Abstract
Herpesvirus envelopment is assumed to follow an uneconomical pathway including primary envelopment at the inner nuclear membrane, de-envelopment at the outer nuclear membrane, and reenvelopment at the trans-Golgi network. In contrast to the hypothesis of de-envelopment by fusion of the primary envelope with the outer nuclear membrane, virions were demonstrated to be transported from the perinuclear space to rough endoplasmic reticulum (RER) cisternae. Here we show by high-resolution microscopy that herpes simplex virus 1 envelopment follows two diverse pathways. First, nuclear envelopment includes budding of capsids at the inner nuclear membrane into the perinuclear space whereby tegument and a thick electron dense envelope are acquired. The substance responsible for the dense envelope is speculated to enable intraluminal transportation of virions via RER into Golgi cisternae. Within Golgi cisternae, virions are packaged into transport vacuoles containing one or several virions. Second, for cytoplasmic envelopment, capsids gain direct access from the nucleus to the cytoplasm via impaired nuclear pores. Cytoplasmic capsids could bud at the outer nuclear membrane, at membranes of RER, Golgi cisternae, and large vacuoles, and at banana-shaped membranous entities that were found to continue into Golgi membranes. Envelopes originating by budding at the outer nuclear membrane and RER membrane also acquire a dense substance. Budding at Golgi stacks, designated wrapping, results in single virions within small vacuoles that contain electron-dense substances between envelope and vacuolar membranes.
Much controversy has arisen about the pathway of herpesvirus capsids from their nuclear origin to the site of their release into the extracellular space (e.g., see references 4, 13, 19, 50, 52, 53, 55, and 56). The current widely accepted view suggests the formation of primary virions comprising capsid, primary tegument, and a primary envelope that originates by budding at the inner nuclear membrane into the perinuclear space (37). For de-envelopment, the primary envelope is assumed to be inserted into the outer nuclear membrane by fusion, releasing capsid and the primary tegument into the cytoplasm (14). In contrast to de-envelopment, many investigations clearly demonstrate that “primary” virions are transported from the perinuclear space into rough endoplasmic reticulum (RER) cisternae (12, 15, 45, 51, 52, 58, 62) and that “primary” wild-type virions can accumulate within the perinuclear space-RER compartment (55). Intraluminal accumulations of virions have also been explained as a failure in de-envelopment, e.g., due to the lack of US3 protein in pseudorabies virus (25). The de-envelopment theory also does not consider that membrane fusion is a fast but well-studied process starting by close apposition of the membranes to allow fusion followed by pore formation (24, 27, 32, 36). Recognizing close apposition and pore formation is imperative to distinguishing fusion from budding and fission. To our knowledge, pore formation between “primary” envelopes and the outer nuclear membrane has not been demonstrated by conventional electron microscopy.
In an attempt to gain more detailed information on interactions between the “primary” envelope and the outer nuclear membrane, we employed a technique that allows arresting of cellular processes in the range of milliseconds (38) and that keeps membranes in place even though they are disintegrating (60). Instead of fusion events at the outer nuclear membrane, we found (i) capsids budding from the cytoplasm into the perinuclear space (62) and (ii) impaired nuclear pores with capsids entering the cytoplasmic matrix in bovine herpesvirus 1 (BHV-1)-infected cells (59). These novel findings implied the existence of at least two different pathways of herpesvirus envelopment, as suggested more than a decade ago (31). One pathway involves budding of capsids at the inner nuclear membrane resulting in fully enveloped virions, which are transported from the perinuclear space via a continuous membrane system directly into Golgi cisternae for packaging into transport vacuoles (62). Capsids following the other pathway escape the nucleus via dilated nuclear pores, approach Golgi complexes from the cytoplasmic side, and are wrapped by Golgi membranes. Wrapping results in an enveloped virion within a small concentric vacuole (59).
For this study, we investigated the pathways of herpes simplex virus type 1 (HSV-1) capsids from the nucleus to the cell periphery, employing microscopic techniques that yield both high temporal and spatial resolution. The high temporal resolution allows the investigation of membrane-bound processes in the millisecond range (49) so that diverse phases of fusion, budding, or fission can be visualized. The high spatial resolution offers an intact cellular architecture of high clarity, enabling subtle distinctions between cellular compartments. A careful examination of hundreds of cells revealed that HSV-1 envelopment follows fundamentally identical pathways to those of BHV-1. Immunofluorescence microscopy demonstrated the translocation of nuclear pore complex (NPC) proteins to the cytoplasm, confirming the idea of nuclear pore impairment drawn on the basis of electron microscopic observation. In addition to BHV-1 capsids, HSV-1 capsids were found to bud at any intracellular membrane, including the outer nuclear membrane, RER membranes, Golgi membranes, and membranes of vacuoles.
MATERIALS AND METHODS
Cells and viruses.
Vero cells (ECACC) and HeLa cells (American Type Culture Collection) were propagated in Dulbecco's modified minimal essential medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum. Vero 2-2 cells, which contain the HSV-1 immediate-early 2 gene (54), were cultivated in Dulbecco's modified minimal essential medium containing penicillin-streptomycin, 10% fetal bovine serum, and G418 (Geneticin; 500 μg/ml; GIBCO-BRL, Basel, Switzerland). Wild-type HSV-1 strain F was grown in Vero cells.
Infection of cells.
Vero cells, HeLa cells, and Vero 2-2 cells were grown for 2 days on 30-μm-thick sapphire disks with a diameter of 3 mm (Bruegger, Minusio, Switzerland) for low-temperature transmission electron microscopy (LTEM) or on coverslips for immunolabeling of the nuclear pore protein Nup153 and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining to detect apoptosis. Sapphire disks were covered with 8- to 10-nm carbon, obtained by evaporation under high-vacuum conditions, to enhance cell growth. Cells were infected with HSV-1 at a multiplicity of infection (MOI) of 1 or 4 in 1 ml of medium per well in six-well plates and kept at 37 C° for 1 h to allow adsorption prior to incubation at 37°C for 8 to 24 h.
Freezing of cells.
Cells grown on sapphire disks were frozen in a high-pressure freezer (HPM010; BAL-TEC Inc., Balzers, Liechtenstein) as described in detail previously (62) early in infection (8 to 10 h) to late in infection (15 to 18 h). The frozen samples were stored in liquid nitrogen until use.
LTEM.
Frozen cells grown on sapphire disks were transferred to a freeze-substitution unit (FS 7500; Boeckeler Instruments, Tucson, Arizona) precooled to −88°C for substitution with acetone and subsequent fixation with 0.25% glutaraldehyde and 0.5% osmium tetroxide at temperatures between −30°C and +2°C as described in detail previously (61) and were embedded in Epon at 4°C. Sections of 50 to 60 nm in thickness were analyzed in a transmission electron microscope (CM12; Philips, Eindhoven, The Netherlands) equipped with a slow-scan charge-coupled device camera (Gatan, Pleasanton, CA) at an acceleration voltage of 100 kV.
Immunolabeling.
