Abstract
Early detection and accurate cancer diagnosis are crucial for improving patient outcomes and survival rates. This review presents a comprehensive and updated synthesis of emerging biomarkers, essential for providing non-invasive, efficient, and reliable methods to identify cancer in its early stages. An extensive literature review focuses on recent studies and advancements in both traditional and emerging biomarkers, including circulating tumor DNA (ctDNA), exosomes, liquid biopsies, microRNAs (miRNAs), and immunotherapy biomarkers, which show promising potential for early cancer detection. Liquid biopsies, nanobiosensors, artificial intelligence, and next-generation sequencing (NGS) are transforming biomarker discovery and application. Key challenges include low concentration and fragmentation, as well as clearance of ctDNA, the complexity of exosome isolation, inter-patient variability in miRNA expression, and the absence of clinical standardization. We also highlight the translational barriers in low-resource settings and suggest strategies for future implementation. We also underscore the limited diagnostic accessibility in low-resource settings, emphasizing the importance of equity in future applications. Future research should prioritize overcoming current challenges, promoting multidisciplinary collaboration, and creating standardized protocols to enhance the clinical utility of this approach.
Keywords: Biomarker, Cancer, Diagnosis, Exosomes, Expression, Immunotherapy, Liquid biopsy, MiRNA
Introduction
Cancer has become one of the most fatal public health concerns throughout the globe. In 2022, there were 20 million new cases of cancer reported, along with 10 million cancer-associated deaths, making cancer the second leading cause of mortality [1].
Early cancer diagnosis significantly increases patient outcomes by enhancing overall and recurrence-free survival rates [2, 3]. An investigation demonstrated that early detection led to a median overall survival of 38 months, compared to 14 months with delayed diagnosis. In addition, it raises quality of life scores from 55 to 75 and lowers severe treatment-related side effects from 18 to 45% [4]. Early cancer detection enables timely interventions and the possibility of less invasive treatments, resulting in more effective disease management and improved patient outcomes [5].
In low-income countries, the rate of cancer-related deaths is 75% higher due to poor health literacy and socioeconomic factors. It results in substantial economic loss, estimated to be more than a trillion dollars annually [6, 7]. According to various studies, patients in low-income countries are 50% less likely to be diagnosed with cancer than patients in high-income countries due to low accessibility to diagnostic and treatment procedures [8].
Cancer is a multigenic and multifactorial disease [1]. Genetic factors, including mutations in proto-oncogenes and tumor suppressor genes, cause elevated cell replication and resistance to apoptosis. At the same time, the non-genetic factors include epigenetic modification, exposure to radiation; chemical carcinogens including lead, arsenic, and fungal toxins; certain viruses and bacteria such as Human papillomavirus (HPV), hepatitis C virus, and human T-cell lymphotropic virus; age; and lifestyle factors such as alcohol consumption, smoking, obesity, and poor nutrition [9].
Cancer is caused by the accumulation of molecular alterations in the genome that lead to changes in the typical physiological properties of cells. Genetic variations can lead to dysregulation of the balance between cell survival and cell death, resulting in increased cell growth and uncontrolled proliferation [10]. The transformed cells can acquire distinctive characteristics, including altered cell morphology, loss of cell adhesion, degradation of the extracellular matrix, increased migration, and enhanced proliferation [10].
Cancer cells replicate in an unregulated manner and spread to other organs of the body. They can also transform healthy cells into cancer cells. Tumors can be categorized as benign and malignant [10]. Benign tumors are low-proliferating cell masses that remain localized in their primary location and do not invade distant areas of the body. These tumors proliferate slowly and do not cause serious problems. Fibroids in the uterus and breast fibroadenoma are the prime examples of benign tumors. However, benign cells can become malignant and require surgical procedures for removal [11].
The tumor cells that can proliferate and metastasize to secondary sites are called malignant tumors, and they are cancerous. Cancer metastasis occurs when cancer cells detach from the primary site and spread through the bloodstream or lymphatic system to secondary regions of the body [12]. Advanced-stage metastatic cancers are the primary cause of death among cancer patients. Various reports have indicated that most cancers can metastasize to some common secondary sites such as bones, liver, lungs, and brain [12].
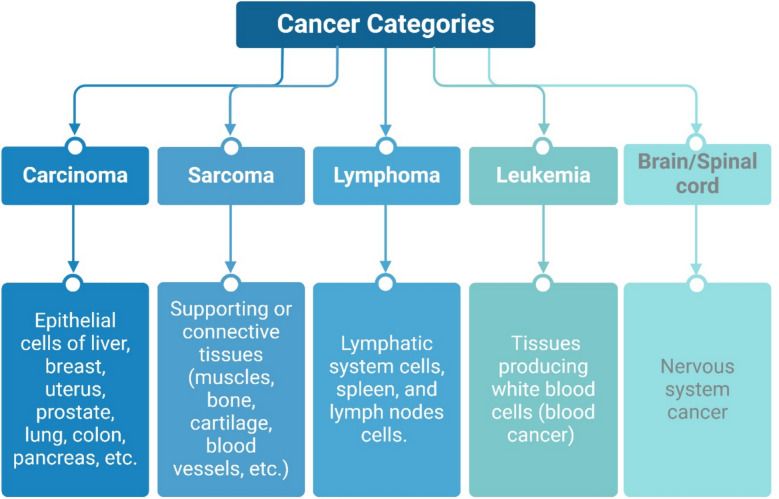
Cancers can be categorized into groups based on the type of cells or tissues undergoing oncogenic transformation. Some of the essential categories of cancer include carcinoma (the most prevalent), sarcoma, lymphoma, leukemia, and brain/spinal cord cancers (Fig. 1). When the epithelial cells that form the outer lining of the internal organs, passageways, and skin become cancerous, it is known as carcinoma. Carcinoma can form on the liver, breast, uterus, prostate, lung, colon, and pancreas, among others [13].
Fig. 1.
Main Cancer Categories. As outlined above, the primary cancer categories are defined based on the specific human tissues they affect
There are various subclasses of carcinoma, including adenocarcinoma, squamous cell carcinoma, ductal carcinoma, and basal cell carcinoma [13]. Sarcoma is a type of cancer mainly arising from supporting or connective tissues, such as muscles, bones, cartilage, and blood vessels [14]. Leukemia, also known as blood cancer, originates in the tissues that produce white blood cells, specifically the bone marrow. The bone marrow produces more immature white blood cells that cannot perform regular functions and accumulate in the blood [15].
Lymphoma is a type of cancer that affects the lymphatic system, spleen, and lymph nodes. Lymphoma occurs when the cells of the immune system, specifically lymphocytes, start to proliferate uncontrollably and remain immature, rendering them unable to perform their immunological functions. Myeloma is a cancer of plasma cells that produce antibodies inside the bone marrow. Lastly, cancers that initiate in the cells of the brain and spinal cord are known as central nervous system cancers. Glioma is the most common type of brain cancer that develops from the glial cells of the brain [12].
Given all the earlier points, this review aims to explore how emerging biomarkers (such as circulating tumor DNA (ctDNA), exosomes, and microRNAs) are shaping the future of early cancer detection. We provide an enhanced, integrative perspective that underscores how these tools function, their current clinical applications, and the challenges that still need to be addressed. Our goal is to give a valuable resource for researchers and clinicians seeking to enhance attention in patient care.
Significance of early detection and diagnosis
Early cancer detection aims to identify carcinogenic changes at the earliest stage, at which therapeutic intervention can result in an improved survival rate and reduced morbidity. This process can occur during the conversion of normal cellular functions to dysregulation and eventually into cancer [11].
Research in early cancer detection has yielded significant health benefits. The implementation of screening regimens for breast, cervical, and colorectal cancers has reduced the frequency of diagnoses at advanced stages and improved patient survival rates [16]. However, there are various malignancies, such as oral cancer, ovarian cancer, and pancreatic cancer, that are still diagnosed at late stages and have poor prognoses. For over a hundred years, the early diagnosis of cancer has held an immense insightful interest from the medical community [17]. Delayed detection and imperfect patient prognoses are the prime causes of poor survival rates among patients with malignancies. Cancers can be treated effectively and have better prognoses if detected at the primary stage.
Timely detection offers multiple clinical advantages. Patients with early diagnosis of cancer not only significantly increase their chances of survival, but such patients also experience better treatment efficacy, enhanced treatment outcomes, better recovery, and improved quality of life. Developing early cancer detection is a complex and comprehensive process. Studies have revealed that certain patients’ behaviors, such as undergoing cancer pre-screening procedures intended to identify cancers in their asymptomatic stage (such as mammography or BRCA screening for breast cancer detection), can prove helpful in early diagnosis of cancer [18].
Increasing public awareness about cancer symptoms and promoting frequent visits to healthcare facilities. Earlier-stage patient diagnoses can substantially improve treatment opportunities. Though early detection of cancer has increased the survival rates in high-income countries, cancer patients in low-income countries still have poor survival rates owing to delayed diagnosis [19].
Advanced-stage diagnosis of cancer is a primary global health concern, particularly in resource-poor settings. Patients with late-stage diagnoses demonstrate ineffective treatment with various adverse effects and have poor clinical outcomes. Till today, approximately 50% of cases of cancer are detected at advanced stages, resulting in multiple health complications and mortality, which can be countered by early cancer detection [11]. In conclusion, intensifying research efforts to develop and expand early detection strategies across various cancer types will help improve patient outcomes.
Role of biomarkers in cancer detection
Biomarkers are crucial in healthcare, as they offer valuable insights into disease diagnosis and prognosis. They can objectively indicate the molecular characteristics of cancer, enabling early diagnosis and management of the disease, which results in improved patient prognosis [20]. These markers also play a role in determining therapeutic options by predicting the clinical outcomes of patients; they can be utilized to assess patients, including risk estimation of cancer, screening for primary-stage cancers, prognosis prediction, monitoring disease status and patients’ responses to therapy, detecting early signs of recurrence, distinguishing one type of cancer from another, and evaluation of patient clinical outcomes [13, 21, 22]. Beyond clinical use, biomarkers enhance drug development by identifying suitable patients and speeding up drug approval [23].
Cancer biomarkers are perhaps one of the most promising tools to diagnose cancer early. Therefore, further research is needed to identify more reliable and accurate indicators of cancer. For example,
RCAS1 (Receptor-Binding Cancer Antigen Expressed on SiSo Cells) is utilized in stomach cancer for disease detection and prognosis [24].
CT antigens (Cancer/Testis Antigens) are utilized in the diagnosis and prognosis of prostate, liver, lung, bladder, and skin cancers [25]. EGFR (epidermal growth factor receptor) is a crucial marker in lung cancer, used for diagnosis and treatment monitoring [26].
CD20 (Cluster of Differentiation 20) is used in blood cancers to determine the appropriate treatment [27].
HCG-β (Human Chorionic Gonadotropin Beta) is essential for diagnosis, staging, recurrence identification, and treatment monitoring in ovarian and testicular cancers [28].
The BCR-ABL fusion gene (Breakpoint Cluster Region-Abelson) is utilized in the diagnosis and treatment of bone marrow and blood cancers for prognostication, treatment determination, and response monitoring [29] (Table 1).
Table 1.
Frequently utilized traditional biomarkers for cancer
| Biomarker | Cancer type | Application | References |
|---|---|---|---|
| CEA | Colon, liver | Screening, identifying recurrence, treatment monitoring, disease prognosis | [30] |
| CA 15-3 | Breast | Treatment monitoring | [31] |
| CA 125 | Ovary | Prognosis, identifying recurrence, treatment monitoring, disease diagnosis | [32] |
| CA 19-9 | Pancreas, colon | Treatment monitoring | [33] |
| AFP | Liver (hepatocellular carcinoma, HCC) | Identifying recurrence, treatment monitoring, disease diagnosis | [34] |
| PSA | Prostate gland | Screening, identifying recurrence, treatment monitoring, disease diagnosis | [35] |
| RCAS1 | Stomach | Detection, prognosis | [24] |
| Her2 | Lung, breast | Monitoring therapy | [36] |
| ER & PR | Breast | Stratification | [37] |
| CT antigens | Prostate, liver, lung, bladder, skin | Diagnosis, prognosis | [25] |
| EGFR | Lung | Diagnosis and monitoring therapy | [26] |
| CD20 | Blood | Treatment determination | [27] |
| HCG-β | Ovary, testis |
Diagnosis, staging, identifying recurrence, treatment monitoring |
[28] |
| BCR-ABL fusion gene | Bone marrow, blood | Prognosis, treatment determination, monitoring | [29] |
AFP Alpha-Fetoprotein, BCR-ABL Breakpoint Cluster Region-Abelson Murine Leukemia Viral Oncogene Homolog, CA 15-3 Cancer Antigen 15-3, CA 125 Cancer Antigen 125, CA 19-9 Cancer Antigen 19-9, CD20 Cluster of Differentiation 20, CEA Carcinoembryonic Antigen, CT antigens Cancer/Testis Antigens, EGFR Epidermal Growth Factor Receptor, ER Estrogen Receptor, HCG-β Human Chorionic Gonadotropin Beta, Her2 Human Epidermal Growth Factor Receptor 2, PR Progesterone Receptor, PSA Prostate-Specific Antigen, RCAS1 Receptor-binding cancer antigen expressed on SiSo cells 1.
Traditional biomarkers for cancer
Traditional diagnostic techniques such as Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scans, biopsies, and ultrasound are not adequate for diagnosing cancer at the earliest stage due to their dependency on the phenotypic features of cancer and its carcinogenic properties. Cancer is a complex disease characterized by various genetic and epigenetic alterations that can disrupt cell signaling, leading to uncontrolled cell proliferation [38]. In response to these limitations, scientific research aims to identify methods or techniques that can diagnose cancer at the earliest stage, provide more accurate prognoses, and improve survival rates.
Over the past few decades, various cancer biomarkers have been applied in oncology. Biomarkers are molecules that emerge biologically in multiple body parts. According to the National Cancer Institute, biomarkers are “a biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or a condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease or condition.”
Cancer biomarkers are detected in malignant tissues, as well as in blood, saliva, urine, and stool; they are produced by the cancer cells detected in malignant tissues, as well as in blood, saliva, urine, and stool; they are produced by the cancer cells or the body in response to the cancer [23]. Tumor biomarkers can play a crucial role in developing specific, sensitive, cost-effective, reproducible, reliable, and effective monitoring and detection techniques for assessing the risk of cancer development, tumor classification, and tumor progression.
Biomarkers support personalized medicine, enabling healthcare providers to monitor the progress, regression, or recurrence of the treatment. Figure 2 indicates the clinical applications of biomarkers in cancer. Some traditional biomarkers include Prostate-Specific Antigen (PSA), Cancer Antigen 19-9 (CA 19-9), Alpha-Fetoprotein (AFP), Carcinoembryonic Antigen (CEA), Cancer Antigen 125 (CA 125), and Cancer Antigen 15-3 (CA 15-3), which have been used to detect cancers and predict patients’ prognoses and treatment outcomes [39].
Fig. 2.
Potential applications of cancer biomarkers. Cancer biomarkers can be applied to various cancer control categories during relevant periods, serving as decision-making molecules at the critical stages of pre-diagnosis, diagnosis, prognosis, and treatment of cancers
Early screening of cancer is an essential public health tool that can allow early detection of cancer and can reduce the incidence rate of cancer, provide higher chances of therapeutic intervention, improve patient response, and prolong the survival rates of cancer patients [40]. Several biomarkers play a crucial role in the early detection of cancers and have been investigated. For example, the AFP protein has been used to screen for liver cancer since 1964, and the BRCA testing is currently being used for the screening of breast cancer [12].
Biomarkers also play an essential role in the diagnosis of cancer. Studies have reported that biomarker panels and other techniques, such as biopsy or endoscopy, can be a promising alternative to conventional, less effective diagnostic approaches [18]. Biomarkers have also been utilized to assess the clinical outcomes of cancer patients, enabling the selection of effective therapeutic regimens tailored to individual patients. Breast cancer can be identified and differentiated into its molecular subtypes based on the expression of proteins such as estrogen receptor (ER), progesterone receptor (PR), and human epithelial growth factor 2 (HER2) [36, 37]. These biomarkers play an essential role in the identification of triple-negative breast cancer (the most aggressive form of breast cancer with poor prognosis and therapeutic options), which lacks the expression of these proteins. Therefore, improving treatment and management options to improve patients’ clinical outcomes [41].
Lastly, the levels of tumor biomarkers can also indicate the recurrence of cancer in patients. Some conventional biomarkers, such as PSA, CA19-9, and CEA, have been applied for tumor diagnosis and prognosis, and they can indicate the recurrence of various cancers, such as prostate cancer, liver cancer, gastric cancer, and breast cancer [30, 42, 43].
Traditional cancer biomarkers used in clinical practices
Few traditional cancer biomarkers are discussed in clinical practices (Fig. 3). In this section, we describe and update the information about the conventional biomarkers.
Fig. 3.
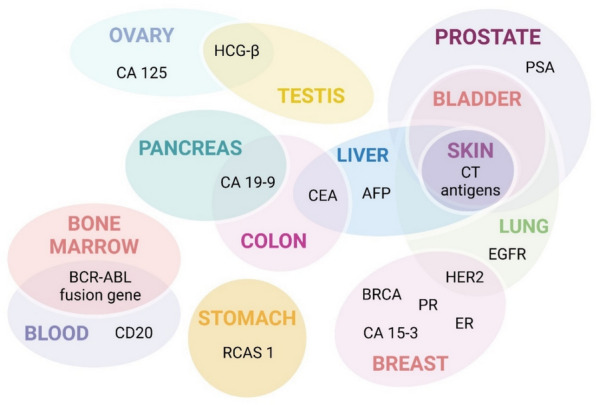
Biomarkers are employed for several cancers. Cancer biomarkers used for clinical application
CA 19-9
CA 19-9 (Cancer Antigen 19-9) is relevant for pancreatic and colon cancers, mainly for monitoring treatment [33]. This cancer antigen was first identified in 1981 in the serum of patients with pancreatic cancer. It is the MUC1 glycoprotein`s Lewis’s antigen [44]. The normal serum level of CA 19-9 is less than 37 U/mL. Over the past few years, this biomarker has been extensively used in clinical settings to diagnose pancreatic cancer [33]. CA 19-9 serum levels can be essential in diagnosing gastric and urothelial cancers [45]. Hence, there is a need to develop precise and sensitive systems that can accurately detect the levels of this biomarker in cancer patients.
CA 125
CA 125 (Cancer Antigen 125) is utilized in ovarian cancer for prognosis, recurrence identification, treatment monitoring, and, in some cases, diagnosis [32]. Elevated levels of CA125 are primarily associated with ovarian cancer [46]. Furthermore, various other types of cancers, such as lung, cervix, pancreas, breast, liver, stomach, uterus, and colon cancers, also cause increased levels of CA125. However, the levels of these markers can also grow in various conditions, such as pregnancy and menstruation.
Multiple reports have indicated that 90% of patients with advanced ovarian cancer and 40% of patients with intra-abdominal tumors exhibit elevated levels of CA125 [47]. However, 50% of patients with primary-stage ovarian cancer are exposed to normal levels of CA125. Increased levels of CA125 can be used to detect the progression of benign tumors into malignant tumors. Furthermore, high levels of this biomarker also correlate with treatment failure and disease recurrence [48].
CA 15-3
CA 15-3 is an essential biomarker for detecting breast cancer [31]. This marker is commonly employed in clinical practices for the monitoring of breast cancer therapy in advanced stages [49, 50]. At various stages of breast cancer, the levels of this biomarker can increase from 10% at the primary stage to 40% at stage 4. Raised values of CA 15-3 are also associated with extensive metastasis. A study demonstrated that breast cancer patients with CA 15-3 levels of 30 U/ml had higher survival rates compared to patients with increased levels of CA 15-3 [31]. Endometriosis, hepatitis, pelvic inflammatory disease, pregnancy, and lactation are conditions other than cancer in which the levels of CA 15-3 are increased [31].
