Abstract
Background
Imogolite is a naturally occurring hollow aluminosilicate nanotube with potential for engineered applications due to its high aspect ratio, hydrophilicity, and polarization. However, these same features raise concerns about potential adverse health effects. These concerns parallel those associated with multi-walled carbon nanotubes (MWCNTs), which are known to cause inflammation, fibrosis, and cardiovascular effects. The purpose of this study was to investigate how surface functionalization of imogolite influences its toxicity and biological response, with the aim of informing safer design of nanomaterials. Female C57BL/6J mice were exposed via intratracheal instillation to 6, 18, or 54 µg of hydroxylated (Imo-OH) or methylated (Imo-CH3) imogolite. Toxicity was assessed at day 1, 28 and 90 post-exposure, with carbon black (Printex90) nanoparticles as a benchmark. Pulmonary inflammation and systemic acute-phase response were assessed as key indicators of chronic health effects.
Results
Physicochemical characterization showed that Imo-OH dispersed as single nanotubes, while Imo-CH3 formed bundles, impacting surface accessibility. Both variants induced strong pulmonary inflammation, but Imo-OH elicited a stronger and more persistent neutrophil influx, lymphocyte recruitment, and acute-phase response. Cytotoxicity was low, though elevated total protein in bronchoalveolar lavage fluid indicated altered alveolar-capillary barrier integrity, especially for Imo-OH. Lung histopathology confirmed more severe lung lesions, macrophage aggregates, and type II pneumocyte hyperplasia in the Imo-OH group. Benchmark dose modeling revealed that Imo-OH’s inflammatory potential surpassed other high aspect ratio nanomaterials.
Conclusions
Both imogolite variants induced pulmonary inflammation and an acute-phase response in mice; however, these effects were markedly reduced for the methylated imogolite (Imo-CH3). In addition to surface functionalization, factors like bundle formation and by-product particles may also influence toxicity. These findings emphasize the pivotal role of surface chemistry—and associated structural properties—in shaping the biological response to nanomaterials, reinforcing the need for thoughtful design strategies to promote safer applications in nanotechnology.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-025-03647-w.
Keywords: Inflammation, Imogolites, HARN, Acute phase response, Safe-by-design, Pulmonary exposure
Background
Imogolite is a naturally occurring hollow, nanoscale aluminosilicate mineral primarily found in volcanic ash soils, but can also be chemically synthesized, enabling the production of both pristine and modified derivatives [1, 2]. Due to its distinctive structural and chemical properties, imogolite finds application in various fields, including catalysis, insulation, gas adsorption, membrane filtration, polymer-based nanocomposites, and flame retardants [3–5]. These applications are largely attributed to its nanotubular morphology, characterized by a high aspect ratio and properties like nanocavities, hydrophilicity, polarization, and a large band gap [6, 7].
However, the high aspect ratio of imogolite nanotubes raises health concerns particularly regarding inhalation exposure in occupational settings. Research on similar high aspect ratio nanotubes, especially multi-walled carbon nanotubes (MWCNTs), has shown that inhalation in rats can lead to chronic inflammation, fibrosis, and lung cancer [8, 9]. In mice, pulmonary exposure to MWCNTs has shown to cause inflammation, fibrosis, genotoxicity, plaque progression, and acute-phase responses [10–17], which are associated with increased risk of cardiovascular disease [18, 19].
To enhance the functionality of imogolite for specific applications, its intrinsic properties are often modified. For instance, the naturally hydroxylated inner surface can be functionalized with methyl to produce hybrid forms [5, 20]. Substituting silica with heavier atoms like germanium (Ge) can also alter material properties, as demonstrated in Ge-substituted imogolites [21]. While such modifications could enhance functionality, they may also affect toxicity, underscoring the need for comprehensive assessments.
Despite its potential, the toxicological data on imogolites remain limited. Ishikawa et al. investigated cellular responses to imogolite assemblies in human (Saos-2) and mouse (MC3T3-E1) osteoblast-like cells [22, 23]. Cells were seeded and cultured on scaffolds of imogolite and the findings indicated biocompatibility with enhanced proliferation and osteoblastic differentiation. Liu et al. assessed the cytotoxicity and genotoxicity of Ge-substituted imogolites in human fibroblast cell cultures, focusing on nanotube length [24]. While uptake was higher for shorter imogolites, no cytotoxicity was observed regardless of the length. However, genotoxicity - DNA damage driven by oxidative stress - where more pronounced with shorter imogolites. Rotoli et al. studied macrophages and airway epithelial cells exposed to imogolites, MWCNTs, and single-walled carbon nanotubes (SWCNTs) [25]. Imogolites were internalized by macrophages, but low cytotoxic and genotoxic effect with no clear dose-response relationship was shown. This low toxicity was attributed to their highly hydrophilic surface and the Al(OH)Al groups at the outer surface and SiOH groups at the inner surface [25]. Van den Brule et al. studied the toxicity of Ge-substituted imogolite in rats pulmonary exposed by intratracheal instillation [26]. They showed that Ge-based imogolites persisted in the lung and promoted lung inflammation, genotoxicity and fibrosis, challenging the conventional 5 μm threshold for fiber-induced pulmonary toxicity of high aspect ratio nanomaterials (HARN) [26]. Collectively, these studies demonstrate the need for standardized toxicity tests that consider particle length, chemical substitution, and surface modifications. Clearly, there is a need to alleviate potential health risk and therefore a Safe and Sustainable by Design (SSbD) strategy is essential. SSbD promotes early integration of safety considerations into material development, aiming to minimize toxicity at both molecular and macroscopic levels.
In this study, we investigate the pulmonary toxicity of two imogolite variants − hydroxylated (Imo-OH) and methylated (Imo-CH3) − in mice following intratracheal instillation. Specifically, we assess how increasing material complexity affects pulmonary inflammation and systemic acute-phase responses, which are key indicators of chronic health effects such as fibrosis, cancer, and cardiovascular disease. Our findings are discussed in the context of the physicochemical properties of these materials and their potential agricultural applications [27].
Methods
Imogolite nanotubes
The imogolites used in this study were synthesized at the PRODIGE facility of CEA, Paris-Saclay [28]. Imo-OH is by itself a multicomponent nanotube composed of various atoms (Si, Al, O, H), with a chemical structure that can be represented from the outer to the inner surface as (HO)3Al2O3Si(OH). Both surfaces are hydrophilic, with the external surface bearing Al2-µOH groups, while the inner surface is lined with Si-OH functionalities. The functionalization of the internal cavity with methyl moieties (Imo-CH3) increases the chemical complexity (Si, Al, O, H, C) [5]. This hybrid imogolite retains a similar outer surface but has a hydrophobic inner cavity fully covered by methyl groups, resulting in a slightly larger diameter than Imo-OH. The selective addition of carbon in the structure modifies the imogolite’s properties.
Both Imo-OH and Imo-CH3 were synthesized through the controlled hydrolysis of aluminum and silicon-based precursors, following protocols previously described [5, 29, 30]. For Imo-OH, tetraethoxysilane and aluminum chloride were used as precursors, and synthesis was performed at semi-pilot scale in 150 L of water. For Imo-CH3, trimethoxymethylsilane and aluminum-sec-butoxide were used as precursors, and synthesis was performed at lab scale in a 1 L Teflon reactor. The protocol is provided in the Supplementary Material.The characterization parameters are shown in Table 1.
Table 1.
Characterization parameters
| Sample | Mean length (Lm, nm) from AFM# or TEM$ Lognormal distribution (Ln, σ) | Si/Al From XPS+ |
|||||
|---|---|---|---|---|---|---|---|
| Inner diameter (nm) | Outer diameter (nm) | BET (m2/g) | Lm (nm) | Ln (nm) | σ | ||
| Imo-OH | 1.70* | 2.90* | 407 | 653# | 590.0 | 0.45 | 0.5 |
| Imo-CH3 | 1.88 | 3.08 | 625 | 309$ | 301.8 | 0.21 | 0.55 |
*The dimension of the diameter measured by SAXS are slightly higher than classical values obtained for Imo-OH where internal and external diameter are closer 1.5 and 2.7 nm, respectively (Liao et al. 2018, Picot et al. 2024). These diameters being determined by SAXS, it is an indication of the presence of proto-imogolites and allophane in equilibrium with the Imo-OH
Instillation suspensions
The instillation suspensions were prepared, following a standard protocol for toxicity studies of nanomaterials [31], at a concentration of 3.24 mg/ml in Nanopure water with 2% mouse serum and probe-sonicated on ice for 16 min at 10% amplitude without pause, using a Branson Sonifier S-450D (Branson Ultrasonics Corp., Danbury, CT, USA) equipped with a disruptor horn (model number 101-147-037). The suspensions were prepared form a stock Imo-OH suspension in milliQ water at 8.46 mg/ml or directly from the dried powder for Imo-CH3. The stock suspensions were then diluted to concentrations of 1.08, 0.36 and 0.12 mg/ml, corresponding to 54, 18 and 6 µg per 50 µl, respectively, and further sonicated for 4 min. Printex90, at a dose of 162 µg per mouse, was included as a reference material with known pulmonary toxicity. The vehicle, consisting of Nanopure water with 2% mouse serum, was similarly sonicated. The instillation volume per mouse was 50 µl.
Animals and exposure
Seven weeks old female C57BL/6JRj mice (Janvier Labs, France), were randomly divided into four experimental groups. The housing conditions followed those described by Jacobsen et al. [32]. In brief, mice were housed under the controlled environmental conditions with a 12-hour light/12-hour dark cycle, a room temperature of 22 °C ± 1 °C, and a relative humidity of 55% ± 5%. They had free access to feed (Altromin1324, Brogaarden, Denmark) and water. The mice were housed in groups of three or six per polypropylene cage with Enviro-dri bedding (Brogaarden, Denmark) along with wood blocks and hides as enrichment items. At the start of experiment, the mice were anaesthetized and administered Imo-OH, Imo-CH3, Printex90 carbon nanoparticles, or vehicle (2% mouse serum (v/v) in water) through a single intratracheal instillation [33–36].
The experiment was conducted in overlapping series with terminations at day 1, 28 and 90 post-exposure. Each series included vehicle control groups and groups of mice exposed to the particle materials. The sample size for each dose group at each time point was n = 6 for markers of lung toxicity. In addition, separate groups of n = 5 animals were allocated for histology, with a single dose group and termination at 28 and 90 days post exposure.
The experimental series also included particle materials not covered in this study. As a result, the cumulative number of vehicle control animals was N = 40 at day 1, and N = 27 at both 28 and 90 days post-exposure. The cumulative number of animals exposed to Printex90 was N = 12 at day 1, and N = 18 at both 28 and 90 days post-exposure.
The study was approved by the Danish Animal Experiment Inspectorate (permission no. 2020-15-0201-00485) and received prior approval from the local Animal Ethics Council.
