Abstract
Human immunodeficiency virus type 1 (HIV-1) Nef activation of p21-activated kinase 2 (PAK-2) was recapitulated in a cell-free system consisting of in vitro-transcribed RNA, rabbit reticulocyte lysate, and microsomal membranes on the basis of the following observations: (i) Nef associated with a kinase endogenous to the rabbit reticulocyte lysate that was identified as PAK-2, (ii) Nef-associated kinase activity was detected with Nefs from HIV-1SF2, HIV-1YU2, and SIVmac239, (iii) kinase activation was not detected with a myristoylation-defective Nef (HIV-1SF2NefG2A) or with a Nef defective in PAK-2 activation but fully competent in other Nef functions (HIV-1SF2NefF195I), and (iv) Nef-associated kinase activation required activated endogenous p21 GTPases (Rac1 or Cdc42). The cell-free system was used to analyze the mechanism of Nef activation of PAK-2. First, studies suggest that the p21 GTPases may act transiently to enhance Nef activation of PAK-2 in vitro. Second, addition of wortmannin to the cell-free system demonstrated that Nef activation of PAK-2 does not require PI 3-kinase activity. Third, ultracentrifugation analysis revealed that whereas the majority of Nef and PAK-2 partitioned to the supernatant, Nef-associated PAK-2 activity partitioned to the membrane-containing pellet as a low-abundance complex. Lastly, Nef activation of PAK-2 in vitro requires addition of microsomal membranes either during or after translation of the Nef RNA. These results are consistent with a model in which activation of PAK-2 by Nef occurs by recruiting PAK-2 to membranes. As demonstrated herein, the cell-free system is a new and important tool in the investigation of the mechanism of PAK-2 activation by Nef.
The Nef proteins encoded by human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) are major determinants of viral pathogenicity. The importance of Nef in viral pathogenesis was first shown in rhesus macaques, where a large deletion of the nef gene severely reduced SIV pathogenicity (28). Furthermore, in macaques infected with SIV containing a nef gene with a premature stop codon, the virus rapidly restored the nef open reading frame, showing that there is substantial selective pressure on the virus to express Nef (28). This finding was supported by the fact that a cohort consisting of one blood donor and eight transfusion recipients infected with Nef-defective HIV type 1 (HIV-1) demonstrated dramatically decreased rates of disease progression (13, 30, 32).
Nef is a 27- to 34-kDa accessory protein expressed at high levels early in the viral life cycle (22, 41, 57). Nef is posttranslationally modified by N-terminal myristoylation, and this modification is essential for its different functions (9, 11, 35, 38). Nef is reported to alter signaling through the T-cell receptor (53), block apoptosis (60), activate Rac1 and Rac2 through DOCK2/ELMO1 (24), activate the serine/threonine kinase p21-activated kinase 2 (PAK-2) (4, 36, 44, 50), down-modulate cell surface receptors CD4 (1, 8, 20, 21) and major histocompatibility complex class I (52), and enhance viral infectivity (3, 11, 57). These last four activities of Nef are genetically separable on the basis of singly defective primary isolates and mutational analysis (19).
The ability of Nef to interact with PAK-2 is conserved in a variety of SIV and HIV-1 Nefs, including SIVmac and SIVcpz, as well as in HIV-1 M, N, and O groups (31). The activation is also known to occur in different cell types and species, including human T cells and monocytic cells (4, 18, 36). Given this widespread conservation, Nef binding and activation of PAK-2 is likely to play an important role in viral pathogenesis, though at this time cellular substrates have yet to be identified.
The cellular consequences of PAK-2 activation depend on the mechanism of activation and the cellular context in which activation occurs (47). For instance, cells respond to hyperosmolarity by inducing PAK-2 translocation to membranes prior to its activation (46). Activation of PAK-2 on membranes is transient and reversible and requires the activity of the p21-GTPase, Cdc42 (46). Cdc42 binds the p21 binding domain of PAK-2, but it is not known whether Cdc42 remains bound following activation (47). Some of the downstream consequences of PAK-2 activation by hyperosmolarity include activation of the stress-activated protein kinases SAPK/JNK and p38 (47). Stimulation of these pathways leads to phosphorylation of transcription factors and other proteins, which can eventually result in growth arrest or immune cell activation (23, 47).
The activators of PAK-2, Cdc42 and Rac1, cycle between an active and an inactive state (47). In the inactive state they are bound to GDP, cytosolic, and complexed to RhoGDI, a GDP dissociation inhibitor (12, 58). After dissociation of RhoGDI, Rac1-GDP or Cdc42-GDP attaches to membrane via a C-terminal prenyl group, allowing binding of a guanine nucleotide exchange factor such as Vav or β-PIX (58). The guanine nucleotide exchange factor opens the nucleotide binding site of the p21-GTPase, allowing dissociation of GDP and binding of GTP (58). Inactivation occurs by hydrolysis of bound GTP to GDP through intrinsic GTPase activity and can be accelerated by a GTPase-activating protein (58).
GTP-bound p21-GTPases are known to be required for PAK-2 activation by Nef on the basis of the following observations: (i) mutation of the p21-binding domain (PBD) of PAK-2 eliminates activation by Nef (45), (ii) cotransfection of Nef with constitutively active p21-GTPases enhances the Nef-associated PAK-2 activity (34, 37), and (iii) reduction of the steady-state level of activated p21-GTPases in the cell through the use of dominant negative (N17) mutants inhibits PAK-2 activation by Nef (34). The role of Nef could be to stabilize an interaction between the activated GTPase and PAK-2, to recruit PAK-2 to a membrane fraction where it can interact with activated GTPases, or to inhibit PAK-2 dephosphorylation.
To examine the mechanism of PAK-2 activation by Nef, a eukaryotic cell-free system consisting of rabbit reticulocyte lysate, microsomal membranes, and in vitro-transcribed Nef RNA was characterized. This system faithfully recapitulated fundamental aspects of PAK-2 activation by Nef, including the need for activated p21 GTPases and for membrane association of Nef. This system was then employed to gain new insights into the mechanism of PAK-2 activation by Nef.
MATERIALS AND METHODS
Plasmid construction.
The open reading frames of HIV-1SF2Nef, HIV-1SF2NefG2A, HIV-1SF2NefF195I, HIV-1YU2Nef, HIV-1NL4-3Nef, SIVmac239Nef, hemagglutinin (HA)-tagged Cdc42Q61L, Myc-tagged Rac1, Myc-tagged Rac1Q61L, and HA-tagged PAK-2 were cloned into the pTNT in vitro expression vector (Promega, Madison, WI). This vector contains a T7 promoter, a β-globin leader sequence, a 3′ untranslated region, and a poly(A) tail for optimal translation in vitro. Cell-free transcription-translation of all of the above-named constructs resulted in functional protein, except for HA-tagged PAK-2.
RNA synthesis.
Constructs were linearized with either BglI or BamHI, purified, and used as templates for in vitro transcription reactions with a T7 transcription kit (Ambion, Austin, TX). The concentration of the resulting capped transcripts was estimated by UV absorbance, and the integrity of the RNA was verified by ethidium bromide staining of a formaldehyde-agarose gel. RNA was stored at −80°C with RNasin (Promega).
Cell-free translation.
Rabbit reticulocyte lysate translation reactions (Promega) with canine pancreatic microsomal membranes (Promega) were performed per the manufacturer's instructions with the following exceptions: reactions were performed with 1.2 pmol RNA; the final volume of the translation reaction mixtures was 15 μl, and the reaction mixtures were incubated for 25 min at 30°C. Rabbit reticulocyte lysates contain isoprenyl transferase but are limiting for the prenyl group (5, 29, 49). Because geranylgeranylation of Rac1 and Cdc42 is required for their membrane insertion and activation and for most of their known cellular functions (2, 10, 17, 25), cell-free translation reactions of p21-GTPase transcripts were supplemented with geranylgeranyl pyrophosphate (Sigma, Saint Louis, MO) (100 μM). In some cases as indicated in the text, cell-free translation reactions were supplemented with exogenous GTP (Sigma) (200 μM) and were performed using [35S]methionine (Amersham Biosciences, Piscataway, NJ). After incubation of the translation reaction mixtures, 2.5 μl of the 15-μl reaction mixture was used to verify protein expression by either Western blot analysis or autoradiography. The remainder of the reaction was immunoprecipitated and used for the in vitro kinase assay.