AFP
AFP is one of the most frequently used traditional biomarkers for cancer diagnosis [34]. It is produced during pregnancy by the fetus. Cancer patients have increased levels of AFP in their serum, which serves as a marker for tumor detection. This biomarker is unique for patients with hepatocellular carcinoma (HCC). AFP is widely accepted for the diagnosis of HCC [51]. Furthermore, AFP levels can also be raised in various other diseases, such as hepatitis, congenital tyrosinemia, ataxia-telangiectasia syndrome, and cancer, such as gastric, nasopharyngeal, and testicular cancers. Hence, evaluating the levels of AFP is critical for diagnosing cancer [52].
PSA
PSA was the first biomarker used for the detection and screening of prostate cancer in healthcare settings [35]. Increased serum levels of PSA are associated with prostate cancer [53]. According to a study, 30% of patients with high PSA were diagnosed with prostate cancer. Other than cancer, increased PSA levels can also indicate prostate inflammation, prostate hyperplasia, and benign cancers [54]. Hence, increased PSA levels may not specifically indicate malignant conditions.
CEA (Carcinoembryonic Antigen) is a biomarker used in the detection of colon and liver cancers, mainly for early detection, recurrence identification, treatment monitoring, and prognosis. CA 15-3 (Cancer Antigen 15-3) is primarily used in breast cancer to monitor treatment response and progression. AFP (Alpha-fetoprotein) is an important marker in liver cancer, especially hepatocellular carcinoma (HCC), and is employed for recurrence detection, treatment tracking, and disease diagnosis. Finally, PSA (Prostate-Specific Antigen) is widely used in the detection of prostate cancer for early detection, recurrence monitoring, treatment evaluation, and diagnosis.
Limitations of traditional biomarkers
Although these traditional biomarkers have been applied for cancer detection for several years, they still have certain limitations. Most traditional biomarkers for cancer detection are proteins, and they are not specific to cancer. Genetic biomarkers can be more reliable than the various protein-based biomarkers; however, the genetic alterations have not yet been determined for every type of cancer. Another hurdle faced when using traditional biomarkers is the issue of sensitivity and specificity. The expression levels of several biomarkers for cancer can also be increased during other diseases or conditions, resulting in higher false-positive rates. For instance, PSA has been used routinely to screen for prostate cancer; however, its levels have also been found to be elevated in other conditions such as prostatitis, low-grade prostate tumors, and benign prostate hyperplasia, which can lead to needless biopsies and overtreatment while killing only a small number of cancer cells. Not every patient with a specific cancer type will have increased expression of a particular biomarker, and neither does every cancer type have its unique biomarkers.
Furthermore, differentiating between aged tissue, pre-cancerous lesions in tissues, and cancer tissues can be complex due to the gradual evolution of cancer. Additionally, samples collected to identify biomarkers through invasive techniques can also encounter technical challenges when detecting lower levels of the desired marker. Moreover, the heterogeneity of cancers is also a limiting factor when applying biomarkers. However, panels of biomarkers are being established to detect the expression patterns of various biomarkers, which can help overcome the difficulties related to tumor heterogeneity. For instance, a biomarker panel of pancreatic cancer cell-initiating protein markers, including ECAM, tetraspanin-8, CD104, and CD44v6, was significantly upregulated in the serum of cancer patients but not in healthy individuals or patients with non-malignant diseases [55]. The FDA-approved biomarker panel for colorectal cancer, Cologuard (Exact Sciences, Madison, WI), comprises 11 different types of biomarkers, including 7 genetic mutations, 2 DNA methylation markers, and a hemoglobin marker for disease detection, with a beta-actin marker serving as an internal control to validate the integrity of the samples.
Transition from traditional biomarkers to circulating tumor DNA (ctDNA)
As we mentioned, traditional cancer detection methods primarily include instrumental examinations, tissue biopsies, and the detection of tumor biomarkers. These methods, while effective, often involve invasive procedures and may lack the sensitivity and specificity required for early detection and real-time monitoring of cancer progression [56, 57].
Evidence suggests that ctDNA has emerged as a promising alternative to traditional biomarkers due to its non-invasive nature and ability to provide detailed genetic and epigenetic information about tumors. ctDNA is a fragment of DNA shed from tumor cells into the bloodstream, carrying tumor-specific genetic alterations such as mutations, methylations, and microsatellite instability [57, 58]. This enables real-time monitoring of tumor dynamics and can be utilized for early cancer detection, prognosis, and treatment guidance [59, 60].
The detection of ctDNA is facilitated by advanced techniques, including high-throughput sequencing, PCR-based methods, and innovative materials such as CRISPR-Cas systems and graphene, which enhance the sensitivity and specificity of ctDNA assays [57, 61]. ctDNA is particularly useful in monitoring tumor progression, assessing treatment response, and detecting minimal residual disease (MRD) in various cancers, including gliomas, breast cancer, and rectal cancer [58, 62, 63]. It also plays a crucial role in immuno-oncology, where it helps predict responses to immune checkpoint inhibitors and distinguish between actual progression and pseudoprogression [64, 65].
ctDNA (circulating tumor DNA)
Circulating tumor DNA (ctDNA) is double-stranded or single-stranded DNA found in the serum or plasma of cancer patients. ctDNA exhibits various cancer-like molecular features, including epigenetic alterations (such as altered methylation), nucleotide mutations, and cancer-derived viral sequences [66–68]; it is cell-free DNA in the blood of cancer patients released from tumor cells through necrosis, apoptosis, and active release.
Over the past few years, scientists have demonstrated various potential applications of ctDNA in clinical settings, including cancer identification, treatment evaluation, and disease monitoring [69]. However, certain biological features of ctDNA remained unclear. Some scientists speculated that the size of ctDNA is shorter than non-cancer cell-free DNA, while others believed its size is larger [14, 70].
Recent findings have clarified this controversy. Studies have shown that the size of ctDNA is shorter than that of non-cancer cell-free DNA. Madhavan et al. and Jiang et al. independently exposed that the plasma of breast cancer and liver cancer patients contained short DNA molecules with high tumor relevance [71]. According to a study, 4–5 mL of plasma or serum from cancer patients includes 5–10 ng/mL of ctDNA. ctDNA undergoes rapid clearance from the bloodstream, with a half-life of approximately 2.5 h. Therefore, to obtain reliable results from ctDNA, it is essential to develop molecular techniques that can precisely and efficiently detect and characterize ctDNA [72]. Applications of ctDNA in cancer analysis are depicted in Fig. 4.
Fig. 4.

Application of circulating tumor DNA in cancer. In the tumor microenvironment, cells undergo necrosis, apoptosis, pyroptosis, and phagocytosis, releasing their DNA as soluble debris known as circulating tumor DNA (ctDNA) (left). This ctDNA is present in liquid biopsies and has clinical applications in cancer diagnosis, prognosis, and monitoring treatment response (right)
Mechanism of ctDNA release
Although the presence of ctDNA has been widely accepted, the mechanisms by which it is released into the bloodstream are still being extensively investigated. It has been suggested that ctDNA can be released through various mechanisms, including necrosis, apoptosis, pyroptosis, phagocytosis, and the active release of living and circulating cancer cells [73, 74]. Hence, ctDNA can have multiple origins rather than just one.
Apoptosis, a form of programmed cell death, is executed by caspases and is crucial for maintaining cellular homeostasis [75]. When cells undergo apoptosis, their contents, including the nucleic acids, are packed into apoptotic bodies, which are then phagocytized and release their DNA content as soluble debris. There is evidence that ctDNA is approximately 166 bp and exhibits ladder-like patterns when visualized by gel electrophoresis, similar to DNA fragments released by cells undergoing apoptosis [76, 77]. This ctDNA feature is due to DNA internucleosomal fragmentation during apoptosis [78].
The release of DNA after necrosis is a complex process, as necrotic cancer cells are known to produce various immune attractants that recruit macrophages, which can effectively eliminate these cells and their contents. This results in the degradation of the tumor cell DNA, and the digested ctDNA is released into the extracellular spaces [79]. For instance, necrotic T-lymphocytes from T-cell leukemia patients released minimal ctDNA; however, when they were co-cultured with macrophages, the amount of ctDNA released into the media increased [80–82].
Circulating tumor cells (CTCs) are also a significant source of circulating tumor DNA (ctDNA) [83]. When the CTCs are in blood circulation, they encounter biophysical challenges, such as the bloodstream’s rapidity, hemodynamic pressure, collisions with blood elements, and interactions with intercellular complexes involving leukocytes and platelets [84]. These phenomena can cause breakage and release of DNA from CTCs. However, this release of ctDNA from CTCs is not clinically relevant due to the scarcity of CTCs, infeasible quantification of the release of ctDNA, and lack of scientific evidence [85]. ctDNA is present in cancer samples where CTCs were undetectable but not otherwise [80]. Additionally, the genome equivalents of ctDNA are approximately 1000 times higher than those of CTCs. Hence, the levels of ctDNA do not relate to the number of CTCs [86].
Clinical applications
ctDNA has potential as a novel biomarker in early cancer detection, prognosis, and monitoring. Kruger et al. evaluated the prognostic significance of KRAS ctDNA in patients with advanced pancreatic cancer [87]. The study aimed to analyze whether KRAS ctDNA can serve as a more precise biomarker than standard biomarkers, such as CEA and CA 19-9. KRAS ctDNA was a more accurate prognostic biomarker for patients with advanced pancreatic cancer [87].
Studies have also shown the efficacy of ctDNA in the identification of HPV ctDNA, which is the leading cause of cervical cancer, in the bloodstream of patients, demonstrating high levels of precision in the diagnosis of HPV-positive cases of cancer [88]. The detection of HPV ctDNA in the serum has been observed to occur years before the clinical diagnosis of HPV-induced oral cancer. The evidence indicates its potential as an early marker for pre-cancerous lesions. Studies have also shown a higher level of specificity in the identification of HPV ctDNA in serum, with various studies indicating no false-positive results. Multiple studies demonstrate that HPV ctDNA can serve as a biomarker to help patients select the appropriate course of action [89].
Genotyping analyzes genetic alterations and has essential applications in managing cancer treatment [90]. Genotyping in the current clinical setting employs genomic DNA isolated from tissue biopsy. However, tissue biopsies have limitations, as they can only provide static or local tumor information, and it is not possible to determine tumor genotypes in real time due to the heterogeneity and constant evolution of cancer [91]. Analysis of ctDNA from patients’ blood can be used to overcome these challenges by demonstrating the genetic alterations of the entire cancer tissue [92]. Furthermore, ctDNA from the same patients at different clinical stages of cancer can be used to dynamically monitor changes in DNA during cancer progression [93]. Hence, liquid biopsy-based analysis of ctDNA can enhance cancer genotyping analysis, which is significant in personalized medicine.
ctDNA can also serve as a marker for disease monitoring and treatment outcomes. Detection of ctDNA can also be used to assess the remission status and relapse of the disease [72]. ctDNA detection analysis successfully detected 47% of patients in stage I cancer and 82% in stage IV cancer, indicating that levels of ctDNA increased as the tumor progressed to advanced stages [94]. Furthermore, a study revealed that levels of ctDNA significantly reduced after surgical removal of the cancer. Moreover, patients with detectable levels of ctDNA after surgery relapsed, while patients with no detectable ctDNA post-surgery had a good prognosis, and cancer remained in remission [68]. These investigations suggest that the detection of ctDNA can predict treatment outcomes and provide a valuable reference for healthcare providers in determining the subsequent treatment protocols [95].
Current research and developments
ctDNA detection has recently drawn interest from the scientific community due to its potential as a sensitive and specific biomarker for cancer. For this purpose, various new approaches are being devised that can effectively detect ctDNA from cancer patients. To address these challenges, new biosensor technologies have been developed that are small, have low sample requirements, and offer rapid detection, high accuracy, and sensitivity, making them essential for biomarker identification and the early detection of cancers [96, 97]. Currently, the detection methods for ctDNA primarily include digital PCR and amplification depth sequencing; however, these techniques have limitations in terms of cost, test time, false-positive results, and portability [98].
Electrical biosensors can offer a more effective alternative to traditional techniques, as they utilize electrical signals to detect and analyze biological samples. The sensors attach a specific identification probe to the target ctDNA [99]. When the probe and specific ctDNA are connected, it produces an electrical signal that can be detected and used for identification [100].
Furthermore, the incorporation of nanotechnology with biosensors for the detection of ctDNA is also currently being investigated. Various nanomaterials have been synthesized for the detection of ctDNA [101]. To detect the ctDNA of PI3KA in the blood of patients with gastric cancer, scientists developed a gold nanoparticle glass electrode and embedded the probe for target DNA. After the hybridization of target ctDNA with the probe, a helical structure forms, resulting in the detachment of the hybridized fragment of DNA, which produces an increased electrical current intensity that the sensor can detect. This technique enabled the real-time analysis of ctDNA from the serum samples of cancer patients [101].
Zhang et al. developed MoS2 nanosheets for the detection of ctDNA. The nanosheet served as a platform for immobilizing DNA probes for target ctDNA. This probe was highly sensitive, with a detection limit of 8 × 10−17 mol/L [15]. PdAu/Fe3O4 nanoparticles were synthesized to detect ctDNA of EGFR L858R using the CRISPR/Cas12a system. The nanostructure had a detection limit of 3.3 aM, and it could accurately identify the target ctDNA mutation due to the role of the CRISPR/Cas12a system [102].
Recent advancements in liquid biopsies have modified how oncologists approach cancer treatment options. Liquid biopsies for the detection of ctDNA can provide relatively effective, easy, and non-invasive ways to identify genetic alterations in cancer patients, which can influence the patient’s prognosis and treatment decisions [103]. Liquid biopsies can be used to analyze various biomarkers in the blood of cancer patients, including CTCs, cfDNA, cfRNA, and ctDNA [104]. The development of new strategies for detecting ctDNA has led to more advanced approaches for treating patients with various cancers [105, 106]. A more detailed discussion of the role of liquid biopsies in the early detection of cancer is provided in a separate section.
Furthermore, Table 2 summarizes the primary challenges of establishing ctDNA as a first-line biomarker for cancer.
Table 2.
Key challenges of ctDNA as biomarkers for cancer
| Challenge | Description | Reference |
|---|---|---|
| Low concentration and fragmentation | ctDNA is present in low concentrations in the bloodstream and is highly fragmented, which makes detection difficult. This necessitates the development of highly sensitive and specific detection methods | [59, 107] |
| Short half-life: | ctDNA fragments have a short half-life, making it challenging to achieve detectable concentrations in body fluids consistently | [59] |
| Lack of standardization: | There is a need for standardized methodologies and cut-off values to differentiate between high and low ctDNA concentrations, which are currently lacking | [59, 65] |
| Detection sensitivity and specificity |
While ctDNA can be detected in a significant percentage of patients with advanced cancers, its detection in early-stage cancers or certain cancer types (e.g., brain, renal, prostate) is less reliable, which limits its use as a universal biomarker ctDNA is present in low concentrations in the bloodstream, making it difficult to detect. The high fragmentation and short half-life of ctDNA further complicate its detection, requiring highly sensitive and specific methods |
[59, 107] |
| Clinical validation |
Further rigorous clinical studies are necessary to validate the utility of ctDNA as both a prognostic and predictive biomarker, particularly regarding minimal residual disease and its potential as a general cancer screening tool While ctDNA shows promise in detecting minimal residual disease and monitoring treatment response, most studies are observational or indication-specific, lacking rigorous clinical validation |
[63, 108] |
| Technological limitations | Current detection technologies, although advanced, still face limitations in accurately identifying and quantifying circulating tumor DNA (ctDNA), necessitating the ongoing development and refinement of biosensing platforms | [109] |
| Tumor heterogeneity | ctDNA reflects the genetic landscape of both primary and metastatic tumor sites, which can vary significantly, making interpreting results for treatment decisions more complicated | [59, 103] |
Exosomes
Extracellular vesicles (EVs) are membrane-bound particles released by cells, playing crucial roles in intercellular communication by transferring proteins, lipids, and nucleic acids. They are mainly categorized into small extracellular vesicles (sEVs), often referred to as exosomes, and large extracellular vesicles (lEVs), which include microvesicles and large oncosomes, each possessing distinct characteristics and functions. The main differences between sEVs and lEVs are shown in Table 3.
Table 3.
Differences between small extracellular vesicles (sEVs) and large extracellular vesicles (lEVs)
| Characteristic | sEVs | lEVs | References |
|---|---|---|---|
| Size |
The mean size is approximately 79 nm Typically, it ranges in diameter from 30 to 150 nm |
Start at a size of 100 nm and can be much larger | [110, 111] |
| Isolation method | Higher centrifugal forces compared to lEVs | Differential centrifugation | [110, 111] |
| Cargo | They transport proteins, mRNA, miRNAs, and lipids, which can modulate the tumor microenvironment, promote immune suppression, and facilitate metastasis | They are enriched with chromosomal DNA, including large fragments of up to 2 million base pairs. This DNA content reflects the genomic alterations of the tumor of origin, making lEVs a valuable source for detecting tumor-derived genomic changes | [112–115] |
| Protein content | sEVs are enriched in proteins associated with cell adhesion and signaling pathways, contributing to their role in promoting cellular adhesion and motility | lEVs are enriched in proteins associated with ribosomes and RNA biogenesis, suggesting various functional roles | [116] |
| Roles in cancer | sEVs are involved in cancer progression by delivering bioactive cargos that reprogram target cells, promoting tumor growth, invasion, and metastasis. They are also being explored as drug carriers and anti-cancer vaccines due to their ability to modulate immune responses and create pre-metastatic niches | lEVs carry most of the tumor DNA circulating in the plasma of cancer patients, such as those with prostate cancer. This makes them a critical component for studying tumor-derived genomic alterations and potentially guiding personalized cancer therapies | [112, 114, 115, 117] |
Furthermore, sEVs are heterogeneous membrane vesicles released by all cell types and are involved in various physiological and pathological processes. They can influence tissue protection and damage by delivering different molecular messengers [118, 119].
Exosomes are a subtype of sEVs formed in late endosomes and released from the cell membrane. They contain proteins, RNA, and lipids from their cells of origin. They are involved in development, signal transduction, and the transfer of infectious material [120].
Exosomes, minute membranous bodies typically ranging from 30 to 160 nm, have garnered significant attention as a therapeutic platform due to their unique capability to deliver and exchange intracellular chemical messages [121]. These nanoscale entities interact with recipient cells to facilitate communication across neighboring cells and distant organs, carrying a diverse array of organic compounds, including proteins, RNA, genomic DNA, and other non-coding RNAs (ncRNAs). Exosomes are part of the broader category of extracellular vesicles (EVs), which originate from cells, including microvesicles/ectosomes, and apoptotic bodies, and are distinguished by size and origin (Fig. 5). Exosomes are natural carriers that efficiently transport various types of cargo between cells with low immunogenicity, making them promising candidates for therapeutic applications, such as mRNA delivery [122].
Fig. 5.

Application of extracellular vesicles in cancer. Exosomes are derived not only from intraluminal vesicles within multivesicular bodies (MVBs) but also from the budding of the plasma membrane. Early endosomes mature into late endosomes or multivesicular bodies (MVBs), which can then be directed to either the secretory or degradative pathways. In contrast, microvesicles are produced by direct budding from the cell membrane, while apoptotic bodies form during programmed cell death. Exosomes are spherical structures enclosed by a lipid bilayer and contain a variety of complex molecules, including proteins, mRNA, miRNA, ncRNA, and DNA (left). The initial biofluid sample contains extracellular vesicles (EVs), suspended proteins, and debris. Subsequently, ultracentrifugation results in a pellet of EVs mixed with proteins, which can be further purified using discontinuous ultracentrifugation or density gradient ultracentrifugation. These methods separate proteins and EVs based on their density. Other techniques include ultrafiltration, size exclusion chromatography, and asymmetrical flow field-flow fractionation for separate molecules based on size or hydrodynamic radius. Precipitation-based isolation utilizes compounds such as polyethylene glycol (PEG) to concentrate particles. In contrast, immunoaffinity isolation employs antibodies to capture specific EV populations, though it does not isolate all EV types (right)
Once regarded merely as carriers of cellular waste, exosomes are now recognized as critical intermediaries in intercellular communication [123]. Emerging evidence suggests that exosomes play a significant role in cancer progression by promoting angiogenesis and metastasis. Within the tumor microenvironment, they facilitate information exchange between fibroblasts and cancer cells, thereby supporting tumor growth. In gliomas, hypoxic conditions induce cancer cells to secrete exosomes that promote the polarization of M2 macrophages, thereby enhancing cancer cell proliferation and survival [124]. These exosomes, rich in Interleucin-6 (IL-6) and miRNA-155-3p, further activate pSTAT3 and induce autophagy in macrophages. Moreover, exosome-enriched miRNAs exhibit dual roles in glioma. While miR-1298-5p in cerebrospinal fluid exosomes suppresses glioma progression, it simultaneously promotes the immunosuppressive function of myeloid-derived suppressor cells, thereby accelerating glioma development [125].
