Abstract
Nonviral producer cell proteins incorporated into retroviral vector surfaces profoundly influence infectivity and in vivo half-life. We report the purification and concentration of lentiviral vectors using these surface proteins as an efficient gene transduction strategy. Biotinylation of these proteins and streptavidin paramagnetic particle concentration enhances titer 400- to 2,500-fold (to 109 CFU/ml for vesicular stomatitis virus G protein and 5 × 108 for amphotropic murine leukemia virus envelope). This method also uses newly introduced membrane proteins (B7.1 and ΔLNGFR) directed to lentiviral surfaces, allowing up to 17,000-fold concentrations. Particle conjugation of lentivirus allows facile manipulation in vitro, resulting in the transduction of 48 to 94% of human acute myeloid leukemia blasts.
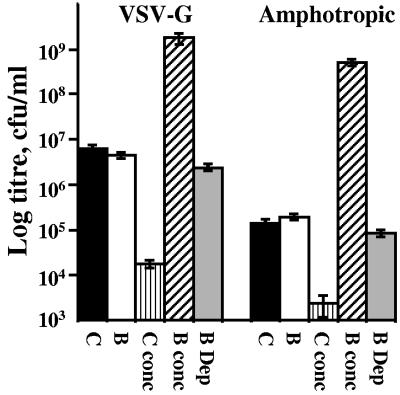
Paramagnetic particles (PMP) are extremely efficient vehicles for the capture and concentration of infectious retroviral vectors (28). This property has since been confirmed for retrovirus (39, 43) and extended to adenoviral (34, 39), adeno-associated (30), baculoviral (37), and lentiviral (23) vectors. We applied magnetic capture (28) to lentivirus pseudotyped with vesicular stomatitis virus G protein (VSV-G) or amphotropic envelopes (Fig. 1). Biotinylation of 293T and 293T-Ampho cells was performed immediately prior to transfection with 3.25 μg pCMVΔR8.91 (46), 1.75 μg pMD.G (31), and 5 μg pLV.bla or 4 μg pCMVΔR8.91 and 6 μg pLV.bla for 293T-Ampho cells. The self-inactivating LV.bla was constructed using the spleen focus-forming virus promoter, a cppt fragment encompassing human immunodeficiency virus (HIV) central polypurine tract/termination sequences (14), and IRES-BLAST (18) in pHR′CMVGFPWSIN-18 (45). Lentiviral vectors were harvested 48 h after transfection, 24 h after replenishment with 10 mM sodium butyrate in Dulbecco modified Eagle medium plus 10% fetal calf serum. After 0.45-μm filtration, lentivirus was used to infect K562 cells or agitated at 4°C with 1.25 × 109 Dynal MPC-E washed streptavidin Magnesphere paramagnetic particles (Promega) per 5 ml supernatant. After 90 min the lentivirus-PMP mix was extensively washed and magnetically concentrated and titers were determined by drug-resistant colony formation in 10 μg/ml Blasticidin S (Invivogen) (28). The biotinylated VSV-G starting titer of 4.4 × 106/ml was concentrated to 1.7 × 109/ml, representing a 400-fold increase, while control vectors (6.3 × 106/ml) were not captured and lost 99% of titer (C conc). Biotinylated amphotropic vectors were concentrated to 5 × 108/ml, 2,600-fold above the control, while capture efficiency indicates that 50% of lentivirus evaded capture.
FIG. 1.
Biotin-dependent capture of 293T-derived lentiviral vectors. For LV.bla vector supernatants from 293T (VSV-G) and 293T-Ampho (amphotropic, stable transfection of the murine leukemia virus 4070A amphotropic envelope-encoding pALF [13] into 293T) cells without (C) and with (B) biotinylation, titers were immediately determined on K562 cells in 4 μg/ml Polybrene. Alternatively, the vectors were captured and magnetically concentrated 100-fold with streptavidin-PMP prior to titration (C conc and B conc). The remaining supernatant following removal of the PMP (B Dep) was also used to infect target cells as an estimate of the efficiency of capture.