Western blot analysis.
HIV-1 and SIV Nef expression was determined with sheep polyclonal anti-HIV-1 or anti-SIV Nef serum (1:3,000) followed by horseradish peroxidase (HRP)-conjugated rabbit anti-sheep immunoglobulin G (IgG; Chemicon International Inc., Temecula, CA) (1:10,000). The presence of glutathione S-transferase (GST), GST-PBD, and GST-PBDH84LH87L in translation reaction mixtures was confirmed by Western blot analysis with mouse monoclonal anti-GST antibodies (B-14; Santa Cruz Biotechnology, Santa Cruz, CA), followed by HRP-conjugated goat anti-mouse IgG (1:10,000; Zymed, San Francisco, CA). PAK-2 expression was detected using a combination of N-19 and C-19 antibodies (sc-1872 and sc-1519; Santa Cruz Biotechnology), both at a 1:100 dilution, followed by HRP-conjugated bovine anti-goat IgG (1:5000).
In vitro kinase assay.
The assay for the Nef-associated kinase was performed essentially as described previously (4, 19, 36, 50), using 12.5 μl of the 15-μl translation reaction mixture. HIV-1 Nef and SIV Nef were immunoprecipitated from the cell-free translation reaction mixtures with sheep polyclonal anti-HIV-1 Nef and anti-SIV Nef antibodies, respectively. PAK-2 was immunoprecipitated with the N20 antibody (Santa Cruz Biotechnology), and the p21-GTPases were immunoprecipitated with either a mouse monoclonal anti-Myc antibody (Zymed) or a mouse monoclonal anti-HA antibody (Covance, Berkeley, CA). Immunoprecipitates were resuspended in kinase assay buffer (50 mM Tris-HCl [pH 7.5], 100 mM sodium chloride, 5 mM magnesium chloride, and 1% [vol/vol] Triton X-100). Subsequently, 30 μCi of [γ-32P]ATP (Perkin Elmer, Boston, MA) was added and reaction mixtures were incubated for 10 min at 30°C. Kinase reactions were stopped by the addition of EDTA (45 mM).
Quantitation of in vitro kinase reactions.
Differences in in vitro kinase reactions were quantitated from at least three different experiments using a Cyclone storage phosphor system (Packard, Meriden, CT).
Substrate phosphorylation assay.
Phosphorylation of myelin basic protein (MBP) (bovine brain; Sigma) and histone 4 (H4) (calf thymus; Roche Diagnostics, Mannheim, Germany) was assayed by the addition of substrate (10 μg) to anti-Nef immunoprecipitates prior to the in vitro kinase assay (39). To eliminate phosphorylation of the substrates by other kinases present in the cell-free translation reactions, a preclearing step was required. Thus, after translation, samples were incubated with sheep serum (10 μl) for 1 h at 4°C; protein A beads were added (80 μl of a 1:1 slurry), and then the samples were rotated for 1 h at 4°C. After a brief centrifugation, the supernatant was removed and immunoprecipitated with sheep polyclonal anti-HIV-1 or anti-SIV Nef antibodies. Immunoprecipitates were washed five times with lysis buffer (50 mM Tris-HCl [pH 8.0], 2 mM EDTA [pH 8.0], 0.5% [vol/vol] IGEPAL CA-630, 10% [vol/vol] glycerol, 100 mM sodium chloride, 25 mM sodium fluoride, 2 mM sodium vanadate, 20 mM β-glycerophosphate, 25 mM benzamidine), once with 1 M magnesium chloride, and five times with kinase assay buffer.
Caspase 3 treatment.
After the kinase assay, caspase 3 digestions were performed as described previously (4). Briefly, kinase reaction mixtures were placed on ice and then washed twice with ice-cold caspase 3 buffer (50 mM HEPES [pH 7.5], 100 mM sodium chloride, 0.1% [vol/vol] Triton X-100, 5 mM dithiothreitol, 20 mM sodium fluoride, 2 mM sodium vanadate, 20 mM β-glycerophosphate). The samples were then mock treated, treated with caspase 3 (kindly provided by Xiaodong Wang) or treated with caspase 3 plus the caspase inhibitor zVAD (kindly provided by Xiaodong Wang) and incubated for 30 min at 37°C. Reactions were stopped by the addition of 1.5× Laemmli protein loading buffer, and the proteins were resolved by polyacrylamide gel electrophoresis.
GST, GST-PBD, and GST-PBDH84LH87L proteins.
The PBD of wild-type PAK-2 and of a PAK-2 mutant with two amino acid changes from histidine to leucine at positions 84 and 87 was PCR amplified using the following primer sequences: 5′-CCGGATCCAAGGAACGGCCAGAAATTTCTCCTCC-3′ and 5′-TTCTCGAGGTAGAACTTTAGGACATCCAGCACAGC-3′. PCR products were isolated and cloned into BamHI/XhoI restriction enzyme sites of pGEX-4T-1 (Amersham Biosciences, Piscataway, NJ). pGEX-4T-1 plasmids expressing GST, GST-PBD, and GST-PBDH84LH87L were transformed into BL21 Escherichia coli cells, and proteins were purified using glutathione Sepharose 4B (Amersham Biosciences) per the manufacturer's instructions.
PI 3-kinase assay.
Phosphatidylinositol (PI) substrate (Avanti Polar Lipids, Alabaster, Alabama) was diluted to a final concentration of 1 μg/μl in buffer (25 mM HEPES [pH 7.4], 1 mM EDTA) and then sonicated for 20 min in a bath sonicator. Cell-free translation reaction mixtures (15 μl) or 293T cellular lysates (600 μg of protein) were immunoprecipitated with anti-PI 3-kinase (class 1a p85α) antibodies (Upstate Cell Signaling, Lake Placid, NY) (2 μl). Immunoprecipitates were washed three times with lysis buffer (137 mM sodium chloride, 20 mM Tris-HCl [pH 7.4], 1 mM calcium chloride, 1 mM magnesium chloride, 0.1 mM sodium orthovanadate, and 1% IGEPAL CA-630) and once with 10 mM Tris, pH 7.4. Immunoprecipitates were resuspended in 45 μl of kinase reaction buffer (10 mM MgCl2, 50 mM Tris, pH 7.4); the phosphatidylinositol substrate (5 μg) and [γ-32P]ATP (20 μCi) were added, and reaction mixtures were incubated at room temperature for 20 min. Reactions were stopped by the addition of 1 N HCl (100 μl), and the lipids were extracted using CHCl3-MeOH (1:1; 200 μl). The organic phase was washed with MeOH-(100 mM HCl, 2 mM EDTA) (1:1; 80 μl), and the aqueous phase was discarded. The lipid product (10 μl) was spotted on a silica gel 150 thin-layer chromatography plate (Whatman, Florham Park, NJ), and the products were resolved by thin layer chromatography using a mobile phase consisting of CHCl3-MeOH-H2O-NH4OH (45:35:8.5:1.5).
Cell culture conditions, plasmid expression constructs, transfections, and inhibitor assays.
293T cells were cultured in Dulbecco's modified Eagle's medium (Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (Cellgro), 100 IU penicillin/ml, 100 μg streptomycin/ml, and 0.2 mg/ml glutamine (Cellgro) and were maintained at 37°C in a humidified incubator with 10% carbon dioxide. Plasmid expression constructs used for transient transfection of 293T cells included pcDNA3.1 empty vector (Invitrogen, Carlsbad, CA) and pcDNA3.1HIV-1SF2Nef. Transfections were performed using Lipofectamine 2000 (Invitrogen) per the manufacturer's instructions. At 36 to 48 h after transfection two different concentrations of the PI 3-kinase inhibitor wortmannin (Calbiochem, San Diego, CA) (10 nM and 100 nM) were added to the cell culture. Cells were incubated 5 h with wortmannin and then harvested for Western blot analysis (200 μg of protein) and the in vitro kinase assay (600 μg of protein).
Ultracentrifugation analysis.
