Abstract
ST-246 is a low-molecular-weight compound (molecular weight = 376), that is potent (concentration that inhibited virus replication by 50% = 0.010 μM), selective (concentration of compound that inhibited cell viability by 50% = >40 μM), and active against multiple orthopoxviruses, including vaccinia, monkeypox, camelpox, cowpox, ectromelia (mousepox), and variola viruses. Cowpox virus variants selected in cell culture for resistance to ST-246 were found to have a single amino acid change in the V061 gene. Reengineering this change back into the wild-type cowpox virus genome conferred resistance to ST-246, suggesting that V061 is the target of ST-246 antiviral activity. The cowpox virus V061 gene is homologous to vaccinia virus F13L, which encodes a major envelope protein (p37) required for production of extracellular virus. In cell culture, ST-246 inhibited plaque formation and virus-induced cytopathic effects. In single-cycle growth assays, ST-246 reduced extracellular virus formation by 10 fold relative to untreated controls, while having little effect on the production of intracellular virus. In vivo oral administration of ST-246 protected BALB/c mice from lethal infection, following intranasal inoculation with 10× 50% lethal dose (LD50) of vaccinia virus strain IHD-J. ST-246-treated mice that survived infection acquired protective immunity and were resistant to subsequent challenge with a lethal dose (10× LD50) of vaccinia virus. Orally administered ST-246 also protected A/NCr mice from lethal infection, following intranasal inoculation with 40,000× LD50 of ectromelia virus. Infectious virus titers at day 8 postinfection in liver, spleen, and lung from ST-246-treated animals were below the limits of detection (<10 PFU/ml). In contrast, mean virus titers in liver, spleen, and lung tissues from placebo-treated mice were 6.2 × 107, 5.2 × 107, and 1.8 × 105 PFU/ml, respectively. Finally, oral administration of ST-246 inhibited vaccinia virus-induced tail lesions in Naval Medical Research Institute mice inoculated via the tail vein. Taken together, these results validate F13L as an antiviral target and demonstrate that an inhibitor of extracellular virus formation can protect mice from orthopoxvirus-induced disease.
Recent concerns over the use of variola (smallpox) virus as a biological weapon have prompted renewed interest in development of small molecule therapeutics that target variola virus replication. Currently, there is no U.S. Food and Drug Administration-approved drug for the prevention or treatment of smallpox infection. While a number of compounds have been shown to inhibit orthopoxvirus replication in vitro, these compounds often lack potency and/or are associated with significant adverse effects, due to their relative nonspecific mechanisms of virus inhibition (3).
The cornerstone of the current national public health response plan to a smallpox bioterrorist attack calls for rapid mass immunization with vaccinia virus. However, concerns about vaccine-related adverse events have compromised implementation of a smallpox immunization program. Individuals with immunodeficiency disorders or certain common skin conditions are unusually susceptible to vaccine-related complications (6, 32). Moreover, the lag period for antibody formation from a vaccine leaves a window of vulnerability. Antiviral therapies can fill this void and provide an excellent complement to vaccination in that they reduce virus titers quickly, regardless of immune status, and lower transmission rates by diminishing the virus reservoir. A small-molecule antiviral drug designed to treat variola virus infection will be a critical component to a smallpox defense strategy.
Currently, only cidofovir [CDV; (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine; Vistide], a drug approved for treatment of cytomegalovirus (CMV) retinitis in AIDS patients, is permitted for use as emergency treatment in the case of a smallpox outbreak (25). CDV, a nucleoside analog, exhibits activity in cell culture against a number of DNA-containing viruses including adenoviruses, herpesviruses, hepadnaviruses, polyomaviruses, papillomaviruses, and orthopoxviruses (7, 9, 10, 26, 36). The 5′-diphosphorylated metabolite of CDV is recognized by viral DNA polymerases and acts as a DNA chain terminator to inhibit virus replication (39). CDV has been shown to be active against orthopoxvirus infection in normal and immunodeficient mice (26, 27, 30).
CDV has well-established shortcomings that may limit the utility of this compound as an antipoxvirus therapeutic. CDV is not orally bioavailable and must be administered intravenously, with dose-limiting nephrotoxicity that can be managed by hydration therapy and probenicid (25). Prodrugs and aerosolized forms of CDV are being investigated in the hope of addressing these shortcomings (1). Alkoxyalkyl analogs of CDV, such as 1-O-hexa-decyloxypropyl-CDV, have been reported to possess 100-fold improved orthopoxvirus antiviral potency over the unmodified nucleosides in cell culture (1). 1-O-Hexadecyloxypropyl-CDV is also orally bioavailable and is active in a lethal cowpox virus infection in mice (31). While these analogs may make CDV dosing more manageable, their toxicological profile and efficacy remain to be fully established.
A number of other drugs developed for unrelated indications have been shown to possess antiorthopoxvirus activity in vitro (3, 23). Most are nucleoside analogs and act by interfering with DNA polymerase activities; they thus may exhibit cross resistance with CDV. While continued development of these compounds as potential treatments for smallpox virus infection is important, the Institute of Medicine of the National Academies has recommended that at least two antiviral compounds that work by distinct mechanisms be stockpiled as a defense against a possible smallpox outbreak (25a). Thus, new compounds that act by mechanisms distinct from that of CDV are needed.
We have discovered a potent and specific inhibitor of orthopoxvirus replication, ST-246 {4-trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide}, which is active against multiple species of orthopoxviruses, including two strains of variola virus and a CDV-resistant cowpox virus variant. Resistance mapping studies indicate that ST-246 targets the cowpox virus V061 gene, which encodes a major envelope protein homologous to the vaccinia virus F13L gene product. Vaccinia virus F13L encodes a 37-kDa palmitylated peripheral membrane protein required for extracellular virus particle formation (16, 17, 19, 21). The protein participates in the envelopment of intracellular mature virus (IMV) particles in virus-modified membranes derived from the trans-Golgi or early-endosome compartments to produce an egress-competent form of virus particle (4). Oral administration of ST-246 in mice at 100 mg/kg of body weight/day for 14 days was tolerated and resulted in plasma concentrations of >4,000 fold above the in vitro concentration that inhibited virus replication by 50% (EC50) at 2 h postadministration. Moreover, oral delivery of ST-246 protected mice from lethal orthopoxvirus challenge and prevented vaccinia virus-induced disease. Taken together, these results establish F13L (p37) as a valid antiviral target and demonstrate that an inhibitor of extracellular virus formation can protect mice from lethal orthopoxvirus infection.
MATERIALS AND METHODS
Cells and viruses.
Vero (CCL-81), BSC-40 (CRL-2761), and SIRC (CCL-60) cell lines were purchased from American Type Culture Collection (ATCC). Ectromelia virus strain Moscow, vaccinia virus strain IHD-J, cowpox virus strain Brighton Red, and a cowpox virus variant derived from the Brighton Red strain that exhibited reduced susceptibility to CDV (designated CPX CDVr) have been described previously (3, 8, 15). Shope fibroma virus (SFV) strain Kasza (VR-364) and vaccinia virus strain NYCBH (VR-1536) were purchased from ATCC. Monkeypox strain Zaire, camelpox, and variola virus strain Bangladesh and strain Butler were propagated at the U.S. Army Medical Research Institute for Infectious Diseases or at the Centers for Disease Control and Prevention, Atlanta, GA. SFV was propagated on SIRC cultures, while all other virus stocks were propagated on BSC-40 or Vero cell cultures. Cell cultures were grown at 37°C in a humid incubator containing 5% CO2 in Dulbecco's modified minimal essential medium containing 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5% fetal bovine serum (FBS; Invitrogen).
