Abstract
The genomes of all members of the Parvovirus genus were found to contain a small open reading frame (ORF), designated SAT, with a start codon four or seven nucleotides downstream of the VP2 initiation codon. Green fluorescent protein or FLAG fusion constructs of SAT demonstrated that these ORFs were expressed. Although the SAT proteins of the different parvoviruses are not particularly conserved, they were all predicted to contain a membrane-spanning helix, and mutations in this hydrophobic stretch affected the localization of the SAT protein. SAT colocalized with calreticulin in the membranes of the endoplasmic reticulum and the nucleus. A knockout mutant (SAT−), with an unmodified VP sequence, showed a “slow-spreading” phenotype. These knockout mutants could be complemented with VP2− SAT+ mutant. The SAT protein is a late nonstructural (NS) protein, in contrast to previously identified NS proteins, since it is expressed from the same mRNA as VP2.
Porcine parvovirus (PPV) is the major causative virus in a syndrome of reproductive failure in swine, which includes stillbirths, mummified fetuses, early embryonic death, and infertility (12, 25). PPV replicates autonomously and has physicochemical properties and genomic sequences which resemble those of minute virus of mice (MVM), H-1 rodent parvovirus, canine parvovirus, and feline panleukopenia virus. These viruses are classified in the Parvovirus genus of the Parvoviridae (41). All parvoviruses have nonenveloped, icosahedral capsids with a diameter of around 25 nm and contain a single-stranded, linear DNA genome of about 4 to 6 kb.
The 5-kb genome of PPV contains two major open reading frames (ORFs). The left ORF encodes the nonstructural protein 1 (NS1 protein) and, via alternative splicing, the NS2 protein (3). NS1 has helicase and nickase activities and is indispensable for viral replication and genome packaging (13, 29). NS1 binds both viral and host DNA, interacts with cell proteins, and induces cell lysis (10) and apoptosis (32, 36). The large right ORF of the parvoviral genome encodes two to three N-terminally extended isoforms of the structural protein. VP1, the minor component of the capsid, possesses a Ca-dependent phospholipase A2 (PLA2) motif within its unique N-terminal region in almost all parvoviruses (48). This enzymatic activity is required for viral infectivity. Interestingly, this region of VP1 resides within the capsid of PPV (7), as was previously observed for MVM (9). It is currently unknown if and when in the viral cycle the PLA2 is externalized to gain access to its substrate. In addition to the two major ORFs, shorter, alternative ORFs were identified at various positions in different parvovirus genomes. Some of them overlap with the VP ORF. For example, two minor ORFs that overlap the 3′ ends of the NS and VP ORFs were recognized in B19 parvovirus (37, 38). Abundant amounts of polyadenylated small mRNAs with these two ORFs are found in infected human erythroid leukemic cells and are translated into 11.5- and 7.5-kDa proteins (14, 23). A short ORF (protein X) was found in the VP1 gene of the human erythrovirus V9 (27). Evidence that an ORF located between nucleotide (nt) 3922 and 4388 is transcribed from a novel promoter p81 (18) has been found for adeno-associated virus 2. Aleutian disease virus (ADV), which in many ways is unique among parvoviruses, has two overlapping ORFs between the two mayor ORFs (1, 6).
We noticed that all members of the Parvovirus genus, as well as ADV, have a short ORF of about 60 codons directly downstream of the initiation codon of VP2. Our objectives were to establish whether these conserved ORFs are translated, to characterize the translation products, and determine whether these putative late NS proteins are essential in parvoviral reproduction.
MATERIALS AND METHODS
Cells.
The PT cell line (3) was used for transfection of the different constructs and propagation of mutant and wild-type viruses. Cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with penicillin (100 IU/ml), streptomycin (50 g/ml), and 8% fetal calf serum (Sigma).
Transfection.
The Lipofectamine-plus (GIBCO-BRL) and Effectine transfection reagent (QIAGEN) kits were used according to the supplier's recommendations for transfection of the different constructs into PT cells.
Site-directed mutagenesis.
All mutations were made with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to the supplier's recommendations. The pN2D infectious clone of the porcine parvovirus NADL2 strain was used for creating mutant viruses as described previously (48).
Insertion of tags.
For the amplification and insertion of enhanced green fluorescent protein (eGFP) into the three frames, the greef25 (GCCGCTCGAGGTGAGCAAGGGCGAGGAGC), greef35 (GCCGCTCGAGG GTGAGCAAGGGCGAGGAG), greef15 (GCCGCTCGAGTGGTGAGCAAGGGCGAGGAG), and greere3 (CGGAATTCTTGTACAGCTCGTCCATGCCG) primers were used. The PCR fragments were digested with XhoI and EcoRI and inserted into the XhoI-EcoRI-digested constructs. Replicating constructs were made using the PNS1825 (cacaaccaataagagacagaatg), PPgrin13 (gcgaattcaactcgagCTGCATTAATAGGGTTGTGTTG), PPgrin23 (gcgaattcaactcgagGATTCATTTCCTGTTGCAGAC), PPgrin33 (gcgaattcaactcgagGTATTGAAATTCTG TTTGATTATTG), and PPgrin43 (gcgaattcaactcgagGATGTATGAGTCTTGATGCGTG) primers. Ins51 was generated by cutting pN2D with AccI enzyme; by insertion of GFP amplified with 5EGFS1 (ATCGCCgtctacGTGAGCAAGGGCGAGGAGC), 5EGFS2 (ATCGCCGTCTACGGTGAGCAAGGGCGAGGAG), 5EGFS3 (ATCGCCGTCTACTGGTGAGCAAGGGCGAGGAG), and 3EGFAS1 (CGGCCGGTAGACTTGTACAGCTCGTCCATGCCG) primers; and by digestion with AccI. For the SAT (for small alternatively translated protein)-FLAG fusion construct, the Ins68FR2 construct was digested with XhoI and EcoRI enzymes, and the GFP fragment was changed to a double-FLAG sequence by using the hybridized FLAGST5 (TCGATTATAAAGACGACGACGATAAGGGAGGAGATTACAAGGACGACGACGATAAAAGTAGTTGATCAT) and FLAGST3 (AATTATGATCAACTACTTTTATCGTCGTCGTCCTTGTAATCTCCTCCCTTATCGTCGTCGTCTTTATAA) oligomers.
Cloning the VP1 and VP2 transcripts.