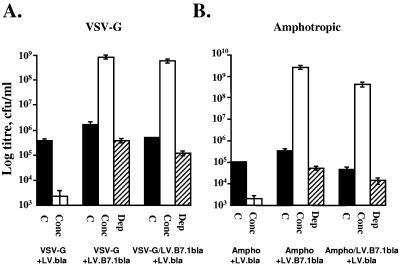
Biotinylation prior to transfection would not modify VSV-G proteins; other biotinylated proteins must therefore associate with lentiviral vectors for biotin-dependent capture. We investigated another surface protein, B7.1, for lentiviral capture (Fig. 2). 293T cells transiently transfected to produce LV.B7.1bla vectors (B7.1 from pWZLIL2/B7F [18] into LV.bla) express the vector-encoded B7.1 (CD80) on the cell surface, providing a potential handle for lentiviral capture. For B7.1-dependent capture 1.25 × 109 PMP were serially conjugated (30 min) with 50 μl of 1-mg/ml protein A-biotin and 100 μl of 500-μg/ml B7.1 binding CTLA4-immunoglobulin (Ig) (15, 20), and lentivirus was manipulated as before. Transient B7.1 expression allowed 490-fold (VSV-G+LV.B7.1bla, Fig. 2A) or 7,000-fold (Ampho+LV.B7.1bla, Fig. 2B) concentration (to 8 × 108 and 2.5 × 109CFU/ml, respectively). Similarly, stable expression of B7.1 by the 293T cells enabled B7.1-mediated concentration of LV.bla, resulting in 1,100- (VSV-G/LV.B7.1bla+LV.bla, Fig. 2A) or 9,000-fold (Ampho/LV.B7.1bla+LV.bla, Fig. 2B) titer increases. B7.1 labeling of lentivirus enabled >70% capture, while B7.1-negative control vectors could not be concentrated.
FIG. 2.
B7.1-dependent capture of lentiviral vectors. Lentivirus was produced from 293T cells by transient transfection of helper functions with either LV.bla vector plasmids (VSV-G+LV.bla and Ampho+LV.bla) or B7.1-encoding LV.B7.1bla vector plasmids (VSV-G+LV.B7.1bla and Ampho+LV.B7.1bla). Alternatively 293T cells expressing B7.1 from a prior infection with the self-inactivating B7.1 vector (VSV-G/LV.B7.1bla and Ampho/LV.B7.1bla) were transfected with LV.bla to result in VSV-G/LV.B7.1bla+LV.bla and Ampho/LV.B71bla+LV.bla. The lentiviral affinity for CTLA4-Ig-conjugated PMP was thus examined for vectors derived from B7.1-negative 293T cells and compared with those derived from 293T cells expressing either transient or integrated stable B7.1. Titers of VSV-G-pseudotyped or amphotropic lentiviral vector supernatants were determined on K562 cells (4 μg/ml Polybrene), either immediately without concentration (C) or following capture and 100-fold concentration with CTLA4-Ig-conjugated PMP (Conc). The remaining supernatant following removal of the PMP (Dep) was used to infect target cells as an estimate of capture efficiency.
Titration of CTLA4-Ig in the B7.1-dependent vector capture assay showed that a fivefold reduction was possible before concentrate titer was reduced (data not shown). We then replaced CTLA4-Ig with 100 μl of 175-μg/ml mouse anti-human B7.1 and protein A with 50 μl of 1-mg/ml biotin-goat anti-mouse IgGFc (Table 1). The similarly efficient B7.1-mediated concentration protocols indicate that the increased titer was not due to fortuitous interactions of protein A, CTLA4-Ig, or B7.1 with target cells (22). Vectors expressing a low-affinity nerve growth factor receptor (NGFR), “LV.LNbla” (ligation of ΔLNGFR [4] upstream of IRES-BLAST in LV.bla), could be similarly captured with anti-NGFR-conjugated PMP (100 μl, 175 μg/ml anti-human NGFR). As with B7.1, this concentration was specific to ΔLNGFR and did not result in the concentration of control LV.bla vectors.
TABLE 1.