Recent advances in exosome research have underscored their involvement in various hallmark features of cancer, including neoplasia, tumor growth, metastasis, paraneoplastic syndromes, and therapy resistance. These roles will be discussed in the following section (Table 4).
Table 4.
An overview of different methods for detecting miRNAs
| Detection Method | Description | Advantages | Limitations | References |
|---|---|---|---|---|
| Quantitative PCR (qPCR) | Measures miRNA levels using specific primers and probes, providing quantitative data | High sensitivity and specificity; widely used and validated | It requires prior RNA extraction and is limited to known miRNAs | [183] |
| Microarray Analysis | It utilizes a chip with probes for multiple miRNAs to profile their expression levels across various samples | It can detect multiple miRNAs simultaneously, making it suitable for broad profiling | Less sensitive for low-abundance miRNAs; complex data analysis | [184] |
| Next-Generation Sequencing (NGS) | Provides comprehensive profiling of miRNAs by sequencing RNA fragments, allowing for the discovery of novel miRNAs | High throughput; can identify novel miRNAs; provides detailed expression profiles | High cost; complex data analysis; requires significant bioinformatics resources | [185] |
| Northern blotting | Detects miRNAs based on their size and abundance using gel electrophoresis followed by hybridization with a labeled probe | It provides size information and is suitable for the specific detection of miRNA | Time consuming; requires large amounts of RNA; less quantitative | [187] |
| In Situ Hybridization (ISH) | Detects miRNA expression in tissue samples using labeled probes that bind to complementary miRNA sequences | Allows localization of miRNA within tissue; provides spatial context | Less quantitative; requires tissue samples | [188] |
| RNA Sequencing (RNA-seq) | A form of NGS focused on sequencing all RNA species, including miRNAs, to analyze their expression and discover novel miRNAs | Comprehensive; detects known and novel miRNAs; high resolution | Expensive; requires bioinformatics expertise; data complexity | [186] |
| Droplet Digital PCR (ddPCR) | Quantifies miRNAs with high precision by partitioning the sample into droplets and performing PCR in each droplet | Highly sensitive and precise; allows absolute quantification | Expensive equipment; limited multiplexing capabilities | [189] |
| Biosensors | Employs various sensing technologies (e.g., electrochemical, optical) to detect specific miRNA sequences in real time | It can be susceptible and enables real-time monitoring | It may require extensive calibration, which is less common in standard laboratories | [190] |
Role in cancer detection
The study of exosomes in cancer has advanced rapidly, highlighting their association with several hallmark features of the disease. Exosomes play crucial roles in neoplasia, tumor growth, metastasis, paraneoplastic syndromes, and therapy resistance, with their impact varying by cancer type, genetics, and stage. Notably, the secretion of exosomes is elevated in cancer patients, making exosomal markers promising targets for cancer detection [126]. Research has demonstrated a correlation between plasma exosome levels and tumor burden. For instance, in stage IV oral squamous cell carcinoma patients, elevated levels of CD63-positive and caveolin 1 (CAV1)-positive exosomes were linked to poorer prognosis [127]. In prostate cancer, increased exosomal CD81 and prostate-specific antigen levels helped distinguish cancer patients from those with benign prostatic hyperplasia and healthy individuals [128].
Innovative diagnostic methods, such as the extracellular array developed by Jakobsen et al., have demonstrated 75% accuracy in distinguishing between non-small cell lung cancer patients and healthy subjects using minimal plasma samples [129]. Mass spectrometry analyses of blood exosomes have identified specific biomarker candidates, such as CLDN4, EPCAM, and GPC1, in pancreatic and colon cancers [130]. Elevated GPC1 + circulating exosome levels can differentiate between healthy individuals and patients with benign pancreatic disease, as well as those with pancreatic cancer, correlating with tumor burden and patient survival [131, 132]. Moreover, the identification of phosphoproteins in exosomes, especially in breast cancer patients, indicates the possibility of improving the process of cancer diagnostics and observation. These findings demonstrate the importance of exosomes in cancer diagnosis and prognosis [133].
For instance, advancements made in recent years demonstrate the potential of functional exosomes in cancer detection. To improve the purification process of HCC-derived EVs, new antibodies have been developed using trans-cyclooctene-conjugated EpCAM, ASGPR1, and CD147 multi-marker cocktails, which can aid in early diagnosis. However, progress in developing such antibody cocktails is based on identifying specific exosome markers in cancer. Circulating protein markers, involving EpCAM and CEA, have been identified in pan-cancer studies, particularly in the context of exosomes derived from colorectal adenomas and cancers. Additionally, studies have demonstrated that CD24 and EGFR are present in ovarian cancer exosomes, making them potential biomarkers. In prostate cancer, the lumen of the prostate gland has been used along with the membrane-specific prostate antigen to identify the specific exosomes, and these levels are often reported to be higher than those in the normal population.
Isolation and analysis techniques
Exosomes can be isolated from the culture media of healthy and diseased cells, as well as from body fluids and tumor tissues. Various procedures have been proposed to identify these exosomes, each with its advantages and disadvantages. The primary source material for isolating exosomes is cell culture medium, and the standard method used is differential ultracentrifugation (UC) because of its high purity of exosomes. UC involves a series of centrifugation steps and provides particle separation based on their relative density. Other procedures used in the purification of exosomes include density gradient centrifugation, polyethylene glycol precipitation, ultracentrifugation, size exclusion chromatography (SEC), and immunoaffinity chromatography [134, 135]. Density gradient fractionation involves the separation of particles based on their buoyant density, enabling the achievement of high levels of purity. The use of polymers for the precipitation of exosomes results in high yields. However, at the same time, it presents a significant challenge in incorporating a large number of non-exosomal substances [134].
Ultrafiltration and size exclusion chromatography separate particles based on their physical size. In ultrafiltration, membranes with defined pore sizes are used. On the other hand, SEC entails the passage of the particles through a column containing porous beads. These methods are typically used in conjunction with UC to enhance the purity of exosomes. The selectivity of immunoaffinity isolation is based on the surface proteins that are unique to the exosomes. This method involves attaching antibodies to beads to either directly capture the targeted exosomes or exclude undesired ones [136]. Immunoaffinity isolation offers high selectivity for the target and contaminants, but the yields obtained from this method are generally lower than those obtained from other methods.
To address the drawbacks associated with the low yield of isolation in individual techniques, it has been proposed to use a combination of methods to enhance both the yield and the purity of isolated exosomes [137]. For example, UC can be employed in conjunction with density gradient fractionation, which first segregates particles according to size and then according to density, allowing for the elimination of non-vesicular contaminants. Consequently, ultrafiltration and SEC are often combined with UC or other methods to obtain highly pure and concentrated samples of exosomes. Due to the synergistic utilization of these techniques for exosome sample enrichment, the studies employing these methodologies generated highly purified exosome preparations for various research and clinical purposes [138–140].
Essentially, exosomal proteins can be identified by methods other than the current standard of Western blotting. Among all the available methods, the enzyme-linked immunosorbent assay (ELISA) is the most commonly used. In recent years, several strategies have been developed to facilitate the detection of exosomal proteins [141]. For example, the nanozymes can be applied to an immunosorbent assay (ISA) to immobilize exosomes by specific surface proteins, and then NAISA catalyzes a chemical reaction to produce a signal. Another standard chemical research analysis method is fluorescence spectrophotometry, which is used to determine the presence and concentration of substances. Among this evidence, there are some improvements in the detection of exosomes, such as exosome immunoassays based on single-molecule array technology (SiMoa) [142]. Overall, assays such as the CD9-CD63 and Epcam-CD63 SiMoa are presented, which are highly sensitive, enabling the method to distinguish between cancerous and non-cancerous plasma samples.
Yoshioka et al. described a new approach for the direct analysis of circulating exosomes from the plasma of colorectal cancer patients. This method utilizes two types of antibodies to capture exosomes and employs photosensitizer beads for direct detection, eliminating the need for purification. The technique can distinguish CD147 and CD9 doubly positive EVs, which colorectal cancer patients secrete. [143, 144].
Additionally, single-exosome profiling and exosome barcoding are advanced techniques used in cancer research to analyze and utilize exosomes, providing detailed insights into their composition and function. These methods hold the potential for improving cancer diagnostics and developing targeted therapies; however, challenges such as variability in exosome content remain [145].
Single-exosome profiling is a technique that aims to analyze the molecular content of individual exosomes, providing detailed insights into their composition and potential as cancer biomarkers [146–148]. Single-exosome profiling can identify specific lipid and protein compositions that may serve as early detection markers for cancers such as hepatocellular carcinoma and lung cancer [147, 148]. The key challenges of this technology include the variability in exosome content resulting from different biological sources and isolation methods, which can complicate the profiling process [146, 149].
On the other hand, exosome barcoding involves tagging exosomes with unique molecular identifiers, allowing for the tracking and analysis of exosome populations in complex biological systems [150]. This technique can enhance the understanding of exosome biodistribution and its role in cancer progression, potentially leading to the development of targeted therapeutic strategies [150, 151]. Barcoding can enhance the specificity of exosome-based diagnostics and therapeutics by facilitating the precise identification and tracking of exosome subpopulations [150].
Liquid biopsy
Techniques for molecular analysis of liquid biopsy samples, including blood, have undergone significant improvements, enabling their clinical utilization in cancer patients and greatly enriching the understanding of cancer development mechanisms. The minimally invasive technique offers a method for obtaining tumor-derived information from various body fluids, with blood being the most commonly used [152]. The concept of liquid biopsies originated with the identification of circulating tumor cells at the beginning of the last decade and has since rapidly evolved to include circulating tumor DNA and other tumor-origin components, such as cell-free circulating RNA (including microRNA and messenger RNA), extracellular vesicles, and tumor-promoting platelets (Fig. 6). Studies on CTCs and ctDNA, the two primary elements of liquid biopsy tests, remain a highly active area of investigation, with ongoing advancements in the findings [153]. On this account, identifying and quantifying these components from body fluids presents a novel strategy for early cancer diagnosis, disease progression, and therapeutic assessment. Thus, the concept of liquid biopsy has become a significant asset in current cancer management.
Fig. 6.

Liquid biopsy in cancer. Liquid biopsies, such as those obtained from saliva, urine, blood, plasma, and breast milk, have potential clinical value in managing tumors (left). These non-invasive or minimally invasive techniques are primarily being developed to identify biomarkers for early diagnosis, prognosis prediction, and targeted treatments (right)
Applications in cancer diagnosis
CTC and its isolation from the heterogeneous population of blood cells were first reported as early as the 1860 s, and discoveries have successively refined techniques for capturing the cells [154]. It has been postulated that CTCs are released from primary tumors and circulate in the bloodstream due to the presence of disseminated cancer cells at other sites. The importance of tumor diagnosis has increased due to the ability to sample tumors and obtain quick information on their status [155]. Unlike other biomarkers, the number of CTCs can more accurately and promptly reflect the tumor’s condition. In breast cancer, lower CTC count has also been correlated with better overall survival, which makes CTC a possible tool for predicting the response to therapy [156, 157]. Additionally, CTCs have been shown to have the potential for early detection of multiple types of cancer and for distinguishing between benign and malignant pulmonary masses. As expected, this diagnostic ability has been validated in the tissue assays, thus affirming the value of CTCs in liquid biopsies [158, 159].
Clinical application of CTCs in cancer detection
CTCs are now the pillar of liquid biopsy research, providing a comprehensive picture of tumors’ genetic and molecular profiles. Clinicians can obtain helpful information about tumor behavior and anticipate reactions to treatment by understanding the characteristics of CTCs. For example, specific gene alterations, such as T790M, are associated with resistance to medicines like gefitinib and erlotinib in lung adenocarcinoma but confer sensitivity to the newer EGFR inhibitors [160, 161]. Similarly, profiling genes such as KRAS and PIK3CA in CRC indicates the prospects for treatment outcomes, as it reflects variations in patient genetic response rates. CTCs also show prominent epigenetic alterations in cancer-related genes [162].
In breast cancer, DNA methylation in the promoter regions of tumor suppressor genes such as SOX17, BRMS1, and CST6 correlates with increased metastasis and poorer prognosis. In prostate cancer (PCa) and colorectal cancer (CRC), methylation changes in genes associated with angiogenesis, such as VEGF and SFRP2, have been detected [163]. These epigenetic alterations in CTCs offer a more effective diagnostic tool than traditional tissue biopsies and can also signal resistance to specific treatments in breast cancer (BC) patients. The methylation profiles of non-coding RNAs, such as miR-200 linked to epithelial-to-mesenchymal transition, are elevated in CTCs from PCa patients [164]. The biomarkers aid in prognosis, monitoring tumor response, and indicating the presence of metastasis. Despite the evolving nature of primary tumors, CTCs offer a real-time snapshot of the tumor’s genetic state, which is crucial for developing targeted therapies [165]. CTCs are also used to create patient-derived tumor models for treatment research. For example, breast cancer xenografts from luminal CTCs have shown the presence of metastatic-inducing cells (MICs), leading to metastases in bone, liver, and lungs in mice [166]. These studies link specific CTC surface markers with increased metastasis and reduced survival rates, thereby aiding in the development of better diagnostic tools for metastatic breast cancer.
Limitations of liquid biopsies
Liquid biopsies are becoming valuable non-invasive methods for diagnosing and monitoring cancer. Nevertheless, they have not yet become standard diagnostic tools and are typically used with traditional tissue biopsies. While liquid biopsies offer significant potential for non-invasive cancer diagnosis, they encounter significant challenges related to sensitivity and specificity, particularly in detecting rare biomarkers [167]. The main limitations include lower sensitivity and precision compared to tissue biopsies, as well as uncertainty regarding whether they capture all genomic clones within a tumor. Despite advancements in single-cell analysis, detecting circulating tumor cells (CTCs), ctDNA, and other tumor-derived elements in blood remains challenging due to their scarcity [168–170]. Their diagnostic sensitivity and accuracy are limited by the low detection rate of CTCs [171]. Moreover, the detection sensitivity of liquid biopsies is influenced by the variability in biomarker types and the technologies employed for their identification [172, 173].
Additionally, variations in extraction methods can lead to inconsistent yields of cell-free DNA (cfDNA) [167]. Pre-analytical variables, such as the type of blood collection tube and processing time, also impact the analysis. Furthermore, the reliability of biomarkers in biofluids remains limited, necessitating optimization and standardization of protocols for effective clinical implementation [174].
Cost-effective strategies are needed for pre-selecting patients due to the low frequency of target mutations in CTCs and ctDNA [175]. The fragile nature of some biomarkers and the lack of standardized isolation protocols further complicate the process, leading to potential false positives and negatives. Microenvironmental factors that affect accuracy can influence biological materials used for LBs. Extracellular vesicles present additional challenges due to the inadequacy of existing enrichment technologies. The variability and low numbers of CTCs make analysis challenging, often necessitating high starting concentrations, which can potentially introduce biases in DNA amplification. Establishing CTC cell lines and xenograft models is costly and complex due to tumor heterogeneity [176]. Despite these challenges, LBs hold significant potential for providing comprehensive tumor profiling and real-time therapeutic information. However, novel techniques and standardized approaches are necessary to realize the clinical applications of these methods fully.
MicroRNAs
MicroRNAs are small, non-coding RNA molecules of 22 nucleotides that play vital roles in development, differentiation, cell proliferation, and cell death, also known as apoptosis.
Participation occurs through specific base-pairing interactions with target mRNAs, which regulate both transcription and post-transcriptional processes. In addition to the direct functions on cancer cells, miRNAs also regulate the other components of the tumor microenvironment. miRNAs were initially implicated in cancer in 2002 when Lee et al. presented evidence of concurrent frequent deletions spanning miRNA-coding genes in around 50% of patients with B-cell chronic lymphocytic leukemia [177, 178]. Following this, the relevance of miRNAs in cancer has been recognized as a critical component influencing tumorigenesis and disease progression. Many miRNAs have been discovered to possess either tumor suppressor or oncogenic abilities, thus highlighting the complex nature of the cancer process [179].
Secreted miRNAs outside the tumor can influence cell crosstalk and interact with the stroma and matrix to create a protective environment for the cancer, thereby compromising immune surveillance. For instance, miR-200c may directly inhibit PTEN and FOG2, thereby promoting the PI3K/Akt pathway and increasing the levels of MDSC, which suppresses the anti-tumor immune response [180, 181]. Moreover, macrophages can release exosomes containing miRNA that promote the invasive ability of cancer cells, with miR-223 being a key agent. Furthermore, the transfer of miR-144 and miR-126 via exosomes contributes to shaping a metabolic environment conducive to cancer progression [182]. miRNA detection techniques comprise various methods. Quantitative PCR (qPCR) offers high sensitivity and specificity for quantifying known miRNAs, but it requires prior RNA extraction [183]. Microarray analysis allows for broad profiling by detecting multiple miRNAs simultaneously, although it is less sensitive for low-abundance miRNAs and involves complex data analysis [184]. Next-generation sequencing (NGS) and RNA sequencing (RNA-seq) provide comprehensive profiling, including the discovery of novel miRNAs, but they are expensive and require significant bioinformatics resources [185, 186]. Northern blotting and in situ hybridization (ISH) detect miRNAs based on size and spatial localization, respectively. Northern blotting is time consuming and less quantitative. At the same time, ISH provides spatial context in tissues but is also less quantitative [187, 188]. Droplet Digital PCR (ddPCR) enables precise quantification with high sensitivity, although it requires costly equipment [189]. Biosensors offer real-time, sensitive detection, but they often need calibration and are less commonly used in laboratories [190].
Regulatory role in cancer
In cancer, the regulation of miRNAs is a highly complex process that may affect both the expression and functionality of these molecules. As studied over the last few decades, miRNAs are essential players in tumor biology; however, the understanding of miRNA biogenesis regulation and the consequences of their deregulation in cancer remains incomplete. The following parameters have been proposed to contribute to cancer-related miRNA dysregulation: genetic amplification or deletion, the activity of specific transcription factors, and epigenetic changes [191]. First, changes in miRNA genes and their numbers, which can be caused by gene amplification or deletion, significantly affect cancer genesis. Notably, miRNAs are in various genomic regions, including intergenic regions and introns of protein-coding genes. For instance, mutations of miR-15a and miR-16a, located on chromosome 13q14, are notable in chronic lymphocytic leukemia. A consequence is the overexpression of oncogenes, such as BCL2 [192]. Consequent to the loss of function, miR-143 and miR-145 are downregulated in lung cancers, while the miR-17–92 cluster is upregulated in T-cell acute lymphoblastic leukemia, leading to lymphomagenesis.
Secondly, there are inputs from transcription factors to stabilize or release miRNA expression [193]. Disruptions in especially essential proto-oncogenes/tumor suppressor genes, such as p53 or c-Myc, will cause alterations in miRNA levels [194]. For instance, p53 regulates miR-34 and miR-145, which are implicated in apoptosis pathways and the cell cycle [195, 196]. On the other hand, c-Myc induces highly reported oncogenic miRNAs, such as the miR-17–92 cluster, while repressing tumor suppressor miRNAs [197]. These studies highlight the intricate role of miRNAs in cancer regulation (Fig. 7).
