ irradiated (100 Gy) and seeded at 2.5 × 104 cells/well in RPMI 1640 medium supplemented with 5% FCS, 50 μM 2-mercaptoethanol, and 10 IU/ml rIL-2 in 96-well plates. T cells were added to the wells of stimulator cells at 2 × 105 cells/well. On day 7 intracellular cytokine staining was performed.
Intracellular cytokine staining and fluorescence-activated cell sorting analysis.
Restimulated T cells were transferred to 24-well plates (1 × 106 cells/well) and incubated for 1 h at 37°C with 5% CO2 in the presence of 1 μg/ml of E7(49-57) peptide and 1 μg/ml (each) anti-CD28 and anti-CD49d monoclonal antibody (BD Biosciences, Heidelberg, Germany). Then, cytokine secretion was blocked by adding 10 μg/ml of brefeldin A (Sigma). After 6 h, the cells were washed with PBS-3% FCS and surface stained with rat anti-mouse CD8+-fluorescein isothiocyanate antibody (BD Pharmingen) and 7-aminoactinomycin D (7-AAD; Sigma) for 30 min on ice. Unbound antibody and 7-AAD were washed with PBS-3% FCS, and the cells were fixed for 15 min on ice and permeabilized with Cytofix/Cytoperm solution (BD Pharmingen) in the presence of 10 μg/ml actinomycin D (AD) (Sigma). AD was added to the solutions used for the following steps to avoid DNA binding of fluorescent 7-AAD that can leak out of stained cells into unstained cells after permeabilization. After cells were washed twice with Perm/Wash buffer (BD Pharmingen) containing AD, intracellular IFN-γ staining was performed with Perm/Wash-AD buffer containing phycoerythrin-conjugated rat anti-mouse IFN-γ antibody (clone XMG1.2; BD Pharmingen) for 30 min on ice. Stained cells were washed twice with Perm/Wash-AD buffer and once with PBS-3% FCS-AD. Cells were then filtered through a cell strainer cap into a 5-ml polystyrene tube (BD Labware) to be immediately analyzed. As a negative control, splenocytes were incubated in the absence of peptide but otherwise treated identically. Flow cytometry was performed using a FACSCalibur (Becton Dickinson, San Jose, CA) instrument, and the CellQuest software (Becton Dickinson) was used for data acquisition and analysis. A total of 1 × 104 events were analyzed for fluorescence intensity. The CD8+ fraction was gated after debris was gated out using the 7-AAD staining.
IFN-γ ELISPOT assay.
The numbers of peptide-specific, interferon IFN-γ-producing cells were determined as follows. Multiscreen-HA filtration plates (96-well; Millipore, Bedford, MA) were coated overnight at 4°C with 5 μg/ml of anti-mouse IFN-γ antibody (clone R4-6A2; BD Pharmingen) in PBS. After being washed with PBS, the plates were blocked with PBS-1% FCS for 1 h at 37°C. Freshly isolated splenocytes were plated on precoated ELISPOT plates at densities of 5 × 104 to 1.25 × 105 live cells per well in 100 μl of culture medium (RPMI 1640, 10% FBS, 50 U/ml penicillin-streptomycin) along with 10 IU/ml of IL-2 (Roche, Mannheim, Germany). The splenocytes were incubated for 24 h at 37 C in 5% CO2 in the presence or absence of 1 μg/ml of HPV-16 E7(49-57) peptide. Then, the plates were washed twice with PBS-0.05% Tween 20, rinsed in PBS, and incubated overnight at 4°C with 2.5 μg/ml biotinylated anti-mouse IFN-γ antibody (clone XMG1.2; BD Pharmingen). Following extensive washing with PBS, the plates were incubated for 2 h at room temperature with streptavidin-alkaline phosphatase conjugate (BD Pharmingen), diluted 1:500. After washing with PBS, the reaction was developed by the addition of 5-bromo-4-chloro-3-3-indolyl phosphate-nitroblue tetrazolium solution (Sigma). Finally, the plates were washed with tap water and allowed to dry overnight. The number of spots was measured with the aid of an ELISPOT reader (Zeiss-Vison C; Zeiss, Oberkochen, Germany).
In vivo CTL assay.
To detect cytotoxic activity in vivo, C57BL/6 mice were immunized at 2-week intervals either with 1 × 106 IFU (i.m.) of Ad-HBsAg(S)EE7Δ1-35 or Ad-E7 wild-type vectors, or with 4 μg of HBsAg(S)16E7Δ49-57 particles. Six days after the fourth injection, we assessed CTL activity in vivo as previously described (1, 13). Single cell suspensions of splenocytes from donor naïve mice (1 × 107 cells/ml) were divided into three identical populations: one was pulsed with peptide E7(49-57) and labeled with 0.08 μM 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSElow target cells), another population was not pulsed with peptide but was labeled with 0.5 μM CFSE (CFSEint control cells), and the third was pulsed with peptide HBsAg(S)(208-215) and labeled with 3 μM CFSE (CFSEhigh target cells). After washing, an equal number of cells from each population was mixed into one suspension and injected intravenously into recipient immunized mice (15 × 106 cells/mouse). At 16 h after injection the mice were sacrificed for their lymph nodes and spleens. Cell suspensions were analyzed by flow cytometry counting up to 1 × 104 CFSE-positive cells. Differential CFSE intensities permitted detection of each cell population. Specific lysis was calculated as follows: % specific lysis = [100 − (ratio primed × 100)/(ratio unprimed)], where ratio = (% CFSElow or CFSEhigh)/(%CFSEint).
RESULTS
Construction and expression of HBsAg(S)/HPV-16 E7 fusions.
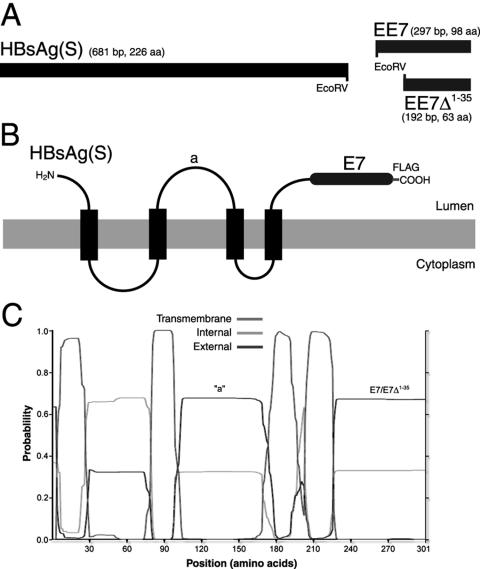
In order to generate constructs encoding chimeric HBsAg(S) proteins, an HBsAg(S) gene was amplified whose stop codon was replaced by the EcoRV cleavage sequence (Fig. 1A), which encodes two additional amino acids (D and I) between HBsAg(S) and the foreign antigen. In contrast to HBsAg, HPV genes have codon biases divergent from human genes, and as consequence their protein expression is restricted in human cells (39). Therefore, we used a synthetic, codon-optimized E7 gene (EE7) previously shown to drive expression of high levels of E7 protein in transient transfections (4). The complete EE7 gene or a truncated mutant devoid of codons 1 to 35 (EE7Δ1-35), both carrying a FLAG tag, were used for fusion at the 3′ end of HBsAg(S) (Fig. 1A). EE7Δ1-35 encodes an E7 polypeptide that has lost its transforming capability as it has no Rb binding domain (4) while still containing relevant E7 antigenic epitopes. The resulting fusion genes, encoding proteins named HBsAg(S)16E7 and HBsAg(S)16E7Δ1-35 bearing wild-type or truncated E7, respectively, were cloned into E1/E3-deleted adenovirus expression vectors [Ad-HBsAg(S)16EE7 and Ad-HBsAg(S)16EE7Δ1-35], which were used in subsequent vaccination studies.