Comparative CTLA4-Ig and antibody-dependent capture of lentiviral pseudotypesa
| Treatment | Titerb | Fold increase | % Capture |
|---|---|---|---|
| V+LV.B7.1bla | |||
| Control | 2.06 ± 0.3 | ||
| 100 μg CTLA4-Ig | 1,330 ± 57 | 645 | 81 |
| 175 μg anti-B7.1 | 1,130 ± 153 | 548 | 60 |
| V + LV.LNbla | |||
| Control | 1.3 ± 0.46 | ||
| 175 μg anti-NGFR | 866 ± 115 | 666 | 52 |
| V + LV.bla | |||
| Control | 0.34 ± 0.07 | ||
| 175 μg anti-NGFR | 0.16 ± 0.05 | 0 | |
| A + LV.B7.1bla | |||
| Control | 0.72 ± 0.015 | ||
| 100 μg CTLA4-Ig | 4,560 ± 288 | 6,307 | 95 |
| 175 μg anti-B7.1 | 2,900 ± 265 | 4,011 | 80 |
| A + LV.LNbla | |||
| Control | 0.57 ± 0.011 | ||
| 175 μg anti-NGFR | 2,070 ± 250 | 3,600 | 0 |
| A + LV.bla | |||
| Control | 0.09 ± 0.01 | ||
| 175 μg anti-NGFR | 0.16 ± 0.07 | 1.7 |
Titers of 293T cell-derived lentiviral vector supernatants (LV.B7.1bla, LV.LNbla, and LV.bla), pseudotyped with VSV-G (V) or amphotropic (A) envelopes, were determined on K562 cells (in 4 μg/ml Polybrene) either before (control) or after capture and 100-fold concentration, using CTLA4-Ig, anti-B7.1 antibody, or anti-NGFR antibody-conjugated PMP. The concentrates and the depleted supernatants remaining after the removal of the PMP were used to infect K562 cells to determine the efficiency of both concentration (fold increase) and capture (percent capture).
Titers are shown as 106 CFU per milliliter for VSV-G envelopes and 105 CFU per milliliter for amphotropic envelopes.
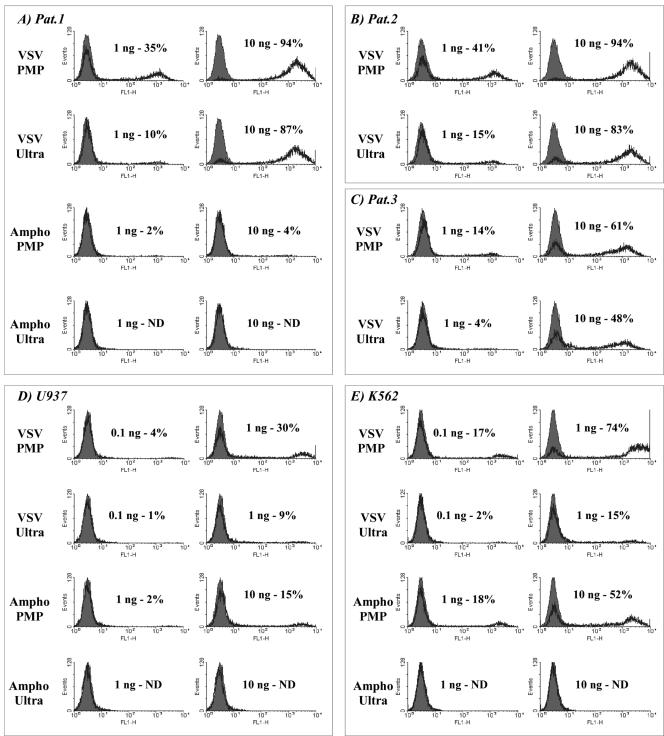
The relative ease of access to sufficient quantities of primary human acute myeloid leukemia (AML) blasts suggests an immunotherapy strategy based on ex vivo genetic modification (19). Observations that allogeneic bone marrow transplantation reduces relapse risk compared with autologous bone marrow transplantation (27) show that AML can be recognized by the immune system and that AML is susceptible to allogeneic antileukemic responses (9, 25). The potentially beneficial immune response against AML blasts expressing costimulators and/or proinflammatory cytokines has prompted efforts to devise efficient strategies for their modification (6, 26, 29, 42). We used B7.1-expressing 293T cells and green fluorescent protein vectors (LV.gfp) to investigate the ability of PMP concentrated vectors to infect AML blasts (6). We compared PMP concentration with ultracentrifugation (8,600 × g, 4°C, overnight, followed by 183,000 × g, 90 min, 4°C), each providing 100-fold volume reductions (Fig. 3). Equimolar p24 adjusted vector concentrations were then used to infect primary AML blasts cultured in X-VIVO medium with 20 ng/ml stem cell factor and 10 ng/ml interleukin-3 (Fig. 3A to C) or U937 (Fig. 3D) and K562 (Fig. 3E) cells in RPMI plus 10% fetal calf serum. After 96 h the cells were analyzed by fluorescence-activated cell sorting and titers were determined from <20% FL-1-positive cell populations. Centrifuged VSV-G lentivirus (1 ng p24) infected 10%, 15%, and 4% of AML samples, compared with PMP rates of 35%, 41%, and 14%, respectively—a 2.7- to 3.5-fold-greater p24-to-infectivity ratio. Thus, the problematic infection of primary AML cells (26) that was alleviated using ultracentrifuged VSV-G enveloped lentivirus (6, 29, 42) can be further improved upon by PMP concentration.
