Abstract
Recognition of pathogens by the innate immune system is mediated by pattern recognition receptors (PRRs), which recognize specific molecular structures of the infectious agents and subsequently trigger expression of genes involved in host defense. Toll-like receptors (TLRs) represent a well-characterized class of membrane-bound PRRs, and the RNA helicase retinoic acid inducible gene I (RIG-I) has recently been described as a novel cytoplasmic PRR recognizing double-stranded RNA (dsRNA). Here we show that activation of signal transduction and induction of cytokine expression by the paramyxovirus Sendai virus is dependent on virus replication and involves PRRs in a cell-type-dependent manner. While nonimmune cells relied entirely on recognition of dsRNA through RIG-I for activation of an antiviral response, myeloid cells utilized both the single-stranded RNA sensing TLR7 and TLR8 and dsRNA-dependent mechanisms independent of RIG-I, TLR3, and dsRNA-activated protein kinase R to trigger this response. Therefore, there appears to be a large degree of cell-type specificity in the mechanisms used by the host to recognize infecting viruses.
Innate defense against infections is activated by pattern recognition receptors (PRRs), which recognize molecular structures conserved on larger groups of pathogens, or so-called pathogen-associated molecules (PAMs) (16). Toll-like receptors (TLRs) constitute a class of membrane-bound PRRs residing extracellularly or in endosomic compartments (1, 8). TLRs are expressed in cell-type-specific patterns, with the most abundant expression levels found on dendritic cells (DCs), monocytes, and macrophages (15). Ligand engagement of TLRs activates signal transduction to nuclear factor (NF)-κB, mitogen-activated protein kinases, and for some TLRs also to interferon (IFN) regulatory factor (IRF) (1). This leads to induction of cytokine expression and maturation of DCs (15). For viruses, PAMs known to be recognized by TLRs include glycoproteins from different viruses (TLR2 and TLR4), double-stranded RNA (dsRNA) (TLR3), single-stranded RNA (ssRNA) (TLR7 and TLR8), and unmethylated viral CpG DNA (TLR9) (5, 26, 27).
The intracellular location of virus replication, together with a number of recent studies from this and other laboratories (14, 20, 24), has raised the question of whether TLRs are the sole class of PRRs responsible for recognition of viruses and activation of the antiviral response. One such intracellular PRR has recently been reported by the identification of the RNA helicase retinoic acid inducible gene I (RIG-I), which binds synthetic and viral dsRNA and activates NF-κB and IRF-3 (39). In addition, the IFN-induced dsRNA-activated protein kinase (PKR) has also long been known to stimulate inflammatory signal transduction upon dsRNA binding (40) and, hence, also represents an attractive candidate for an intracellular sensor of viral replication (10).
In this work, we have examined the employment of cellular PRRs in activation of the antiviral response during infection with the paramyxovirus Sendai virus (SeV) in different cell types. Our results show that nonimmune cells primarily respond to intracellular accumulation of viral dsRNA via RIG-I, while myeloid cells are capable of sensing SeV both through the ssRNA receptors TLR7 and TLR8 and through a dsRNA-dependent mechanism independent of RIG-I, PKR, and TLR3. These results thus illustrate that cell-type-specific mechanisms are employed in the process of pathogen recognition during viral infections.
MATERIALS AND METHODS
Cells, virus, and reagents.
The parental cell lines used in this study were U937 (human and myeloid), A549 (human and epithelial), HEK293 (human and epithelial), RAW 264.7 (murine and myeloid), and NIH 3T3 (murine and fibroblast). U937 cells were grown in RPMI 1640 medium with 2 mM l-glutamine, 10 mM HEPES, and 10% fetal calf serum (FCS), while A549, HEK293, RAW 264.7, and NIH 3T3 cells were grown in Dulbecco's modified Eagle's medium with 5% FCS. All media were supplemented with penicillin, 200 IU/ml, and streptomycin, 200 μg/ml.
Stable cell lines derived from the parental cell lines were either generously donated by colleagues (K. A. Fitzgerald provided HEK293-TLR2, HEK293-TLR3, HEK293-TLR4/MD2, and HEK293-TLR9 [17], and J. A. Corbett provided RAW-pBK and RAW-PKR-M7 [23]), obtained from commercial sources (HEK293-TLR7 and HEK293-TLR8 [InvivoGen]), or generated in the laboratory (RAW 264.7-pcDNA3, RAW 264.7-dnMyD88, RAW 264.7-dnTRIF, RAW 264.7-dnMal, RAW 264.7-dnRIG-I, and RAW 264.7-NS1) following the protocol previously described (24). The transfected cells were grown under the same conditions as the parental cell lines, plus additional selection (for HEK293, 500 μg/ml G418 [Roche] or 10 μg/ml blasticidin [InvivoGen], and for RAW 264.7, 350 to 400 μg/ml G418).
The viruses used were SeV (strain Cantell) and encephalomyocarditis virus (EMCV) (strain EMC), which were propagated in embryonated hen eggs as described previously (31) and Vero cells, respectively. UV inactivation of SeV was performed by exposing the virus to UV light for 10 min. The uninfected hen egg allantoic fluid did not stimulate proinflammatory cytokine expression in human macrophages and DCs, and the virus preparation did not contain lipopolysaccharide (LPS) (data not shown).
The nonviral stimuli and inhibitors used were tumor necrosis factor alpha (TNF-α) (Genzyme), LPS (Sigma), ODN2006, ODN1826, polyIC, Pam3CSK4, and R848 (all InvivoGen), and chloroquine (Sigma-Aldrich).