Collection of plasma, tissue and BAL cells
Mice were euthanized at 1 day, 28 days, or 3 months post-exposure via intramuscular injection of 0.1 ml ZRF-solution (Zoletil 250 mg, Rompun 20 mg/ml, Fentanyl 50 mg/ml in sterile isotonic saline). Heart blood was withdrawn via intracardiac puncture, stabilized with K2EDTA and centrifuged to collect the plasma, which were stored at − 80 °C. Small pieces of lung and liver tissues were snap-frozen in liquid nitrogen and stored in cryotubes at − 80 °C until RNA isolation for mRNA expression analysis. Bronchoalveolar lavage fluid (BALF) was collected by flushing the lungs twice with 1 ml saline through the trachea. The BALF was kept on ice until separation of fluid and cells by centrifugation at 400 x g for 10 min at 4 °C. The separated BAL cells were re-suspended in HAMF12 medium with 10% fetal bovine serum. The total number of BAL cells and cell viability were measured using the NucleoCounter NC-200 (Chemometec, Allerød, Denmark). BAL differential cell counts of macrophages, neutrophils, lymphocytes, and eosinophils were assessed by counting 200 cells per sample under a light microscope [31].
Total protein and LDH in BAL fluid
The Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific Inc.) was used to measure total protein content in BALF [14]. The Lactate Dehydrogenase Assay Kit (Sigma-Aldrich MAK464 from Merck KGaA, Darmstadt, Germany) was used to measure LDH activity in BALF.
mRNA expression of Saa3 in lung tissue and Saa1 in liver tissue
Gene expression levels were measured by quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR) as previously described [15, 37, 38]. The acute phase response was assessed by measuring Saa3 and Saa1 mRNA expression levels in lung and liver tissues, respectively. Total RNA was isolated using the Maxwell® 16 LEV simplyRNA Tissue Kit (AS1280, Promega, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was prepared using TaqMan® reverse transcription reagents (Applied Biosystems, USA) following the manufacturer’s instructions. Total RNA and cDNA concentrations were measured with the NanoDrop 2000c (ThermoFisher, USA). Gene expression levels were determined using predesigned TaqMan expression assays with 18 S rRNA as the endogenous control (Applied Biosystems, USA). Samples were run in triplicates on the ViiA7 Real-Time PCR detector (Applied Biosystems, USA). Negative controls were included in each analysis run. The relative expression of the target genes was measured using the comparative CT method.
SAA3 protein levels in plasma
The Mouse SAA3 ELISA kit (Millipore EZMSAA3 from Merck KGaA, Darmstadt, Germany) was used to measure SAA3 protein levels in plasma following the manufacturer’s protocol [15]. At the day 1 post-exposure time point, all doses were analyzed, while only the highest dose (54 µg/mouse) was analyzed at the day 28 post-exposure time point. Control groups (n = 6) were randomly selected to represent the different exposure days.
Histological examination of lung and liver tissue
At necropsy on days 28 and 90 post-exposure, lungs and liver samples were collected from six vehicle control mice and five mice from Imo-OH and Imo-CH3 high-dose groups (54 µg/animal). For lung fixation, the trachea was cannulated, and 4% neutral buffered formaldehyde was infused at a constant pressure of 25 cm before opening the thorax. The lungs were then excised and immersed in the same fixative. Liver samples were collected after inspecting the abdominal cavity and fixed by immersion in 4% neutral buffered formaldehyde. Tissue sampling and trimming followed established guidelines for organ processing in rodents [39, 40]. After fixation, lung and liver samples were embedded in paraffin, sectioned at 2–3 μm thickness, and stained with hematoxylin and eosin (HE) for histological examination. Light microscopy analysis was conducted on all control and high-dose groups by a single operator, first with knowledge of treatment groups, followed by a blinded evaluation [41]. The diagnostic nomenclature for microscopic changes adhered to INHAND recommendations for the lungs [42] and liver [43].
For focal mononuclear cell infiltrations, laden macrophage aggregates, and macrophage-dense areas, both the number and severity of changes were reported separately for the left and the right lung (cranial, middle accessory, and caudal lobes). Mononuclear cell infiltrations were defined as regions with an increased density of mononuclear cells compared to the surrounding tissue, forming distinct shapes and sizes. Only infiltrates containing more than 20 cells were counted. These infiltrations primarily consisted of lymphocytes but occasionally included other inflammatory cells and were observed near blood vessels, bronchioles, alveolar ducts, interstitial regions or subpleural areas. Laden macrophage aggregates were defined as clusters of alveolar macrophages in close proximity at a single site. In contrast, areas of laden macrophages were defined as lung regions with an increased density of alveolar macrophages compared to the background, but where macrophages were not clustered together.
In the liver, inflammatory cell infiltrates (focal mononuclear cell infiltrations) were categorized as small (≤ 10 inflammatory cells, occasionally accompanied by necrotic hepatocytes with distinct eosinophilic cytoplasm) or large (> 10 inflammatory cells, often surrounding necrotic hepatocytes with distinct eosinophilic cytoplasm, with or without apoptotic bodies or debris). Liver changes were assessed for severity, including microvesicular steatosis, an apparent increase in binucleate hepatocytes and Kupffer cells, hyperplasia of connective tissue near bile ductules, and oval cell hyperplasia.
The severity of histopathological changes in both the lungs and liver was graded on a five-point scale Grade 1: minimal/very few/very small; Grade 2: mild/few/small; Grade 3: moderate/moderate number/moderate size; Grade 4: marked/many/large; Grade 5: massive/extensive number/extensive size.
Statistical analyses, exploratory data analyses
Statistical analyses were performed using the software package GraphPad Prism 8.0.2 (GraphPad Software Inc., La Jolla, CA, USA). Data were tested for normality using the Shapiro-Wilk test, for variance homogeneity using the Brown-Forsythe test, and each dose group was compared with the vehicle control group by ordinary one-way ANOVA with Dunnett’s multiple comparison test. Data that did not fulfill normality and variance homogeneity criteria were analyzed by the nonparametric Kruskal-Wallis test. Pairwise statistical comparisons between Imo‑OH and Imo‑CH3 for each dose group were performed using Sidak’s multiple comparisons test. Gene expression data were logarithmically transformed before analysis. P-values ≤ 0.05 were considered significant.
Statistical analyses, benchmark dose analyses (BMD)
Benchmark dose analyses were performed with the ToxicR R package (v. 23.4.1.1.0). The neutrophil values were log2-transformed prior to the BMD analysis for each dose, including control. If the value was empty, it was replaced with the total number of cells/400, as that was the lowest limit of the detection range. The BMD analysis used the robust median and median absolute deviation (MAD) values as inputs, as well as n or the number of observations. BMDs were obtained as a weighted average of exponential 3, exponential 5, Hill’s and power models fitted with the Laplace method and a benchmark response (BMR) of one standard deviation. The mass dose levels, and the surface area dose levels were modelled separately. The BMD and the 95% lower and upper limits were obtained (BMDL and BMDU). BMD values 20-fold greater or lesser than the measured concentrations were set to these limits.
Results
Imogolite characterization
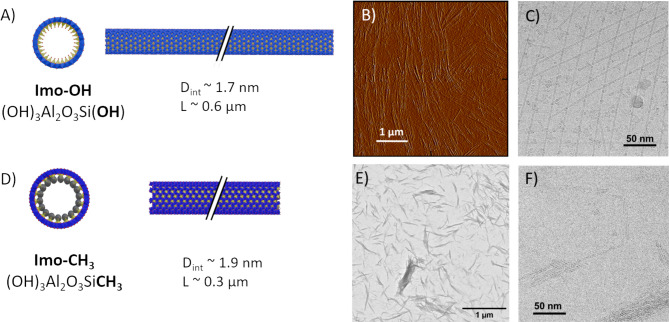
Imogolites were analyzed prior to their use using a combination of techniques (atomic force microscopy (AFM), transmission electron microscopy (TEM), small-angle X-ray scattering (SAXS), transmission electron cryomicroscopy (cryoTEM)) to determine chemical composition, physical dimensions, specific surface areas and, agglomeration state (Table 1; Fig. 1, Fig. S1-S6, Table S1 and S2). X-ray photoelectron spectroscopy (XPS) identified the characteristic Si/Al ratio of imogolites, interpretation of XPS for imogolites is rather difficult because of the wall polarization [7]. Infrared spectroscopy (IR) identified the characteristic doublet between 1000–900 cm−1 (Si-O-Al stretching) of the nanotube structure and the typical bands at 781 and 1275 cm−1 of Si-C and Si-CH3 respectively for the Imo-CH3 [30, 44] (See Fig. S1 and Fig. S2 in the supplementary material for the detailed IR and XPS spectra, respectively). XPS spectra (survey and core level) of Imo-OH and Imo-CH3, obtained from dried powder and the table of the peak attribution issued from fitting are shown in the supplementary material (Fig. S3, S4, S5, and Table S2). The deconvolution of Si 2p core level spectra and C 1 s spectra clearly identified the differences between both imogolite types and evidence the Si-C bound typical in Imo-CH3. SAXS patterns (Fig. S6) describe a dispersion of nanotubes which can be fitted using a homogeneous cylinder core-shell model including the association of 1 to 4 tubes in a bundle, to extract the internal and outer diameters [7, 45]. The parameters used for fitting the SAXS patterns are shown in Table S2. For Imo-OH, the obtained values are slightly higher than classical values reported in the literature, indicating the presence of allophanes and proto-imogolites [29, 46]. These smaller particles (nanospheres and nanotiles) with imogolite-like structure are in equilibrium with the imogolites. They are clearly visible in the cryoTEM images (Fig. 1C), although in many images they represent only a small fraction of the sample by mass. The SAXS patterns also indicate that Imo-CH3 tends to form bundles, while Imo-OH is more easily dispersed as single nanotubes (Fig. S2). This feature is confirmed in the cryoTEM images, where Imo-CH3 predominantly appears as bundles (Fig. 1F). The precise quantification of the bundles size distribution could be done following a systematic X-ray scattering and cryoTEM approach [47]. However, for this study, we limit the analysis to the accessible specific surface area to take into account the formation of bundles. The formation of bundles has a significant impact on the specific surface areas accessible to biological moieties. Specific surface areas are typically measured by BET analysis on dried powder; however, Dhaini et al. demonstrated that these measurements underestimate the true surface area due to the formation of large bundles, which prevents access to the nitrogen N2 gas molecules [48]. Additionally, they emphasized the different contributions of intratube, intertubes, and interbundles surfaces to the total surface area, as well as differences in their accessibilities. Therefore, the use of BET-measured surface areas should be interpreted with caution when comparing the effects of the imogolite suspensions.
Fig. 1.