Different GFP fusion constructs were obtained by using recombinant PCR to bridge the intron by amplification of pN2D using the PP40tr5 primer (start VP mRNA, GCTCTAGACATCAGTGAAAACTTCGCCAG) with either VP1mrna3 primer (bridges donor 2 with acceptor GTATCCTGGTAGAGTTAGTCCTCTTGCTCTTTTTGCAGGAGGCG) or VP2mrna3 primer (bridges donor 1 with acceptor GTATCCTGGTAGAGTTAGTCCTTATTCAAGGTTTGTTGTGGGTGC) for the upstream fragment of VP1 and VP2, respectively. The common downstream fragments of VP1 and VP2 were obtained by PCR using either the VP2mrna5 primer (CCTTGAATAAGGACTAACTCTACCAGGATAC) for VP2 or the VP1mrna5 primer (GAGCAAGAGGACTAACTCTACCAGGATAC) for VP1 and the PPV PR36 reverse primer (AGTTAGTAGTTTTGGAGGCAGTCC). The two overlapping fragments were then fused by recombinant PCR, digested with EcoRI and XbaI, and cloned into the NheI- and EcoRI-digested pCDNA 3.1 plasmid.
Immunoprecipitation.
About 1 million cells were lysed in 200 μl IP lysis buffer (0.5% NP-40, 1% Triton, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl, pH 7.4). The lysate was clarified by centrifugation (13, 000 × g; 5 min) and the supernatant was incubated with 5 μl polyclonal anti-eGFP antibody for 2 h at 4°C. Then, 10 μl of protein G-agarose (Roche) was added to the sample. Following an additional 1-h incubation, the protein G-agarose beads were centrifuged (8, 000 × g; 5 s) and washed twice with ice-cold lysis buffer. Finally, 20 μl 2× sodium dodecyl sulfate (SDS) loading buffer was added to the beads.
SDS-PAGE and Western blotting.
Immunodetection of the proteins after SDS-polyacrylamide gel electrophoresis (PAGE), was carried out by using anti-eGFP antibody (Clontech) in a 1,000-fold dilution and alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Bio-Rad) in a 2,000-fold dilution. The alkaline phosphatase was revealed using the nitroblue tetrazolium chloride-5-bromo-4-chloro-3-indolylphosphate colorimetric substrate according to the supplier's recommendations (Roche Diagnostics).
Immunofluorescence virus titration.
Cells were fixed with 3% formaldehyde and permeabilized with 3% Triton X-100. Calreticulin-fused DsRed2 fluorescent protein was used for endoplasmic reticulum (ER) labeling (pDsRed2-ER plasmid; Clontech). The 3C9 (CRL-17; ATCC) anti-PPV capsid-specific monoclonal antibody (MAb) was used for titration of viral stocks by three parallel, independent dilutions and plating experiments with PT cells. Confocal images were taken with the Bio-Rad MRC 1000 laser microscope with an argon-krypton light source. Anti-eGFP and anti-Flag antibodies were visualized with Alexa Fluor-labeled secondary antibodies (Molecular Probes).
RESULTS
Small conserved ORF.
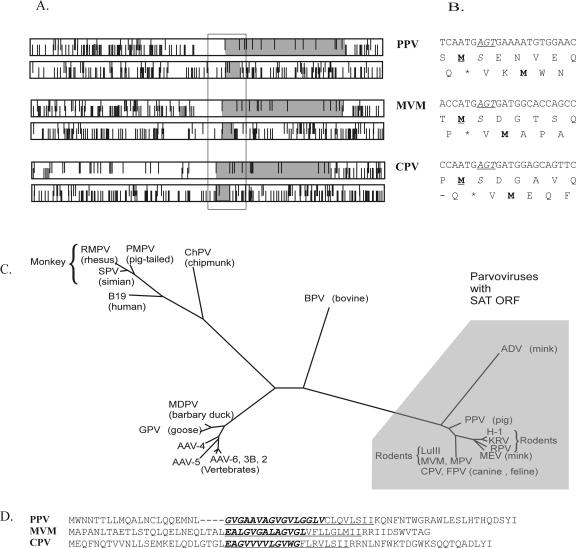
Sequence analysis revealed a small, conserved alternative ORF overlapping the amino-terminal portion of the VP2 ORF of all viruses of the Parvovirus genus (Fig. 1A). This small ORF can also be found in the genome of the ADV but not in other parvoviruses (Fig. 1C). Among members of the Parvovirus genus, the ORF extends between 50 to 64 amino acids and starts with an ATG codon 4 nt downstream of the VP2 protein start codon. Exceptionally in PPV, the ATG codon is positioned 7 nt after the VP2 initiation codon (Fig. 1B). We designated this ORF of the Parvovirus genus members as small alternatively translated protein (SAT)-ORF. The primary sequence of SAT proteins is not particularly conserved. However, all SAT proteins were predicted by different topology prediction programs (33, 44, 45) to be membrane proteins containing a single membrane-spanning α-helix in approximately the same position. In each case, the N-terminal region of the hydrophobic helix shared the same G-rich coding region with the so-called glycine stretch in the VP2 protein (Fig. 1D).
FIG. 1.
(A) Position of the long VP2 and short SAT ORFs (gray boxes) in the coding frames of genomes of PPV, MVM, and canine parvovirus. Bars on the top and bottom represent start and stop codons, respectively. (B) The N-terminal coding region of VP2 from the same viruses is shown with the first amino acid of VP2 in boldface and underlined and the fully conserved serine in VP2 in italics, whereas the conserved codon is in italics and underlined. The first amino acid of the SAT is also in boldface type. (C) Unrooted phylogenetic tree of NS protein sequences from parvoviruses as determined previously (15) with those having the SAT ORF shaded. (D) The alignment of SAT proteins of some parvoviruses showed few conserved amino acids. Amino acids sharing the common coding region of the G-stretch are in boldface type and italics and the predicted membrane-spanning helices are underlined.
Anti-SAT antisera.
The SAT-ORF was expressed in Escherichia coli in the pBAD2TEV expression vector (7) as a thioredoxin-SAT-six-His fusion protein. However, the sera failed to recognize SAT, although they readily recognized the SAT fusion partners. Serum raised by peptide immunization against the first 16 amino acids of SAT also failed to recognize the immunizing peptide. Although disappointing, immunization failure is not unusual with small hydrophobic proteins (5).
GFP-SAT fusion proteins.