FIG. 3.
Comparative infectivity of lentivirus in primary and established leukemia cells after concentration by either PMP or ultracentrifugation. Lentiviral vectors expressing enhanced green fluorescent protein (LV.gfp) were prepared from stable B7.1-expressing 293T cells (VSV-G) or 293T-Ampho (Ampho) and concentrated 100-fold either by CTLA4-Ig-conjugated PMP capture (PMP) or by ultracentrifugation (Ultra). After enzyme-linked immunosorbent assay determination of p24 Gag the viral concentrates were used at equimolar p24 levels to infect three cryopreserved primary AML samples (A to C; patients 1 to 3, respectively) and the established leukemia cell lines U937 and K562 (D and E, respectively) all in the presence of 4 μg/ml Polybrene. Fluorescence-activated cell sorting analysis of enhanced green fluorescent protein expression was carried out 96 h after infection (black line, enhanced green fluorescent protein; shaded area, background). No enhanced green fluorescent protein expression was detected for primary AML samples 2 and 3 following inoculation with amphotropic virus concentrated by either strategy (data not shown). ND, not detected.
The amphotropic PMP concentrates provided low-level infection in only one AML sample (Fig. 3A, patient 1, 10 ng p24, 4%), even though the K562 and U937 titers confirmed the infectivity of amphotropic-PMP concentrates, 10 ng of p24 infecting 52% and 15% of cells, respectively. The inability of amphotropic lentivirus to transduce AML was unexpected, as it is the most efficient for cytokine-mobilized human CD34+ cell transduction (24).
Paramagnetic particle-conjugated virus is highly infectious, demonstrating substantially higher levels of infectivity than are explainable by concentration alone. The ΔLNGFR-labeled vectors demonstrated this to a remarkable degree where depletion (percent capture) was evident only for VSV-G pseudotypes. Despite the fact that ΔLNGFR-labeled amphotropic lentivirus did not appear to be efficiently captured (as judged by depletion), a 3,600-fold vector titer increase was observed. The high amphotropic/ΔLNGFR concentrate titer suggests that the lentivirus become several orders of magnitude more infectious when anchored to the PMP. Unexpectedly large increases in titer have also been observed for other vector/particle complexes and postulated to result from rapid settling of the PMP-conjugated vectors onto target cells, promotion of additional vector-target cell interactions (23, 28, 30, 34, 39), and the removal of inhibitory factors (41). We addressed these anomalies by preincubating B7.1-labeled LV.gfp lentivirus with CTLA4-Ig/PMP for 90 min prior to infection. This increased the effective titer of amphotropic lentivirus by >150-fold to 2 × 107/ml, and when combined with a 100-fold reduction in volume the titer increased to 1.9 × 109/ml. This suggests that increased titer is substantially derived from improved viral presentation to target cells rather than the purification from supernatant-derived inhibitors of infection.
The presence of nonviral proteins on lentiviral surfaces is consistent with numerous studies showing host-derived proteins copackaging with HIV virions (1, 16, 17, 32, 36). Remodeling of lentiviral surfaces, as exemplified by B7.1 and ΔLNGFR, allows new antibody-antigen or receptor-ligand interactions for concentration. Although VSV-G pseudotypes remain infective after ultracentrifugation (5), there are limitations in scale-up and contaminant coconcentration (10, 41) and an apparent limit of 2,000-fold to concentration (11, 12, 24, 38, 44). Moreover, vectors from different sources (2) and with alternative or reengineered targeting envelopes (3, 21, 33, 40) may be particularly sensitive to centrifugation (35). Thus, magnetic concentration not only is a useful purification technology but also allows the use of additional factors for capture and/or targeting strategies that are not dependent on the modification of viral envelope proteins (7, 8).
