Abstract
Objective
To determine the incidence of acute kidney injury (AKI) following abdominal surgery, assess its outcome associations, and identify factors associated with postoperative AKI development.
Methods
We performed a systematic search of PubMed, Embase, and Cochrane Database of Systematic Reviews, from January 2004, to December 2024. We included studies reporting AKI based on consensus criteria (RIFLE, AKIN, or KDIGO) in adult abdominal surgery patients.
Results
A total of 162 studies (675361 patients) were included. The pooled AKI incidence was 16% (95% CI: 14-17%), with significant variation by surgical procedure. Meta-analysis showed AKI was significantly associated with increased short-term mortality (risk ratio [RR], 6.46; 95% CI: 4.63–9.00) and long-term mortality (RR, 6.36; 95% CI: 3.32–12.16). Mortality risk demonstrated stage-dependent increase, with RR of 2.74 (95%CI: 1.77–4.24), 8.01 (95%CI: 3.18–20.18), and 15.73 (95%CI: 5.52–44.81) for AKI stages 1, 2, and 3, respectively. AKI was associated with prolonged hospital stay (weighted mean difference 4.72 days; 95%CI: 3.43–6.02), also showeing stage-dependent increase of 5.03, 11.16, and 14.46 days for stages 1, 2, and 3, respectively. Twenty-five risk factors were associated with AKI. Meta-analysis of randomized controlled trials revealed that individualized blood pressure target management significantly reduced AKI incidence (RR, 0.67; 95% CI: 0.52–0.88).
Conclusions
AKI remains a common and important complication after abdominal surgery, with severity showing a graded association with mortality and hospital stay. Individualized blood pressure management demonstrates promise in AKI prevention.
Registration
PROSPERO CRD42022304083.
Keywords: Acute kidney injury, abdominal surgery, incidence, risk factor, systematic review
KEY MESSAGES
The incidence of postoperative AKI after abdominal surgery is 16% (95% CI: 14–17%), varying by specific abdominal surgical procedure but not significantly different over time or by consensus definition.
AKI severity shows a strong graded association with both short-term and long-term mortality, as well as prolonged hospital stays.
Twenty-five factors were identified, providing valuable information for clinical risk assessment.
Meta-analysis of randomized trials reveals that individualized blood pressure target management significantly reduces AKI incidence, while other single perioperative interventions (crystalloids versus colloids, restrictive versus liberal fluid management, cardiac output-guided therapy, hemodynamic monitoring) show no significant protective effects.
Background
Acute kidney injury (AKI) occurs in approximately 17% of hospitalized patients worldwide, representing its average global incidence [1]. Majority occur as a sequela of abdominal surgery [2]. The development of postoperative AKI is associated with longer hospital length of stay (LOS), mortality, post-discharge readmission rates, and development of chronic kidney disease (CKD) [3,4]. Patients with AKI have a significantly higher risk of developing new or progressive CKD (hazard ratio 2.67, 95% confidence interval [CI]1.99–3.58; 17.76 vs 7.59 cases per 100 person-years) and end-stage kidney disease (hazard ratio 4.81, 95% CI 3.04–7.62; 0.47 vs 0.08 cases per 100 person-years), relative to non-AKI patients [5]. Thus, AKI should be recognized as a potential indicator of adverse effects associated with post-abdominal surgery. A previous systematic review of 19 studies with 82,514 patients reported that the incidence of AKI after major abdominal surgery was 13.4% [6]. Advances in perioperative management, supportive interventions in the intensive care unit, and surgical techniques have been developed since 2016. However, dozens of studies have reported a high incidence of AKI, and some even occurred in patients undergoing non-major abdominal surgery [7–11].
Different mechanisms are involved in the development of postoperative AKI with a complex and multifactorial etiology. Renal hypoperfusion, high intra-abdominal pressure, sepsis, and/or nephrotoxic exposure are classical mechanisms of postoperative AKI [12–16]. This has led to a lack of a single and effective renoprotective strategy to protect surgical patients from AKI. Recently, a series of studies have explored and identified risk factors associated with an increased risk of postoperative AKI, such as older age [17], higher body mass index (BMI) [18,19], hypertension [19,20], diabetes mellitus [8,21], red blood cell (RBC) suspension transfusion [8,11,20], and the use of synthetic colloid [10,20,22], among others. Notably, conflicting results exist in the literature regarding certain risk factors. For instance, intraoperative RBC transfusion was identified as a significant risk factor in Kim et al.’s study (odds ratio [OR] = 1.58; 95%CI: 1.06–2.35) [20] but failed to reach statistical significance in Mahmooth et al. research (OR = 2.2; 95%CI: 0.6–8.06) [11]. Similarly, diabetes showed no statistical significance in the STARSurg Collaborative study (OR = 1.16; 95%CI: 0.79–1.7) [23] but emerged as a significant risk factor in Ji Hoon Sim’s research (OR = 2.77; 95%CI: 1.16–6.58) [24].
Perioperative hemodynamic changes play a crucial role in the development of postoperative AKI. Salmasi et al. demonstrated that intraoperative mean arterial pressure (MAP) <65 mmHg or >20% decrease from baseline was progressively associated with postoperative AKI risk, with longer exposure times correlating with higher risk [25]. The subsequent analysis of the POISE-2 trial revealed that sustained postoperative systolic blood pressure below 90 mmHg was associated with increased AKI risk, particularly when combined with anemia [26]. However, the optimal perioperative blood pressure management strategy remains controversial. The recent POISE-3 trial compared an avoid-hypotension strategy (targeting intraoperative MAP ≥80 mmHg) with an avoid-hypertension strategy (targeting MAP ≥60 mmHg) and found no significant difference in postoperative AKI incidence [27]. These findings highlight the complexity of perioperative management. Although several validated postoperative AKI risk assessment tools exist (such as the NSQIP calculator, Thakar score, and SPARK scoring system), they lack comprehensive systematic evaluation specifically for the abdominal surgery population.
Therefore, we conducted this systematic review and meta-analysis to determine the proportion of postoperative AKI according to contemporary consensus criteria [28–30] and to further elucidate associated influence factors, providing evidence for the prevention and management of postoperative AKI.
Methods
The following three consensus definitions of AKI were used: Risk, Injury, Failure, Loss, End-Stage (RIFLE), Acute Kidney Injury Network (AKIN), and Kidney Disease Improving Global Outcomes (KDIGO) [28–30]. Given that the three consensus definitions mentioned above were the result of continuous change, improvement, and integration, if multiple definitions are used for a study, we extract AKI data in the following order of priority: KDIGO > AKIN > RIFLE.
Previous meta-analyses have demonstrated that the incidence of AKI after liver transplant (LT) and open abdominal aortic aneurysm (OAAA) surgery is higher (47 and 24%, respectively) than that after other abdominal procedures (such as colorectal, hepatobiliary, and gastrointestinal procedures) [6]. If analyzed together, the incidence of AKI after LT and OAAA would carry excessive weight; therefore, they were not included in the main analysis. The data are presented in the supplemental file (Supplementary Table S1).
Systematic literature search
As the consensus definition of AKI was designed and published in or after 2004 [29], we conducted a systematic search of PubMed, Embase, and the Cochrane Database of Systematic Reviews, from January 1, 2004 to December 31, 2024. The following search themes were used: (((Surgical Procedures, Operative[MeSH]) OR (General Surgery[MeSH])) AND ((Acute Kidney Injury[MeSH]) OR (Renal Insufficiency[MeSH]))) OR ((Risk[MeSH]) OR (Incidence[MeSH])). If more than one publication contained overlapping research subjects, we decided which publication would be included based on the completeness of reporting results. This study was conducted according to the recommendations of the PRISMA reporting guideline [31]. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022304083).
Inclusion criteria
Adult patient undergoing abdominal cavity surgery
The RIFLE, AKIN, or KDIGO diagnosis and classification criteria were used to evaluate postoperative AKI.
Data for OR and the corresponding CI are available for the risk factor of AKI after abdominal surgery.