(A) Schematic representation of Imo-OH is shown with its chemical formula, internal diameter (Dint), and average length (L), including both transversal and side view. Atoms are represented as spheres in red, light blue, and blue corresponding to oxygen, silicon, and aluminum, respectively. (B) Example of an AFM images and (C) a cryoTEM image of Imo-OH is shown. (D) Schematic representation of Imo-CH3 with its chemical formula, the internal diameter (Dint) and the average length (L), including both transversal and side view with. Atoms are represented as spheres in red, yellow, blue and grey, corresponding to oxygen, silicon, aluminum and carbon, respectively. (E) Example of a TEM images and (F) a cryoTEM image of Imo-CH3 is shown
Inflammation
Both types of imogolite triggered a significant pulmonary inflammatory response, primarily characterized by an increase in the total cell number (Supplemental Table S3), largely driven by a dose-dependent increase in neutrophils on day 1 post-exposure (Fig. 2A). Imo-OH induced a strong inflammatory response across all dose levels, while Imo-CH3 showed dose-dependency with a similar significant effect at the highest dose (Fig. 2A). Imo-OH also exhibited a prolonged inflammatory response, with notably elevated neutrophil levels persisting through day 28 and 90 post-exposure (Fig. 2B and C and Supplementary Table S3). Additionally, Imo-OH caused a significant increase in lymphocytes and eosinophils on day 1 post-exposure (Supplemental Table S3), this effect was only observed at the middle dose, resulting in a non-monotonic (inverted) dose-response pattern. This pattern aligns with findings from previous studies on carbon nanotube exposure [49, 50]. Elevated lymphocyte levels were also observed on day 28 (middle dose) and day 90 (highest dose) post-exposure (Supplemental Table S3).
Fig. 2.
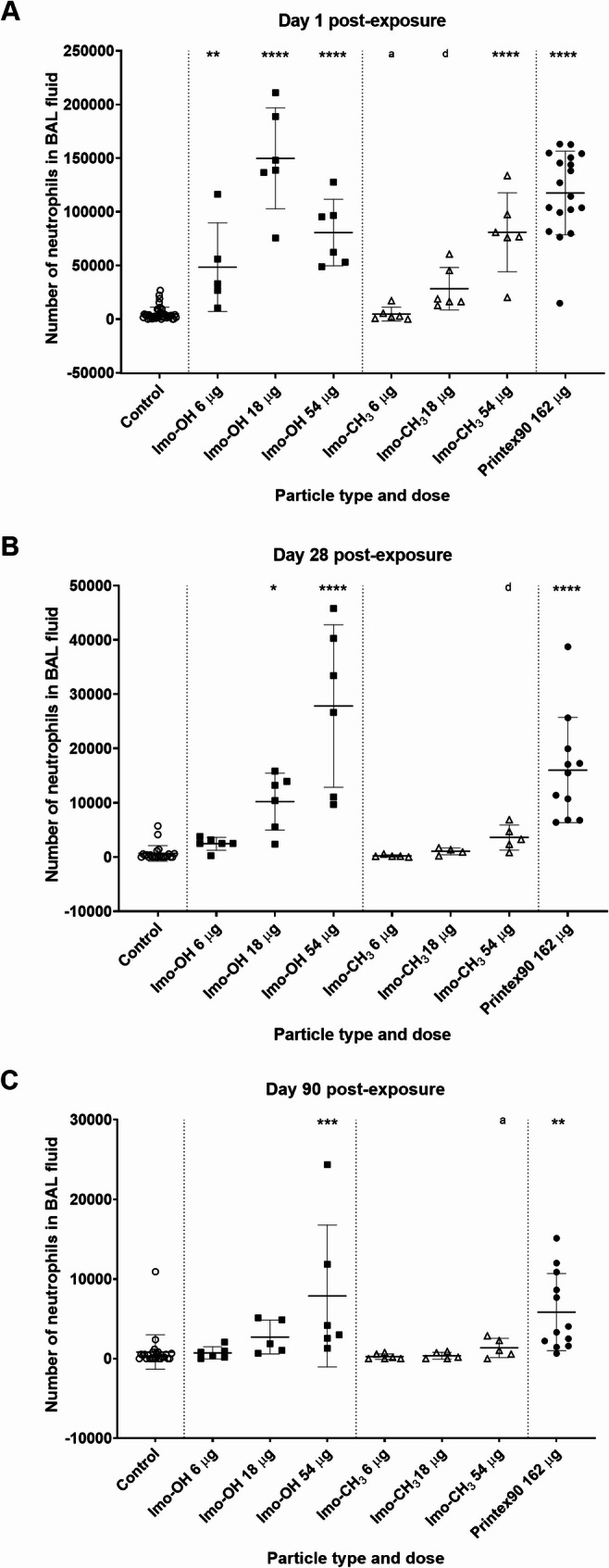
Neutrophil influx (A) 1, (B) 28 and (C) 90 days post-exposure to imogolites. Data are shown as individual values, mean ± SD. Stars indicate comparison with the control (Dunnett’s multiple comparison method), and letters indicate comparison to the same dose of Imo-OH (Sidak’s multiple comparison test). Significance levels are as follows: * or a, P ≤ 0.05; ** or b, P ≤ 0.01; *** or c, P ≤ 0.001; **** or d, P ≤ 0.0001
Cytotoxicity and total protein levels in BAL fluid
Cytotoxicity remained largely unaffected, there were no significant change in cell viability at any post-exposure time points (Supplemental Fig. S7) and increased LDH activity was only significantly increased at the highest dose of Imo-OH at day 28 post-exposure (Supplemental Fig. S8B).
At day 1 post-exposure, Imo-OH elevated total protein in BAL fluid across all doses, whereas Imo-CH3 induced a significant effect only at the high dose (Fig. 3A). Overall, the total protein levels at day 28 post-exposure (Fig. 3B) were reduced across all dose groups compared to day 1 post-exposure (Fig. 3A). In the 54 µg Imo‑CH3 group, protein levels at day 28 post-exposure have returned to values comparable to the control group, resulting in a lack of significant difference at this time point. However, the percentage decreases observed at 28 post-exposure are similar to those seen at day 1 post-exposure (e.g., 26% decrease for 54 µg Imo‑CH3 and 32% for 54 µg Imo‑OH). Notably, Imo-OH showed a sustained response at day 28 post-exposure, unlike Imo-CH3 (Fig. 3B). Day 90-post exposure were not analyzed.
Fig. 3.
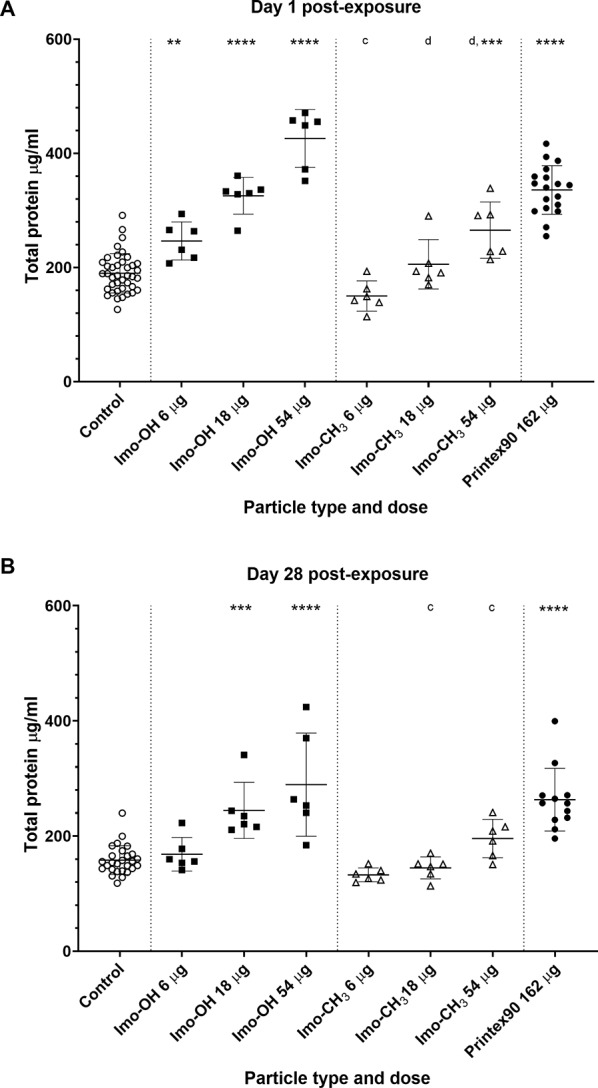
Total protein level in BAL fluid (A) 1 and (B) 28 days post-exposure to imogolites. Data are shown as individual values, mean ± SD. Stars indicate comparison with the control (Dunnett’s multiple comparison method), and letters indicate comparison to the same dose of Imo-OH (Sidak’s multiple comparison test). Significance levels are as follows: * or a, P ≤ 0.05; ** or b, P ≤ 0.01; *** or c, P ≤ 0.001; **** or d, P ≤ 0.0001
The total protein levels was closely mirrored by the neutrophil influx as also previously observed for MWCNTs ([14], Supplemental Fig. S9).
Acute phase response
At day 1 post-exposure, plasma levels of the acute phase protein SAA3 were significantly elevated for both imogolites at the highest dose (Fig. 4A). Imo-OH also induced a significant increase at the middle dose and maintained a significantly elevated level at the high dose through day 28 post-exposure (Fig. 4B). The elevated SAA3 protein level observed in a single low-dose Imo-OH sample appears to be an outlier (Fig. 4A). We retained this data point for transparency, as we have no technical or biological explanation for its deviation. Due to the high cost of the SAA3 protein assay kit, we prioritized sample inclusion from key experimental groups and excluded Printex90 from this analysis. Based on prior experience, SAA3 plasma protein levels typically return to baseline levels by day 28 following exposure to lower doses [15, 16]. Therefore, at day 28 post-exposure, we focused on the highest doses of Imo-OH and Imo‑CH3 to assess the persistence of systemic effects, and the lower doses were excluded from the analysis.
Fig. 4.
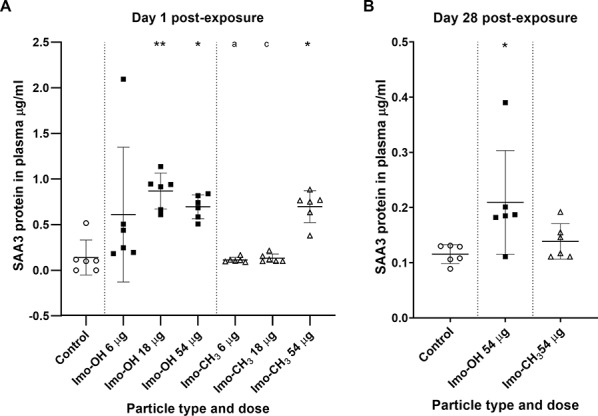
SAA3 protein in plasma (A) 1 and (B) 28 days post-exposure to imogolites. Data are shown as individual values, mean ± SD. Stars indicate comparison with the control (Dunnett’s multiple comparison method), and letters indicate comparison to the same dose of Imo-OH (Sidak’s multiple comparison test). Significance levels are as follows: * or a, P ≤ 0.05; ** or b, P ≤ 0.01; *** or c, P ≤ 0.001; **** or d, P ≤ 0.0001
At the gene expression level, Saa3 mRNA was significantly increased in lung tissue of mice at day 1 post-exposure by both types of imogolite (Fig. 5A). At day 28 post-exposure, the increase remained statistically significant; however, for Imo-CH3, this was observed only at the high dose (Fig. 5B). At day 90 post-exposure, Saa3 mRNA levels had returned to control levels (Supplemental Fig. S10).