Acknowledgments
This work was supported by Leukemia Research UK and the Biotechnology and Biological Sciences Research Council.
We thank Didier Trono for pCMVΔR8.91 and pHR′CMVGFPWSIN-18, Adrian Thrasher for pHR′SIN-cppt-SEW, Yasu Takeuchi for pALF, and Claudio Bordignon for ΔLNGFR.
REFERENCES
- 1.Arthur, L. O., J. W. Bess, R. C. Sowder, R. E. Benveniste, D. L. Mann, J. C. Chermann, and L. E. Henderson. 1992. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science 258:1935-1938. [DOI] [PubMed] [Google Scholar]
- 2.Beer, C., A. Meyer, K. Muller, and M. Wirth. 2003. The temperature stability of mouse retroviruses depends on the cholesterol levels of viral lipid shell and cellular plasma membrane. Virology 308:137-146. [DOI] [PubMed] [Google Scholar]
- 3.Beyer, W. R., M. Westphal, W. Ostertag, and D. von Laer. 2002. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J. Virol. 76:1488-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordignon, C., C. Bonini, S. Verzeletti, N. Nobili, D. Maggioni, C. Traversari, R. Giavazzi, P. Servida, E. Zappone, E. Benazzi, M. Bernadi, F. Porta, G. Ferrari, F. Mavilio, S. Rossini, R. M. Blaese, and F. Candotti. 1995. Clinical protocol: transfer of the HSV-tk gene into donor peripheral blood lymphocytes for in vivo modulation of donor anti-tumor immunity after allogeneic bone marrow transplant. Hum. Gene Ther. 6:813-819. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis-virus G glycoprotein pseudotyped retroviral vectors—concentration to very high-titre and efficient gene-transfer into mammalian and non-mammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, L., N. Hardwick, D. Darling, J. Galea-Lauri, J. Gäken, S. Devereux, M. Kemeny, G. Mufti, and F. Farzaneh. 2005. IL-2/B7.1 fusagene transduction of AML blasts by a self-inactivating lentiviral vector stimulates T cell responses in vitro: a strategy to generate whole cell vaccines for AML. Mol. Ther. 11:120-131. [DOI] [PubMed] [Google Scholar]
- 7.Chandrashekran, A., M. Y. Gordon, and C. Casimir. 2004. Targeted retroviral transduction of c-kit+ hematopoietic cells using novel ligand display technology. Blood 104:2697-2703. [DOI] [PubMed] [Google Scholar]
- 8.Chandrashekran, A., M. Y. Gordon, D. Darling, F. Farzaneh, and C. Casimir. 2004. Growth factor displayed on the surface of retroviral particles without manipulation of envelope proteins is biologically active and can enhance transduction. J. Gene Med. 6:1189-1196. [DOI] [PubMed] [Google Scholar]
- 9.Charbonnier, A., B. Gaugler, D. Sainty, M. Lafage-Pochitaloff, and D. Olive. 1999. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce differentiation of cytotoxic T cells against autologous leukemias. Eur. J. Immunol. 29:2567-2578. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., L. Reeves, N. Sanburn, J. Croop, D. A. Williams, and K. Cornetta. 2001. Packaging cell line DNA contamination of vector supernatants: implication for laboratory and clinical research. Virology 282:186-197. [DOI] [PubMed] [Google Scholar]
- 11.Coil, D. A., J. H. Strickler, S. K. Rai, and A. D. Miller. 2001. Jaagsiekte sheep retrovirus Env protein stabilizes retrovirus vectors against inactivation by lung surfactant, centrifugation, and freeze-thaw cycling. J. Virol. 75:8864-8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman, J. E., M. J. Huentelman, S. Kasparov, B. L. Metcalfe, J. F. R. Paton, M. J. Katovich, S. L. Semple-Rowland, and M. K. Raizada. 2003. Efficient large-scale production and concentration of HIV-1 based lentiviral vectors for use in vivo. Physiol. Genomics 12:221-228. [DOI] [PubMed] [Google Scholar]
