Abstract
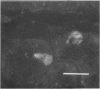
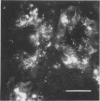
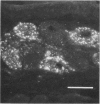
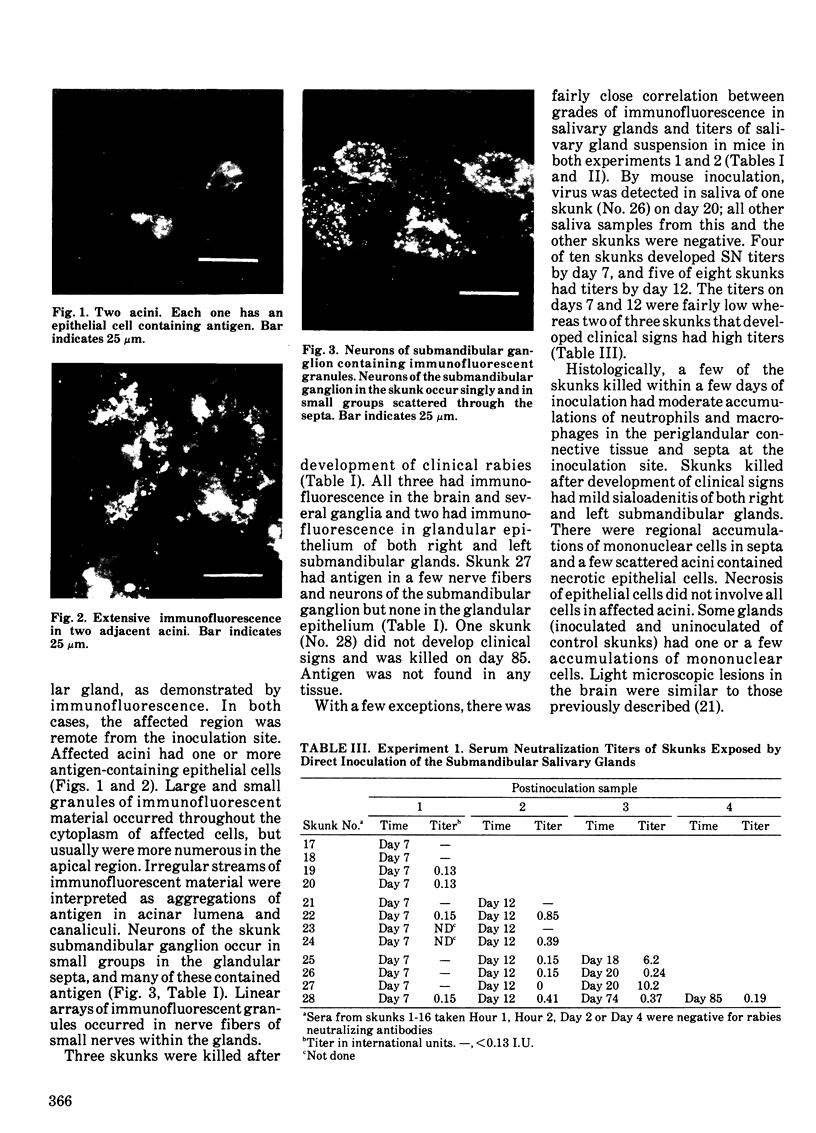
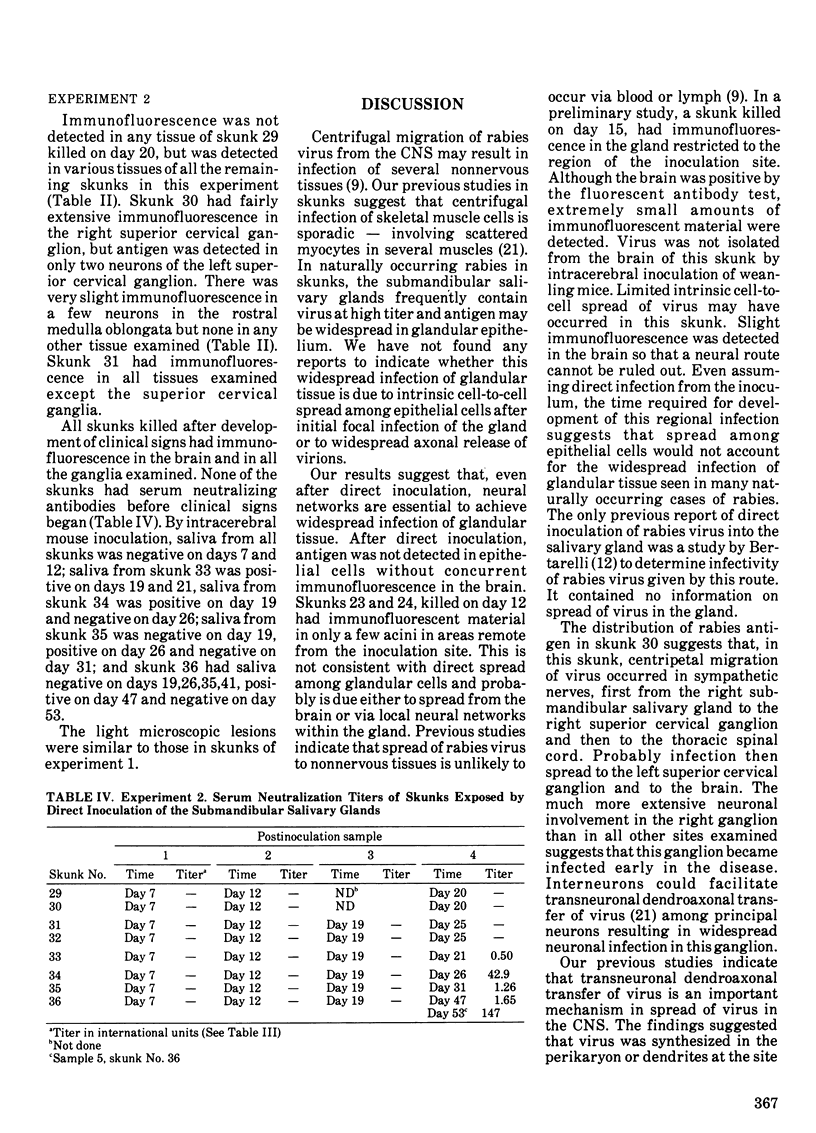
Striped skunks (Mephitis mephitis) were inoculated into the right submandibular salivary gland with street rabies virus. They were killed at various times after inoculation and several tissues were examined by immunofluorescence and light microscopy. Right and left superior cervical, nodose and trigeminal ganglia, medulla oblongata and at least three regions of right and left submandibular salivary glands were examined by the fluorescent antibody technique. Intracerebral titrations of salivary gland suspensions were made in weanling white Swiss mice. Immunofluorescent material (inoculum) was detected in septa and connective tissue surrounding secretory units of the right submandibular gland immediately after inoculation, but otherwise antigen was not detected in either right or left submandibular glands without coincident antigen in the medulla oblongata. This occurred first on day 12 in areas of the gland remote from the inoculation site. Titers of virus were low at this time. Serum neutralizing antibodies occurred by day 7 in a few skunks. The time of development and distribution of antigen strongly suggest that, even after direct inoculation, neural networks are necessary for development of widespread infection of the salivary gland. The early occurrence of serum neutralizing antibodies in some of the skunks suggests that the immune response was activated by virus in the inoculum since immunofluorescence was not detected in any tissue at this time.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRAL L., SERIE C. Etudes expérimentales sur la rage en Ethiopie. Ann Inst Pasteur (Paris) 1957 Oct;93(4):475–488. [PubMed] [Google Scholar]