Fig. 7.

MicroRNA in cancer. MicroRNA (miRNA) genes are transcribed by RNA polymerase II (Pol II) to produce primary transcripts (pri-miRNAs). These are processed by the Drosha complex, generating ~ 70 nucleotide (nt) pre-miRNAs. Pre-miRNAs are recognized by exportin 5 (XPO5) for export to the cytoplasm, where the enzyme Dicer, TRBP (TAR RNA-binding protein; also known as TARBP2), and Argonaute (AGO) 1–4 further process pre-miRNA into miRNA duplexes. The miRNA duplex is incorporated into the RNA-induced silencing complex (RISC), where one strand is removed, leaving a single-stranded miRNA. This miRNA binds to the 3′ UTR of target mRNAs, primarily through its seed sequence, inducing post-transcriptional gene silencing by promoting mRNA degradation and inhibiting translation (top). MicroRNAs are associated with the hallmarks of cancer, influencing specific cellular functions across various cancer types. Frequently, a single microRNA or a set of microRNAs can influence multiple hallmarks, with a dominant mechanism that may vary depending on the tissue type, highlighting the diverse pathways they regulate. The upregulation or downregulation of these microRNAs plays a crucial role in cancer progression (bottom)
Potential as diagnostic biomarkers
Initially, extracellular miRNAs (ECmiRNAs) were dismissed as byproducts of cellular damage or apoptosis, with no significant biological function [198].
However, recent insights reveal that their release into the extracellular space is regulated, suggesting they serve as signaling molecules in cell communication. The discovery of ECmiRNAs in microvesicles and exosomes, along with their distinct expression patterns in various biofluids associated with disease states, supports their potential as non-invasive biomarkers for early disease detection, including cancer. miRNAs are essential regulators of many physiological and developmental processes, and their abnormal expression often indicates disease, including cancer [199]. The dysregulation can be attributed to genomic changes, such as gene amplifications or deletions, which may indicate the presence of cancer. Numerous studies have demonstrated a connection between altered ECmiRNA levels and cancer, highlighting their potential as a diagnostic tool [200].
Circulating miRNAs hold promise as a diagnostic tool for lung cancer. Elevated levels of miR-21 and miR-210 have been associated with lung cancer and can distinguish early-stage patients from healthy controls. Reduced levels of miR-486-5p in patients with solitary pulmonary nodules, along with increased miR-31 expression, which correlates with shorter survival, further highlight the diagnostic value of miRNAs.
Other promising miRNAs include members of the miR-34 family, miR-944, miR-3662, miR-448, and miR-4478, which have demonstrated varying effectiveness in distinguishing between lung cancer patients and healthy individuals [201–203]. Gastric cancer, the second leading cause of cancer-related deaths, also benefits from miRNA-based diagnostic approaches. Early research revealed lower plasma levels of miR-17-5p, miR-21, miR-106a, and miR-106b in patients with gastric cancer. Subsequent studies have highlighted miR-221-3p, −376c-3p, and −744-5p, which change significantly before clinical symptoms appear [204]. These studies highlight the potential use of miRNAs as effective diagnostic and prognostic markers.
Recent findings on uncovering the diagnostic power of extracellular miRNAs and the functional significance of exosomes in cancer progression are also informing therapeutic strategies. Insights into tumor immune interactions and the manipulation of their environment, particularly the immune system, as discussed below, have led to breakthroughs in immunotherapy, which aims to restore the body’s natural defenses against cancer. These developments underscore the importance of integrating biomarker discovery with treatment innovation as both fields converge to offer more targeted and personalized approaches in oncology.
Immunotherapy biomarkers
Conventional cancer therapies can impair the immune system, which can facilitate metastasis of leftover tumor cells and can also encourage relapse of cancer [205] (Fig. 8). Cancer treatments have targeted immune cells for decades [206]. Later in the 1980 s, immunotherapy was added to cancer treatment options, along with chemotherapy, radiotherapy, and surgery [207].
Fig. 8.
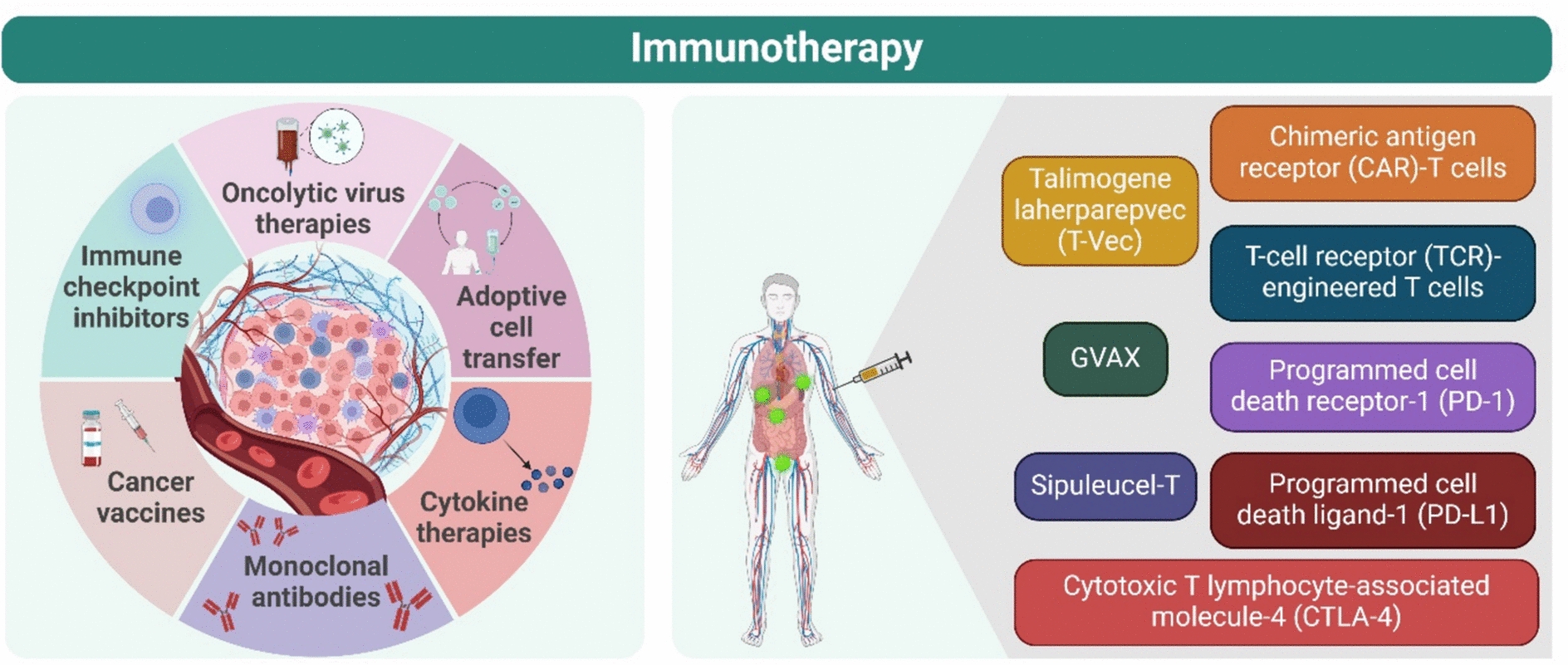
Immunotherapy biomarkers. Major immunotherapy categories included oncolytic virus therapies, immune checkpoint inhibitors (ICIs), cancer vaccines, cytokine therapies, cancer immunotherapy, adoptive cell transfer, and monoclonal antibodies. Oncolytic virus therapies utilize modified viruses to infect and destroy cancer cells, triggering immune responses. For instance, talimogene laherparepvec (T-Vec), a modified herpes virus, is approved for treating advanced melanoma, showcasing the progress made in virus-based cancer treatments. Cancer vaccines activate the immune system by targeting tumor-specific antigens. Critical research identified melanoma antigens that trigger T-cell responses, leading to the development of vaccines targeting these proteins. Dendritic cell (DC)-based vaccines are especially promising, as DCs efficiently present antigens to T cells, enhancing immune responses. Sipuleucel-T, approved for prostate cancer, is one example. Whole tumor cell vaccines, such as GVAX, have also shown potential in treating multiple cancer types. Cytokine therapies: cytokines, immune system messengers, play a crucial role in cancer immunotherapy. Interleukin-2 (IL-2) and interferon-alpha (IFN-α) are key cytokines that stimulate immune responses against cancer. IL-2 helps expand T cells, and high doses have been effectively used to treat metastatic cancers. IFN-α boosts anti-tumor immunity by promoting T-cell activity and tumor cell death. However, their use as monotherapies is limited by toxicity, leading to combination therapies for better outcomes. Adoptive Cell Transfer (ACT) utilizes a patient’s immune cells, particularly T cells, to fight cancer. T cells are expanded or genetically modified outside the body and reinfused to target tumors. Chimeric antigen receptor (CAR)-T cells and T-cell receptor (TCR)-engineered cells are two advanced ACT approaches, both revealing significant success in treating cancers such as leukemia and melanoma. CAR-T cells recognize cancer cell antigens, while TCR-T cells target tumor-specific proteins, resulting in promising clinical outcomes. Immune Checkpoint Inhibitors (ICIs) are a breakthrough in immunotherapy, blocking cancer’s ability to evade immune detection. These therapies target molecules such as CTLA-4, PD-1, and PD-L1, thereby suppressing immune responses. Blocking these pathways reinvigorates T cells to attack tumors. Ipilimumab, a CTLA-4 inhibitor, was the first approved ICI, followed by PD-1 and PD-L1 inhibitors, which have shown remarkable efficacy in treating various cancers
Immunotherapy acts by stimulating the immune system to recognize and destroy malignant cells. It enhances both innate and adaptive immunity, which can halt the progression of cancer cells [208–210]. Dendritic cells (DCs), natural killer (NK) cells, macrophages, and monocytes comprise the innate immune system [211]. These cells intercede with the release of cytokines in an instant immune response. The cytokines can facilitate the direct lysis of cancer cells, mediate antigen processing through the phagocytosis of cancer cells, activate anti-tumor immune responses (mediated by T cells), and activate the release of cytoplasmic granules that can directly eliminate cancer cells [212–214]. Adaptive immune cells, comprising B and T cells, mediate long-lived, antigen-specific reactions and possess operational memory [215].
Immune checkpoint inhibitors (ICIs) have substantially altered the treatment of cancer. ICIs halt inhibitory signals in T cells, while T cells’ immune activity is reinstated by it [216]. Monoclonal antibodies are administered in ICI treatment that targets the negative regulators of T-cell function, specifically immune checkpoint controllers such as cytotoxic T-lymphocyte-associated protein 4 (CTLA4), programmed death-ligand 1 (PD-L1), and programmed cell death-1 (PD-1). ICIs have been permitted for handling numerous cancer types, non-small cell lung cancer (NSCLC) (anti-PD-1/PD-L1), including melanoma (anti-PD1 and anti-CTLA4), Merkel cell carcinoma (anti-PD-L1), renal cell carcinoma (anti-PD-1), bladder cancer (anti-PD-L1), and head and neck squamous cell carcinoma (anti-PD-L1). Despite the therapeutic potential of immunotherapy against numerous malignancies, only a tiny percentage of patients benefit from ICIs [217].
Biomarkers for response prediction
PD-L1 expression is usually lower in normal tissues; however, many tumor cells overexpress PD-L1 to avoid immune system responses [218].
This overexpression by tumor cells can suppress the immune activity of T cells. In a group of 42 patients treated with anti-PD-1 antibodies, which included 10 patients with NSCLC, 2 with prostate cancer, 7 with colorectal cancer, 18 with melanoma, and 5 with renal cell cancer, PD-L1-positive tumors displayed a substantially better-engrossed response compared to negative tumors [219].
A study explained that PD-L1 expression in ≥ 50% of tumor cells was associated with better efficacy of pembrolizumab in late-stage NSCLC patients [220]. Similarly, studies involving patients with metastatic renal cell carcinoma (RCC) or relapsed head and neck cancer treated with nivolumab have indicated that PD-L1 expression levels may predict nivolumab’s effectiveness [221]. However, nivolumab benefited patients with late-stage RCC, regardless of PD-L1 nivolumab [222]. However, in patients with formerly treated NSCLC, atezolizumab treatment is associated with a clinically significant improvement in overall survival compared to docetaxel, regardless of PD-L1 expression [223]. These findings suggest that the effectiveness of treatment (anti-PD-1/PD-L1) may not depend on PD-L1 expression [224].
Tumor mutation burden (TMB) is considered an auspicious biomarker for predicting ICI responses, but specific problems are associated with it. There is no standardized assay for PD-L1, and it lacks an authenticated cut-off point (optimum). The cut-off point differs depending on the type of tumor [225]. A team of researchers is currently endeavoring to establish standardized assays to determine TM and solve these troubles. Another benefit of TMB compared to PD-L1 is that TMB appears to be more efficient in predicting responses to various types of immunotherapy, including anti-CTLA4 antibodies such as ipilimumab, adoptive T-cell transfer therapy, and PD-1/PD-L1 inhibitors [225].
DNA mismatch repair (MMR) pathway involving various genetic variations can pave the path to microsatellite instability (MSI), which is a specified type of TMB (high) tumors and has a large amount of CD8+ tumor-infiltrating lymphocytes (TILs) and indoleamine 2,3-dioxygenase (IDO)+ tumor cells have been identified in colorectal cancer with MMR deficiency (dMMR) [226]. Five clinical trials involving various tumor types have demonstrated that patients with dMMR/MSI-H can achieve robust responses to pembrolizumab [227]. Also, dMMR can induce alterations in the DNA polymerase gene (POLE/POLD1) epsilon/delta 1, increasing the overall neoantigen and mutation load. Hence, it can act as a prognostic marker and risk factor in identifying patients who can benefit from ICIs [228].
Challenges and opportunities
ICIs have transformed the treatment of various cancer types, but there remain challenges, such as most patients developing resistance and failing to respond. The administration of immunotherapy can cause side effects, most importantly, reactions that damage healthy organs [229].
Various challenges are associated with using these biomarkers (PDL-1). The explanations of PD-L1 expression vary in different reports. Although many studies elucidate PD-L1 expression in cancer cells, some studies highlight the importance of PD-L1 expression in tumor-infiltrating immune cells [230]. Another limitation associated with it is the lack of consensus regarding the threshold of PD-L1 expression. According to some studies, the predictive value of PD-L1 expression has been demonstrated for response prediction to immune checkpoint inhibitors; however, this may vary depending on the threshold used [231]. In the case of lung cancer and brain metastasis, varying levels of PD-L1 expression were observed in different patients. In patients with lung cancer, PD-L1 expression can remain for 6 months or more after disease diagnosis [232].
TMB, compared to High Microsatellite Instability (MSI-H)/dMMR and PD-L1 measurement, is quite expensive; its availability in routine settings is a problem and requires considerably prolonged periods. However, when compared to MSI-H/dMMR and PD-L1 measurements, blood-based TMB can be uncovered, which can benefit patients if tumor tissue is not accessible or cannot be obtained [225]. Challenges such as adverse immune system responses and high at a cost. Consequently, recognizing biomarkers to predict the benefit of patients from IC is vital [231].
Advanced technologies in biomarker detection
Biomarker detection technology (Fig. 9) has undergone a significant transformation with the introduction of next-generation sequencing, which enables the comprehensive assessment of genetic variants, epigenetic changes, and gene expression studies. It facilitates the concurrent sequencing of various genes, providing a comprehensive mutational landscape. It helps detect tumor mutations in numerous cancers, aiding personalized treatment decisions [233].
Fig. 9.
Cancer biomarkers and their advanced technologies for detection
Digital PCR (dPCR) is a technology that approaches excellent precision and sensitivity in detecting RNA biomarkers and small-abundance DNA. This method is mainly used to validate NGS results and detect rare mutations. These panels (dPCR) can multiplex numerous targets, providing exact and rapid biomarker detection [234]. The dPCR has been widely applied in multiple biomedical explorations, including infectious diseases, oncology, and other rare diseases, due to its high sensitivity and accuracy [235].
Other advanced proteomic methods, like spectrometry, help detect and quantify biomarkers (protein based). The procedures can identify protein–protein interactions and post-translational modifications, providing insights into disease mechanisms and therapeutic targets [236]. The findings obtained from these studies can be used to interpret disease pathways, diagnose and treat various diseases, aid in the development of drugs, and provide a basis for discoveries in biology [237, 238].
A liquid biopsy is a non-invasive test that evaluates circulating tumor cells (CTCs), exosomes, and circulating tumor DNA (ctDNA) in blood samples. These techniques provide a real-time examination of the tumor’s dynamics, identifying emerging resistance mutations and minimal residual disease [239].
Another technique, known as single-cell sequencing, analyzes transcriptomic and genetic heterogeneity at the single-cell level. Practical procedures for isolating a single cell have undergone significant improvements in recent years, transitioning from more straightforward techniques such as cell-search antibody-based isolation or flow-sorting of cells and manual micromanipulation to high-throughput methods that utilize microfluidics, emulsion-based platforms, dielectrophoresis (DEP) arrays, and the 10X Genomics Chromium Single Cell Manager System. These advancements may provide immense benefits by substantially increasing the throughput sensitivity and precision of engaged approaches. Understanding the tumor microenvironment and identifying rare cell populations can help elucidate therapeutic resistance and disease progression [240].
Next-generation sequencing (NGS)
Millions of RNA and DNA sequences can be obtained through next-generation sequencing (NGS). This sequencing method is also known as high-throughput and massively parallel sequencing [241]. This technology enables the rapid, simultaneous sequencing of millions of samples, surpassing traditional sequencing techniques in terms of throughput and efficiency. With higher sample multiplexing, low-frequency variants can be detected via increased sensitivity, and high sample volumes can be processed with lower turnaround times and operational costs. After Sanger sequencing, NGS is a revolutionary technique. Human sequencing requires billions of dollars and several years of operation, but NGS can achieve the same results in a few days at a cost of around $1000 [242].
NGS is a vital part of precision medicine and has a broad-spectrum applicability in laboratory medicine. It can be employed in prognosis, diagnosis, and the selection of therapy for infectious diseases, oncology, and constitutional disorders [243, 244]. It can also identify several genomic alterations typically observed in cancer, including CNVs, SNVs, small indels, and fusion genes in solid malignancies or those in hematological disorders [245].
In addition to exome, genome, and transcriptomic sequencing, targeted gene sequencing is utilized in laboratories dealing with cancer diagnosis to ensure that depth, character, coverage, and reporting are undertaken optimally, and turnaround times are adequate. Panel’s genes can be used for breast cancer or acute myeloid leukemia [246]. Likewise, larger panels effectively address various cancers for academic and teaching hospitals, as well as other commercial laboratories. Custom-designed panels for blood cancers and solid tumors were used to detect CNVs, SNVs, and fusions in 367 samples, revealing an 88% impact in lymphomas, 96% in CNS tumors, and 62% in other types of tumors [245]. Numerous custom-designed next-generation sequencing (NGS) panels in clinical laboratories supplement those approved by the US Food and Drug Administration (FDA). These customized panels reveal an expanding repertoire of biomarkers for cancer diagnosis, prognosis, and therapeutic decision-making [247].
Among these, NPM1 is the most mutated gene in adult-type AML. NPM1 mutations are associated with favorable outcomes, particularly when they occur independently of concurrent DNMT3A and FLT3-ITD mutations [246]. Conversely, bi-allelic CEBPA mutations are associated with a favorable prognosis, while RUNX1 mutations are linked to worse overall survival [248]. Patients harboring FLT3-ITD mutations face an elevated risk of relapse and shorter survival compared to those without this mutation [249]. In 2017, the US FDA approved midostaurin, an inhibitor of multiple protein kinases, for treating newly diagnosed adult patients with AML who harbor FLT3 mutations. The same year, the FDA approved enasidenib for adult AML patients with relapsed or refractory disease characterized by an IDH2 gene mutation. The following year, in 2018, ivosidenib received FDA approval as the first treatment option for adult patients with relapsed or refractory AML with an IDH1 mutation [249].