For the demonstration of nuclear pores by confocal microscopy, cells grown on coverslips were infected with HSV-1 at an MOI of 1 and incubated at 37°C. Cells were fixed in methanol-acetone (1:1) at −20°C for 15 to 30 min at 2 to 12 h postinfection, rehydrated in phosphate-buffered saline (PBS) at room temperature (RT) for 5 min, treated with 0.1% Triton X-100 for 5 to 10 min, and after being blocked with 3% bovine serum albumin in PBST (PBS and 0.05% Tween 20), incubated with polyclonal antibodies raised in sheep against the nuclear pore protein Nup153 (Immunoquest, Cleveland, Great Britain) at 4°C overnight. After being washed with PBST containing 3% milk, cells were incubated with donkey anti-sheep secondary antibodies conjugated to Texas Red (Jackson ImmunoResearch, Philadelphia, PA) and with 1 μg/ml DAPI (4′,6′-diamidino-2-phenylindole) in PBST containing 3% bovine serum albumin at RT for 1 h. To ascertain if the changes in nuclear pore proteins were related to HSV-1 infection, ICP4 was stained with monoclonal mouse antibodies (Advanced Biotechnologies, Columbia, MD) and sheep anti-mouse antibodies coupled to fluorescein isothiocyanate (FITC; Boehringer, Mannheim, Germany). After being washed with PBST containing 3% milk and finally with PBS, samples were fixed with 2% formaldehyde for 15 min, washed in water, and embedded in VectaShield hard medium (Vector Laboratories, Burlingame, CA). For controls, mock-infected cells were used, and the secondary antibodies were omitted.
TUNEL and N-hydroxysuccinimide-biotin staining.
For recognition of apoptosis or necrosis, cells grown on coverslips were infected with HSV-1 at an MOI of 1 and incubated for 8 h. Cells were then incubated with 0.1 mg/ml N-hydroxysuccinimide-biotin (Pierce) in PBS for 15 min on ice and fixed with 2% formaldehyde in PBS. Samples were permeabilized with 0.1% Triton X-100 in PBS for 1 min at RT and stained for DNA strand breaks using a terminal transferase kit (Roche, Basel, Switzerland) for the incorporation of dUTP-FITC (Roche) overnight at 37°C, as described previously (28). Subsequently, cells were incubated at RT for 1 h with 0.5 μg/ml streptavidin-Texas Red (Amersham Biosciences, Little Chalfont, Great Britain) and 1 μg/ml DAPI in PBS. Samples were embedded in VectaShield hard medium. For the induction of apoptosis, uninfected cells were incubated with staurosporin (1 μg/ml) at 37°C for 4 h.
Confocal microscopy.
Samples were analyzed using a confocal laser scanning microscope (SP2; Leica, Mannheim, Germany). Images were deconvolved by employing a blind deconvolution algorithm using the program suite Huygens Essential (SVI, Hilversum, The Netherlands).
RESULTS
The goal of this study was to analyze the interactions of capsids with the nuclear membranes and Golgi membranes and to clarify the origin of capsids occurring within the cytoplasm. Therefore, we infected Vero cells, Vero 2-2 cells, and HeLa cells grown on sapphire disks with HSV-1 and arrested virus production and egress after incubation at 37°C for 8 to 17 h by immediate freezing. After freeze substitution and embedding, serial sections were cut parallel to the smooth surface from which the sapphire disks were removed. This procedure enabled examinations of virus-cell interactions in an in situ situation with a delay of less than 15 seconds between removal of the sapphire disks from the incubation chamber and freezing (62). All processes of envelopment at the nuclear envelope, RER, and Golgi complex could be readily visualized due to the well-preserved ultrastructure.
Nuclear envelope.
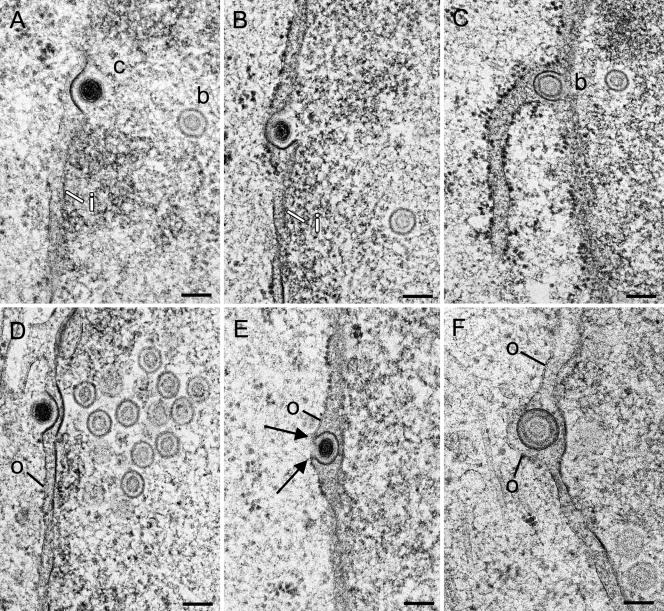
Capsids escaping the nucleus via budding at the inner nuclear membrane were found at various stages of the process. At initial stages, capsids were in close apposition to the inner nuclear membrane, which was slightly thickened and indented into the perinuclear space. The thickening always expanded exactly from one side, where the membrane indentation started, to the other side, forming a protrusion in which the capsid was located (Fig. 1A). At intermediate to late stages, capsids embedded in tegument and surrounded by a dense envelope were within the perinuclear space, with the dense envelope still continuing into the inner nuclear membrane (Fig. 1B and C). Similar phenotypes of capsid-membrane interactions were found at the outer nuclear membrane (Fig. 1D and E). A comparison with fusion (Fig. 2) led to the conclusion that these phenotypes of capsid-membrane interactions are very likely to represent budding events. This idea is strongly supported by the contour of the outer nuclear membrane in relation to the viral envelope showing indications of fission (Fig. 1F). Interestingly, the incidence of events at the outer nuclear membrane was much higher than that at the inner nuclear membrane, at least late in infection (Table 1). Virions were rarely found within the perinuclear space early in infection. Their number progressively increased during the course of incubation (Table 1).
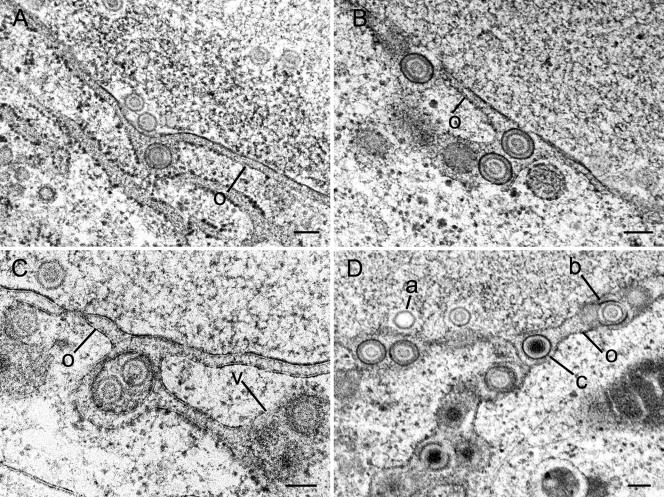
FIG. 1.
Budding capsids at inner and outer nuclear membranes in Vero and Vero 2-2 cells infected with HSV-1 at 10 h (B), 12 h (A, C, D, and E), and 15 h (F) postincubation. (A) C-capsid (c) at the beginning of budding at the inner nuclear membrane (i), which is thickened by an electron-dense substance at the site of budding. (B) Budding C-capsid with about three-fourths of the envelope being part of the inner nuclear membrane. Tegument is present only between the capsid and the thickened membrane. (C) Budding B-capsid (b), probably at the inner nuclear membrane, that is pushed into the perinuclear space at the site of an associated RER cisterna. (D) C-capsid at the beginning of budding at the outer nuclear membrane. Both the inner and outer nuclear membranes are thickened by an electron-dense substance. At the nuclear periphery is a small cluster of B-capsids. (E) C-capsid close to completion of budding at the outer nuclear membrane (o). The membrane (about four-fifths of the perimeter) around the capsid is thickened and turns in a sharp loop (arrows) into the normal outer nuclear membrane. (F) B-capsid within the perinuclear space containing tegument and a dense envelope, probably immediately after fission from the outer nuclear membrane, which is slightly indented. Bars, 100 nm.