- Atanasiu P., Gamet A., Tsiang H., Dragonas P., Lépine P. Nouvelles données sur les voies d'élimination du virus rabique chez les animaux infectés. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2434–2436. [PubMed] [Google Scholar]
- Charlton K. M., Casey G. A. Experimental rabies in skunks: immunofluorescence light and electron microscopic studies. Lab Invest. 1979 Jul;41(1):36–44. [PubMed] [Google Scholar]
- Charlton K. M., Casey G. A. Experimental rabies in skunks: persistence of virus in denervated muscle at the inoculation site. Can J Comp Med. 1981 Oct;45(4):357–362. [PMC free article] [PubMed] [Google Scholar]
- Constantine D. G., Emmons R. W., Woodie J. D. Rabies virus in nasal mucosa of naturally infected bats. Science. 1972 Mar 17;175(4027):1255–1256. doi: 10.1126/science.175.4027.1255. [DOI] [PubMed] [Google Scholar]
- Correa-Giron E. P., Allen R., Sulkin S. E. The infectivity and pathogenesis of rabiesvirus administered orally. Am J Epidemiol. 1970 Feb;91(2):203–215. doi: 10.1093/oxfordjournals.aje.a121129. [DOI] [PubMed] [Google Scholar]
- DEAN D. J., EVANS W. M., MCCLURE R. C. PATHOGENESIS OF RABIES. Bull World Health Organ. 1963;29:803–811. [PMC free article] [PubMed] [Google Scholar]
- Dean D. J., Abelseth M. K. Laboratory techniques in rabies: the fluorescent antibody test. Monogr Ser World Health Organ. 1973;(23):73–84. [PubMed] [Google Scholar]
- Debbie J. G., Trimarchi C. V. Pantropism of rabies virus in free-ranging rabid red fox Vulpes fulva. J Wildl Dis. 1970 Oct;6(4):500–506. doi: 10.7589/0090-3558-6.4.500. [DOI] [PubMed] [Google Scholar]
- Dierks R. E., Murphy F. A., Harrison A. K. Extraneural rabies virus infection. Virus development in fox salivary gland. Am J Pathol. 1969 Feb;54(2):251–273. [PMC free article] [PubMed] [Google Scholar]