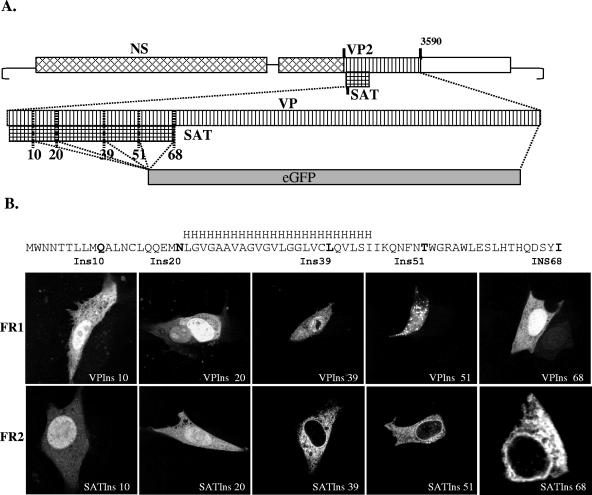
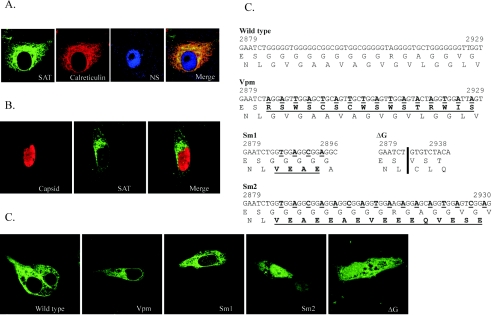
To demonstrate its existence and to identify the product of this ORF, GFP was inserted into the infectious clone of PPV in five different positions of the SAT-ORF in all three open reading frames (Fig. 2). These 15 different constructs were transfected into PT cells; after 24 h, the cells were fixed and monitored for GFP expression. All five positive control constructs in which the GFP was inserted into the VP frame (frame 1 [FR1]) resulted in GFP expression in PT cells (Fig. 2B). GFP expression could be detected by fluorescence in both the cytoplasm and the nucleus. As expected, no GFP was detected in any of the five inserts in frame 3 (no ORF). In contrast, all five constructs in which GFP was inserted in frame with the SAT-ORF (FR2) expressed GFP, although the distribution of the protein in the cell showed marked differences, depending on the insertion points (Fig. 2B). The two constructs in which GFP was inserted before the predicted membrane-spanning helix showed cytoplasmic and nuclear distribution, similar to that of the VP2-GFP constructs. However, fusion proteins in which the GFP was inserted at the 3′ end or after the predicted membrane-spanning helix coding sequence were excluded from the nucleus and the cell membrane. To demonstrate that this localization of SAT was not the result of some unrecognized interaction of the fusion tag, another construct was made where the GFP fusion tag of the full-length SAT was replaced by a double FLAG tag. This fusion protein showed exactly the same localization as the GFP-labeled SAT (Fig. 3A). The nuclear membrane staining and the characteristic pattern in the cytoplasm suggested that these proteins resided in the ER-nuclear membrane system. Cotransfection of the FLAG fusion protein construct with the pDsRed2-ER plasmid expressing the calreticulin-RFP fusion protein ER marker demonstrated that the two proteins colocalized and that SAT indeed localized in the ER-nuclear membrane compartment (Fig. 3A). The localization of GFP-tagged SAT with viral NS and capsid proteins was also investigated. No colocalization was observed, since both the NS and the VP proteins were localized in the nucleus (Fig. 3A and B).
FIG. 2.
Topology of the SAT and VP fusion proteins. (A) Schematic representation of mutant viruses (boxes symbolize the coding regions of the PPV genome). The small grid box and the gray box represent the SAT coding region and the eGFP-substituted parts of the genome, respectively. Vertical bars indicate the initiation codon of SAT, VP2, and the five positions in the SAT coding region (numbered by the amino acids position of the insertion site in SAT) where eGFP was inserted in three frames. (B) Confocal microscopy images of the different length VP and SAT-eGFP fusion proteins expressed by the recombinant viral clones. The position of the predicted membrane-spanning helix is indicated with an H series. The insertion points of the GFP are in boldface in the SAT sequence and labeled with the amino acid position of the insertion site in SAT. The images in the top row (FR1) show cells with eGFP-VP fusion proteins, whereas images in the bottom row show eGFP-SAT-positive cells transfected with viral constructs in which the eGFP was inserted in the SAT frame (FR2). None of the clones were positive for eGFP when the eGFP was inserted in the third frame (not shown).
FIG. 3.
The localization of SAT. (A) SAT localizes in the ER. The pFLAG68 viral construct expressing FLAG-labeled SAT was cotransfected with the pDsRed2-ER vector expressing the ER marker calreticulin fused to DsRed2 fluorescent protein. Anti-FLAG (mouse) and anti-NS1 (rabbit) antibodies were labeled with Alexa Fluor-488 and Alexa Fluor-647 giving green and blue fluorescence, respectively. The extensive colocalization of SAT and calreticulin resulted in orange-yellow staining in the merged image. (B) SAT does not colocalize with the viral capsid proteins. The pSATins68 viral construct expressing GFP-labeled SAT was cotransfected with the infectious clone of the wild-type virus pN2D. Anti-GFP and anti-capsid antibodies were labeled with Alexa Fluor-488 and Alexa Fluor-568. (C) Changes in the G-stretch sequence of mutants derived from pSATIns68. The changes in the nucleotide and protein sequences are boldface type and underlined; the nucleotide sequences are numbered according to their original position in the PPV NADL-2 strain. The position of deletion in the ΔG clone is indicated by a vertical bar. (D) Localization of the G-stretch-modified SAT proteins. The G-stretch mutant constructs listed in panel C were transfected and the expression of SAT-GFP fusion proteins was followed with Alexa Fluor-488-labeled anti-GFP antibody. Images are labeled with the names of the corresponding mutants.
Role of membrane-spanning helix in localization of SAT protein.
The inability of the membrane-spanning helix-truncated SAT versions to localize in the ER suggested that this helix is the main determining factor of the confinement of SAT into this specific cellular compartment. Two types of mutants were made to investigate the role of the predicted membrane-spanning helix in SAT localization. In one type, the amino acid sequence of SAT in the helix region was partially or completely changed without changing the amino acid sequence of VP; in the other, the full helix was deleted (Fig. 3C). Five amino acid changes sufficed for leakage of the SAT protein into the nucleus (Fig. 3D, panel Sm1), whereas more changes or deletion of the amino acid sequence of the predicted membrane-spanning helix of SAT completely changed its localization. To exclude the possibility that the observed differences in SAT localization were the consequence of some nonspecific effect of the nucleotide sequence modification, we changed the amino acid sequence of VP in this region without changing the SAT amino acid sequence (Fig. 3D). This mutant SAT localized as the wild type, confirming that changes in SAT localization can be attributed to the amino acid changes in the membrane-spanning helix.
