Abstract
Heparan sulfate proteoglycans (HSPGs) are used by a number of viruses to facilitate entry into host cells. For the retrovirus human T-cell leukemia virus type 1 (HTLV-1), it has recently been reported that HSPGs are critical for efficient binding of soluble HTLV-1 SU and the entry of HTLV pseudotyped viruses into non-T cells. However, the primary in vivo targets of HTLV-1, CD4+ T cells, have been reported to express low or undetectable levels of HSPGs. For this study, we reexamined the expression of HSPGs in CD4+ T cells and examined their role in HTLV-1 attachment and entry. We observed that while quiescent primary CD4+ T cells do not express detectable levels of HSPGs, HSPGs are expressed on primary CD4+ T cells following immune activation. Enzymatic modification of HSPGs on the surfaces of either established CD4+ T-cell lines or primary CD4+ T cells dramatically reduced the binding of both soluble HTLV-1 SU and HTLV-1 virions. HSPGs also affected the efficiency of HTLV-1 entry, since blocking the interaction with HSPGs markedly reduced both the internalization of HTLV-1 virions and the titer of HTLV-1 pseudotyped viral infection in CD4+ T cells. Thus, HSPGs play a critical role in the binding and entry of HTLV-1 into CD4+ T cells.
The human T-cell leukemia virus type 1 (HTLV-1) retrovirus, the first disease-causing human retrovirus isolated (60), is the etiologic agent of a severe lymphocytic neoplasia called adult T-cell leukemia (60, 81) and of an inflammatory neurological disease (HTLV-1-associated myelopathy/tropical spastic paraparesis [HAM/TSP]) (20, 54). Adult T-cell leukemia is a malignancy of CD4+ T cells, and HTLV-1 has a preferential tropism for CD4+ T cells in asymptomatic patients (25, 62). In HAM/TSP patients, both CD4+ and CD8+ T cells serve as viral reservoirs (50). The closely related retrovirus HTLV-2, which is believed to share a common receptor with HTLV-1 (71, 72), also infects both CD4+ and CD8+ T cells in vivo (30, 61). HTLV transmission appears to require the passage of cells between individuals, and optimal infection is believed to require contact between T cells.
Since primary T cells are difficult to infect with HTLV-1 in vitro, the majority of the work over the past 20 years has examined the requirements for HTLV envelope (Env)-mediated binding and fusion with established (often non-T) cell lines. These studies revealed that in contrast to the limited in vivo tropism of HTLV, cell surface molecules capable of specifically binding HTLV SU are widely expressed. All vertebrate cell lines tested to date, including cells which previously had been scored as negative in HTLV Env pseudotype and fusion assays, are capable of binding soluble SU (33, 36, 39, 43, 53, 77). In contrast, primary quiescent T cells do not bind soluble HTLV SU; binding is observed rapidly following activation of the cells (36, 43) or the treatment of quiescent CD4+ T cells with transforming growth factor beta (TGF-β) (35).
Recently, glucose transporter 1 (GLUT-1) was shown to bind soluble forms of the HTLV-1 and HTLV-2 SU proteins in both leukemic T-cell and non-T-cell lines and to be critical for efficient entry of HTLV-2 pseudotyped virions into a non-T adherent cell line (42). A subsequent paper reported that the overexpression of GLUT-1 in a relatively resistant cell line, MDBK, increased the titers of HTLV-1 and HTLV-2 pseudotyped particles (13). However, it is not clear whether GLUT-1 is sufficient for entry or whether other molecules are critical for HTLV Env-mediated binding and/or fusion.
Indeed, studies from several laboratories have identified additional molecules on the cell surface that may be critical for HTLV Env-mediated entry. The binding of HTLV-1 virions can be blocked by treating a CD4+ T-cell line with antagonists of type 2 adenosine receptors (24). Earlier studies reported that a monoclonal antibody (MAb) (34-23) directed against an antigen that maps to chromosome 17 blocked HTLV-1 entry (19). Recently, heparan sulfate proteoglycans (HSPGs) have been reported to play a role in HTLV-1 binding (52, 58).
HSPGs, a type of glycosaminoglycan consisting of a core protein with O-linked heparan sulfate (HS) polysaccharide chains, are widely expressed on the surfaces of mammalian cells and are critical for the cellular attachment of many viruses (76). These include several members of the herpesvirus, flavivirus, adenovirus, papillomavirus, and retrovirus families (3, 18, 21, 46, 68, 75). For most viruses, the initial virus-cell interactions use HSPGs as binding receptors or attachment factors for the viral envelope. Rarely, HSPGs can function as fusion receptors. For example, an interaction of the herpes simplex virus glycoprotein gD with a 3-O-sulfotransferase-modified form of HSPG induces fusion (69).
Two recent studies indicated that HSPGs can play a role in HTLV Env-mediated entry into adherent, non-T-cell lines. Both the binding of soluble HTLV-1 SU and the titer of HTLV-1 pseudotyped viruses were dramatically reduced when HSPGs were removed from the cell surface (58). The titer of HTLV-1 pseudotyped viruses was also reduced when transductions were performed in the presence of dextran sulfate (58) and osteoprotegerin (52), two substances which block interactions with HSPGs. These studies indicate that HTLV binding and entry, at least on adherent cell lines, reflect interactions with HSPGs as well as with other specific cell surface molecules.
The interpretation of the relevance of these studies to HTLV-1 infectivity in vivo is complicated by the fact that primary CD4+ T cells have been reported to express low or undetectable levels of HSPGs (12, 28, 65, 77). Thus, it is not clear whether the requirements for HTLV-1 Env-mediated fusion are identical for established cell lines and primary T cells or which, if any, of the reported candidates for HTLV-1 receptors are relevant for HTLV Env-mediated binding and entry into primary T cells (55).
For this study, the role of HSPGs in HTLV-1 binding and entry into T cells was examined. First, we show that the expression of HSPGs on both T-cell lines and activated T cells is transiently regulated during active growth. Removal of the heparan sulfate chains of HSPGs on the surfaces of activated CD4+ T cells and established T-cell lines eliminated the majority of binding of soluble HTLV-1 SU. Blocking interactions with HSPGs also significantly decreased the binding and internalization of HTLV-1 virions and reduced the titer of HTLV-1 Env pseudotyped viruses.
MATERIALS AND METHODS
Cells and cell culture.
MOLT4 and SupT1, obtained from American Type Culture Collection (ATCC, Manassas, VA), were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS). HEK 293-T/17, a highly transfectable subclone of a 293 cell line transformed with the simian virus 40 large T antigen (56), was maintained in Dulbecco's modified Eagle's medium supplemented with 10% FCS. MT-2, a CD3-negative HTLV-1 producer cell line (48), was maintained in RPMI 1640 supplemented with 10% FCS.
To isolate CD4+ T lymphocytes, cord blood samples were obtained from healthy volunteer donors during vaginal births at Frederick Memorial Hospital (Frederick, MD), and leukopaks of peripheral blood from healthy donors were collected according to NIH-approved Institutional Review Board protocols. CD4+ T lymphocytes were isolated and cultured as previously described (35). Experiments involving naïve, unactivated CD4+ T cells were performed using cord blood lymphocytes as the source of cells, since adult peripheral blood contains activated and memory T cells. CD4+ T cells were activated either by culturing with 20 units/ml of interleukin-2 (IL-2; Zeptometrix, Buffalo, NY) and 1 μg/ml phytohemagglutinin (PHA; Abbott Diagnostics, Abbott Park, IL) or by culturing with anti-CD3 and anti-CD28 antibody beads at a ratio of 4.4 beads/cell (a gift of Carl June, University of Pennsylvania, Philadelphia, PA). Monocytes were isolated by countercurrent elutriation and activated by using either 10 μg/ml of lipopolysaccharide (LPS; Difco, Detroit, MI) or 10 ng/ml human granulocyte-macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ).
Reagents.
The monoclonal antibody clones specific for HSPG, F58-10E4 (immunoglobulin M [IgM]) and F69-3G10 (IgG2b), heparan sulfate lyase (HS lyase; also referred to as heparitinase III), and chondroitinase ABC lyase, were obtained from Seikagaku Corp. (Tokyo, Japan). F58-10E4 recognizes a constitutively expressed epitope of native HSPGs. F69-3G10 recognizes an epitope that is exposed following cleavage of HSPGs by HS lyase. Heparin was obtained from Sigma-Aldrich (St. Louis, MO). Soluble SU proteins, an HTLV-1 SU immunoadhesin (HTSU-IgG), and a negative control avian retrovirus ALSV-A SU immunoadhesin (SUA-rIgG) were generated as previously described (35).