FIG. 2.
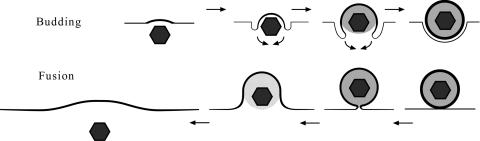
Schematic drawing of budding and fusion. Budding induces thickening of the membrane that is pulled behind the capsid. The space between capsids and the envelope is progressively filled by tegument. The resulting virion is finally released by fission. Fusion starts by close apposition of the virion to the membrane followed by pore formation between the envelope and the membrane. Capsid and tegument are rapidly released, and the membrane is flattened because there are no forces to keep it bent. The thickened membrane would persist at the entire length equaling the viral surface.
TABLE 1.
Number of virus particles during envelopment or of impaired nuclear pores in 20 cellular profiles chosen at random from ultrathin sections of Vero cells incubated for 12 or 15 h after infection with HSV-1 at an MOI of 1
| Pathwaya | Phenotypeb | No. of particles with phenotype or indicated value
|
||
|---|---|---|---|---|
| 8 h | 12 h | 15 h | ||
| NE | Budding IM | 1 | 7 | |
| NE/CE | Virions in PNS | 3 | 81 | |
| NE/CE | Virions in RER | 0 | 18 | |
| NE/CE | Virions in Golgi cisternae | 6 | 2 | |
| NE/CE | Virions in P vacuoles | 47 | 90 | |
| Number of P vacuoles | 19 | 25 | ||
| CE | Cytoplasmic capsids | 0 | 61 | |
| CE | Budding at OM | 1 | 16 | |
| CE | Budding at RER | 0 | 1 | |
| CE | Budding at Golgi cisternae | 15 | 2 | |
| CE | Budding at Golgi fragments | 2 | 5 | |
| CE | Budding at P vacuoles | 14 | 21 | |
| CE | W vacuoles | 41 | 16 | |
| CE | Impaired nuclear pores | 1 | 3 | |
| Ratio of Nup153 dislocation to ICP4 particles | 1 | |||
NE, nuclear envelopment; CE, cytoplasmic envelopment.
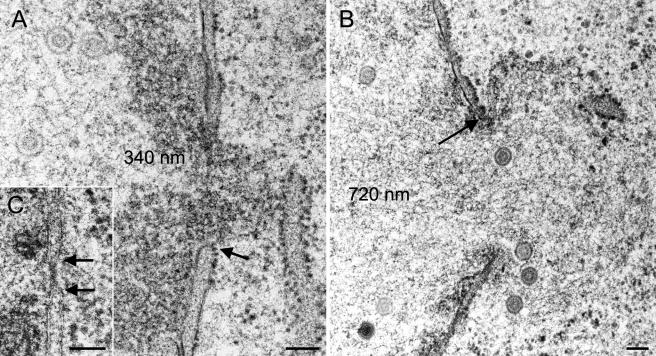
The capsid-membrane interactions at the outer nuclear membrane that are more likely budding than fusion events question the theory of de-envelopment of primary virions at the outer nuclear membrane. If the viral envelope does not fuse with the outer nuclear membrane, then cytoplasmic capsids must escape the nucleus via another route, e.g., nuclear pores. Intact isolated nuclear pores measure 125 nm in diameter and are occupied by the NPC (40), which controls nuclear import and export of proteins up to 28 nm (41). In sections where nuclear membranes are hit perpendicular to the nuclear surface, details of both nuclear membranes and nuclear pores can be visualized. Intact nuclear pores measured 100 to 120 nm in diameter in both mock-infected and HSV-1-infected cells (Fig. 3A, inset). After 12 h of infection, nuclear pores were measured to be 300 to 500 nm in width (Fig. 3A and B), and occasionally up to 1,000 nm. Nuclear material with or without capsids (Fig. 3B) protruded through dilated pores into the cytoplasm. However, nuclear material never merged with the cytoplasmic matrix. The number of impaired nuclear pores in a given section through a nucleus was low for up to 12 h postinfection. It increased when infection proceeded (Table 1). Loss of integrity of the nuclear envelope was only considered an impaired nuclear pore when the inner nuclear membrane obviously continued into the outer nuclear membrane in at least one location (Fig. 3A). This probably has led to an underestimation of the actual number of impaired nuclear pores.
FIG. 3.
Impairment of nuclear pores in Vero 2-2 cells and HeLa cells infected with HSV-1 after 12 h (A) and 14 h (B) of incubation. (A) Dilated nuclear pore with intact nuclear membrane (arrow) and nuclear substance protruding into the cytoplasm. (B) B-capsids escaping through an impaired nuclear pore with intact membrane at the border (arrow). (C) Intact nuclear pore of a mock-infected HeLa cell (17 h) with a distinctly visible nuclear pore complex and bordering membranes (arrows). Bars, 100 nm.
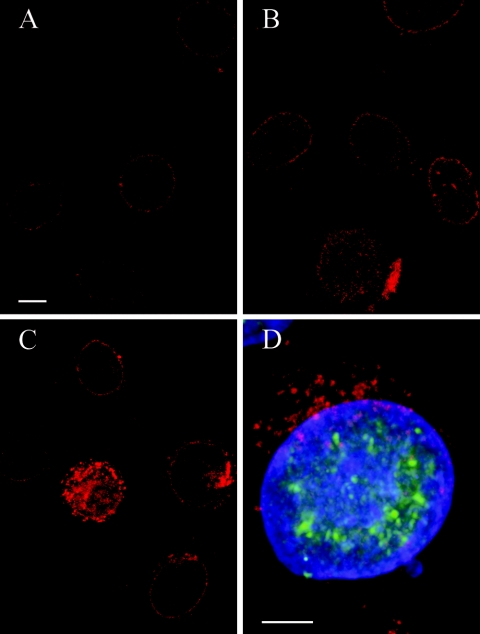
In order to confirm nuclear pore impairment and to clarify the fate of NPCs, we visualized the NPC protein Nup135 (42) by immunolabeling. Confocal microscopy revealed a regular distribution of fluorescence signals at the nuclear periphery in mock-infected cells and cells incubated with HSV-1 for 2 and 4 h (Fig. 4A). Fluorescence signals were gradually irregular at the nuclear surface and concomitantly accumulated in the cytoplasm from 6 to 8 h of incubation (Fig. 4B and C). The codetection of HSV-1 ICP4 demonstrated that all cells infected with HSV-1 showed this pattern of fluorescence signals (Fig. 4D), indicating a dislocation of Nup153 from nuclear pore complexes into the cytoplasm. Neither TUNEL nor biotin staining revealed any indications of apoptosis or necrosis, respectively, in cells incubated with HSV-1 for 8 h, whereas apoptosis was apparent in control cells incubated for only 4 h with staurosporin (data not shown).
FIG. 4.
Confocal microscopy of HeLa cells after immunolabeling of pore complex protein Nup153 (Texas Red) and of HSV-1 immediate-early protein ICP4 (D) and subsequent DAPI staining. (A) Faint Texas Red signals are regularly distributed at the nuclear surface in cells incubated for 4 h with HSV-1. (B) Signal intensity is increased at the nuclear surface in cells incubated for 6 h. (C) Irregular accumulation of Texas Red signals at the nuclear periphery and in the cytoplasm at 8 h postinfection. (D) Merger of Texas Red, FITC, and DAPI staining demonstrating that dislocation of Nup153 from the nuclear periphery into the cytoplasm occurred only in cells expressing HSV-1 virus protein ICP4 (FITC). Bars, 5 μm.