Initiation of SAT protein translation.
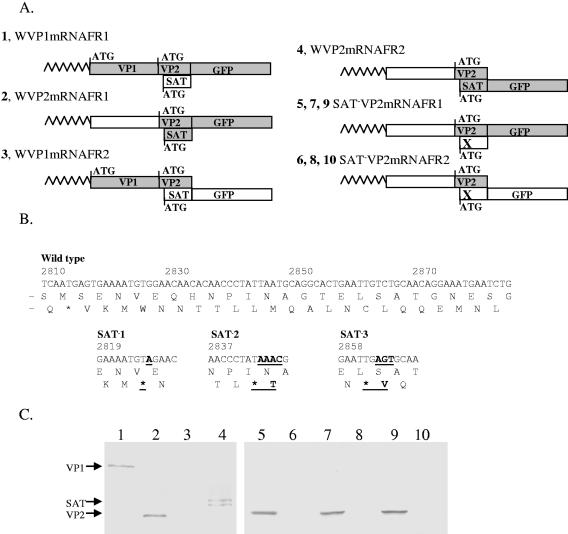
The first AUG in mRNAs usually initiates translation in eukaryotes. However, there are numerous exceptions (17, 20, 21, 34) to this rule. One of the exceptions is when two AUGs are just a few nucleotides from each other (19, 47). In this case, both AUGs can function as a start codon for protein synthesis. The closeness of the potential start codons of the SAT proteins and VP2 indicated that they could be translated from the same mRNA. To investigate this hypothesis, we cloned the spliced versions of VP1-GFP and VP2-GFP fusion constructs into pCDNA3.1 vectors and expressed them in PT cells (Fig. 4A). These vectors contained the full-length 5′ nontranslated leader sequences of either VP1 or VP2 mRNAs and the translated region where the GFP was fused either to the VP frame or to the SAT frame. The VP2 mRNA of both the VP- and SAT-fused versions of three SAT knockout mutants (Fig. 4B) was also cloned and expressed. The expressed proteins were immunoprecipitated with a polyclonal anti-GFP antibody and run on SDS-PAGE, followed by Western blotting using an anti-GFP MAb. A single band could be detected on the blot when VP1 mRNA was expressed and GFP was fused to the VP frame (corresponding to the appropriate size of the VP1-GFP fragment) (Fig. 4C, lane 1). No protein was detected from the VP1 mRNA when GFP was fused to the SAT frame. These findings strongly suggest that only the VP1 start codon is used for translation initiation of the VP1 mRNA. In contrast, proteins translated from both the VP and SAT frames from the VP2 mRNA (Fig. 4C, lanes 2 and 4). Protein translation was not observed from the SAT frame of the VP2 mRNA of the knockout mutants (Fig. 4C, lanes 6, 8, and 10), indicating that SAT is indeed translated from the VP2 mRNA and that the introduction of a stop codon into the SAT-ORF inhibits SAT synthesis.
FIG. 4.
Western blot of VP1- and VP2-mRNA constructs expressing different GFP fusion proteins in pCDNA3.1. (A) Four constructs were created in pcDNA3.1, expressing the complete 5′ end of either the VP1 or VP2 mRNA of the wild-type PPV (constructs 1 to 4, labeled with a W), and six were created expressing the 5′ end of the VP2 mRNA from three SAT− mutants (in every case the SAT ORF was knocked out by a different stop codon (indicated by an X) (constructs 5 to 10). In all constructs, GFP was fused to either the VP frame (constructs 1, 2, 5, 7, and 9) or the SAT frame (constructs 3, 4, 6, 8, and 10). The predicted nontranslated ORFs are illustrated with open boxes, while the translated ones are illustrated with gray boxes. Shark's tooth lines represent the nontranslated leaders of the mRNAs. Start codons are shown with ATGs and bars. (B) Positions of sequence modifications in the three SAT knockout mutants. In every mutant, another stop codon was introduced into the SAT reading frame without changing the VP2 protein sequence. The nucleotide and amino acid changes are in boldface type and underlined, and the names of the mutants are in boldface type (SAT−1 to -3). (C) GFP-tagged proteins of all 10 constructs were immunoprecipitated with anti-GFP rabbit polyclonal sera, run on SDS-PAGE gels, blotted, and developed with an anti-GFP MAb. Wild-type constructs are shown in lanes 1 to 4, and SAT−1 to -3 constructs are shown in lanes 5 to 6, 7 to 8, and 9 to 10, respectively.
The usage of a GFP-expressing plasmid as an internal reference in transfection enabled an estimation of the translation ratio of SAT and VP2. Based on the densitometric comparison of GFP-tagged proteins, the ratio of the alternative translation of SAT was around 60% of the VP2 proteins (VP2 and SAT) (Fig. 5B).
FIG. 5.
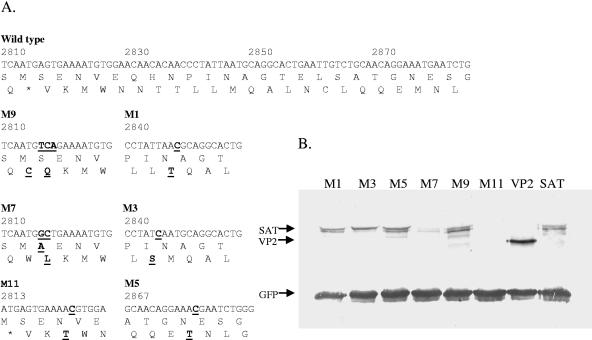
Western blot of mutant SAT constructs. (A) Six mutants of the WVP2mRNAFR2 construct (A4) (Fig. 4) were created. The 5′ end of the wild-type PPV VP2 and SAT ORF are shown with the mutants. The changes are indicated with boldface type and underlined. The methionines of SAT are mutated in M1, M5, and M11. Ser-2 coding in VP2 was changed in M9 and M7, whereas Leu-19 of SAT was changed to S in M3. (B) The mutant GFP-tagged constructs were cotransfected with the GFP-expressing pEGFPN1 plasmid for normalization and immunoprecipitated. To compare the expression levels of VP2 and SAT, the WVP2mRNAFR2 construct (SAT) and VP2mRNAFR1 construct (VP2) were tested.