Fig. 5.

Pulmonary Saa3 mRNA expression levels (A) 1 and (B) 28 days post-exposure to imogolites. All values are log transformed and presented as individual values, mean ± SD. Stars indicate comparison with the control (Dunnett’s multiple comparison method), and letters indicate comparison to the same dose of Imo-OH (Sidak’s multiple comparison test). Significance levels are as follows: * or a, P ≤ 0.05; ** or b, P ≤ 0.01; *** or c, P ≤ 0.001; **** or d, P ≤ 0.0001
Pearson correlations between neutrophil numbers, pulmonary Saa3 mRNA, and plasma SAA3 protein levels were analyzed at day 1 post-exposure (Supplemental Fig. S11). Significant correlations were found between neutrophil numbers and Saa3 mRNA levels (r = 0.79, p-value < 0.0001) (Fig. S11A ), between Saa3 mRNA levels and SAA3 protein levels (r = 0.55, p-value 0.0003) (Fig. S11B), and between neutrophil numbers and SAA3 protein levels (r = 0.78, p-value < 0.0001) (Fig. S11C), indicating a high level of inter-correlation consistent with previous findings across different types of nanomaterials [51].
In liver tissue, Saa1 mRNA levels were significantly increased on day 1 post-exposure in response to Imo-OH across all dose levels and in response to the high dose of Imo-CH3 (Fig. 6). Gene expression at later time points in liver tissue was not analyzed.
Fig. 6.
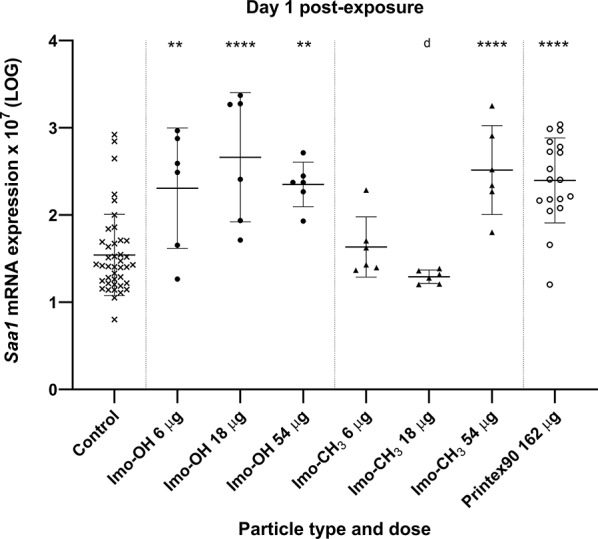
Hepatic Saa1 mRNA expression levels a day post-exposure to imogolites. All values are log transformed and presented as individual values, mean ± SD. Stars indicate comparison with the control (Dunnett’s multiple comparison method), and letters indicate comparison to the same dose of Imo-OH (Sidak’s multiple comparison test). Significance levels are as follows: * or a, P ≤ 0.05; ** or b, P ≤ 0.01; *** or c, P ≤ 0.001; **** or d, P ≤ 0.0001
Histological examination of lung and liver tissue
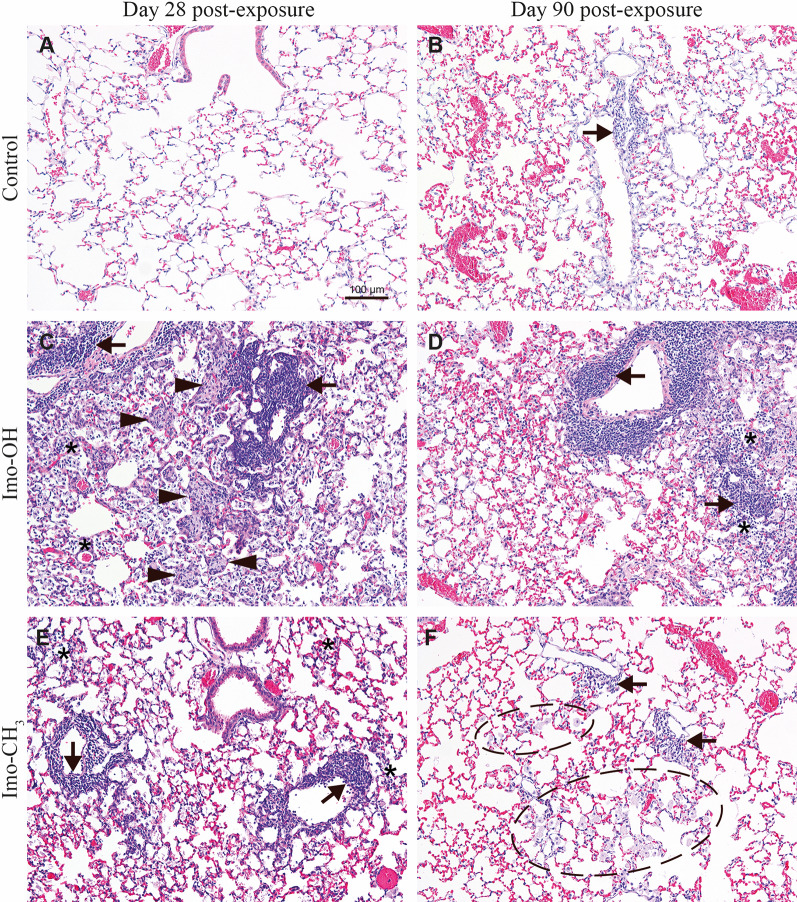
In the lungs of the mice exposed to Imo-OH and Imo-CH3, multifocal mononuclear cell infiltrations at blood vessels, bronchiole or located interstitial or subpleural, laden macrophage aggregates and areas of laden macrophages (examples shown in Supplemental Fig. S12), and areas with debris in alveoli were observed 28 days post-exposure. Overall, the changes were more abundant and severe in mice exposed to Imo-OH group than in mice exposed to Imo-CH3 (Table 2 and Supplemental Table S4). Figure 7 depicts examples of the microscopic changes in the lungs of mice after exposure to a high dose of imogolites in comparison to control mice. There were no changes in the control animals on day 28 post-exposure (Fig. 7A) and minimal to mild mononuclear cell infiltrations were sporadically observed in the control animals on day 90 post-exposure (Fig. 7B). The changes appeared more severe in mice exposed to Imo-OH at both time points post-exposure (Fig. 7C and D) compared to mice exposed to Imo-CH3 (Fig. 7E and F). The aggregates of laden macrophages in mice exposed to Imo-OH often had a differentiated appearance with no clear foamy structure of the cytoplasm while in mice exposed to Imo-CH3 their foamy appearance was preserved. The macrophage aggregates and areas were localized either at the bronchioalveolar junctions, alveolar ducts or in alveoli.
Table 2.
Type and incidence of microscopic changes in the lungs of mice 28 and 90 days after a single intratracheal exposure to a vehicle (2% mouse serum in nanopure water; controls) or to Imo-OH, Imo-CH3 (54 µg/animal)
| Day post exposure Type of change |
Day 28 | Day 90 | ||||
|---|---|---|---|---|---|---|
| Control n = 6 |
Imo-OH n = 5 |
Imo-CH3 n = 5 |
Control n = 6 |
Imo-OH n = 5 |
Imo-CH3 n = 5 |
|
| Mononuclear cell infiltrationB peribronchial, perivascular, interstitial or subpleural (focal or multifocal) | 0/6A |
5/5** 0/0/0/0/5F |
5/5** 0/0/1/2/2 |
3/6 0/1/1/1/0 |
5/5 0/0/1/0/4 |
5/5 0/2/1/0/2 |
| Mononuclear cell infiltration in single alveoli, focal | 0/6 |
5/5** 0/1/1/0/3 |
4/5 0/0/2/1/1 |
1/6 1/0/0/0/0 |
5/5* 0/2/3/0/0 |
3/5 3/0/0/0/0 |
| Single, laden alveolar macrophages | 0/6 |
5/5** 1/1/1/1/1 |
5/5** 3/1/0/1/0 |
0/6 |
5/5** 2/3/1/0/0 |
5/5** 5/0/0/0/0 |
|
Area of laden alveolar macrophagesC (focal or multifocal) |
0/6 |
5/5** 1/0/3/0/1 |
5/5** 0/0/2/3/0 |
0/6 |
5/5* 1/0/2/1/1 |
4/5 0/1/1/2/0 |
|
Small aggregates of laden macrophages D (focal or multifocal) |
0/6 |
4/5 1/3/0/0/0 |
3/5 1/2/0/0/0 |
0/6 |
3/5 1/2/0/0/0 |
0/5 |
|
Large aggregates of laden macrophages D (focal or multifocal corresponding to severity grade G3-G5) |
0/6 |
5/5** 0/0/0/0/5 |
1/5 0/0/1/0/0 |
0/6 |
3/5 0/0/2/1/0 |
0/5 |
| Debris in alveoli | 0/6 |
5/5** 0/1/0/0/4 |
5/5** 2/0/0/1/2 |
0/6 |
5/5** 2/0/0/0/3 |
3/5 1/0/0/1/1 |
| Hypertrophy/hyperplasia of type II pneumocytes | 0/6 |
4/5 2/1/1/0/0 |
0/5 | 0/6 |
1/5 1/0/0/0 |
0/5 |
| Desquamation of bronchiole epitheliumE | 0/6 |
5/5** 5/0/0/0/0 |
0/5 | 0/6 |
3/5 3/0/0/0/0 |
2/5 2/0/0/0/0 |
* p > 0.05, ** p < 0.01 Fisher exact test. The data on incidence and severity originate from the whole lung of each animal i.e. from the left, right cranial, right middle, right accessory and right caudal lobes
A Incidence of each change is expressed by the number of animals with a given change of a total animals examined in the group
B Mononuclear cell infiltrations: an area of lung tissue where the density of mononuclear cells is increased compared to the background of the surrounding, so that the mononuclear cells collection has a shape and size. Only the infiltrations of more than 20 cells were counted. The infiltrations consist usually of lymphocytes but other inflammatory cells like neutrophils or eosinophils can also be present in the infiltration. The infiltrations are observed near blood vessels, or bronchioles or alveolar ducts, interstitial or subpleural
C Area of laden macrophages: an area of tissue where the density of macrophages is higher than background but the laden macrophages are situated not close to each other
D Laden macrophage aggregates: groups of alveolar laden macrophages at one site close to each other. The aggregates of severity grade 1 to 2 are considered as small and the aggregates of severity grades from 3 to 5 are considered as big
E This change was not recorded, when there was an indication of a tangential cut of bronchioles. A tangential cut can result in an artifact resembling a desquamation of bronchiolar epithelium
F Severity of a given change was evaluated in a semi-quantitative way using a five grade scoring system. Grade 1: minimal/very few/very small; grade 2: mild/few/small; grade 3: moderate/moderate number/moderate size; grade 4: marked/many/large; grade 5: massive/extensive number/extensive size. Results for severity are presented as a number of animals per group for which a given change was assigned to a certain grade either G1/G2/G3/G4/G5. When a given change was seen in several sites of the lungs, each focus was assigned a severity grade, and the highest severity grade was chosen to represent the animal in this table. For example, the severity reported as 0/1/2/0/0 means that 1 animal in the group had a given change of the maximum severity graded as G2 (small) and 2 animals had a given change of the maximum severity graded as G3 (moderate size)
Fig. 7 .