After incubation, cell-free translation reaction mixtures were diluted in ice-cold spin solution (final volume = 200 μl; 150 mM potassium acetate, 1 mM magnesium acetate, 2 mM dithiothreitol). As a positive control, half of the reaction mixture was taken and placed on ice. The remaining 100 μl was placed in prechilled noncollapsible 11- by 34-mm polycarbonate centrifuge tubes (Beckman, Palo Alto, CA) and ultracentrifuged at 48,000 × g for 30 min at 4°C using an MLA-130 fixed-angle rotor (Beckman) and an Optima MAX (Beckman) ultracentrifuge. The supernatant and pellet fractions were taken (after rinsing the pellet fraction with ice-cold spin solution) and immunoprecipitated along with the total reaction mixture for the in vitro kinase assay and Western blot analysis.
RESULTS
Expression of Nef in vitro and association of Nef with a kinase endogenous to the cell-free translation system.
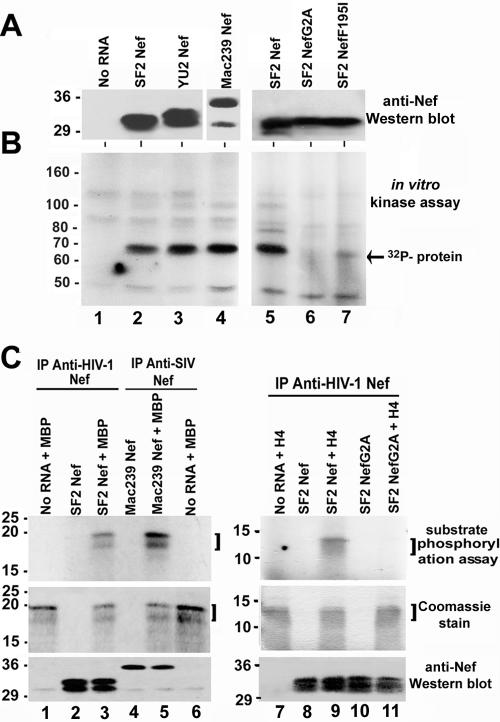
Since most of the known Nef functions in cell lines require Nef myristoylation and membrane insertion (9, 11, 38, 40), we anticipated that to study Nef function in vitro, myristoylation and a source of membranes would be necessary. The rabbit reticulocyte lysate translation system was chosen because it contains N-myristoyl-transferase and myristic acid (5, 15, 16, 55), and the reaction mixtures were supplemented with microsomal membranes. Cell-free translation reactions were first optimized for RNA concentration, time of incubation, and magnesium and GTP concentration (data not shown). Different Nefs were expressed by cell-free translation, including HIV-1SF2 (subtype B), HIV-1YU2 (primary isolate; subtype B), and SIVmac239 (pathogenic isolate). As depicted in Fig. 1A, a portion of the cell-free translation reaction mixture was used to verify protein expression by Western blotting using either an anti-HIV-1 Nef antibody (subtype B) or an anti-SIV (mac239) Nef antibody as appropriate. Nef protein was detected for each of the Nef RNAs used in this system but not in a reaction lacking exogenous Nef RNA (Fig. 1A, lane 1). Translation of the Nef proteins in some instances resulted in a doublet, which was probably a result of initiation from the second in-frame initiation codon present in some Nefs and may also reflect lysate specific differences in recognition of the initiation codon (26, 42). The remainder of the cell-free translation reaction mixture was immunoprecipitated with saturating amounts of anti-Nef antibodies and used in an in vitro kinase assay. Preliminary studies demonstrated that approximately 90% of the total Nef protein translated in the cell-free reactions is immunoprecipitated under the conditions used (data not shown). All three Nefs tested associated with an endogenous kinase activity, as determined by the presence of a 32P-labeled band after the in vitro kinase assay (Fig. 1B, lanes 2 to 4) that was not present in immunoprecipitates from a translation reaction to which no Nef RNA was added (Fig. 1B, lane 1). The Nef-associated kinase activity was not detected in immunoprecipitates from a translation reaction containing Nef with a defective myristoylation signal (HIV-1SF2NefG2A; lane 6). Nef-associated kinase activity was also significantly lower with a Nef mutant previously shown to be defective in PAK-2 kinase activation but not in other Nef functions (HIV-1SF2NefF195I; lane 7) (19). These results demonstrate the presence of a kinase activity in the cell-free translation system that associates with Nef.
FIG. 1.
Association of HIV-1 and SIV Nef with a kinase endogenous to the cell-free translation system. Cell-free translations were performed without exogenous RNA (lane 1) or with HIV-1SF2Nef RNA (lanes 2 and 5), HIV-1YU2Nef RNA (lane 3), SIVmac239Nef RNA (lane 4), HIV-1SF2NefG2A RNA (lane 6), or HIV-1SF2NefF195I RNA (lane 7). (A) Western blots with subtype B anti-HIV-1 (left and right panels) or anti-SIVmac239 (middle panel, lane 4) Nef antibodies. (B) In vitro kinase assays of the anti-Nef immunoprecipitations described for panel A (the arrow points toward the most prominent 32P-labeled band). Kinase assays were performed at least three different times with similar results. (C) Substrate phosphorylation assays using MBP (left panels) or H4 (right panels). Upper panel, autoradiograph of the substrate phosphorylation assay; middle panel, a Coomassie stain of the same gel; lower panel, Western blot using a combination of anti-HIV-1 and anti-SIV Nef antibodies. The data from four different experiments indicate that MBP is a better substrate for PAK-2 phosphorylation than PAK-2 itself, and the data from two different experiments indicate that H4 and PAK-2 are phosphorylated to similar levels (PAK-2 data not shown).
Phosphorylation of MBP and H4 by the Nef-associated kinase.
To characterize the Nef-associated kinase activity present in the cell-free translation reactions, the exogenous substrates MBP and H4 were included in the kinase reaction. Cell-free translation reactions were carried out with HIV-1SF2 Nef, SIVmac239Nef, or HIV-1SF2NefG2A transcripts. Reaction mixtures were immunoprecipitated with anti-Nef antibodies, and substrate phosphorylation assays were performed with MBP or H4 (Fig. 1C). 32P-labeled substrates were detected in reaction mixtures containing MBP and HIV-1SF2Nef (lane 3), MBP and SIVmac239Nef (lane 5), and H4 and HIV-1SF2Nef (lane 9). There were no specific 32P-labeled products in reaction mixtures lacking added RNA (lanes 1 and 7), reaction mixtures without added substrate (lanes 2, 4, 8, and 10), or reaction mixtures with a myristoylation-defective Nef (lane 11). Importantly, the 32P-labeled bands comigrated with the position of the MBP or H4 substrates, as determined by Coomassie staining of the polyacrylamide gels (Fig. 1C, middle panels). These results demonstrate that the Nef-associated kinase can transphosphorylate model substrates.
Identification of the kinase associated with Nef in the cell-free translation reactions as PAK-2.
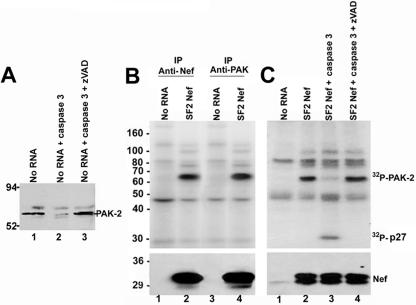
The kinase associated with Nef in the translation reactions migrates to a position in the polyacrylamide gel similar to that seen with activated PAK-2 (44, 45). To examine whether PAK-2 is an endogenous component of the cell-free translation system, translation reaction mixtures containing microsomal membranes were first analyzed by Western blotting using anti-PAK antibodies. As shown in Fig. 2A, an immuno-cross-reactive band of the appropriate molecular weight was detected (lane 1). To determine whether the immuno-cross-reactive band was PAK-2, an enzymatic digestion with caspase 3 was carried out because PAK-2 but not PAK-1 contains a site for caspase 3 cleavage (48, 56). As shown in Fig. 2A, the intensity of the immuno-cross-reactive band was significantly reduced after treatment with caspase 3 (lane 2). The specificity of this cleavage was confirmed by the addition of the caspase inhibitor, zVAD, to the caspase digestion, which blocked cleavage of this band (lane 3). These results are consistent with the presence of PAK-2 in the cell-free translation system.