The DNA plasmids used for transfection experiments were pEGFP-RIG-I helicase, pcDNA3-HA-PKRΔE7 (19), pCAGGS-NS1 (35), pcDNA3-MyD88(TIR) (28), pCMV-Myc-TRIFΔNΔC (38), pDC304-Mal-P/H125 (11), κB luciferase reporter gene (12), and pGL2-IP-10(÷243) (29). For generation of the pEGFP-DN-RIG-I construct, cDNA from human A549 cells was PCR amplified with primers 5′-GACA AAGCTTCG GAATGCCAGAATCTTAGTGAGAATTCATGT-3′ and 5′-GACA GGTACC TCATTTGGACATTTCTGCTGGATCAAA-3′. The resulting PCR fragment encompasses the helicase domain of RIG-I encoded by nucleotides 652 to 2778 (39), flanked by HindIII and KpnI restriction sites which were provided by the PCR primers. The PCR fragment was cloned in frame into the enhanced green fluorescent protein expression vector pEGFP-C1 (Clontech) by using the HindIII and KpnI sites of both insert and vector.
Transient transfections and reporter gene assay.
HEK293 cells were seeded at a density of 2.0 × 104 cells per well in 96-well plates. Transfections were performed the following day with Lipofectamine 2000 (Invitrogen) by following the manufacturer's instructions. Briefly, DNA and Lipofectamine 2000, each diluted in Dulbecco's modified Eagle's medium and left for 2 to 3 min, were mixed and incubated for 20 min. Twenty microliters of the DNA-Lipofectamine 2000 solution were added to each well. We used 80 ng/well of NF-κB reporter plasmid and 40 ng/well of IP-10 reporter plasmid. In some experiments, the cells were also transfected with the indicated amounts of plasmid DNA encoding NS1 or dominant negative (DN) mutants of RIG-I or PKR. The total amount of transfected DNA was kept constant by using pcDNA3 as irrelevant DNA. The ratio of Lipofectamine 2000 (μl) to DNA (μg) was kept at 2.5. Twenty-four hours posttransfection, the cells were stimulated as described for the specific experiment and left for 20 h before wash, cell lysis, and measurement of luciferase activity by the Luciferase 1000 assay system (Promega). To normalize the data, we cotransfected with Renilla in select experiments and measured luciferase and Renilla using Dual-Glo luciferase (Promega). We observed that SeV infection reduced Renilla activity by 10 to 20% but found no significant interwell variation (less that 15%) between wells receiving the same treatment. The data presented in this paper are shown as unnormalized luciferase activities.
ELISA.
Human interleukin-8 (IL-8) and IP-10 and murine RANTES/CCL5 and IL-6 were detected by enzyme-linked immunosorbent assay (ELISA). Maxisorp plates were coated overnight at room temperature with 100 μl of antibody (Ab) (anti-IL-8, 4 μg/ml; anti-IP-10, 2 μg/ml; anti-RANTES, 2 μg/ml; or anti-IL-6, 2 μg/ml [all from R&D Systems]) in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 0.02% NaN3 [pH 9.6]). After blocking for at least 1 h at room temperature with 300 μl of 1% bovine serum albumin (BSA) in blocking buffer (phosphate-buffered saline with 5% sucrose and 0.05% NaN3 [pH 7.4]), successive culture supernatants or recombinant murine IL-8, IP-10, RANTES (all from R&D Systems), or IL-6 (Genzyme) was added to the wells (100 μl each) and incubated overnight at 4°C. Subsequently, wells were incubated at room temperature for 2 h with 100 μl biotinylated, anti-human detection Ab (IL-8, 20 ng/ml, or IP-10, 100 ng/ml [both from R&D Systems]) or anti-murine detection Ab (RANTES, 100 ng/ml, or IL-6, 200 ng/ml [both from R&D Systems]) in a 0.1% suspension of BSA in Tris-buffered saline (TBS) (20 mM tromethamine [Trizma], 150 mM NaCl, and 0.05% Tween 20 [pH 7.3]). Streptavidin-horseradish peroxidase (R&D Systems) diluted at 1:200 in TBS with 0.1% BSA was added, and the mixture was incubated for 20 min. For color development, we added H2O2 and tetramethylbenzidine (R&D Systems) and plates were incubated in the dark for an appropriate amount of time. The color reaction was stopped with 50 μl 5% H2SO4, and the absorbance was measured at 450 nm with 570 nm as a reference. Between each step, the plates were washed three times with phosphate-buffered saline-0.05% Tween 20, pH 7.4.
IFN-α/β bioassay.
IFN-α/β bioactivity was measured by an L929-cell-based bioassay. L929 cells (2 × 104 cells/well in 100 μl) in modified Eagle's medium with 5% FCS were incubated overnight at 37°C in successive twofold dilutions of samples or murine IFN-α/β as the standard. Subsequently, vesicular stomatitis virus (VSV/V10) was added to the wells and the cells were incubated for 2 to 3 days. The dilution mediating 50% protection was defined as 1 U/ml of IFN-α/β.
Isolation of RNA and RT-PCR.
To isolate RNA, cells were lysed in Trizol (Invitrogen) and phase separated by addition of chloroform and centrifugation at 12,000 × g for 12 min (4°C). The aqueous phase was isolated, and RNA was precipitated with isopropanol and pelleted by centrifugation for 12 min at 15,300 × g (4°C). Finally, the RNA pellet was washed with ethanol and redissolved in RNase-free water. Two micrograms of RNA was subjected to reverse transcription (RT) with oligo(dN)6 (Roche) as primer and Expand reverse transcriptase (Roche). The cDNA was amplified by PCR with the following primers: for SeV, 5′-TCT GTT GAA GGC TGT CAT GC-3′ (sense) and 5′-GAA TGG GTT ATC CGG GAG TT-3′ (antisense), and for human β-actin, 5′-AAA AGC CAC CCC ACT TCT CT-3′ (sense) and 5′-CTC AAG TTG GGG GAC AAA AA-3′ (antisense). The products for SeV and human β-actin spanned 174 and 201 bp, respectively.