Microscopic changes in the lungs of mice (A, C, E) 28 and (B, D, F) 90 days after a single intratracheal exposure to a vehicle (2% mouse serum in nanopure water; controls) or to Imo-OH or Imo-CH3 (54 µg/animal). Asterisks: mononuclear cell infiltrations in alveoli on C, D and E. Arrows: foci of mononuclear cell infiltrations around or at blood vessels in B C, D, E, and F. Arrowheads: aggregates of laden macrophages in C. Ellipse: area of laden macrophages in F.
On day 90 post-exposure the mononuclear cell infiltrations, and areas of laden macrophages and debris were still present in both imogolite exposed groups. These changes appeared more severe in the Imo-OH group compared to the Imo-CH3 group (Fig. 7D and F). The aggregates of laden macrophages were only seen in the Imo-OH group (Fig. 7D).
Hypertrophy/hyperplasia of type II pneumocytes of minimal to moderate severity was observed in Imo-OH group at day 28 post-exposure (Fig. 8A; Table 2). Some of the type II pneumocytes appeared slightly larger than in controls at day 90 post-exposure (Fig. 8B). Hypertrophy/hyperplasia was not observed in the control groups or in mice exposed to Imo-CH3 at any time points.
Fig. 8.
Imo-OH, A: day 28 post exposure. Hypertrophy/hyperplasia of type II pneumocytes (arrows) and increased presence of laden alveolar macrophages (arrowheads) variably associated with mononuclear inflammatory cell infiltrate (asterisks). B: day 90 post exposure. Some of type II pneumocytes were slightly larger than normal (long arrows). Debris (+) in alveoli and alveolar ducts
Microscopic examination of the liver samples from imogolite-exposed mice 28 and 90 days post-exposure did not reveal any morphological differences from controls in the type, incidence and severity of the observed changes (Supplemental Table S5 and S6).
Comparisons of imogolites with other nanotubes
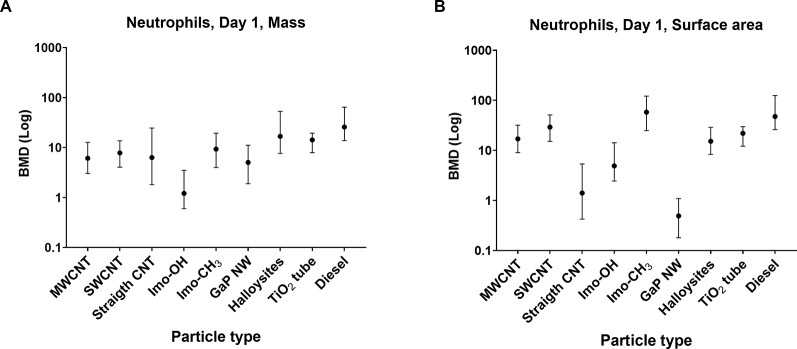
The inflammatory potential of imogolites was compared with other high-aspect-nanomaterials (HARNs) using benchmark dose (BMD) modelling of neutrophil influx on day 1 (Fig. 9). The BMD for Imo-OH-induced inflammation was compared to BMD values for previously published HARNs including entangled MWCNTs, SWCNTs, long and straight MWCNTs [16, 17], long and straight GaP nanowires [52], short and straight nanofibers such as halloysite fibers [53] and TiO2 nanotubes [54]), as well as diesel exhaust particles, that were included in the analysis as a benchmark material with known toxicity [55–57]. The mass-based BMD for Imo-OH was lower than that of other HARNs, whereas Imo-CH3 BMD values were comparable to that of other HARNs and lower than the BMD for diesel exhaust particles. For the surface area-based BMD, Imo-OH had the lowest BMD, along with straight CNTs and GaP nanowires.
Fig. 9.
Benchmark dose (BMD) modelling of neutrophil influx 1 day post-exposure for the studied imogolites, entangled MWCNTs (n=20), SWCNTs (n=7), straight and long MWCNTs (n=2), straight and long GaP NWs (n=1), straight and short Halloysite fibers (n=2), TiO2 tube (n=1) and diesel engine exhaust particles (n=7). The mass dose levels (A), and the surface area dose levels (B) were modelled separately
Previously, we investigated the inflammatory effects of highly hydroxylated materials in vivo, comparing them to corresponding functionalized materials without free OH groups using methodologies similar to those in the present study [58, 59]. A comparison of neutrophil influx at day 1 post-exposure, at equivalent mass doses, revealed a significant reduction when mice were exposed to reduced graphene oxide (rGO) versus graphene oxide (GO) and carboxylated nanofibrillated cellulose (AS (-COOH)) versus unmodified nanofibrillated cellulose (Fine NFC) (Fig. 10). Notably, GO and Fine NFC were highly hydroxylated, whereas rGO and AS (-COOH) were not.
Fig. 10.

Comparison of neutrophil influx in mice lungs after intratracheal instillation to the same mass dose levels of imogolites, graphene oxides and nanocelluloses at day 1 post-exposure. Original data for graphene oxide (GO) and reduced graphene oxide (rGO) is published in Bengtson et al. 2017 and nanofibrillated cellulose (NFC) and carboxylated cellulose (AS (-COOH)) is published in Hadrup et al. 2019
Discussion
In this study, we assessed the pulmonary toxicity of imogolites with a focus on inflammatory responses and systemic acute phase response. Pulmonary inflammation and systemic responses are critical endpoints linked to long-term health effects such as fibrosis, cancer, and cardiovascular disease [60, 61].
Although inhalation studies are the gold standard of toxicity testing of inhalation exposure, intratracheal instillation, used in this study to expose mice to imogolites, is an established method for hazard comparison. It allows precise control of the dose deposited in the lungs and ensures even distribution across lung lobes, as demonstrated in previous studies [14, 62, 63]. For imogolites, van den Brule et al. demonstrated that intratracheal instillation is a powerful method to study the lung toxicity of nanometer long Ge-based imogolites [26]. Moreover, the correspondence between inhalation and instillation exposures as previously shown for CNT-induced neutrophil influx [64] and lung distribution of metal oxides [63] supports the relevance of this approach for toxicity studies.
Both imogolite variants triggered a robust acute inflammatory response in the lungs, characterized by substantial neutrophil influx (Fig. 2). However, only Imo-OH exposure resulted in persistently elevated neutrophil levels at later time points. The increased neutrophil influx is associated to elevated protein levels in BAL fluid (Fig. 3). Mechanistically, the increased protein levels can be attributed to damage to the alveolar-capillary barrier, allowing protein leakage into the lungs; epithelial cell damage, leading to the release of intracellular proteins; and cytokine secretion by immune cells, which exacerbates tissue injury and increases vascular permeability. These findings align with previous studies demonstrating strong correlations between neutrophil influx and protein levels after airway exposure to nanomaterials [14, 63]. Similarly, Van den Brule et al. showed a correlated increase of neutrophil influx and protein level for Ge-based imogolite although not hightlighted by the authors [26]. Additionally, the elevated protein levels observed in this study are consistent with AOP173, where increased membrane permeability is recognized as a key event [65]. While AOP173 specifically describes membrane permeability changes, the presence of elevated proteins in BAL fluid can be considered a supporting indicator of this key event. Increased membrane permeability is a critical early step in the development of fibrosis, as described in AOP173, which outlines how inhalation of fibrous materials such as carbon nanotubes (CNTs) may induce fibrosis [65].
The systemic acute phase response, assessed in terms of SAA3 plasma protein levels, showed significant increases for both imogolites at the highest dose on day 1 post-exposure (Fig. 4). Notably, Imo-OH also caused a significant elevation at the middle dose on day 1 and sustained increased SAA3 levels through day 28, indicating a prolonged systemic response. This pattern reflects that while Saa3 gene expression at day 28 was significantly elevated, the Saa3 mRNA levels were much lower than at day 1 post-exposure (Fig. 5). We have previously shown that highly increased Saa3 mRNA levels are required before increased SAA3 protein levels may be detected in plasma [51]. The transient elevation of Saa3 mRNA levels at Day 1 and 28 reflects the acute phase nature of the response. These increases highlight an early and intermediate transcriptional activation following exposure, which resolves by day 90 post-exposure, indicating recovery. This pattern underscores the utility of Saa3 mRNA levels as a sensitive marker for early and subacute systemic responses to nanomaterial exposure.
Systemic effects were also confirmed by the upregulation of hepatic Saa1 mRNA 1 day after pulmonary exposure (Fig. 6), consistent with previous studies on various nanomaterials [15, 37, 51, 54, 66–68]. Hepatic Saa1 upregulation may be triggered either by direct translocation of nanomaterials to the liver [20, 52, 68, 69] or indirectly via cytokines and acute phase mediators released from the inflamed lung [15, 70]. The transient nature of this response is well-documented: the hepatic acute phase response typically peaks around 1 day post-exposure and resolves relatively quickly, whereas the pulmonary response persists [15]. Accordingly, Saa1 expression was measured only at the early post-exposure time point in this study. Despite evidence of a hepatic acute phase response, no histopathological liver changes were observed 28 days post-exposure. These findings support the liver’s role as a secondary target in the systemic response to inhaled nanomaterials without progression to overt pathology, as acute phase reactions are functional and do not necessarily lead to structural damage.
The acute phase response is particularly concerning due to its systemic implications, including an increased risk of cardiovascular disease [18, 19]. Elevated plasma SAA3 levels promotes plaque formation, as demonstrated in susceptible mouse models [70–72], linking particle and fiber-induced pulmonary acute phase response to atherosclerosis and thereby cardiovascular disease. The systemic effects observed in this study highlight the potential long-term health risks of sustained pulmonary inflammation and acute phase responses following exposure to imogolites as observed for both Imo-OH and Imo-CH3.
The inflammation observed in this study is consistent with the common understanding of pulmonary responses to particulate exposure. Pulmonary inflammation represents an early key event associated with adverse health outcomes, including cancer, fibrosis, and chronic obstructive pulmonary disease [61, 71, 72]. A hallmark of this inflammatory response is the influx of neutrophils into the lung, which, in this study, correlated with elevated levels of the acute phase protein SAA3 in plasma and Saa3 mRNA expression in lung tissue (Fig. S6). Such correlations have been observed across exposure methods (inhalation and instillation), post-exposure time points, and nanomaterial types [15, 51, 54, 58, 59, 67, 73, 74].