Flow cytometric analysis.
To examine the level of cell surface expression of HSPG, 106 cells were spun down, resuspended in 100 μl of phosphate-buffered saline with 2% (vol/vol) human serum from type AB blood donors (PBS-2% HuAB), and incubated for 15 min on ice. Cells were then washed with PBS-2% HuAB and incubated on ice with 2 μg of either the appropriate anti-HSPG antibody or an isotype control. After 30 min, cells were washed, resuspended in 100 μl of PBS-2% skim milk-0.01% sodium azide, and incubated with 10 μg of a goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated antibody directed against the appropriate isotype. After 25 min on ice, the samples were washed, resuspended in 400 μl of PBS, and immediately analyzed by flow cytometry. Fifty thousand live cell events were measured on a FACScan cytometer (BD Pharmingen, San Diego, CA) and analyzed using Flowjo software (Treestar, Aurora, CA).
For studies involving enzymatic removal of the HS chains of cell surface HSPGs by HS lyase, 106 cells were spun down and resuspended in 200 μl of HS lyase buffer (20 mM Tris, pH 7.4, 0.01% bovine serum albumin, and 4 mM CaCl2). Cells were then incubated for 2 h at 37°C with HS lyase (10 to 20 mU, as indicated in text) or in buffer only. Cells were washed twice with PBS-5% HuAB-0.01% sodium azide (PBS-5% HuAB), resuspended in 400 μl of PBS, and then analyzed by flow cytometry as described above.
The specific binding of HTSU-IgG to target cells was examined as recently described (35). Briefly, target cells were fixed in 4% paraformaldehyde for 30 min on ice, washed with PBS, and then resuspended in PBS-2% FCS-0.02% sodium azide. The target cells (106) were then incubated on ice for 30 min with immunoadhesin (either HTSU-IgG or, as a negative control, SUA-IgG) to a final volume of 0.3 ml. The cells were washed, incubated for 30 min on ice with a FITC-conjugated antibody specific for rabbit immunoglobulins (Biosource, Camarillo, CA), washed again, resuspended in PBS, and analyzed by flow cytometry.
Virion binding assay.
The specific binding of HTLV virions to target cells was examined using a modification (35) of a previously described method (24). Briefly, virus was concentrated from the supernatant of an HTLV-1-producing cell line (MT-2), and the amount of HTLV-1 in the sample was determined using an enzyme-linked immunosorbent assay for the matrix protein MA (p19) (Zeptometrix Corp.). For the viral binding assay, target cells (106 in 0.2 ml of PBS) were then incubated at 22°C with and without (negative control) 100 μl of the viral preparation containing 5 μg of HTLV-1 virions. After 30 min, cells were washed in PBS, resuspended in 200 μl of PBS, and incubated for 30 min on ice with 1.0 μg of monoclonal antibody directed against HTLV SU (anti-gp-46 MAb clone 65/6C2.2.34; Zeptometrix Corp.). The cells were then washed and resuspended in 100 μl of PBS with a FITC-conjugated goat anti-mouse antibody for 30 min. After the incubation, the cells were washed and immediately analyzed by flow cytometry.
Virion internalization assay.
To generate virus for the internalization assay, the HTLV-1 producer cell line MT-2 was suspended at a concentration of 2 × 106 cells/ml. Sixteen hours later, the culture was centrifuged, and the virus-containing supernatant was collected and filtered through a 0.45-μm filter. Target cells were incubated with 250 ng of virus at 37°C for 2 h, washed twice in serum-free medium, and then incubated in trypsin for 10 min at 37°C to remove virus bound to the cell surface. The cells were then washed once in medium with 20% FCS and once in ice-cold PBS and then resuspended in 1 ml of paraformaldehyde (4% [wt/vol] in PBS). After incubation for 15 min at room temperature, cells were washed once in permeabilization wash buffer consisting of PBS, 0.5% saponin, 0.1% sodium azide, and 2% human AB sera, resuspended in 300 μl of permeabilization buffer (PBS with 2.5% saponin), and incubated at 22°C. After 20 min, 5 μl of human AB sera and either 4 μg of an anti-HTLV p19 (MA) mouse antibody (Zeptometrix Corp.) or an IgG1 isotype control (BD Pharmingen) were added, and the cells were incubated at 4°C for 30 min. Cells were then washed once in permeabilization wash buffer, resuspended in 100 μl of permeabilization buffer containing 3 μl (0.6 μg) of FITC-conjugated anti-mouse IgG1 antibody, and incubated for 20 min at 4°C. The efficiency of permeabilization was determined using a FITC-conjugated anti-actin antibody. Cells were then washed twice in permeabilization buffer, resuspended in 500 μl of sheath fluid (balanced salt solution to prevent clumping), and analyzed by flow cytometry.
Western blot analysis of HSPGs on T cells.
CD4+ T cells were treated either with or without 10 mU of HS lyase as described above. Cells were pelleted, washed twice with PBS, and lysed for 1 hour on ice in digitonin lysis buffer (PBS with 0.25% deoxycholate, 1% digitonin, and 10 μM protease inhibitor cocktail [Sigma]). After removal of the debris by centrifugation at 4°C for 10 min at 16,000 × g, the protein concentration was determined (protein assay reagent; Bio-Rad). Equal amounts of protein (200 to 300 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% Tris-glycine gels (Invitrogen) and then transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA). The membranes were blocked with 10% nonfat dry milk-TBST (Tris-buffered saline with 0.1% Triton X-100) for 30 min at room temperature and then incubated overnight at 4°C with the monoclonal antibody F69-3G10 diluted in 5% nonfat dry milk-TBST. The membranes were then washed twice with TBST, hybridized with horseradish peroxidase-conjugated anti-mouse IgG for 1 h at room temperature, and washed three times with TBST. Bands were visualized using ECL reagent (Amersham Biosciences Corp., Piscataway, NJ) and exposed to film (Kodak).
Generation of pseudotyped retroviral vectors and viral transduction.
Pseudotyped retroviral vectors were generated and their relative titers determined as previously described (35). Briefly, retroviral vectors were generated by cotransfecting 293-T cells with an human immunodeficiency virus (HIV)-based retroviral vector encoding enhanced green fluorescent protein (EGFP) and a plasmid encoding the appropriate Env protein. Transductions were performed by resuspending the cells in fivefold dilutions of supernatant containing the pseudotyped viruses and transducing them using a modification of the spin infection method. Four days later, the cells were analyzed by flow cytometry for the expression of EGFP to determine the percentage of transduced cells. Relative titers of the pseudotyped viruses were determined from the dilution with the lowest percentage that was between 5% and 40% positive for EGFP. The relative titers were determined using the following formula: dilution factor × (% positive − % positive in negative control) × (2 × 105).
Cell-cell transmission of HTLV-1.
Cell-cell transmission of HTLV-1 was performed essentially as previously described (57). Briefly, primary CD4+ T cells isolated from cord blood were activated with anti-CD3/anti-CD28 antibody beads. Four days later, 5 × 106 cells were incubated for 2 h at 37°C in either buffer alone or buffer containing HS lyase. From both samples, 1 × 106 cells were analyzed for the presence of HSPGs to verify the activity of the HS lyase. The remainder were used as targets in a cell-cell transmission assay, as previously described (57). Briefly, 16 × 106 cells of the HTLV-1 producer cell line MT-2 were irradiated at 15,000 rads and then mixed at a 2:1 ratio of irradiated MT-2 cells to activated cord blood CD4+ T cells. Since HSPGs can be recycled to the cell surface and since irradiated MT-2 cells transmit virus for 2 to 4 days after irradiation, 25 μg/ml of soluble heparin was added to the medium to reduce binding to HSPGs. The cells were stained with anti-CD3 antibody, and flow cytometry was then used to determine the percentage of CD3+ target cells containing HTLV-1 virions as described above.
RESULTS
Expression of HSPGs in CD4+ T-cell lines.
For several cell lines, it has been reported that the majority of binding of soluble HTLV-1 Env to the cell surface occurs through attachment to HSPGs (52, 58). Removal of the HS chains of HSPGs dramatically reduces the ability of HTLV-1 Env pseudotyped viruses to transduce those cells (58). Since those studies examined the role of HSPGs in non-T adherent cell lines, we investigated the role of HSPGs in the binding and entry of HTLV into CD4+ T cells, the primary in vivo target of this virus.