Oligonucleotide DNA precipitation.
A549, U937, and HEK293 cells were stimulated with live SeV or UV-treated SeV as indicated. At the appropriate times postinfection, the cells were harvested, washed, and lysed in a buffer containing 10 mM HEPES, 400 mM KCl, 10% glycerol, 2 mM EDTA, 1 mM EGTA, 0.01% Triton X-100, 0.5 mM dithiothreitol, 1 mM NaVO4, and protease inhibitors (Complete; Roche). Cleared cell lysates were incubated with streptavidin-agarose beads (Neutravidin; Pierce) coupled to 5′-biotinylated 5′ 6-bp extended oligonucleotides (DNA Technology). The oligonucleotides used were as follows: IFNβ PRDI-III (5′-GGA TCC GAA AAC TGA AAG GGA GAA GTG AAA GTG-3′ and 5′-GGA TCC CAC TTT CAC TTC TCC CTT CTT TCA GTT TTC-3′) for IRF-3 precipitation, and IFNβ PRDII (5′-GG ATC CGG AAT TTC CCG GAA TTT CCC-3′ and 5′-GGA TCC GGG AAA TTC CGG GAA ATT CC-3′) for NF-κB p65 precipitation. The binding reactions were performed for 2 h at 4°C in binding buffer containing 10 mM HEPES, 133 mM KCl, 10% glycerol, 2 mM EDTA, 1 mM EGTA, 0.01% Triton X-100, 0.5 mM dithiothreitol, 1 mM NaVO4, and protease inhibitors. After being washed, the oligonucleotide-bound proteins were released in sodium dodecyl sulfate sample buffer, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto membranes (Bio-Rad). Membranes were blocked with 5% skim milk in TBS containing 0.05% Tween 20. Rabbit-anti-IRF3 antibody or goat anti-p65 antibody (both from Santa Cruz Biotechnology), diluted 1:2,000 in TBS containing 0.05% Tween 20, were allowed to bind overnight at 4°C. Peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:2,000 dilution) or anti-goat (both from DAKO) was allowed to bind for 1 h at room temperature. The proteins were visualized on Fuji Super RX film by the enhanced chemiluminescence system (Pierce).
Statistics.
The data are presented as means ± standard errors of the means (SEM). Statistical significance was estimated with Student's t test for unpaired observations. P values of <0.05 were considered significant.
RESULTS
SeV induces cytokine expression in human and murine myeloid and nonimmune cells through a mechanism dependent on viral replication.
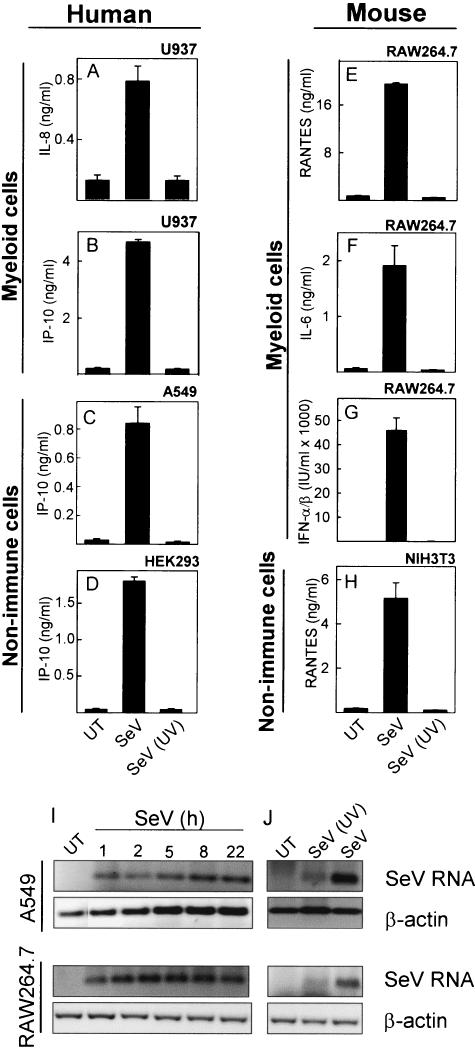
Previous studies have shown that SeV is a very potent inducer of cytokine and chemokine expression in many different cell types (25, 30). To examine if this property of SeV was carried by the viral particle or was dependent on viral replication, we inactivated SeV by UV treatment for 10 min and treated human and murine myeloid and nonimmune cells with infectious and UV-inactivated virus. At subsequent time points, supernatants were harvested and the levels of different cytokines and chemokines were measured. For all cell lines tested and all cytokines and chemokines measured, we found that the ability of SeV to activate gene expression was inhibited if the virus had been inactivated by UV light prior to addition to the cells (Fig. 1A to H).
FIG. 1.
Induction of cytokine expression by SeV. U937 (A and B), A549 (C), HEK293 (D), RAW 264.7 (E to G), and NIH 3T3 (H) cells were seeded in 96-well plates and left overnight to settle. The cells were left untreated (UT) or treated with infectious (SeV) or UV-inactivated [SeV (UV)] SeV at a multiplicity of infection (MOI) of 1. Supernatants were harvested 16 to 24 h later, and cytokine levels were measured by ELISA or bioassay. The results are shown as means of triplicate cultures ± SEM. Essentially similar results were observed for two to five independent experiments. (I and J) A549 and RAW 264.7 cells were seeded and left overnight to settle prior to treatment with infectious and UV-inactivated SeV at an MOI of 1. After the indicated times of infection, total RNA was harvested and accumulation of viral RNA and cellular β-actin was assessed by RT-PCR. Similar results were obtained for two independent experiments.