No language restriction
Exclusion criteria
Urological procedure
Caesarean section
Data on AKI is not available for the abdominal surgery group
AKI diagnosis was based on diagnostic coding or the receipt of renal replacement therapy
Type of article: Case study, review, editorial, non-human study, letter, and conference paper
Factors associated with postoperative AKI
Based on existing literature [32] and clinical experience, we selected factors associated with postoperative AKI encompassing eight major categories: demographic characteristics, preoperative status assessment, comorbidities, perioperative medication exposure, surgery-related factors, intraoperative management factors, laboratory parameters, and pathological conditions.
Outcome measures
The primary outcomes of this study include the incidence of AKI in patients following abdominal surgery and the OR and corresponding CI for the influence factors of post-operative AKI. The secondary outcome was the number of patients with AKI according to stage, hospital LOS, and mortality. Studies reporting hospital or < 30-day mortality were defined as short-term mortality. Long-term mortality was defined as mortality > 30 days. If both univariate and multivariate analyses for OR were conducted, the latter was the first choice because the results were adjusted for confounding factors.
Study selection and data extraction
Each study was independently evaluated by two reviewers (J.L. and S.H.L.), first for title and abstract screening and second for full-text careful reading. Data from the included primary studies were independently extracted by two reviewers (J.L. and S.H.L.) using pre-formulated forms. Disagreements were resolved by a third author (M. Z.). Two reviewers (H.T. and F.X.) reviewed the manuscript.
Quality assessment
We used the Risk of Bias in Non-randomized Studies of Exposures (ROBINS-E) tool to assess the risk of bias in observational studies across seven domains: (Domain 1) bias due to confounding, (Domain 2) bias in measurement of exposure, (Domain 3) bias in selection of participants, (Domain 4) bias due to post-exposure interventions, (Domain 5) bias due to missing data, (Domain 6) bias in measurement of outcomes, and (Domain 7) bias in selection of reported results. Each study was rated as having low, moderate, high or very high risk of bias for each domain and overall [33]. The risk of bias in the randomized controlled trials (RCT) was assessed using the Cochrane risk-of-bias tool. All quality assessments were conducted independently by two reviewers (J.L. and S.H.L.).
Analysis
Statistical analyses were performed using Stata/SE (version 17.0; StataCorp, LLC). The random-effects model for meta-analysis was utilized to calculate the overall pooled proportion of AKI among all included studies, the risk ratio (RR) of mortality in patients with AKI, the weighted mean difference in hospital LOS, and the ORs of factors associated with postoperative AKI. Subgroup analysis was conducted for studies with different definitions of AKI, surgical settings, and years of publication. To explore the impact of each study on the overall findings, we conducted a leave-one-out meta-analysis by removing each study from the meta-analysis. Potential publication bias was assessed using the Egger’s test. Between-study heterogeneity was assessed using the I2 index. If the I2 statistic was 50% or higher, heterogeneity was considered significant.
Results
Characteristics of the included studies
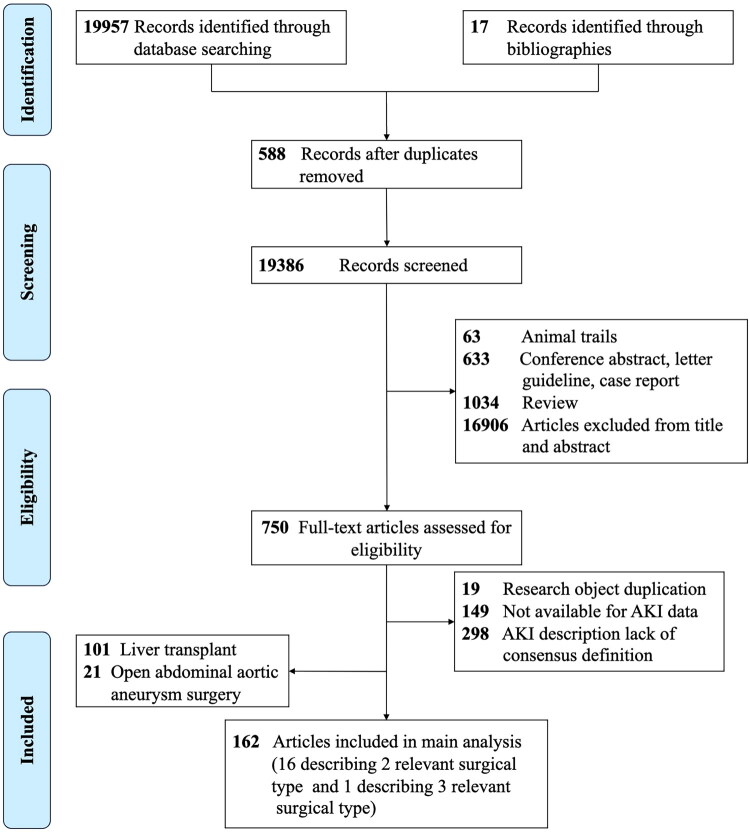
The study selection process is illustrated in Figure 1. In total, 19974 studies were screened. A total of 750 studies were identified for full-text review, which were excluded because of a lack of consensus on the definition of AKI (n = 298), unstated incidence of AKI (n = 149), and research object duplication (n = 19) (Supplementary Table S2). In total, 162 studies satisfied the pre-specified inclusion criteria (Supplementary Table S3) [7,9–11,17–22,24,25,34–183]. Of the 162 included studies, 158 were observational studies [7,9–11,17–20,22, 24,25,34–79,81–86,88–111,113–183], and four were RCT studies[21,80,87,112]. Risk of bias assessment using ROBINS-E revealed that most included studies had low to moderate risk of bias across the seven domains (Supplementary Table S4). The majority of studies demonstrated low risk in participant selection (D3), outcome measurement (D6), and selective reporting (D7). However, moderate risk was commonly observed in confounding bias (D1) and missing data (D5). A small number of studies were classified as having high overall risk of bias, primarily due to inadequate control of confounding factors and high attrition rates. Four of the included RCTs reported the generation of random sequences. Two studies described allocation concealment [21,87]. One study masked the trial to all participants and investigators [21]. Two studies masked the trial to outcome assessors [21,80]. All studies included the same outcome indicators in their reporting as the initially planned research project indicators, and no omissions in the outcome indicators were identified (Supplementary Figure S1). Sixteen studies reported that patients underwent two types of surgery [25,38,42,47,51,64,68,72,73,86,96,139,162,171,172,179]. One study reported that patients underwent three types of surgery, and the number of AKI events in patients undergoing gynecological surgery was 0. These issues were addressed in two separate studies. The included studies reported the following surgical settings: mixed abdominal surgery (72 studies), digestive surgery (46 studies), gynecological surgery (18 studies), hepatobiliary surgery (22 studies), pancreatic surgery (11 studies), cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with cisplatin (CRS and HIPEC) (eight studies), and bariatric surgery (three studies).
Figure 1.
Flow diagram for study selection.
Incidence of AKI
The meta-analysis of the pooled incidence of AKI was 16% (14–17%, 95% CI), with subgroup analyses presented in Table 1 and the forest plot of individual study incidence rates shown in Supplementary Figure S2. The between-study heterogeneity was considerable (I2 = 99.90%). We conducted a leave-one-out meta-analysis, which revealed that the results were robust. Egger’s test (t = 4.81, p < 0.01) suggested the presence of publication bias; however, the trim-and-fill imputed no missing data. The nonparametric trim-and-fill analysis of 180 studies showed that no studies needed to be fille. Visual inspection of funnel plots with contour lines at 1, 5, and 10% significance levels further confirmed no significant publication bias in this meta-analysis (Supplementary Figure S3).
Table 1.