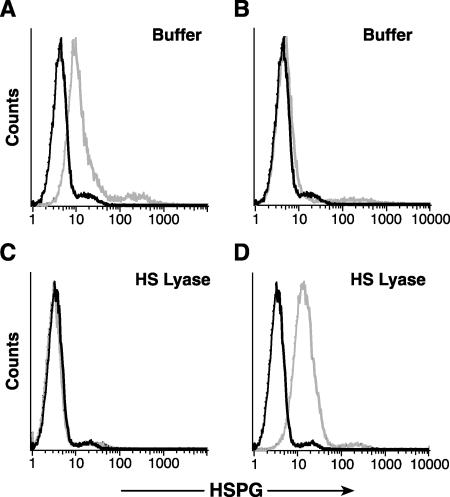
Since the reported HSPG expression levels for various established leukemia-lymphoma CD4+ T-cell lines have been heterogeneous, we chose to study two cell lines previously reported to express cell surface HSPGs, SupT1 (67) and MOLT4 (3). The MAb F58-10E4, which specifically recognizes intact HSPGs on the cell surface, bound MOLT4 cells at levels significantly above that of the isotype control (Fig. 1A), while the F69-3G10 antibody did not bind these cells (Fig. 1B). The enzyme HS lyase specifically removes the heparan sulfate chain from the core proteoglycan and thus eliminates the epitope recognized by F58-10E4. When MOLT4 cells were treated with 20 mU of HS lyase, F58-10E4 binding was reduced almost to the background level (Fig. 1C). The F69-3G10 antibody recognizes an epitope on HSPGs that is revealed following cleavage by HS lyase. As expected, F69-3G10 did not bind at significant levels to untreated cells but bound at high levels to the cells after the HS lyase treatment (Fig. 1B and D). An analysis of SupT1 gave similar results (data not shown). Thus, some CD4+ T-cell lines express significant amounts of cell surface HSPGs that can be removed by enzymatic treatment with HS lyase.
FIG. 1.
HSPGs are expressed on CD4+ T-cell lines. MOLT4 cells were washed, resuspended in HS lyase buffer, and incubated with (bottom panels) or without (top panels) 20 mU of HS lyase. Cells were stained with a monoclonal antibody specific for intact HSPGs (F58-10E4) (panels A and C) or with a monoclonal antibody specific for an epitope generated by the cleavage of HSPGs with HS lyase (F69-3G10) (panels B and D). The data shown are from a representative experiment out of 10 performed. Light lines, anti-HSPG antibody; dark lines, isotype control.
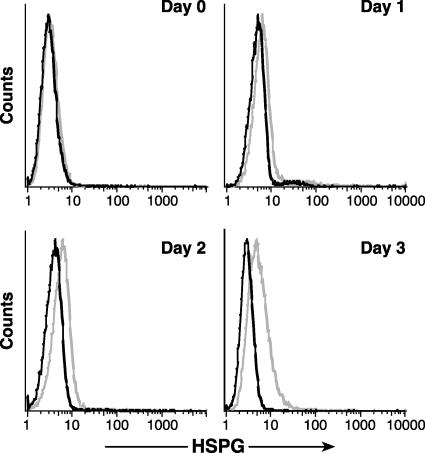
Furthermore, HSPG expression on these CD4+ T-cell lines was transient. When MOLT4 cells were grown to saturation density, at which there was little or no cell growth (approximately 3 × 106/ml), there was no detectable binding of F58-10E4 (Fig. 2, day 0). After subculturing of the cells at a concentration of 3 × 105 cells/ml, the level of F58-20E4 binding was determined daily. Actively growing MOLT4 cells bound increasing levels of the antibody up to day 3 (Fig. 2). Similar results were obtained with SupT1 cells (data not shown). These results suggest that cell surface HSPG expression on CD4+ T-cell lines is transient and related to active cell growth.
FIG. 2.
HSPGs are transiently expressed during CD4+ T-cell growth. MOLT4 cells were grown to confluence and analyzed by flow cytometry for the presence of HSPGs as described above. The remainder of the culture was split 1:10, harvested 1, 2, or 3 days later, and analyzed for HSPG expression. The data shown are from a representative experiment out of three performed. Light lines, anti-HSPG antibody; dark lines, isotype control.
HSPGs are expressed on primary CD4+ T cells following activation.
Several studies have reported that primary CD4+ T lymphocytes express little or no detectable levels of HSPG (12, 28, 40, 65). The observation that the cell surface expression of HSPGs on T-cell lines increased with active cell division suggested that T-cell activation could induce transient cell surface expression of HSPGs.
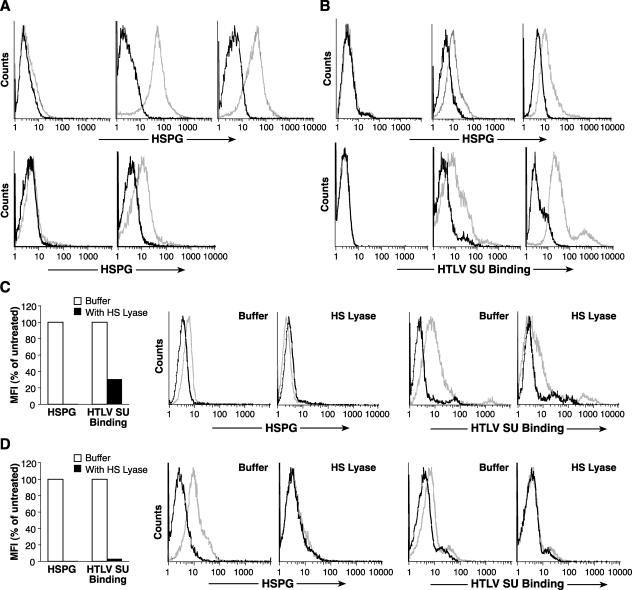
To examine this, lymphocytes were isolated from peripheral blood and enriched for CD4+ T cells. Since monocytes require activation to express detectable levels of HSPGs (12), monocytes were isolated from the same sample as a control for activation-induced HSPG expression. For both cell types, freshly harvested cells and cells activated for 18 h of culture were examined for the expression of HSPGs as described above. As expected from previous work, freshly isolated unactivated monocytes did not bind F58-10E4 (Fig. 3A, top left panel), while monocytes activated with GM-CSF (Fig. 3A, top middle panel) or LPS (Fig. 3A, top right panel) bound high levels of F58-10E4. Similarly, while freshly isolated CD4+ lymphocytes did not bind F58-10E4 (Fig. 3A, bottom right panel), significant levels of F58-10E4 binding were observed after 18 h of activation (Fig. 3A, bottom right panel). The level observed for activated CD4+ lymphocytes was lower than that observed for the activated monocytes (Fig. 3A). Further studies revealed that the level of HSPG expression on T cells peaked 3 to 5 days after activation and fell to background levels as the cells became quiescent (data not shown).
FIG. 3.
Activation of primary CD4+ T cells induces cell surface HSPG expression. (A) Monocytes and CD4+ T cells were isolated from adult peripheral blood Leukopaks as described in Materials and Methods. Cells were assayed for the level of HSPG as described in the text, either immediately or after 18 h of activation. Panels: top left, unstimulated monocytes; top middle; monocytes 18 h after stimulation with GM-CSF; top right, monocytes 18 h after stimulation with LPS; bottom left, unstimulated CD4+ T cells; bottom right, CD4+ T cells 18 h after stimulation with PHA and IL-2. (B) CD4+ T cells were isolated from cord blood as described in Materials and Methods, and flow cytometry was performed either immediately (left) or 3 days after activation with anti-CD3/anti-CD28 antibody beads (middle) or PHA and IL-2 (right). Top, cell surface expression of HSPGs was determined using the F58-10E4 antibody; bottom, expression levels of HTLV SU binding proteins were determined using the soluble form of the HTLV-1 SU protein (HTSU-IgG) or, as a negative control, SUA-IgG. (C) MOLT4 cells were incubated with or without 20 mU of HS lyase, and the levels of HSPGs and HTLV SU binding were assayed as described above. For both samples, the amount of specific binding was determined by subtracting the MFI of control (isotype control or SUA-IgG) binding from the MFI of specific (F58-10E4 or HTSU-IgG) binding. The MFI shown is expressed as a percentage of the MFI of untreated cells. (D) CD4+ T cells, isolated from adult peripheral blood and activated for 2 days with PHA and IL-2, were incubated with or without 10 mU of HS lyase. The levels of HSPGs and HTLV SU binding were determined as described for panel C. For panels C and D, the data shown are from a representative experiment out of nine performed. Symbols for HSPG analysis: light lines, F58-10E4; dark lines, IgM isotype control. Symbols for HTLV SU binding: light lines, HTSU-IgG; dark lines, SUA-IgG.