Mutations in DNMT3A are typically linked to a decrease in overall survival, while TET2 mutations often correlate with a poor prognosis in cytogenetically normal AML [250]. Nonetheless, patients with TET2 mutations might respond favorably to hypomethylating agents (Papaemmanuil et al., 2019). Mutations in ASXL1, KMT2A, or TP53 are generally associated with an adverse prognosis. The 2017 World Health Organization (WHO) classification identifies AML with NPM1, CEBPA, and RUNX1 mutations as distinct entities (biomarkers) [251].
In another notable application of clinical NGS, the treatment of lung cancer has seen significant advancements. Specific gene mutations, such as EGFR, ALK, and ROS1, are targetable drivers for patients with NSCLC who harbor EGFR mutations. The US FDA has approved several inhibitors, including afatinib, gefitinib, erlotinib, and osimertinib [252]. Furthermore, lung cancer patients with ALK rearrangements, such as EML4-ALK, may benefit from FDA-approved ALK inhibitors like crizotinib, alectinib, and ceritinib. ROS1 mutations are detected in 1–2% of NSCLC patients, who may respond well to crizotinib and entrectinib, which received FDA approval in 2016 and 2019. According to the 2018 guidelines issued by the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology, testing for EGFR, ALK, and ROS1 mutations is mandatory in all advanced-stage lung cancer cases. If these tests are negative, further testing for mutations in BRAF, MET, RET, ERBB2, and KRAS should be conducted. The implementation of NGS technology in lung cancer testing has proven reliable, cost-effective, and efficient [246].
NGS technologies, including amplicon-based platforms, targeted panels, and liquid biopsies, are integral to developing cancer biomarkers. They provide comprehensive genetic insights, including genetic mutations, which enable personalized treatment strategies and improve diagnostic accuracy across various cancer types. These technologies are particularly beneficial in cases where traditional tissue biopsies are challenging or infeasible.
The Lucence Liquid-HALLMARK (LHM) is an amplicon-based ctDNA platform targeting 80 cancer-related genes and 10 fusions. It is beneficial in cases where tissue samples are unavailable, such as in advanced cholangiocarcinoma, showing an 80.4% discovery rate for molecular alterations [253]. On the other hand, the Tempus xF 105 Gene Panel is used for ctDNA assessment in pancreatic cancer. This NGS panel facilitates the monitoring of treatment response and the detection of mutations, including those in TP53 and KRAS. It provides a non-invasive method to evaluate treatment efficacy and disease progression [254].
Additionally, the Ion Torrent Cancer Hotspot Panel is used for childhood solid tumors. This technology detects mutations and deletions in cfDNA, demonstrating its utility in non-invasive diagnostics and post-surgical evaluation [255].
The Endoscopic Ultrasound-Guided Tissue Acquisition (EUS-TA) is used to obtain tissue samples for NGS in pancreatic cancer. It enables the sequencing of multiple genes from limited samples, facilitating the identification of mutations such as KRAS, which are crucial for diagnosis and treatment planning [256]. MSK-IMPACTTM Platform is an FDA-authorized platform that profiles ctDNA from cerebrospinal fluid in central nervous system tumors, identifying somatic alterations and tracking tumor evolution, which is crucial for treatment decisions and prognosis [257].
Role in biomarker discovery
Advancements in proteomics technology have led to its application in numerous diseases, particularly in the case of cancer. Substantial progress has been made in detecting clinically appropriate biomarkers and new therapeutic targets [258, 259]. The proteomics method has been widely applied in cancer research. Proteomics-based methods have enabled the identification of biomarkers and the expression patterns of different proteins, which can help assess tumor characteristics, such as prediction, prognosis, and classification, and identify potential responders to various therapies. This knowledge can be acquired from multiple sample types and utilized to develop different cancer therapeutics [260].
The application of dPCR has been widely studied in infectious diseases and oncology. Drugs based on various mechanisms and therapeutic approaches have been developed to shift from baseline to improved levels for both diseases. This suggests that specific biomarkers are effective predictors of use, and the dPCR technique makes a substantial contribution [261]. Most uses of dPCR focus on identifying molecules from samples (tumor tissue) in oncology, which is usually called the “gold standard” of genotyping of various tumors [262]. dPCR demonstrated suitable performance for accurately measuring the expression of numerous genes, including BRAF, HER2, TERT, and NRAS, among others, at both the DNA and RNA levels from formalin-fixed paraffin-embedded (FFPE) tissues. dPCR can analyze even the tiniest remnants of genetic mutations, chromosomal rearrangements, copy number variations, and rare alleles [263–265].
Cell-free DNA (cfDNA) is one of the most investigated analytes in liquid biopsies. The integrity and quantity of cfDNA in circulation have been shown to distinguish cancer patients from healthy individuals. The levels of cfDNA are higher in cancer patients when compared to healthy individuals [266] and appear to increase with stage and cause metastasis [267]. The cfDNA with a high concentration in these patients is assumed to exhibit a surplus distribution of genetic material from cancerous cells; however, this can also occur due to flawed circulating DNA clearance through phagocytes [268].
Metabolomics-based biomarkers in cancer
In recent years, metabolomics-based biomarkers have attracted growing interest in cancer research. This discipline opens a very promising avenue for detecting the disease earlier, making more accurate diagnoses, and closely monitoring how each patient responds to treatment. Metabolomics focuses on the detailed study of metabolites, which are the end products of cellular metabolism. As they directly reflect the physiological state of the body, they have become a valuable source of information, especially in the field of oncology, where detecting cancer early and tailoring treatment to each person’s characteristics can significantly impact clinical outcomes.
Although many potential biomarkers have been identified through metabolomic studies in different types of cancer, such as gastrointestinal and lung cancers, most of them have not yet been applied in clinical practice. Many are still in the early stages of research and have not passed the necessary technical and clinical validation stages, nor have they demonstrated their real usefulness in medical settings [269–272].
One of the strengths of metabolomics is that it enables us to observe the metabolome, a type of “chemical fingerprint” that results from the interaction between the genome, transcriptome, proteome, and the physiological state of the individual. This perspective has enabled the detection of apparent differences between healthy and cancerous tissues, as well as in fluids such as blood and urine. However, many of these findings come from pilot studies with small groups, so it remains to be seen whether the same results are replicated in larger and more diverse populations [270, 273, 274].
In the case of gastrointestinal cancer, initial studies have shown that certain panels of metabolomic biomarkers achieve very high levels of diagnostic accuracy, with AUROC values exceeding 0.9. Despite these encouraging results, none have yet been approved for clinical use, clearly reflecting the gap between advances in the laboratory and their actual medical application [269].
Concerning lung cancer, metabolomics is being investigated as a complement to current screening methods, such as low-dose computed tomography (LD-CT), which, although useful, has a high false-positive rate. Incorporating metabolomic biomarkers could enhance the specificity of these tests; however, they have not yet been validated in real-world early detection scenarios [273, 274].
In pancreatic cancer (one of the most aggressive and difficult to treat forms of cancer because it is often detected at advanced stages) metabolomics is also being explored to improve early diagnosis. However, there are still no established metabolomic biomarkers that can be routinely used for this purpose [275].
One of the significant obstacles in this field is the so-called “translational gap,” i.e., the gap that prevents discoveries made in research settings from being translated into clinical tools. This is due, in part, to the need for larger, prospective studies to confirm initial findings, as well as the complexity of integrating new biomarkers into everyday medical practice [269, 271, 272].
Advances are also needed in the standardization of techniques used in metabolomic studies and in the validation of biomarkers in diverse populations to ensure that they are reliable and instrumental in different clinical contexts. Research must focus on closing these gaps so that advances in metabolomics can be turned into tangible benefits for patients [271, 273, 274].
Tumor-educated platelets as biomarkers for cancer
Tumor-educated platelets, known as TEPs, have begun to attract attention as potential biomarkers for cancer detection and prognosis. These platelets are believed to be capable of transporting RNA and other tumor-derived materials, which has made them more accessible through liquid biopsies taken from the blood. In recent years, several studies have explored their potential in this field.
The central idea is that tumor cells can transfer RNA to platelets, which would make TEPs an indirect source of information about the tumor. However, recent research has questioned this hypothesis. In a study of melanoma patients, the RNA content of extracellular vesicles (EVs) and platelets was compared. The results showed that EVs were more effective than TEPs in detecting tumor-specific mutations, such as BRAF V600E. This suggests that, although tumor RNA can be transferred to platelets, the levels would be so low that they are challenging to detect with current technologies [276].
Another critical challenge is the purity of the samples. The process of isolating TEPs and other biomarkers present in blood can be affected by contamination with other components, such as tumor-derived extracellular vesicles (tdEVs). This mixture complicates the interpretation of results and raises questions about whether current protocols effectively allow for the collection of pure biomarkers. Therefore, it is essential to be cautious when analyzing data from TEPs [277].
In addition to their value as potential sources of tumor RNA, several studies have analyzed the prognostic role of specific platelet-related indices. For example, it has been observed that the combination of platelet count and lymphocyte–monocyte ratio (COP-LMR) can predict clinical outcome in patients with non-small cell lung cancer. Those with high scores on this index tended to have shorter overall and progression-free survival, indicating its potential usefulness as a prognostic marker [278, 279].
Similarly, the platelet–neutrophil ratio has been identified as a possible indicator of poor response to neoadjuvant chemoradiotherapy in rectal cancer. However, this ratio failed to predict a complete pathological response [280]. Despite these promising findings, several obstacles remain to the application of PETs in clinical practice. These include low sensitivity for detecting tumor RNA and the difficulty of obtaining pure samples. To move forward, it will be key to continue investigating exactly how tumor material is transferred to platelets and to improve isolation techniques to achieve more reliable biomarkers. It will also be necessary to evaluate their usefulness in different types of cancer and at various stages of the disease [276, 277].
AI-based multi-omics biomarkers in cancer
The incorporation of artificial intelligence (AI) into multi-omic data analysis is transforming the way biomarkers are discovered in cancer. Multi-omic approaches—ranging from genome analysis and gene expression to the study of proteins and patterns obtained through medical imaging—allow cancer to be observed from multiple angles at once. This comprehensive view has been key to identifying new biomarkers that not only help diagnose the disease but also predict its progression and choose the most appropriate treatment for each case. In this context, artificial intelligence (AI)-based tools offer a decisive advantage: their ability to handle enormous amounts of complex data and find connections that would otherwise go unnoticed. As a result, new doors are opening to better understand how cancer works and discover potential therapeutic targets that could make a difference in treatment.
Within AI, techniques such as machine learning and deep learning are playing an increasingly important role in the analysis of multi-omic data. These methodologies make it possible to integrate different types of information—such as genetic data, gene expression levels, or imaging patterns—to identify biomarkers and meta-biomarkers that can predict, for example, the response to immunotherapeutic treatments such as immune checkpoint inhibitors (ICIs) [281–283]. In addition, some AI-based models have shown great potential for identifying therapeutic targets and synthetic lethality pairs, which is key to the development of personalized therapies [284].
Understanding cancer in all its complexity requires combining multiple types of data, and this is where specialized tools make a difference. Platforms such as MultiAssayExperiment (MAE) and Multi-omics Statistical Approaches (MuSA) have been designed to integrate and analyze heterogeneous information, including radiogenomic datasets, in order to improve both the diagnosis and prognosis of cancer [283, 285]. Recently, a type of model known as graph neural networks (GNNs) has also begun to be used, which allows existing biological knowledge to be incorporated to classify molecular subtypes of cancer more accurately [282].
However, despite all the advances in this field, bringing these AI-driven multi-omic approaches into clinical practice still represents a major challenge. Most of the studies conducted to date have been developed in observational or retrospective contexts, which makes it difficult to apply them directly in everyday medicine. For these tools to be routinely incorporated into hospitals and clinics, large-scale clinical trials are essential to confirm their accuracy and usefulness in real-world settings [286, 287]. Furthermore, the incorporation of radiomic data into these integrated multi-omic analysis schemes is still at a very early stage, so more work is needed to develop and evaluate them properly [285]. Designing prospective studies will be a fundamental step in ensuring that AI-based biomarkers effectively reach the clinic and benefit patients [281].
Nanobiosensors in cancer diagnostics
In recent years, nanobiosensors have become a crucial tool for cancer diagnosis. Their impact has been further enhanced when combined with artificial intelligence (AI), as this technology enhances their ability to detect biomarkers associated with different types of cancer [288]. Due to their high sensitivity and accuracy, these sensors enable the identification of disease at very early stages, which is crucial for improving patient prognosis [289]. The incorporation of artificial intelligence has opened up the possibility of analyzing enormous volumes of information and discovering complex patterns that would otherwise go unnoticed with traditional methods [290]. This collaboration between nanotechnology and AI is marking the beginning of a new era in cancer diagnosis, one that is much more accurate and tailored to each person’s individual characteristics.
There are various types of nanobiosensors, each with specific applications. For example, sensors based on nanomaterials—such as graphene—take advantage of their unique properties, such as high electrical conductivity and their great ability to attract and retain molecules, making them particularly effective at detecting minute amounts of biomarkers in complex biological samples [288, 291]. On the other hand, electrochemical immunosensors can help identify tumors that respond better to treatments with DNA-damaging agents, allowing for more personalized and effective therapies [292]. Aptamer-based sensors are also noteworthy, as they have shown notable improvements in sensitivity and selectivity, two fundamental characteristics when seeking to detect biomarkers in liquid biopsies. In addition, these sensors are minimally invasive and allow real-time monitoring of the patient’s condition [288].
Despite the enormous potential offered by these technologies, their clinical application still faces several challenges. Among the most important are the need to obtain consistently reproducible results and to overcome certain technical difficulties, such as controlling the deposition of materials during the manufacturing process. Even so, advances in this field continue apace. Research is ongoing to resolve these obstacles, and interest in integrating artificial intelligence into these systems is growing every day. It is hoped that this combination will enable the development of increasingly accurate, accessible, and easy-to-use diagnostic tools [291].
Challenges and improvements
Despite the rapid development of dPCR technology, several challenges persist regarding its widespread adoption in clinical settings. The primary challenge is that the novel dPCR prototype machines still rely on the sophisticated and costly platforms in academic laboratories, restricting their availability to a broader client base. Besides, its equipment is complicated for fluorescent reading units and thermocycling modules. Therefore, in the future, the evolving biosensors and operational materials should be amalgamated into dPCR machines to strengthen the concept of inventions [262].
Various OMICs approaches (proteomics, metabolomics) should guide the detection and classification of a range of cancer-related cell populations (biomarkers). Nevertheless, those methodologies are incompatible with obtaining the heterogeneous types of cancer cell populations. Heterogeneity at inter- or intra-tumoral levels can render these biomarkers entirely unfit. Similarly, homogenous populations of cancer cells, which are enriched and cleansed by a set of well-recognized surface markers, repeatedly screen for exceptional heterogeneity. It can be more profoundly observed in hematological cancers [293].
The challenge with the use of liquid biopsy is that high levels of cfDNA are not restricted to cancer and can be detected in non-pathological and other pathological states, including surgery, trauma, and exercise [294]; this can hamper its proper application in the diagnosis of cancer. Interpreting the integrity, cancer patients demonstrate increased fragmentation of cfDNA (< 100 bp) when compared to healthy subjects [295], even though a study disclosed contrary results in the case of thyroid carcinoma, displaying that this type of method still does not have the specificity and sensitivity required for diagnosis [296].
In the case of single-cell sequencing, the molecular profiling of data, specifically the assessment of heterogeneity related to the population of cancer cells, is incomplete. After the killing of the primary clones in cancer therapy, the other clones can substitute them and cause the recurrence of cancer [297]. The cancer niches with dynamic alterations encompass various cell types. Detecting, classifying, and separating the different cancer niche sections is challenging. Hence, an elevated-resolution approach is required to realistically monitor and thoroughly examine the heterogeneity of various cancer cells. Cancer niche cells are considered and anticipated to conduct a wave of unexpected discoveries [298].
Challenges and opportunities
The constraints associated with discovering biomarkers begin immediately after sample collection and continue through the processing of representative tissue, transportation, the specificity and sensitivity of assays, reference standards, and post-analytical analysis at the end [299]. Another major drawback is that the maximum number of these biomarkers are proteins in nature and exhibit the limitation of not being specific to a particular cancer type. Genetic biomarkers are considered more reliable than protein-based biomarkers; however, genetic alterations have not been detected in every kind of cancer [300].
Cancer heterogeneity is also a significant concern in recognizing biomarkers that can be correctly detected and are applicable universally. The absence of specificity or sensitivity may indicate false-positive results, whereas biomarkers may not be able to identify various types of cancer. Distinct alterations cause difficulty in establishing precise cut-off scores for risk assessment diagnosis. In clinical trials and regulatory approval processes, time-consuming and expensive authentication procedures and standardization techniques in various laboratories, which are vital for ensuring the comparability and reliability of results, can be challenging [301].
The accessibility and expense of tests can restrict the widespread application of biomarkers. Regardless, biomarker exploration in cancer continues to advance, with ongoing efforts to identify and validate novel biomarkers. Generally, efforts in the initial stage of cancer diagnosis are focused on leveraging progress in molecular profiling, genomics, computational analysis, non-invasive techniques, and collaborative research to enhance timely diagnosis, expand diagnostic precision, and facilitate personalized therapeutic methods [302]. With the improvement of technology and the deepening of our recognition of cancer biology, the likelihood of a quicker and more exact diagnosis of cancer is progressively reachable.
NGS technologies, commonly employed in biomarker studies, have limitations that include short reads and a reliance on clonal PCR to generate adequate signals for their findings. We have a probability of lowering mortality and morbidity related to cancer in the future substantially, but repeated advancements, with governing support, can be the key to realizing the maximum ability of biomarkers in cancer diagnosis and therapeutics [303].
Novel methods with long reads, such as those from Oxford Nanopore and Pacific Biosystems, which are accountable for single-molecule sequencing, would hypothetically be improved and require less starting material. However, their elevated fault rate prevents them from being the technique of excellence. GS is established to play a substantially vital role in laboratory medicine because there is no specific requirement for an upfront enrichment step, and it provides uniform coverage of the entire genome. The analysis, specifically variant structural analysis, data storage, and variant interpretation, remains challenging in GS [304].
Future perspectives
The expanded use of AI to find predictive biomarkers for ICIs’ effectiveness in different cancers can also be applied to many other areas, including the success of chemotherapy and other targeted cancer treatments. AI procedures can provide new insights from composite statistics, but the development of AI-built “software biomarkers” is hindered by retrospective datasets, varied AI systems, and opaque decision-making [281].
Integrating OMICs that combine various datasets from proteomics, genomics, metabolomics, and different “omics” areas will lead to a more inclusive knowledge of biomarkers in cancer diagnostics. This unified approach can comprehend breakthroughs in new biomarkers and enhance the precision of cancer diagnostics [305].
Over the past few years, digital imaging and communication systems in diagnostics have become more essential for multimodal tomography, using complex techniques, innovative hardware, and new protocols. Medical imaging technology is advancing, which has enhanced access to comprehensive, interchangeable evidence and facilitated the standardization of primarily diagnostic execution, thereby giving imaging a more central role in personalized treatment and diagnosis. Traditional cancer investigation is painful and time consuming for the patient [306].
Distinct, measurable metrics (radio genomics) can be used to represent different opinions on the clinical concern, which can be connected to appropriate clinical performance metrics to forecast potential benefits to human health. By combining data from these quantitative biomarkers obtained from various imaging techniques, we can begin to understand how new strategies might influence tumor cells and their microenvironments in targeted therapies and drug discovery through imaging biomarkers and precision medicine [307].