FIG. 2.
Identification of the kinase present in the cell-free translation reaction mixtures as PAK-2. (A) Western blot analysis of the cell-free translation reactions by use of anti-PAK-2 antibodies. Lane 1 was mock treated, lane 2 was treated with caspase 3, and lane 3 was treated with caspase 3 plus the caspase inhibitor zVAD. (B) Cell-free translation reactions were performed without exogenous RNA or with HIV-1SF2Nef RNA. After incubation, reaction mixtures were immunoprecipitated with sheep anti-Nef antibodies (lanes 1 and 2) or the anti-PAK antibody N20 (lanes 3 and 4) and an in vitro kinase assay was performed (upper panel). (Lower panel) Nef protein expression was verified by Western blot analysis. (C) Translation reactions were performed without exogenous RNA or with HIV-1SF2Nef RNA. (Upper panel) Reaction mixtures were immunoprecipitated with anti-Nef antibodies, and an in vitro kinase assay was carried out. After the kinase assay a caspase 3 enzymatic digestion was performed. Lanes 1 and 2 were mock treated, lane 3 was incubated with caspase 3, and lane 4 was incubated with caspase 3 and the caspase inhibitor zVAD. 32P-labeled PAK-2 and the diagnostic 32P-p27 fragment are indicated (32P-p27 migrates as a 32-kDa band). (Lower panel) Nef protein expression was verified by Western blotting. These experiments were performed at least three different times with similar results.
In cell lines, Nef activation of PAK-2 occurs in a low-abundance complex (4, 43, 45) that can only be detected by the very sensitive in vitro kinase assay (51). Thus, to determine whether the kinase endogenous to the cell-free translation system is a member of the PAK family, cell-free translation reaction mixtures were immunoprecipitated with either an anti-Nef antibody or with an antibody that recognizes both PAK-1 and PAK-2 (44, 45), and an in vitro kinase assay was performed. As shown in Fig. 2B, upper panel, kinase activity was detected in reaction mixtures containing HIV-1SF2Nef RNA when either anti-Nef or anti-PAK antibodies were used for immunoprecipitation (lanes 2 and 4), suggesting that the Nef-associated kinase is immuno-cross-reactive with members of the PAK family of serine/threonine kinases. Kinase activity was not detected when cell-free translation reaction mixtures lacking exogenous Nef RNA were immunoprecipitated with either anti-Nef or anti-PAK antibodies (lanes 1 and 3), demonstrating that the endogenous kinase is inactive without exogenous Nef. Finally, to distinguish between PAK-1 and PAK-2, caspase 3 enzymatic digestions were performed after the in vitro kinase reactions. PAK-2 is cleaved by caspase 3 into an N-terminal fragment (p27) that contains most of the kinase regulatory domain and a C-terminal fragment containing the catalytic domain (4, 56). Autophosphorylation of serine residues in the N-terminal regulatory region following activation results in the presence of a 32-kDa fragment in the in vitro kinase assay (32P-p27) (4, 56). Incubation of caspase 3 with the products of an in vitro kinase reaction mixture containing HIV-1SF2Nef resulted in cleavage of the Nef-associated kinase and the appearance of the expected 32P-p27 fragment of PAK-2 (Fig. 2C, lane 3). In contrast, no cleavage was detected in reaction mixtures lacking caspase 3 (lane 2) or when a caspase inhibitor, zVAD, was coincubated with caspase 3 (lane 4). Altogether, these results identify the kinase associated with Nef in the cell-free translation reactions as PAK-2.
Role of the p21-GTPases Rac1 and Cdc42 in PAK-2 activation by Nef in vitro.
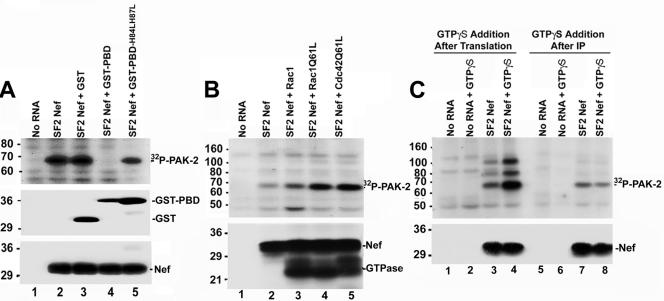
PAK-2 activation by Nef in 293T cells requires activated Rac1 or Cdc42 (34, 37, 45). To assess the role of p21-GTPases in the in vitro activation of PAK-2 by Nef, cell-free translation reaction mixtures containing HIV-1SF2Nef RNA were incubated with a protein that binds the activated (GTP-bound) forms of Cdc42 and Rac1 (6, 7) and sequesters them from any potential involvement in PAK-2 activation. This protein contains the p21-binding domain of PAK-2 fused to GST. As shown in Fig. 3A, addition of GST-PBD resulted in complete inhibition of the PAK-2 kinase activity associated with Nef (lane 4). In contrast, when a fusion protein containing a PBD defective in binding activated GTPases (GST-PBDH84LH87L) was used in this assay PAK-2 kinase activity was detected (lane 5).
FIG. 3.
Role of p21-GTPases (Rac1 and Cdc42) in PAK-2 activation by Nef in vitro. (A) Cell-free translation reaction mixtures without exogenous RNA or with HIV-1SF2Nef RNA were incubated with no exogenous protein, GST (2 μg), GST-PBD (1 μg), or GST-PBDH84LH87L (2 μg) as indicated. (Top panel) Reaction mixtures were immunoprecipitated with anti-Nef antibodies and an in vitro kinase assay was performed. (Middle panel) A portion of the reaction mixtures was analyzed by Western blotting with anti-GST antibodies. The positions of GST-PBD/GST-PBDH84LH87L and GST proteins are indicated. (Lower panel) Anti-Nef Western blot. (B) Cell-free translation reactions were performed without exogenous RNA or with HIV-1SF2Nef, Myc-Rac1, Myc-Rac1Q61L, or HA-Cdc42Q61L transcripts. Translation reaction mixtures were supplemented with geranylgeranyl pyrophosphate, GTP, and [35S]methionine. Cell-free reaction mixtures expressing Nef were combined with a reaction mixture lacking exogenous RNA or with reaction mixtures expressing a p21 GTPase as indicated and incubated for 15 min. After incubation, a portion of the reaction was used to verify Nef and p21 GTPase protein expression by autoradiography. The remainder of the reaction was immunoprecipitated with anti-HIV-1 Nef antibodies and used in the in vitro kinase assay. (Upper panel) In vitro kinase assay; (lower panel) autoradiograph to verify protein expression. The positions of HIV-1SF2Nef and the p21-GTPases are indicated. (C) Cell-free translation reactions were carried out without exogenous RNA or with HIV-1SF2Nef RNA. (Upper panel, lanes 1 to 4) After incubation of the translation reactions for 25 min, GTPγS (final concentration, 200 μM; lanes 2 and 4) or an equal volume of water (lanes 1 and 3) was added, and reaction mixtures were incubated an additional 10 min at 30°C. Reaction products were immunoprecipitated with anti-Nef antibodies, and an in vitro kinase assay was performed. (Lanes 5 to 8) After translation, reaction mixtures were immunoprecipitated with anti-Nef antibodies. Immunoprecipitates were washed and resuspended. Subsequently, either GTPγS (final concentration, 200 μM; lanes 6 and 8) or an equal volume of water (lanes 5 and 7) was added and immunoprecipitates were incubated 10 min at 30°C. After washing in ice-cold kinase assay buffer, in vitro kinase reactions were performed. (Lower panel) HIV-1SF2Nef protein expression was verified by Western blotting. These experiments were performed three different times with similar results.