Perinuclear space, RER, Golgi complex, and transport vacuoles.
If capsids are not released from the perinuclear space by de-envelopment, then two other pathways may be possible, namely, the release of virions by vesicle formation at the outer nuclear membrane and intraluminal transportation into RER cisternae. We did not find any indications for vesicle formation. Virions, however, were found in RER cisternae adjacent to the perinuclear space (Fig. 5A to D), indicating intraluminal transportation. All of these virions consisted of capsid, tegument, and a distinctly thickened, dense envelope. Interestingly, capsids were also in close apposition at the cytoplasmic sides of RER cisternae, where the membrane was slightly thickened, or in indentations delineated by a distinctly thickened, dense membrane (Fig. 6C and D). These capsid-membrane interactions are also considered likely to represent stages of budding because of their similarity to the events at the inner nuclear membrane. If this is true, then budding will result in fully enveloped virions that need to be transported within RER cisternae in order to be released finally into the extracellular space.
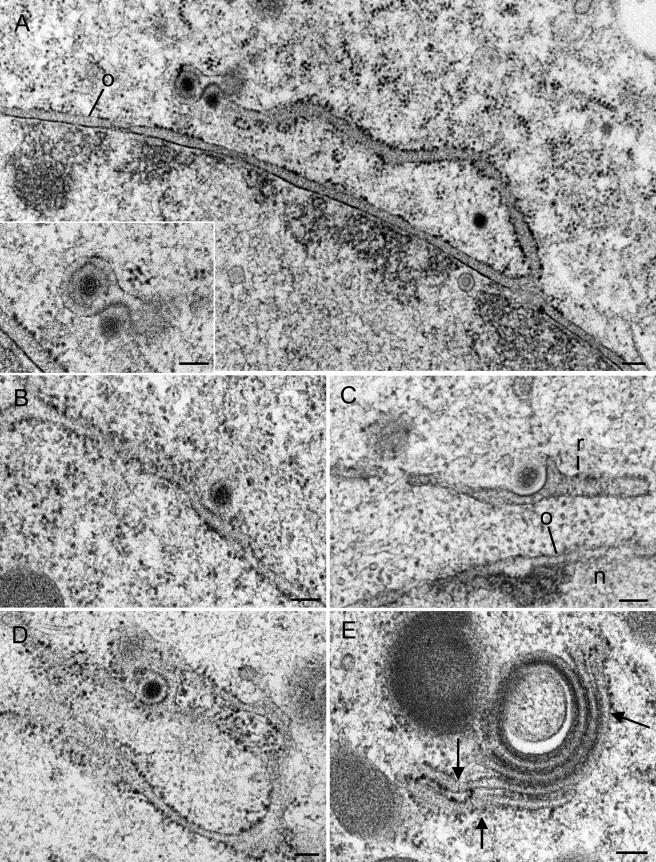
FIG. 5.
Virions within perinuclear space and associated RER cisternae 12 h (B), 15 h (C), and 17 h (A and D) after infection of Vero and Vero 2-2 cells with HSV-1. (A to C) Single virions comprising one or two (C) B-capsids, tegument, and dense envelope within the perinuclear space (A) and associated RER cisternae (B and C) that continue into a vacuole-like structure (v) sectioned tangentionally in panel C. Note the dilation of the perinuclear space and RER at sites where virions are present. (D) Virions containing B- and C-capsids queue within the perinuclear space and associated RER cisternae. a, A-capsid; o, outer nuclear membrane. Bars, 100 nm.
FIG.6.
Budding capsids in RER cisternae of Vero 2-2 cells at 12 h postinfection (A, C, and D) and of HeLa cells at 16 h postinfection (B and E) with HSV-1. (A) RER membrane continuous with the outer nuclear membrane (o) is associated with Golgi membranes surrounding a virion (inset). A cytoplasmic capsid is in an early phase of budding at the site where RER and Golgi membranes are associated. (B to D) Capsids in initial (B), early (C), and late (D) phases of budding into RER cisternae, for which the membrane situated close to the nucleus (n) has only a few ribosomes (r) in panel C. Note that all membrane capsid buds are thickened by a dense substance. (E) Golgi complex with narrow cisternae filled with a dense substance, probably tegument. Note the continua between Golgi and RER membranes (arrows). Bars, 100 nm.
In the quest for destinations of intraluminal virion transportation, we found (i) continua between RER and Golgi membranes (Fig. 6E) and (ii) virions within laterally dilated Golgi cisternae with strong indications of fission (Fig. 7A and B) resembling the packaging process of proteins in the secretory pathway (39). The fission of lateral cisternae would lead to vacuoles containing virions. Vacuoles containing one or several virus particles consisting of capsid, tegument, and an envelope with distinct spikes (Fig. 7C and D) were frequently found after incubation for more than 12 h (Table 1).
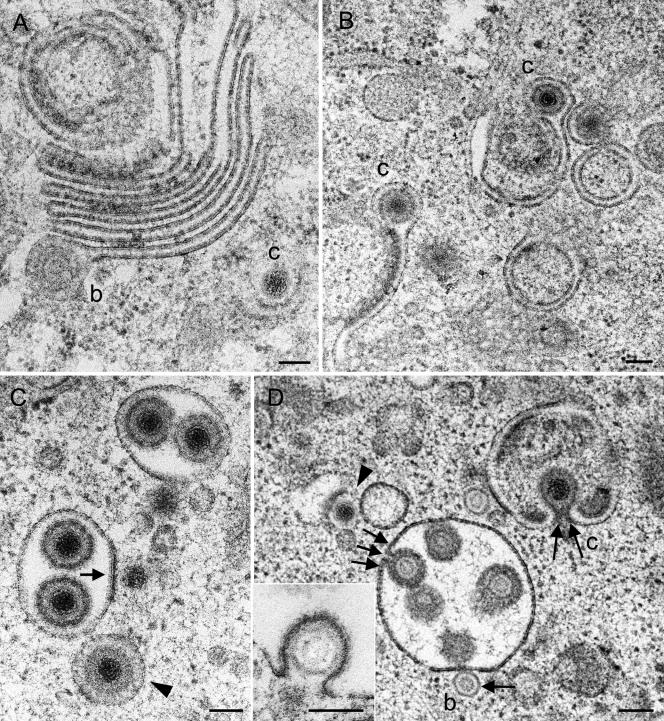
FIG. 7.
Golgi complex and packaging-derived vacuoles or cross-sectioned Golgi cisternae in Vero 2-2 cells incubated for 12 h (A, B, and C) and 15 h (D) with HSV-1. (A) Large Golgi complex bearing a virion with a B-capsid (b) at the lateral end of a Golgi cisterna in a late stage of packaging. C-capsid (c) budding is shown at a banana-shaped membrane. (B) Two single Golgi cisternae with C-capsids late in packaging. (C) Two vacuoles derived from packaging or cross-sectioned Golgi cisternae, each containing two virions, and a vacuole (arrowhead) with a virion exactly in the center, with the space between the envelope and the vacuolar membrane containing a dense substance that might have resulted from wrapping or packaging. C-capsids are in close apposition to the packaging-derived vacuoles, one of which is in an initial phase of budding (arrow). (D) B-capsid in an initial phase (arrow) and C-capsid in a late phase (two arrows) of budding into vacuoles or cross-sectioned Golgi cisternae. One virion (three arrows) is shown either immediately prior to completion of budding or in an early stage of fusion. A C-capsid (arrowhead) in an early phase of budding at a banana-shaped membrane is shown. The inset shows a B-capsid close to completion of budding into a vacuole. Bars, 100 nm.