- 13.Cosset, F.-L., Y. Takeuchi, J.-L. Battini, R. A. Weiss, and M. K. L. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaison, C., K. Parsley, G. Brouns, M. Scherr. K. Battmer, C. Kinnon, M. Grez, and A. J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13:803-813. [DOI] [PubMed] [Google Scholar]
- 15.Dohring, C., L. Angman, G. Spagnoli, and A. Lanzavecchia. 1994. T-helper-cell-independent and accessory-cell-independent cytotoxic responses to human tumor-cells transfected with a B7 retroviral vector. Int. J. Cancer 57:754-759. [DOI] [PubMed] [Google Scholar]
- 16.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules in human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75:6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortin, J.-F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gäken, J., J. Jiang, K. Daniel, E. van Berkel, C. Hughes, M. Kuiper, D. Darling, M. Tavassoli, J. Galea-Lauri, K. Ford, M. Kemeny, S. Russell, and F. Farzaneh. 2000. Fusagene vectors: a novel strategy for the expression of multiple genes from a single cistron. Gene Ther. 7:1979-1985. [DOI] [PubMed] [Google Scholar]
- 19.Galea-Lauri, J. 2002. Immunological weapons against acute myeloid leukaemia. Immunology 107:20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galea-Lauri, J., D. Darling, S.-U. Gan. L. Krivochtchapov, J. Gäken, B. Souberbeille, and F. Farzaneh. 1999. Expression of a variant CD28 on a sub-population of human NK cells: implications for B7-mediated stimulation of NK cells. J. Immunol. 163:62-70. [PubMed] [Google Scholar]
- 21.Gatlin, J., M. W. Melkus, A. Padgett, P. F. Kelly, and J. V. Garcia. 2001. Engraftment of NOD/SCID mice with human CD34+ cells transduced by concentrated oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. J. Virol. 75:9995-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giguere, J.-F., S. Bounou, J.-S. Paquette, J. Madrenas, and M. J. Tremblay. 2004. Insertion of host-derived costimulatory molecules CD80 (B7.1) and CD86 (B7.2) into human immunodeficiency virus type 1 affects the virus life cycle. J. Virol. 78:6222-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haim, H., I. Steiner, and A. Panet. 2005. Synchronized infection of cell cultures by magnetically controlled virus. J. Virol. 79:622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanawa, H., P. F. Kelly, A. C. Nathwani, D. A. Persons, J. A. Vandegriff, P. Hargrove, E. F. Vanin, and A. W. Neinhuis. 2002. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 5:242-251. [DOI] [PubMed] [Google Scholar]
- 25.Harrison, B. D., J. A. Adams, M. Briggs, M. L. Brereton, and J. A. Yin. 2001. Stimulation of autologous proliferative and cytotoxic T-cell responses by “leukaemic dendritic cells” derived from blast cells in acute myeloid leukaemia. Blood 97:2764-2771. [DOI] [PubMed] [Google Scholar]
- 26.Hirst, W. J. R., A. Buggins, D. Darling, J. Gäken, F. Farzaneh, and G. J. Mufti. 1997. Enhanced immune costimulatory activity of primary acute myeloid leukaemia blasts after gene transfer of B7.1. Gene Ther. 4:691-699. [DOI] [PubMed] [Google Scholar]
- 27.Ho, A. Y. L., A. Paglucia, M. Kenyon, J. E. Parker, A. Mijovic, S. Devereux, and G. J. Mufti. 2004. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukaemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzmab (FBC) conditioning. Blood 104:1616-1623. [DOI] [PubMed] [Google Scholar]
- 28.Hughes, C., J. Galea-Lauri, F. Farzaneh, and D. Darling. 2001. Streptavidin paramagnetic particles provide a choice of three affinity-based capture and magnetic concentration strategies for retroviral vectors. Mol. Ther. 3:623-630. [DOI] [PubMed] [Google Scholar]