The contribution of exogenous Rac1 and Cdc42 to PAK-2 activation by Nef was then evaluated. In Fig. 3B, translation reactions either without added RNA or with RNA expressing HIV-1SF2Nef, wild-type Rac1, constitutively active Rac1 (Q61L), or constitutively active Cdc42 (Q61L) were performed. After incubation, cell-free translation reaction mixtures expressing Nef were combined with reaction mixtures containing no exogenous RNA (Fig. 3B, lane 2) or with reaction mixtures expressing a p21 GTPase (Fig. 3B, lanes 3 to 5), and the reaction mixtures were incubated an additional 15 min. After incubation, part of the reaction was immunoprecipitated with anti-HIV-1 Nef antibodies and an in vitro kinase assay was performed (Fig. 3B, upper panel), and part of the cell-free translation reaction mixture was used to verify Nef and p21 GTPase protein expression (Fig. 3B, lower panel). Expression of wild-type Rac1 had a modest effect on the PAK-2 kinase activity associated with Nef (1.6- ± 0.4-fold difference; compare lanes 2 and 3 of Fig. 3B). Expression of the constitutively active GTPases resulted in a 3.0- ± 0.3-fold increase in the PAK-2 activity associated with Nef (Fig. 3B; compare lane 2 with lanes 4 and 5). The effect of exogenous GTPγS on PAK-2 activation by Nef in vitro was then evaluated. GTPγS is a nonhydrolyzable GTP analog that is expected to increase the steady-state level of activated GTPases endogenous to the cell-free translation system. Addition of GTPγS after incubation of the translation reaction mixtures resulted in a 4.8- ± 0.5-fold increase in the PAK-2 activity associated with Nef (Fig. 3C; compare lanes 3 and 4). Consistent with its role in activating p21s and not on PAK-2 directly, GTPγS had no discernible effect on PAK-2 activity when added after immunoprecipitation of the cell-free translation reaction mixtures (Fig. 3C; compare lanes 7 and 8). Altogether, these results demonstrate the requirement for activated GTPases in PAK-2 activation by Nef in vitro and indicate that activated GTPases are limiting in the cell-free system.
Analysis of the presence of p21-GTPases in the Nef/PAK-2 complex.
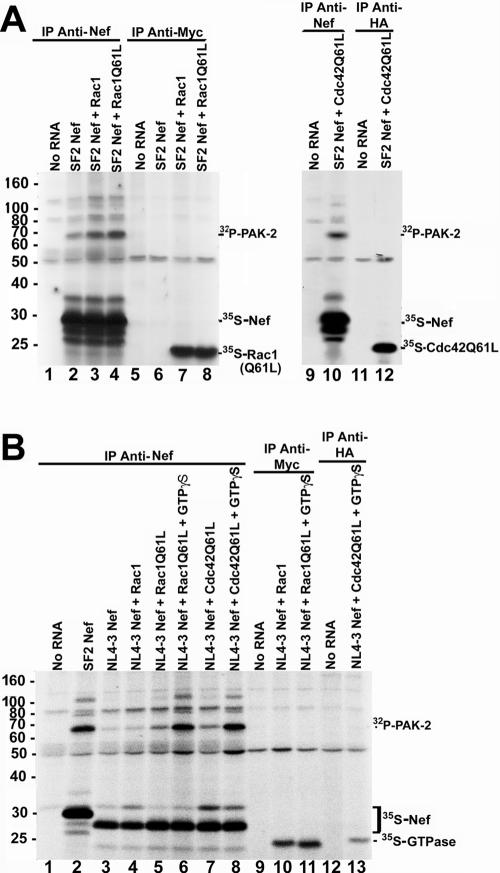
To address whether activated p21-GTPases are components of the Nef/PAK-2 complex, cell-free reaction mixtures expressing HIV-1SF2Nef were incubated with reaction mixtures expressing wild-type or constitutively active tagged p21-GTPases in the presence of [35S]methionine. Half of the reaction mixture was immunoprecipitated with anti-Nef antibodies and half with anti-tag antibodies (anti-Myc for Myc-tagged Rac1 and Rac1Q61L and anti-HA for HA-tagged Cdc42Q61L). In similarity to the results in Fig. 3B, addition of exogenous GTPases enhanced the PAK-2 activity immunoprecipitated with Nef (Fig. 4A, lanes 2 to 4). However, although the anti-Nef immunoprecipitations efficiently brought down 35S-Nef (Fig. 4A, lanes 2 to 4), no 35S-labeled p21 GTPases were present in the anti-Nef immunoprecipitates. When translation reaction mixtures were immunoprecipitated with anti-Myc or anti-HA antibodies, the [35S]methionine-labeled GTPases were clearly immunoprecipitated. However, in these reactions no PAK-2 activity or 35S-Nef was immunoprecipitated (Fig. 4A, lanes 7, 8, and 12). Since the stability of the putative Nef/PAK-2/p21-GTPase complex could be different depending on the Nef used for these experiments, a similar experiment was performed with HIV-1NL4-3Nef (Fig. 4B). HIV-1NL4-3Nef was chosen because the requirement of p21-GTPases in HIV-1NL4-3Nef activation of PAK-2 has been characterized in the literature (43, 45). Consistent with in vivo studies (4, 43), activation of PAK-2 by HIV-1NL4-3Nef results in much weaker kinase activity compared to that seen with HIV-1SF2Nef (Fig. 4B; compare lanes 2 and 3). p21-GTPases enhanced the phosphorylation activity of the HIV-1NL4-3Nef/PAK-2 complex (lanes 4 to 5 and 7), and the addition of GTPγS resulted in a further increase in the PAK-2 activity associated with HIV-1NL4-3Nef (lanes 6 and 8). As with HIV-1SF2Nef immunoprecipitates, no 35S-labeled p21 GTPases were detected in the HIV-1NL4-3Nef immunoprecipitates. Furthermore, no PAK-2 kinase activity or [35S]Nef was found in the anti-HA or anti-Myc immunoprecipitations (lanes 10 to 11 and 13). The observations that the p21-GTPases are required for PAK-2 activation by Nef in vitro but that neither Rac1 nor Cdc42 immunoprecipitates with the active Nef/PAK-2 complex suggest a transient role for the activated GTPases in PAK-2 activation by Nef in vitro.
FIG. 4.
The active Nef/PAK-2 complex formed in the cell-free system can be immunoprecipitated with anti-Nef antibodies but not with anti-p21-GTPase antibodies. (A) Cell-free translation reactions were performed either without exogenous RNA or with RNA expressing HIV-1SF2Nef, Myc-Rac1, Myc-Rac1Q61L, or HA-Cdc42Q61L. Translation reaction mixtures were supplemented with geranylgeranyl pyrophosphate, GTP, and [35S]methionine. Cell-free reaction mixtures expressing Nef were combined either with a reaction mixture lacking added RNA or with reaction mixtures expressing a p21 GTPase as indicated. Reaction mixtures were incubated for 15 min and immunoprecipitated with anti-Nef, anti-Myc, or anti-HA antibodies, and then an in vitro kinase assay was performed. The positions of immunoprecipitated 32P-PAK-2, 35S-Nef, and 35S-p21-GTPases (either Myc-Rac1, Myc-Rac1Q61L, or HA-Cdc42Q61L) are indicated. (B) Cell-free translation reactions were performed without exogenous RNA or with RNA expressing HIV-1SF2Nef, HIV-1NL4-3Nef, Myc-Rac1, Myc-Rac1Q61L, or HA-Cdc42Q61L. Translation reaction mixtures were supplemented with geranylgeranyl pyrophosphate, GTP, and [35S]methionine. After incubation, cell-free reaction mixtures expressing HIV-1NL4-3Nef were combined with a reaction mixture lacking exogenous RNA or with reaction mixtures expressing a p21 GTPase as indicated. In some cases as indicated in the figure, GTPγS (200 μM) or an equal volume of water was added to the reaction mixtures, and reaction mixtures were incubated an additional 15 min at 30°C. Reaction mixtures were immunoprecipitated using anti-Nef, anti-Myc, or anti-HA antibodies as indicated, and an in vitro kinase assay was performed. The positions of 32P-PAK-2, immunoprecipitated 35S-Nef, and immunoprecipitated 35S-p21-GTPases are indicated on the right.
Role of PI 3-kinase on PAK-2 activation by Nef in the cell-free translation system.