Fate of cytoplasmic capsids.
Table 1 shows that large numbers of capsids gained access to the cytoplasm. If the capsid-membrane interactions at the outer nuclear membrane and RER membranes do indeed represent budding events, then a small portion of cytoplasmic capsids will be enveloped in this way. A much larger number of capsids were found to interact at Golgi cisternae (Fig. 8A, C, and F), Golgi-derived vacuoles (Fig. 7A and D), and banana-shaped membranes of probable Golgi origin (Fig. 8B, C, E, and G). All of these capsid-membrane interactions were of great similarity to those at the inner and outer nuclear membranes and RER membranes. They are hence considered very likely to be budding events. Budding at Golgi stacks differs significantly from budding at any other membrane, as shown in great detail for BHV-1 (59). The deriving vacuoles are small, containing only one virion with a dense envelope. The space between envelope and vacuolar membranes is filled with a dense substance (Fig. 7C and 8D). Possibly, small vacuoles derived by packaging appear similar (Fig. 8B and C).
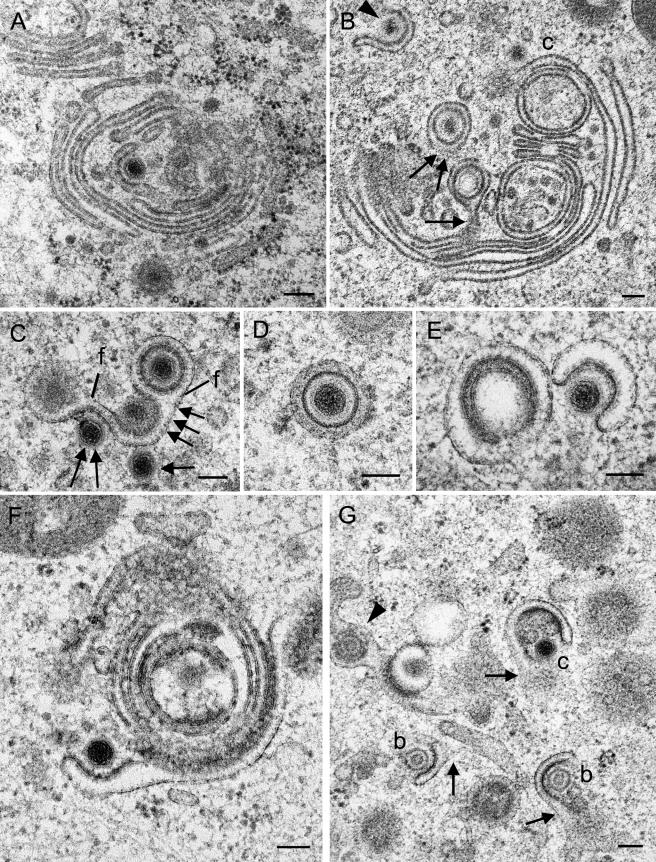
FIG.8.
Budding capsids at Golgi membranes in Vero 2-2 cells at 12 h (A, C, F, and G) and in HeLa cells at 15 h (E) and 17 h (B and D) postinfection. (A) C-capsid at initial phase of wrapping at a slightly thickened membrane in a central region of a large Golgi complex. (B) Virions inside concentric vacuoles close to the completion of fission. The vacuolar membrane is still connected to the origin in the section plane (arrow) and probably below the section plane (two arrows). A C-capsid (c) approaches a large Golgi cisterna, and another capsid (arrowhead) is midway through being wrapped by a short Golgi cisterna. (C) C-capsids in initial (arrow), early (two arrows), and late (three arrows) phases of wrapping by a Golgi cisterna, from which two virions are dispatched by fission (f) as a result of packaging. (D) Virion with a dense envelope inside a concentric vacuole, possibly derived by wrapping because of the dense substance between the envelope and the vacuolar membrane. (E) C-capsid in an early phase of wrapping by a Golgi cisterna or Golgi-derived vacuole with a surface that is not sufficient to form the substance between the envelope and the surrounding vacuolar membrane, at least in this section plane. (F) C-capsid in an early phase of budding at a banana-shaped cisterna of a large Golgi complex. (G) C-capsid and two B-capsids budding at small membrane pieces that might be connected (arrows) to each other or to other membrane entities outside of this section plane. At the left is a tangentially cut virion within a Golgi cisterna (arrowhead). Bars, 100 nm.
Golgi fields were large, consisting of up to six stacks (Fig. 7A and 8A, B, and F) in HSV-1-infected cells incubated for more than 8 h. They were, however, small in mock-infected cells (not shown), and only small entities were commonly seen in HSV-1-infected cells incubated for more than 12 h (Fig. 8B, C, E, and G).
DISCUSSION
High-resolution microscopy of HSV-1-infected cells revealed (i) budding of capsids at the inner nuclear membrane that was distinctly thickened by a dense substance; (ii) the presence of virus particles consisting of capsid, tegument, and a thick, dense envelope within the perinuclear space and RER cisternae; (iii) capsid-membrane interactions at the outer nuclear membrane resembling budding capsids at the inner nuclear membrane; (iv) budding capsids at RER membranes; (v) fission of Golgi sacs containing virions with clearly discernible spikes; (vi) the presence of two distinctly different vacuoles within the cytoplasm containing virions; (vii) budding at vacuoles and/or dilated Golgi cisternae; (viii) budding at banana-shaped membranous entities; (ix) impairment of nuclear pores; and (x) dislocation of the nuclear pore complex protein Nup153.
Budding of capsids at the inner nuclear membrane is generally accepted as the pathway of capsids to exit the nucleus. The resulting virus particle is a fully enveloped virion containing tegument (14). A crucial phenomenon is the thickening of the inner nuclear membrane at the site of budding, which has been described for many members of the herpesvirus family (4, 50). Its significance is unclear. Thickening is obviously an initial event in budding, as clearly shown for BHV-1 envelopment (59, 62). Thickening of the envelope remains in virions that have been released into the perinuclear space after fission of the envelope from the inner nuclear membrane (2-4, 6, 7, 10, 15, 30, 44, 51, 55). Interestingly, our study shows thickening also at the outer nuclear membrane, where the membrane is bent into the perinuclear space and a capsid is located in the depression. For both the inner and outer nuclear membranes, the length of the thickening was always related to the degree of bending and always expanded exactly from one side where the membrane turns around the capsid to the other side. The envelopes of virions within the perinuclear space are assumed to fuse with the outer nuclear membrane, whereby tegument and capsid are released into the cytoplasm. If this idea were true, then the length of the thickening would be expected to equal the perimeter of the viral envelope (Fig. 2). This was not found once in more than 50 events in this study and in an equal number of events in studies of BHV-1 envelopment. In the two reports describing similar events at the outer nuclear membrane (3, 13), the length of the thickened membrane was also shorter than the perimeter of the entire virion would be. This discrepancy might be due to a rapid removal of the substance responsible for the thickening, or the phenotypes of this capsid-membrane interaction may represent various stages of budding rather than fusion. Rapid removal is considered unlikely because it can be assumed that capsid and tegument are rapidly released after fusion is complete and that the membranes would flatten because there would be no need and no forces to keep them bent around an empty depression. Membrane fusion starts by close apposition followed by pore formation (20, 27, 32, 36). The envelopes of virions within the perinuclear space were occasionally seen in close apposition to the outer nuclear membrane but also to the inner nuclear membrane, as shown in detail for BHV-1 (59, 62). Membrane fusion is rapid, as demonstrated for influenza virus, which fuses with liposomes within approximately 1 min (24). Budding, on the other hand, is a more complicated process involving thickening of the membrane, deposition of tegument, pulling of the membrane behind the capsid, which is concomitantly pushed into the perinuclear space, and fission of the envelope from the nuclear membrane. Budding is thus assumed to take much longer than fusion and, consequently, the probability of hitting a budding event will be much larger than that of hitting a fusion event. To our knowledge, fusion has very rarely been shown by conventional electron microscopy. The capsid-membrane interaction at the outer nuclear membrane was more frequently encountered than budding events at the inner nuclear membranes. Capsid-membrane interactions similar to those at the nuclear membranes were also found at RER membranes, Golgi stacks, large Golgi-derived vacuoles or dilated Golgi cisternae, and small membranous banana-shaped entities that have been referred to as the trans-Golgi network (37). The similarity of the phenotypes of capsid-membrane interactions at all of these membranes, the lack of evidence of pore formation, and the large number of events at the outer nuclear membrane strongly indicate that all of these phenotypes represent stages of the very same process, namely, budding.