Meta-analysis of the proportion of patients developing postoperative AKI.
| No. of studies | No. of patients | Incidence of AKI | 95%CI | Heterogeneity |
||
|---|---|---|---|---|---|---|
| I 2 | τ 2 | |||||
| All patients | 180 | 675361 | 16% | 14–17% | 99.90% | 0.02 |
| AKI definition | ||||||
| AKIN | 36 | 62285 | 13% | 10–16% | 99.41% | 0.01 |
| KDIGO | 127 | 538532 | 15% | 13–18% | 99.91% | 0.02 |
| RIFLE | 17 | 74544 | 21% | 13–29% | 99.84% | 0.03 |
| Surgery type | ||||||
| Mixed abdominal surgery | 72 | 422095 | 19% | 12–22% | 99.94% | 0.02 |
| Digestive surgery | 46 | 139470 | 15% | 12–18% | 99.80% | 0.01 |
| Gynecological surgery | 18 | 87066 | 6% | 4–8% | 98.78% | 0.00 |
| Hepatobiliary surgery | 22 | 15338 | 12% | 9–14% | 97.29% | 0.00 |
| Pancreatic surgery | 11 | 7067 | 11% | 8–14% | 94.07% | 0.00 |
| CRS and HIPEC | 8 | 2083 | 25% | 11–38% | 98.8% | 0.04 |
| Bariatric surgery | 3 | 2242 | 11% | 4–17% | 95.87% | 0.00 |
| Year of study publication | ||||||
| 2021–2024 | 78 | 412038 | 11% | 8–15% | 98.22% | 0.00 |
| 2011–2020 | 98 | 247500 | 17% | 14–20% | 99.84% | 0.02 |
| 2004–2010 | 4 | 15823 | 18% | 3–33% | 99.81% | 0.02 |
Abbreviations: AKI, acute kidney injury; CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy.
As shown in Table 1, the incidence of AKI varied significantly among different types of abdominal surgery (p < 0.01). Gynecological procedures demonstrated the lowest AKI incidence at 6% (95% CI: 4–8%), which was notably lower than other surgical categories. Pancreatic surgery (11%, 95% CI: 8–14%), bariatric surgery (11%, 95% CI: 4–17%), and hepatobiliary surgery (12%, 95% CI: 9–14%) showed relatively low AKI incidence rates. Digestive surgery exhibited an AKI incidence of 15% (95% CI: 12–18%), while mixed abdominal procedures showed a rate of 19% (95% CI: 12–22%). Notably, CRS and HIPEC demonstrated the highest AKI incidence at 25% (95% CI: 11–38%), but the wide confidence interval suggests limited precision in this estimate. Importantly, our analysis revealed no statistically significant differences in AKI incidence rates across different AKI definition criteria (p = 0.12) or among studies from different publication years (p = 0.23).
Furthermore, we compared the 122 studies that we analyzed between LT and OAAA and found a remarkable difference in the incidence of AKI (p < 0.01). The incidences of AKI in the LT and OAAA groups were 46 and 33%, respectively. Of the 66 studies that reported the number of patients with AKI according to stage, 72% of patients with AKI were in stage 1 or RIFLE-R, 16% of patients with AKI were in stage 2 or RIFLE-I, and 12% of patients with AKI were in stage 3 or RIFLE-F (Supplementary Table S5) [11,17, 18,20,22,34–36,41,43,46,49,50,54,61,66,69,76,80,83,87–91,93,95–97,100,109,110,112,115,118–120,123, 125,126,128,131,133,134,138,143–147,149,150,154–160,175,181,182].
Length of hospital stay
A total of 33 studies reported LOS (Supplementary Table S6) [19,35,37,39,41,44,54,65,69,74, 79,82,83,91,92,97, 100,106,107,109,111,115,117,119,124,128,133,134,138,147,149,156,157]. Three studies were not included in the meta-analysis: two reported the mean length of stay without standard deviation [82,107], and another defined patients without AKI as non-AKI and AKI stage 1 [83]. Meta-analysis results suggested that patients with AKI had longer hospital stays than non-AKI patients (weighted mean difference [WMD], 4.72 days; 95%CI: 3.43–6.02) (Figure 2). The between-study heterogeneity was significant (I2 = 93.24). Additionally, we performed a leave-one-out meta-analysis, which revealed that the results were robust.
Figure 2.
Forest plot of hospital LOS of patients who developed postoperative AKI and those who did not.
Mortality
A total of 32 studies documented the mortality of patients with or without AKI (Supplementary Table S7) [19,35,37,39–41,44,54,65,74,78,82,83,91,92,97,104,106,109–111,115,117,128,133,134,147,149,150,152, 155,156]. Three studies were not included in the meta-analysis: two reported no deaths in non-AKI patients [91,106] and one defined patients without AKI as non-AKI and AKI stage 1 [83]. Meta-analysis of the pooled RR for short-term, long-term, and overall mortality for patients with AKI compared to patients without AKI is 6.46 (95%CI, 4.63–9.00), 6.36 (95%CI, 3.32–12.156), and 6.43 (95%CI, 4.80–98.62) respectively (Figure 3). The I2 values for short-term, long-term, and overall mortalities were 62.17, 92.94, and 82.27%, respectively. A leave-one-out meta-analysis showed that these results were robust.
Figure 3.
Forest plot of the risk ratio of the association between AKI and mortality.
AKI staging, timing, and clinical outcomes
Among the included studies, 9 studies reported mortality outcomes [35,41,54,83,90,92,97,150,155], 4 studies included length of hospital stay data [35,90, 97,155], and 3 studies provided complications data stratified by AKI stages [35,150,155]. Meta-analysis demonstrated a graded association between AKI stage and clinical outcomes. It should be noted that these analyses were conducted as post-hoc analyses, rather than a pre-planned analyses.
Subgroup analysis by AKI stages showed a stepwise increase in mortality association compared to patients without AKI: Stage 1 (RR, 2.74; 95%CI: 1.77–4.24), Stage 2 (RR, 8.01; 95%CI: 3.18–20.18), and Stage 3 (RR, 15.73; 95%CI: 5.52–44.81) (Figure S4). Similarly, analysis by AKI stages demonstrated progressive increases in hospital LOS with increasing AKI severity: compared to patients without AKI: Stage 1 patients stayed 5.03 (95%CI, 3.61–6.46) days longer, Stage 2 patients 11.16 (95%CI, 9.06–13.26) days longer, and Stage 3 patients 14.46 (95%CI, 6.48–22.45) days longer (p < 0.001) (Figure S5).
Three studies reported complications stratified by AKI stages (Table S8). Despite limited data, they consistently demonstrated higher complication rates in patients with more severe AKI stages, with Stage 3 AKI patients having the highest rates of surgical, pulmonary, and cardiovascular complications. Regarding timing, AKI diagnosis varied significantly among included studies, ranging from immediately after surgery to 30 days postoperatively. Three studies provided detailed temporal analysis: early AKI (within 48 h, 7.9%) was primarily associated with intraoperative factors, while late AKI (48 h–7 days, 3.2%) was related to both preoperative comorbidities and intraoperative factors [182]. Studies distinguishing transient versus persistent AKI found that non-transient AKI (persisting beyond postoperative day 1) was associated with higher complication rates and persistent renal dysfunction [178]. In bariatric surgery, early AKI occurred in 71 patients (within 72 h) versus 30 with late-onset AKI (72 h–30 days), the latter primarily due to volume depletion and complications, increasing readmission rates [100].
Factors associated with postoperative AKI
Our meta-analysis identified multiple factors associated with postoperative AKI, but these findings should be interpreted with caution as some of these factors were not adjusted for potential confounding variables.