FIG. 1.
Schematic representation of HBsAg(S)16EE7 fusion genes and proteins and their expression in mammalian cells. (A) HBsAg(S)/E7 fusion genes used in this study. The stop codon of HBsAg(S) was replaced by an EcoRV site, which allows for in-frame 3′ end fusion of coding sequences. Constructs were derived by fusion of HBsAg(S) to either a complete EE7 gene or a truncated mutant devoid of the first 35 codons (EE7Δ1-35). A FLAG tag (not represented) was added at the 3′ end of the EE7 sequences. (B) Topology of the HBsAg(S)-HPV-16E7 fusion proteins in the ER membrane. The luminal side of the protein is that exposed on the surface of the extruded particles. (C) Transmembrane domain prediction of the HBsAg-E7 fusion proteins according to the algorithm developed by Sonnhammer et al. (37). The positions of the “a” determinant and the E7 domains are indicated.
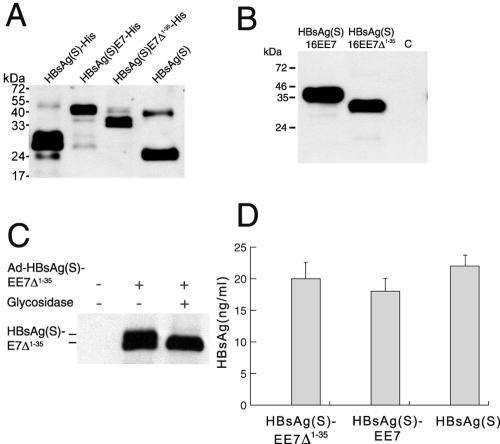
The topology of the fusion proteins in the endoplasmic reticulum (ER) and VLPs, in agreement with previous experimental data (3, 15) and current algorithms for the prediction of transmembrane domains (37), consists of three hydrophobic regions with four transmembrane domains and luminally exposed N- and C-terminal segments (Fig. 1B and C). The first and second transmembrane domains are separated by a 50-residue cytoplasmic loop, and the second and third are separated by a 60-residue luminal domain containing the major B-cell epitope (the “a” determinant) (15). Accordingly, the E7 polypeptides in the fusion proteins were expected to appear on the luminal side of the ER (Fig. 1B and C) and, therefore, on the outside of the chimeric VLPs. Supporting the likelihood of the appearance of E7 on the surface of the C terminus was the fact that intact VLPs containing the HBsAg(S)16E7 or HBsAg(S)16E7Δ1-35 fusion proteins bearing a His tag at the carboxyl terminus of E7 were readily purified on Ni-nitrilotriacetic acid agarose columns upon expression in yeast cells. These particles were comparable in structure to wild-type HBsAg(S) particles, as shown by electron microscopy of negatively stained fractions (data not shown). In addition, recombinant HBsAg(S)16E7 and HBsAg(S)16E7Δ1-35 reacted in Western blots with specific anti-HBsAg antibodies (Fig. 2A), as well as with anti-FLAG and anti-E7 antibodies (not shown), as proteins with apparent molecular sizes of 40 and 33 kDa, respectively. Proteins of the same size were seen in blots of human HEK 293T cells that had been transfected with mammalian expression plasmids encoding HBsAg/E7 fusion proteins with a C-terminal FLAG tag (Fig. 2B). Like wild-type HBsAg(S), the fusion proteins were also glycosylated and appeared as doublets, of which the upper glycosylated band disappeared after endoglycosydase H treatment (Fig. 2C). Similar results were obtained with cells infected with the Ad-HBsAg/E7 vectors (data not shown). These data suggested that particle assembly as well as posttranslational processing and folding of HBsAg(S) was not altered by the fusion of E7 to its carboxyl terminus.
FIG. 2.
Expression and secretion of HBsAg/E7 fusion proteins. (A) Expression of HBsAg/E7 fusion proteins in yeast. Western blotting using anti-HBsAg antibodies was carried out to detect His-tagged wild-type and chimeric HBsAg(S) proteins purified from yeast on Ni-nitrilotriacetic acid agarose. A wild-type HBsAg(S) with no His tag (Engerix B) was included. Note the higher molecular mass of HBsAg(S)-His compared to the nontagged protein. (B) Western blot analysis of HEK 293T cells transfected with pIRES-neo2 plasmids encoding FLAG-tagged HBsAg(S)16E7 or HBsAg(S)16E7Δ1-35, as indicated. Cellular extracts prepared 48 h after transfection were separated in a 15% acrylamide gel and hybridized with anti-FLAG M2 monoclonal antibodies. Note the thickness of the bands due to the presence of glycosylated and nonglycosylated forms running close to each other. The apparent molecular masses are 40 and 33 kDa, respectively. C, control cells transfected with pIRES-neo2 empty vector. (C) The HBsAg(S)/E7 fusion protein is glycosylated. Western blot analysis of lysates from HEK 293T cells infected with Ad-HBsAg(S)EE7Δ1-35 before and after treatment with Endo H and detected with anti-FLAG antibodies is shown. (D) Detection of HBsAg particles in supernatants of HeLa cells transfected with plasmids encoding either HBsAg(S)16E7, HBsAg(S)16E7Δ1-35 or wild-type HBsAg(S). Transfection efficiencies were normalized by cotransfection with a plasmid expressing green fluorescent protein. At 48 h after transfection, cell supernatants were subjected to ELISA (Monolisa). A standard curve was obtained using known amounts of a commercial HBsAg(S) particle preparation (Engerix B). Data are the means ± standard deviations of three independent transfections.
HBsAg(S)/E7 proteins assemble into VLPs and are secreted by mammalian cells.
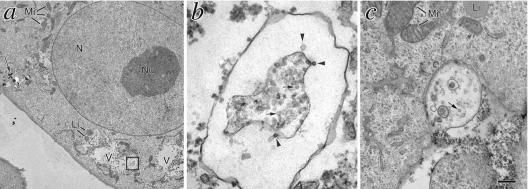
To determine whether the HBsAg(S)16E7 and HBsAg(S)16E7Δ1-35 fusion proteins assembled into VLPs and were secreted by mammalian cells, we transfected HeLa cells and 72 h later collected the supernatants and analyzed them for the presence of HBsAg using a sensitive commercial assay (Monolisa). As shown in Fig. 2D, cells transfected with plasmid encoding the fusion proteins secreted HBsAg(S) particles at levels comparable to cells transfected with a plasmid encoding wild-type HBsAg(S), suggesting that the fusion protein was properly exocytosed. Assembly of the fusion proteins into VLPs in mammalian cells was shown by electron microscopy of Vero cells expressing HBsAg(S)16E7 or HBsAg(S)16E7Δ1-35 to reveal large vacuoles (Fig. 3a, V) containing particles of about 20 to 25 nm in diameter (Fig. 3b), similar to those formed naturally during human infection with HBV or in cells expressing HBsAg(S) (26). Vacuoles containing HBsAg(S)16E7 VLPs were seen releasing their content to the extracellular space (Fig. 3c). The pattern of assembly was comparable among those HBsAg particles bearing truncated E7Δ1-35 (Fig. 3) or full-length E7 (not shown).