- Fekadu M., Shaddock J. H., Baer G. M. Intermittent excretion of rabies virus in the saliva of a dog two and six months after it had recovered from experimental rabies. Am J Trop Med Hyg. 1981 Sep;30(5):1113–1115. doi: 10.4269/ajtmh.1981.30.1113. [DOI] [PubMed] [Google Scholar]
- Fischman H. R., Schaeffer M. Pathogeneis of experimental rabies as revealed by immunofluorescence. Ann N Y Acad Sci. 1971 Jun 21;177:78–97. doi: 10.1111/j.1749-6632.1971.tb35035.x. [DOI] [PubMed] [Google Scholar]
- Howard D. R. Rabies virus titer from tissues of naturally infected skunks (Mephitis mephitis). Am J Vet Res. 1981 Sep;42(9):1595–1597. [PubMed] [Google Scholar]
- Moreno J. A., Baer G. M. Experimental rabies in the vampire bat. Am J Trop Med Hyg. 1980 Mar;29(2):254–259. doi: 10.4269/ajtmh.1980.29.254. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Winn W. C., Bauer S. P. Comparative pathogenesis of rabies and rabies-like viruses: infection of the central nervous system and centrifugal spread of virus to peripheral tissues. Lab Invest. 1973 Jul;29(1):1–16. [PubMed] [Google Scholar]
- Parker R. L., Wilsnack R. E. Pathogenesis of skunk rabies virus: quantitation in skunks and foxes. Am J Vet Res. 1966 Jan;27(116):33–38. [PubMed] [Google Scholar]
- VAUGHN J. B., GERHARDT P., PATERSON J. C. Excretion of street rabies virus in saliva of cats. JAMA. 1963 Jun 1;184:705–708. doi: 10.1001/jama.1963.73700220001013. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Otani S., Shiraki H. A study of the evolution of viral infection in experimental herpes simplex encephalitis and rabies by means of fluorescent antibody. Acta Neuropathol. 1965 Nov 18;5(2):288–306. doi: 10.1007/BF00686524. [DOI] [PubMed] [Google Scholar]
- Zalan E., Wilson C., Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand. 1979 Jul;7(3):213–220. doi: 10.1016/s0092-1157(79)80024-4. [DOI] [PubMed] [Google Scholar]