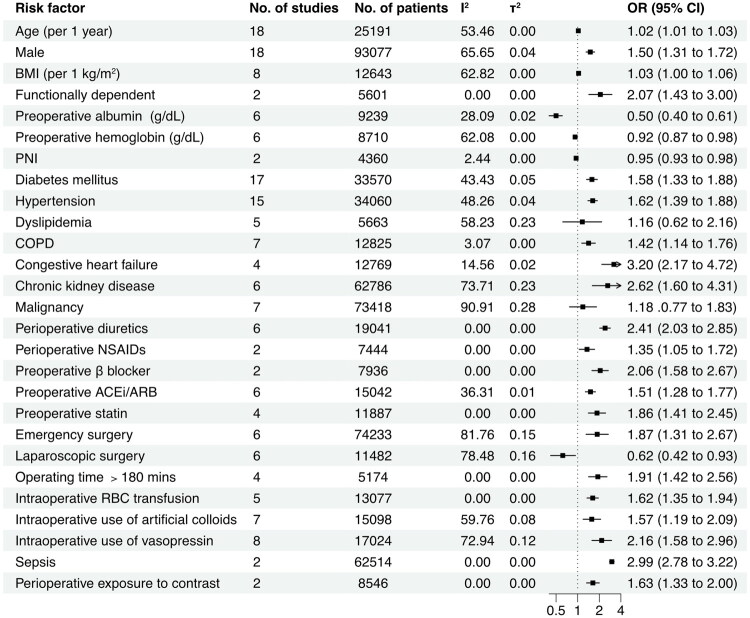
As shown in Figure 4, the pooled analysis of risk factors for AKI after abdominal surgery indicates the following showed positive associations with AKI: age (per 1-year increment) (OR, 1.02; 95% CI: 1.01–1.03; p < 0.01), male (vs. female, OR, 1.50; 95% CI: 1.31–1.72; p < 0.01), BMI (per 1 kg/m2 increment) (OR, 1.03; 95% CI: 1.00–1.06; p = 0.02), diabetes mellitus (OR, 1.58; 95% CI: 1.33–1.88; p < 0.01), congestive heart failure (OR, 3.20; 95% CI: 2.17–4.72; p < 0.01), hypertension (OR, 1.62; 95% CI: 1.39–1.88; p < 0.01), chronic obstructive pulmonary disease (COPD) (OR, 1.42; 95% CI: 1.14–1.76; p < 0.01), CKD (OR,2.62; 95% CI: 1.60–4.31; p < 0.01), sepsis (OR, 2.99; 95% CI: 2.78–3.22; p < 0.01), perioperative exposure to contrast agents (OR, 1.63; 95% CI: 1.33–2.00; p < 0.01), perioperative use of diuretics (OR, 2.41; 95% CI: 2.03–2.85; p < 0.01), perioperative nonsteroidal anti-inflammatory drugs (NSAIDs) (OR, 1.35; 95% CI: 1.05–1.72; p = 0.02), preoperative use of β blockers (OR, 2.06; 95% CI: 1.58–2.67; p < 0.01), preoperative use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEi/ARB) (OR, 1.51; 95% CI: 1.28–1.77; p < 0.01), preoperative use of statins (OR, 1.86; 95% CI: 1.41–2.45; p < 0.01), emergency surgery (OR, 1.87; 95% CI: 1.31–2.67; p < 0.01), intraoperative red blood cell transfusion (OR, 1.62; 95% CI: 1.35–1.94; p < 0.01), intraoperative use of artificial colloids (OR, 1.57; 95% CI: 1.19–2.09; p < 0.01), intraoperative use of vasopressin (OR,2.16; 95% CI: 1.58–2.96; p < 0.01), and surgery duration over 180 min (OR, 1.91; 95% CI: 1.42–2.56; p < 0.01). Hemoglobin (per 1 g/dL increase, OR, 0.92; 95% CI: 0.87–0.98; p = 0.01), albumin (per 1 g/dL increase, OR, 0.50; 95% CI: 0.40–0.61; p < 0.01), prognostic nutritional index (PNI) (OR, 0.95; 95% CI: 0.93–0.98; p < 0.01), and laparoscopic surgery (OR, 0.62; 95% CI: 0.42–0.93; p = 0.02) showed negative associations with AKI postoperative AKI. Dyslipidemia (OR, 1.16; 95% CI: 0.62–2.16; p = 0.65) and malignancy (OR, 1.18; 95% CI: 0.77–1.83; p = 0.65) demonstrated no significant association with postoperative AKI. A detailed summary of all the identified influence factors is provided in Supplementary Table S9.
Figure 4.
Forest plot of the risk factors for postoperative AKI.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NSAID, non-steroidal anti-inflammatory drug; RBC, red blood cell. PNI, prognostic nutritional index, PNI= [10 × serum albumin (g/dL)] + [0.005 × total lymphocyte count (per mm3)].
Effects of perioperative interventions on postoperative AKI
To comprehensively evaluate the effects of perioperative interventions on postoperative AKI, we additionally retrieved RCTs related to perioperative management. It should be noted that these studies included various surgery types (such as abdominal surgery, orthopedic surgery, urological surgery, etc.), which do not fully meet the original inclusion criteria of this study and thus are presented separately as supplementary analyses. A total of 30 RCTs were included, and detailed baseline characteristics and risk of bias are provided in (supplementary file Table S10, Figure S1) [2,27,87,112,184–209].
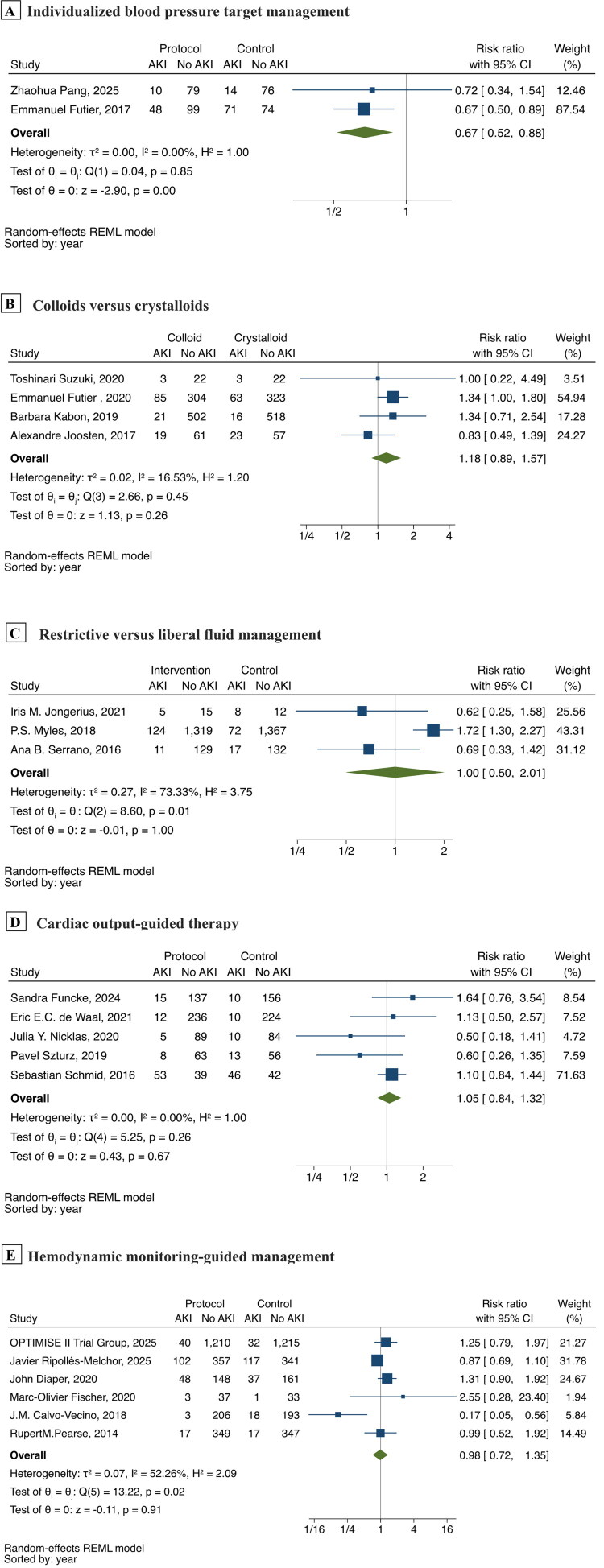
Among these, studies related to perioperative hemodynamic management strategies were eligible for meta-analysis (Figure 5). The results showed that individualized blood pressure target management (n = 471) significantly reduced postoperative AKI incidence (RR,0.67; 95% CI: 0.52–0.88) [185,207]; the POISE-3 trial (n = 7006) compared hypotension-avoidance strategy (MAP ≥ 80mmHg) versus hypertension-avoidance strategy (MAP ≥ 60mmHg) and found no significant difference in AKI incidence (15.1 vs 14.4%) [27]; compared with crystalloids, colloids (n = 2042) did not significantly increase the risk of postoperative AKI (RR, 1.18; 95% CI: 0.89–1.57) [2,193,197,198]; restrictive vs. liberal fluid management (n = 3211) showed no significant difference in AKI incidence (RR, 1.00; 95% CI: 0.50–2.01) [87,192,208]; cardiac output-guided therapy (n = 1308) demonstrated no protective effect against AKI (RR, 1.05; 95% CI: 0.84–1.32) [112,187,189,191,202]; hemodynamic monitoring-guided management (n = 5032) also showed no significant benefit (RR, 0.98; 95% CI: 0.72–1.35) [184,196,201,203, 204,206].