FIG. 3.
Transmission electron microscopy analysis of Vero cells transfected to express HBsAg(S)16E7Δ1-35. (a) Cells transfected with pIRESneo2-HBsAg(S)16EE7Δ1-35. This section corresponds to a low plane where the cell contains a number of large vacuolated structures surrounding the nucleus. Boxed area is magnified in panel b showing particles being extruded (arrowheads) into the lumen of a larger structure that contains a great number of particles (arrows). (c) A vesicle underneath the plasma membrane releasing its particle content to the extracellular medium. Li, lipid droplets; Mi, mitochondria; N, nucleus; Nu, nucleolus; V, large vacuoles containing 22-nm VLPs. Bar, 2 μm (a), 100 nm (b), and 225 nm (c).
The intracellular localization of the HBsAg(S)16E7 and HBsAg(S)16E7Δ1-35 proteins was also studied by confocal microscopy. Series of confocal sections of cells showed a perinuclear pattern (Fig. 4A) and vesicular distribution throughout the cytoplasm, especially in the basal planes (Fig. 4A, xy1, and B). Likely, these vesicles carried HBsAg(S)16E7 particles to the cell surface for exocytosis. Careful analysis of confocal sections revealed small fluorescent spots of HBsAg(S)16E7 in vacuolated structures in nontransfected cells in the vicinity of HBsAg(S)16E7-expressing cells (Fig. 4A, arrowheads). Such spots could represent the endocytosis of HBsAg(S)16EE7 particles or their aggregates. Altogether, these results are in agreement with those shown in Fig. 2C and are consistent with appropriate packaging and secretion of the fusion proteins.
FIG. 4.
Intracellular localization of the HBsAg(S)16E7 fusion protein analyzed by confocal microscopy. A representative Vero cell expressing HBsAg(S)16EE7 stained with an anti-FLAG antibody (green). Cells were counterstained with propidium iodide (red). Each photograph represents a single confocal section within a series in the xy and xz planes. (A) Three consecutive sections in the xy plane through the basal (xy1), medial (xy2), and apical (xy3) levels. The HBsAg(S)16E7 protein accumulates in large perinuclear structures derived from the ER and Golgi. In addition, a punctuate pattern of smaller vesicles containing fusion protein is seen scattered throughout the cytoplasm. These vesicles appear to migrate to the periphery, where they are exocytosed. Arrowheads point to HBsAg(S)16E7 protein into vesicular structures in neighboring untransfected cells. These are particle aggregates likely coming from the cell in the center, which was the only one transfected in this field. (B) Three cross-sections of the cells in the plane xz (perpendicular to the plane of adherence) at the levels indicated in xy2, showing large stained structures that are in tight contact with the nucleus and small vesicles that migrate distally to the periphery of the cell. The arrowhead in xz3 points to HBsAg(S)16E7 fusion protein captured by an untransfected cell. Bar, 20 μm.
Antibody responses against E7 and HBsAg(S) in mice inoculated with Ad-HBsAg(S)/E7.
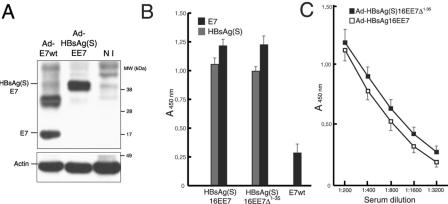
In a first series of immunization experiments, we tested the antibody responses to HPV-16 E7 and HBsAg after vaccination with vectors encoding the HBsAg/E7 fusion proteins. Because preliminary experiments using plasmid DNA under physiological conditions (4 μg/dose of endotoxin-free plasmid administered i.m. without adjuvant four times at 2-week intervals) resulted in weakly positive reactions (data not shown), subsequent experiments were carried out with the recombinant adenovirus vaccines. BALB/c mice (n = 10 per group) were immunized with three doses of 106 IFU/dose of Ad-HBsAg(S)16EE7 or Ad-HBsAg(S)16EE7Δ1-35 or of 107 IFU/dose of Ad-E7wt, which carries a wild-type E7 gene. A 10-fold higher dose of the latter was used to achieve a level of expression of E7 protein equivalent to that obtained with the Ad vectors encoding the fusion genes, which carried codon-optimized E7 sequences. This was necessary because production of recombinant Ad-EE7 virus was not possible, most likely due to the toxicity of excessive expression of E7 (4; T. Kovalik, personal communication). However, infection experiments in vitro showed that cells infected with a 10-fold higher dose of Ad-E7wt expressed levels of E7 protein comparable to those of cells infected with Ad-HBsAg(S)16EE7 (Fig. 5A).
FIG. 5.
Antibody reactivity to E7 and HBsAg(S) in vaccinated mice determined by ELISA. (A) Expression of E7 and HBsAg(S)E7 in HEK 293 cells infected with either Ad-E7wt (MOI of 100) or Ad-HBsAg(S)EE7 (MOI of 10). At 48 h after infection, the cells were lysed with SDS loading buffer and equal amounts were loaded on a 4 to 20% gradient gel. After blotting, the E7 and HBsAg(S)E7 proteins were detected with anti-E7 and anti-actin antibodies. The upper band in the lane Ad-E7wt represents likely a dimer. NI, noninfected control cells. (B) Groups of BALB/c mice were inoculated at 2-week intervals with 106 IFU of either Ad-HBsAg(S)16EE7, Ad-HBsAg(S)16EE7Δ1-35 or Ad-E7wt, as indicated. Results correspond to serum samples taken 2 weeks after the third inoculation. The sera were diluted 1:200 and assayed on plates coated with either recombinant E7 or HBsAg protein as described in Materials and Methods. Data are the means ± standard deviations of 10 serum samples. (C) Extinction curves of anti-E7 antibody titers of sera derived from the mice immunized with Ad-HBsAg(S)16EE7 or Ad-HBsAg(S)16EE7Δ1-35 vectors in panel A showing slightly higher titer values for the latter and extinction values for both beyond a dilution of 1:3,200. The ELISA plates were coated with the indicated proteins as described in Materials and Methods. The results show the mean OD and the standard deviation.