Conclusion
The use of diagnostic, predictive, and prognostic biomarker models are the basis for personalized therapeutics in cancer treatment. Biomarkers must be quality-assured, robust, and reproducible, and the compilation of specific biomarkers cannot describe the entire scenario of oncological therapy. Although advancements have been made in molecular imaging and blood-based assessments, biomarker examination in oncology still primarily relies on molecular tissue laboratory analysis. A precise molecular characterization of cancer cells in the malignant tumor requires the identification of specialized markers and the use of appropriate enrichment techniques. New genomics, transcriptomics, proteomics, and epigenomics techniques, combined with the development of new single-cell methodologies, will help in accurately identifying biomarkers.
Acknowledgements
Gabriela Figueroa-González acknowledges the financial support from PAPIIT IA206724 (DGAPA-UNAM), Octavio Daniel Reyes-Hernández from PAPIIT IN221824 (DGAPA-UNAM) and Gerardo Leyva-Gomez from PAPIIT IN204722 and PAPIME PE205524 (DGAPA-UNAM). This article is a product of a Postdoctoral Program Scholarship of Consejo Nacional de Ciencia y Tecnología (CONACyT), “Estancias Posdoctorales por México 2022; Estancia Posdoctoral Académica Inicial 2022” assigned to Sheila Irais Peña-Corona (CVU:495850). We appreciate the professional assistance of María de los Dolores Campos Echeverría and Nieves Herrera-Mundo (UNAM).
Abbreviations
- ACT
Adoptive Cell Transfer
- AFP
Alpha-Fetoprotein
- AGO
Argonaute
- BCR-ABL
Breakpoint Cluster Region-Abelson Murine Leukemia Viral Oncogene Homolog
- CA 125
Cancer Antigen 125
- CA 15-3
Cancer Antigen 15-3
- CA 19-9
Cancer Antigen 19-9
- CAR
Chimeric Antigen Receptor
- CAR-T cells
Chimeric Antigen Receptor T cells
- CEA
Carcinoembryonic Antigen
- CNVs
Copy Number Variations
- CNS
Central Nervous System
- CT scans
Computed Tomography scans
- CT antigens
Cancer/Testis Antigens
- CTLA4
Cytotoxic T-Lymphocyte-Associated Protein 4
- CTCs
Circulating Tumor Cells
- ctDNA
Circulating Tumor DNA
- DC
Dendritic Cell
- DCs
Dendritic Cells
- DEP
Dielectrophoresis
- dMMR
Deficient Mismatch Repair
- dPCR
Digital PCR
- EGFR
Epidermal Growth Factor Receptor
- ER
Estrogen Receptor
- ECmiRNAs
Extracellular miRNAs
- FFPE
Formalin-Fixed Paraffin-Embedded
- HCG-β
Human Chorionic Gonadotropin Beta
- HCC
Hepatocellular Carcinoma
- HER2
Human Epidermal Growth Factor Receptor 2
- IC
Immune Checkpoint
- ICIs
Immune Checkpoint Inhibitors
- IDO
Indoleamine 2,3-Dioxygenase
- IFN-α
Interferon-alpha
- IL-2
Interleukin-2
- IL-6
Interleukin-6
- MRI
MAGNETIC Resonance Imaging
- MICs
Metastatic-Inducing Cells
- miRNA
MicroRNA
- MMR
Mismatch Repair
- MSI
Microsatellite Instability
- MSI-H
High Microsatellite Instability
- ncRNAs
Non-Coding RNAs
- NGS
Next-Generation Sequencing
- NK cells
Natural Killer Cells
- NSCLC
Non-Small Cell Lung Cancer
- PD-1
Programmed Cell Death-1
- PD-L1
Programmed Death-Ligand 1
- PD-1/PD-L1
Programmed Death-1/Programmed Death-Ligand 1
- PEG
Polyethylene Glycol
- POLE
Polymerase Epsilon
- POLD1
Polymerase Delta 1
- Pol II
RNA Polymerase II
- PR
Progesterone Receptor
- pri-miRNAs
Primary Transcripts
- PSA
Prostate-Specific Antigen
- RCC
Renal Cell Carcinoma
- RCAS1
Receptor-Binding Cancer Antigen Expressed on SiSo Cells 1
- RISC
RNA-Induced Silencing Complex
- RNA
Ribonucleic Acid
- SiMoa
Single-Molecule Array Technology
- SNVs
Single Nucleotide Variants
- T-Vec
Talimogene Laherparepvec
- TCR
T-Cell Receptor
- TILs
Tumor-Infiltrating Lymphocytes
- TMB
Tumor Mutation Burden
- TRBP
TAR RNA-Binding Protein
- UC
Differential Ultracentrifugation
- WHO
World Health Organization
- XPO5
Exportin 5
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas that is revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Prof. Dr. Javad Sharifi-Rad serves as an Associate Editor for European Journal of Medical Research. However, he was not involved in the peer-review or editorial decision-making process for this manuscript. The authors declare no other competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Khushbukhat Khan, Email: khan2011@hotmail.com.
Lashyn N. Kiyekbayeva, Email: lashynk@mail.ru
Monica Butnariu, Email: monicabutnariu@yahoo.com.
Javad Sharifi-Rad, Email: javadsharifirad@uees.edu.ec.
Gerardo Leyva-Gómez, Email: leyva@quimica.unam.mx.
References
- 1.Institute NC. Cancer statistics 2024 [Available from: https://www.cancer.gov/about-cancer/understanding/statistics.
- 2.Buchanan AH, Lennon AM, Rego SP, Choudhry OA, Elias PZ, Sadler JR, et al. Long-term clinical outcomes of cancers diagnosed following detection by a blood-based multi-cancer early detection (MCED) test. J Clin Oncol. 2023;41(16_suppl):3037. [Google Scholar]
- 3.Isakova Dilnoza B, Farmankulova YR. Innovative Technologies in Early Diagnosis of Precancer and Cancer Diseases of the Cervix. J Med Genet Clin Biol. 2024;1(9):610. [Google Scholar]
- 4.Adil S, Faiz M, Jawad A, Haseeb A, Muhammad A, Adnan A. The role of early diagnosis and intervention in improving outcomes for lung cancer. Innov Res Appl Biol Chem Sci. 2024;2(1):126–32. [Google Scholar]
- 5.Garg P, Mohanty A, Ramisetty S, Kulkarni P, Horne D, Pisick E, et al. Artificial intelligence and allied subsets in early detection and preclusion of gynecological cancers. Biochimica et Biophysica Acta (BBA). 2023;1878(6): 189026. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 7.Anandasabapathy S, Asirwa C, Grover S, Mungo C. Cancer burden in low-income and middle-income countries. Nat Rev Cancer. 2024;24(3):167–70. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Ssentongo P, Sharma R. Editorial: cancer burden, prevention and treatment in developing countries. Front Public Health. 2023. 10.3389/fpubh.2022.1124473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S, Zhu W, Thompson P, Hannun YA. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun. 2018;9(1): 3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel A. Benign vs malignant tumors. JAMA Oncol. 2020;6(9):1488. [DOI] [PubMed] [Google Scholar]
- 11.Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, et al. Early detection of cancer. Science. 2022;375(6586): eaay9040. [DOI] [PubMed] [Google Scholar]
- 12.Rao Bommi J, Kummari S, Lakavath K, Sukumaran RA, Panicker LR, Marty JL. Recent trends in biosensing and diagnostic methods for novel cancer biomarkers. Biosensors. 2023;13(3): 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sukumar J, Gast K, Quiroga D, Lustberg M, Williams N. Triple-negative breast cancer: promising prognostic biomarkers currently in development. Expert Rev Anticancer Ther. 2021;21(2):135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouliere F, Robert B, Arnau Peyrotte E, Del Rio M, Ychou M, Molina F, et al. High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE. 2011;6(9): e23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Dai Z, Liu X, Yang J. High-performance electrochemical sensing of circulating tumor DNA in peripheral blood based on poly-xanthurenic acid functionalized MoS2 nanosheets. Biosens Bioelectron. 2018;105:116–20. [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, et al. Colorectal cancer statistics 2017. CA A Cancer J Clin. 2017;67(3):177–93. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman JD, Fisher PG, Gibbs P. Early detection of cancer: past, present, and future. Am Soc Clin Oncol Educ Book. 2015;35(1):57–65. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Tao L, Qiu J, Xu J, Yang X, Zhang Y, et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther. 2024;9(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loud JT, Murphy J. Cancer screening and early detection in the 21(st) century. Semin Oncol Nurs. 2017;33(2):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarhadi VK, Armengol G. Molecular biomarkers in cancer. Biomolecules. 2022;12(8):1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirata I. Evaluation of the usefulness of the simultaneous assay of fecal hemoglobin (Hb) and transferrin (Tf) in colorectal cancer screening-for the establishment of the Hb and Tf two-step cutoff assay (HTTC assay). Diagnosis Berl. 2020;7(2):133–9. [DOI] [PubMed] [Google Scholar]
- 22.Moradi A, Srinivasan S, Clements J, Batra J. Beyond the biomarker role: prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 2019;38(3):333–46. [DOI] [PubMed] [Google Scholar]
- 23.Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol. 2012;6(2):140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazmierczak W, Lazar A, Tomaszewska R, Popiela TJ, Koper K, Wicherek L, et al. Analysis of the intensity of immune cell infiltration and immunoreactivity of RCAS1 in diffuse large B-cell lymphoma of the palatine tonsil and its microenvironment. Cell Tissue Res. 2015;361(3):823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow S, Berek JS. Development of therapeutic vaccines for ovarian cancer. Vaccines. 2020;8(4):657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison G, Zhu G, Moulis A, Dearden S, Speake G, McCormack R. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Appl Immunohistochem Mol Morphol. 2013;21(2):116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtzman JT, Wilson H, Rao CV. A proposed role for hCG in clinical obstetrics. Semin Reprod Med. 2001;19(1):63–8. [DOI] [PubMed] [Google Scholar]
- 29.Gaafar TM, Raafat II, Aly AA, Mohamed NA, Farid RJ, Saad NE, et al. Detection of BCR/ABL translocation in bone marrow derived mesenchymal stem cells in Egyptian CML patients. Open Access Maced J Med Sci. 2015;3(2):231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Kleist S. The clinical value of the tumor markers CA 19/9 and carcinoembryonic antigen (CEA) in colorectal carcinomas: a critical comparison. Int J Biol Markers. 1986;1(1):3–8. [DOI] [PubMed] [Google Scholar]
- 31.Bast RC Jr., Ravdin P, Hayes DF, Bates S, Fritsche H Jr., Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(6):1865–78. [DOI] [PubMed] [Google Scholar]
- 32.Tuxen MK, Sölétormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev. 1995;21(3):215–45. [DOI] [PubMed] [Google Scholar]
- 33.Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: Why, how, and who? Ann Surg. 2013;257(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson PJ, Williams R. Serum alpha-fetoprotein estimations and doubling time in hepatocellular carcinoma: influence of therapy and possible value in early detection. J Natl Cancer Inst. 1980;64(6):1329–32. [DOI] [PubMed] [Google Scholar]
- 35.Vickers AJ, Brewster SF. PSA velocity and doubling time in diagnosis and prognosis of prostate cancer. Br J Med Surg Urol. 2012;5(4):162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawaki M, Taira N, Uemura Y, Saito T, Baba S, Kobayashi K, et al. Adjuvant trastuzumab without chemotherapy for treating early HER2-positive breast cancer in older patients: a propensity score-adjusted analysis of a prospective cohort study. Breast. 2022;66:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(23):3726–34. [DOI] [PubMed] [Google Scholar]
- 38.Das S, Dey MK, Devireddy R, Gartia MR. Biomarkers in cancer detection, diagnosis, and prognosis. Sensors. 2024;24(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altintas Z, Tothill I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens Actuators B Chem. 2013;188:988–98. [Google Scholar]
- 40.Soung MC. Screening for cancer: When to stop? A practical guide and review of the evidence. Med Clin North Am. 2015;99(2):249–62. [DOI] [PubMed] [Google Scholar]
- 41.Höller A, Nguyen-Sträuli BD. Diagnostic and prognostic biomarkers of luminal breast cancer: Where are we now? Breast Cancer Targets Ther. 2023;15:525–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayanthi V, Das AB, Saxena U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens Bioelectron. 2017;91:15–23. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhury SG, Ray R, Karmakar P. Exosomal miRNAs—a diagnostic biomarker acting as a guiding light in the diagnosis of prostate cancer. Funct Integr Genomics. 2022;23(1): 23. [DOI] [PubMed] [Google Scholar]
- 44.Li B, Li Y, Li C, Yang J, Liu D, Wang H, et al. An ultrasensitive split-type electrochemical immunosensor based on controlled-release strategy for detection of CA19-9. Biosens Bioelectron. 2023;227: 115180. [DOI] [PubMed] [Google Scholar]
- 45.Badheeb M, Abdelrahim A, Esmail A. Pancreatic tumorigenesis: precursors, genetic risk factors and screening. Curr Oncol. 2022;29(11):8693–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neesham D. Ovarian cancer screening. Aust Fam Physician. 2007;36(3):126–8. [PubMed] [Google Scholar]
- 47.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK collaborative trial of ovarian cancer screening (UKCTOCS). Lancet Oncol. 2009;10(4):327–40. [DOI] [PubMed] [Google Scholar]
- 48.Kui Wong N, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, et al. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem. 2003;278(31):28619–34. [DOI] [PubMed] [Google Scholar]
- 49.Hasan D. Diagnostic impact of CEA and CA 15-3 on chemotherapy monitoring of breast cancer patients. J Circ Biomark. 2022;11:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tampellini M, Berruti A, Gerbino A, Buniva T, Torta M, Gorzegno G, et al. Relationship between CA 15–3 serum levels and disease extent in predicting overall survival of breast cancer patients with newly diagnosed metastatic disease. Br J Cancer. 1997;75(5):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferraro S, Bussetti M, Bassani N, Rossi RS, Incarbone GP, Bianchi F, et al. Definition of outcome-based prostate-specific antigen (PSA) thresholds for advanced prostate cancer risk prediction. Cancers. 2021;13(14): 3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan Q, Song J, Yang W, Wang H, Huo Q, Yang J, et al. The effect of CA125 on metastasis of ovarian cancer: old marker new function. Oncotarget. 2017;8(30):50015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith DS, Humphrey PA, Catalona WJ. The early detection of prostate carcinoma with prostate specific antigen: the Washington University experience. Cancer. 1997;80(9):1852–6. [PubMed] [Google Scholar]
- 54.Jaffee EM, Dang CV, Agus DB, Alexander BM, Anderson KC, Ashworth A, et al. Future cancer research priorities in the USA: a Lancet Oncology Commission. Lancet Oncol. 2017;18(11):e653–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madhavan B, Yue S, Galli U, Rana S, Gross W, Müller M, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2015;136(11):2616–27. [DOI] [PubMed] [Google Scholar]
- 56.Mishra M, Ahmed R, Das DK, Pramanik DD, Dash SK, Pramanik A. Recent advancements in the application of circulating tumor DNA as biomarkers for early detection of cancers. ACS Biomater Sci Eng. 2024;10(8):4740–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen X, Pu H, Liu Q, Guo Z, Luo D. Circulating tumor DNA-a novel biomarker of tumor progression and its favorable detection techniques. Cancers (Basel). 2022;14(24): 6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMahon JT, Studer M, Ulrich B, Revuelta Barbero JM, Pradilla I, Palacios-Ariza MA, et al. Circulating tumor DNA in adults with glioma: a systematic review and meta-analysis of biomarker performance. Neurosurgery. 2022;91(2):231–8. [DOI] [PubMed] [Google Scholar]
- 59.Pessoa LS, Heringer M, Ferrer VP. ctDNA as a cancer biomarker: a broad overview. Crit Rev Oncol Hematol. 2020;155: 103109. [DOI] [PubMed] [Google Scholar]
- 60.Chin RI, Chen K, Usmani A, Chua C, Harris PK, Binkley MS, et al. Detection of solid tumor molecular residual disease (MRD) using circulating tumor DNA (ctDNA). Mol Diagn Ther. 2019;23(3):311–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han X, Wang J, Sun Y. Circulating tumor DNA as biomarkers for cancer detection. Genom Proteom Bioinform. 2017;15(2):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: a systematic review and meta-analysis. Cancer Treat Rev. 2022;104: 102362. [DOI] [PubMed] [Google Scholar]
- 63.Morais M, Pinto DM, Machado JC, Carneiro S. CtDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: a systematic review. Eur J Surg Oncol. 2022;48(1):218–27. [DOI] [PubMed] [Google Scholar]
- 64.Stadler JC, Belloum Y, Deitert B, Sementsov M, Heidrich I, Gebhardt C, et al. Current and future clinical applications of ctDNA in immuno-oncology. Cancer Res. 2022;82(3):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vellanki PJ, Ghosh S, Pathak A, Fusco MJ, Bloomquist EW, Tang S, et al. Regulatory implications of ctDNA in immuno-oncology for solid tumors. J Immunother Cancer. 2023;11(2): e005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freidin MB, Freydina DV, Leung M, Montero Fernandez A, Nicholson AG, Lim E. Circulating tumor DNA outperforms circulating tumor cells for KRAS mutation detection in thoracic malignancies. Clin Chem. 2015;61(10):1299–304. [DOI] [PubMed] [Google Scholar]
- 67.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4(136): 136ra68. [DOI] [PubMed] [Google Scholar]
- 68.Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7(30):48832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez-Herrero E, Serna-Blasco R, Robado de Lope L, González-Rumayor V, Romero A, Provencio M. Circulating Tumor DNA as a cancer biomarker: an overview of biological features and factors that may impact on ctDNA Analysis. Front Oncol. 2022;12:943253. [DOI] [PMC free article] [PubMed]
- 70.Gao YJ, He YJ, Yang ZL, Shao HY, Zuo Y, Bai Y, et al. Increased integrity of circulating cell-free DNA in plasma of patients with acute leukemia. Clin Chem Lab Med. 2010;48(11):1651–6. [DOI] [PubMed] [Google Scholar]
- 71.Madhavan D, Wallwiener M, Bents K, Zucknick M, Nees J, Schott S, et al. Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res Treat. 2014;146(1):163–74. [DOI] [PubMed] [Google Scholar]
- 72.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mouliere F, Rosenfeld N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proc Natl Acad Sci U S A. 2015;112(11):3178–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Vaart M, Pretorius PJ. The origin of circulating free DNA. Clin Chem. 2007;53(12):2215. [DOI] [PubMed] [Google Scholar]
- 75.Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel). 2020;9(1):473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Udomruk S, Orrapin S, Pruksakorn D, Chaiyawat P. Size distribution of cell-free DNA in oncology. Crit Rev Oncol Hematol. 2021;166: 103455. [DOI] [PubMed] [Google Scholar]
- 77.Mouliere F, Chandrananda D. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. 2018;10(466): eaat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stejskal P, Goodarzi H, Srovnal J, Hajdúch M, van ’t Veer LJ, Magbanua MJM. Circulating tumor nucleic acids: biology, release mechanisms, and clinical relevance. Mol Cancer. 2023;22(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–37. [DOI] [PubMed] [Google Scholar]
- 80.Papadopoulos N. Pathophysiology of ctDNA Release into the Circulation and Its Characteristics: What Is Important for Clinical Applications. Recent Results Cancer Res Fortschritte der Krebsforschung Progres dans les recherches sur le Cancer. 2020;215:163–80. [DOI] [PubMed] [Google Scholar]
- 81.Choi JJ, Reich CF 3rd, Pisetsky DS. The role of macrophages in the in vitro generation of extracellular DNA from apoptotic and necrotic cells. Immunology. 2005;115(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol Ther. 2019;20(8):1057–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L, Liang Y, Li S, Zeng F, Meng Y, Chen Z, et al. The interplay of circulating tumor DNA and chromatin modification, therapeutic resistance, and metastasis. Mol Cancer. 2019;18(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chistiakov DA, Chekhonin VP. Circulating tumor cells and their advances to promote cancer metastasis and relapse, with focus on glioblastoma multiforme. Exp Mol Pathol. 2018;105(2):166–74. [DOI] [PubMed] [Google Scholar]
- 85.Gold B, Cankovic M, Furtado LV, Meier F, Gocke CD. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. J Mol Diagn JMD. 2015;17(3):209–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472–84. [DOI] [PubMed] [Google Scholar]
- 87.Kruger S, Heinemann V, Ross C, Diehl F, Nagel D, Ormanns S, et al. Repeated mutKRAS ctDNA measurements represent a novel and promising tool for early response prediction and therapy monitoring in advanced pancreatic cancer. Ann Oncol. 2018;29(12):2348–55. [DOI] [PubMed] [Google Scholar]