Three methionines can be found in the PPV SAT-ORF and at least two different versions of SAT can be distinguished on the Western blot. These three methionines were mutated to threonine to investigate whether the SAT proteins are initiated at different AUGs. Mutation of the second and third AUG did not have any visible effect (M1 and M5) (Fig. 5). However, mutation of the first AUG completely eliminated all protein translation from the SAT frame (M11) (Fig. 5). This suggested that the multiple bands might be the result of posttranslational modifications rather then multiple initiations.
The N-terminal regions of VP2 and SAT are not particularly conserved in parvoviruses. In spite of this, the first 8 nt of VP2 show an almost complete conservation (ATGAGTG/AA), which results in a fully conserved serine at the second amino acid position of the VP2 and two stop codons overlapping with the methionine and serine codons (in the second and third frames). To investigate the role of this conserved sequence in the SAT translation, two mutants were created (Fig. 5). Changing the DNA sequence of the fully conserved serine-2 of VP2 (AGT to TCA), which also eliminates the overlapping stop codons, did not change the translation pattern of the SAT. However, mutating the conserved serine to alanine (AGT to GCT, as in construct M7) (Fig. 5) dramatically reduced the translation of SAT. Interestingly, the L8S (M3) (Fig. 5) mutation of SAT seemed to interfere with the normal modification process because this eliminated the lower bands of SAT.
SAT-related phenotype.
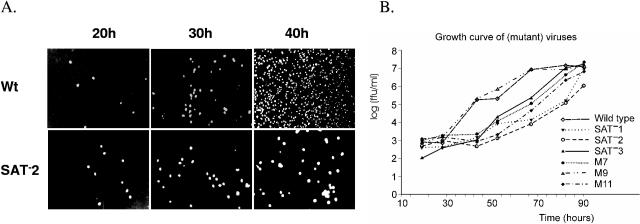
To investigate the role of SAT in the PPV life cycle, six mutant viruses were created introducing mutations M7, M9, M11, and SAT−1 to -3 into the infectious clone of PPV (Table 1). The transfected mutant clones yielded infectious virions (designated M7V, M9V, M11V, and SAT−1 to -3V), suggesting that SAT, at least in tissue culture, is not essential. There was no significant difference between the wild-type virus and the mutant viruses in the specific infectivity (genome equivalents/fluorescent focus units [FFU]) or the infectious titer of the viral stocks (∼107 FFU/ml) if the infection was allowed to proceed completely. However, compared to the wild-type virus and mutant M9, there was a significant delay in the spreading of five mutant viruses, containing mutations (SAT−1 to -3, M7, and M11) that eliminated or significantly reduced SAT expression. After 40 h at a low multiplicity of infection (MOI) (0.02 FFU/cell of virus), 99% of the cells were infected in the case of wild-type virus while this was still significantly <10% for the SAT−2 mutant (Fig. 6A). This delay of the spreading manifested itself in lagging growth curves for all mutants that do not express SAT (Fig. 6B).
TABLE 1.
Summary of effects of mutations
| Mutation | Protein sequence change
|
SAT expression | Plaque | SAT complementation | |
|---|---|---|---|---|---|
| VP2 | SAT | ||||
| Wild type | No change | No change | Normal | Large | NAa |
| SAT1 | No change | W2Stop | No | Small | Yes |
| SAT2 | No change | L8Stop | No | Small | Yes |
| SAT3 | No change | C14Stop | No | Small | Yes |
| M7 | S2A | No change | Reduced | Small | Weak |
| M9 | No change | No change | Normal | Large | NA |
| M11 | No change | M1T | No | Small | Yes |
NA, not applicable.
FIG. 6.
Effect of SAT mutations on virus spreading. (A) SAT− mutants spread more slowly in tissue culture than the wild-type virus. PT cells at 50% confluence were infected with wild-type virus or SAT−2 at an MOI of 0.02, and the advance of infection was monitored. Cells were fixed at the appropriate time points, and infected nuclei were visualized by Alexa Fluor-488-labeled capsid-specific antibody. (B) Virus growth in cells infected with wild-type and six mutant viruses. PT cells at 50% confluence were infected at an MOI of 0.02. Aliquots of the culture medium were removed, and titers were determined as described in Materials and Methods. The standard variation in every case was <0.7 log.
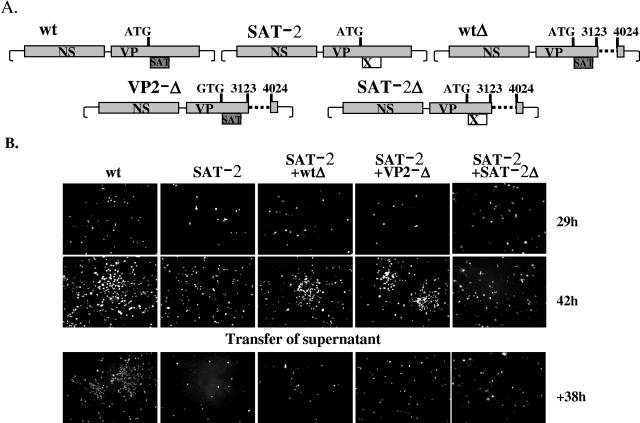
Complementation experiments were done to test whether the “slow-spreading” phenotype of the SAT− mutants was really due to the loss of the SAT (Fig. 7). The PPV life cycle takes about 18 to 24 h in PT cells. Foci of infected cells can be detected around the transfected cells about 40 h after transfection of the wild-type infectious clone. In contrast, only a few cells were infected at the same time, and no foci could be observed in the case of the SAT−2 mutant (Fig. 7A and B). When the SAT−2 mutant was cotransfected with either of the two VP2 deletion constructs (wtΔ and VP2−Δ), both containing a complete and functional SAT frame, the foci of infected cells could be detected just as soon as in the case of wild-type virus. However, foci could not be observed when SAT−2 was cotransfected with the SAT−2Δ construct. Thus, the wild-type SAT supplied in trans could complement the “slow” phenotype of the mutant virus. To exclude the possibility that foci in the cotransfection experiments were the result of recombination between two constructs, virus stocks from all transfections were seeded and cells were monitored for the presence of foci. Only the wild-type stock was able to form foci, whereas the SAT− mutant stocks raised from either cotransfection or transfection alone were not able to do so at 38 h postinfection, demonstrating that the foci detected during cotransfection were due to complementation and not to recombination (Fig. 7). Similar complementation experiments were executed with SAT−1, SAT−3, M11, and M7 mutants. The first three mutants gave similar results to SAT−2, while the M7 mutant yielded somehow fewer and significantly smaller foci.