The mice received Ad at 2-week intervals. Two weeks after the second and third inoculations, sera were collected and tested for antibodies to E7 and HBsAg by ELISA on plates coated with either His-tagged E7 protein expressed in bacteria or commercially available HBsAg (Engerix B). The mice developed progressively higher titers of anti-E7 and anti-HBsAg(S) IgG antibodies in serum. As shown in Fig. 5B, after the third inoculation, values for anti-E7 at a serum dilution of 1:200 were over 1 optical density unit at 450 nm (OD450), with a tendency to be slightly higher in mice immunized with Ad-HBsAg(S)16EE7Δ1-35 (Fig. 5B and C). The extinction curves of anti-E7 titers showed values within the linear range of the ELISA system used and extinction coefficients beyond 6,400 (Fig. 5C). Such kinetics of antibody responses is in agreement with previous reports of immunization with adenovirus vectors expressing HPV-16 L1 (23). In contrast, the anti-E7 titer of sera derived from mice immunized with Ad-E7wt was nearly fivefold lower. In addition, the mice immunized with recombinant Ad encoding the fusion proteins developed antibody reactivity against HBsAg with OD450 values of about 1 (equivalent to an anti-HBsAg titer of 2,500 mIU/ml in our assay), somewhat lower than those against E7. OD450 values above 1 OD unit persisted for at least 6 months for both anti-E7 and anti-HBsAg titers (data not shown). Comparable results were obtained with C57BL/6 mice immunized in the same way (data not shown). The results indicate that recombinant Ad encoding HBsAg/E7 fusion proteins are able to induce higher antibody titers for E7 than for HBsAg, suggesting an enhancement effect by HBsAg on E7 immunogenicity.
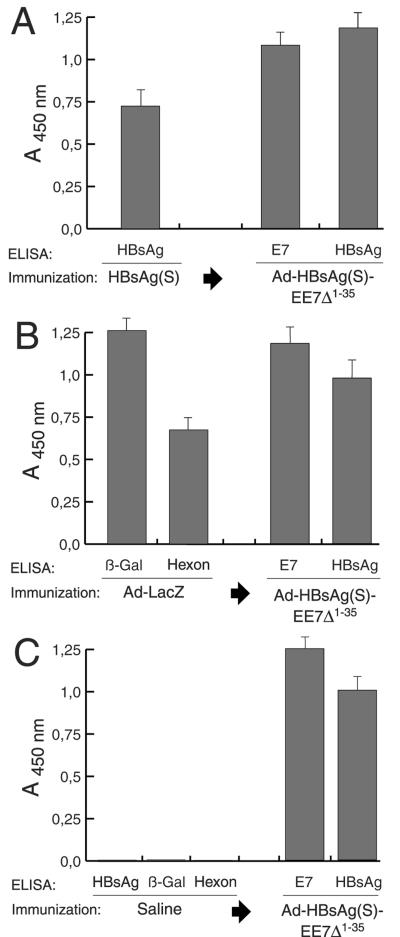
Antibody responses in mice preimmunized with HBsAg or adenovirus.
A large percentage of the human population carries anti-HBsAg(S) antibodies in serum as a result of natural infection with HBV or vaccination. Therefore, we next determined if preexisting immunity to HBsAg(S) could interfere with the immune responses to Ad encoding the HBsAg(S)16E7Δ1-35 fusion protein. BALB/c mice (n = 10 per group) were immunized with alum-adjuvanted HBV vaccine based on recombinant HBsAg (Engerix-B) following an established protocol (17). Two weeks after the third boost, the serum reactivity to HBsAg(S) was tested and a response was obtained, which was absent in the control mice injected with saline (Fig. 6A and C, respectively). Subsequent immunization with Ad-HBsAg(S)16EE7Δ1-35 as described in the previous section induced anti-E7 antibody titers slightly lower than those of control mice immunized with the same virus (Fig. 6A, middle column). In these mice, the anti-HBsAg titer increased further and was indeed significantly higher than the anti- HBsAg titer seen in the control mice (Fig. 6C) and even higher than the anti-E7 titer.
FIG. 6.
Antibody reactivity to E7 and HBsAg(S) in mice preimmunized with HBsAg or Ad-LacZ determined by ELISA. (A) BALB/c mice (n = 10) were first immunized i.m. with HBsAg(S) protein. Serum reactivity to HBsAg(S) was tested after 2 weeks after the third boost (left column). Subsequently, the mice were inoculated three times at 2-week intervals with Ad-HBsAg(S)16EE7Δ1-35. Serum samples were taken 2 weeks after the last inoculation and antibody titers against E7 and HBsAg were tested. (B) Vaccination of C57BL/6 mice (n = 10) preimmunized with Ad-LacZ. Mice were inoculated with Ad-LacZ at days 0, 14, and 28, beginning 2 weeks after the last boost antibody responses to the β-galactosidase and hexon proteins were tested. Subsequent vaccination with Ad-HBsAg(S)16EE7Δ1-35 induced additional antibody response against E7 and boosted the anti-HBsAg(S) (third and fourth columns from the left, respectively) (compare with panels A and C). (C) A control group of 10 C57BL/6 mice were injected with saline three times at 2-week intervals. Two weeks after the last injection the mice were tested for HBsAg, β-galactosidase, and Ad hexon antibodies. Then the mice were inoculated with Ad-HBsAg(S)16EE7Δ1-35 and tested as above for E7 and HBsAg antibodies. ELISA plates were coated with the indicated proteins as described in Materials and Methods. Data are the mean of 10 serum samples; the error bars represent one standard deviation from the mean values. Sera were diluted 1:200, and the cutoff value was 0.045 throughout.
Because immunity to Ad may also be present in a subset of the population, in another series of experiments we tested whether previous immunity against Ad could interfere with immunization with the Ad vectors. To this end, a group of mice was first immunized with an Ad-LacZ vector of the same serotype as the Ad-HBsAg(S)/E7 recombinant. Two weeks after the third inoculation, high to moderate titers of antibodies against β-galactosidase and the hexon protein of Ad were detected (Fig. 6B), which were absent in saline-injected control mice (Fig. 6C). Ad vector neutralization titers in these mice, defined as the reciprocal dilution of serum required to reduce infectivity of Ad-HBsAg(S)/E7 to 50% with preimmune serum as the baseline control, were found to be in the range of 16 to 32. One week later, the Ad-LacZ preimmunized mice were vaccinated with Ad-HBsAg(S)16EE7Δ1-35 as above. These mice developed antibody responses against both E7 and HBsAg(S), which were comparable to those of saline-injected mice inoculated likewise with Ad-HBsAg(S)16EE7Δ1-35 (Fig. 6C), indicating that preexisting antibodies against the Ad vector were not able to affect infection by Ad-HBsAg(S)16EE7Δ1-35.
E7-specific CD8+/IFN-γ T-lymphocyte responses in mice vaccinated with Ad encoding HBsAg/E7 fusion proteins.