Figure 5.
Meta-analysis of perioperative hemodynamic interventions on postoperative acute kidney injury.
Forest plots showing risk ratios and 95% CI for different perioperative hemodynamic intervention strategies. (A) Individualized blood pressure target management; (B) Colloids versus crystalloids; (C) Restrictive versus liberal fluid management; (D) Cardiac output-guided therapy; (E) Hemodynamic monitoring-guided management.
Additionally, several other perioperative interventions were evaluated in RCTs, but due to the heterogeneity of interventions, these studies could not be quantitatively combined. Regarding perioperative respiratory management, a large RCT found no significant difference in AKI incidence between low tidal volume and conventional tidal volume ventilation [209]. Similarly, other interventions, including individualized positive end-expiratory pressure, open lung strategy, and varied inspired oxygen concentrations, showed no significant impact on postoperative AKI rates compared to standard care [188,190,195]. In terms of fluid management, a study of 90 patients undergoing emergency laparotomy found that isotonic sodium bicarbonate infusion, compared with balanced salt solution, improved perioperative acid-base balance and significantly reduced postoperative AKI incidence (9 vs 24%) [186]. Another study that preoperative intravenous iron supplementation to treat anaemia showed no significant impact on postoperative AKI rates [194]. Other perioperative interventions, such as preoperative focused cardiac ultrasound for individualized anesthesia and perioperative aspirin administration, also did not shown significant differences in their effects on postoperative AKI [199,205].
Discussion
This systematic review summarizes the results of 162 studies conducted on 675361 adult patients who underwent abdominal surgery. The incidence of AKI after abdominal surgery is approximately 16%. Twenty-seven variables influencing the development of AKI were analyzed. The study identified twenty-one risk factors associated with an increased risk of AKI and four protective factors associated with a decreased risk of AKI following abdominal surgery. Moreover, two factors showed no significant association.
Through a meta-analysis, we found a strong association between postoperative AKI and short-term (RR, 6.46; 95%CI 4.63–9.00), long-term (RR, 6.36; 95%CI 3.32–12.16), and overall (RR, 6.43; 95%CI 4.63–9.00) mortalities. Although there was significant heterogeneity, consistent evidence suggests an association between AKI and risk of death. Some reports have proposed a relationship between post-abdominal surgery mortality and AKI. Vaca et al. reported that patients with postoperative AKI experienced increased hospital mortality (adjusted OR, 6.29; 95% CI 2.32–17.08) [19]. Beghdadi et al. reported similar results (adjusted OR, 5.09; 95%CI 1.13–22.8) [58]. This suggests that AKI has important predictive significance for the prognosis of patients after abdominal surgery [6]. Further analysis revealed a graded association between AKI stage and clinical outcomes. Among studies reporting detailed staging data, adverse outcomes increased progressively with AKI severity. The relative risk of mortality was nearly threefold higher in Stage 1 AKI patients compared to those without AKI (RR, 2.74; 95%CI: 1.77–4.24), eightfold higher in Stage 2 (RR, 8.01; 95%CI: 3.18–20.18), and nearly sixteen-fold higher in Stage 3 (RR, 15.73; 95%CI: 5.52–44.81). Hospital length of stay demonstrated a similar trend, with Stage 1, 2, and 3 AKI patients staying 5.03, 11.16, and 14.46 days longer respectively than patients without AKI (p < 0.01). Complications analysis further supported these findings, with three studies consistently showing increasing complication rates with AKI severity, particularly highest rates of surgical, pulmonary, and cardiovascular complications in Stage 3 AKI patients. This gradient relationship further confirms that AKI serves as an important marker of perioperative harm [6]. Notably, among 66 studies reporting AKI staging, 72% of AKI patients were in early stages. Previous research has shown that even minor postoperative increases in serum creatinine are associated with increased mortality and prolonged hospital stay [117], emphasizing the importance of early identification and intervention.
The temporal patterns of AKI demonstrated important clinical implications. While the diagnostic time window varied considerably among studies (immediate postoperative period to 30 days), detailed temporal analysis from three studies showed that early AKI was primarily associated with intraoperative factors, while late AKI was related to both preoperative comorbidities and intraoperative factors. Notably, non-transient AKI (persisting beyond postoperative day 1) was associated with higher complication rates and persistent renal dysfunction compared to very transient AKI (immediate postoperative period only).
Regarding some special cases of AKI, we reported that the incidence of AKI was lower than that of cardiac surgery–associated [210] and sepsis-associated AKI [211]. The incidences of AKI after LT and OAAA were 46 and 33%, respectively. O’Connor et al. suggested that AKI associated with such procedures should be considered in isolation [6]. The incidence of AKI after LT and OAAA procedures and subgroup analyses by year of publication and certain patient characteristics were not described in a pre-specified manner; these analyses can only be considered exploratory. Owing to the wide variation in baseline patient characteristics between studies, we did not conduct subgroup analyses according to whether the study population had CKD. Some reports confirmed the association between preoperative CKD and AKI following abdominal surgery [19,89]. This was confirmed by subsequent analysis of the risk factors for AKI after abdominal surgery.
We systematically screened the included studies to identify factors associated with postoperative AKI. Non-modifiable factors showing associations with postoperative AKI include advanced age [20–22,24, 35,37,50,52–54,74,75,79,88,90,92,94,111], male sex [10,17–20,22,24,37,41,44,53,74,78,90,100,111,116,121], and comorbidities such as diabetes mellitus [17–22,24,35,52,53,69,79,90,91,94,100,111], hypertension [17,19–22,24,41,52,53,74,79,90,94,100,111], COPD [19–21,41,54,74,90], congestive heart failure [20,35, 74,90], CKD [11,19,39,54,78,111], and sepsis [74,78]. Certain medications also show associations with AKI occurrence, including ACEi/ARB [20,44,94,106,109,121], NSAIDs [20,22,85], β-blockers [20,94], statins [20,44, 94,106,109,121], perioperative exposure to contrast [22,41], and diuretic use [17,20,41,53,94,111]. Preoperative nutritional status show associations with postoperative AKI. Higher preoperative albumin [20,24,44,53,109,117], hemoglobin [20,37,44,53,79,117], and PNI [24,53] levels show negative associations with AKI occurrence, whereas functional dependency [17,74] show positive associations.
Surgery-related factors are also documented in the literature. Emergency surgery poses higher AKI risk compared to elective surgery [20,35,37,78,90,94]. Minimally invasive laparoscopic surgery is associated with lower AKI risk compared to open abdominal surgery [19,37,53,88,116,121]. However, in patients with sepsis, the lower AKI risk associated with laparoscopic surgery should not be the sole determinant of the surgical approach selection. These factors should serve as early warning signs to enable prompt intervention. Our study also identified associations between AKI and intraoperative blood transfusions [11,20,24,41,53] and surgery duration (>180 min) [11,18,69,92].