If the capsid-membrane interactions at the outer nuclear membrane and at RER membranes are budding rather than fusion events, then the question arises of how virions are released from the perinuclear space and how cytoplasmic capsids gain access to the cytoplasm. It was suggested, though never shown, that virions are released from the perinuclear space by vesicle formation (4, 6, 9, 15, 45, 56). Alternatively, virions of many members of the herpesvirus family were clearly demonstrated to be present in RER cisternae (5, 12, 15, 45, 51, 52, 55, 58). The envelopes of virions were often in close contact with the membranes of the perinuclear space and RER cisternae. Membranes in close apposition start to fuse immediately, provided that the fusion machinery is present (33, 34). Consequently, the viral envelope would probably fuse at the very moment it comes into close apposition with the outer nuclear membrane. It is difficult to understand why virions able to be transported through RER cisternae should have the ability to fuse with RER membranes. The presence of virions within RER cisternae and the lack of images convincingly demonstrating fusion of viral envelopes with the outer nuclear membrane imply intraluminal transportation of virions from the perinuclear space into RER cisternae. The ability of intraluminal transportation might be due to insertion of the substance responsible for thickening of the viral envelope in the course of budding. This substance of unknown nature is speculated to consist of antifusion proteins (62). A possible candidate could be glycoprotein K (gK) (21, 22). It has been assumed that gK facilitates the egress of virus particles (8, 35), possibly in concert with UL20 (8), UL31, and UL34 (48), and down regulates undesirable fusions of the viral envelope with membranes during translocation (46). gK, however, was also postulated to prevent reinfection (26) and to be involved in cell-cell fusion (1, 8, 35).
Preventing the viral envelope from fusing is probably a necessity for two reasons. First, cell membranes per se have the ability to perform fusion. It should be borne in mind, however, that fusion and fission for intracellular trafficking take place at the cytoplasmic side and not at the luminal side (34, 43). Second, gB is believed to play a significant role in fusion during cell entry. gB and other glycoproteins were localized at the inner and outer nuclear membranes (11, 12, 23, 53, 55, 56). Viral envelopes originating by budding at the nuclear membranes consequently acquire gB, which was presumed to be masked in virions during transport through the RER system (55). Glycoproteins C and D, however, were demonstrated to be on viral particles in the perinuclear space (23).
There is no doubt that virions are transported within the RER compartment. The destination of virions, however, has led to some confusion (52, 55). We recently demonstrated continua between the perinuclear space and Golgi cisternae in BHV-1-infected MDBK cells, suggesting that the destination of virus transportation through RER cisternae is the Golgi cisternae. Continua between RER and Golgi membranes were also found in this study. Since the perinuclear space continues into RER cisternae, it is reasonable to assume that the perinuclear space is continuous with Golgi cisternae via the RER. The Golgi complex is the machinery for the packaging of products in the secretory pathway (39). Indeed, the events found at lateral sides of Golgi cisternae clearly resemble packaging processes, namely, fission of vacuoles of various sizes containing one or several virions with clearly visible spikes, as described for the envelopment of BHV-1 (62).
Capsid-membrane interactions with phenotypes identical to those at nuclear membranes and RER membranes were also found at Golgi stacks, large Golgi-derived vacuoles or dilated Golgi cisternae, and small banana-shaped membrane entities that were referred to as the trans-Golgi network (37). The trans-Golgi network is proposed to be the only location where capsids acquire tegument and the final envelope (37), a process designated wrapping. Our data and those of others (3, 19, 55) do not support this theory. The small banana-shaped membrane entities with budding capsids (Fig. 5E and F) were not necessarily vacuoles at the beginning of budding. They also may be connected to the Golgi complex, as is apparent in Fig. 5G. Membranes needed for the viral envelope and the vacuolar membrane might thus be recruited directly from the remaining Golgi membrane. In this case, capsids would bud at small vacuoles as assumed previously (37), and the vacuoles would have to increase in size by the insertion of membrane constituents derived from Golgi membranes. On the basis of current knowledge, the transportation of membrane constituents would be maintained by vesicular trafficking involving fission of coated pits from Golgi membranes leading to coated vesicles, uncoating of vesicles, and fusion of uncoated vesicles where the membrane capsids bud. To our knowledge, such vesicular trafficking has not been shown so far, although the vesicles are easily recognized even by conventional electron microscopy (63). We have also not found budding capsids close to completion at such small membrane entities, and to our knowledge, this has not been reported to date. The lack of evidence for the completion of budding leads to the idea that budding at such small membrane entities arrests because of an insufficient availability of membranes. We found budding capsids at banana-shaped entities and small vacuole-like structures exclusively late in infection in those cells in which virus multiplication had progressed to a substantial extent, as judged by the number of enveloped virions within the cytoplasm. It is reasonable to assume that the amount of cell membranes, especially Golgi membranes, is reduced late in infection because they have been used for the formation of envelopes and transport vacuoles. On the other hand, budding at large vacuoles and/or dilated Golgi cisternae occurred frequently in HSV-1-infected cells, in contrast to the case for BHV-1-infected cells (59). Budding capsids at large Golgi-derived vacuoles were also demonstrated for cytomegalovirus (19) and for simian agent 8 (3). Both vacuoles and dilated Golgi cisternae are considered parts of the trans-Golgi network.
Budding at Golgi stacks differs from budding at any other site insofar as a second membrane is involved. Budding starts with thickening of the membrane by insertion of a dense substance, followed by bending of the membrane around the tegument deposited between the capsid and membrane, as shown in detail for BHV-1 (59). The result is a small concentric vacuole containing a single virion. The space between the envelope and the vacuolar membrane is occupied by a dense substance that is considered to have the same function as the dense substance at the envelopes of virions within the perinuclear space and RER cisternae, i.e., preventing fusion of the envelope with the vacuolar membrane. Preventing fusion of the envelope with the vacuolar membrane might also be a necessity for large vacuoles. In fact, a substance at low concentrations was also observed in large vacuoles.