- 88.Mishra M, Ahmed R, Das DK, Pramanik DD, Dash SK, Pramanik A. Recent advancements in the application of circulating tumor DNA as biomarkers for early detection of cancers. ACS Biomater Sci Eng. 2024;10:4740–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aulakh SS, Silverman DA, Young K, Dennis SK, Birkeland AC. The promise of circulating tumor DNA in head and neck cancer. Cancers. 2022;14(12):2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stachler MD, Rinehart E, Lindeman N, Odze R, Srivastava A. Novel molecular insights from routine genotyping of colorectal carcinomas. Hum Pathol. 2015;46(4):507–13. [DOI] [PubMed] [Google Scholar]
- 91.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(6):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamakawa T, Kukita Y, Kurokawa Y, Miyazaki Y, Takahashi T, Yamasaki M, et al. Monitoring gastric cancer progression with circulating tumour DNA. Br J Cancer. 2015;112(2):352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224): 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reinert T, Schøler LV, Thomsen R, Tobiasen H, Vang S, Nordentoft I, et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. 2016;65(4):625–34. [DOI] [PubMed] [Google Scholar]
- 96.Pollard TD, Ong JJ, Goyanes A, Orlu M, Gaisford S, Elbadawi M, et al. Electrochemical biosensors: a nexus for precision medicine. Drug Discov Today. 2021;26(1):69–79. [DOI] [PubMed] [Google Scholar]
- 97.Zou Z, Qi P, Qing Z, Zheng J, Yang S, Chen W, et al. Technologies for analysis of circulating tumour DNA: progress and promise. TrAC Trends Anal Chem. 2017;97:36–49. [Google Scholar]
- 98.Sharifi M, Hasan A, Attar F, Taghizadeh A, Falahati M. Development of point-of-care nanobiosensors for breast cancers diagnosis. Talanta. 2020;217: 121091. [DOI] [PubMed] [Google Scholar]
- 99.Antiochia R. Developments in biosensors for CoV detection and future trends. Biosens Bioelectron. 2021;173: 112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang K, Peng Z, Lin X, Nian W, Zheng X, Wu J. Electrochemical biosensors for circulating tumor DNA detection. Biosensors. 2022;12(8): 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rahman M, Cui D, Zhou S, Zhang A, Chen D. A graphene oxide coated gold nanostar based sensing platform for ultrasensitive electrochemical detection of circulating tumor DNA. Anal Methods. 2020;12(4):440–7. [Google Scholar]
- 102.Liu F, Peng J, Lei Y-M, Liu R-S, Jin L, Liang H, et al. Electrochemical detection of ctDNA mutation in non-small cell lung cancer based on CRISPR/Cas12a system. Sens Actuators, B Chem. 2022;362: 131807. [Google Scholar]
- 103.Dang DK, Park BH. Circulating tumor DNA: current challenges for clinical utility. J Clin Invest. 2022. 10.1172/JCI154941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paoletti C, Hayes DF. Circulating tumor cells. Novel Biomarke Contin Breast Cancer. 2016;2016:235–58. [DOI] [PubMed] [Google Scholar]
- 105.Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. New England J Med. 2021;384(25):2394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–23. [DOI] [PubMed] [Google Scholar]
- 107.Farshchi F, Hasanzadeh M. Efficient diagnosis of cancer using biosensing of circulating tumor DNAs(ctDNA): recent progress and challenges. Microchem J. 2023;193: 109076. [DOI] [PubMed] [Google Scholar]
- 108.Cohen SA, Liu MC, Aleshin A. Practical recommendations for using ctDNA in clinical decision making. Nature. 2023;619(7969):259–68. [DOI] [PubMed] [Google Scholar]
- 109.Bettegowda C, Sausen M, Leary R, Kinde I, Agrawal N, Bartlett B, et al. Detection of circulating tumor dna in early and late stage human malignancies. Neuro-Oncology. 2014;16(suppl_3):iii7-iii. [DOI] [PMC free article] [PubMed]
- 110.Soukup J, Moško T, Kereïche S, Holada K. Large extracellular vesicles transfer more prions and infect cell culture better than small extracellular vesicles. Biochem Biophys Res Commun. 2023;687: 149208. [DOI] [PubMed] [Google Scholar]
- 111.Soukup J, Mosko T, Kereiche S, Holada K. Large Extracellular Vesicles Induce Stronger Prion Infection in Cell Culture than Small Extracellular Vesicles 2023. [DOI] [PubMed]
- 112.Vagner T, Spinelli C, Minciacchi VR, Balaj L, Zandian M, Conley A, et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J Extracell Vesicles. 2018;7(1): 1505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gao Y, Qin Y, Wan C, Sun Y, Meng J, Huang J, et al. Small extracellular vesicles: a novel avenue for cancer management. Front Oncol. 2021;11: 638357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider N, Hermann PC, Eiseler T, Seufferlein T. Emerging roles of small extracellular vesicles in gastrointestinal cancer research and therapy. Cancers (Basel). 2024;16(3): 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jimenez L, Yu H, McKenzie AJ, Franklin JL, Patton JG, Liu Q, et al. Quantitative proteomic analysis of small and large extracellular vesicles (EVs) reveals enrichment of adhesion proteins in small EVs. J Proteome Res. 2019;18(3):947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benito-Martín A, Jasiulionis MG, García-Silva S. Extracellular vesicles and melanoma: new perspectives on tumor microenvironment and metastasis. Front Cell Dev Biol. 2022;10: 1061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Masawa ME, Alshawsh MA, Ng CY, Ng AMH, Foo JB, Vijakumaran U, et al. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: a systematic review and meta-analysis of animal studies. Theranostics. 2022;12(15):6455–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shaihov-Teper O, Ram E, Brzezinski R, Volvovitch D, Zuroff E, Amunts S, et al. Small extracellular vesicles from epicardial fat of patients with atrial fibrillation induce inflammation, fibrosis and re-entrant arrhythmias. Eur Heart J. 2020;41(Supplement_2): ehaa946.3612. [Google Scholar]
- 120.Schwab A, Meyering SS, Lepene B, Iordanskiy S, van Hoek ML, Hakami RM, et al. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol. 2015;6: 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chung IM, Rajakumar G, Venkidasamy B, Subramanian U, Thiruvengadam M. Exosomes: current use and future applications. Clin Chim Acta. 2020;500:226–32. [DOI] [PubMed] [Google Scholar]
- 122.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. [DOI] [PubMed] [Google Scholar]
- 123.Zubkova E, Kalinin A. Autophagy-dependent secretion: crosstalk between autophagy and exosome biogenesis. Curr Issues Mol Biol. 2024;46(3):2209–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu J, Zhang J, Zhang Z, Gao Z, Qi Y, Qiu W, et al. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 2021;12(4): 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo X, Sui R, Piao H. Exosomes-mediated crosstalk between glioma and immune cells in the tumor microenvironment. CNS Neurosci Ther. 2023;29(8):2074–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li W, Li C, Zhou T, Liu X, Liu X, Li X, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodríguez Zorrilla S, Pérez-Sayans M, Fais S, Logozzi M, Gallas Torreira M, García García A. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers (Basel). 2019;11(3): 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Logozzi M, Angelini DF. Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: a prospective study. Cancers. 2019;11(10):1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles. 2015;4(1):26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Castillo J, Bernard V, San Lucas FA, Allenson K, Capello M, Kim DU, et al. Surfaceome profiling enables isolation of cancer-specific exosomal cargo in liquid biopsies from pancreatic cancer patients. Ann Oncol. 2018;29(1):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li J, Chen Y, Guo X, Zhou L, Jia Z, Peng Z, et al. GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J Cell Mol Med. 2017;21(5):838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen IH, Xue L, Hsu CC, Paez JS, Pan L, Andaluz H, et al. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci USA. 2017;114(12):3175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y, Ma H, Zhang X, Xiao X, Yang Z. The increasing diagnostic role of exosomes in inflammatory diseases to leverage the therapeutic biomarkers. J Inflamm Res. 2024;17:5005–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2: 20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7(1): 1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hendrix A, Lippens L, Pinheiro C, Théry C, Martin-Jaular L, Lötvall J, et al. Extracellular vesicle analysis. Nat Rev Methods Primers. 2023;3(1): 56. [Google Scholar]
- 138.Lobb RJ, Becker M, Wen Wen S, Wong CS, Wiegmans AP, Leimgruber A, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4(1):27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 2022;15(1): 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang X, Borg EGF, Liaci AM, Vos HR, Stoorvogel W. A novel three step protocol to isolate extracellular vesicles from plasma or cell culture medium with both high yield and purity. J Extracell Vesicles. 2020;9(1): 1791450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Di H, Mi Z, Sun Y, Liu X, Liu X, Li A, et al. Nanozyme-assisted sensitive profiling of exosomal proteins for rapid cancer diagnosis. Theranostics. 2020;10(20):9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei P, Wu F, Kang B, Sun X, Heskia F, Pachot A, et al. Plasma extracellular vesicles detected by single molecule array technology as a liquid biopsy for colorectal cancer. J Extracell Vesicles. 2020;9(1):1809765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5(1):3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tenchov R, Sasso JM, Wang X, Liaw W-S, Chen C-A, Zhou QA. Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16(11):17802–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Agnihotram R, Dhar R, Dhar D, Purushothaman K, Narasimhan AK, Devi A. Fusion of exosomes and nanotechnology: cutting-edge cancer theranostics. ACS Appl Nano Mater. 2024;7(8):8489–506. [Google Scholar]
- 146.Sancho-Albero M, Jarne C, Savirón M, Martín-Duque P, Membrado L, Cebolla VL, et al. High-performance thin-layer chromatography-densitometry-tandem ESI-MS to evaluate phospholipid content in exosomes of cancer cells. Int J Mol Sci. 2022;23(3): 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smolarz M, Widlak P. Serum exosomes and their miRNA load-a potential biomarker of lung cancer. Cancers (Basel). 2021;13(6): 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanchez JI, Jiao J, Kwan SY, Veillon L, Warmoes MO, Tan L, et al. Lipidomic profiles of plasma exosomes identify candidate biomarkers for early detection of hepatocellular carcinoma in patients with cirrhosis. Cancer Prev Res (Phila). 2021;14(10):955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tiruvayipati S, Wolfgeher D, Yue M, Duan F, Andrade J, Jiang H, et al. Variability in protein cargo detection in technical and biological replicates of exosome-enriched extracellular vesicles. PLoS ONE. 2020;15(3): e0228871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hwang DW, Jo MJ, Lee JH, Kang H, Bao K, Hu S, et al. Chemical modulation of bioengineered exosomes for tissue-specific biodistribution. Adv Ther (Weinh). 2019;2(11):1900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ku J, Flojo R, Zhang P, Lu B. 5110 Development of Exosome-mediated Suicide Gene Therapy for Enhanced Cancer Treatment. J Endocr Soc. 2024;8(Supplement_1):bvae163.2236.
- 152.Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;21(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022;15(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ko J, Baldassano SN, Loh PL, Kording K, Litt B, Issadore D. Machine learning to detect signatures of disease in liquid biopsies—a user’s guide. Lab Chip. 2018;18(3):395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhan Q, Liu B, Situ X, Luo Y, Fu T, Wang Y, et al. New insights into the correlations between circulating tumor cells and target organ metastasis. Signal Transduct Target Ther. 2023;8(1):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(31):3483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kotsifaki A, Maroulaki S, Armakolas A. Exploring the immunological profile in breast cancer: recent advances in diagnosis and prognosis through circulating tumor cells. Int J Mol Sci. 2024;25(9): 4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mascalchi M, Maddau C, Sali L, Bertelli E, Salvianti F, Zuccherelli S, et al. Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions. J Cancer. 2017;8(12):2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Agnoletto C, Corrà F, Minotti L, Baldassari F, Crudele F, Cook WJJ, et al. Heterogeneity in circulating tumor cells: the relevance of the stem-cell subset. Cancers. 2019;11(4):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Passaro A, Jänne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2(4):377–91. [DOI] [PubMed] [Google Scholar]
- 162.Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16(7):409–24. [DOI] [PubMed] [Google Scholar]
- 163.Janin M, Davalos V. Cancer metastasis under the magnifying glass of epigenetics and epitranscriptomics. Cancer Metastasis Rev. 2023;42(4):1071–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pixberg CF, Raba K, Müller F, Behrens B, Honisch E, Niederacher D, et al. Analysis of DNA methylation in single circulating tumor cells. Oncogene. 2017;36(23):3223–31. [DOI] [PubMed] [Google Scholar]
- 165.Chimonidou M, Strati A, Malamos N, Kouneli S, Georgoulias V, Lianidou E. Direct comparison study of DNA methylation markers in EpCAM-positive circulating tumour cells, corresponding circulating tumour DNA, and paired primary tumours in breast cancer. Oncotarget. 2017;8(42):72054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bamodu OA, Chung CC, Pisanic TR 2nd, Wu ATH. The intricate interplay between cancer stem cells and cell-of-origin of cancer: implications for therapeutic strategies. Front Oncol. 2024;14:1404628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Woo H, Joosse SA, Harten F, Hilpert F, Pantel K, Kim MS. Abstract A004: full spectrum isolation technique-based dual circulating rare cells analysis to maximize early cancer diagnosis. Clin Cancer Res. 2024;30(21_Supplement): A004-A. [Google Scholar]
- 168.Nagasawa S, Kashima Y, Suzuki A, Suzuki Y. Single-cell and spatial analyses of cancer cells: toward elucidating the molecular mechanisms of clonal evolution and drug resistance acquisition. Inflamm Regen. 2021;41(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miles LA, Bowman RL. Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature. 2020;587(7834):477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bai Y, Zhao H. Liquid biopsy in tumors: opportunities and challenges. Ann Transl Med. 2018;6(Suppl 1):S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pandey S, Yadav P. Liquid biopsy in cancer management: integrating diagnostics and clinical applications. Pract Lab Med. 2025;43: e00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Eslami-S Z, Cortés-Hernández LE, Cayrefourcq L, Alix-Panabières C. The different facets of liquid biopsy: a kaleidoscopic view. Cold Spring Harb Perspect Med. 2020;10(6): a037333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arechederra M, Ávila MA, Berasain C. Liquid biopsy for cancer management: a revolutionary but still limited new tool for precision medicine. Adv Lab Med Ava en Med de Laboratorio. 2020;1(3): 20200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lyu N, Hassanzadeh-Barforoushi A, Rey Gomez LM, Zhang W, Wang Y. SERS biosensors for liquid biopsy towards cancer diagnosis by detection of various circulating biomarkers: current progress and perspectives. Nano Convergence. 2024;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Neumann MHD, Bender S, Krahn T, Schlange T. CtDNA and CTCs in liquid biopsy - current status and where we need to progress. Comput Struct Biotechnol J. 2018;16:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Revelo AE, Martin A, Velasquez R, Kulandaisamy PC, Bustamante J, Keshishyan S, et al. Liquid biopsy for lung cancers: an update on recent developments. Ann Transl Med. 2019;7(15):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99(24):15524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang S, Li D. Role of microRNAs in triple-negative breast cancer and new therapeutic concepts (Review). Oncol Lett. 2024;28(3): 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20(1):5–20. [DOI] [PubMed] [Google Scholar]
- 180.Mei S, Xin J, Liu Y, Zhang Y, Liang X, Su X, et al. Microrna-200c promotes suppressive potential of myeloid-derived suppressor cells by modulating PTEN and FOG2 expression. PLoS ONE. 2015;10(8): e0135867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Klicka K, Grzywa TM, Mielniczuk A, Klinke A, Włodarski PK. The role of miR-200 family in the regulation of hallmarks of cancer. Front Oncol. 2022;12: 965231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Z, Wang Q, Qin F, Chen J. Exosomes: a promising avenue for cancer diagnosis beyond treatment. Front Cell Dev Biol. 2024;12: 1344705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Voss G, Edsjö A, Bjartell A, Ceder Y. Quantification of microrna editing using two-tailed RT-qPCR for improved biomarker discovery. RNA. 2021;27(11):1412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ekiz Kanik F, Celebi I, Sevenler D, Tanriverdi K, Lortlar Ünlü N, Freedman JE, et al. Attomolar sensitivity microRNA detection using real-time digital microarrays. Sci Rep. 2022;12(1): 16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Benesova S, Kubista M. Small RNA-sequencing: approaches and considerations for miRNA analysis. Diagnostics. 2021;11(6): 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ergin S, Kherad N, Alagoz M. RNA sequencing and its applications in cancer and rare diseases. Mol Biol Rep. 2022;49(3):2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yaylak B, Akgül B. Experimental MicroRNA Detection Methods. Methods Mol Biol (Clifton, NJ). 2022;2257:33–55. [DOI] [PubMed] [Google Scholar]
- 188.Paulsen IW, Bzorek M, Olsen J, Grum-Schwensen B, Troelsen JT, Pedersen OB. A novel approach for microRNA in situ hybridization using locked nucleic acid probes. Sci Rep. 2021;11(1):4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cirillo PDR, Margiotti K. Quantification of circulating microRNAs by droplet digital PCR for cancer detection. BMC Res Notes. 2020;13(1): 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Low SS, Ji D, Chai WS. Recent progress in nanomaterials modified electrochemical biosensors for the detection of microRNA. Micromachines. 2021;12(11):1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Balatti V, Croce CM. Microrna dysregulation and multi-targeted therapy for cancer treatment. Adv Biol Regul. 2020;75: 100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: a signature for cancer progression. Biomed Pharmacother. 2021;138: 111528. [DOI] [PubMed] [Google Scholar]
- 193.Maldonado E, Morales-Pison S. Role of the mediator complex and MicroRNAs in breast cancer etiology. Genes. 2022;13(2): 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tao J, Zhao X, Tao J. C-MYC-miRNA circuitry: a central regulator of aggressive B-cell malignancies. Cell Cycle. 2014;13(2):191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Feng Z, Zhang C, Wu R, Hu W. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011;3(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Navarro F, Lieberman J. MiR-34 and p53: new insights into a complex functional relationship. PLoS ONE. 2015;10(7): e0132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xiang J, Wu J. Feud or friend? The role of the miR-17-92 cluster in tumorigenesis. Curr Genomics. 2010;11(2):129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vu LT, Gong J, Pham TT, Kim Y, Le MTN. MicroRNA exchange via extracellular vesicles in cancer. Cell Prolif. 2020;53(11): e12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Felekkis K, Papaneophytou C. The circulating biomarkers league: combining miRNAs with cell-free DNAs and proteins. Int J Mol Sci. 2024;25(6): 3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ideozu JE, Zhang X, Rangaraj V, McColley S, Levy H. Microarray profiling identifies extracellular circulating miRNAs dysregulated in cystic fibrosis. Sci Rep. 2019;9(1): 15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao K, Cheng J, Chen B, Liu Q, Xu D, Zhang Y. Circulating microRNA-34 family low expression correlates with poor prognosis in patients with non-small cell lung cancer. J Thorac Dis. 2017;9(10):3735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Powrózek T, Krawczyk P, Kowalski DM, Winiarczyk K, Olszyna-Serementa M, Milanowski J. Plasma circulating microRNA-944 and microRNA-3662 as potential histologic type-specific early lung cancer biomarkers. Transl Res. 2015;166(4):315–23. [DOI] [PubMed] [Google Scholar]