Next, HSPG expression levels on primary CD4+ lymphocytes following two different methods of T-cell activation were compared. CD4+ T cells isolated from cord blood were activated either with IL-2 and PHA or with anti-CD3/anti-CD28 antibody beads for 4 days. Τhe levels of cell surface expression of HSPGs and HTLV SU binding proteins were determined. For both methods of activation, significant levels of binding to F58-10E4 were observed (Fig. 3B, top middle and top right panels). As expected from previous work (35, 51), quiescent CD4+ T cells did not bind HTLV SU (Fig. 3B, bottom left panel). In contrast, activation by either method induced high levels of binding of the soluble HTLV SU protein (Fig. 3B, bottom middle and bottom right panels).
HTLV-1 SU binds to HSPGs on CD4+ T cells.
The studies described above indicate that activated, proliferating CD4+ T cells have significant amounts of HSPGs on their cell surfaces. Previously, we showed that a soluble form of HTLV-1 SU protein (HTSU-IgG) bound specifically to the cell surfaces of activated CD4+ T cells (36, 51, 80). We next examined whether reducing the amount of HSPGs on the cell surface reduced binding of the soluble HTLV-1 SU protein.
MOLT4 cells, after 3 days of subculturing, were either left untreated or treated with HS lyase, and the amount of binding of HTSU-IgG was determined by flow cytometry analysis. To verify that the HSPGs were removed by the enzyme treatment, the level of binding of F58-10E4 was also determined. Treatment of the MOLT4 cells with HS lyase reduced the level of HSPGs to below the level detectable by the antibody (Fig. 3C). Binding of HTLV-1 SU was dramatically reduced, but not eliminated, when the HSPGs were removed (Fig. 3C). Similar experiments performed on activated CD4+ T cells revealed that enzymatic modification of the HSPGs from the cell surface had an even more dramatic effect on the level of binding of soluble HTLV-1 SU protein (Fig. 3D). Flow cytometry analysis following double staining with HTSU-IgG and F58-10E4 revealed that the activated CD4+ T cells with the highest levels of HSPG expression bound the highest levels of HTLV-1 SU (data not shown). Thus, it appears that HTLV SU binding to the T-cell surface predominantly reflects binding to HSPGs.
Multiple HSPGs are present on activated CD4+ T cells.
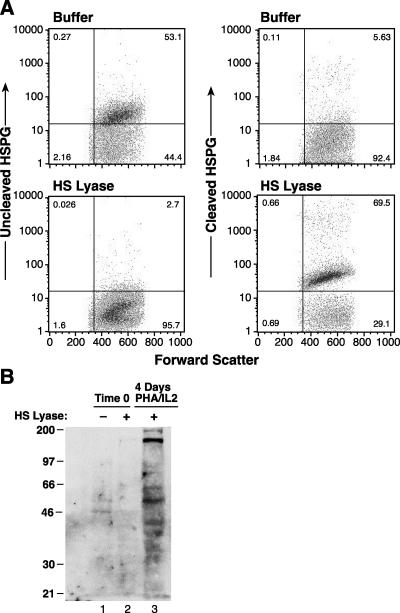
To further investigate the expression of HSPGs on activated CD4+ T cells, Western blot analyses were performed using the F69-3G10 antibody. Previously, this antibody was shown to recognize residual unsaturated glucuronate stubs generated following cleavage by HS lyase, and it was previously used to investigate the species of HSPGs present on the cell surface (10, 14, 38). HS lyase treatment of activated CD4+ T cells eliminated F58-10E4 binding (Fig. 4A, top panels) and caused the appearance of F69-3G10 binding (Fig. 4A, bottom panels). The HS stub-bearing core proteins were visible as multiple bands between 200 kDa and 35 kDa (Fig. 4B, lane 3), indicating that several different members of HSPG families are present on activated CD4+ T cells. As expected from previous results, freshly isolated CD4+ T cells (HS lyase treated and untreated) did not show any reactivity with F69-3G10 (Fig. 4B, lanes 1 and 2). As expected from previous studies (14), activated cells not treated with HS lyase did not bind F69-3G10 above background levels (Fig. 4A, top left panel) and were not used for Western analyses.
FIG. 4.
Expression of HSPGs on the cell surfaces of CD4+ T cells. (A) CD4+ T cells isolated from cord blood lymphocytes were activated for 4 days with PHA and IL-2. The cells were then incubated either with (bottom panels) or without (top panels) 10 mU of HS lyase, and flow cytometry was performed as described in Materials and Methods. Left panels, staining with F58-10E4; right panels, staining with F69-3G10. The data shown are from a representative experiment out of five performed. (B) CD4+ T cells isolated from cord blood lymphocytes, either quiescent or 4 days after activation with PHA and IL-2, were treated with 10 mU of HS lyase. Cells were lysed, and proteins were separated by electrophoresis and subjected to Western blot analysis using the F69-3G10 antibody as described in Materials and Methods.
HTLV-1 virions bind to HSPGs on CD4+ T cells.
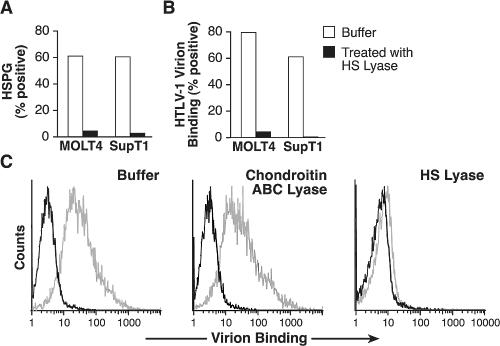
To complement the studies of the binding of soluble HTLV SU protein, the role of HSPGs on the binding of HTLV-1 virions was examined using CD4+ T-cell lines (MOLT4 and SupT1) either treated or not treated with HS lyase. The cells were then incubated with concentrated cell-free HTLV-1 virions, and the relative amounts of virion binding were determined using a recently described flow-based assay (24, 35). Enzymatic removal of heparan sulfate chains reduced the binding of HTLV-1 virions by >95% for both MOLT4 and SupT1 cells (Fig. 5A and B). The binding of HTLV-1 virions to activated CD4+ T lymphocytes was also examined. Since some viruses use both HSPG and another glycosaminoglycan, chondroitin sulfate proteoglycan, to facilitate entry, we compared the effects of HS lyase and chondroitin ABC lyase, which cleaves the chondroitin sulfate chain from chondroitin sulfate proteoglycans. Enzymatic modification of HSPGs reduced the binding of HTLV-1 virions by >90% (Fig. 5C, right panel). In contrast, virion binding was not significantly reduced by 1 U of chondroitin sulfate ABC lyase (Fig. 5C, middle panel).
FIG. 5.
Binding of HTLV-1 virions to CD4+ T cells involves interaction with HSPGs. (A and B) SupT1 and MOLT4 cells incubated either with or without HS lyase. Some cells were assayed for the ability to bind F58-10E4, and the remainder were exposed to concentrated HTLV-1 virions, with the amount of virion binding determined as described in Materials and Methods. The percentage of cells positive for virion binding was determined by subtracting the amount of anti-SU antibody binding observed in the absence of virus from that observed in the cells exposed to the virions. (A) Binding of F58-10E4 antibody. (B) Binding of HTLV-1 virions. (C) Activated CD4+ T cells isolated from adult peripheral blood were incubated for 2 h at 37°C in either buffer alone (left) or with buffer containing 1 U of chondroitin ABC lyase (middle) or 10 mU of HS lyase (right). Cells were then exposed to HTLV-1 virions, and the amount of virion binding was determined as described for panel B. Dark lines, binding in the absence of virions; light lines, binding in the presence of virions. The data shown are from a representative experiment out of five (A and B) or three (C) performed.