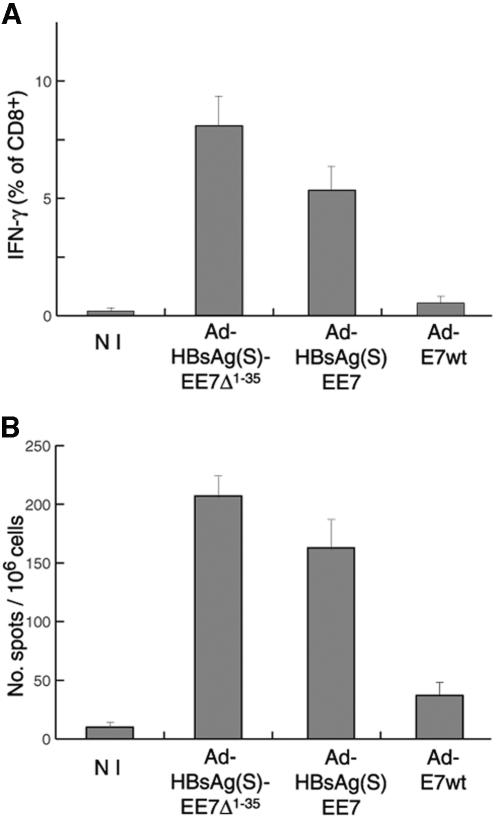
The level of E7-specific CD8+ T cells in splenocytes from mice immunized with Ad encoding HBsAg/E7 fusion proteins was determined by intracellular cytokine staining and IFN-γ ELISPOT tests upon in vitro restimulation. Groups of C57BL/6 mice (n = 10 per group) were inoculated four times at 2-week intervals with 106 IFU of either Ad-HBsAg(S)16EE7 or Ad-HBsAg(S)16EE7Δ1-35 or 107 IFU of Ad-E7wt. One week after the last immunization, the mice were sacrificed, and the splenocytes were processed and restimulated as described in the Materials and Methods section.
We used for restimulation a well-characterized HPV-16 E7 peptide, which is a MHC-I CTL epitope in H2b mice [E7(49-57), RAHYNIVTF]. This peptide should enhance the in vivo induced CD8+ T-cell response without altering its hierarchy (46). We analyzed the restimulated lymphocytes by IFN-γ staining, followed by flow cytometry and by IFN-γ ELISPOT, and measured the percentage of E7-specific CD8+ T cells. In all groups tested, 80% of mice responded with high levels of specific CD8+ T cells. As illustrated in Fig. 7A for each group of vaccinated mice, the intracellular IFN-γ response in T lymphocytes upon in vitro restimulation was higher in the mice immunized with Ad encoding the fusion proteins compared to mice vaccinated with Ad-E7wt. This result was consistently reproduced with the IFN-γ ELISPOT tests (Fig. 7B). The stronger difference between the vectors expressing HBsAg/E7 and wild-type E7 protein seen with the intracellular IFN-γ staining (∼20- to 36-fold) compared to the ELISPOT (∼5- to 7-fold) may be due to differences in sensitivity between the two methods. A tendency for Ad-HBsAg(S)16EE7Δ1-35 to elicit significantly higher (∼35%) numbers of E7-specific CD8+ T cells than Ad-HBsAg(S)16EE7 was also observed. The IFN-γ ELISPOT assay confirmed this tendency, albeit with a somewhat lower difference (∼20% higher) (Fig. 7B).
FIG. 7.
Cytotoxic responses induced in C57BL/6 mice vaccinated with Ad vectors. (A) Intracellular flow cytometry analysis of E7-specific CD8+ T cells. Mice (n = 10 per group) were immunized with Ad vectors encoding HBsAg/E7 fusion proteins or Ad-E7wt. Splenocytes were restimulated for 7 days with E7(49-57) peptide. Intracellular cytokine staining was performed after a 6-h block with brefeldin A in the presence of E7(49-57) and anti-CD28 and anti-CD49d costimulator molecules. Nonstimulated controls were treated similarly but in the absence of E7(49-57). CD8+ lymphocytes were gated and analyzed by fluorescence-activated cell sorting for IFN-γ production. Percentages of IFN-γ-positive CD8+ cells are given. NI, nonimmunized control group. Nonstimulated cells gave values similar to those of the nonimmunized group. (B) T-cell response determined by IFN-γ ELISPOT of splenocytes from mice immunized with the indicated Ad vectors after overnight restimulation in vitro with E7(49-57) peptide. NI, nonimmunized control. Reactivity values from nonstimulated samples have been subtracted. Data are the means ± standard deviations of three independent assays.
Cytotoxic T-cell responses induced by Ad-HBsAg(S)/E7 vectors in vivo.
To evaluate the ability of Ad vectors encoding HBsAg/E7 fusion protein to induce efficient cytotoxic responses in vivo, we challenged immunized C57BL/6 (H2b) mice with splenocytes from naïve mice loaded with E7 and HBsAg(S) peptides. Groups of C57BL/6 mice (n = 10 per group) were vaccinated four times at 2-week intervals with 1 × 106 IFU of Ad-HBsAg(S)16EE7Δ49-57 or Ad-16E7wt or with 4 μg/dose of recombinant HBsAg(S)16E7Δ49-57 VLPs with no adjuvant. For loading isogenic naïve T lymphocytes, we used two well-studied MHC-I peptides, the HPV-16 E7(49-57) and the Kb-binding 8-mer HBsAg(208-215) (ILSPFLPL) peptide, an epitope generated by exogenous as well as endogenous processing of HBsAg(S) (35). After loading with peptide, we labeled the splenocytes with three different concentrations of the vital dye CFSE (1), low, high and intermediate, to allow differentiation between E7, HBsAg(S), and peptide-unloaded (control) populations of splenocytes, which we mixed in a 1:1:1 proportion and injected intravenously as indicator cells into vaccinated and control naïve mice. At 16 h after adoptive transfer, we followed the fate of peptide-loaded spleen cells in vivo by flow cytometry analysis of T lymphocytes from the spleen and lymph nodes, which revealed the three distinct CFSE-labeled indicator cell populations.
Quantitative analysis of specific lysis (Fig. 8) was performed as described elsewhere (13). The E7(49-57)-loaded population was markedly reduced in mice vaccinated with Ad-HBsAg(S)16EE7Δ1-35, as deduced from the higher percentages of specific lysis seen in both spleen (35%) and regional lymph nodes (40%) with this vector (Fig. 8). In contrast, the mean specific lysis obtained in the same tissues with Ad-E7wt was 2% and 4%, respectively. These values were even lower than the lysis seen in mice vaccinated with HBsAg(S) particles (9% and 12%, respectively). The HBsAg(208-215) peptide induced markedly lower specific lysis than the E7(49-57) peptide under these experimental conditions. Higher percentages of specific lysis in the regional lymph nodes were consistently observed in all animals tested, which may reflect an accumulation of E7- and HBsAg-specific cytotoxic T cells in this tissue. These results further confirmed the ability of the Ad vectors encoding HBsAg/E7 fusion protein to induce cytotoxic responses in vivo.
FIG. 8.
Cytotoxic responses induced by vaccination with Ad encoding HBsAg/E7 fusion proteins determined by antigen-specific kill of indicator cell populations. C57BL/6 mice (n = 10 per group) were immunized at 2-week intervals with Ad-HBsAg(S)16EE7Δ49-57, Ad-16E7wt, or recombinant HBsAg(S)16E7Δ49-57 VLPs. Six days after the fourth boost immunization, splenocytes from naïve mice were loaded with either E7(49-57) or HBsAg(S)(208-215) peptides or left unloaded and subsequently labeled with CFSE to low, high, and intermediate concentrations, respectively. Equal amounts of labeled cells were mixed, and 15 × 106 cells of the mixture was injected intravenously into control naïve and vaccinated mice. Lymphocytes from spleens and regional lymph nodes were taken 20 h after injection and analyzed by flow cytometry. Represented are the mean percentages ± standard deviations of specific CTL activities in the different groups of mice calculated as described in Materials and Methods.