- 203.Powrózek T, Krawczyk P, Kowalski DM, Kuźnar-Kamińska B, Winiarczyk K, Olszyna-Serementa M, et al. Application of plasma circulating microRNA-448, 506, 4316, and 4478 analysis for non-invasive diagnosis of lung cancer. Tumor Biol. 2016;37(2):2049–55. [DOI] [PubMed] [Google Scholar]
- 204.Amiri-Dashatan N, Koushki M, Naghi-Zadeh M, Razzaghi MR, Mohaghegh Shalmani H. Prognostic value of microRNA-125a/b family in patients with gastric cancer: a meta-analysis. Gastroenterol Hepatol Bed Bench. 2021;14(Suppl1):S1–9. [PMC free article] [PubMed] [Google Scholar]
- 205.Garcia-Mayea Y, Mir C, Masson F, Paciucci R, LLeonart M, editors. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin Cancer Biol; 2020: Elsevier. [DOI] [PubMed]
- 206.Min H-Y, Lee H-Y. Mechanisms of resistance to chemotherapy in non-small cell lung cancer. Arch Pharm Res. 2021;44(2):146–64. [DOI] [PubMed] [Google Scholar]
- 207.Fujitani T, Takahara T, Hattori H, Imajo Y, Ogasawara H. Radiochemotherapy for non-Hodgkin’s lymphoma in palatine tonsil. Cancer. 1984;54(7):1288–92. [DOI] [PubMed] [Google Scholar]
- 208.Póvoa V, Rebelo de Almeida C, Maia-Gil M, Sobral D, Domingues M, Martinez-Lopez M, et al. Innate immune evasion revealed in a colorectal zebrafish xenograft model. Nature Commun. 2021;12(1):1156. [DOI] [PMC free article] [PubMed]
- 209.Möckl L. The emerging role of the mammalian glycocalyx in functional membrane organization and immune system regulation. Front Cell Dev Biol. 2020;8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chulpanova DS, Kitaeva KV, Green AR, Rizvanov AA, Solovyeva VV. Molecular aspects and future perspectives of cytokine-based anti-cancer immunotherapy. Front Cell Dev Biol. 2020;8:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Germic N, Frangez Z, Yousefi S, Simon H-U. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019;26(4):715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Borghaei H, Smith MR, Campbell KS. Immunotherapy of cancer. Eur J Pharmacol. 2009;625(1–3):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim N, Lee HH, Lee H-J, Choi WS, Lee J, Kim HS. Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch Pharm Res. 2019;42:591–606. [DOI] [PubMed] [Google Scholar]
- 214.Liang W, Ferrara N. Iron metabolism in the tumor microenvironment: contributions of innate immune cells. Front Immunol. 2021;11: 626812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Otoshi T, Nagano T, Tachihara M, Nishimura Y. Possible biomarkers for cancer immunotherapy. Cancers (Basel). 2019;11(7):935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen Q, Li T, Yue W. Drug response to PD-1/PD-L1 blockade: based on biomarkers. Onco Targets Ther. 2018. 10.2147/OTT.S168313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, et al. PD-L1 on host cells is essential for PD-L1 blockade–mediated tumor regression. J Clin Invest. 2018;128(2):580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. [DOI] [PubMed] [Google Scholar]
- 221.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389(10066):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kaderbhaï C, Tharin Z, Ghiringhelli F. The role of molecular profiling to predict the response to immune checkpoint inhibitors in lung cancer. Cancers (Basel). 2019;11(2):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang Y, Sun Z, Mao X, Wu H, Luo F, Wu X, et al. Impact of mismatch-repair deficiency on the colorectal cancer immune microenvironment. Oncotarget. 2017;8(49):85526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Overman MJ, Lonardi S, Wong KYM, Lenz H-J, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair–deficient/microsatellite instability–high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–9. [DOI] [PubMed] [Google Scholar]
- 228.Wang Z, Zhao J, Wang G, Zhang F, Zhang Z, Zhang F, et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78(22):6486–96. [DOI] [PubMed] [Google Scholar]
- 229.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–68. [DOI] [PubMed] [Google Scholar]
- 230.Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 232.Mansfield A, Aubry M, Moser J, Harrington S, Dronca R, Park S, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kamali H, Golmohammadzadeh S, Zare H, Nosrati R, Fereidouni M, Safarpour H. The recent advancements in the early detection of cancer biomarkers by DNAzyme-assisted aptasensors. J Nanobiotechnol. 2022;20(1):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tian M, Wu R, Xiang C, Niu G, Guan W. Recent Advances in Fluorescent Probes for Cancer Biomarker Detection. Molecules. 2024;29(5):1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater. 2021;6(4):351–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ahmad A, Imran M, Ahsan H. Biomarkers as biomedical bioindicators: approaches and techniques for the detection, analysis, and validation of novel Biomarkers of diseases. Pharmaceutics. 2023;15(6):1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zaslavsky BY, Uversky VN, Chait A. Solvent interaction analysis as a proteomic approach to structure-based biomarker discovery and clinical diagnostics. Expert Rev Proteomics. 2016;13(1):9–17. [DOI] [PubMed] [Google Scholar]
- 238.Goeminne LJ, Gevaert K, Clement L. Experimental design and data-analysis in label-free quantitative LC/MS proteomics: a tutorial with MSqRob. J Proteomics. 2018;171:23–36. [DOI] [PubMed] [Google Scholar]
- 239.Taylor CR. Introduction to predictive biomarkers: definitions and characteristics. Pred Biomark Oncol Appl Prec Med. 2019;2019:3–18. [Google Scholar]
- 240.Perera-Bel J, Leha A, Beissbarth T. Bioinformatic methods and resources for biomarker discovery, validation, development, and integration. Pred Biomark Oncol Appl Prec Med. 2019;2019:149–64. [Google Scholar]
- 241.Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17(6):333–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schwarze K, Buchanan J, Fermont JM, Dreau H, Tilley MW, Taylor JM, et al. The complete costs of genome sequencing: a microcosting study in cancer and rare diseases from a single center in the United Kingdom. Genet Med. 2020;22(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lalonde E, Wertheim G, Li MM. Clinical impact of genomic information in pediatric leukemia. Front Pediatr. 2017;5:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gwinn M, MacCannell D, Armstrong GL. Next-generation sequencing of infectious pathogens. JAMA. 2019;321(9):893–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Surrey LF, MacFarland SP, Chang F, Cao K, Rathi KS, Akgumus GT, et al. Clinical utility of custom-designed NGS panel testing in pediatric tumors. Genome Med. 2019;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Miller TE, Yang M, Bajor D, Friedman JD, Chang RY, Dowlati A, et al. Clinical utility of reflex testing using focused next-generation sequencing for management of patients with advanced lung adenocarcinoma. J Clin Pathol. 2018;71(12):1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Coccaro N, Anelli L, Zagaria A, Specchia G, Albano F. Next-generation sequencing in acute lymphoblastic leukemia. Int J Mol Sci. 2019;20(12):2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dufour A, Schneider F, Metzeler KH, Hoster E, Schneider S, Zellmeier E, et al. Acute myeloid leukemia with biallelic CEBPA gene mutations and normal karyotype represents a distinct genetic entity associated with a favorable clinical outcome. J Clin Oncol. 2010;28(4):570–7. [DOI] [PubMed] [Google Scholar]
- 249.Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Coccaro N, Tota G, Anelli L, Zagaria A, Specchia G, Albano F. Digital PCR: a reliable tool for analyzing and monitoring hematologic malignancies. Int J Mol Sci. 2020;21(9):3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jaffe R. WHO classification of tumours of haematopoietic and lymphoid tissues. World Health Organization Classification of Tumours. 2008. p. 358–60.
- 252.Shaw AT, Solomon BJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2015;372(7):683–4. [DOI] [PubMed] [Google Scholar]
- 253.Kwok GW, Poh J, Cher CY, Pinweha P. Utility of an amplicon-based ctDNA platform for advanced cholangiocarcinomas (CCA) in Asia. J Clin Oncol. 2024;42(16_suppl):e16206.
- 254.Shah D, Wells A, Dawravoo K, Abad J, D'Souza A, Suh G, et al. Abstract PR014: Evaluating circulating tumor DNA by targeted next generation sequencing as a biomarker in the treatment of localized pancreatic cancer. Cancer Res. 2022;82(22_Supplement):PR014.
- 255.Kurihara S, Ueda Y, Onitake Y, Sueda T, Ohta E, Morihara N, et al. Circulating free DNA as non-invasive diagnostic biomarker for childhood solid tumors. J Pediatr Surg. 2015;50(12):2094–7. [DOI] [PubMed] [Google Scholar]
- 256.Song TJ. A new era of endoscopic ultrasound-guided tissue acquisition for next-generation sequencing for pancreatic cancer. Gut Liver. 2020;14(3):279–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hickman R, Miller AM, Holle BM, Jee J, Liu S-Y, Ross D, et al. BIOM-46. Real-world experience with circulating tumor dna in cerebrospinal fluid in patients with central nervous system tumors. Neuro Oncol. 2024;26(Supplement_8):viii29-viii30. [DOI] [PMC free article] [PubMed]
- 258.Prieto P, Jaén RI, Calle D, Gómez-Serrano M, Núñez E, Fernández-Velasco M, et al. Interplay between post-translational cyclooxygenase-2 modifications and the metabolic and proteomic profile in a colorectal cancer cohort. World J Gastroenterol. 2019;25(4):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Faria SS, Morris CF, Silva AR, Fonseca MP, Forget P, Castro MS, et al. A timely shift from shotgun to targeted proteomics and how it can be groundbreaking for cancer research. Front Oncol. 2017;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hanash S, Taguchi A. Application of proteomics to cancer early detection. Cancer J. 2011;17(6):423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sorolla A, Wang E, Golden E, Duffy C, Henriques ST, Redfern AD, et al. Precision medicine by designer interference peptides: applications in oncology and molecular therapeutics. Oncogene. 2020;39(6):1167–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang L, Parvin R, Fan Q, Ye F. Emerging digital PCR technology in precision medicine. Biosens Bioelectron. 2022;211: 114344. [DOI] [PubMed] [Google Scholar]
- 263.McEvoy AC, Wood BA, Ardakani NM, Pereira MR, Pearce R, Cowell L, et al. Droplet digital PCR for mutation detection in formalin-fixed, paraffin-embedded melanoma tissues: a comparison with sanger sequencing and pyrosequencing. J Mol Diagn. 2018;20(2):240–52. [DOI] [PubMed] [Google Scholar]
- 264.Laprovitera N, Riefolo M, Porcellini E, Durante G, Garajova I, Vasuri F, et al. MicroRNA expression profiling with a droplet digital PCR assay enables molecular diagnosis and prognosis of cancers of unknown primary. Mol Oncol. 2021;15(10):2732–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shah PS, Murarka S, Joshi A, Mehta B, Parmar V, Shah N, et al. Single-day HER2neu amplification assessment using chip-based digital PCR in formalin-fixed paraffin-embedded breast carcinoma tissue. Breast Cancer (London). 2018;2018:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Balaji SA, Shanmugam A, Chougule A, Sridharan S, Prabhash K, Arya A, et al. Analysis of solid tumor mutation profiles in liquid biopsy. Cancer Med. 2018;7(11):5439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pisetsky DS, Fairhurst A-M. The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity. 2007;40(4):281–4. [DOI] [PubMed] [Google Scholar]
- 269.Savva KV, Das B, Antonowicz S, Hanna GB, Peters CJ. Progress with metabolomic blood tests for gastrointestinal cancer diagnosis—an assessment of biomarker translation. Cancer Epidemiol Biomarkers Prev. 2022;31(12):2095–105. [DOI] [PubMed] [Google Scholar]
- 270.Mazzone PJ. Beyond the usual suspects. J Thorac Oncol. 2012;7(10):1477–8. [DOI] [PubMed] [Google Scholar]
- 271.Mamas M, Dunn WB, Neyses L, Goodacre R. The role of metabolites and metabolomics in clinically applicable biomarkers of disease. Arch Toxicol. 2011;85(1):5–17. [DOI] [PubMed] [Google Scholar]
- 272.Hassanein M, Callison JC, Callaway-Lane C, Aldrich MC, Grogan EL, Massion PP. The state of molecular biomarkers for the early detection of lung cancer. Cancer Prev Res (Phila). 2012;5(8):992–1006. [DOI] [PMC free article] [PubMed]
- 273.Widłak P, Jelonek K, Kurczyk A, Żyła J, Sitkiewicz M, Bottoni E, et al. Serum metabolite profiles in participants of lung cancer screening study; comparison of two independent cohorts. Cancers (Basel). 2021;13(11):2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jelonek K, Widłak P. Metabolome-based biomarkers: their potential role in the early detection of lung cancer. Contemp Oncol (Pozn). 2018;22(3):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nguyen V, Hurton S, Ayloo S, Molinari M. Advances in pancreatic cancer: the role of metabolomics. JOP J Pancr. 2015;16(3):244–8. [Google Scholar]
- 276.Brinkman K, Meyer L, Bickel A, Enderle D, Berking C, Skog J, et al. Extracellular vesicles from plasma have higher tumour RNA fraction than platelets. J Extracell Vesicles. 2020;9(1):1741176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rikkert LG, van der Pol E, van Leeuwen TG, Nieuwland R, Coumans FAW. Centrifugation affects the purity of liquid biopsy-based tumor biomarkers. Cytometry A. 2018;93(12):1207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lim JU, Yeo CD, Kang HS, Park CK, Kim JS, Kim JW, et al. Prognostic value of platelet count and lymphocyte to monocyte ratio combination in stage IV non-small cell lung cancer with malignant pleural effusion. PLoS ONE. 2018;13(7): e0200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liu W, Ha M, Yin N. Combination of platelet count and lymphocyte to monocyte ratio is a prognostic factor in patients undergoing surgery for non-small cell lung cancer. Oncotarget. 2017;8(42):73198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Morais M, Fonseca T, Machado-Neves R, Honavar M, Coelho AR, Lopes J, et al. Can pretreatment blood biomarkers predict pathological response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer? Future Oncol. 2021;17(35):4947–57. [DOI] [PubMed] [Google Scholar]
- 281.Prelaj A, Miskovic V, Zanitti M, Trovo F, Genova C, Viscardi G, et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: a systematic review. Ann Oncol. 2023;35:29–65. [DOI] [PubMed] [Google Scholar]
- 282.Xiao S, Lin H, Wang C, Wang S, Rajapakse JC. Graph Neural Networks With Multiple Prior Knowledge for Multi-Omics Data Analysis. IEEE J Biomed Health Inform. 2023;27(9):4591–600. [DOI] [PubMed] [Google Scholar]
- 283.Zanfardino M, Castaldo R, Pane K, Affinito O, Aiello M, Salvatore M, et al. MuSA: a graphical user interface for multi-OMICs data integration in radiogenomic studies. Sci Rep. 2021;11(1):1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Murcia Pienkowski V, Skoczylas P, Zaremba A, Kłęk S, Balawejder M, Biernat P, et al. Harnessing the power of AI in precision medicine: NGS-based therapeutic insights for colorectal cancer cohort. Front Oncol. 2024;14:1407465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zanfardino M, Franzese M, Pane K, Cavaliere C, Monti S, Esposito G, et al. Bringing radiomics into a multi-omics framework for a comprehensive genotype-phenotype characterization of oncological diseases. J Transl Med. 2019;17(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kuntz S, Krieghoff-Henning E, Kather JN, Jutzi T, Höhn J, Kiehl L, et al. Gastrointestinal cancer classification and prognostication from histology using deep learning: systematic review. Eur J Cancer. 2021;155:200–15. [DOI] [PubMed] [Google Scholar]
- 287.Breen J, Allen K, Zucker K, Adusumilli P, Scarsbrook A, Hall G, et al. Artificial intelligence in ovarian cancer histopathology: a systematic review. NPJ Precis Oncol. 2023;7(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shahazi R, Saddam A, Islam M, Rahman M, Paimard G, Kumer A, et al. Recent progress in Nanomaterial based biosensors for the detection of cancer biomarkers in human fluids. Nano Carbons. 2024;2:1254. [Google Scholar]
- 289.Raheja IK, Kumar P, Rajashekarappa KK, Mahadevan GD. Nanobiosensors for Early Detection of Cancer: a Recent Update. Biomed Mater Devices. 2024.
- 290.Liu Z, Zhou Y, Lu J, Gong T, Ibáñez E, Cifuentes A, et al. Microfluidic biosensors for biomarker detection in body fluids: a key approach for early cancer diagnosis. Biomarker Res. 2024;12(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mukherjee S, Mukherjee A, Bytesnikova Z, Ashrafi AM, Richtera L, Adam V. 2D graphene-based advanced nanoarchitectonics for electrochemical biosensors: applications in cancer biomarker detection. Biosens Bioelectron. 2024;250: 116050. [DOI] [PubMed] [Google Scholar]
- 292.Kanagavalli P, Elkaffas RA, Mohideen MIH, Eissa S. Electrochemical immunosensor for the predictive cancer biomarker SLFN11 using reduced graphene oxide/MIL-101(Cr)-NH2 composite. Int J Biol Macromol. 2025;285: 138174. [DOI] [PubMed] [Google Scholar]
- 293.Radpour R. Tracing and targeting cancer stem cells: new venture for personalized molecular cancer therapy. World J Stem Cells. 2017;9(10):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Braig D, Becherer C, Bickert C, Braig M, Claus R, Eisenhardt AE, et al. Genotyping of circulating cell-free DNA enables noninvasive tumor detection in myxoid liposarcomas. Int J Cancer. 2019;145(4):1148–61. [DOI] [PubMed] [Google Scholar]
- 295.Sinha S, Brown H, Tabak J, Fang Z, du Tertre MC, McNamara S, et al. Multiplexed real-time polymerase chain reaction cell-free DNA assay as a potential method to monitor stage IV colorectal cancer. Surgery. 2019;166(4):534–9. [DOI] [PubMed] [Google Scholar]
- 296.Salvianti F, Giuliani C, Petrone L, Mancini I, Vezzosi V, Pupilli C, et al. Integrity and quantity of total cell-free DNA in the diagnosis of thyroid cancer: correlation with cytological classification. Int J Mol Sci. 2017;18(7):1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wang E, Zou J, Zaman N, Beitel LK, Trifiro M, Paliouras M, editors. Cancer systems biology in the genome sequencing era: part 1, dissecting and modeling of tumor clones and their networks. Semin Cancer Biol; 2013: Elsevier. [DOI] [PubMed]
- 298.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 299.Purkayastha K, Dhar R, Pethusamy K, Srivastava T, Shankar A, Rath GK, et al. The issues and challenges with cancer biomarkers. J Cancer Res Ther. 2023;19(Suppl 1):S20–35. [DOI] [PubMed] [Google Scholar]
- 300.Howe F, Barton S, Cudlip S, Stubbs M, Saunders D, Murphy M, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med An Off J Int Soc Magn Reson Med. 2003;49(2):223–32. [DOI] [PubMed] [Google Scholar]
- 301.Almendro V, Marusyk A, Polyak K. Cellular heterogeneity and molecular evolution in cancer. Annu Rev Pathol. 2013;8(1):277–302. [DOI] [PubMed] [Google Scholar]
- 302.Schrader KA, Cheng DT, Joseph V, Prasad M, Walsh M, Zehir A, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2(1):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Chen G, Ning B, Shi T. Single-cell RNA-seq technologies and related computational data analysis. Front Genet. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Petersen B-S, Fredrich B, Hoeppner MP, Ellinghaus D, Franke A. Opportunities and challenges of whole-genome and-exome sequencing. BMC Genet. 2017;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Passaro A, Al Bakir M, Hamilton EG, Diehn M, André F, Roy-Chowdhuri S, et al. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell. 2024;187(7):1617–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Varoquaux G, Cheplygina V. Machine learning for medical imaging: methodological failures and recommendations for the future. NPJ Dig Med. 2022;5(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chiu F-Y, Yen Y. Imaging biomarkers for clinical applications in neuro-oncology: current status and future perspectives. Biomarker Res. 2023;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.