These toxicological findings are confirmed by the histopathological evaluation of lung tissue, where all changes, both in terms of incidence and severity score, appeared significantly more predominant in the Imo-OH group compared to the Imo-CH3 group at both day 28 and day 90 post-exposure (Fig. 7). Interestingly, hypertrophy/hyperplasia of alveolar epithelial type II cells was observed in the lung after exposure to Imo-OH but not to Imo-CH3, further highlighting the toxicity differences between the two imogolites (Fig. 8).
The inflammatory potential of imogolites was assessed in comparison to other HARN using benchmark dose (BMD) modeling (Fig. 9). Overall, imogolites elicited an acute inflammatory response that was broadly comparable to the BMDs observed for other HARNs, including SWCNTs, short and thin MWCNT, long and straight MWCNTs, and other HARNs included in the analysis. This indicates that imogolites induce a similar level of inflammatory response as these materials. Notably, Imo-OH exhibited the lowest BMD by mass, and when assessed by surface area, it ranked among the lowest, alongside straight CNTs and GaP nanowires. Diesel engine combustion particles were included as a benchmark material because of their well-documented toxicity and occupational exposure limits for diesel engine exhaust in many countries, based on chronic inhalation studies in rats and human epidemiological data [75–77]. Previous studies from our group have reported inflammatory, acute-phase response, and genotoxic effects following pulmonary exposure in mice (Bendtsen et al. 2019; Bendtsen et al. 2020; Kyjovska et al. 2015). In the present BMD analysis, diesel engine combustion particles demonstrated a higher BMD compared to all HARNs tested. This suggests that HARNs – including imogolites, and particularly Imo-OH − have a stronger inflammatory potency than diesel combustion particles.
The observed differences in toxicity between Imo-OH and Imo-CH3 are noteworthy. Imo-OH induced stronger and more persistent inflammatory responses and systemic effects compared to Imo-CH3.
This distinction cannot be explained by differences in BET surface area, as normalization (407 m²/g for Imo-OH vs. 625 m²/g for Imo-CH3) would only amplify the disparity in their toxicity. Moreover, as underlined by Dhaini et al., the proportion of bundles can strongly influence the BET specific surface and as a consequence the accessible surface of the imogolite, leading to underestimation of the specific surface area of Imo-CH3. This underlines the need for methods able to give an absolute value of the specific surfaces accessible in the given experimental conditions. Thus, Imo-CH3 induces less inflammation than Imo-OH despite the larger specific surface area, which may be underestimated due to agglomeration. Another important point is the amount of residual small particles (protoimogolite and allophane) in equilibrium with the nanotubes. For Imo-OH, the proto-imogolites are well dispersed, while for Imo-CH3, the proto-imogolites are in majority stuck on the external surface of the nanotube with probably a lower reactivity. Going from -OH to -CH3 in the inner cavity also impacts the length distribution, as the studied samples exhibit a longer average length for Imo-OH (ca. 600 nm) than for Imo-CH3 (ca. 300 nm). However, both Imo-OH and Imo-CH3 are short as compared to fiber paradigm-predicted toxicity, which is relevant for fibers longer than 3–5 μm [78].
Another strong difference is the variation in the chemical composition, particularly the functional groups present on their surfaces. Imo-OH has a higher degree of hydroxylation, but at the inner surface of the tube, so it is unclear how this contributes to its increased toxicity, in particular because the inner diameter is less than 2 nm and the Imo-OH inner cavity contains confined structured water strongly linked by H-bond, while Imo-CH3 contains weakly H-bonded water molecules like in CNT [79]. However, the link between hydroxylation and toxicity is supported by other studies as illustrated in Fig. 10. A highly hydroxylated graphene oxide (GO) induced significantly stronger acute pulmonary inflammation and a more robust acute phase response than reduced graphene oxide (rGO), where the surface hydroxylation was significantly reduced [58]. Similarly, carboxylated nanofibrillated cellulose were shown to reduce neutrophil influx and systemic acute phase responses compared to unmodified nanofibrillated cellulose, suggesting that carboxylation of the OH groups reduces the nanofibrillated cellulose-induced toxicity [59]. These findings indicate that modifying the surface chemistry of highly hydroxylated nanomaterials could be an effective strategy for reducing their toxicity while maintaining their functional properties, representing a safe and sustainable by design (SSbD framework) approach. However, as stated previously, the hydroxyl part of the inner surface of the tube is not easily accessible, indicating an indirect effect of going from -OH to -CH3 causing the toxicity differences. Beyond the difference in bundle formation, in the presence of isolated proto-imogolite, changing OH by CH3 also modifies the length of the nanotube and the direct band gap (from 5.83 ± 0.3 eV to 5.4 ± 0.2 eV, respectively [7]. This variation could have consequences on the production of ROS and, consequently, influence toxicity. Liu et al. have shown that toxicity is associated with ROS production for short nanotubes whereas longer nanotubes exhibit toxicity through a different mechanism [24]. A recently published QSAR model can predict neutrophil influx based on physicochemical properties and modeling using materials with similar characteristics [80]. It would be highly relevant to assess whether this modeling approach also identifies surface hydroxylation as a pro-inflammatory property.
Finally, the application of traditional, state-of-the-art cell culture analyses for comparing further the indicated toxicity differences of the two imogolite forms in vitro were unexpectedly unsuccessful as the imogolites solidified the cell culture medium (unpublished results).
In conclusion, this study highlights significant differences in the toxicity profiles of Imo-OH and Imo-CH3 following pulmonary exposure in mice, demonstrating that blocking hydroxyl groups may serve as a safe-by-design strategy to reduce imogolite-induced inflammation and acute phase response, even when the reduced toxicity results from indirect effects not directly related to hydroxyl interactions with biological media. Importantly, indirect effects arising from changing OH with CH3 within the inner cavity, such as changes in length, bundle formation, the presence of small by-product particles, or alterations in band gap are also critical factors to consider when predicting the evolution of toxicity. However, benchmark comparisons indicate that, upon intratracheal instillation, imogolites exhibit a high inflammatory potential, comparable to other HARNs. These findings emphasize the importance of carefully considering the physicochemical properties of nanomaterials in the development of safer products, particularly in occupational settings where pulmonary exposure risks are elevated.
Supplementary Information
Acknowledgements
The excellent technical assistance from Michael Guldbrandsen, Eva Terrida, Yasmin, and Noor Irmam from the National Research Centre for the Working Environment is greatly appreciated. Józef Szarek, Department of Pathophysiology, Forensic Veterinary Medicine and Administration, Faculty of Veterinary Medicine, University of Warmia and Mazury in Olsztyn, Poland is acknowledged for validation of the histological assessment and Ana Criado from the European Food Safety Agency for discussing the changes in selected histological slides. Frédéric Gobeaux is warmly acknowledged for the CryoTEM images and his help in the data treatment. Cryo-TEM observations were made thanks to “Investissements d’Avenir” LabEx PALM (ANR-10-LABX-0039-PALM). Vincent Mertens from CEA/NIMBE/LEDNA was greatly acknowledged for the BET analysis. Jocelyne Leroy is greatly acknowledged for the XPS analysis. Antoine Thill and Pierre Picot are greatly acknowledged for the fruitful discussion and remarks on imogolite characterizations. The SWAXS Lab from NIMBE and LLB is acknowledge for the access to the machine.
Author contributions
SSP and UV designed and coordinated the study and contributed to data interpretation and discussion. PHD interpreted the data, performed statistical analyses, prepared data visualizations and wrote the first draft. AM and TB performed the histological evaluation and contributed to data interpretation and discussion. FT and AF coordinated the distribution and characterization of imogolites and contributed to data interpretation and discussion. DG have performed the imogolite synthesis and characterizations. AF performed the AFM analysis, FT have done the SAXS analysis and prepared data visualization. PK and RG performed the BMD modeling and contributed to the discussion. All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement 953183 HARMLESS and by the Focused Research Effort on Chemicals in the Working Environment (FFIKA2) from the Danish Government.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All procedures complied with the EC Directive 86/609/EEC and Danish law regulating experiments with animals (The Danish Ministry of Justice, Animal Experiments Inspectorate, permit no. 2020-15-0201-00485).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Govan J, Arancibia-Miranda N, Escudey M, Bonelli B, Tasca F. Imogolite: a nanotubular aluminosilicate: synthesis, derivatives, analogues, and general and biological applications. Mater Chem Front. 2021. 10.1039/D1QM00617G [Google Scholar]
- 2.Picot P, Gobeaux F, Coradin T, Thill A. Dual internal functionalization of imogolite nanotubes as evidenced by optical properties of nile red. Appl Clay Sci. 2019. 10.1016/j.clay.2019.105133 [Google Scholar]
- 3.Paineau E. Imogolite nanotubes: a flexible nanoplatform with multipurpose applications. Appl Sci. 2018. 10.3390/app8101921 [Google Scholar]
- 4.Bonelli B. Chap. 12 - Surface chemical modifications of imogolite. Dev Clay Sci. 2016;7:279–307. [Google Scholar]
- 5.Bottero I, Bonelli B, Ashbrook SE, Wright PA, Zhou WZ, Tagliabue M, Armandi M, Garrone E. Synthesis and characterization of hybrid organic/inorganic nanotubes of the imogolite type and their behaviour towards methane adsorption. Phys Chem Chem Phys. 2011;13(2):744–50. [DOI] [PubMed] [Google Scholar]