Animals.
Female A/NCr mice 4 to 6 weeks of age and weighing 12 to 20 g each were obtained from the National Cancer Institute, Frederick, Md., and were used in experiments conducted with ectromelia virus. Naval Medical Research Institute (NMRI) mice (Harlan) and BALB/c mice 4 to 6 weeks of age and weighing 15 to 20 g each were used in experiments conducted with vaccinia virus. All animals were housed in filter-top microisolator cages and fed commercial mouse chow and water ad libitum. Pharmacokinetic and tolerability assessments were conducted with female BALB/c mice. Animal husbandry and experimental procedures were in accordance with U.S. Public Health Service policy and were approved by the Institutional Animal Care and Use Committee or by the Ethical Committee on Vertebrate Animal Experiments of the University of Leuven.
HTS.
A high-throughput screening (HTS) format was used to evaluate compounds for their ability to inhibit vaccinia virus-induced cytopathic effect (CPE). The HTS consisted of Vero cell monolayers seeded in 96-well plates (104 cells per well) that were infected with vaccinia virus at approximately 0.1 PFU per cell. At 3 days postinfection, the cultures were fixed with 5% glutaraldehyde and stained with 0.1% crystal violet in 5% methanol. Virus-induced CPE was quantified spectrophotometrically by absorbance at 570 nm.
The signal-to-noise ratio of the 96-well assay and well-to-well and assay-to-assay variability were evaluated in six independent experiments. The signal-to-noise ratio (ratio of signal of cell control wells [signal] to virus control wells [noise]) was 9.2 ± 1.8. The well-to-well and assay-to-assay variability was <20%. Using this assay, the EC50s for CDV and phosphonoacetic acid were determined to be 84 ± 15 μM and 985 ± 85 μM, respectively. These values were within the range of published values for these compounds (24, 37).
The HTS assay was used to evaluate 356,240 compounds for their ability to inhibit vaccinia virus-induced CPE. Compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted in medium such that the final concentration in each well was 5 μM compound and 0.5% DMSO. The compounds were added robotically to the culture medium using the Biomek FX robot system. Following compound addition, the cultures were infected with vaccinia virus. After 3 days, plates were processed and CPE was quantified. Compounds were scored as “hits” if they inhibited vaccinia virus-induced CPE by >50% at the test concentration (5 μM). Based on this definition, 759 compounds tested positive for inhibition, giving a hit rate of approximately 0.2%.
Antiviral compounds.
ST-246 was synthesized by ViroPharma, Inc., Exton, PA. ST-246 was dissolved at 10 mM in DMSO and further diluted in tissue culture medium to achieve final concentrations for in vitro experiments. For oral (p.o.) dosing, ST-246 was suspended in aqueous 0.75% methylcellulose (Sigma) containing 1% Tween 80 (Sigma). For intravenous (i.v.) dosing, ST-246 was solubilized in aqueous 50% polyethylene glycol, 20% methanol, and 1% Tween 80. CDV was purchased from a retail pharmacy and diluted in sterile, distilled water or phosphate-buffered saline (PBS).
Inhibitory potency.
Vero cell monolayers were seeded in 96-well plates (104 cells per well). Dose-response curves were generated by measuring virus-induced cytopathic effects in the presence of a range of compound concentrations. Eight compound concentrations (5, 1.5, 0.5, 0.15, 0.05, 0.015, 0.005, and 0.0015 μM) were used to generate inhibition curves suitable for calculating the EC50 from virus-induced CPEs. Compound dilutions were prepared in DMSO prior to addition to the cell culture medium. The final DMSO concentration in all samples was 0.5%. Cell monolayers were infected with selected orthopoxviruses at the cell culture infectious dose that caused a 90% cytopathic effect at 3 days postinfection. This dose of virus was approximately 0.05 PFU/cell. At 3 days postinfection, the assay was terminated by fixing the cells in a 5% glutaraldehyde solution; the level of CPE was visualized by staining the monolayers with 0.1% crystal violet. Virus-induced cytopathic effects were quantified by measuring absorbance at 570 nm. EC50s were calculated by fitting the data to a four-parameter logistic model (variable-slope, nonlinear regression model) to generate a dose-response curve using XLfit 4.1 (IBDS, Emeryville, CA). The linear correlation coefficient squared (R2) for fitting data to this model was typically >0.97. From this curve, the concentration of compound that inhibited virus-induced CPE by 50% was calculated.
Cytotoxicity assay.
Cytotoxicity was determined by measuring mitochondrial dehydrogenase activity in dividing cells by the MTT [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (17). Subconfluent monolayers of Vero cells were incubated in the presence of increasing concentrations of compound. Eight compound concentrations (50, 15, 5.0, 1.5, 0.5, 0.15, 0.05, and 0.015 μM) were used to generate inhibition curves suitable for calculating the cytotoxic concentration of compound that inhibited cell viability by 50% (CC50). At 72 h after the addition of compound, medium containing 0.5-mg/ml MTT was added to the cultures and incubated for 90 min at 37°C to form an insoluble formazan product caused by reduction of the tetrazolium salt by mitochondrial dehydrogenase (17). The formazan product was solubilized in a solution containing 0.004 N HCl in isopropanol and was quantified by measuring absorbance at 490 nm. The concentration of compound that inhibited the MTT assay signal by 50% (i.e., CC50) was calculated by fitting the absorbance data to a four-parameter logistic model (variable-slope, nonlinear regression model) to generate a dose-response curve.
Antiviral specificity.
Herpes simplex virus type 1 (HSV-1; strain KOS), CMV (strain AD-169), respiratory syncytial virus (RSV; strain A), rotavirus, Rift Valley fever virus (RFV; strain MP12), Tacaribe virus (strain TRVL 11573), lymphocytic choriomeningitis virus (LCMV; strain Armstrong E350), and bovine viral diarrhea virus (strain NADL) were obtained from the ATCC. All assays were carried out in the appropriate medium containing 3% heat-inactivated FBS. Ninety-six-well cell culture plates were seeded 24 h prior to use with 1.5 × 104 (Vero), 2.0 × 104 (MDBK), 2.2 × 104 (HEp-2), or 4.5 × 104 (MRC-5) cells per well. Cells were infected at the cell culture infectious dose causing 90% cytopathic effects at 3 days postinfection for HSV-1, RSV, rotavirus, RFV, Tacaribe, and bovine viral diarrhea virus or at titers that generated an enzyme-linked immunosorbent assay (ELISA) signal of 2.5 for CMV and LCMV at the end of the respective incubation periods. Compound was added to duplicate wells of cells at final concentrations of 50, 25, 12.5, 6.3, 3.1, 1.6, 0.8, and 0 μM. The final concentration of DMSO in the assays was 0.5%. As controls, uninfected cells and cells receiving virus without compound were included on each assay plate. In addition, reference agents, when available, were included on each assay plate: ganciclovir (Sigma) for HSV-1 and CMV and ribavirin (Sigma) for LCMV and RSV. At the end of the incubation period (3 days for HSV-1, rotavirus, RFV, tacaribe virus, and LCMV; and 4 days for CMV and RSV) virus-infected cells were processed for crystal violet staining (cytopathic effect assay) or ELISA by standard techniques. LCMV- and CMV-specific ELISAs were carried out using a 1:20 dilution of mouse monoclonal antibody raised against LCMV nuclear protein (a generous gift of Juan Carlos de la Torre, The Scripps Research Institute, La Jolla, Calif.) and a 1:200 dilution of CMV-specific cocktail of monoclonal antibodies generated from protein 52 and unique long gene 44 products (Dako), respectively. Goat anti-mouse horseradish peroxidase-conjugated monoclonal antibody (Bio-Rad) at a 1:4,000 dilution for LCMV and at a 1:400 dilution for CMV was used as the secondary antibody for both ELISAs.