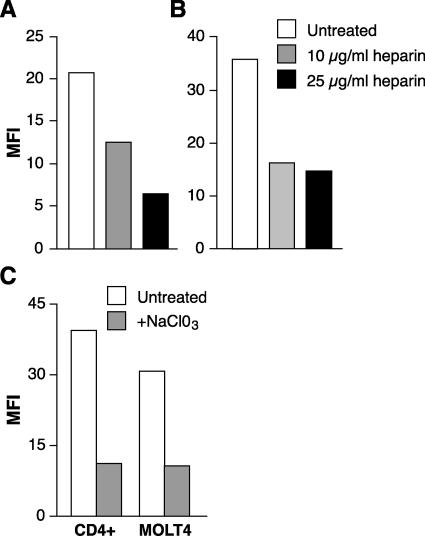
To further validate our results obtained with HS lyase, we also examined whether the binding of HTLV virions was decreased by other approaches previously shown to inhibit interactions of viral envelope proteins with HSPGs. First, the effect of soluble heparin, a polyanionic reagent with the same repeating disaccharide as the heparan sulfate side chain of HSPGs, was examined. Soluble heparin has previously been shown to block viral interactions with HSPGs (2, 8, 29, 49, 53, 67, 82). SupT1 and CD4+ T cells were exposed to concentrated HTLV virions in the presence of 0, 10, or 25 μg/ml of soluble heparin. Virion binding in the presence of heparin was dramatically reduced (69% and 59% at 25 μg/ml for SupT1 and CD4+ T cells, respectively) (Fig. 6A and B). As another approach, we examined the effect of treating activated CD4+ T cells and MOLT4 cells with sodium chlorate, which prevents sulfate donation to newly synthesized polysaccharide chains (11, 17, 73). Blocking sulfation of polysaccharide chains of HSPGs markedly inhibited HTLV virion binding to both the activated CD4+ T cells and the CD4+ cell line MOLT4 (Fig. 6C).
FIG. 6.
Blocking HSPG interactions reduces HTLV-1 virion binding to T cells. Concentrated HTLV-1 virions were incubated for 30 min in the presence of 0, 10, or 25 μg/ml of soluble heparin and then incubated with SupT1 (A) or activated CD4+ T cells isolated from adult peripheral blood (B), and the amount of virion binding was determined as described in Materials and Methods. (C) MOLT4 cells and activated CD4+ T cells isolated from adult peripheral blood were cultured either in RPMI containing 30 mM sodium chlorate or in RPMI alone for 3 days. The cells were then analyzed for virus binding. The data shown are from a representative experiment out of five (A and B) or three (C) performed.
HSPGs enhance HTLV-1 Env-mediated entry into CD4+ T cells.
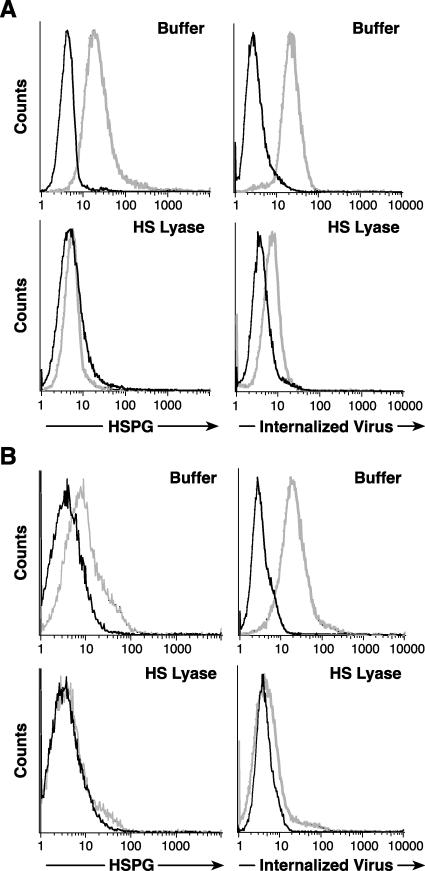
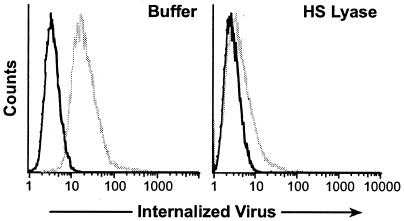
The studies described above indicate that HSPGs are critical for efficient binding of both HTLV-1 virions and soluble HTLV-1 SU on activated CD4+ T cells. We next examined whether blocking interactions between HTLV-1 Env and HSPGs also decreased the entry of HTLV-1 virions into T cells. First, the role of HSPGs in the internalization of HTLV-1 virions was examined. MOLT4 cells, either untreated or treated with HS lyase, were exposed to virus from MT-2, a cell line that produces high levels of HTLV-1. After 2 h, bound virions were removed by washing and a trypsin treatment, which completely removes cell surface-bound HTLV-1 Env from the cell surface (data not shown). The amount of internalized virions was then determined by flow cytometry following intracellular staining for HTLV-1 MA (p19), a protein in the viral core. As before, the HS lyase treatment decreased the binding of F58-10E4 to background levels (Fig. 7A, left panels). When MOLT4 cells were exposed to HTLV-1 virions, most of the untreated cells (88%) internalized the virus. The amount of virus internalized following HS treatment was reduced by >85%, as judged by the mean fluorescence intensity (MFI) (Fig. 7A, right panels).
FIG. 7.
HSPGs enhance HTLV-1 Env-mediated entry into CD4+ T cells. (A) MOLT4 cells were incubated either with (bottom) or without (top) 20 mU of HS lyase as described in the legends to the previous figures. (Left) HSPG expression was determined as described above. Dark lines, isotype control; light lines, F58-10E4. (Right) The extent of internalization was determined 2 h after exposing the cells to HTLV-1 virions, as described in Materials and Methods. Dark lines, mouse IgG1 (isotype control); light lines, anti-HTLV MA (p19) antibody. Without HS lyase treatment, the MFI was 17.8, and 89% of the cells were positive for internalized virus. With HS lyase treatment, the MFI was 2.6, and 8.9% of the cells were positive. (B) CD4+ T cells were isolated from cord blood lymphocytes, activated for 3 days with anti-CD3/anti-CD28 antibody beads, and then treated with 10 mU of HS lyase (bottom) or left untreated (top). (Left) HSPG binding to F58-10E4 (light lines) or isotype control (dark lines). (Right) Binding of anti-MA (p19) antibody (light lines) or isotype control (dark lines). The data shown are from a representative experiment out of 14 performed.
The role of HSPGs on the internalization of HTLV-1 virions into primary CD4+ T cells was also examined. No significant expression of HSPGs could be detected following enzymatic treatment of the cells (Fig. 7B, bottom left panel). As seen with MOLT4 cells, a high percentage (82%) of the activated T cells had internalized HTLV-1 virions. The amount of virus internalized following this treatment was reduced by >90%, as measured by MFI (Fig. 7B, right panels). To date, we have examined more than 12 samples isolated from both cord and adult peripheral blood and observed that entry was decreased between 60 and 97% following modification of HSPGs. These observations suggest that HSPGs play an important role in the efficient entry of HTLV-1 virions into CD4+ T cells.
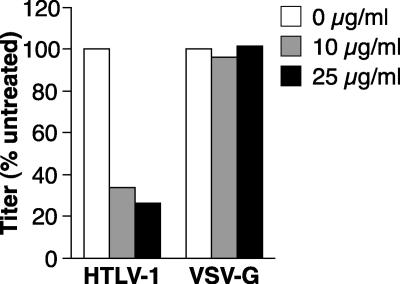
As another approach to examine the role of HSPGs in HTLV-1 Env-mediated entry, we examined the effect of soluble heparin treatment on the titer of HTLV-1 pseudotyped viral transduction into CD4+ T cells. HIV-based indicator proviruses, pseudotyped with either HTLV-1 Env or vesicular stomatitis virus glycoprotein (VSV-G), were generated and used to transduce SupT1 cells in the presence of either 0, 10, or 25 μg/ml of soluble heparin, as described in Materials and Methods. Four days later, the titers of the pseudotyped viruses were determined. Soluble heparin significantly reduced the titer of the HTLV-1 Env pseudotyped virus (Fig. 8). The titer in the presence of heparin (25 μg/ml) was reduced to about 25% that of the control. The effect of heparin was a specific effect on HTLV-1 Env-mediated interactions with the target cell, since the titer of the VSV-G pseudotyped virus was not significantly affected. We also observed for SupT1 cells that a 70% reduction in F58-10E4 binding resulted in a 60% decrease in the titer of HTLV-1 Env pseudotyped virus (data not shown). Thus, HSPGs play a significant role in HTLV-1 Env-mediated entry into CD4+ T cells.
FIG. 8.