- 29.Koya, R. C., N. Kasahara, V. Pullarkat, A. M. Levine, and R. Stripecke. 2002. Transduction of acute myeloid leukemia cells with third generation self-inactivating lentiviral vectors expressing CD80 and GM-CSF: effects on proliferation, differentiation, and stimulation of allogeneic and autologous anti-leukemia immune responses. Leukemia 16:1645-1654. [DOI] [PubMed] [Google Scholar]
- 30.Mah, C., T. J. Fraites, I. Zolotukhin, S. H. Song, T. R. Flotte, J. Dobson, C. Batich, and B. J. Byrne. 2002. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther. 6:106-112. [DOI] [PubMed] [Google Scholar]
- 31.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of non dividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 32.Nyambi, P. N., M. K. Gorny, L. Bastiani, G. van der Groen, C. Williams, and S. Zoller-Pazner. 1998. Mapping epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J. Virol. 72:9384-9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen, J. C. 1998. Gene transfer vectors derived from equine infectious anaemia virus. Gene Ther. 5:1481-1487. [DOI] [PubMed] [Google Scholar]
- 34.Pandori, M. W., D. A. Hobson, and T. Sano. 2002. Adenovirus-microbead conjugates possess enhanced infectivity: a new strategy for localized gene therapy. Virology 299:204-212. [DOI] [PubMed] [Google Scholar]
- 35.Pham, L., H. Ye, F.-L. Cosset, S. J. Russell, and K. W. Peng. 2001. Concentration of viral vectors by co-precipitation with calcium phosphate. J. Gene Med. 3:188-194. [DOI] [PubMed] [Google Scholar]
- 36.Pickl, W. F., F. X. Pimentel-Muinos, and B. Seed. 2001. Lipid rafts and pseudotyping. J. Virol. 75:7175-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raty, J. K., K. J. Airenne, A. T. Marttilla, V. Marjomaki, V. P. Haytonen, P. Lehtolainen, O. H. Laitinen, A. J. Mahonen, M. S. Kulomaa, and S. Yla-Hertuala. 2002. Enhanced gene delivery by avidin-displaying baculovirus. Mol. Ther. 9:282-291. [DOI] [PubMed] [Google Scholar]
- 38.Reiser, J. 2000. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 7:910-913. [DOI] [PubMed] [Google Scholar]
- 39.Scherer, F., M. Anton, U. Schillinger, J. Henkel, C. Bergemann, A. Kruger, B. Gansbacher, and C. Plank. 2002. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 9:102-109. [DOI] [PubMed] [Google Scholar]
- 40.Stitz, J., C. J. Buchholz, M. Engelstadter, W. Uckert, U. Bloemer, I. Schmitt, and K. Cichutek. 2000. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology 273:16-20. [DOI] [PubMed] [Google Scholar]
- 41.Strang, B. L., Y. Ikeda, F.-L. Cosset, M. K. L. Collins, and Y. Takeuchi. 2004. Characterisation of HIV-1 vectors with gammaretrovirus envelope glycoproteins produced from stable packaging cells. Gene Ther. 11:591-598. [DOI] [PubMed] [Google Scholar]
- 42.Stripecke, R., A. A. Cardoso, K. A. Pepper, D. G. Skelton, X. J. Yu, L. Mascarenhas, K. I. Weinberg, L. M. Nadler, and D. B. Kohn. 2000. Lentiviral vectors for efficient delivery of CD80 and granulocyte-macrophage-colony-stimulating factor in human acute lymphoblastic leukemia cells and acute myeloid leukemia cells to induce antileukemic responses. Blood 96:1317-1326. [PubMed] [Google Scholar]
- 43.Tai, M.-F., K.-M. Chi, K.-H. W. Lau, D. J. Baylink, and S. T. Chen. 2003. Generation of magnetic retroviral vectors with magnetic nanoparticles. Rev. Adv. Mater. Sci. 5:319-323. [Google Scholar]
- 44.Zhang, B., H. Q. Xia, G. Cleghorn, G. Gobe, M. West, and M. Q. Wei. 2001. A highly efficient and consistent method for harvesting large volumes of high-titre lentiviral vectors. Gene Ther. 8:1745-1751. [DOI] [PubMed] [Google Scholar]
- 45.Zufferey, R., T. Dull, R. J. Mandel, A. Bukovsky, D. Quiroz, L. Naldini, and D. Trono. 1998. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 72:9873-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15:871-875. [DOI] [PubMed] [Google Scholar]