Virus yield assay.
Viral replication kinetics were measured with BSC-40 monolayers seeded in 24-well plates (2 ×105 cells per well) that were infected with 2 PFU/cell of vaccinia virus strain IHD-J. After a 1-h absorption period, the inoculum was removed, and the cultures were washed with 1 ml of 1× PBS supplemented with 1% FBS. The cultures were incubated in 0.5 ml medium in the presence and absence of 5 μM ST-246; at 3-h intervals for up to 24 h postinfection, the infected cell medium was removed and stored at −80°C, and the cells were scraped into 0.5 ml of 1× PBS supplemented with 1% FBS and stored at −80°C. The cells were thawed at 37°C, and intracellular virus was released by sonication at 20% power for 40 s at 0°C using a 550 Sonic Dismembrator (Fisher Scientific). The cell debris was removed by centrifugation at 300 × g for 5 min at 4°C. The virus titer in the culture medium (extracellular virus) and cell-associated fraction (intracellular virus) were measured by plaque assay on BSC-40 monolayers.
ST-246-resistant virus isolation.
Cowpox virus variants with reduced susceptibility to ST-246 were isolated by plating 107 PFU of wild-type cowpox virus on Vero cell monolayers in the presence of 10 μM ST-246 and isolating virus from plaques that formed at 3 days postinfection. Virus isolates exhibiting reduced susceptibility to ST-246 were plaque purified three times in the presence of compound prior to large-scale stock preparation. The EC50 of the resistant variant was shifted from 0.01 μM for wild-type virus-infected cells to >40 μM for the ST-246-resistant variants. This shift in EC50 generated a selective index (SI) value of 1, indicating that the EC50 for the compound-resistant variant was equal to the CC50.
Marker rescue.
Marker rescue techniques were used to map the genetic locus that correlated with reduced susceptibility to ST-246 (41). Overlapping DNA fragments (10 to 15 kb in length with 0.2- to 3-kb overlap) that span the entire length of the ST-246-resistant viral genome were generated by PCR using 17 sets of primers. PCR was performed using the Expand Hi Fidelity system (Roche Diagnostics, Inc.). Reaction mixtures contained 80 ng of cowpox genomic DNA; 1× buffer; 200 μM dATP, dCTP, dGTP, and dTTP; 300 nM each primer; and 3.5 units of enzyme per reaction mixture. The reaction mixtures were incubated for 1 cycle at 94°C for 1 min; for 10 cycles, each consisting of 94°C for 1 min, 54°C for 1 min, and 68°C for 12 min; and for 20 cycles, each consisting of 94°C for 1 min, 54°C for 1 min, and 68°C for 12 min, with an additional 5 s added to each cycle. This was followed by incubation at 4°C.
Individual gel-purified PCR DNA products (0.7 μg) were cotransfected with full-length wild-type cowpox viral DNA (1 μg) into BGMK cells seeded at 2 × 105 cells per 35-mm-diameter dish infected 1 h prior to transfection with 2 PFU/cell of SFV. The function of SFV is to provide the necessary transacting factors to initiate replication of the transfected cowpox virus DNA. Transfections were performed using Lipofectamine 2000 and OptiMEM (Invitrogen) according to the manufacturer's recommendations. At 3 days posttransfection, progeny virus was plated onto BSC-40 cells in the presence and absence of 10 μM ST-246 compound. BSC-40 cells are permissive for cowpox virus infection but not for SFV infection. Thus, only cowpox virus produced from the transfection will form plaques on BSC-40 monolayers, and only recombinants that contain the resistant gene will form plaques in the presence of drug selection. PCR DNA products that increased the plaque number in the presence of ST-246 relative to nonspecific DNA controls were considered positive for rescue of the resistant phenotype.
Pharmacokinetic analyses.
ST-246 was formulated for p.o. dosing of mice, with an aqueous suspension in 0.75% methyl cellulose containing 1% Tween 80. ST-246 concentrations were measured with plasma harvested from blood collected from mice at approximately 0.5, 1, 2, 4, 6, 8, 12, and 24 h after dosing. Compound concentrations were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a Quatro Micro LC-MS/MS instrument. LC was performed with a Phenomenex Luna C18 column (3-μm particle size; 100 mm by 2 mm) at a flow rate of 0.2 ml/min with a mobile phase containing 80% acetonitrile, 19.7% water, and 0.3% acetic acid. WINNONLIN (Pharsight, Inc.) was used to estimate pharmacokinetic values.
RESULTS
Identification of ST-246.
A HTS assay was developed to quantify vaccinia and cowpox virus-induced CPE in Vero cell cultures. This CPE-based assay was used to evaluate 356,240 low-molecular-weight compounds (molecular weight, <500) for their ability to inhibit orthopoxvirus-induced CPE. Compounds that inhibited virus-induced CPE by >50% relative to untreated virus controls at a compound concentration of 5 μM were evaluated further. The current lead compound, ST-246, was selected from a group of analogs that were synthesized as part of a chemical optimization program designed to improve potency and metabolic stability of an initial hit from the HTS (Fig. 1).
FIG. 1.
Chemical structure of ST-246.
ST-246 antiviral potency, selectivity, and spectrum of activity.
The antiviral potency and selectivity of ST-246 was measured in CPE assays against a panel of DNA- and RNA-containing viruses. In these assays, the EC50 for inhibition of vaccinia virus was determined to be 0.01 μM, while the EC50 values for inhibition of unrelated viruses were >40 μM (Table 1). These results demonstrate that ST-246 is a potent and specific inhibitor of poxvirus replication in cell culture.
TABLE 1.
ST-246 antiviral spectrum of activity and selectivity
| Virus (strain) | Family | Classification | EC50 (μM)a |
|---|---|---|---|
| Vaccinia virus (NYCBH) | Orthopoxviridae | Double-stranded DNA | 0.01 |
| Variola virus (Butler) | Orthopoxviridae | Double-stranded DNA | 0.02 |
| Variola virus (Bangladesh) | Orthopoxviridae | Double-stranded DNA | 0.05 |
| Cowpox virus (Brighton Red) | Orthopoxviridae | Double-stranded DNA | 0.05 |
| Ectromelia virus (Moscow) | Orthopoxviridae | Double-stranded DNA | 0.07 |
| Monkeypox virus (Zaire) | Orthopoxviridae | Double-stranded DNA | 0.01 |
| Camelpox virus | Orthopoxviridae | Double-stranded DNA | 0.01 |
| Herpes simplex virus type 1 | Herpesviridae | Double-stranded DNA | >40 |
| Cytomegalovirus | Herpesviridae | Double-stranded DNA | >40 |
| Respiratory syncytial virus | Paramyxoviridae | Negative single-strand RNA | >40 |
| Rotavirus | Reoviridae | Double-stranded RNA | >40 |
| Bovine viral diarrhea virus | Flaviviridae | Positive single-strand RNA | >40 |
| Rift Valley fever virus | Bunyaviridae | Negative single-strand RNA | >40 |
| Tacaribe virus | Arenaviridae | Ambisense RNA | >40 |
| Lymphocytic choriomenigitis virus | Arenaviridae | Ambisense RNA | >40 |
EC50 values are the averages of at least two independent determinations.