PI 3-kinase is an upstream activator of the guanine nucleotide exchange factors Vav and β-PIX (14, 27, 58), and it has been implicated in the activation of PAK-2 by Nef (33, 60). Immunoprecipitation of a cell-free translation reaction mixture lacking exogenous RNA with anti-PI 3-kinase antibodies (class 1a; p85α) followed by a PI 3-kinase assay resulted in 32P-labeled phosphatidylinositol 3-phosphate (data not shown). However, 32P-labeled phosphatidylinositol 3-phosphate was not detected when a cell-free translation reaction was incubated with the PI 3-kinase inhibitor wortmannin (25 nM) (data not shown). These results demonstrate the presence of active PI 3-kinase in the cell-free translation system. To examine the role of PI 3-kinase in PAK-2 activation by Nef in vitro, wortmannin was added prior to incubation of the translation reaction mixtures (data not shown). No discernible effect of wortmannin on PAK-2 activation by Nef in vitro was observed. To examine whether these results were only pertinent to the cell-free system, 293T cells were transfected with an HIV-1SF2Nef expression vector and the effect of two different concentrations of wortmannin (10 nM and 100 nM) was examined. Despite the fact that PI 3-kinase activity was inhibited by wortmannin, wortmannin did not have an effect on PAK-2 kinase activation by HIV-1SF2Nef in 293T cells (data not shown). These results indicate that PI 3-kinase activity is not required for PAK-2 activation by HIV-1SF2Nef either in the cell-free translation system or in 293T cells.
Role of membranes in the in vitro activation of PAK-2 by Nef.
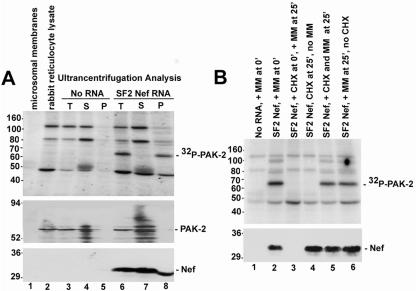
To examine the role of membranes in the activation of PAK-2 by Nef, Western blot analysis was first used to determine whether PAK-2 was present in the rabbit reticulocyte lysate or in the microsomal membranes (Fig. 5A, middle panel). Consistent with previous observations (54), PAK-2 was detected in the rabbit reticulocyte lysate (lane 2). Also in Fig. 5A, the results of ultracentrifugation analysis of cell-free translation reaction mixtures containing rabbit reticulocyte lysate, microsomal membranes, and either no exogenous RNA (lanes 3 to 5) or HIVSF2Nef RNA (lanes 6 to 8) are shown. In translation reactions lacking exogenous Nef RNA, PAK-2 activity was not detected in the total (lane 3), supernatant (lane 4), or pellet (lane 5) fractions. This is in contrast to the results of ultracentrifugation analysis of translation reaction mixtures containing HIV-1SF2Nef RNA. In comparisons of the total (lane 6), supernatant (lane 7), and pellet (lane 8) fractions, the in vitro kinase assay indicated that all of the Nef-associated PAK-2 activity partitioned to the membrane-containing pellet (upper panel, lane 8). However, Western blot analysis using anti-Nef or anti-PAK-2 antibodies demonstrated that the majority of PAK-2 and Nef partitioned to the supernatant (middle and lower panels, lane 7). These results are consistent with data in cell lines showing that activation of PAK-2 by Nef occurs in a low-abundance complex associated with cellular membranes (4, 43, 45).
FIG. 5.
Role of membranes in PAK-2 activation by Nef in the cell-free translation system. (A) Lane 1, microsomal membranes; lane 2, rabbit reticulocyte lysate. In lanes 3 to 5, a cell-free translation reaction containing both rabbit reticulocyte lysate and microsomal membranes was performed without exogenous RNA. After translation, half of the reaction mixture was removed and used as a positive control (T, total) (lane 3). The remainder of the reaction mixture was ultracentrifuged, and the supernatant (S) (lane 4) and pellet (P) (lane 5) fractions were collected. In lanes 6 to 8, a cell-free translation reaction containing HIV-1SF2Nef RNA was carried out. After incubation, half of the reaction mixture was removed and used as the positive control (T) (lane 6). The remainder of the reaction mixture was ultracentrifuged, and the supernatant (S) (lane 7) and pellet (P) (lane 8) fractions were collected. All samples were analyzed by in vitro kinase assay (upper panel) and Western blot analysis using anti-PAK-2 antibodies (middle panel) and anti-Nef antibodies (lower panel). (B) Cell-free translation reactions were performed containing either no added RNA (lane 1) or HIV-1SF2Nef RNA (lanes 2 to 6). In lanes 1 to 2, translation reactions were performed as previously described; i.e., microsomal membranes were added prior to incubation of the translation reaction mixtures. In lanes 3, 5, and 6, microsomal membranes were added after the 25-min translation incubation. In lanes 4 to 5, cycloheximide was also added to inhibit further translation. To examine the efficiency of translation inhibition, cycloheximide (1 mM) was added prior to the 25-min translation incubation (lane 3). In all cases, reaction mixtures were incubated an additional 15 min prior to in vitro kinase assay (upper panel) and Western blot analysis with anti-Nef antibodies (lower panel). Experiments represented in panels A and B were performed three different times with similar results.
To examine the requirement for microsomal membranes for Nef activation of PAK-2 in vitro and whether membranes are required for cotranslational processing of Nef or for an event that occurs posttranslationally, rabbit reticulocyte lysate reaction mixtures containing HIVSF2Nef RNA but lacking microsomal membranes were incubated for 25 min (Fig. 5B). After incubation, cycloheximide was added to inhibit further translation, microsomal membranes were added, and reaction mixtures were incubated an additional 15 min. For comparison, translation reactions were also performed as described above; i.e., the microsomal membranes were added prior to incubation of the translation reaction mixtures (lanes 1 to 2). Cycloheximide efficiently inhibited translation as determined by the absence of Nef protein when cycloheximide was added prior to incubation of a translation reaction mixture containing Nef RNA (lane 3). As shown in Fig. 5B (compare lanes 2, 5, and 6), Nef-associated PAK-2 activity was observed regardless of whether the microsomal membranes were added during Nef translation or after Nef translation was inhibited by the addition of cycloheximide. Altogether, these results are consistent with a model in which Nef recruits PAK-2 to membranes where kinase activation can occur.
DISCUSSION
Here we report the activation of a kinase endogenous to the rabbit reticulocyte lysate translation system by HIV-1 and SIV Nef. This kinase was identified as PAK-2 on the basis of the following evidence: (i) PAK-2 has been shown to be present in rabbit reticulocytes (54), (ii) rabbit PAK-2 and human PAK-2 amino acid sequences are 91% identical, (iii) the electrophoretic mobility of the Nef-associated kinase from the cell-free translation reaction mixtures was similar to that seen with PAK-2 (44, 45), (iv) the Nef-associated kinase activity in the cell-free translation system phosphorylated model substrates known to be phosphorylated by the Nef-associated kinase MBP and H4 (39), (v) Nef-associated kinase activity was immunoprecipitated with anti-PAK antibodies, and (vi) caspase 3 digestion after the in vitro kinase assay resulted in the cleavage of the Nef-associated kinase and the appearance of the diagnostic 32P-labeled 27-kDa fragment (4, 56). Activation of PAK-2 by HIV-1SF2Nef was not observed with a mutant that lacks the N-terminal myristoylation signal and that was previously shown to be defective in PAK-2 kinase activation (HIV-1SF2NefG2A) (51, 59). A kinase-defective Nef mutant (HIV-1SF2NefF195I) (19) also demonstrated significantly reduced PAK-2 activation. Nefs including SIVmac239Nef and HIV-1YU2Nef activated PAK-2 in vitro. PAK-2 activation by Nef was inhibited by addition of a fusion protein containing the p21-binding domain of PAK-2, indicating that it is dependent on the presence of activated p21-GTPases. These results are identical to the results of Nef activation of PAK-2 in cell lines (4, 19, 34, 36, 37, 45) and demonstrate the ability of the cell-free system to faithfully recapitulate fundamental aspects of activation of PAK-2 by Nef.
The cell-free system was then used to investigate the molecular basis of PAK-2 activation by Nef. First, the interaction between Nef, PAK-2, and the p21-GTPases was examined. The results indicate that although Rac1 and Cdc42 are required for PAK-2 activation by Nef, they did not coimmunoprecipitate with active PAK-2 in this system. Since in this system the p21-GTPases appear to dissociate from PAK-2 after activation, these results suggest that the role of Nef in PAK-2 activation is not likely to stabilize a complex between PAK-2 and an activated GTPase.