- 6.Poli E, Elliott JD, Ratcliff LE, Andrinopoulos L, Dziedzic J, Hine ND, Mostofi AA, Skylaris CK, Haynes PD, Teobaldi G. The potential of imogolite nanotubes as (co-)photocatalysts: a linear-scaling density functional theory study. J Phys Condens Matter. 2016;28(7): 074003. [DOI] [PubMed] [Google Scholar]
- 7.Pignie MC, Patra S, Huart L, Milosavljevic AR, Renault JP, Leroy J, Nicolas C, Sublemontier O, Le Caer S, Thill A. Experimental determination of the curvature-induced intra-wall polarization of inorganic nanotubes. Nanoscale. 2021;13(46):19650–62. [DOI] [PubMed] [Google Scholar]
- 8.Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, Fukushima S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol. 2016;13(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol Sci. 2010;113(1):226–42. [DOI] [PubMed] [Google Scholar]
- 10.Nikota J, Banville A, Goodwin LR, Wu D, Williams A, Yauk CL, Wallin H, Vogel U, Halappanavar S. Stat-6 signaling pathway and not interleukin-1 mediates multi-walled carbon nanotube-induced lung fibrosis in mice: insights from an adverse outcome pathway framework. Part Fibre Toxicol. 2017;14(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christophersen DV, Jacobsen NR, Andersen MH, Connell SP, Barfod KK, Thomsen MB, Miller MR, Duffin R, Lykkesfeldt J, Vogel U, et al. Cardiovascular health effects of oral and pulmonary exposure to multi-walled carbon nanotubes in ApoE-deficient mice. Toxicology. 2016;371:29–40. [DOI] [PubMed] [Google Scholar]
- 12.Rahman L, Jacobsen NR, Aziz SA, Wu D, Williams A, Yauk CL, White P, Wallin H, Vogel U, Halappanavar S. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: investigating the mechanisms of pulmonary carcinogenesis. Mutat Res Genet Toxicol Environ Mutagen. 2017;823:28–44. [DOI] [PubMed] [Google Scholar]
- 13.Poulsen SS, Saber AT, Mortensen A, Szarek J, Wu D, Williams A, Andersen O, Jacobsen NR, Yauk CL, Wallin H, et al. Changes in cholesterol homeostasis and acute phase response link pulmonary exposure to multi-walled carbon nanotubes to risk of cardiovascular disease. Toxicol Appl Pharmacol. 2015;283(3):210–22. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen SS, Jackson P, Kling K, Knudsen KB, Skaug V, Kyjovska ZO, Thomsen BL, Clausen PA, Atluri R, Berthing T, et al. Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology. 2016;10(9):1263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulsen SS, Knudsen KB, Jackson P, Weydahl IE, Saber AT, Wallin H, Vogel U. Multi-walled carbon nanotube-physicochemical properties predict the systemic acute phase response following pulmonary exposure in mice. PLoS One. 2017;12(4): e0174167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danielsen PH, Poulsen SS, Knudsen KB, Clausen PA, Jensen KA, Wallin H, Vogel U. Physicochemical properties of 26 carbon nanotubes as predictors for pulmonary inflammation and acute phase response in mice following intratracheal lung exposure. Environ Toxicol Pharmacol. 2024;107: 104413. [DOI] [PubMed] [Google Scholar]
- 17.Solorio-Rodriguez SA, Williams A, Poulsen SS, Knudsen KB, Jensen KA, Clausen PA, Danielsen PH, Wallin H, Vogel U, Halappanavar S. Single-walled vs. multi-walled carbon nanotubes: influence of physico-chemical properties on toxicogenomics responses in mouse lungs. Nanomaterials. 2023. 10.3390/nano13061059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saber AT, Jacobsen NR, Jackson P, Poulsen SS, Kyjovska ZO, Halappanavar S, Yauk CL, Wallin H, Vogel U. Particle-induced pulmonary acute phase response may be the causal link between particle inhalation and cardiovascular disease. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(6):517–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadrup N, Zhernovkov V, Jacobsen NR, Voss C, Strunz M, Ansari M, Schiller HB, Halappanavar S, Poulsen SS, Kholodenko B, et al. Acute phase response as a biological mechanism-of-action of (nano)particle-induced cardiovascular disease. Small. 2020;16(21): e1907476. [DOI] [PubMed] [Google Scholar]
- 20.Amara MS, Paineau E, Rouzière S, Guiose B, Krapf MEM, Taché O, Launois P, Thill A. Hybrid, tunable-diameter, metal oxide nanotubes for trapping of organic molecules. Chem Mater. 2015;27(5):1488–94. [Google Scholar]
- 21.Thill A, Maillet P, Guiose B, Spalla O, Belloni L, Chaurand P, Auffan M, Olivi L, Rose J. Physico-chemical control over the single- or double-wall structure of aluminogermanate imogolite-like nanotubes. J Am Chem Soc. 2012;134(8):3780–6. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa K, Abe S, Yawaka Y, Suzuki M, Watari F. Osteoblastic cellular responses to aluminosilicate nanotubes, imogolite using Saos-2 and MC3T3-E1 cells. J Ceram Soc Jpn. 2010;118(1378):516–20. [Google Scholar]
- 23.Ishikawa K, Akasaka T, Yawaka Y, Watari F. High functional expression of osteoblasts on imogolite, aluminosilicate nanotubes. J Biomed Nanotechnol. 2010;6(1):59–65. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Chaurand P, Di Giorgio C, De Méo M, Thill A, Auffan M, Masion A, Borschneck D, Chaspoul F, Gallice P, et al. Influence of the length of Imogolite-like nanotubes on their cytotoxicity and genotoxicity toward human dermal cells. Chem Res Toxicol. 2012;25(11):2513–22. [DOI] [PubMed] [Google Scholar]
- 25.Rotoli BM, Guidi P, Bonelli B, Bernardeschi M, Bianchi MG, Esposito S, Frenzilli G, Lucchesi P, Nigro M, Scarcelli V, et al. Imogolite: an aluminosilicate nanotube endowed with low cytotoxicity and genotoxicity. Chem Res Toxicol. 2014;27(7):1142–54. [DOI] [PubMed] [Google Scholar]
- 26.van den Brule S, Beckers E, Chaurand P, Liu W, Ibouraadaten S, Palmai-Pallag M, Uwambayinema F, Yakoub Y, Avellan A, Levard C, et al. Nanometer-long Ge-imogolite nanotubes cause sustained lung inflammation and fibrosis in rats. Part Fibre Toxicol. 2014;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picot P, Thill A, USE OF ALUMINOSILICATE POLYMERS AS AN ACTIVE INGREDIENT AGAINST PHYTOPATHOGENIC MICROORGANISMS. WO/2024/002971. https://patentscope.wipo.int/search/en/WO2024002971 2024.
- 28.Thill A. PRODIGE Facility https://iramis.cea.fr/en/nimbe/lions/nanotubes-d-imogolites-aluminosilicates-et-aluminogermanates-synthese-et-proprietes/. 2015.
- 29.Picot P, Lange T, Testard F, Gobeaux F, Thill A. Evidence and importance of intermediate nanostructures in the journey from molecular precursors to allophane and imogolite nanocrystals. Appl Clay Sci. 2023. 10.1016/j.clay.2023.107013 [Google Scholar]
- 30.Picot P, Tache O, Malloggi F, Coradin T, Thill A. Behaviour of hybrid inside/out Janus nanotubes at an oil/water interface. A route to self-assembled nanofluidics? Faraday Discuss. 2016;191:391–406. [DOI] [PubMed] [Google Scholar]
- 31.Danielsen PH, Bendtsen KM, Knudsen KB, Poulsen SS, Stoeger T, Vogel U. Nanomaterial- and shape-dependency of TLR2 and TLR4 mediated signaling following pulmonary exposure to carbonaceous nanomaterials in mice. Part Fibre Toxicol. 2021;18(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobsen NR, Moller P, Jensen KA, Vogel U, Ladefoged O, Loft S, Wallin H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part Fibre Toxicol. 2009;6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson P, Lund SP, Kristiansen G, Andersen O, Vogel U, Wallin H, Hougaard KS. An experimental protocol for maternal pulmonary exposure in developmental toxicology. Basic Clin Pharmacol Toxicol. 2011;108(3):202–7. [DOI] [PubMed] [Google Scholar]
- 34.Saber AT, Jacobsen NR, Mortensen A, Szarek J, Jackson P, Madsen AM, Jensen KA, Koponen IK, Brunborg G, Gutzkow KB, et al. Nanotitanium dioxide toxicity in mouse lung is reduced in sanding dust from paint. Part Fibre Toxicol. 2012;9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadrup N, Bengtson S, Jacobsen NR, Jackson P, Nocun M, Saber AT, Jensen KA, Wallin H, Vogel U. Influence of dispersion medium on nanomaterial-induced pulmonary inflammation and DNA strand breaks: investigation of carbon black, carbon nanotubes and three titanium dioxide nanoparticles. Mutagenesis. 2017;32(6):581–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadrup N, Guldbrandsen M, Terrida E, Bendtsen KMS, Hougaard KS, Jacobsen NR, Vogel U. Intratracheal instillation for the testing of pulmonary toxicity in mice-effects of instillation devices and feed type on inflammation. Anim Models Exp Med. 2025. 10.1002/ame2.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallin H, Kyjovska ZO, Poulsen SS, Jacobsen NR, Saber AT, Bengtson S, Jackson P, Vogel U. Surface modification does not influence the genotoxic and inflammatory effects of TiO2 nanoparticles after pulmonary exposure by instillation in mice. Mutagenesis. 2017;32(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saber AT, Halappanavar S, Folkmann JK, Bornholdt J, Boisen AM, Moller P, Williams A, Yauk C, Vogel U, Loft S, et al. Lack of acute phase response in the livers of mice exposed to diesel exhaust particles or carbon black by inhalation. Part Fibre Toxicol. 2009;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruehl-Fehlert C, Kittel B, Morawietz G, Deslex P, Keenan C, Mahrt CR, Nolte T, Robinson M, Stuart BP, Deschl U. Revised guides for organ sampling and trimming in rats and mice–Part 1. Exp Toxicol Pathol. 2003;55(2–3):91–106. [PubMed] [Google Scholar]
- 40.Kittel B, Ruehl-Fehlert C, Morawietz G, Klapwijk J, Elwell MR, Lenz B, O’Sullivan MG, Roth DR, Wadsworth PF, Group R, et al. Revised guides for organ sampling and trimming in rats and mice–Part 2. A joint publication of the RITA and NACAD groups. Exp Toxicol Pathol. 2004;55(6):413–31. [DOI] [PubMed] [Google Scholar]
- 41.Haschek WM, Rousseaux CG, Wallig MA. Fundementals of toxicologic pathology. London, UK. (: Academic; 1998. p. 13. Chap. 1. [Google Scholar]
- 42.Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, March T, Nagano K, Pino M, Rittinghausen S, et al. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol. 2009;37(7 Suppl):S5–73. [DOI] [PubMed] [Google Scholar]
- 43.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Kuttler K, Deschl U, et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010;38(7 Suppl):S5–81. [DOI] [PubMed] [Google Scholar]
- 44.Liao YU, Picot P, Brubach JB, Roy P, Le Caër S, Thill A. Self-supporting thin films of imogolite and imogolite-like nanotubes for infrared spectroscopy. Appl Clay Sci. 2018;164:58–67. [Google Scholar]
- 45.Picot P, Gobeaux F, Charpentier T, Belloni L, Takahara A, Wada SI, Thill A. Diameter and chirality of natural and synthetic imogolite. Appl Clay Sci. 2024. 10.1016/j.clay.2024.107497 [Google Scholar]
- 46.D’Angelo A, Paineau E, Rouzière S, Elkaim É, Goldmann C, Toquer D, Rols S, Launois P. The atomic structure of imogolite nanotubes: A 50 years old issue reinvestigated by X-ray scattering experiments and molecular dynamics simulations. Appl Clay Sci 2023;242:107043.