The antiviral effect exerted by ST-246 was not due to cytotoxicity, since ST-246 had no measurable effect on cell viability in cytotoxicity assays. The compound concentration that inhibited uninfected Vero cell proliferation by 50% (CC50) was >40 μM, which was above the solubility limit for this compound in tissue culture medium. This resulted in an SI of >4,000 (SI = CC50/EC50). For comparison, the SI for CDV evaluated in parallel was approximately 5 (CC50 = 400 μM/EC50 = 80 μM). Thus, relative to CDV, ST-246 was >8,000-fold-more potent in CPE-based assays (Fig. 2).
FIG. 2.
A comparison of antiviral potency of ST-246 and CDV in Vero cell culture. Vero cells were incubated with increasing concentrations of ST-246 or CDV and infected with vaccinia virus strain NYCBH at 0.05 PFU/cell. The cultures were fixed and stained with crystal violet at 3 days postinfection. The extent of virus-induced CPE was quantified by measuring absorbance at 470 nm. The percent inhibition was calculated relative to untreated infected and mock-infected controls.
ST-246 exhibited potent antiviral activity against a broad spectrum of orthopoxviruses in CPE assays (Table 1). The EC50 values for inhibition of viral replication ranged from 0.01 μM for vaccinia virus to 0.07 μM for ectromelia virus and were dependent on the species of orthopoxvirus tested. ST-246 was also active against a CDV-resistant (CDVr) cowpox virus (EC50 = 0.05 μM), suggesting that the mechanism by which ST-246 inhibits virus replication is distinct from that of CDV. Importantly, ST-246 was found to be active against two strains of variola virus (Table 1). This result validates the use of vaccinia and cowpox viruses as surrogates for variola virus. Taken together, these results demonstrate that ST-246 is a potent and specific inhibitor of orthopoxvirus replication that is active against a broad spectrum of orthopoxviruses.
ST-246 inhibits plaque formation.
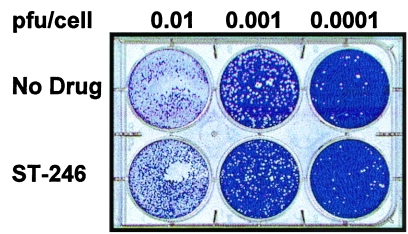
To begin to understand the antiviral mechanism of action of ST-246, the effects of compound on virus plaque formation were measured. Vero cell monolayers were infected with cowpox virus at different multiplicities of infection in the presence and absence of ST-246; plaque formation and virus-induced CPE were measured at 3 days postinfection. In the presence of ST-246, plaque formation and virus-induced CPE were completely inhibited (Fig. 3). In contrast, in the absence of ST-246, plaques were readily visible on cell monolayers infected with cowpox virus at 0.0001 or 0.001 PFU/cell, and complete CPE were observed on cell monolayers infected with cowpox virus at 0.01 PFU/cell (Fig. 3). These results suggest that ST-246 inhibits orthopoxvirus plaque formation and virus-induced CPE.
FIG. 3.
Plaque formation of wild-type cowpox virus in the presence and absence of ST-246. Vero cell monolayers (106 cells/well) were infected with cowpox virus at 0.0001, 0.001, and 0.01 PFU/cell in the presence and absence of 5 μM ST-246. At 3 days postinfection, the cultures were fixed in 5% glutaraldehyde and stained with crystal violet.
ST-246 inhibits extracellular virus production.
The ability of ST-246 to inhibit plaque formation suggests that ST-246 can also inhibit virus replication. To determine the effects of ST-246 on virus replication, virus yield assays were conducted to measure the amount of intracellular and extracellular virus produced in the presence and absence of compound. BSC-40 cells were infected with 2 PFU/cell of vaccinia virus and incubated in the presence and absence of 5 μM ST-246. At selected times postinfection, extracellular virus in the medium and cell-associated virus were quantified by plaque assay. The results show that ST-246 reduced extracellular virus titers by approximately 10 fold at 24 h postinfection, while having little effect on the level of intracellular virus titers relative to untreated controls (Fig. 4). These results suggest that ST-246 inhibits extracellular virus formation.
FIG. 4.
The effects of ST-246 on production of intracellular and extracellular virus. BSC-40 cells were infected with 2 PFU/cell of vaccinia virus strain IHD-J in the presence and absence of 5 μM ST-246. At 3-h intervals for 24 h postinfection, the cultures were harvested and separated into extracellular and intracellular virus fractions. The virus titers in each fraction were measured by plaque assay of BSC-40 cell monolayers. The error bars represent the standard deviation of the means of triplicate virus yield determinations.
Isolation of drug-resistant virus variants.
Isolation of virus variants that are resistant to the inhibitory effects of a compound constitutes strong evidence that the compound is acting by a true antiviral mechanism and not on a cellular target. Drug-resistant virus variants are also useful tools to elucidate the mechanism of action of antiviral compounds. Drug-resistant virus variants were isolated from a wild-type cowpox virus stock by plating 107 PFU of cowpox virus on Vero cell monolayers in the presence of 10 μM ST-246. Virus isolated from plaques that formed at 3 days postinfection were plaque purified in the presence of compound prior to further characterization.
To determine the level of susceptibility to ST-246, the plating efficiency of the resistant variant was measured on Vero cell monolayers infected at different multiplicities of infection in the presence and absence of 10 μM ST-246, and plaque formation and virus-induced cytopathic effects was measured at 3 days postinfection. The results show that the ST-246-resistant variant formed plaques in the presence of 10 μM ST-246 at multiplicities of infection of 0.001 and 0.0001 PFU/cell and at high multiplicities (0.01 PFU/cell) produced complete cytopathic effects (Fig. 5). The plaque size and extent of virus-induced CPE were similar in the presence and absence of compound and equivalent to that of wild-type virus in the absence of compound (Fig. 3). The EC50 for inhibition of the resistant variant (>40 μM) was more than 800-fold higher than the EC50 for inhibition of wild-type cowpox virus (0.050 μM). This shift in EC50 generated an SI of 1, indicating that the EC50 for the drug-resistant variant was equal to the CC50. The ST-246-resistant variant was not cross resistant to CDV, indicating that ST-246 likely inhibits a viral target different from that of CDV (data not shown).
FIG. 5.
Plaque formation of an ST-246-resistant cowpox virus variant in the presence and absence of ST-246. Vero cell monolayers (106 cells/well) were infected with a cowpox virus variant that exhibited reduced susceptibility to ST-246 at 0.0001, 0.001, and 0.01 PFU/cell in the presence and absence of 5 μM ST-246. At 3 days postinfection, the cultures were fixed in 5% glutaraldehyde and stained with crystal violet.
ST-246 resistance maps to the cowpox virus V061 gene.
Marker rescue techniques were used to map the genetic locus that conferred reduced susceptibility to ST-246 (41). Overlapping DNA fragments (8 to 15 kb) that spanned the entire length of the ST-246-resistant viral genome were generated by PCR. Individual gel-purified PCR fragments were cotransfected with full-length wild-type cowpox viral DNA into BGMK cells that were infected with SFV. Cowpox virus recombinants were detected by plating progeny virus from the transfection onto BSC-40 cells in the presence and absence of inhibitor compound. Using this marker rescue procedure, the resistance phenotype was mapped to a PCR fragment that spanned nucleotide positions bp 57353 to 58630 that contained the coding sequence for the cowpox virus V061 gene. Sequence analysis of the ST-246-resistant allele identified a single base change, resulting in a glycine residue being replaced by cysteine at amino acid position 277 in the protein. This change was reengineered back into wild-type cowpox and vaccinia virus genomes, and the resulting recombinants were found to be resistant to ST-246. These results suggest that the cowpox virus V061 gene product is the target of ST-246. The cowpox virus V061 gene is homologous to the vaccinia virus F13L gene, which encodes a major envelope protein required for the formation of extracellular virus (4).