DISCUSSION
Antigen-specific T-cell reactivity is an essential component of cellular immunity against self proteins, viral agents, and tumor antigens. The improvement of methods for the enhancement of the immunogenicity of TSAs is a prerequisite for the development of new immunotherapy strategies. The replicative cycle of tumor viruses like HBV and HPV appears to have adapted to the host in a way that allows infected tumor cells to remain undamaged by the immune system (15, 39). Thus, in approximately 10% of adult women HPV-16 persists in the uterine cervix for more than 2 years. These women are at high risk to develop intraepithelial neoplastic lesions that may progress to invasive cancer over a period of 1 to 2 decades. During chronic infection, HPV-16 remains integrated into the host genome in a nonreplicative state. However, it provides constitutive expression of two nuclear factors, E6 and E7, with transforming capacity (43, 45). These proteins represent TSAs with eventually high antigenic capacity, albeit their competence to induce effective cellular immune responses is somehow compromised in the tumor context. Thus, for instance, immunocompetent cervical cancer patients develop poor E7-specific cellular responses to endogenous as well as vaccine-administered E7 (39).
The aim of this work was to enhance the immunogenicity of the HPV-16 E7 protein by fusion to HBsAg as an immunogenic carrier in order to develop a therapeutic vaccine for cervical cancer caused by HPV-16. HBsAg was chosen because it is a safe protein that has been used during the last 2 decades as a vaccine against HBV. We reasoned that a chimeric HBsAg/E7 protein should be safe, since E7 that is anchored to an integral membrane protein is no longer able to reach the nucleus where it has its transforming effects. Furthermore, a truncated mutant of E7 without the Rb domain was also tested, which would provide additional safety in case proteolytic cleavage releases E7 from the fusion protein. In addition, we sought to develop an immunization approach that could be scaled up and applied to cervical cancer immunotherapy. Therefore, the use of adjuvants not permitted in human vaccines was avoided. Instead, we preferred vaccinating the mice with doses of recombinant Ad that, when scaled up for human application, would be in the range of 109 to 1010 IFU/dose, a range that is considered adequate and safe (25). Inoculation of mice with recombinant Ad up to 1010 IFU/dose has been used for immunization (38); however, if the dose/body weight ratio is to be maintained, this would imply inoculating patients with 1013 IFU/dose, which may have toxic effects (25).
Fusion to the C terminus of HBsAg(S) of either the complete or a truncated E7 did not affect the capacity of this protein to self-assemble into VLPs, as shown by electron microscopy. This was a major concern, since large polypeptides like the green fluorescent protein block particle secretion when fused to the carboxyl terminus of HBsAg (A. Cid-Arregui and E. Herráez-Hernández, unpublished results; S. Urban, personal communication). In addition, HBsAg/E7 protein was detected in the supernatants of transfected cells. The fact that intact VLPs could be purified from yeast cells transformed with plasmids encoding His-tagged HBsAg(S)16E7 proteins further confirmed the proper assembly of this protein into particles. Since insertions at the N terminus or near the major antigenic site of HBsAg(S) (the “a” determinant) have been found to reduce or abolish particle secretion depending on the inserted sequence (7, 9, 24), the C-terminal tail of HBsAg appears to be of choice for insertion of large foreign antigens.
A fundamental feature of virus vector-based immunization is the in situ production of exogenous proteins, which mimics in this respect a viral infection. De novo synthesis ensures presentation of virus-encoded antigens by MHC-I molecules and induction of CTLs. Moreover, released VLPs from infected cells can induce antibodies and also prime specific CD8+ CTLs (33). Accordingly, mice vaccinated with Ad vectors encoding HBsAg/E7 developed antibody and CTL responses against both E7 and HBsAg. We found that antibody (IgG) titers were higher for E7 than for HBsAg. Nonetheless, the anti-HBsAg titers obtained were comparable to those described in previous reports on DNA-mediated immunization to HBsAg (28). Given the low immunogenicity of E7 (39), our results suggest a synergistic effect of the fusion protein. This notion was further supported by the fact that the antibody response to E7 elicited by the Ad-HBsAg/E7 vaccines was higher than that induced by immunization with Ad-E7wt, in spite of the fact that this virus was inoculated at doses one order of magnitude higher. This result was somehow expected, since in contrast to E7wt, the HBsAg/E7 fusion proteins undergo secretion, which facilitates their presentation by antigen-presenting cells. A trend was noted for the Ad encoding HBsAg/truncated E7 to induce slightly higher anti-E7 titers than the virus encoding the fusion protein with the complete E7, which may be due to differences in secretion between both proteins (see below). Even though antibody responses may not play an important role in HPV-16 anti-tumor responses, they may be of interest in combined prophylactic/therapeutic vaccines carrying HPV capsid epitopes.
A major concern when using HBsAg as vaccine carrier and Ad as vector is that a significant percentage of the human population carries anti-HBsAg and/or anti-Ad antibodies as a consequence of either vaccination or natural infection. However, our results indicate that preexisting immunity neither to HBsAg(S) nor to Ad following prevaccination with recombinant protein or Ad-LacZ significantly affected the efficiency of the Ad-HBsAg(S)/E7 vectors to induce antibody responses to E7 and HBsAg. In agreement with this, relatively low neutralization titers to Ad-HBsAg(S)/E7 virus were found in mice preimmunized with three doses of Ad-LacZ, which may be explained by the low viral doses used in this study (106 IFU/dose). Further, the intramuscular administration of the vaccine may have facilitated evasion from circulating neutralizing antibodies as it has been shown for oral administration of Ad vectors (42). Prevaccination with HBsAg particles, however, gave rise to higher antibody titers to this protein than those obtained without prevaccination or prevaccinating with Ad-LacZ, as could be expected from a prime-boost protocol.
Most importantly, immunization with Ad-HBsAg(S)/E7 vectors induced E7-specific CD8+ cytotoxic T-cell reactivity both in vitro and in vivo. Significant CD8+ T-cell responses to E7 by intracellular cytokine staining for IFN-γ were seen after vaccination with Ad encoding the fusion proteins and to a remarkably lesser extent with Ad-E7wt, in contrast to control animals where no response was seen. The CD8+ responses were higher in mice immunized with Ad encoding truncated E7 fusion protein. This was in agreement with the antibody responses obtained with these viruses and may reflect the apparently higher efficiency with which HBsAg(S)16E7Δ1-35 is secreted (Fig. 2D). Even more interesting was the ability of the Ad-HBsAg(S)16EE7Δ1-35 vaccine to induce a high percentage of specific kill of E7-loaded cells as detected in spleen and regional lymph nodes, while only a fourfold lower HBsAg-specific kill against a well-defined HBsAg epitope was seen with the same vector. These results suggest that there is no competitive immunodominance of HBsAg CTL epitopes that could reduce the anti-E7 response. An additional finding of the in vivo specific kill experiments was that immunization with HBsAg/E7 recombinant protein induced lower responses than the Ad vector expressing the same protein, suggesting that genetic immunization with low doses of recombinant Ad is more efficient than protein immunization. These results further strengthen the concept of an enhancement of the E7 immunogenicity by HBsAg.