The key question, however, is where cytoplasmic capsids originate. Careful examination of the nuclear surface revealed the dilation of nuclear pores from 160 nm early in infection up to about 1,000 nm late in infection, through which capsids can gain direct access to the cytoplasm, a pathway that was also documented for BHV-1 (59) and simian agent 8 (3). The dilation of nuclear pores was accompanied by a loss of visible NPC structures by LTEM. The dilation of nuclear pores and changes in the nuclear surface were shown by scanning electron microscopy of cells infected with BHV-1 (59) and HSV-1 (18). To clarify the fate of NPCs, we used antibodies against the NPC protein Nup153 for immunolabeling. Nup153 is a component of the basket docking at core proteins (29) and is involved in anchoring NPC to nuclear membranes (57). Confocal microscopy revealed a dislocation of Nup153 from the nuclear periphery into the cytoplasm from 2 to 8 h of incubation, indicating a breakdown of NPCs starting early in infection. Nup153 was shown to be down regulated early in infection with HSV-1 (47). The dislocation of Nup153 was not accompanied by apparent indications of apoptosis by TUNEL staining (65) or by electron microscopy. The degradation of Nup153 independent of apoptosis was also reported for cells infected with rhinovirus (17) or poliovirus (16).
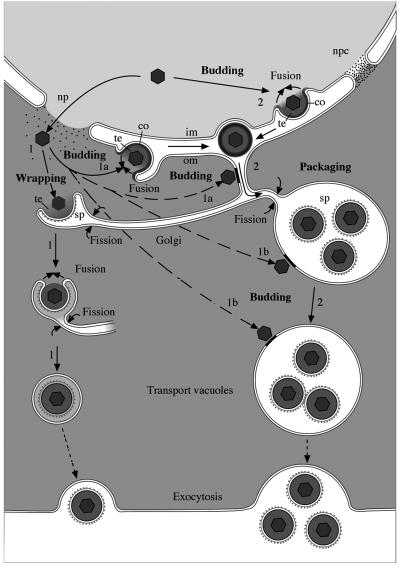
In conclusion, the data obtained by a close examination of well-preserved HSV-1-infected cells support the hypothesis drawn earlier for BHV-1-infected cells that the envelopment of members of the α-herpesvirus family follows two diverse pathways (Fig. 9). These pathways include (i) nuclear envelopment followed by intraluminal transportation to Golgi cisternae for the formation of transport vacuoles and (ii) cytoplasmic envelopment of capsids that have exited the nucleus via impaired nuclear pores. Cytoplasmic envelopment takes place at the outer nuclear membrane and RER membranes, which is followed by intraluminal transportation for the formation of transport vacuoles, or at Golgi membranes, which results in virions situated in transport vacuoles. BHV-1 (64) and HSV-1 (unpublished data) were shown to enter cells by a unique fusion pathway. The ability of the viral envelope to fuse is considered likely to be present immediately after the envelope is formed. Fusion is hence assumed to be prevented by the insertion of antifusion proteins at the viral envelope for intraluminal transportation or between the viral envelope and vacuolar membranes for vacuolar transportation. The nature of this substance needs to be investigated.
FIG. 9.
Schematic drawing of the pathways of HSV-1 envelopment. (1) Cytoplasmic envelopment. Capsids leave the nucleus via impaired nuclear pores (np) and approach Golgi membranes from the cytoplasmic side, inducing budding. Since the entire cisterna is involved, a sphere-like structure comprising two membranes arises. The inner membrane becomes the viral envelope, and the outer one becomes the vacuolar membrane. Fusion of the envelope with the vacuolar membrane is considered likely to be prevented by “antifusion” proteins at high concentrations. Alternatively, capsids escaping the nucleus via impaired nuclear pores can also bud at the outer nuclear membrane (1a), RER membranes (1a), membranes of dilated Golgi cisternae (1b), and membranes of Golgi-derived vacuoles (1b) already containing virions. Virions originating by budding at the outer nuclear membrane and RER membranes need to be transported to Golgi cisternae for packaging. (2) Nuclear envelopment. Capsids bud through the inner nuclear membrane and are transported from the perinuclear space via RER cisternae into Golgi cisternae for packaging into transport vacuoles of various sizes containing one or more virions. Fusion of the viral envelope with cell membranes of the compartments the virion is transported through is considered likely to be prevented by proteins of an unknown nature, which are condensed (co) at the viral envelope. The “antifusion proteins” are speculated to be removed from the envelope in Golgi cisternae but are assumed to remain in the transport vacuoles (possibly together with additional proteins) at low concentrations to prevent the fusion of viral envelopes with the membranes of large transport vacuoles. im, inner nuclear membrane; om, outer nuclear membrane; npc, nuclear pore complex; sp, spikes; te, tegument.
Acknowledgments
We thank Bernard Roizman for critical discussions of the data and Jeanne Peter for drawing Fig. 2 and 9.
This study was supported by the Research Fund of the University of Zürich.
REFERENCES
- 1.Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant and downmodulates their cell surface expression. J. Virol. 78:8015-8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, J. D., C. Cunningham, D. Nalwanga, and A. Davison. 1997. The U(L)15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the U(L)15 gene product. J. Virol. 71:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borchers, K., and M. Oezel. 1993. Simian agent 8 (SA8): morphogenesis and ultrastructure. Zentbl. Bakteriol. 279:526-536. [DOI] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume, G., F. Farabegoli, S. Di Gaeta, and B. Roizman. 1991. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J. Virol. 65:1589-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Church, G. A., and D. W. Wilson. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong, C. K., R. B. Tenser, G. D. Hsiung, and P. A. Gross. 1973. Ultrastructural studies of the envelopment and release of guinea pig herpes-like virus in cultured cells. Virology 52:468-477. [DOI] [PubMed] [Google Scholar]
- 8.Foster, T. P., J. M. Melancon, T. L. Olivier, and K. G. Kousoulas. 2004. Herpes simplex virus type 1 glycoprotein K and the UL20 protein are interdependent for intracellular trafficking and trans-Golgi network localization. J. Virol. 78:13262-13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershon, A., L. Cosio, and P. A. Brunell. 1973. Observations on the growth of varicella-zoster virus in human diploid cells. J. Gen. Virol. 18:21-31. [DOI] [PubMed] [Google Scholar]
- 10.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert, R., and H. P. Ghosh. 1993. Immunoelectron microscopic localization of herpes simplex virus glycoprotein gB in the nuclear envelope of infected cells. Virus Res. 28:217-231. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert, R., K. Ghosh, L. Rasile, and H. P. Ghosh. 1994. Membrane anchoring domain of herpes simplex virus glycoprotein gB is sufficient for nuclear envelope localization. J. Virol. 68:2272-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granzow, H. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2004. The pseudorabies virus US3 protein is a component of primary and of mature virions. J. Virol. 78:1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granzow, H., F. Weiland, A. Jons, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustin, K. E., and P. Sarnow. 2001. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 20:240-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustin, K. E., and P. Sarnow. 2002. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 76:8787-8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haines, H., and R. J. Baerwald. 1976. Nuclear membrane changes in herpes simplex virus-infected BHK-21 cells as seen by freeze-fracture. J. Virol. 17:1038-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. (Erratum, 77: 8179.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughson, F. M. 1997. Enveloped viruses: a common mode of membrane fusion? Curr. Biol. 7:R565-R569. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson, L., C. Roop-Beauchamp, and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 69:4556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayachandra, S., A. Baghian, and K. G. Kousoulas. 1997. Herpes simplex virus type 1 glycoprotein K is not essential for infectious virus production in actively replicating cells but is required for efficient envelopment and translocation of infectious virions from the cytoplasm to the extracellular space. J. Virol. 71:5012-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen, H. L., and B. Norrild. 1998. Herpes simplex virus type 1-infected human embryonic lung cells studied by optimized immunogold cryosection electron microscopy. J. Histochem. Cytochem. 46:487-496. [DOI] [PubMed] [Google Scholar]