To correlate the cytokine data with accumulation of viral genetic material, we harvested RNA from A549 and RAW 264.7 cells at different time points postinfection and assessed the levels of SeV RNA by RT-PCR. As seen in Fig. 1I, viral RNA was detectable at all time points examined in both cell lines from 1 to 22 h postinfection. In addition, UV inactivation of the virus strongly inhibited accumulation of viral RNA during infection (Fig. 1J). However, close examination of the gel revealed a faint band in the lane with UV-inactivated SeV. This might be due to incomplete UV inactivation or, alternatively, detection of the incoming viral RNA.
Collectively, these data show that SeV RNA accumulates in cells during infection with this virus and that induction of cytokine expression in both myeloid and nonimmune cells occurs through mechanisms dependent on viral replication.
SeV infection leads to activation of IRF-3 and NF-κB in both myeloid and nonimmune cells.
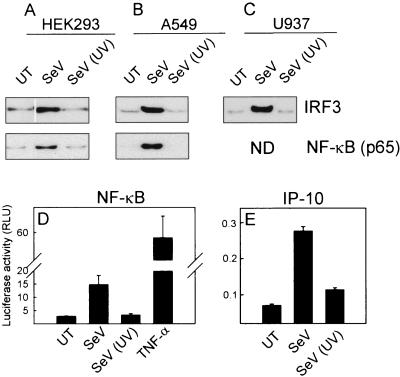
To correlate cytokine expression with activation of transcription factors, we treated HEK293, A549, and U937 cells with live and UV-inactivated SeV. Five hours later, the cells were lysed and DNA binding activities of IRF-3 and NF-κB were measured by oligoprecipitation assay. As seen in Fig. 2A to C, both transcription factors were activated by the replicating virus, but not by the inactivated virus, in all cell types tested. To further support this, we transfected HEK293 cells with two luciferase reporter gene plasmids controlled by NF-κB- or IRF-dependent promoters (the IP-10 promoter requires a functional IFN-stimulated response element for SeV-dependent activation [7]) and treated with infectious and inactivated SeV. Again we observed that UV treatment of SeV abrogated the ability of this virus to activate NF-κB and IRFs (Fig. 2D and E). Thus, SeV triggers activation of inflammatory signal transduction in both myeloid and nonimmune cells through a mechanism dependent on viral replication.
FIG. 2.
Activation of IFN-3 and NF-κB by SeV in myeloid and nonimmune cells. (A to C) HEK293 (A), A549 (B), and U937 (C) cells were seeded and left overnight to settle before treatment with SeV or UV-inactivated SeV [Sev (UV)] as indicated at an MOI of 1. Five hours posttreatment, the cells were lysed and activation of IRF-3 and NF-κB was assayed by DNA oligoprecipitation assays. ND, not done. (D and E) HEK293 cells were transfected with the indicated luciferase reporter constructs and left untreated (UT) or treated with either live or UV-inactivated SeV (MOI of 1) or TNF-α (100 U/ml). Sixteen hours posttreatment, the cells were lysed and luciferase activity was measured. Similar results were obtained for two to four independent experiments. RLU, relative luciferase units.
Role for TLRs in SeV-induced cytokine expression in myeloid cells but not in nonimmune cells.
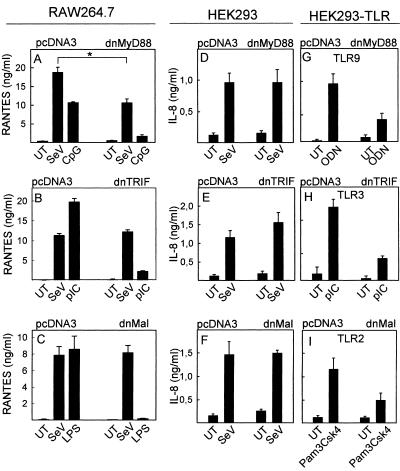
SeV is an ssRNA virus, and we speculated that ssRNA or dsRNA produced during replication could potentially serve as ligands for TLR7 and TLR8 (9, 13, 22) or for TLR3 (2), respectively, to trigger antiviral defense. Therefore, we stably transfected the murine myeloid cell line RAW 264.7 with DN mutants of the TLR adaptor proteins MyD88, TRIF, and Mal and infected the cells with SeV as well as a positive control known to use the adaptor protein targeted in the specific cell lines. The cells expressing DN-MyD88 displayed about a 40% reduced response to SeV (Fig. 3A), whereas the DN-TRIF and DN-Mal cell lines were unaltered in the response to SeV (Fig. 3B and C). These data hence indicated a minor but significant role for TLRs in SeV-induced cytokine expression in myeloid cells. In support of this, we also found that the vaccinia virus-encoded TLR antagonist proteins A46R and A52R (6) partially inhibited SeV-induced RANTES expression in RAW 264.7 cells but totally prevented LPS from triggering expression of this chemokine (data not shown).
FIG. 3.
Role of TLR adaptor molecules in cytokine expression induced by SeV. (A to C) RAW 264.7 cells were stably transfected with empty vector (pcDNA3) or plasmids encoding dnMyD88 (A), dnTRIF (B), or dnMal (C). The cells were seeded in 96-well plates and left overnight to settle. The cells were treated with SeV at an MOI of 1 or LPS (100 ng/ml), CpG (ODN1826, 1 μM), or polyIC (pIC) (25 μg/ml). Supernatants were harvested 20 h later, and RANTES was measured by ELISA. (D to I) HEK293, HEK293-TLR2, HEK293-TLR3, and HEK293-TLR9 cells were transiently transfected with empty vector (pcDNA3) or plasmids encoding dnMyD88 (D and G), dnTRIF (E and H), or dnMal (F and I) as indicated. The cells were seeded in 96-well plates and left overnight to settle before treatment with SeV at an MOI of 1 or LPS (100 ng/ml), CpG (ODN2006, 1 μM), or polyIC (25 μg/ml) for 20 h. Supernatants were harvested for measurements of IL-8 done by ELISA. The results are shown as means of triplicate cultures ± SEM. Essentially similar results were observed for two to three independent experiments. UT, untreated.