Proper hemodynamic management is essential for optimal renal perfusion [119]. While our observational studies found significant associations between synthetic colloids use and increased postoperative AKI risk [17,20,22,24,37,53,111], and identified vasopressors as potential independent risk factors [11,20, 22,37,41,75,82,111], subsequent randomized controlled trials have provided higher-level evidence. Meta-analyses showed that individualized blood pressure target management significantly reduced postoperative AKI incidence (RR, 0.67; 95% CI: 0.52–0.88), though the POISE-3 trial found that a fixed hypotension-avoidance strategy (MAP ≥ 80 versus ≥60mmHg) did not show similar benefits. Compared with crystalloids, colloids did not significantly increase the risk of postoperative AKI (RR, 1.18; 95% CI: 0.89–1.57).
Fluid management strategies remain controversial. Observational studies have been limited by heterogeneity in fluid management variables, with studies examining different parameters such as total fluid input and fluid balance [19,21,24,53,85], making systematic comparison and meta-analysis challenging. Mahmooth et al. found no significant difference between nonrestrictive, restrictive, and ultra restrictive fluid protocols [11]. Meta-analysis further confirmed this finding, showing no significant difference in AKI incidence across fluid volume management strategies (RR, 1.00; 95% CI 0.50–2.01). Additionally, neither cardiac output-guided therapy (RR, 1.05; 95% CI: 0.84–1.32) nor advanced hemodynamic monitoring-guided perioperative management (RR, 0.98; 95% CI: 0.72–1.35) demonstrated protective effects against AKI.
Studies of other perioperative interventions have provided important insights. Regarding respiratory management, low tidal volume ventilation (6 mL/kg) compared with conventional tidal volume (10 mL/kg) did not significantly reduce postoperative incidence of AKI. Interventions such as individualized PEEP, open lung strategy, and different inspired oxygen concentrations showed no significant differences in postoperative incidence of AKI. However, in emergency laparotomy patients, isotonic sodium bicarbonate infusion compared with balanced salt solution significantly reduced postoperative AKI incidence. While preoperative rehabilitation exercise programs improved the Comprehensive Complications Index and quality of life in elderly patients, they showed no significant effect on postoperative AKI incidence.
These findings suggest that although observational studies have identified multiple potential risk factors, many perioperative interventions have not shown expected benefits in randomized controlled trials. This discrepancy may reflect the complex pathophysiology of postoperative AKI and the limitations of single interventions in prevention. Therefore, proper risk stratification and individualized management are paramount for optimizing the care of patients at risk for serious postoperative complication [17]. Future research should focus on multimodal intervention strategies while considering patient-specific characteristics and risk factors.
Based on these identified risk factors and our comprehensive analysis, we propose the following clinical implications to enhance the practical value of our findings. Drawing from the risk and protective factors identified in this study, clinicians can implement stratified management strategies: Perioperative physicians can conduct preoperative risk assessments and implement preventive measures for high-risk patients (such as elderly patients, those with chronic kidney disease, hypertension, or diabetes); anesthesiologists should focus on restoring circulating volume, maintaining circulating pressure, and ensuring optimal oxygen delivery; surgeons may consider minimally invasive techniques for high-risk patients to reduce inflammatory response. For identified high-risk patients, postoperative care should include increased frequency of urine output monitoring and renal function assessment to enable early detection and intervention of potential AKI.
Our study results provide foundational data for developing AKI risk scoring systems. Future research can integrate predictive risk factors to construct risk scoring systems; validate the accuracy and clinical utility of scoring tools through large-sample prospective study; and develop digital decision support tools to help clinicians quickly assess patients’ AKI risk and formulate corresponding preventive measures. Such risk stratification tools will help optimize medical resource allocation, enabling closer monitoring and early intervention for high-risk patients.
Strengths and limitations
This study has several strengths. To our knowledge, this is the first systematic review and meta-analysis to evaluate the risk factors of AKI after abdominal surgery. This study is the most extensive and includes the largest systematic review and meta-analysis to assess the incidence of AKI. Subgroup analyses were conducted to explore sources of heterogeneity. The correlation with clinical practice is the ultimate strength of this study, and the results could lead to the early identification of patients at high risk of AKI and controllable risk factors associated with postoperative AKI by clinical practitioners.
This study has three limitations. First, although our research subjects were explicitly restricted to patients undergoing abdominal surgery, with a clear consensus definition of postoperative AKI, and most included studies had a low to moderate risk of bias, significant heterogeneity was observed in the incidence of AKI, adverse outcomes associated with AKI, and risk factors for AKI. This high degree of heterogeneity warrants further exploration as it may affect the generalizability of our findings. The heterogeneity primarily stems from multiple differentiating factors among the included studies. Abdominal surgery encompasses various procedures of different complexity levels, ranging from minimally invasive laparoscopic cholecystectomy to complex pancreaticoduodenectomy. Each procedure type varies in terms of surgical trauma, operative duration, and physiological impact. Our subgroup analysis revealed significant differences in AKI incidence rates across different surgical types (p < 0.01), potentially reflecting variations in hemodynamic changes, inflammatory responses, and organ perfusion status specific to each surgical setting. Moreover, although all studies employed standardized AKI definition criteria (RIFLE, AKIN, or KDIGO), practical application may have varied, including differences in serum creatinine measurement timing, accuracy of urine output monitoring, and methods for determining baseline renal function. The publication timeline span of included studies also reflects the evolution of perioperative management strategies, encompassing changes in fluid management approaches, advances in anesthetic techniques, and improvements in perioperative monitoring. Therefore, our reported 16% incidence rate of postoperative AKI should be interpreted as an overall estimate based on available evidence, rather than a universal single value applicable to all abdominal surgery patients. Clinicians should consider their specific practice environment and individual patient characteristics when assessing postoperative AKI risk. Second, although a comprehensive search of libraries was conducted, potential studies were excluded. Third, the proportion of studies reporting detailed AKI staging data was relatively limited in our research, which restricted our comprehensive assessment of the impact of AKI at different severity levels. We recommend that future prospective studies adopt standardized AKI staging reporting formats and document detailed time windows of AKI occurrence.
Conclusions
AKI remains a common and important complication following abdominal surgery, with an overall incidence of 16%. This incidence varies significantly by surgical procedure but remains stable across different time periods and AKI diagnostic criteria. This study establishes a clear stage-dependent relationship between AKI severity and clinical outcomes, affecting both mortality risk and length of hospital stay. While individualized blood pressure management shows promise in reducing AKI risk, other perioperative interventions have not demonstrated significant protective effects. These findings emphasize the need for focused attention on blood pressure management within a broader, multimodal approach to AKI prevention, suggesting that future research should explore how to optimize and combine various preventive strategies.
Supplementary Material
Funding Statement
This work was supported by the Chongqing Science and Technology Bureau (CSTB2024NSCQ-MSX1221).
Disclosure statement
No conflicts of interest to declare.
Data availability statement
The data that support the findings of this systematic review and meta-analysis are available from the corresponding author upon reasonable request. The data consist of the extracted information from published studies included in our analysis. All primary sources are cited within the manuscript and listed in the references.