Pharmacokinetic analyses and tolerability.
Prior to conducting animal efficacy evaluations, preliminary studies were conducted with mice to characterize plasma ST-246 concentrations and to assess compound tolerability. Oral administration of ST-246 at 30 and 100 mg/kg/day with mice generated peak plasma compound concentrations of 32.5 and 62.2 μg/ml, respectively (Table 2). This ST-246 concentration was greater than >4,000-fold above the in vitro EC50 of 0.05 μM (0.014 μg/ml). The area under the plasma concentration-time curve (AUC) and elimination half-life (t1/2) were similar for the 30- and 100-mg/kg/day dosing schedules. There was no overt toxicity (i.e., clinical signs or body weight changes) observed with mice given repeated oral ST-246 dosages of 100 mg/kg/day. Pharmacokinetic parameters were also determined following i.v. administration and indicated that the rate of systemic clearance of ST-246 was low (data not shown). Consistent with the low clearance rate, comparison of the AUC values after a single i.v. dose (2 mg/kg) and p.o. dose (10 mg/kg) of ST-246 showed an absolute oral bioavailability of ST-246 of 31%. This indicates that ST-246 was well absorbed and had good systemic availability following oral suspension administration.
TABLE 2.
Pharmacokinetic parameters of ST-246 in micea
| Dosage (mg/kg) | Tmax (h) | Cmax (μg/ml) | AUC (0-t) (μg · h/ml) | t1/2 (h) |
|---|---|---|---|---|
| 30 | 2.7 | 32.5 | 284.2 | 2.5 |
| 100 | 1.0 | 62.2 | 287.6 | 2.5 |
Mice were administered ST-246 by oral gavage. Plasma drug concentrations were measured by LC-MS/MS over a 24-h time period. Tmax, time of maximal concentration; Cmax, maximal concentration.
In vivo antiviral efficacy.
Given that ST-246 was found to be orally bioavailable and well tolerated by mice, the antiviral efficacy of ST-246 was evaluated with several murine models of orthopoxvirus disease. These models were designed to determine the potential of ST-246 for treating different aspects of orthopoxvirus disease including local replication, systemic lesional disease, and lethal systemic infection. Compound dosing regimens were selected to maximize compound exposure without causing adverse effects.
Vaccinia virus lethal mouse model.
ST-246 was tested for antiviral activity in mice that were inoculated via the intranasal route with vaccinia virus (Fig. 6). This route of infection resulted in virus replication in tissues of the respiratory tract and subsequent systemic spread to vital organs to produce lethal disease. Thus, antiviral compounds that inhibit localized replication, as well as systemic spread, can be evaluated with this model.
FIG. 6.
The effects of ST-246 in a lethal intranasal vaccinia virus challenge model. Mice (six animals per dose group) were inoculated on day 0 with 4 × 105 PFU (10× LD50) of vaccinia virus strain IHD-J with the exception of naïve and scarified groups, which remained uninfected and were used as controls to assess the immune status of animals that survived initial viral challenge. Mice were treated with ST-246 at 50 mg/kg b.i.d. or with vehicle administered by gavage at 8-h intervals for 14 days. A control group of mice were treated with CDV at 100 mg/kg administered by i.p. injection. The percent survival (A) or percent starting body weight for each treatment condition (B) was plotted as a function of days postinfection.
Weanling BALB/c mice were challenged with 4 × 105 PFU (10× LD50) of vaccinia virus strain IHD-J delivered by intranasal inoculation. Mice were treated with placebo (vehicle), ST-246 administered by oral gavage at 50 mg/kg twice a day (b.i.d.) for 14 days, or CDV administered as a single intraperitoneal (i.p.) injection at 100 mg/kg. Two additional groups of mice, naïve and scarified, were included as controls in this study to assess the immune status of mice that survived infection as a result of compound treatment. Mice in the naïve group were left untreated, while mice in the scarified group received a sublethal dose of vaccinia virus strain Copenhagen, which served as a vaccine against subsequent viral challenge.
Mice were monitored for disease symptoms and euthanized when moribund. In mice treated with ST-246 or CDV, the degree of weight loss, which was used as a measure of disease progression, was less than that of placebo-treated mice, suggesting that compound treatment reduced disease severity (Fig. 6B). Moreover, mice treated with ST-246 or CDV survived the viral challenge, while placebo-treated mice succumbed to infection by day 6 postinfection (Fig. 6A). No evidence of disease was observed 2 weeks after cessation of drug therapy, indicating that virus was cleared and that ST-246 did not simply suppress virus replication and delay disease onset.
To assess the immune status of mice that survived infection, animals were inoculated with a lethal dose (10× LD50) of vaccinia virus (IHD-J) on day 28 following the initial viral challenge. The naïve animal group succumbed to infection by day 6 postinfection, while the scarified mouse group was resistant to viral challenge. All compound-treated mice that survived infection were also resistant to subsequent viral challenge, suggesting that these animals had acquired protective immunity (data not shown). Antibody titers specific for vaccinia virus L1R protein were measured by ELISA and were found to be comparable between the ST-246 treatment group and the scarified group (data not shown). The protective effect observed with compound-treated mice is unlikely caused by the presence of residual ST-246, since the terminal half-life of compound in mice is 2.5 h and residual compound levels at 2 weeks postadministration would be expected to be below therapeutic levels. These results suggest that ST-246-treated mice mounted a protective immune response to vaccinia virus infection.
Ectromelia virus lethal mouse model.
Ectromelia virus is a natural orthopoxvirus of laboratory mice that causes a generalized disease termed mousepox (13). Infection of mice with ectromelia virus produces a primary viremia and systemic disease similar to smallpox infection in humans (13). The degree of systemic spread can be quantified by measuring virus levels in the liver and spleen.
A lethal ectromelia virus mouse model has been established to evaluate the efficacy of antiviral compounds and was used to measure the antiviral activity of ST-246 (8). Weanling A/NCr mice were challenged by intranasal inoculation with a dose of ectromelia virus that was 40,000 fold above the LD50. Mice were treated with either placebo (vehicle) or ST-246 at 50 mg/kg b.i.d. for 14 days, administered by oral gavage. CDV was administered as a single i.p. dose at 100 mg/kg and was used as a positive control in this experiment. Mice were monitored for disease symptoms and sacrificed when moribund. Mice treated with ST-246 or CDV were protected from lethal ectromelia virus challenge, while placebo-treated mice succumbed to infection within 7 days postinfection (Table 3).
TABLE 3.
Effect of ST-246 on A/NCr mice infected with ectromelia virus
| Treatmenta | Effect
|
|
|---|---|---|
| Mean time to death (days) | Mortality at day 21 p.i.b | |
| ST-246 | 0/5 (0) | |
| CDV | 0/5 (0) | |
| Placebo | 7.0 | 5/5 (100) |
Day 0, cages of mice (five mice/cage) were infected by the intranasal route with 5 μl of ECTV at 40,000× LD50 dose at ≈4 h after the first compound treatment. ST-246 (50 mg/kg) or placebo was administered to mice by gavage (0.1 ml) on day 0 through day 14 at 8-h intervals and twice daily. CDV was administered as a single i.p. injection at 100 mg/kg.
Values in parentheses are percent mortality.