FIG. 7.
Complementation. (A) Schematic representation of the genotypes of four viral constructs used in a complementation study. In Δwt, the nucleotide sequence between the BstXI and SacI site was deleted; in addition, in VP2-Δ the start codon of VP2 was mutated. The positions of the restriction sites are indicated by vertical bars and numbers, whereas the dashed line represents the deletion. The VP2 start codon in the VP2-Δ mutant was changed to GTG. (B) Complementation of the SAT− virus with viral constructs expressing SAT. The SAT− viral construct was transfected into 80% confluent PT cells alone or together with Δwt or VP2−Δ constructs. At 42 h, cells were fixed and monitored for infection. The 42-h supernatant of the transfected cells was diluted to a low MOI to allow spreading and plated to test for the presence of wild-type virus.
DISCUSSION
Presence of SAT-ORF.
The relatively large number of parvoviruses with known sequences enables us to utilize comparative sequence analysis as an effective tool to uncover common genetic features and better understand how these viruses function with their limited genome size. The analysis of the VP2 N-terminal coding region of some closely related but still diverse groups of parvoviruses, i.e., the members of the Parvovirus and Amdovirus genera (41), revealed a conserved, small ORF overlapping the 5′ end of the VP2 ORF. Every known member of the two genera possesses it, and no other parvoviruses do so. The N-terminal half of the hydrophobic helix coded by these ORFs shares a guanine-rich coding region with a glycine-rich portion of the VP proteins. This G stretch is thought to be required for the flexibility to expose of the N-terminal parts of VP proteins during capsid maturation and infection. Although a glycine-rich sequence can be found near the N terminus of the major capsid component in almost all parvoviruses, such sequences are much less extended than in the members of Parvovirus and Amdovirus genera. This suggests that the development of the extensive G stretch in the VP proteins of these viruses resulted from a double evolutionary pressure. This particular region of the DNA may have to accommodate coding for the flexible VP region and for an α-helix hydrophobic stretch of amino acids of SAT. Usage of three codons (GG/T/CG) from the possible four in a glycine repeat satisfies these criteria because such usage results in valine (GTG), alanine (GCG), and glycine (GGG), all of which are well accommodated in a hydrophobic helix.
Translation from proximal AUGs.
An ATG can be found 4 nt downstream of the VP2 start codon in the SAT frame for all of the members of the Parvovirus genus except for PPV, which is 7 nt downstream of the VP2 ATG. Kozak (19) provided evidence of adherence to the first-AUG rule for translation in eukaryotic mRNAs and of a context-dependent leaky scanning mechanism. Exceptions, such as reinitiation, internal initiation (internal ribosome entry site), and leaky scanning, have all been documented (17, 20, 21, 34). Interestingly, the synthesis of NS1 and NS2 of some insect parvoviruses is initiated at AUG codons that are spaced by 4 nt (16, 42). RNA-6 of influenza virus B also directs the synthesis of two proteins (integral membrane glycoprotein and neuramidase) from two initiation codons with an identical spacing sequence of 4 nt (35, 47). It was suggested that linear scanning by the 40S ribosomal subunit may break down if two AUGs are in close apposition, as increasing the spacing to 46 nt prevented initiation from the second AUG (19). In most cases (insect parvoviruses NS1/NS2 and Parvovirus genus members VP2/SAT), the 5′-proximal AUG codon has a suboptimal context. However, it is noteworthy that the context of the first AUG in influenza virus RNA-6, with an A in position −3, is adequate (GenBank no. NC_004284). In the case of PPV, the VP2 ATG initiation codon is in a poor context, whereas the start codon of SAT, 7 nt downstream of the VP2 ATG, is in a favorable context. The different forms of the SAT proteins detected on the Western blot seem to result from posttranslational modification rather than alternative initiation, because mutations of the other ATGs in the SAT frame have no effect on the protein pattern. In almost all members of the Parvovirus genus, all the methionine codons in the SAT frame overlap with a stop codon in the third noncoding frame. The reason of this phenomenon is unknown.
SAT phenotype.
Inserting stop codons in the SAT frame or mutating the initiation codon of SAT abolished SAT protein translation and resulted in a “slow-spreading” phenotype of the mutant viruses in tissue culture. A similar phenotype was observed for MVM after modification of the VP2 N-terminal sequence (43). The S2 serine of VP2 is fully conserved among parvoviruses and the most phosphorylated amino acids in MVM VP2. The phosphorylation is important for the migration of the capsid from the nucleus to the cytoplasm (24). However, the sequence is not only conserved at protein but also at the DNA level. This is surprising, considering the six possible serine codons and that there is no other conserved amino acid (except methionine) in the region. The full conservation of the serine codon suggested that it might be necessary to provide for the most optimal context of SAT initiation. The fact that changing the serine-2 codon in PPV VP2 (mutation M9) did not have a dramatic effect on SAT synthesis and virus infectivity contradicted this idea. Nonetheless, modifications in the VP2 sequence could have an influence on the SAT synthesis or posttranslational modification, and the emerging phenotype could be the result of the modification of either VP2 or SAT or both. The S2A mutation in PPV VP2 (mutation M7) severely down-regulated the SAT synthesis, probably by placing the VP2 AUG initiation codon in a more favorable Kozak context (changing adenine in the +4 position to guanine). However, the phenotype of M7 (S2A) could be less complemented by SAT than that of the SAT knockout mutants, suggesting that changes in the VP2 sequence also contributed to the impaired spreading.
Possible role of SAT.
PPV SAT seems to be posttranslationally modified and localized in the ER. The nature of modification and its role in the function of the protein remain to be clarified. Membrane proteins maintain their residency in the ER by either static retention caused by the hydrophobic helix or dynamic retention by retrieval of the escaped proteins from other compartments (2, 8, 28, 46). The use of truncations and mutations demonstrated that the predicted helix plays a crucial role in the localization of SAT, but no dynamic retention signal on any of the SAT proteins could be identified. Thus, the SAT may be retained in the ER only by static interaction. Several viral proteins from members of other virus families are known to localize in the ER. They can mediate immune evasion by arresting major histocompatibility complex class I molecules in the endoplasmic reticulum (26, 31). Some viral proteins were shown to generate ER stress responses, like unfolded protein response (11, 40) and ER overload response (30), which can induce several pathways of cell death through caspase-12, NH2-terminal Jun kinase, or Gadd153/chop (22, 39). It is tempting to speculate that SAT might facilitate cell lysis through ER stress.