- 24.Kanaseki, T., K. Kawasaki, M. Murata, Y. Ikeuchi, and S. Ohnishi. 1997. Structural features of membrane fusion between influenza virus and liposome as revealed by quick-freezing electron microscopy. J. Cell Biol. 137:1041-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klupp, B. G. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 26.Klupp, B. G., J. Baumeister, P. Dietz, H. Granzow, and T. C. Mettenleiter. 1998. Pseudorabies virus glycoprotein gK is a virion structural component involved in virus release but is not required for entry. J. Virol. 72:1949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozlovsky, Y., L. V. Chernomordik, and M. M. Kozlov. 2002. Lipid intermediates in membrane fusion: formation, structure, and decay of hemifusion diaphragm. Biophys. J. 83:2634-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kressel, M., and P. Groscurth. 1994. Distinction of apoptotic and necrotic cell death by in situ labelling of fragmented DNA. Cell Tissue Res. 278:549-556. [DOI] [PubMed] [Google Scholar]
- 29.Krull, S., J. Thyberg, B. Bjorkroth, H. R. Rackwitz, and V. C. Cordes. 2004. Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket. Mol. Biol. Cell 15:4261-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lecatsas, G., and G. Poste. 1973. Mechanism of envelopment of herpesvirus by the nuclear envelope. Onderstepoort J. Vet. Res. 40:71-72. [PubMed] [Google Scholar]
- 31.Lee, K., J. Bao, J. Wang, W. Zhao, S. Liu, J. Si, Y. Wang, W. Zhang, and J. Jiang. 1987. Ultrastructural study of the morphogenesis of herpes simplex virus type 2 in organ cultured human uterine cervix and the interaction between virus and host cell. J. Electron Microsc. Tech. 7:73-84. [Google Scholar]
- 32.May, S. 2002. Structure and energy of fusion stalks: the role of membrane edges. Biophys. J. 83:2969-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer, A. 1999. Intracellular membrane fusion: SNAREs only? Curr. Opin. Cell Biol. 11:447-452. (Erratum, 11: 753.) [DOI] [PubMed] [Google Scholar]
- 34.Mayer, A. 2002. Membrane fusion in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 18:289-314. [DOI] [PubMed] [Google Scholar]
- 35.Melancon, J. M., R. E. Luna, T. P. Foster, and K. G. Kousoulas. 2005. Herpes simplex virus type 1 gK is required for gB-mediated virus-induced cell fusion, while neither gB and gK nor gB and UL20p function redundantly in virion de-envelopment. J. Virol. 79:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melikyan, G. B., and L. V. Chernomordik. 1997. Membrane rearrangements in fusion mediated by viral proteins. Trends Microbiol. 5:349-355. [DOI] [PubMed] [Google Scholar]
- 37.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller, M. 1992. The integrating power of cryofixation-based electron microscopy in biology. Acta Microscopica 1:37-46. [Google Scholar]
- 39.Palade, G. 1975. Intracellular aspects of the process of protein synthesis. Science 189:347-358. [DOI] [PubMed] [Google Scholar]
- 40.Pante, N., and U. Aebi. 1996. Molecular dissection of the nuclear pore complex. Crit. Rev. Biochem. Mol. Biol. 31:153-199. [DOI] [PubMed] [Google Scholar]
- 41.Pante, N., and U. Aebi. 1996. Sequential binding of import ligands to distinct nucleopore regions during their nuclear import. Science 273:1729-1732. [DOI] [PubMed] [Google Scholar]
- 42.Pante, N., R. Bastos, I. McMorrow, B. Burke, and U. Aebi. 1994. Interactions and three-dimensional localization of a group of nuclear pore complex proteins. J. Cell Biol. 126:603-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters, C., T. L. Baars, S. Buhler, and A. Mayer. 2004. Mutual control of membrane fission and fusion proteins. Cell 119:667-678. [DOI] [PubMed] [Google Scholar]
- 44.Pol, J. M., F. Wagenaar, and A. Gielkens. 1991. Morphogenesis of three pseudorabies virus strains in porcine nasal mucosa. Intervirology 32:327-337. [DOI] [PubMed] [Google Scholar]
- 45.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hubinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 46.Rajcani, J., and A. Vojvodova. 1998. The role of herpes simplex virus glycoproteins in the virus replication cycle. Acta Virologica 42:103-118. [PubMed] [Google Scholar]
- 47.Ray, N., and L. W. Enquist. 2004. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J. Virol. 78:3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riehle, U., and M. Hoechli. 1973. The theory and technique of high-pressure freezing, p. 31. In E. L. Benedetti and P. Favard (ed.), Freeze-etching, techniques and applications. Société Francaises de Microscopie Electronique, Paris, France.
- 50.Roizman, B., and A. E. Sears. 1996. Herpes simplex viruses and their replication, p. 2231-2295. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 51.Roller, R. J., Y. Zhou, R. Schnetzer, J. Ferguson, and D. DeSalvo. 2000. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J. Virol. 74:117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz, J., and B. Roizman. 1969. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology 38:42-49. [DOI] [PubMed] [Google Scholar]
- 53.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 55.Stannard, L. M., S. Himmelhoch, and S. Wynchank. 1996. Intra-nuclear localization of two envelope proteins, gB and gD, of herpes simplex virus. Arch. Virol. 141:505-524. [DOI] [PubMed] [Google Scholar]
- 56.Torrisi, M. R., C. Di Lazzaro, A. Pavan, L. Pereira, and G. Campadelli-Fiume. 1992. Herpes simplex virus envelopment and maturation studied by fracture label. J. Virol. 66:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walther, T. C., M. Fornerod, H. Pickersgill, M. Goldberg, T. D. Allen, and I. W. Mattaj. 2001. The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. EMBO J. 20:5703-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wild, P., M. Engels, C. Senn, K. Tobler, U. Ziegler, E. M. Schraner, E. Loepfe, M. Ackermann, M. Mueller, and P. Walther. 2005. Impairment of nuclear pores in bovine herpesvirus 1-infected MDBK cells. J. Virol. 79:1071-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wild, P., A. Gabrieli, E. M. Schraner, A. Pellegrini, U. Thomas, P. M. Frederik, M. C. Stuart, and R. Von Fellenberg. 1997. Reevaluation of the effect of lysozyme on Escherichia coli employing ultrarapid freezing followed by cryoelectron microscopy or freeze substitution. Microsc. Res. Tech. 39:297-304. [DOI] [PubMed] [Google Scholar]
- 61.Wild, P., E. M. Schraner, H. Adler, and B. Humbel. 2001. Enhanced resolution of membranes in cultured cells by cryoimmobilization and freeze-substitution. Microsc. Res. Tech. 53:313-321. [DOI] [PubMed] [Google Scholar]
- 62.Wild, P., E. M. Schraner, D. Cantieni, E. Loepfe, P. Walther, M. Muller, and M. Engels. 2002. The significance of the Golgi complex in envelopment of bovine herpesvirus 1 (BHV-1) as revealed by cryobased electron microscopy. Micron 33:327-337. [DOI] [PubMed] [Google Scholar]
- 63.Wild, P., E. M. Schraner, and E. Eggenberger. 1984. Quantitative aspects of membrane shifts in rat parathyroid cells initiated by decrease in serum calcium. Biol. Cell 50:263-272. [DOI] [PubMed] [Google Scholar]
- 64.Wild, P., E. M. Schraner, J. Peter, E. Loepfe, and M. Engels. 1998. Novel entry pathway of bovine herpesvirus 1 and 5. J. Virol. 72:9561-9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler, U., and P. Groscurth. 2004. Morphological features of cell death. News Physiol. Sci. 19:124-128. [DOI] [PubMed] [Google Scholar]