To measure ST-246 antiviral activity and to determine if compound inhibited systemic virus spread, groups of five mice were infected with ectromelia virus (100× LD50) and treated with either placebo, ST-246, or CDV. Virus titers were measured in the liver, spleen, and lung of selected dose groups of mice at 4, 6, and 8 days postinfection. In mice treated with ST-246, virus titers in the liver, spleen, and lungs of infected animals were below the limit of detection at day 8 postinfection, with the exception of one mouse where a low level (5 × 102 PFU/ml) of virus was detected in the spleen (Fig. 7). In contrast, virus titers in liver, spleen, and lung tissue in placebo-treated animals at day 8 postinfection were 6.3 × 107, 5.3 × 107, and 1.9 × 105 PFU/ml, respectively (Fig. 7). Virus titers in liver, spleen, and lungs were also reduced below the limits of detection in the presence of ST-246 at day 4 and 6 postinfection (data not shown). These results suggest that ST-246 inhibited systemic virus spread and protected mice from lethal ectromelia virus infection.
FIG. 7.
The effects of ST-246 and CDV on virus titers in liver, spleen, and lung at day 8 postinfection. A/NCr mice were inoculated with 50 PFU (100× LD50) of ectromelia virus via intranasal administration. CDV-treated mice were injected on day 0 with a single dose by the intraperitoneal route. ST-246- or placebo-dosed mice were treated by gavage on day 0 through day 14 at 8-h intervals and twice daily. At day 8 postinfection, five mice in each group were sacrificed, and tissue infectivity was measured in target organs by plaque assay.
Preliminary studies were conducted to determine the optimal dose level and dosing duration that provided protective efficacy against lethal ectromelia virus challenge. A/NCr mice were infected with 100× LD50 of ectromelia virus delivered by footpad inoculation and treated with placebo or ST-246 administered at 10 and 50 mg/kg b.i.d. for 5 or 14 days postinfection. All mice treated for 14 days with either 10 mg/kg or 50 mg/kg of ST-246 were protected from lethal infection, while approximately 80% of the mice treated for 5 days at 50 mg/kg were protected from lethal virus infection (Table 4). These results suggest that the optimal dosing duration is between 5 and 14 days postinfection and that the minimal amount of compound that provides protection in this model is <10 mg/kg.
TABLE 4.
Optimal dosing parameters for ST-246 antiviral efficacy in A/NCr mice
| Treatmenta | Duration (days) | Drug dose (mg/kg) | Day of death, range | Mean time to death ± SD | Mortality at day 21 p.i.b |
|---|---|---|---|---|---|
| Placebo | 14 | 7-8 | 7.8 ± 0.5 | 10/10 (100) | |
| ST-246 | 14 | 10 | 0/10 (0) | ||
| ST-246 | 5 | 50 | 11-18 | 14.5 ± 4.9 | 2/10 (20) |
| ST-246 | 14 | 50 | 0/10 (0) |
On day 0, mice were inoculated in the left rear footpad with 25 PFU of ECTV virus in 25 μl (≈100× LD50). At 1 h postinfection, mice were dosed with ST-246 or placebo by gavage twice daily for the indicated duration.
Values in parentheses are percent mortality.
Vaccinia virus-mouse tail lesion model.
ST-246 was tested for antiviral activity in a tail lesion model designed to mimic the primary viremic phase of virus infection and the systemic lesional disease of smallpox and disseminated vaccinia. NMRI mice (six mice per compound-treated groups and eight mice per placebo-treated group) were inoculated via the tail vein with 4 × 103 PFU per mouse of vaccinia virus. Mice were treated with either placebo (vehicle) or ST-246 administered by oral gavage at 15 mg/kg and 50 mg/kg b.i.d. for 5 days. Virus replication was quantified at day 8 postinfection by counting the number of vaccinia virus-induced tail lesions. Mice treated with ST-246 showed a dose-dependent reduction in the numbers of tail lesions at day 8 postinfection relative to placebo-treated control animals (Fig. 8). On average, animals treated with ST-246 at 50 mg/kg had only 1% of the number of lesions relative to the placebo-treated control group. These results demonstrate that ST-246 can inhibit vaccinia virus-induced lesion formation following i.v. viral inoculation.
FIG. 8.
The effects of ST-246 on vaccinia virus-induced tail lesion formation. NMRI mice (eight mice per placebo-treated group and six mice per compound-treated groups) were inoculated with 4,000 PFU of vaccinia virus administered by tail vein injection. Mice received placebo, ST-246 (50 mg/kg), or ST-246 (15 mg/kg) b.i.d. by gavage starting at 2 h postinoculation and continuing for 5 days. CDV was administered at 25 mg/kg as a single i.p. injection. Photographs of the tail lesions in placebo and ST-246 50-mg/kg dose groups at day 8 postinfection are shown. Lesions for all dose groups were quantified at day 8 postinfection (graph). The error bars represent the standard deviation of the mean lesion number.
DISCUSSION
ST-246 is a novel orally bioavailable antiviral compound that is a potent and specific inhibitor of orthopoxvirus extracellular virus formation which blocks virus spread in vitro and in vivo. ST-246 was discovered during chemical optimization of an initial “hit” that came from a high-throughput screen of 356,240 low-molecular-weight compounds that was designed to identify inhibitors of vaccinia virus replication. Chemical optimization of the initial hit increased metabolic stability and improved antiviral potency decreasing the EC50 from 0.8 μM for the initial hit to 0.01 μM for ST-246. This increase in potency resulted in a compound that was >8,000-fold more potent than CDV (EC50 = 80 μM) (Fig. 2). Moreover, ST-246 was active against a CDV-resistant strain of cowpox virus, suggesting that the mechanism of virus inhibition is different from that of CDV.
ST-246 was found to be active against multiple species of orthopoxviruses, including two strains of variola virus, suggesting that ST-246 inhibits a conserved viral target essential for orthopoxvirus replication. The compound was inactive against unrelated DNA- and RNA-containing viruses, demonstrating specificity for inhibition of orthopoxvirus replication. These results indicate that ST-246 is a potent and specific inhibitor of a conserved target essential for orthopoxvirus replication.
Marker rescue of an ST-246-resistant cowpox virus variant mapped the genetic locus that correlated with reduced compound susceptibility to a single amino acid change within the cowpox virus V061 gene. Changing this amino acid in the wild-type cowpox virus genome resulted in a virus resistant to ST-246, suggesting that the V061 gene product is the target of ST-246 antiviral action. In support of this hypothesis, a vaccinia virus recombinant in which the F13L gene (V061 homolog) was deleted exhibited reduced susceptibility to ST-246 (G. Yang et. al., unpublished data). Furthermore, this recombinant produced small plaques and displayed a phenotype similar to that of wild-type virus grown in the presence of compound. Taken together, these results suggest that ST-246 targets the cowpox virus V061 gene product.
Our current understanding of V061 function comes largely from work conducted with vaccinia virus and the F13L gene homolog. Vaccinia virus F13L encodes a highly conserved 37-kDa peripheral membrane protein that plays a central role in the envelopment of IMV particles to produce an egress-competent form of virus particle (4, 21). The p37-dependent membrane-wrapping activity requires a conserved histidine-lysine-aspartate (HKD) phospholipase motif (19, 33, 38). Vaccinia virus recombinants with changes at the conserved K and D residues exhibited defects in IMV wrapping, alterations in intracellular localization, and inhibition of extracellular particle formation (19, 33). This result suggests that phospholipase activity is important for F13L function. Indeed, purified p37 has been shown to have broad-spectrum lipase activity; treatment of infected cells with butanol-1, a known inhibitor of phospholipase D activity, inhibited vaccinia virus plaque formation (2, 19). The role of lipase activity in p37 function is unclear but may involve p37-induced membrane alterations, since transfection studies have shown that deletion of the HKD motif inhibited the formation of p37-induced vesicles (19. These results suggest that F13L phospholipase activity plays a central role in F13L function and is required for p37-specific extracellular particle production.