For comparison, we also examined how the DN mutants affected SeV-induced cytokine expression in HEK293 cells. With these cells, however, we observed no effect of disruption of TLR signaling on SeV-induced IL-8 expression (Fig. 3D to F). As a control, transfection of HEK293-TLR9, HEK293-TLR3, and HEK293-TLR2 cells with DN-MyD88, DN-TRIF, and DN-Mal, respectively, showed that the DN mutants did work under the experimental conditions used (Fig. 3G to I).
TLR7 and TLR8 enhance responsiveness to SeV infection.
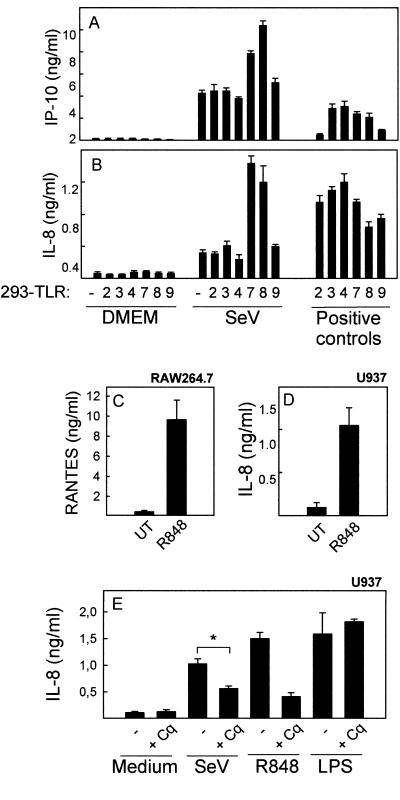
In order to identify which TLR(s) was responsible for the MyD88-dependent response in myeloid cells, HEK293 cells stably transfected with each of the human TLRs were infected with SeV or stimulated with known agonists for the different TLRs. As seen in Fig. 4A and B, and confirming the data from Fig. 1D and Fig. 3D to F, SeV infection triggered expression of IL-8 and IP-10 in HEK293 cells. Although this was not affected by expression of TLR2, -3, -4, or -9, cells expressing TLR7 or TLR8 displayed a significant elevation in the levels of virus-induced cytokine expression. In corroboration with this, HEK293-TLR8 cells also activated the NF-κB reporter gene twice as potently as did HEK293-pcDNA3 cells after SeV infection (data not shown). In order to examine if the myeloid cells used in this study did express functional TLR7 and TLR8, we stimulated RAW 264.7 and U937 cells with R848, which is a synthetic ligand for TLR7 and TLR8, and looked for expression of cytokines. As seen in Fig. 4C and D, both of these myeloid cell lines responded very potently to this treatment. Similar findings have been done with primary human macrophages (J. Melchjorsen, unpublished data; 33), hence demonstrating the authenticity of myeloid cells as potent responders to ligands for TLR7 and TLR8. To further examine a potential role of TLR7 and TLR8 in the response of myeloid cells to SeV, we treated U937 cells with chloroquine, which inhibits TLR7 and TLR8 by preventing acidification of endosomes (22), and infected with SeV or stimulated with R848 or LPS. Supernatants were harvested, and expression of IL-8 was examined. All three treatments led to induction of IL-8 expression (Fig. 4E), but they displayed different degrees of inhibition by chloroquine. IL-8 expressions induced by R848 and SeV were strongly and partially inhibited, respectively, whereas the response to LPS was not affected by chloroquine. Collectively, TLR7 and TLR8 are capable of sensing SeV infection and are expressed on myeloid cells, which display a reduced response to SeV infection in the presence of chloroquine.
FIG. 4.
Identification of TLRs responsible for cytokine expression induced by SeV infection. (A and B) HEK293 cells stably transfected with empty vector (pcDNA3) or plasmids encoding human TLR2, -3, -4, -7, -8, or -9 were seeded in 96-well plates and left overnight to settle before infection with SeV at an MOI of 1 or treatment with well-described TLR ligands: for HEK293-TLR2, Pam3CSK4 (300 ng/ml); for HEK293-TLR3, polyIC (25 μg/ml); for HEK293-TLR4, LPS (100 ng/ml); for HEK293-TLR7 and HEK293-TLR8, R848 (1 μg/ml); and for HEK293-TLR9, CpG (ODN2006, 5 μM). Supernatants were harvested 18 h posttreatment, and the levels of IP-10 and IL-8 were measured by ELISA. The results are shown as means of triplicate cultures ± SEM. Essentially similar results were observed for two independent experiments. (C and D) RAW 264.7 and U937 cells were seeded and left overnight to settle before treatment with 1 μg/ml of R848. Twenty hours later, supernatants were harvested and levels of RANTES (C) and IL-8 (D) were measured by ELISA. The results are shown as means of triplicate cultures ± SEM. Essentially similar results were observed for three independent experiments. UT, untreated. (E) U937 cells were treated with 10 μM of chloroquine (Cq) 15 min prior to infection with SeV (MOI of 1) or treatment with 1 μg/ml of R848 or 100 ng/ml of LPS. Twenty hours later, supernatants were harvested and IL-8 levels were measured by ELISA. The results are shown as means of triplicate cultures ± SEM. Essentially similar results were observed for two independent experiments. *, P value of <0.05.
TLR-independent activation of myeloid and nonimmune cells by SeV.