Taken together, our data support the notion that vaccines based on chimeric HBsAg(S) carrying C-terminally fused E7 could be an effective immunotherapy for cervical cancers caused by HPV-16 and at the same time provide immunization against HBV. Preclinical studies under way will determine whether the Ad-HBsAg(S)/E7 vectors of this study are also able to induce effective cell-mediated immunity in humans. In summary, a vaccine approach based on the combination of HBsAg(S) as carrier, codon-optimized sequences, and adenovirus delivery appears to be safe and efficient for the improvement of antibody and CTL responses to tumor antigens and could be useful to develop therapeutically relevant polytopic vaccines targeting viral- and tumor-specific antigens.
Acknowledgments
We gratefully acknowledge M. von Knebel-Doeberitz for support, S. Vilaró for helpful discussions, T. Kowalik for the Ad-E7wt vector, H. Delius and W. Hunziker for plasmid sequencing, W. Weinig for synthesis of oligonucleotides, B. Hub for assistance with the electron microscopy, and K. Müller, D. Pfeiffer, I. Kupin, and D. Muth for excellent technical assistance.
A. B.-A. was supported by the Instituto Costarricense Contra el Cancer (ICCC) and the DKFZ. E. H.-H. was the recipient of a short-term EMBO fellowship.
REFERENCES
- 1.Aichele, P., K. Brduscha-Riem, S. Oehen, B. Odermatt, R. M. Zinkernagel, H. Hengartner, and H. Pircher. 1997. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity 6:519-529. [DOI] [PubMed] [Google Scholar]
- 2.Bontkes, H. J., T. D. de Gruijl, A. J. van den Muysenberg, R. H. Verheijen, M. J. Stukart, C. J. Meijer, R. J. Scheper, S. N. Stacey, M. F. Duggan Keen, P. L. Stern, S. Man, L. K. Borysiewicz, and J. M. Walboomers. 2000. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int. J. Cancer. 88:92-98. [PubMed] [Google Scholar]
- 3.Bruss, V., and D. Ganem. 1991. Mutational analysis of hepatitis B surface antigen particle assembly and secretion. J. Virol. 65:3813-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cid-Arregui, A., V. Juarez, and H. zur Hausen. 2003. A synthetic E7 gene of human papillomavirus type 16 that yields enhanced expression of the protein in mammalian cells and is useful for DNA immunization studies. J. Virol. 77:4928-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson, E. J., R. L. Faulkner, P. Sehr, M. Pawlita, L. J. Smyth, D. J. Burt, A. E. Tomlinson, J. Hickling, H. C. Kitchener, and P. L. Stern. 2004. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7). Vaccine 22:2722-2729. [DOI] [PubMed] [Google Scholar]
- 6.Davis, H. L., M. L. Michel, and R. G. Whalen. 1993. DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody. Hum. Mol. Genet. 2:1847-1851. [DOI] [PubMed] [Google Scholar]
- 7.Delpeyroux, F., N. Chenciner, A. Lim, M. Lambert, Y. Malpiece, and R. E. Streeck. 1987. Insertions in the hepatitis B surface antigen. Effect on assembly and secretion of 22-nm particles from mammalian cells. J. Mol. Biol. 195:343-350. [DOI] [PubMed] [Google Scholar]
- 8.Delpeyroux, F., N. Chenciner, A. Lim, Y. Malpiece, B. Blondel, R. Crainic, S. van der Werf, and R. E. Streeck. 1986. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science 233:472-475. [DOI] [PubMed] [Google Scholar]
- 9.Delpeyroux, F., E. Van Wezel, B. Blondel, and R. Crainic. 1990. Structural factors modulate the activity of antigenic poliovirus sequences expressed on hybrid hepatitis B surface antigen particles. J. Virol. 64:6090-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dermime, S., D. E. Gilham, D. M. Shaw, E. J. Davidson, K. Meziane el, A. Armstrong, R. E. Hawkins, and P. L. Stern. 2004. Vaccine and antibody-directed T cell tumour immunotherapy. Biochim. Biophys. Acta 1704:11-35. [DOI] [PubMed] [Google Scholar]
- 11.Dubois, M. F., C. Pourcel, S. Rousset, C. Chany, and P. Tiollais. 1980. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc. Natl. Acad. Sci. USA 77:4549-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evander, M., K. Edlund, A. Gustafsson, M. Jonsson, R. Karlsson, E. Rylander, and G. Wadell. 1995. Human papillomavirus infection is transient in young women: a population-based cohort study. J. Infect. Dis. 171:1026-1030. [DOI] [PubMed] [Google Scholar]
- 13.Feuerer, M., P. Beckhove, N. Garbi, Y. Mahnke, A. Limmer, M. Hommel, G. J. Hammerling, B. Kyewski, A. Hamann, V. Umansky, and V. Schirrmacher. 2003. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat. Med. 9:1151-1157. (First published 10 August 2003; 10.1038/nm914.) [DOI] [PubMed] [Google Scholar]
- 14.Frazer, I. H., M. Quinn, J. L. Nicklin, J. Tan, L. C. Perrin, P. Ng, V. M. O'Connor, O. White, N. Wendt, J. Martin, J. M. Crowley, S. J. Edwards, A. W. McKenzie, S. V. Mitchell, D. W. Maher, M. J. Pearse, and R. L. Basser. 2004. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine 23:172-181. [DOI] [PubMed] [Google Scholar]
- 15.Ganem, D. 1996. Hepadnaviridae and their replication, p. 1199-1233. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fundamental virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 16.Hallez, S., P. Simon, F. Maudoux, J. Doyen, J. C. Noel, A. Beliard, X. Capelle, F. Buxant, I. Fayt, A. C. Lagrost, P. Hubert, C. Gerday, A. Burny, J. Boniver, J. M. Foidart, P. Delvenne, and N. Jacobs. 2004. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol. Immunother. 53:642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, X. S., H. S. Chen, K. Chu, M. Rivkina, and W. S. Robinson. 1996. Costimulatory protein B7-1 enhances the cytotoxic T cell response and antibody response to hepatitis B surface antigen. Proc. Natl. Acad. Sci. USA 93:7274-7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho, G. Y., R. Bierman, L. Beardsley, C. J. Chang, and R. D. Burk. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423-428. [DOI] [PubMed] [Google Scholar]
- 19.Hui, J., M. Mancini, G. Li, Y. Wang, P. Tiollais, and M. L. Michel. 1999. Immunization with a plasmid encoding a modified hepatitis B surface antigen carrying the receptor binding site for hepatocytes. Vaccine 17:1711-1718. [DOI] [PubMed] [Google Scholar]
- 20.Jochmus, I., W. Osen, A. Altmann, G. Buck, B. Hofmann, A. Schneider, L. Gissmann, and H. G. Rammensee. 1997. Specificity of human cytotoxic T lymphocytes induced by a human papillomavirus type 16 E7-derived peptide. J. Gen. Virol. 78:1689-1695. [DOI] [PubMed] [Google Scholar]