- 47.Amara MS, Rouzière S, Paineau E, Bacia-Verloop M, Thill A, Launois P. Hexagonalization of aluminogermanate imogolite nanotubes organized into closed-packed bundles. J Phys Chem C. 2014;118(17):9299–306. [Google Scholar]
- 48.Dhaini A, Geneste A, Raad FA, Picot P, Martin-Gassin G, Prelot B, Gassin PM, Trens P, Thill A, Zajac J. Dried hybrid imogolite nanotubes as solids with a changeable surface area: an insight into textural properties based on the correlation between nitrogen gas adsorption, immersion calorimetry into water, and small angle X-ray scattering. Phys Chem Chem Phys. 2024;26(36):23835–45. [DOI] [PubMed] [Google Scholar]
- 49.Poulsen SS, Saber AT, Williams A, Andersen O, Kobler C, Atluri R, Pozzebon ME, Mucelli SP, Simion M, Rickerby D, et al. MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol Appl Pharmacol. 2015;284(1):16–32. [DOI] [PubMed] [Google Scholar]
- 50.Poulsen SS, Jacobsen NR, Labib S, Wu D, Husain M, Williams A, Bogelund JP, Andersen O, Kobler C, Molhave K, et al. Transcriptomic analysis reveals novel mechanistic insight into murine biological responses to multi-walled carbon nanotubes in lungs and cultured lung epithelial cells. PLoS One. 2013;8(11):e80452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutierrez CT, Loizides C, Hafez I, Brostrom A, Wolff H, Szarek J, Berthing T, Mortensen A, Jensen KA, Roursgaard M, et al. Acute phase response following pulmonary exposure to soluble and insoluble metal oxide nanomaterials in mice. Part Fibre Toxicol. 2023;20(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berthing T, Lard M, Danielsen PH, Abariute L, Barfod KK, Adolfsson K, Knudsen KB, Wolff H, Prinz CN, Vogel U. Pulmonary toxicity and translocation of gallium phosphide nanowires to secondary organs following pulmonary exposure in mice. J Nanobiotechnol. 2023;21(1):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barfod KK, Bendtsen KM, Berthing T, Koivisto AJ, Poulsen SS, Segal E, Verleysen E, Mast J, Hollander A, Jensen KA, et al. Increased surface area of Halloysite nanotubes due to surface modification predicts lung inflammation and acute phase response after pulmonary exposure in mice. Environ Toxicol Pharmacol. 2020;73: 103266. [DOI] [PubMed] [Google Scholar]
- 54.Danielsen PH, Knudsen KB, Strancar J, Umek P, Koklic T, Garvas M, Vanhala E, Savukoski S, Ding Y, Madsen AM, et al. Effects of physicochemical properties of TiO(2) nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice. Toxicol Appl Pharmacol. 2020;386:114830. [DOI] [PubMed] [Google Scholar]
- 55.Bendtsen KM, Brostrom A, Koivisto AJ, Koponen I, Berthing T, Bertram N, Kling KI, Dal Maso M, Kangasniemi O, Poikkimaki M, et al. Airport emission particles: exposure characterization and toxicity following intratracheal instillation in mice. Part Fibre Toxicol. 2019;16(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bendtsen KM, Gren L, Malmborg VB, Shukla PC, Tuner M, Essig YJ, Krais AM, Clausen PA, Berthing T, Loeschner K, et al. Particle characterization and toxicity in C57BL/6 mice following instillation of five different diesel exhaust particles designed to differ in physicochemical properties. Part Fibre Toxicol. 2020;17(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kyjovska ZO, Jacobsen NR, Saber AT, Bengtson S, Jackson P, Wallin H, Vogel U. DNA strand breaks, acute phase response and inflammation following pulmonary exposure by instillation to the diesel exhaust particle NIST1650b in mice. Mutagenesis. 2015;30(4):499–507. [DOI] [PubMed] [Google Scholar]
- 58.Bengtson S, Knudsen KB, Kyjovska ZO, Berthing T, Skaug V, Levin M, Koponen IK, Shivayogimath A, Booth TJ, Alonso B, et al. Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide. PLoS One. 2017;12(6): e0178355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hadrup N, Knudsen KB, Berthing T, Wolff H, Bengtson S, Kofoed C, Espersen R, Hojgaard C, Winther JR, Willemoes M, et al. Pulmonary effects of nanofibrillated celluloses in mice suggest that carboxylation lowers the inflammatory and acute phase responses. Environ Toxicol Pharmacol. 2019;66:116–25. [DOI] [PubMed] [Google Scholar]
- 60.Gromelski M, Stolinski F, Jagiello K, Rybinska-Fryca A, Williams A, Halappanavar S, Vogel U, Puzyn T. AOP173 key event associated pathway predictor - online application for the prediction of benchmark dose lower bound (BMDLs) of a transcriptomic pathway involved in MWCNTs-induced lung fibrosis. Nanotoxicology. 2022;16(2):183–94. [DOI] [PubMed] [Google Scholar]
- 61.Halappanavar S, van den Brule S, Nymark P, Gate L, Seidel C, Valentino S, Zhernovkov V, Hogh Danielsen P, De Vizcaya A, Wolff H, et al. Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale. Part Fibre Toxicol. 2020;17(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mikkelsen L, Sheykhzade M, Jensen KA, Saber AT, Jacobsen NR, Vogel U, Wallin H, Loft S, Moller P. Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO(2). Part Fibre Toxicol. 2011;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Battista V, Danielsen PH, Gajewicz-Skretna A, Kedziorski A, Seiffert SB, Ma-Hock L, Berthing T, Mortensen A, Sundermann A, Skjolding LM, et al. Oxide-perovskites for automotive catalysts biotransform and induce multicomponent clearance and hazard. ACS Nano. 2024;18(47):32672–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gate L, Knudsen KB, Seidel C, Berthing T, Chezeau L, Jacobsen NR, Valentino S, Wallin H, Bau S, Wolff H, et al. Pulmonary toxicity of two different multi-walled carbon nanotubes in rat: comparison between intratracheal instillation and inhalation exposure. Toxicol Appl Pharmacol. 2019;375:17–31. [DOI] [PubMed] [Google Scholar]
- 65.Halappanavar S, Sharma M, Solorio-Rodriguez SA, Wallin H, Vogel U, Sullivan K, Clippinger AJ. Substance interaction with the pulmonary resident cell membrane components leading to pulmonary fibrosis. OECD series on adverse outcome pathways. Volume 33. Paris: OECD Publishing; 2023.
- 66.Bourdon JA, Halappanavar S, Saber AT, Jacobsen NR, Williams A, Wallin H, Vogel U, Yauk CL. Hepatic and pulmonary toxicogenomic profiles in mice intratracheally instilled with carbon black nanoparticles reveal pulmonary inflammation, acute phase response, and alterations in lipid homeostasis. Toxicol Sci. 2012;127(2):474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutierrez CT, Loizides C, Hafez I, Biskos G, Loeschner K, Brostrom A, Roursgaard M, Saber AT, Moller P, Sorli JB, et al. Comparison of acute phase response in mice after inhalation and intratracheal instillation of molybdenum disulphide and tungsten particles. Basic Clin Pharmacol Toxicol. 2023;133(3):265–78. [DOI] [PubMed] [Google Scholar]
- 68.Modrzynska J, Berthing T, Ravn-Haren G, Jacobsen NR, Weydahl IK, Loeschner K, Mortensen A, Saber AT, Vogel U. Primary genotoxicity in the liver following pulmonary exposure to carbon black nanoparticles in mice. Part Fibre Toxicol. 2018;15(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modrzynska J, Berthing T, Ravn-Haren G, Kling K, Mortensen A, Rasmussen RR, Larsen EH, Saber AT, Vogel U, Loeschner K. In vivo-induced size transformation of cerium oxide nanoparticles in both lung and liver does not affect long-term hepatic accumulation following pulmonary exposure. PLoS One. 2018;13(8): e0202477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saber AT, Mortensen A, Szarek J, Koponen IK, Levin M, Jacobsen NR, Pozzebon ME, Mucelli SP, Rickerby DG, Kling K, et al. Epoxy composite dusts with and without carbon nanotubes cause similar pulmonary responses, but differences in liver histology in mice following pulmonary deposition. Part Fibre Toxicol. 2016;13(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halappanavar S, Ede JD, Mahapatra I, Krug HF, Kuempel ED, Lynch I, Vandebriel RJ, Shatkin JA. A methodology for developing key events to advance nanomaterial-relevant adverse outcome pathways to inform risk assessment. Nanotoxicology. 2021;15(3):289–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halappanavar S, Nymark P, Krug HF, Clift MJD, Rothen-Rutishauser B, Vogel U. Non-animal strategies for toxicity assessment of nanoscale materials: role of adverse outcome pathways in the selection of endpoints. Small. 2021;17(15): e2007628. [DOI] [PubMed] [Google Scholar]
- 73.Saber AT, Lamson JS, Jacobsen NR, Ravn-Haren G, Hougaard KS, Nyendi AN, Wahlberg P, Madsen AM, Jackson P, Wallin H, et al. Particle-induced pulmonary acute phase response correlates with neutrophil influx linking inhaled particles and cardiovascular risk. PLoS One. 2013;8(7): e69020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Ianni E, Moller P, Mortensen A, Szarek J, Clausen PA, Saber AT, Vogel U, Jacobsen NR. Organomodified nanoclays induce less inflammation, acute phase response, and genotoxicity than pristine nanoclays in mice lungs. Nanotoxicology. 2020;14(7):869–92. [DOI] [PubMed] [Google Scholar]
- 75.Ge C, Peters S, Olsson A, Portengen L, Schuz J, Almansa J, Ahrens W, Bencko V, Benhamou S, Boffetta P, et al. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. A pooled exposure-response analysis of 14 case-control studies. Am J Respir Crit Care Med. 2020;202(3):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vermeulen R, Silverman DT, Garshick E, Vlaanderen J, Portengen L, Steenland K. Exposure-response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts. Environ Health Perspect. 2014;122(2):172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wils RS, Flachs EM, Stokholm ZA, Gustavsson P, Plato N, Kolstad HA, Sejbaek CS, Brauer C, Schlunssen V, Hougaard KS et al. Occupational exposure to diesel engine exhaust and first-time acute myocardial infarction - a nationwide register-based cohort study 1976–2018. Eur J Prev Cardiol 2025;32(8):682-695. [DOI] [PubMed]
- 78.Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos - similarities and differences. Adv Drug Deliv Rev. 2013;65(15):2078–86. [DOI] [PubMed] [Google Scholar]
- 79.Liao YY, Picot P, Laine M, Brubach JB, Roy P, Thill A, Le Caër S. Tuning the properties of confined water in standard an hybrid nanotubes: an infrared spectroscopic study. Nano Res. 2018;11(9):4759–73. [Google Scholar]
- 80.Miszczak M, Khan K, Danielsen PH, Jensen KA, Vogel U, Grafstrom R, Gajewicz-Skretna A. Dynamic QSAR modeling for predicting in vivo genotoxicity and inflammation induced by nanoparticles and advanced materials: a time-dose-property/response approach. J Nanobiotechnol. 2025;23(1): 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.