Second, since PI 3-kinase activity has been postulated as being essential for PAK-2 activation by Nef (33, 60), the presence of active PI 3-kinase in the cell-free translation system was examined. The results demonstrate that active PI 3-kinase is present in the cell-free system. Although wortmannin resulted in complete inhibition of the PI 3-kinase activity in the cell-free translation system, the same concentration of wortmannin had no effect on PAK-2 kinase activation by Nef. These experiments were then repeated in 293T cells. Despite its ability to efficiently inhibit cellular PI 3-kinase activity, wortmannin did not have an effect on PAK-2 activation by HIV-1SF2Nef in 293T cells. Thus, our results indicate that PI 3-kinase is not absolutely required for PAK-2 activation by Nef, in contrast to two previously published reports (33, 60). At this point the difference between our results and the previously published studies appears to consist of their use of a CD8-Nef fusion protein instead of HIV-1SF2Nef (33, 60) and of COS cells instead of 293T cells (33).
Third, the role of membranes in PAK-2 activation by Nef was examined. PAK-2 activation by Nef in vitro absolutely required the presence of microsomal membranes, directly demonstrating the importance of membranes in the activation of PAK-2 by Nef in the cell-free translation system. Furthermore, Western blot analysis demonstrated that PAK-2 is a component of the rabbit reticulocyte lysate, but not of the microsomal membranes, offering a unique opportunity to examine whether Nef activates PAK-2 by translocation to the membrane fraction via its N-terminal myristoyl group. Ultracentrifugation analysis revealed that after incubation of a cell-free translation reaction mixture that contained rabbit reticulocyte lysate, microsomal membranes, and HIV-1SF2Nef RNA, all of the Nef-associated PAK-2 kinase activity partitioned to the membrane-containing pellet, whereas the majority of the Nef and PAK-2 proteins were found in the supernatant. These results are similar to the situation in cell lines, where the active Nef/PAK-2 complex was found in a low-abundance complex associated with membranes (4, 43, 45). Furthermore, the results indicate that the microsomal membranes play a posttranslational role in PAK-2 activation by Nef, since Nef-associated PAK-2 activity was observed when the microsomal membranes were added after Nef translation was inhibited with cycloheximide. Altogether, these results are consistent with a model in which Nef activates PAK-2 by recruiting the kinase to membranes.
The cell-free system described herein has several experimental advantages that will be useful in further elucidating the mechanism of PAK-2 activation by Nef. This includes the ability to control the order and timing of component addition, the possibility of performing immunodepletion and add-back experiments, and the option of directly examining the effects of various inhibitors of enzyme activity without the possibility of nonspecific cellular perturbations. Another advantage of the cell-free system is that it is significantly less complex than systems employing intact cells, possibly allowing for the determination of which proteins implicated in Nef activation of PAK-2 are actually required for this Nef function. For example, if a certain protein is not present in the cell-free translation system, hypotheses could be made that either this protein does not play a direct role in PAK-2 activation by Nef or that there are alternative cellular mechanisms.
In summary, a cell-free eukaryotic system was established and validated to study the activation of PAK-2 by Nef. Using this system, we provided evidence that the p21 GTPases play a transient role in Nef activation of PAK-2 and that Nef activation of PAK-2 does not require PI 3-kinase activity. Furthermore, consistent with the current model of PAK-2 activation by Nef in cell lines we show that Nef likely activates PAK-2 by recruiting the kinase to membranes in the cell-free system. The cell-free system described herein faithfully recapitulates key aspects of PAK-2 activation by Nef and should be a valuable tool in further studies of the molecular analysis of PAK-2 activation by Nef.
Acknowledgments
We are especially grateful to Xiaodong Wang for the caspase 3 and the zVAD. We thank Yuankai Lin for construction of the GST-PBD and GST-PBDH84LH87L bacterial expression constructs and Jonathan Chernoff for providing the GTPase and the PAK-2 constructs. We are also greatly appreciative of B. Barylko, J. Albanesi, Bangdong Wei, Eduardo O'Neill, and Vivek K. Arora for their advice and expertise and to Melanie H. Cobb, David W. Russell, Nicolai Van Oers, Stephen R. Hammes, David M. Margolis, Eduardo O'Neill, and Paul Denton for their critical reviews of the manuscript.
This work was supported by National Institutes of Health grant AI-33331 (J.V.G.). Alexa Raney was supported in part by National Institutes of Health training grant 5 F32 AI058541-02, and Laura Baugh was supported in part by National Institute of Allergy and Infectious Diseases training grant 5T32 A1005284.
REFERENCES
- 1.Anderson, S., D. C. Shugars, R. Swanstrom, and J. V. Garcia. 1993. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J. Virol. 67:4923-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando, S., K. Kaibuchi, T. Sasaki, K. Hiraoka, T. Nishiyama, T. Mizuno, M. Asada, H. Nunoi, I. Matsuda, Y. Matsuura, et al. 1992. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J. Biol. Chem. 267:25709-25713. [PubMed] [Google Scholar]
- 3.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4:189-199. [DOI] [PubMed] [Google Scholar]
- 4.Arora, V. K., R. P. Molina, J. L. Foster, J. L. Blakemore, J. Chernoff, B. L. Fredericksen, and J. V. Garcia. 2000. Lentivirus Nef specifically activates Pak2. J. Virol. 74:11081-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckler, G. S., D. Thompson, and T. Van Oosbree. 1995. In vitro translation using rabbit reticulocyte lysate. Methods Mol. Biol. 37:215-232. [DOI] [PubMed] [Google Scholar]
- 6.Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198-13204. [DOI] [PubMed] [Google Scholar]
- 7.Benard, V., and G. M. Bokoch. 2002. Assay of Cdc42, Rac, and Rho GTPase activation by affinity methods. Methods Enzymol. 345:349-359. [DOI] [PubMed] [Google Scholar]
- 8.Benson, R. E., A. Sanfridson, J. S. Ottinger, C. Doyle, and B. R. Cullen. 1993. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J. Exp. Med. 177:1561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briggs, S. D., B. Scholtz, J. M. Jacque, S. Swingler, M. Stevenson, and T. E. Smithgall. 2001. HIV-1 Nef promotes survival of myeloid cells by a Stat3-dependent pathway. J. Biol. Chem. 276:25605-25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou, M. M., and J. Blenis. 1996. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell 85:573-583. [DOI] [PubMed] [Google Scholar]
- 11.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang, T. H., X. Xu, U. G. Knaus, M. J. Hart, and G. M. Bokoch. 1993. GDP dissociation inhibitor prevents intrinsic and GTPase activating protein-stimulated GTP hydrolysis by the Rac GTP-binding protein. J. Biol. Chem. 268:775-778. [PubMed] [Google Scholar]
- 13.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 14.Deane, J. A., and D. A. Fruman. 2004. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 22:563-598. [DOI] [PubMed] [Google Scholar]
- 15.Deichaite, I., L. P. Casson, H. P. Ling, and M. D. Resh. 1988. In vitro synthesis of pp60v-src: myristylation in a cell-free system. Mol. Cell. Biol. 8:4295-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denker, B. M., E. J. Neer, and C. J. Schmidt. 1992. Mutagenesis of the amino terminus of the alpha subunit of the G protein Go. In vitro characterization of alpha o beta gamma interactions. J. Biol. Chem. 267:6272-6277. [PubMed] [Google Scholar]
- 17.Desrosiers, R. R., F. Gauthier, J. Lanthier, and R. Beliveau. 2000. Modulation of Rho and cytoskeletal protein attachment to membranes by a prenylcysteine analog. J. Biol. Chem. 275:14949-14957. [DOI] [PubMed] [Google Scholar]