- 21.Kadish, A. S., G. Y. Ho, R. D. Burk, Y. Wang, S. L. Romney, R. Ledwidge, and R. H. Angeletti. 1997. Lymphoproliferative responses to human papillomavirus (HPV) type 16 proteins E6 and E7: outcome of HPV infection and associated neoplasia. J. Natl. Cancer Inst. 89:1285-1293. [DOI] [PubMed] [Google Scholar]
- 22.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 23.Kowalczyk, D. W., A. P. Wlazlo, S. Shane, and H. C. Ertl. 2001. Vaccine regimen for prevention of sexually transmitted infections with human papillomavirus type 16. Vaccine 19:3583-3590. [DOI] [PubMed] [Google Scholar]
- 24.Lee, I. H., C. H. Kim, and W. S. Ryu. 1996. Presentation of the hydrophilic domains of hepatitis C viral E2 envelope glycoprotein on hepatitis B surface antigen particles. J. Med. Virol. 50:145-151. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein, D. L., and W. S. Wold. 2004. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 11:819-829. [DOI] [PubMed] [Google Scholar]
- 26.Liu, C. C., D. Yansura, and A. D. Levinson. 1982. Direct expression of hepatitis B surface antigen in monkey cells from an SV40 vector. DNA 1:213-221. [DOI] [PubMed] [Google Scholar]
- 27.Liu, M., B. Acres, J. M. Balloul, N. Bizouarne, S. Paul, P. Slos, and P. Squiban. 2004. Gene-based vaccines and immunotherapeutics. Proc. Natl. Acad. Sci. USA 101(Suppl. 2):14567-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel, M. L., H. L. Davis, M. Schleef, M. Mancini, P. Tiollais, and R. G. Whalen. 1995. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc. Natl. Acad. Sci. USA 92:5307-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel, M. L., M. Mancini, E. Sobczak, V. Favier, D. Guetard, E. M. Bahraoui, and P. Tiollais. 1988. Induction of anti-human immunodeficiency virus (HIV) neutralizing antibodies in rabbits immunized with recombinant HIV-hepatitis B surface antigen particles. Proc. Natl. Acad. Sci. USA 85:7957-7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netter, H. J., T. B. Macnaughton, W. P. Woo, R. Tindle, and E. J. Gowans. 2001. Antigenicity and immunogenicity of novel chimeric hepatitis B surface antigen particles with exposed hepatitis C virus epitopes. J. Virol. 75:2130-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ressing, M. E., W. J. van Driel, E. Celis, A. Sette, M. P. Brandt, M. Hartman, J. D. Anholts, G. M. Schreuder, W. B. ter Harmsel, G. J. Fleuren, B. J. Trimbos, W. M. Kast, and C. J. Melief. 1996. Occasional memory cytotoxic T-cell responses of patients with human papillomavirus type 16-positive cervical lesions against a human leukocyte antigen-A *0201-restricted E7-encoded epitope. Cancer Res. 56:582-588. [PubMed] [Google Scholar]
- 32.Rogoff, H. A., M. T. Pickering, F. M. Frame, M. E. Debatis, Y. Sanchez, S. Jones, and T. F. Kowalik. 2004. Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol. Cell. Biol. 24:2968-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schirmbeck, R., K. Melber, T. Mertens, and J. Reimann. 1994. Antibody and cytotoxic T-cell responses to soluble hepatitis B virus (HBV) S antigen in mice: implication for the pathogenesis of HBV-induced hepatitis. J. Virol. 68:1418-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schirmbeck, R., D. Stober, S. El Kholy, P. Riedl, and J. Reimann. 2002. The immunodominant, Ld-restricted T cell response to hepatitis B surface antigen (HBsAg) efficiently suppresses T cell priming to multiple Dd-, Kd-, and Kb-restricted HBsAg epitopes. J. Immunol. 168:6253-6262. [DOI] [PubMed] [Google Scholar]
- 35.Schirmbeck, R., J. Wild, and J. Reimann. 1998. Similar as well as distinct MHC class I-binding peptides are generated by exogenous and endogenous processing of hepatitis B virus surface antigen. Eur. J. Immunol. 28:4149-4161. [DOI] [PubMed] [Google Scholar]
- 36.Smyth, L. J., M. I. Van Poelgeest, E. J. Davidson, K. M. Kwappenberg, D. Burt, P. Sehr, M. Pawlita, S. Man, J. K. Hickling, A. N. Fiander, A. Tristram, H. C. Kitchener, R. Offringa, P. L. Stern, and S. H. Van Der Burg. 2004. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin. Cancer Res 10:2954-2961. [DOI] [PubMed] [Google Scholar]
- 37.Sonnhammer, E. L. L., G. von Heijne, and A. Krogh. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences, p. pp175-182. In J. Glasgow, T. Litteljohn, F. Major, R. Lathrop, D. Sankoff, and C. Sensen (ed.), Proceedings of the 6th International Conference on Intelligent Systems for Molecular Biology. AAAI Press, MenloPark, Calif. [PubMed]
- 38.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tindle, R. W. 2002. Immune evasion in human papillomavirus-associated cervical cancer. Nat. Rev. Cancer 2:59-65. [DOI] [PubMed] [Google Scholar]
- 40.Villa, L. L., R. L. Costa, C. A. Petta, R. P. Andrade, K. A. Ault, A. R. Giuliano, C. M. Wheeler, L. A. Koutsky, C. Malm, M. Lehtinen, F. E. Skjeldestad, S. E. Olsson, M. Steinwall, D. R. Brown, R. J. Kurman, B. M. Ronnett, M. H. Stoler, A. Ferenczy, D. M. Harper, G. M. Tamms, J. Yu, L. Lupinacci, R. Railkar, F. J. Taddeo, K. U. Jansen, M. T. Esser, H. L. Sings, A. J. Saah, and E. Barr. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 6:271-278. [DOI] [PubMed] [Google Scholar]
- 41.von Brunn, A., K. Fruh, H. M. Muller, H. W. Zentgraf, and H. Bujard. 1991. Epitopes of the human malaria parasite P. falciparum carried on the surface of HBsAg particles elicit an immune response against the parasite. Vaccine 9:477-484. [DOI] [PubMed] [Google Scholar]
- 42.Xiang, Z. Q., G. P. Gao, A. Reyes-Sandoval, Y. Li, J. M. Wilson, and H. C. Ertl. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 77:10780-10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.zur Hausen, H. 1999. Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin. Cancer Biol. 9:405-411. [DOI] [PubMed] [Google Scholar]
- 44.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288:F55-78. [DOI] [PubMed] [Google Scholar]
- 45.zur Hausen, H. 1991. Viruses in human cancers. Science 254:1167-1173. [DOI] [PubMed] [Google Scholar]
- 46.Zwaveling, S., S. C. Ferreira Mota, J. Nouta, M. Johnson, G. B. Lipford, R. Offringa, S. H. van der Burg, and C. J. Melief. 2002. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J. Immunol. 169:350-358. [DOI] [PubMed] [Google Scholar]