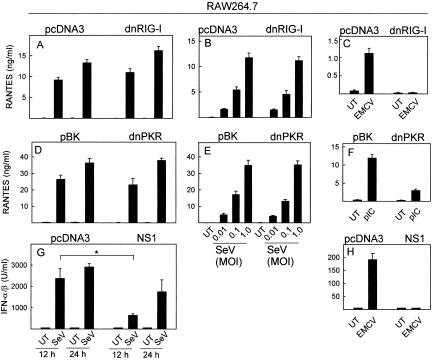
Since the entire SeV-induced response in nonimmune cells and the majority of the response in myeloid cells were independent of TLRs, we also wanted to identify which other PRRs could be involved. Accumulation of dsRNA is a hallmark of viral infections, and cells are in possession of several mechanisms to sense dsRNA (27). To address the potential involvement of dsRNA and dsRNA-recognizing PRRs in the response to SeV infection, RAW 264.7 and HEK293 cells were transfected with constructs encoding DN mutants of RIG-I and PKR as well as the influenza A virus NS1 protein, which binds and sequesters dsRNA (35). Expression of the DN mutants of RIG-I or PKR did not affect the ability of SeV to stimulate expression of RANTES, IL-6, TNF-α, or IFN-α/β in RAW 264.7 cells at a wide range of virus concentrations (Fig. 5A to B and D to E; also data not shown), although induction of RANTES expression by EMCV and polyIC was strongly inhibited in the RAW-dnRNG-I and RAW-dnPKR cells, respectively (Fig. 5C and 5F), thus demonstrating the integrity of the cell lines used. When we examined the ability of SeV to induce IFN-α/β in RAW 264.7 cells expressing NS1, we observed a significant reduction after 12 h of infection, which was not apparent after 24 h (Fig. 5G). Expression of IFN-α/β in response to EMCV infection, on the other hand, was totally abrogated in cells expressing NS1 (Fig. 5H).
FIG. 5.
Role of dsRNA, PKR, and RIG-I in induction of the antiviral response in myeloid cells. (A to C) RAW 264.7 cells stably transfected with DN-RIG-I or a control plasmid were seeded and left overnight to settle before infection with SeV (A, MOI of 1; B, MOI of 0.01 to 1) or EMCV (C, MOI of 1). After 12 h (A and B) or 24 h (A and C) of infection, supernatants were harvested and RANTES was measured by ELISA. (D to F) RAW 264.7 cells stably transfected with DN-PKR or a control plasmid were seeded and left overnight to settle before infection with SeV (D, MOI of 1; E, MOI of 0.01 to 1) or treatment with polyIC (pIC) (F, MOI of 1). After 12 h (D and E) or 24 h (D and F), supernatants were harvested and RANTES was measured by ELISA. (G and H) RAW 264.7 cells stably transfected with NS1 or a control plasmid were seeded and left overnight to settle before infection with SeV (G) or EMCV (H), both at an MOI of 1. After the indicated periods of incubation, supernatants were harvested and IFN-α/β was measured by bioassay. Essentially similar results were observed for three to four independent experiments. UT, untreated.
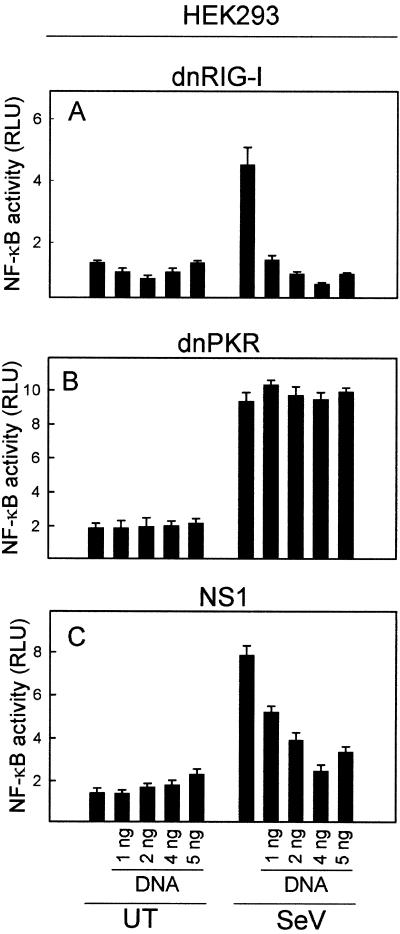
In HEK293 cells, expression of DN-RIG-I totally abrogated activation of NF-κB in response to SeV infection (Fig. 6A), whereas no effect was observed when the cells expressed DN-PKR (Fig. 6B). Finally, expression of the NS1 protein nearly led to a total block of SeV-induced NF-κB activation in HEK293 cells (Fig. 6C).
FIG. 6.
Role of dsRNA, PKR, and RIG-I in induction of the antiviral response in HEK293 cells. The cells were transiently transfected with the NF-κB reporter construct and increasing amounts of DN-RIG-I (A), DN-PKR (B), or NS1 (C) and either left untreated (UT) or infected with SeV at a multiplicity of infection of 1. Sixteen hours later, the cells were lysed and luciferase activity was measured. Essentially similar results were observed for two to three independent experiments. RLU, relative luciferase units.
Thus, accumulation of dsRNA during SeV infection contributes to activation of myeloid cells through a mechanism independent of both PKR and RIG-I, whereas in nonimmune cells, the response is totally dependent on RIG-I and the presence of dsRNA.