F13L colocalizes to the trans Golgi, plasma and endosomal membranes (20). The protein shuttles between these various compartments through a clathrin-mediated endosomal pathway (20). Golgi colocalization and intracellular trafficking requires palmitylation of a cysteine residue at position 185 within F13L, as well as the HKD phospholipase motif (16, 33). Changing this cysteine residue to alanine alters the intracellular distribution of p37 (16). Moreover, virus variants expressing this mutated F13L allele produce small plaques and reduced levels of extracellular virus, suggesting that palmitylation and/or intracellular distribution of F13L is essential for activity (16).
The intracellular distribution of F13L is also affected by N1-isonicotinoly-N2-3-methyl-4-chlorobenzoylhydrazine (IMCBH), a pharmacological inhibitor of orthopoxvirus replication that is active in cell culture (22). Like ST-246, IMCBH targets F13L and inhibits extracellular virus particle formation in cell culture; however, IMCBH is not active in vivo (18, 22, 29, 34). In the presence of IMCBH, F13L is redistributed to the cytoplasm, reminiscent of the phenotype observed in cells infected with vaccinia virus variants containing a C185A change (18). ST-246 was also found to cause changes in the intracellular localization of F13L (G. Yang and Y. Chen, unpublished observations). The phenotype of IMCBH-resistant variants was mapped to a single Asp-to-Tyr change at amino acid position 280 within the F13L gene (34). This change is located in the vicinity of the glycine-to-cysteine change at position 277 in the CPX V061 gene that correlates with resistance to ST-246. This region of F13L is highly conserved among orthopoxviruses (Fig. 9). Thus, two compounds with divergent chemical structures appear to target F13L and alter intracellular distribution. The mechanism by which ST-246 alters F13L intracellular localization and inhibits F13L activity is currently under investigation.
FIG. 9.
A sequence comparison of F13L orthologs. (A) Graphic representation of F13L, illustrating the location of known sites of palmitoylation, resistance to the antiviral compounds ST-246 (G277C) and IMCBH (D280Y), and an HKD phospholipase motif implicated in F13L function. (B) Amino acid sequence comparison of F13L orthologs from amino acid positions 245 to 305 showing the sequence conservation surrounding the sites of resistance to ST-246 (amino acid position 277) and IMCBH (amino acid position 280), identified by asterisks. The shaded amino acids at positions 250 and 291 indicate deviations from the consensus sequence.
Extracellular virus particles are responsible for efficient cell-to-cell spread and long range dissemination of vaccinia virus (35). Virus variants containing defects in genes required for production of extracellular virus particles produce small plaques and are attenuated for virus spread (35). ST-246 treatment of infected cells generates a similar phenotype; in the presence of compound, plaque formation and virus-induced cytopathic effects are inhibited. Furthermore, in single-round virus growth assays, ST-246 treatment reduced extracellular virus production by 10 fold without affecting production of intracellular virus, suggesting that ST-246 inhibits virus egress (Fig. 4). Consistent with this hypothesis, a recombinant vaccinia virus that lacks functional F13L shows a dramatic reduction in the amount of extracellular virus formed during productive infection of cultured cells (4). Taken together, these results suggest that ST-246 inhibits F13L activity and prevents the formation of extracellular virus.
Extracellular virus particles are essential for systemic virus spread in the host and play an important role in viral pathogenesis (28). Virus strains that produce higher proportions of extracellular virus particles as part of the infection cycle are more virulent in mice, with the exception of strain WR, which has been adapted to replicate in mouse brain tissue and is likely more neurovirulent (28). Virus variants defective in extracellular virus particle production are attenuated for replication in mice (11, 42). Moreover, vaccines derived from extracellular virus antigens are completely protective in a rabbitpox model of orthopoxvirus infection, suggesting that these particles play a significant role in disease progression (5). Given the role of extracellular virus in pathogenesis, therapeutics like ST-246 that target extracellular virus particle production would be expected to provide complete protection against systemic orthopoxvirus disease. Indeed, this concept was validated in a recent report describing the antiviral effects of a cancer therapeutic, CI-1033, which inhibits the cellular tyrosine kinase Erb-1 (40). Erb-1 is one of several host kinases recruited by vaccinia virus to sites of actin tail assembly and is involved in release of extracellular virus from infected-cell membranes (14). At concentrations 200 fold above the 50% inhibitory concentration for inhibition of Erb-1 activity, CI-1033 reduced plaque size in cell culture and virus titers in mice infected with vaccinia virus (40). While CI-1033 has demonstrated antiviral activity, this compound targets cellular enzymes involved in cell growth and has the potential for toxic side effects. Indeed, CI-1033 has been shown to increase cytotoxicity of compounds by inhibiting drug efflux in cell culture (12).
ST-246 targets a specific viral enzyme with no known human homolog and thus is highly specific for orthopoxvirus replication. Oral administration of ST-246 protected mice from challenge with 40,000 times the LD50 of ectromelia virus. In addition, ST-246 reduced ectromelia virus titers in lung, liver, and spleens of infected mice by at least 5 orders of magnitude relative to placebo-treated mice, suggesting that ST-246 was efficient at inhibiting systemic virus spread. While ST-246 is highly active, maximal protective effect required 14 days of dosing, with 5-day dosing periods protecting 80% of the mice from lethal viral challenge. Similarly, 5-day dosing with ST-246 at 50 mg/kg b.i.d. reduced virus titers in liver but failed to protect mice from lethal cowpox virus infection (J. W. Huggins, unpublished data).
The requirement for extended dosing durations for protective efficacy is consistent with a compound that targets a virulence factor. Unlike CDV, which accumulates inside cells and appears to target virus replication, ST-246 inhibits virus spread by targeting an enzyme involved in extracellular virus formation. Thus, maximum therapeutic benefit requires the continued presence of ST-246 to inhibit virus spread and allow the host immune response to clear the infection. While host immunity is important for protective efficacy, ST-246 has been shown to inhibit virus replication with the same efficiency as CDV in athymic (nu/nu) mice, suggesting that T-cell immunity is not critical for compound efficacy (J. Neyts et al., unpublished data). By targeting a virulence factor, ST-246 reduces disease progression without diminishing development of protective immunity. Indeed, mice that survived infection as a result of ST-246 treatment were resistant to subsequent challenge with a lethal dose of virus. Taken together, these results validate F13L as an antiviral target and demonstrate that an inhibitor of extracellular virus production can protect mice from lethal orthopoxvirus infection. The fact that ST-246 is orally bioavailable, well tolerated, and active against a highly conserved orthopoxvirus target makes this compound a strong candidate for development as a smallpox antiviral drug that could be stockpiled as part of the Strategic National Stockpile for use in treatment and prevention of smallpox virus infection in the event of a bioterrorist attack.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases grants 1 R43 AI056409-01 and R44 AI056409-02 to R.J. and NO1-AI-15436 to R.L.M.B.
We thank Richard Condit, Bertram Jacobs, Paula Traktman, and Sylvie Laquerre for helpful discussions and critical analysis of the data. We also thank Tove' Bolken and Chelsea Byrd for critical reviews of the manuscript.