Soluble heparin decreases titers of HTLV-1 pseudotyped viruses in CD4+ T cells. HTLV-1 or VSV-G pseudotyped virus particles were generated as described in Materials and Methods. The virus preparations were incubated in RPMI containing 0, 10, or 25 μg/ml of soluble heparin for 30 min. SupT1 cells were resuspended at 2 × 105/ml in RPMI, and then 0.5 ml of cells was mixed with an equal volume of the pseudotyped virus in the presence or absence of heparin, as indicated. The cells were transduced by spinoculation and then harvested 4 days later, and the viral titers were determined as described in Materials and Methods.
The role of HSPGs in the cell-cell transmission of HTLV-1 virions into primary CD4+ T cells was also examined. For this experiment, MT-2 cells were irradiated and cultured overnight with activated CD4+ T cells that had either been treated with HS lyase or left untreated. Soluble heparin was also added to the cultures to further prevent Env-HSPG interactions. The amount of HTLV-1 virions internalized in the CD4+ T cells and the number of cells positive for internalized virions were then determined by gating on the CD3+ population. HTLV-1 virions had internalized in >90% of the activated CD4+ T cells, as judged by the presence of the HTLV-1 MA protein. Blocking the interactions with HSPGs by HS lyase and soluble heparin dramatically reduced the cell-cell transmission of HTLV-1 (Fig. 9).
FIG. 9.
Blocking HSPG interactions blocks cell-cell transmission of HTLV-1. Cord blood CD4+ T cells were activated for 4 days with anti-CD3/anti-CD28 antibody beads and then either treated with HS lyase or incubated in buffer alone. MT-2 cells were irradiated and then incubated for 30 min with 25 μg/ml of soluble heparin or left untreated, as described in Materials and Methods. The appropriate irradiated MT-2 cells (with heparin for the HS lyase-treated targets and without heparin for the untreated targets) were then exposed to the CD4+ target cells at a ratio of 2:1. To distinguish the target cells from the MT-2 cells, the cells were first stained with an antibody directed against a cell surface antigen (CD3) that is expressed at high levels on primary CD4+ T cells and at undetectable levels on MT-2 cells. The cells were then permeabilized and stained for the HTLV-1 MA (p19) protein. (Left) Untreated; (Right) treated with HS lyase and soluble heparin. Dark lines, staining with mouse IgG1 (isotype control); light lines, staining with anti-HTLV p19 antibody.
DISCUSSION
Previous studies (52, 58) demonstrated a role for HSPGs in HTLV-1 entry into adherent non-T-cell lines, which generally express high levels of HSPGs. The relevance of these results to the in vivo infection of CD4+ T cells, the primary target cell of HTLV-1, has not been directly examined. This is most likely due to previous reports that CD4+ T cells express low or undetectable levels of HSPGs (12, 28, 65, 77). In this study, we report that while quiescent primary CD4+ T cells do not express detectable levels of HSPGs, HSPGs are expressed on primary CD4+ T cells following immune activation, although at a lower level than on activated macrophages (12, 28, 65) and adherent cell lines (52, 58).
The role of HSPGs on HTLV-1 Env-mediated binding to CD4+ T cells and the T-cell lines MOLT4 and SupT1 was then examined. We observed that enzymatic removal of HSPGs from the cell surface dramatically reduced (75 to 95%) the binding of both soluble SU and HTLV-1 virions to these cells. The induction of cell surface expression of HSPGs following T-cell activation is consistent with previous reports that primary T cells only bind soluble HTLV SU after immune activation (36, 43). Similarly, the treatment of quiescent primary CD4+ T cells with TGF-β induces the cell surface expression of HSPGs (data not shown) coincident with the induction of binding of HTLV SU and virions (35). Furthermore, incubation of the cells with sodium chlorate, which blocks sulfation of the HSPGs (11, 73), and soluble heparin, which blocks HSPG interactions (2, 8, 29, 49, 53, 67, 82), also dramatically reduced the binding of HTLV virions. The effect of HSPGs on the efficiency of HTLV-1 Env-mediated entry was then examined. Following exposure of both MOLT 4 and activated T cells to cell-free HTLV-1 virions, the majority of the cells contained viral cores. The amount of virus internalized following HS lyase treatment was reduced by >85%. For cell-to-cell transmission, the transmission of virus to the recipient CD4+ T cells was similarly inhibited by HS lyase treatment. The titers of HTLV-1 pseudotyped viruses were reduced by blocking interactions with HSPGs, either by soluble heparin (Fig. 8) or by the removal of HSPGs (data not shown). Soluble heparin also blocks the binding of HTLV-1 SU and dramatically reduces the titers of HTLV pseudotyped viruses on several different human and nonhuman adherent cell lines (unpublished data). Thus, HSPGs play a significant role in HTLV-1 Env-mediated entry into CD4+ T cells.
HSPGs have previously been shown to play a critical role in the infectivity of many viruses, including herpesviruses, papillomaviruses, dengue virus, hepatitis C virus, and certain retroviruses, including HIV (reviewed in reference 76). The precise function of HSPGs in HTLV-1 infectivity is not yet clear. For most viruses, an interaction with HSPGs is the first of a series of events required for viral entry and infection, with HSPGs functioning as either an attachment factor or a binding receptor. For some viruses, this binding, while not essential for viral entry, can dramatically increase the efficiency of viral entry by concentrating virus on the cell surface and thereby can increase the probability of interactions with the fusion receptors. For other viruses, HSPGs can be an essential component of the cellular receptor, either as a binding receptor, as in the entry of HIV into brain microvascular endothelial cells (2, 7), or as a fusion receptor, as in the case of herpes simplex virus type 1 (74). The importance of HSPG binding for a given virus can vary with different cell types, depending on the relative abundance of the HSPG and other binding and fusion receptors on the cell surface. For certain HIV strains, HSPGs are not important for entry into CD4+ T cells, presumably because of high levels of CD4 on the surfaces of these cells. In contrast, it has been reported that HSPGs are critical for the efficient infection of macrophages, which express low levels of CD4+ and high levels of HSPGs (65). Our observations that activated CD4+ T cells express HSPGs raise the possibility that these molecules can contribute to infection by HIV of T cells possessing lower levels of CD4.
For some viruses, changes in the envelope proteins can generate variants that differ in their ability to use HSPGs for entry. A neurotropic variant of the leukemogenic murine retrovirus Friend murine leukemia virus was shown to be caused by a two-amino-acid change in Env which increased the affinity for HSPG and thereby altered the tropism of the virus to include brain capillary endothelial cells (34). Alphaviruses appear to utilize HSPGs only after cell culture adaptation (4, 26, 37, 70); this may also be true for flaviviruses (41) and pestiviruses (27). However, preliminary studies suggest that the use of HSPGs by HTLV-1 does not reflect lab adaptation of the virus. HTLV-1 isolated from cells of an infected patient and the strain of HTLV-1 used in these studies showed a similar dramatic decrease (85 and 84%, respectively) following the removal of HSPGs from the cell surface (data not shown).
What are the HSPGs involved in HTLV-1 binding? HSPGs are a diverse group of molecules that include three major classes, namely, the transmembrane syndecan family, the phosphatidylinositol-anchored glypican family, and the extracellular perlecan family (5, 31). The HS stub-bearing core proteins recognized by F69-3G10 following HS cleavage of activated T cells are visible as multiple bands, suggesting that a mixture of the syndecan and glypican families are present on the cell surface. Similar results have been observed for other cell types (9, 10, 12, 47). We are currently further characterizing the HSPGs expressed on the surfaces of CD4+ T cells and examining whether HTLV-1 Env binds to one or several of these HSPGs. It has been reported that all syndecans can bind HIV (18) and that syndecans 2 and 4 function as attachment molecules during HIV infection of macrophages (12). Syndecans on the surfaces of cells that do not express CD4 (and thus are nonpermissive for HIV infection) can capture, protect, and transmit HIV to T cells (18). It is possible that HSPGs may function to capture, protect, and transmit HTLV-1 in a similar fashion.
The elimination of HSPGs from the cell surface reduced but did not eliminate the binding of HTLV-1 SU to T cells (Fig. 3) or adherent cells (58; unpublished observations). Since HSPGs can be cleaved by trypsin, this is consistent with previous observations that treatment with trypsin eliminated the majority of SU binding and reduced the titers of HTLV pseudotyped viruses (32, 36, 53, 64, 78). These data suggest the presence of one or more additional molecules on the cell surface that bind HTLV SU.