DISCUSSION
Cellular recognition of infectious agents allows the organism to mount a host-defense response against the infecting pathogen. The cellular receptors (PRRs) responsible for sensing infections recognize specific PAMs and trigger signal transduction leading to cytokine expression and maturation of DCs. In this work, we have examined the cellular usage of PRRs for activation of the inflammatory response against the paramyxovirus SeV in both myeloid and nonimmune cells, and we show the following results. (i) SeV induced activation of NF-κB and IRF-3 as well as expression of a range of cytokines and chemokines in both myeloid cells and nonimmune cells. This was inhibited by UV treatment of the virus. (ii) Expression of a DN mutant of the TLR adaptor protein MyD88 partially inhibited SeV-induced cytokine expression in RAW 264.7 cells (myeloid cells) but had no effect in HEK293 cells (nonimmune cells). (iii) Myeloid cells expressed TLR7 and TLR8, overexpression of which elevated the response to SeV infection in HEK293 cells, and chloroquine treatment reduced the inflammatory response to SeV in myeloid cells. (iv) Expression of the dsRNA binding protein NS1 of influenza A virus and a DN mutant of RIG-I totally abrogated the cellular response of HEK293 cells to SeV infection. By contrast, in RAW 264.7 cells, expression of NS1 led to a partial inhibition of the SeV-induced response, whereas expression of DN-RIG-I did not affect the response.
We found that the capacity of SeV to elicit an antiviral cellular response in all cell lines tested was entirely dependent on the ability of the virus to replicate. This strongly indicates that a viral replication product is responsible for viral triggering of the cellular response. Expression of the influenza virus-derived dsRNA binding protein NS1 totally abrogated the response to SeV in HEK293 cells and partially inhibited the response in the myeloid cell line RAW 264.7. Thus, the data support the conclusion that UV treatment inhibits viral production of dsRNA, which is sensed by the cells to induce antiviral gene expression. A recent study has shown that maturation of DCs in response to SeV infection is also sensitive to UV treatment of the virus (20), thus demonstrating that this phenomenon is seen in a broad range of cell types.
In our study, we used UV treatment of the virus to distinguish between events induced by replicating virus and the virus particle per se. However, we cannot formally exclude that events in the infection process, which may be affected by UV treatment, also contribute to our findings, and hence it remains a possibility that parts of the replication cycle other than viral gene expression contribute to triggering the response observed. For instance, it has been reported by others that strong UV treatment of the Newcastle disease paramyxovirus destroys the hemadsorption and cell binding activity of the virus (41). However, we inactivated the virus by using only low loses of light; furthermore, our finding that the dsRNA binding protein NS1 prevented the inflammatory response completely in nonimmune cells and partially in myeloid cells also points to a central role of viral gene expression in induction of cytokine expression by invoked SeV infection.
As to the cellular PRR responsible for triggering the dsRNA-dependent response to SeV infection, recent work has shown that this effect is independent of TLRs or PKR in both conventional and plasmacytoid DCs (14, 20). We found that expression of a DN mutant of the RNA helicase RIG-I totally abrogated the cellular response to SeV in HEK293 cells but had no effect in RAW 264.7 cells. These data suggest that dsRNA is sensed by RIG-I or a related RNA helicase in HEK293 cells, triggering activation of signal transduction pathways, which stimulate expression of antiviral and inflammatory genes. It has recently been reported that activation of NF-κB and IRF-3 by Newcastle disease paramyxovirus is also suppressed by expression of DN-RIG-I (39) and that the V protein of a number of paramyxoviruses binds and inhibits the RNA helicase MDA-5 (3). Therefore, RNA helicases represent a major cellular sensor of replicating paramyxovirus and probably also other virus infections associated with accumulation of dsRNA (34).
In myeloid cells, a dsRNA-dependent TLR3-, PKR-, and RIG-I-independent mechanism was found to contribute to stimulation of the antiviral response. This mechanism, which remains to be identified, may constitute a novel cellular system to sense viral dsRNA. Recently, an evolutionarily conserved mechanism of dsRNA-dependent signaling to IRF-3 via Fas-associated death domain protein and receptor interacting protein 1 was reported (4), and it will be interesting to learn the role of this pathway in myeloid cells and also which PRRs act upstream of this signal transduction cascade. It is also possible that the dsRNA-dependent mechanism seen in myeloid cells works in concert with the viral nucleoprotein to trigger the SeV-induced response. It has been reported that the ribonuclear protein of vesicular stomatitis virus (37) and the nucleocapsid of the measles paramyxovirus induce IRF-3 activation (36), and others have shown that the latter associates with RNA when expressed recombinantly (32). Answers to these questions require further investigations.
Our finding that expression of NS1 only partially inhibited the response to SeV infection in myeloid cells, together with the observation that DN-MyD88 also reduced the response in these cells, suggested the existence of a dsRNA-independent and TLR-dependent response in myeloid cells. Accordingly, ectopic expression of TLR7 and TLR8 (but not TLR2, -3, -4, or -9) in HEK293 cells elevated SeV-induced activation of NF-κB and expression of IL-8 and IP-10. Moreover, a synthetic ligand for TLR7 and TLR8 potently activated gene expression in both murine RAW 264.7 cells and human U937 cells, hence showing that myeloid cells do indeed express TLR7 and TLR8. Finally, inhibition of TLR7 and TLR8 by chloroquine significantly reduced the response of U937 cells to SeV. Murine TLR7 and human TLR8 have recently been reported to recognize ssRNA (9, 13, 22), and since SeV is an ssRNA virus, this is likely to be the viral trigger of the TLR-dependent response in myeloid cells. However, human TLR7 does not respond to ssRNA yet did elevate the response to SeV infection. The data presented here thus show that virus infections are associated with activation of human TLR7 via ligands that remain to be identified (18).