REFERENCES
- 1.Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-14C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 2.Baek, S. H., J. Y. Kwak, S. H. Lee, T. Lee, S. H. Ryu, D. J. Uhlinger, and J. D. Lambeth. 1997. Lipase activities of p37, the major envelope protein of vaccinia virus. J. Biol. Chem. 272:32042-32049. [DOI] [PubMed] [Google Scholar]
- 3.Baker, R. O., M. Bray, and J. W. Huggins. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 57:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J. Virol. 65:5910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulter, E. A., H. T. Zwartouw, D. H. Titmuss, and H. B. Maber. 1971. The nature of the immune state produced by inactivated vaccinia virus in rabbits. Am. J. Epidemiol. 94:612-620. [DOI] [PubMed] [Google Scholar]
- 6.Bray, M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 58:101-114. [DOI] [PubMed] [Google Scholar]
- 7.Bronson, J. J., L. M. Ferrara, M. J. Hitchcock, H. T. Ho, K. L. Woods, I. Ghazzouli, E. R. Kern, K. F. Soike, and J. C. Martin. 1990. (S)-1-(3-hydroxy-2-(phosphonylmethoxy)propyl)cytosine (HPMPC): a potent antiherpesvirus agent. Adv. Exp. Med. Biol. 278:277-283. [DOI] [PubMed] [Google Scholar]
- 8.Buller, R. M., G. Owens, J. Schriewer, L. Melman, J. R. Beadle, and K. Y. Hostetler. 2004. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology 318:474-481. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, and A. Holy. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira, C. B., D. Stevenson, L. LaBree, P. J. McDonnell, and M. D. Trousdale. 1996. Evaluation of cidofovir (HPMPC, GS-504) against adenovirus type 5 infection in vitro and in a New Zealand rabbit ocular model. Antiviral Res. 31:165-172. [DOI] [PubMed] [Google Scholar]
- 11.Engelstad, M., and G. L. Smith. 1993. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194:627-637. [DOI] [PubMed] [Google Scholar]
- 12.Erlichman, C., S. A. Boerner, C. G. Hallgren, R. Spieker, X. Y. Wang, C. D. James, G. L. Scheffer, M. Maliepaard, D. D. Ross, K. C. Bible, and S. H. Kaufmann. 2001. The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10-hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux. Cancer Res. 61:739-748. [PubMed] [Google Scholar]
- 13.Fenner, F., and R. M. L. Buller. 1997. Mousepox, p. 535-553. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 14.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401:926-929. [DOI] [PubMed] [Google Scholar]
- 15.Grosenbach, D. W., S. G. Hansen, and D. E. Hruby. 2000. Identification and analysis of vaccinia virus palmitylproteins. Virology 275:193-206. [DOI] [PubMed] [Google Scholar]
- 16.Grosenbach, D. W., and D. E. Hruby. 1998. Analysis of a vaccinia virus mutant expressing a nonpalmitylated form of p37, a mediator of virion envelopment. J. Virol. 72:5108-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heo, D. S., J. G. Park, K. Hata, R. Day, R. B. Herberman, and T. L. Whiteside. 1990. Evaluation of tetrazolium-based semiautomatic colorimetric assay for measurement of human antitumor cytotoxicity. Cancer Res. 50:3681-3690. [PubMed] [Google Scholar]
- 18.Hiller, G., H. Eibl, and K. Weber. 1981. Characterization of intracellular and extracellular vaccinia virus variants: N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine interferes with cytoplasmic virus dissemination and release. J. Virol. 39:903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain, M., and B. Moss. 2002. Similarities in the induction of post-Golgi vesicles by the vaccinia virus F13L protein and phospholipase D. J. Virol. 76:7777-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husain, M., and B. Moss. 2003. Intracellular trafficking of a palmitoylated membrane-associated protein component of enveloped vaccinia virus. J. Virol. 77:9008-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Husain, M., A. Weisberg, and B. Moss. 2003. Topology of epitope-tagged F13L protein, a major membrane component of extracellular vaccinia virions. Virology 308:233-242. [DOI] [PubMed] [Google Scholar]
- 22.Kato, N., H. J. Eggers, and H. Rolly. 1969. Inhibition of release of vaccinia virus by N1-isonicotinoly-N2-3-methyl-4-chlorobenzoylhydrazine. J. Exp. Med. 129:795-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern, E. R. 2003. In vitro activity of potential anti-poxvirus agents. Antiviral Res. 57:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalezari, J. P., R. J. Stagg, B. D. Kuppermann, G. N. Holland, F. Kramer, D. V. Ives, M. Youle, M. R. Robinson, W. L. Drew, and H. S. Jaffe. 1997. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann. Intern. Med. 126:257-263. [DOI] [PubMed] [Google Scholar]
- 25a.LeDuc, J. W., I. Damon, D. A. Relman, J. Huggins, and P. B. Jahrling. 2002. Smallpox research activities: U.S. interagency collaboration, 2001. Emerg. Infect. Dis. 8:743-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neyts, J., and E. De Clerq. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 41:242-246. [DOI] [PubMed] [Google Scholar]
- 27.Neyts, J., P. Leyssen, E. Verbeken, and E. De Clerq. 2004. Efficacy of cidofovir in a murine model of disseminated progressive vaccinia. Antimicrob. Agents Chemother. 48:2267-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 29.Payne, L. G., and K. Kristenson. 1979. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J. Virol. 32:614-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quenelle, D. C., D. J. Collins, and E. R. Kern. 2003. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob. Agents Chemother. 47:3275-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quenelle, D. C., D. J. Collins, W. B. Wan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redfield, R. R., D. C. Wright, W. D. James, T. S. Jones, C. Brown, and D. S. Burke. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316:673-676. [DOI] [PubMed] [Google Scholar]
- 33.Roper, R. L., and B. Moss. 1999. Envelope formation is blocked by mutation of a sequence related to the HKD phospholipid metabolism motif in the vaccinia virus F13L protein. J. Virol. 73:1108-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmutz, C., L. G. Payne, J. Gubser, and R. Wittek. 1991. A mutation in the gene encoding the vaccinia virus 37,000-Mr protein confers resistance to an inhibitor of virus envelopment and release. J. Virol. 65:3435-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 36.Snoeck, R., M. Bossens, D. Parent, B. Delaere, H. Degreef, M. Van Ranst, J. C. Noel, M. S. Wulfsohn, J. F. Rooney, H. S. Jaffe, and E. De Clercq. 2001. Phase II double-blind, placebo-controlled study of the safety and efficacy of cidofovir topical gel for the treatment of patients with human papillomavirus infection. Clin. Infect. Dis. 33:597-602. [DOI] [PubMed] [Google Scholar]
- 37.Sridhar, P., and R. C. Condit. 1983. Selection for temperature-sensitive mutations in specific vaccinia virus genes: isolation and characterization of a virus mutant which encodes a phosphonoacetic acid-resistant, temperature-sensitive DNA polymerase. Virology 128:444-457. [DOI] [PubMed] [Google Scholar]
- 38.Sung, T. C., R. L. Roper, Y. Zhang, S. A. Rudge, R. Temel, S. M. Hammond, A. J. Morris, B. Moss, J. Engebrecht, and M. A. Frohman. 1997. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 16:4519-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong, X., J. L. Smith, and M. S. Chen. 1997. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob. Agents Chemother. 41:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, H., S. K. Kim, M. Kim, P. A. Reche, T. J. Morehead, I. K. Damon, R. M. Welsh, and E. L. Reinherz. 2005. Antiviral chemotherapy facilitates control of poxvirus infections through inhibition of cellular signal transduction. J. Clin. Investig. 115:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao, X. D., and D. H. Evans. 2003. High-frequency genetic recombination and reactivation of orthopoxviruses from DNA fragments transfected into leporipoxvirus-infected cells. J. Virol. 77:7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, W. H., D. Wilcock, and G. L. Smith. 2000. Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J. Virol. 74:11654-11662. [DOI] [PMC free article] [PubMed] [Google Scholar]