References
- 1.Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/s0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 2.Futier E, Garot M, Godet T, et al. Effect of hydroxyethyl starch vs saline for volume replacement therapy on death or postoperative complications among high-risk patients undergoing major abdominal surgery. JAMA. 2020;323(3):225–236. doi: 10.1001/jama.2019.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261(6):1207–1214. PubMed Central PMCID: PMC4247993. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selby NM, Taal MW.. Long-term outcomes after AKI-a major unmet clinical need. Kidney Int. 2019;95(1):21–23. doi: 10.1016/j.kint.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 5.See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–172. doi: 10.1016/j.kint.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor ME, Kirwan CJ, Pearse RM, et al. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med. 2016;42(4):521–530. doi: 10.1007/s00134-015-4157-7. [DOI] [PubMed] [Google Scholar]
- 7.Slagelse C, Gammelager H, Iversen LH, et al. Renin-angiotensin system blocker use and the risk of acute kidney injury after colorectal cancer surgery: a population-based cohort study. BMJ Open. 2019;9(11):e032964. PubMed Central PMCID: PMC6887015. doi: 10.1136/bmjopen-2019-032964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gameiro J, Duarte I, Marques F, et al. Transient and persistent AKI and outcomes in patients undergoing major abdominal surgery. Nephron. 2020;144(5):236–244. doi: 10.1159/000506397. [DOI] [PubMed] [Google Scholar]
- 9.Joliat GR, Labgaa I, Demartines N, et al. Acute kidney injury after liver surgery: does postoperative urine output correlate with postoperative serum creatinine? HPB (Oxford). 2020;22(1):144–150. doi: 10.1016/j.hpb.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Shim J-W, Ro H, Lee CS, et al. Male patients may be more vulnerable to acute kidney injury after colorectal surgery in an enhanced recovery program: a propensity score matching analysis. World J Surg. 2021;45(6):1642–1651. doi: 10.1007/s00268-021-06041-3. [DOI] [PubMed] [Google Scholar]
- 11.Mahmooth Z, Jajja MR, Maxwell D, et al. Ultrarestrictive intraoperative intravenous fluids during pancreatoduodenectomy is not associated with an increase in post-operative acute kidney injury. Am J Surg. 2020;220(2):264–269. doi: 10.1016/j.amjsurg.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Brienza N, Malcangi V, Dalfino L, et al. A comparison between fenoldopam and low-dose dopamine in early renal dysfunction of critically ill patients. Crit Care Med. 2006;34(3):707–714. doi: 10.1097/01.CCM.0000201884.08872.A2. [DOI] [PubMed] [Google Scholar]
- 13.Demarchi ACdS, de Almeida CTP, Ponce D, et al. Intra-abdominal pressure as a predictor of acute kidney injury in postoperative abdominal surgery. Ren Fail. 2014;36(4):557–561.56177. doi: 10.3109/0886022X.2013.876353. [DOI] [PubMed] [Google Scholar]
- 14.Poston JT, Koyner JL.. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. PubMed Central PMCID: PMC6890472. doi: 10.1136/bmj.k4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luque Y, Louis K, Jouanneau C, et al. Vancomycin-associated cast nephropathy. J Am Soc Nephrol. 2017;28(6):1723–1728. PubMed Central PMCID: PMC5461798. doi: 10.1681/ASN.2016080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehran R, Dangas GD, Weisbord SD.. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146–2155. doi: 10.1056/NEJMra1805256. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Li G, Mohan S, et al. Intraoperative data enhance the detection of high-risk acute kidney injury patients when added to a baseline prediction model. Anesth Analg. 2021;132(2):430–441. PubMed Central PMCID: PMC7855510. doi: 10.1213/ANE.0000000000005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurreck A, Gronau F, Alberto Vilchez ME, et al. Sodium thiosulfate reduces acute kidney injury in patients undergoing cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with cisplatin: a single-center observational study. Ann Surg Oncol. 2022;29(1):152–162. PubMed Central PMCID: PMC8677645. doi: 10.1245/s10434-021-10508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zorrilla-Vaca A, Mena GE, Ripolles-Melchor J, et al. Risk factors for acute kidney injury in an enhanced recovery pathway for colorectal surgery. Surg Today. 2021;51(4):537–544. doi: 10.1007/s00595-020-02107-2. [DOI] [PubMed] [Google Scholar]
- 20.Kim BR, Yoon S, Song GY, et al. The impact of total intravenous anesthesia versus inhalation anesthesia on acute kidney injury after major abdominal surgery: a propensity score analysis. J Anesth. 2021;35(1):112–121. doi: 10.1007/s00540-020-02882-9. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Hu J, Xiao H, et al. Effect of continuous intraoperative infusion of methoxamine on renal function in elderly patients undergoing gastrointestinal tumor surgery: a randomized controlled trial. BMC Anesthesiol. 2020;20(1):148. PubMed Central PMCID: PMC7293117. doi: 10.1186/s12871-020-01064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh TK, Oh AY, Ryu JH, et al. Retrospective analysis of the association between intraoperative magnesium sulfate infusion and postoperative acute kidney injury after major laparoscopic abdominal surgery. Sci Rep. 2019;9(1):2833. PubMed Central PMCID: PMC6391431. doi: 10.1038/s41598-019-39106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean KA, Ahmed WUR, Akhbari M, STARSurg Collaborative ., et al. Perioperative intravenous contrast administration and the incidence of acute kidney injury after major gastrointestinal surgery: prospective, multicentre cohort study. Br J Surg. 2020;107(8):1023–1032. doi: 10.1002/bjs.11453. [DOI] [PubMed] [Google Scholar]
- 24.Sim JH, Jun I-G, Moon Y-J, et al. Association of preoperative prognostic nutritional index and postoperative acute kidney injury in patients who underwent hepatectomy for hepatocellular carcinoma. J Pers Med. 2021;11(5):428. PubMed Central PMCID: PMC8157861. doi: 10.3390/jpm11050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65. doi: 10.1097/aln.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 26.Turan A, Rivas E, Devereaux PJ, et al. Relative contributions of anaemia and hypotension to myocardial infarction and renal injury: Post hoc analysis of the POISE-2 trial. Eur J Anaesthesiol. 2023;40(5):365–371. doi: 10.1097/eja.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 27.Investigators P-T, Study G.. A sub-study of the POISE-3 randomized trial examined effects of a perioperative hypotension-avoidance strategy versus a hypertension-avoidance strategy on the risk of acute kidney injury. Kidney Int. 2025;107(1):155–168. doi: 10.1016/j.kint.2024.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Section 2: AKI definition. Kidney Int Suppl (2011). 2012;2(1):19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellomo R, Ronco C, Kellum JA, Acute Dialysis Quality Initiative w ., et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. PubMed Central PMCID: PMC522841. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. PubMed Central PMCID: PMC2206446. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. PubMed Central PMCID: PMC8005924. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molinari L, Sakhuja A, Kellum JA.. Perioperative renoprotection: general mechanisms and treatment approaches. Anesth Analg. 2020;131(6):1679–1692. PubMed Central PMCID: PMC8366579. doi: 10.1213/ANE.0000000000005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Morgan RL, Rooney AA, et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int. 2024;186:108602. PubMed Central PMCID: PMC11098530. doi: 10.1016/j.envint.2024.108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis. 2016;67(6):872–880. PubMed Central PMCID: PMC4775458. doi: 10.1053/j.ajkd.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaught AJ, Ozrazgat-Baslanti T, Javed A, et al. Acute kidney injury in major gynaecological surgery: an observational study. BJOG. 2015;122(10):1340–1348. PubMed Central PMCID: PMC4334755. doi: 10.1111/1471-0528.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao CT, Lin YF, Tsai HB, et al. Acute kidney injury network staging in geriatric postoperative acute kidney injury patients: shortcomings and improvements. J Am Coll Surg. 2013;217(2):240–250. doi: 10.1016/j.jamcollsurg.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira C, Rosa R, Rodrigues N, et al. Acute kidney injury after major abdominal surgery: a retrospective cohort analysis. Crit Care Res Pract. 2014;2014:132175. PubMed Central PMCID: PMC3955689. doi: 10.1155/2014/132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun LY, Wijeysundera DN, Tait GA, et al. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 39.Kambakamba P, Slankamenac K, Tschuor C, et al. Epidural analgesia and perioperative kidney function after major liver resection. Br J Surg. 2015;102(7):805–812. doi: 10.1002/bjs.9810. [DOI] [PubMed] [Google Scholar]