- 18.Foster, J. L., S. J. Anderson, A. L. Frazier, and J. V. Garcia. 1994. Specific suppression of human CD4 surface expression by Nef from the pathogenic simian immunodeficiency virus SIVmac239open. Virology 201:373-379. [DOI] [PubMed] [Google Scholar]
- 19.Foster, J. L., R. P. Molina, T. Luo, V. K. Arora, Y. Huang, D. D. Ho, and J. V. Garcia. 2001. Genetic and functional diversity of human immunodeficiency virus type 1 subtype B Nef primary isolates. J. Virol. 75:1672-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia, J. V., J. Alfano, and A. D. Miller. 1993. The negative effect of human immunodeficiency virus type 1 Nef on cell surface CD4 expression is not species specific and requires the cytoplasmic domain of CD4. J. Virol. 67:1511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 22.Greenway, A. L., G. Holloway, D. A. McPhee, P. Ellis, A. Cornall, and M. Lidman. 2003. HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication. J. Biosci. 28:323-335. [DOI] [PubMed] [Google Scholar]
- 23.Huang, Z., J. Ling, and J. A. Traugh. 2003. Localization of p21-activated protein kinase gamma-PAK/Pak2 in the endoplasmic reticulum is required for induction of cytostasis. J. Biol. Chem. 278:13101-13109. [DOI] [PubMed] [Google Scholar]
- 24.Janardhan, A., T. Swigut, B. Hill, M. P. Myers, and J. Skowronski. 20. January 2004, posting date. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLOS Biol. 2:E6. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce, P. L., and A. D. Cox. 2003. Rac1 and Rac3 are targets for geranylgeranyltransferase I inhibitor-mediated inhibition of signaling, transformation, and membrane ruffling. Cancer Res. 63:7959-7967. [PubMed] [Google Scholar]
- 26.Kaminchik, J., N. Bashan, A. Itach, N. Sarver, M. Gorecki, and A. Panet. 1991. Genetic characterization of human immunodeficiency virus type 1 nef gene products translated in vitro and expressed in mammalian cells. J. Virol. 65:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kane, L. P., and A. Weiss. 2003. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol. Rev. 192:7-20. [DOI] [PubMed] [Google Scholar]
- 28.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 29.Kinsella, B. T., R. A. Erdman, and W. A. Maltese. 1991. Posttranslational modification of Ha-ras p21 by farnesyl versus geranylgeranyl isoprenoids is determined by the COOH-terminal amino acid. Proc. Natl. Acad. Sci. USA 88:8934-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhoff, F., M. Schindler, N. Bailer, G. H. Renkema, K. Saksela, V. Knoop, M. C. Muller-Trutwin, M. L. Santiago, F. Bibollet-Ruche, M. T. Dittmar, J. L. Heeney, B. H. Hahn, and J. Munch. 2004. Nef proteins from simian immunodeficiency virus-infected chimpanzees interact with p21-activated kinase 2 and modulate cell surface expression of various human receptors. J. Virol. 78:6864-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 33.Linnemann, T., Y. H. Zheng, R. Mandic, and B. M. Peterlin. 2002. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology 294:246-255. [DOI] [PubMed] [Google Scholar]
- 34.Lu, X., X. Wu, A. Plemenitas, H. Yu, E. T. Sawai, A. Abo, and B. M. Peterlin. 1996. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol. 6:1677-1684. [DOI] [PubMed] [Google Scholar]
- 35.Luo, T., J. R. Downing, and J. V. Garcia. 1997. Induction of phosphorylation of human immunodeficiency virus type 1 Nef and enhancement of CD4 downregulation by phorbol myristate acetate. J. Virol. 71:2535-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo, T., and J. V. Garcia. 1996. The association of Nef with a cellular serine/threonine kinase and its enhancement of infectivity are viral isolate dependent. J. Virol. 70:6493-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manninen, A., M. Hiipakka, M. Vihinen, W. Lu, B. J. Mayer, and K. Saksela. 1998. SH3-domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology 250:273-282. [DOI] [PubMed] [Google Scholar]
- 38.Niederman, T. M., W. R. Hastings, and L. Ratner. 1993. Myristoylation-enhanced binding of the HIV-1 Nef protein to T cell skeletal matrix. Virology 197:420-425. [DOI] [PubMed] [Google Scholar]
- 39.Nunn, M. F., and J. W. Marsh. 1996. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 70:6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng, B., and M. Robert-Guroff. 2001. Deletion of N-terminal myristoylation site of HIV Nef abrogates both MHC-1 and CD4 down-regulation. Immunol. Lett. 78:195-200. [DOI] [PubMed] [Google Scholar]
- 41.Piguet, V., and D. Trono. 1999. The Nef protein of primate lentiviruses. Rev. Med. Virol. 9:111-120. [DOI] [PubMed] [Google Scholar]
- 42.Poulin, L., and J. A. Levy. 1992. The HIV-1 nef gene product is associated with phosphorylation of a 46 kD cellular protein. AIDS 6:787-791. [DOI] [PubMed] [Google Scholar]
- 43.Pulkkinen, K., G. H. Renkema, F. Kirchhoff, and K. Saksela. 2004. Nef associates with p21-activated kinase 2 in a p21-GTPase-dependent dynamic activation complex within lipid rafts. J. Virol. 78:12773-12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renkema, G. H., A. Manninen, D. A. Mann, M. Harris, and K. Saksela. 1999. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 9:1407-1410. [DOI] [PubMed] [Google Scholar]
- 45.Renkema, G. H., A. Manninen, and K. Saksela. 2001. Human immunodeficiency virus type 1 Nef selectively associates with a catalytically active subpopulation of p21-activated kinase 2 (PAK2) independently of PAK2 binding to Nck or β-PIX. J. Virol. 75:2154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roig, J., Z. Huang, C. Lytle, and J. A. Traugh. 2000. p21-activated protein kinase gamma-PAK is translocated and activated in response to hyperosmolarity. Implication of Cdc42 and phosphoinositide 3-kinase in a two-step mechanism for gamma-PAK activation. J. Biol. Chem. 275:16933-16940. [DOI] [PubMed] [Google Scholar]
- 47.Roig, J., and J. A. Traugh. 2001. Cytostatic p21 G protein-activated protein kinase gamma-PAK. Vitam Horm. 62:167-198. [DOI] [PubMed] [Google Scholar]
- 48.Rudel, T., and G. M. Bokoch. 1997. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science 276:1571-1574. [DOI] [PubMed] [Google Scholar]
- 49.Sanford, J. C., J. Yu, J. Y. Pan, and M. Wessling-Resnick. 1995. GDP dissociation inhibitor serves as a cytosolic acceptor for newly synthesized and prenylated Rab5. J. Biol. Chem. 270:26904-26909. [DOI] [PubMed] [Google Scholar]
- 50.Sawai, E. T., A. Baur, H. Struble, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1994. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl. Acad. Sci. USA 91:1539-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawai, E. T., A. S. Baur, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1995. A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J. Biol. Chem. 270:15307-15314. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 53.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 54.Tahara, S. M., and J. A. Traugh. 1981. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Identification and characterization of a protein kinase activated by proteolysis. J. Biol. Chem. 256:11558-11564. [PubMed] [Google Scholar]
- 55.Utsumi, T., J. Kuranami, E. Tou, A. Ide, K. Akimaru, M. C. Hung, and J. Klostergaard. 1996. In vitro synthesis of an N-myristoylated fusion protein that binds to the liposomal surface. Arch. Biochem. Biophys. 326:179-184. [DOI] [PubMed] [Google Scholar]
- 56.Walter, B. N., Z. Huang, R. Jakobi, P. T. Tuazon, E. S. Alnemri, G. Litwack, and J. A. Traugh. 1998. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. J. Biol. Chem. 273:28733-28739. [DOI] [PubMed] [Google Scholar]
- 57.Wei, B. L., V. K. Arora, J. L. Foster, D. L. Sodora, and J. V. Garcia. 2003. In vivo analysis of Nef function. Curr. HIV Res. 1:41-50. [DOI] [PubMed] [Google Scholar]
- 58.Welch, H. C., W. J. Coadwell, L. R. Stephens, and P. T. Hawkins. 2003. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 546:93-97. [DOI] [PubMed] [Google Scholar]
- 59.Wiskerchen, M., and C. Cheng-Mayer. 1996. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology 224:292-301. [DOI] [PubMed] [Google Scholar]
- 60.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti- apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]