It is of note that although we showed a contribution from TLR7 and TLR8 to induction of gene expression in myeloid cells, this induction was still sensitive to inactivation of the virus, which is in contrast to what has been reported for viral activation of plasmacytoid DCs via TLR7 and TLR9 (9, 21). However, we have recently shown that herpes simplex virus activates macrophages via a mechanism dependent on both TLR9 and a UV-sensitive signal (24). This indicates that in myeloid cells, either it is not the incoming viral genomic material that activates TLR7, -8, and -9 but rather de novo-synthesized RNA/DNA or, alternatively, a second signal which is UV sensitive cooperates with the TLRs to amplify the signal.
Altogether, in this work we have investigated the mechanisms through which cells recognize SeV infection and trigger induction of antiviral gene expression. The data presented suggest that while nonimmune cells are armed only with a system to sense cytoplasmic accumulation of SeV dsRNA via the RNA helicasae RIG-I, myeloid cells can recognize both ssRNA and dsRNA of SeV through TLR7 and TLR8 and through a novel unidentified mechanism, respectively.
Acknowledgments
This work was supported by grants from The Danish Health Science Research Council (grants no. 22-02-0144 and no. 22-03-0193), The Lundbeck Foundation, and The Carlsberg Foundation. J.M. was supported by a fellowship from the Faculty of Health Sciences, University of Aarhus.
The technical assistance by Birthe Søby, Kirsten Stadel Petersen, and Elin Jacobsen is greatly appreciated.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balachandran, S., E. Thomas, and G. N. Barber. 2004. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432:401-405. [DOI] [PubMed] [Google Scholar]
- 5.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, G., A. S. Nazar, H. S. Shin, P. Vanguri, and M. L. Shin. 1998. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent κB site but not IRF-1 or viral transcription. J. Interferon Cytokine Res. 18:987-997. [DOI] [PubMed] [Google Scholar]
- 8.Crozat, K., and B. Beutler. 2004. TLR7: a new sensor of viral infection. Proc. Natl. Acad. Sci. USA 101:6835-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 10.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald, K. A., E. M. Palsson-McDermott, A. G. Bowie, C. A. Jefferies, A. S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M. T. Harte, D. McMurray, D. E. Smith, J. E. Sims, T. A. Bird, and L. A. O'Neill. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78-83. [DOI] [PubMed] [Google Scholar]
- 12.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. O'Neill. 2003. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 14.Hornung, V., J. Schlender, M. Guenthner-Biller, S. Rothenfusser, S. Endres, K. K. Conzelmann, and G. Hartmann. 2004. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935-5943. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 16.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 17.Latz, E., A. Visintin, E. Lien, K. A. Fitzgerald, B. G. Monks, E. A. Kurt-Jones, D. T. Golenbock, and T. Espevik. 2002. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277:47834-47843. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J., T. H. Chuang, V. Redecke, L. She, P. M. Pitha, D. A. Carson, E. Raz, and H. B. Cottam. 2003. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 100:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, S., and A. E. Koromilas. 2001. Dominant negative function by an alternatively spliced form of the interferon-inducible protein kinase PKR. J. Biol. Chem. 276:13881-13890. [DOI] [PubMed] [Google Scholar]
- 20.Lopez, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 21.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggi, L. B., Jr., M. R. Heitmeier, D. Scheuner, R. J. Kaufman, R. M. Buller, and J. A. Corbett. 2000. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J. 19:3630-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173:6890-6898. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen, M., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2:349-355. [DOI] [PubMed] [Google Scholar]
- 26.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogensen, T. H., and S. R. Paludan. 2005. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J. Mol. Med. 83:180-192. [DOI] [PubMed] [Google Scholar]
- 28.Muzio, M., J. Ni, P. Feng, and V. M. Dixit. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278:1612-1615. [DOI] [PubMed] [Google Scholar]
- 29.Ohmori, Y., and T. A. Hamilton. 1993. Cooperative interaction between interferon (IFN) stimulus response element and κB sequence motifs controls IFN γ- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J. Biol. Chem. 268:6677-6688. [PubMed] [Google Scholar]
- 30.Pirhonen, J., S. Matikainen, and I. Julkunen. 2002. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J. Immunol. 169:5673-5678. [DOI] [PubMed] [Google Scholar]
- 31.Pirhonen, J., T. Sareneva, M. Kurimoto, I. Julkunen, and S. Matikainen. 1999. Virus infection activates IL-1 β and IL-18 production in human macrophages by a caspase-1-dependent pathway. J. Immunol. 162:7322-7329. [PubMed] [Google Scholar]
- 32.Schoehn, G., M. Mavrakis, A. Albertini, R. Wade, A. Hoenger, and R. W. Ruigrok. 2004. The 12 A structure of trypsin-treated measles virus N-RNA. J. Mol. Biol. 339:301-312. [DOI] [PubMed] [Google Scholar]
- 33.Siren, J., J. Pirhonen, I. Julkunen, and S. Matikainen. 2005. IFN-α regulates TLR-dependent gene expression of IFN-α, IFN-β, IL-28, and IL-29. J. Immunol. 174:1932-1937. [DOI] [PubMed] [Google Scholar]
- 34.Sumpter, R., Jr., Y.-M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.tenOever, B. R., M. J. Servant, N. Grandvaux, R. Lin, and J. Hiscott. 2002. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J. Virol. 76:3659-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.tenOever, B. R., S. Sharma, W. Zou, Q. Sun, N. Grandvaux, I. Julkunen, H. Hemmi, M. Yamamoto, S. Akira, W.-C. Yeh, R. Lin, and J. Hiscott. 2004. Activation of TBK1 and IKKɛ kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J. Virol. 78:10636-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 40.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. G. Williams. 2000. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol. Cell. Biol. 20:1278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng, J., P. Fournier, and V. Schirrmacher. 2002. Stimulation of human natural interferon-α response via paramyxovirus hemagglutinin lectin-cell interaction. J. Mol. Med. 80:443-451. [DOI] [PubMed] [Google Scholar]