SAT protein is probably toxic to the cells in the long term, because no SAT-expressing, stable cell lines could be established, even after repeated attempts. NS proteins from different parvoviruses can induce cell death (apoptosis or lysis) (4, 10, 36), and with this they facilitate viral spreading. SAT seems to be another molecular tool to overcome the defense system of the cell and complement the effect of NS proteins via an alternative pathway.
Members of the Parvovirus genus are not alone in having alternative ORFs in their VP region. In fact, all the five groups (genera) of closely related parvoviruses contain genus-specific evolutionary preserved alternative ORFs at different positions in their genome. The protein X ORF found in the genome of V9 erythrovirus (GenBank accession no. NC_004295.1; nucleotide 2586 to 2831), for example, is found in all members of the Erythrovirus genus and is predicted in every case to contain two transmembrane helices. Although the p81 transcript seems to be a more or less unique feature of the adeno-associated virus 2 genome, a large genus-specific ORF (GenBank accession no. AF043303.1; nucleotide 2717 to 3340) can be found to overlap the VP ORF of all members of the Dependovirus genus. The probability that these alternative ORFs occurred randomly during the evolution of these viruses is marginal, and the best explanation for their presence in the genome is that they encode other proteins. The presence of genus-specific ORFs and the experimental data presented here confirm that the function of the right side of the parvovirus genome is generally more complicated than previously assumed and not restricted to the encoding the capsid proteins.
Acknowledgments
We are grateful to Micheline Letarte for technical support, to Jürg P. F. Nüesch for the anti-NS antibodies, and to Sonja M. Best, Sandra Fernandes, and Ivan R. Nabi for the discussions.
This work was supported by a grant to P.T. from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Alexandersen, S., M. E. Bloom, and S. Perryman. 1988. Detailed transcription map of Aleutian mink disease parvovirus. J. Virol. 62:3684-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barre, L., J. Magdalou, P. Netter, S. Fournel-Gigleux, and M. Ouzzine. 2005. The stop transfer sequence of the human UDP-glucuronosyltransferase 1A determines localization to the endoplasmic reticulum by both static retention and retrieval mechanisms. FEBS J. 272:1063-1071. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron, J., J. Menezes, and P. Tijssen. 1993. Genomic organization and mapping of transcription and translation products of the NADL-2 strain of porcine parvovirus. Virology 197:86-98. [DOI] [PubMed] [Google Scholar]
- 4.Best, S. M., J. F., Shelton, J. M. Pompey, J. B. Wolfinbarger, and M. E. Bloom. 2003. Caspase cleavage of the nonstructural protein NS1 mediates replication of Aleutian mink disease parvovirus. J. Virol. 77:5305-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betakova, T., E. J. Wolffe, and B. Moss. 2000. The vaccinia virus A14.5L gene encodes a hydrophobic 53-amino-acid virion membrane protein that enhances virulence in mice and is conserved among vertebrate poxviruses. J. Virol. 74:4085-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom, M. E., S. Alexandersen, S. Perryman, D. Lechner, and J. B. Wolfinbarger. 1988. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J. Virol. 62:2903-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canaan, S., Z. Zádori, F. Ghomashchi, J. Bollinger, M. Sadilek, M. E. Moreau, P. Tijssen, and M. H. Gelb. 2004. Interfacial enzymology of parvovirus phospholipases A2. J. Biol. Chem. 279:14502-14508. [DOI] [PubMed] [Google Scholar]
- 8.Cosson, P., and F. Letourneur. 1994. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263:1629-1631. [DOI] [PubMed] [Google Scholar]
- 9.Cotmore, S. F., M. A. D'Abramo, Jr., C. M. Ticknor, and P. Tattersall. 1999. Controlled conformational transitions in the MVM virion expose the VP1 N-terminus and viral genome without particle disassembly. Virology 254:169-181. [DOI] [PubMed] [Google Scholar]
- 10.Daeffler, L., R. Horlein, J. Rommelaere, and J. P. Nuesch. 2003. Modulation of minute virus of mice cytotoxic activities through site-directed mutagenesis within the NS coding region. J. Virol. 77:12466-12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimcheff, D. E., S. Askovic, A. H. Baker, C. Johnson-Fowler, and J. L. Portis. 2003. Endoplasmic reticulum stress is a determinant of retrovirus-induced spongiform neurodegeneration. J. Virol. 77:12617-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne, H. W., J. L. Gobble, J. F. Hokanson, D. C. Kradel, and G. R. Bubash. 1965. Porcine reproductive failure associated with a newly identified “SMEDI” group of picorna viruses. Am. J. Vet. Res. 26:1284-1297. [PubMed] [Google Scholar]
- 13.Faisst, S., and J. Rommelaere. 2000. Parvoriruses: from molecular biology to pathology and therapeutic uses. Contributions to microbiology, vol. 4. S. Karger Publishers, Basel, Switzerland.