One likely candidate would be GLUT-1, which has been reported to bind HTLV-1 and HTLV-2 SU (42). The titers of HTLV-1 (13) and HTLV-2 (42) Env pseudotyped viruses were enhanced in adherent cells by overexpressing GLUT-1. The data presented in the current report do not address the role of the widely expressed GLUT-1 protein in HTLV binding and entry. Similarly, previous reports of the effect of GLUT-1 on the efficiency of HTLV Env-mediated entry did not rule out a significant role for HSPGs, since the target cells used in those studies expressed high levels of HSPGs (15, 63). The relative contributions of HSPGs and GLUT-1 to HTLV-1 infection of T cells are currently under investigation.
As part of the current study, we established a flow cytometry method to allow us to measure the internalization of HTLV-1 virions. These studies revealed that following exposure to either cell-free or cell-associated HTLV-1, nearly all of the CD4+ T cells contained viral cores. This contrasts sharply with the extremely low level of productive infection observed when CD4+ T cells are exposed to HTLV-1 virions and is consistent with previous reports that HTLV-1 has a postfusion block to replication (22, 59). Recent evidence shows that some viruses enter cells through either a productive cytoplasmic pathway (reviewed in reference 16) or a less productive endocytotic degradative pathway (6, 23, 44, 45, 66). It seems likely that at least some of the virions entered through a productive HTLV Env-mediated fusion pathway rather than a degradative one, since the effect of the removal of HS chains from the cell surface HSPGs on viral internalization paralleled the effect on the titers of HTLV-1 Env pseudotyped viruses in both T cells and adherent cells (58). It has recently been reported that a CD4-mediated reciprocal relationship between productive fusion and degradation exists for HIV entry into T cells (66). Alternatively, since syndecans and DC-SIGN can protect HIV from degradation (8, 79), HSPGs may similarly protect HTLV-1 during entry. We are currently investigating the fate of these internalized virions.
HTLV-1 is primarily transmitted by breast-feeding or sexual contact via cell-to-cell contact between T cells. Our observation that blocking HSPGs on CD4+ T cells can inhibit the cell-to-cell spread of HTLV-1 suggests that therapeutics based on blocking these electrostatic interactions may be beneficial (1).
Acknowledgments
We thank Vineet KewalRamani, Sandra Ruscetti, Chris Grant, and Steve Jacobson for providing reagents, helpful suggestions, and encouragement.
This publication was funded in whole or in part with federal funds from the National Cancer Institute under contract NCI-CO-12400.
The content of this publication does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1.Altmeyer, R. 2004. Virus attachment and entry offer numerous targets for antiviral therapy. Curr. Pharm. Des. 10:3701-3712. [DOI] [PubMed] [Google Scholar]
- 2.Argyris, E. G., E. Acheampong, G. Nunnari, M. Mukhtar, K. J. Williams, and R. J. Pomerantz. 2003. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol. 77:12140-12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. [DOI] [PubMed] [Google Scholar]
- 4.Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93-103. [DOI] [PubMed] [Google Scholar]
- 5.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 6.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobardt, M. D., P. Salmon, L. Wang, J. D. Esko, D. Gabuzda, M. Fiala, D. Trono, B. Van der Schueren, G. David, and P. A. Gallay. 2004. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J. Virol. 78:6567-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobardt, M. D., A. C. Saphire, H. C. Hung, X. Yu, B. Van der Schueren, Z. Zhang, G. David, and P. A. Gallay. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27-39. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury, M. G., and C. R. Parish. 1991. Characterization of lymphocyte receptors for glycosaminoglycans. Immunology 72:231-238. [PMC free article] [PubMed] [Google Scholar]
- 10.Carter, N. M., S. Ali, and J. A. Kirby. 2003. Endothelial inflammation: the role of differential expression of N-deacetylase/N-sulphotransferase enzymes in alteration of the immunological properties of heparan sulphate. J. Cell Sci. 116:3591-3600. [DOI] [PubMed] [Google Scholar]
- 11.Chung, C. S., J. C. Hsiao, Y. S. Chang, and W. Chang. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72:1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clasper, S., S. Vekemans, M. Fiore, M. Plebanski, P. Wordsworth, G. David, and D. G. Jackson. 1999. Inducible expression of the cell surface heparan sulfate proteoglycan syndecan-2 (fibroglycan) on human activated macrophages can regulate fibroblast growth factor action. J. Biol. Chem. 274:24113-24123. [DOI] [PubMed] [Google Scholar]
- 13.Coskun, A. K., and R. E. Sutton. 2005. Expression of glucose transporter 1 confers susceptibility to human T-cell leukemia virus envelope-mediated fusion. J. Virol. 79:4150-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David, G., X. M. Bai, B. Van der Schueren, J. J. Cassiman, and H. Van den Berghe. 1992. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J. Cell Biol. 119:961-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Parseval, A., and J. H. Elder. 2001. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 75:4528-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 17.Fjeldstad, K., M. E. Pedersen, T. T. Vuong, S. O. Kolset, L. M. Nordstrand, and K. Prydz. 2002. Sulfation in the Golgi lumen of Madin-Darby canine kidney cells is inhibited by brefeldin A and depends on a factor present in the cytoplasm and on Golgi membranes. J. Biol. Chem. 277:36272-36279. [DOI] [PubMed] [Google Scholar]
- 18.Gallay, P. 2004. Syndecans and HIV-1 pathogenesis. Microbes Infect. 6:617-622. [DOI] [PubMed] [Google Scholar]
- 19.Gavalchin, J., N. Fan, P. G. Waterbury, E. Corbett, B. D. Faldasz, S. M. Peshick, B. J. Poiesz, L. Papsidero, and M. J. Lane. 1995. Regional localization of the putative cell surface receptor for HTLV-I to human chromosome 17q23.2-17q25.3. Virology 212:196-203. [DOI] [PubMed] [Google Scholar]
- 20.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 21.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green, P. L., and I. S. Y. Chen. 2001. Human T-cell leukemia virus types 1 and 2, p. 1941-1969. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 23.Grewe, C., A. Beck, and H. R. Gelderblom. 1990. HIV: early virus-cell interactions. J. Acquir. Immune Defic. Syndr. 3:965-974. [PubMed] [Google Scholar]
- 24.Hague, B. F., T. M. Zhao, and T. J. Kindt. 2003. Binding of HTLV-1 virions to T cells occurs by a temperature and calcium-dependent process and is blocked by certain type 2 adenosine receptor antagonists. Virus Res. 93:31-39. [DOI] [PubMed] [Google Scholar]
- 25.Hanon, E., P. Goon, G. P. Taylor, H. Hasegawa, Y. Tanaka, J. N. Weber, and C. R. Bangham. 2001. High production of interferon gamma but not interleukin-2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood 98:721-726. [DOI] [PubMed] [Google Scholar]
- 26.Heil, M. L., A. Albee, J. H. Strauss, and R. J. Kuhn. 2001. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J. Virol. 75:6303-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulst, M. M., H. G. van Gennip, and R. J. Moormann. 2000. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein E(rns). J. Virol. 74:9553-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim, J., P. Griffin, D. R. Coombe, C. C. Rider, and W. James. 1999. Cell-surface heparan sulfate facilitates human immunodeficiency virus type 1 entry into some cell lines but not primary lymphocytes. Virus Res. 60:159-169. [DOI] [PubMed] [Google Scholar]
- 29.Ida, H., A. Kurata, K. Eguchi, I. Yamashita, M. Nakashima, M. Sakai, Y. Kawabe, T. Nakamura, and S. Nagataki. 1994. Mechanism of inhibitory effect of dextran sulfate and heparin on human T-cell lymphotropic virus type I (HTLV-I)-induced syncytium formation in vitro: role of cell-to-cell contact. Antiviral Res. 23:143-159. [DOI] [PubMed] [Google Scholar]
- 30.Ijichi, S., M. B. Ramundo, H. Takahashi, and W. W. Hall. 1992. In vivo cellular tropism of human T cell leukemia virus type II (HTLV-II). J. Exp. Med. 176:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iozzo, R. V. 1998. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 67:609-652. [DOI] [PubMed] [Google Scholar]