- 14.Fan, M. M., L. Tamburic, C. Shippam-Brett, D. B. Zagrodney, and C. R. Astell. 2001. The small 11-kDa protein from B19 parvovirus binds growth factor receptor-binding protein 2 in vitro in a Src homology 3 domain/ligand-dependent manner. Virology 291:285-291. [DOI] [PubMed] [Google Scholar]
- 15.Farkas, S. L., Z. Zádori, M. Benkõ, S. Essbauer, B. Harrach, and P. Tijssen. 2004. A parvovirus isolated from royal python (Python regius) is a member of the genus Dependovirus. J. Gen. Virol. 85:555-561. [DOI] [PubMed] [Google Scholar]
- 16.Fediere, G., M. El-Far, Y. Li, M. Bergoin, and P. Tijssen. 2004. Expression strategy of densonucleosis virus from Mythimna loreyi. Virology 320:181-189. [DOI] [PubMed] [Google Scholar]
- 17.Hellen, C. U., and P. Sarnow. 2001. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 15:1593-1612. [DOI] [PubMed] [Google Scholar]
- 18.Hermonat, P. L., A. D. Santin, J. De Greve, M. De Rijcke, B. M. Bishop, L. Han, M. Mane, and N. Kokorina. 1999. Chromosomal latency and expression at map unit 96 of a wild-type plus adeno-associated virus (AAV)/Neo vector and identification of p81, a new AAV transcriptional promoter. J. Hum. Virol. 2:359-368. [PubMed] [Google Scholar]
- 19.Kozak, M. 1995. Adherence to the first-AUG rule when a second AUG codon follows closely upon the first. Proc. Natl. Acad. Sci. USA 92:2662-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozak, M. 1999. Initiation of translation in prokaryotes and eukaryotes. Gene 234:187-208. [DOI] [PubMed] [Google Scholar]
- 21.Kozak, M. 2002. Pushing the limits of the scanning mechanism for initiation of translation. Gene 299:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, X. D., H. Lankinen, N. Putkuri, O. Vapalahti, and A. Vaheri. 2005. Tula hantavirus triggers pro-apoptotic signals of ER stress in Vero E6 cells. Virology 333:180-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo, W., and C. R. Astell. 1993. A novel protein encoded by small RNAs of parvovirus B19. Virology 195:448-555. [DOI] [PubMed] [Google Scholar]
- 24.Maroto, B., J. C. Ramirez, and J. M. Almendral. 2000. Phosphorylation status of the parvovirus minute virus of mice particle: mapping and biological relevance of the major phosphorylation sites. J. Virol. 74:10892-10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mengeling, W. L., and R. C. Cutlip. 1976. Reproductive disease experimentally induced by exposing pregnant gilts to porcine parvovirus. Am. J. Vet. Res. 37:1393-1400. [PubMed] [Google Scholar]
- 26.Misaghi, S., Z. Y. Sun, P. Stern, R. Gaudet, G. Wagner, and H. Ploegh. 2004. Structural and functional analysis of human cytomegalovirus US3 protein. J. Virol. 78:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen, Q. T., C., Sifer, V. Schneider, X. Allaume, A. Servant, F. Bernaudin, V. Auguste, and A. Garbarg-Chenon. 1999. Novel human erythrovirus associated with transient aplastic anemia. J. Clin. Microbiol. 37:2483-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson, T., and G. Warren. 1994. Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus. Curr. Opin. Cell Biol. 6:517-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuesch, J. P., S. F. Cotmore, and P. Tattersall. 1995. Sequence motifs in the replicator protein of parvovirus MVM essential for nicking and covalent attachment to the viral origin: identification of the linking tyrosine. Virology 209:122-135. [DOI] [PubMed] [Google Scholar]
- 30.Pahl, H. L., and P. A. Baeuerle. 1997. The ER-overload response: activation of NF-κB. Trends Biochem. Sci. 22:63-67. [DOI] [PubMed] [Google Scholar]
- 31.Park, B., Y. Kim, J. Shin, S. Lee, K. Cho, K. Fruh, S. Lee, and K. Ahn. 2004. Human cytomegalovirus inhibits tapasin-dependent peptide loading and optimization of the MHC class I peptide cargo for immune evasion. Immunity 1:71-85. [DOI] [PubMed] [Google Scholar]
- 32.Rayet, B., J. A. Lopez-Guerrero, J. Rommelaere, and C. Dinsart. 1998. Induction of programmed cell death by parvovirus H-1 in U937 cells: connection with the tumor necrosis factor alpha signalling pathway. J. Virol. 72:8893-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rost, B., G. Yachdav, and J. Liu. 2003. The PredictProtein Server. Nucleic Acids Res. 32:W321-W326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryabova, L. A., M. M. Pooggin, and T. Hohn. 2002. Viral strategies of translation initiation: ribosomal shunt and reinitiation. Prog. Nucleic Acid Res. Mol. Biol. 72:1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw, M. W., P. W. Choppin, and R. A. Lamb. 1983. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc. Natl. Acad. Sci. USA 80:4879-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sol, N., J. Le Junter I. Vassias, J. M. Freyssinier,. A. Thomas, A. F. Prigent, B. B. Rudkin, S. Fichelson, and F. Morinet. 1999. Possible interactions between the NS-1 protein and tumor necrosis factor alpha pathways in erythroid cell apoptosis induced by human parvovirus B19. J. Virol. 73:8762-8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St. Amand, J., and C. R. Astell. 1993. Identification and characterization of a family of 11-kDa proteins encoded by the human parvovirus B19. Virology 192:121-131. [DOI] [PubMed] [Google Scholar]
- 38.St. Amand, J., C. Beard, K. Humphries, and C. R. Astell. 1991. Analysis of splice junctions and in vitro and in vivo translation potential of the small, abundant B19 parvovirus RNAs. Virology 183:133-142. [DOI] [PubMed] [Google Scholar]
- 39.Su, H. L., C. L. Liao, and Y. L. Lin. 2002. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J. Virol. 76:4162-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tardif, K. D., K. Mori, R. J. Kaufman, and A. Siddiqui. 2004. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J. Biol. Chem. 279:17158-17164. [DOI] [PubMed] [Google Scholar]
- 41.Tattersall, P. 2005. The evolution of parvoviral taxonomy, p. 5-14. In J. Kerr, S. F. Cotmore, M. E. Bloom, R. M. Linden, and C. R. Parrish (ed.), The parvoviruses, in press. Hodder Arnold, London, United Kingdom.
- 42.Tijssen, P., Y. Li, M. El-Far, J. Szelei, M. Letarte, and Z. Zádori. 2003. Organization and expression strategy of the ambisense genome of densonucleosis virus of Galleria mellonella. J. Virol. 77:10357-10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tullis, G. E., L. R. Burger, and D. J. Pintel. 1992. The trypsin-sensitive RVER domain in the capsid proteins of minute virus of mice is required for efficient cell binding and viral infection but not for proteolytic processing in vivo. Virology 191:846-857. [DOI] [PubMed] [Google Scholar]
- 44.Tusnády, G. E., and I. Simon. 1998. Principles governing amino acid composition of integral membrane proteins: applications to topology prediction. J. Mol. Biol. 283:489-506. [DOI] [PubMed] [Google Scholar]
- 45.Tusnády, G. E., and I. Simon. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849-850. [DOI] [PubMed] [Google Scholar]
- 46.Vainauskas, S., and A. K. Menon. 2005. Endoplasmic reticulum localization of Gaa1 and PIG-T, subunits of the glycosylphosphatidylinositol (GPI) transamidase complex. J. Biol. Chem. 280:16402-16409. [DOI] [PubMed] [Google Scholar]
- 47.Williams, M. A., and R. A. Lamb. 1989. Effect of mutations and deletions in a bicistronic mRNA on the synthesis of influenza B virus NB and NA glycoproteins. J. Virol. 63:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zádori, Z., J. Szelei, M. C. Lacoste, S. Gariepy, P. Raymond, M. Allaire, I. R. Nabi, and P. Tijssen. 2001. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 1:291-302. [DOI] [PubMed] [Google Scholar]