- 32.Jassal, S. R., M. D. Lairmore, A. J. Leigh-Brown, and D. W. Brighty. 2001. Soluble recombinant HTLV-1 surface glycoprotein competitively inhibits syncytia formation and viral infection of cells. Virus Res. 78:17-34. [DOI] [PubMed] [Google Scholar]
- 33.Jassal, S. R., R. G. Pohler, and D. W. Brighty. 2001. Human T-cell leukemia virus type 1 receptor expression among syncytium-resistant cell lines revealed by a novel surface glycoprotein-immunoadhesin. J. Virol. 75:8317-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jinno-Oue, A., M. Oue, and S. K. Ruscetti. 2001. A unique heparin-binding domain in the envelope protein of the neuropathogenic PVC-211 murine leukemia virus may contribute to its brain capillary endothelial cell tropism. J. Virol. 75:12439-12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones, K. S., S. Akel, C. Petrow-Sadowski, Y. Huang, D. C. Bertolette, and F. W. Ruscetti. 2005. Induction of human T cell leukemia virus type I receptors on quiescent naive T lymphocytes by TGF-beta. J. Immunol. 174:4262-4270. [DOI] [PubMed] [Google Scholar]
- 36.Jones, K. S., M. Nath, C. Petrow-Sadowski, A. C. Baines, M. Dambach, Y. Huang, and F. W. Ruscetti. 2002. Similar regulation of cell surface human T-cell leukemia virus type 1 (HTLV-1) surface binding proteins in cells highly and poorly transduced by HTLV-1-pseudotyped virions. J. Virol. 76:12723-12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusano, Y., Y. Yoshitomi, S. Munesue, M. Okayama, and K. Oguri. 2004. Cooperation of syndecan-2 and syndecan-4 among cell surface heparan sulfate proteoglycans in the actin cytoskeletal organization of Lewis lung carcinoma cells. J. Biochem. (Tokyo) 135:129-137. [DOI] [PubMed] [Google Scholar]
- 39.Li, Q. X., D. Camerini, Y. Xie, M. Greenwald, D. R. Kuritzkes, and I. S. Chen. 1996. Syncytium formation by recombinant HTLV-II envelope glycoprotein. Virology 218:279-284. [DOI] [PubMed] [Google Scholar]
- 40.Manakil, J. F., P. B. Sugerman, H. Li, G. J. Seymour, and P. M. Bartold. 2001. Cell-surface proteoglycan expression by lymphocytes from peripheral blood and gingiva in health and periodontal disease. J. Dent. Res. 80:1704-1710. [DOI] [PubMed] [Google Scholar]
- 41.Mandl, C. W., H. Kroschewski, S. L. Allison, R. Kofler, H. Holzmann, T. Meixner, and F. X. Heinz. 2001. Adaptation of tick-borne encephalitis virus to BHK-21 cells results in the formation of multiple heparan sulfate binding sites in the envelope protein and attenuation in vivo. J. Virol. 75:5627-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 43.Manel, N., S. Kinet, J. L. Battini, F. J. Kim, N. Taylor, and M. Sitbon. 2003. The HTLV receptor is an early T-cell activation marker whose expression requires de novo protein synthesis. Blood 101:1913-1918. [DOI] [PubMed] [Google Scholar]
- 44.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez, I., and J. A. Melero. 2000. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J. Gen. Virol. 81:2715-2722. [DOI] [PubMed] [Google Scholar]
- 47.Mertens, G., J. J. Cassiman, H. Van den Berghe, J. Vermylen, and G. David. 1992. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J. Biol. Chem. 267:20435-20443. [PubMed] [Google Scholar]
- 48.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 49.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagai, M., M. B. Brennan, J. A. Sakai, C. A. Mora, and S. Jacobson. 2001. CD8(+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 98:1858-1861. [DOI] [PubMed] [Google Scholar]
- 51.Nath, M. D., F. W. Ruscetti, C. Petrow-Sadowski, and K. S. Jones. 2003. Regulation of the cell-surface expression of an HTLV-I binding protein in human T cells during immune activation. Blood 101:3085-3092. [DOI] [PubMed] [Google Scholar]
- 52.Okuma, K., K. P. Dalton, L. Buonocore, E. Ramsburg, and J. K. Rose. 2003. Development of a novel surrogate virus for human T-cell leukemia virus type 1: inhibition of infection by osteoprotegerin. J. Virol. 77:8562-8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 54.Osame, M., S. Izumo, A. Igata, M. Matsumoto, T. Matsumoto, S. Sonoda, M. Tara, and Y. Shibata. 1986. Blood transfusion and HTLV-I associated myelopathy. Lancet ii:104-105. [DOI] [PubMed] [Google Scholar]
- 55.Overbaugh, J. 2004. HTLV-1 sweet-talks its way into cells. Nat. Med. 10:20-21. [DOI] [PubMed] [Google Scholar]
- 56.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Persaud, D., J. L. Munoz, S. L. Tarsis, E. S. Parks, and W. P. Parks. 1995. Time course and cytokine dependence of human T-cell lymphotropic virus type 1 T-lymphocyte transformation as revealed by a microtiter infectivity assay. J. Virol. 69:6297-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinon, J. D., P. J. Klasse, S. R. Jassal, S. Welson, J. Weber, D. W. Brighty, and Q. J. Sattentau. 2003. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 77:9922-9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poiesz, B. J., M. J. Poiesz, and D. Choi. 2003. The human T-cell lymphoma/leukemia viruses. Cancer Investig. 21:253-277. [DOI] [PubMed] [Google Scholar]
- 60.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prince, H. E., D. M. Weber, and E. R. Jensen. 1991. Spontaneous lymphocyte proliferation in HTLV-I/II infection reflects preferential activation of CD8 and CD16/56 cell subsets. Clin. Immunol. Immunopathol. 58:419-430. [DOI] [PubMed] [Google Scholar]
- 62.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rong, J., H. Habuchi, K. Kimata, U. Lindahl, and M. Kusche-Gullberg. 2000. Expression of heparan sulphate l-iduronyl 2-O-sulphotransferase in human kidney 293 cells results in increased d-glucuronyl 2-O-sulphation. Biochem. J. 346:463-468. [PMC free article] [PubMed] [Google Scholar]
- 64.Sagara, Y., C. Ishida, Y. Inoue, H. Shiraki, and Y. Maeda. 1998. 71-Kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J. Virol. 72:535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaeffer, E., V. B. Soros, and W. C. Greene. 2004. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 78:1375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Secchiero, P., D. Sun, A. L. De Vico, R. W. Crowley, M. S. Reitz, Jr., G. Zauli, P. Lusso, and R. C. Gallo. 1997. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J. Virol. 71:4571-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shafti-Keramat, S., A. Handisurya, E. Kriehuber, G. Meneguzzi, K. Slupetzky, and R. Kirnbauer. 2003. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J. Virol. 77:13125-13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 70.Smit, J. M., B. L. Waarts, K. Kimata, W. B. Klimstra, R. Bittman, and J. Wilschut. 2002. Adaptation of alphaviruses to heparan sulfate: interaction of Sindbis and Semliki Forest viruses with liposomes containing lipid-conjugated heparin. J. Virol. 76:10128-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sommerfelt, M. A., and R. A. Weiss. 1990. Receptor interference groups of 20 retroviruses plating on human cells. Virology 176:58-69. [DOI] [PubMed] [Google Scholar]
- 72.Sommerfelt, M. A., B. P. Williams, P. R. Clapham, E. Solomon, P. N. Goodfellow, and R. A. Weiss. 1988. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science 242:1557-1559. [DOI] [PubMed] [Google Scholar]
- 73.Song, B. H., G. C. Lee, M. S. Moon, Y. H. Cho, and C. H. Lee. 2001. Human cytomegalovirus binding to heparan sulfate proteoglycans on the cell surface and/or entry stimulates the expression of human leukocyte antigen class I. J. Gen. Virol. 82:2405-2413. [DOI] [PubMed] [Google Scholar]
- 74.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 75.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spillmann, D. 2001. Heparan sulfate: anchor for viral intruders? Biochimie 83:811-817. [DOI] [PubMed] [Google Scholar]
- 77.Sutton, R. E., and D. R. Littman. 1996. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J. Virol. 70:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trejo, S. R., and L. Ratner. 2000. The HTLV receptor is a widely expressed protein. Virology 268:41-48. [DOI] [PubMed] [Google Scholar]
- 79.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 80.Wielgosz, M. M., D. A. Rauch, K. S. Jones, F. W. Ruscetti, and L. Ratner. 2005. Cholesterol dependence of HTLV-I infection. AIDS Res. Hum. Retrovir. 21:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang, Y. J., T. Hatziioannou, T. Zang, D. Braaten, J. Luban, S. P. Goff, and P. D. Bieniasz. 2002. Envelope-dependent, cyclophilin-independent effects of glycosaminoglycans on human immunodeficiency virus type 1 attachment and infection. J. Virol. 76:6332-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]