- 40.Causey MW, Maykel JA, Hatch Q, et al. Identifying risk factors for renal failure and myocardial infarction following colorectal surgery. J Surg Res. 2011;170(1):32–37. doi: 10.1016/j.jss.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Kim CS, Oak CY, Kim HY, et al. Incidence, predictive factors, and clinical outcomes of acute kidney injury after gastric surgery for gastric cancer. PLoS One. 2013;8(12):e82289. PubMed Central PMCID: PMC3857284. doi: 10.1371/journal.pone.0082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biteker M, Dayan A, Tekkeşin Aİ, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg. 2014;207(1):53–59. doi: 10.1016/j.amjsurg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249(5):851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 44.Tomozawa A, Ishikawa S, Shiota N, et al. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth. 2015;62(7):753–761. doi: 10.1007/s12630-015-0397-9. [DOI] [PubMed] [Google Scholar]
- 45.Brunelli SM, Waikar SS, Bateman BT, et al. Preoperative statin use and postoperative acute kidney injury. Am J Med. 2012;125(12):1195–1204 e3. PubMed Central PMCID: PMC3597342. doi: 10.1016/j.amjmed.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correa-Gallego C, Berman A, Denis SC, et al. Renal function after low central venous pressure-assisted liver resection: assessment of 2116 cases. HPB (Oxford). 2015;17(3):258–264. PubMed Central PMCID: PMC4333788. doi: 10.1111/hpb.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol. 2014;25(11):2625–2632. PubMed Central PMCID: PMC4214537. doi: 10.1681/ASN.2014010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coca SG, King JT, Jr., Rosenthal RA, et al. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78(9):926–933. PubMed Central PMCID: PMC3082138. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armstrong T, Welsh FK, Wells J, et al. The impact of pre-operative serum creatinine on short-term outcomes after liver resection. HPB (Oxford). 2009;11(8):622–628. PubMed Central PMCID: PMC2799614. doi: 10.1111/j.1477-2574.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho E, Kim SC, Kim MG, et al. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: a prospective observational study. BMC Nephrol. 2014;15(1):169. PubMed Central PMCID: PMC4221681. doi: 10.1186/1471-2369-15-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao B-C, Zhuang P-P, Lei S-H, et al. Pre-operative N-terminal pro-B-type natriuretic peptide for prediction of acute kidney injury after noncardiac surgery: a retrospective cohort study. Eur J Anaesthesiol. 2021;38(6):591–599. doi: 10.1097/EJA.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Hu S, Li S, et al. Systemic immune-inflammation index predicts postoperative acute kidney injury in hepatocellular carcinoma patients after hepatectomy. Medicine (Baltimore). 2021;100(14):e25335. PubMed Central PMCID: PMC8036044. doi: 10.1097/MD.0000000000025335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sim JH, Bang JY, Kim SH, et al. Association of preoperative prognostic nutritional index and postoperative acute kidney injury in patients with colorectal cancer surgery. Nutrients. 2021;13(5):1604. PubMed Central PMCID: PMC8170895. doi: 10.3390/nu13051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inácio R, Gameiro J, Amaro S, et al. Intraoperative oliguria does not predict postoperative acute kidney injury in major abdominal surgery: a cohort analysis. J Bras Nefrol. 2021;43(1):9–19. PubMed Central PMCID: PMC8061965. doi: 10.1590/2175-8239-JBN-2019-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y, Yang HY, Zhang HL, et al. High-density lipoprotein cholesterol concentration and acute kidney injury after noncardiac surgery. BMC Nephrol. 2020;21(1):149. PubMed Central PMCID: PMC7183648. doi: 10.1186/s12882-020-01808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trongtrakul K, Patumanond J, Kongsayreepong S, et al. Acute kidney injury risk prediction score for critically-ill surgical patients. BMC Anesthesiol. 2020;20(1):140. PubMed Central PMCID: PMC7271390. doi: 10.1186/s12871-020-01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimoto M, Murashima M, Kokubu M, et al. Positive association between intra-operative fluid balance and post-operative acute kidney injury in non-cardiac surgery: the NARA-AKI cohort study. J Nephrol. 2020;33(3):561–568. doi: 10.1007/s40620-019-00688-x. [DOI] [PubMed] [Google Scholar]
- 58.Beghdadi N, Reitano E, Cochennec F, et al. Predictors of mortality following emergency open colectomy for ischemic colitis: a single-center experience. World J Emerg Surg. 2020;15(1):40. PubMed Central PMCID: PMC7325045. doi: 10.1186/s13017-020-00321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Argalious MY, Mao G, Davison RK, et al. Association of intraoperative tidal volumes and acute kidney injury after noncardiac surgery. Anesth Analg. 2020;130(4):925–932. doi: 10.1213/ANE.0000000000004254. [DOI] [PubMed] [Google Scholar]
- 60.Murashima M, Nishimoto M, Kokubu M, et al. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci Rep. 2019;9(1):20260. PubMed Central PMCID: PMC6937243. doi: 10.1038/s41598-019-56615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaudery H, MacDonald N, Ahmad T, et al. Acute kidney injury and risk of death after elective surgery: prospective analysis of data from an international cohort study. Anesth Analg. 2019;128(5):1022–1029. doi: 10.1213/ANE.0000000000003923. [DOI] [PubMed] [Google Scholar]
- 62.Wu Q, Yang H, Bo H, et al. Predictive role of estimated glomerular filtration rate prior to surgery in postsurgical acute kidney injury among very elderly patients: a retrospective cohort study. Ren Fail. 2019;41(1):866–874. PubMed Central PMCID: PMC6758700. doi: 10.1080/0886022X.2019.1662440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S, Wang S, Priyanka P, et al. Acute kidney injury in critically ill patients after noncardiac major surgery: early versus late onset. Crit Care Med. 2019;47(6):e437–e44. PubMed Central PMCID: PMC6522312. doi: 10.1097/CCM.0000000000003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li N, Qiao H, Guo J-F, et al. Preoperative hypoalbuminemia was associated with acute kidney injury in high-risk patients following non-cardiac surgery: a retrospective cohort study. BMC Anesthesiol. 2019;19(1):171. PubMed Central PMCID: PMC6719349. doi: 10.1186/s12871-019-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kopitkó C, Medve L, Gondos T.. The value of combined hemodynamic, respiratory and intra-abdominal pressure monitoring in predicting acute kidney injury after major intraabdominal surgeries. Ren Fail. 2019;41(1):150–158. PubMed Central PMCID: PMC6442204. doi: 10.1080/0886022X.2019.1587467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koerner CP, Lopez-Aguiar AG, Zaidi M, et al. Caution: increased acute kidney injury in enhanced recovery after surgery (ERAS) protocols. Am Surg. 2019;85(2):156–161. doi: 10.1177/000313481908500221. [DOI] [PubMed] [Google Scholar]
- 67.Kee YK, Kim H, Jhee JH, et al. Incidence of and risk factors for delayed acute kidney injury in patients undergoing colorectal surgery. Am J Surg. 2019;218(5):907–912. doi: 10.1016/j.amjsurg.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 68.Iyigun M, Aykut G, Tosun M, et al. Perioperative risk factors of acute kidney injury after non-cardiac surgery: a multicenter, prospective, observational study in patients with low grade American Society of Anesthesiologists physical status. Am J Surg. 2019;218(3):457–461. doi: 10.1016/j.amjsurg.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 69.Grass F, Lovely JK, Crippa J, et al. Early acute kidney injury within an established enhanced recovery pathway: uncommon and transitory. World J Surg. 2019;43(5):1207–1215. doi: 10.1007/s00268-019-04923-1. [DOI] [PubMed] [Google Scholar]
- 70.Dedinská I, Mikolajčík P, Skálová P, et al. Acute kidney injury after liver resection in elderly patients. BMC Nephrol. 2019;20(1):272. PubMed Central PMCID: PMC6639960. doi: 10.1186/s12882-019-1449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonavia A, Javaherian M, Skojec AJ, et al. Angiotensin axis blockade, acute kidney injury, and perioperative morbidity in patients undergoing colorectal surgery: A retrospective cohort study. Medicine (Baltimore). 2019;98(33):e16872. PubMed Central PMCID: PMC6831354. doi: 10.1097/MD.0000000000016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vernooij LM, van Klei WA, Machina M, et al. Different methods of modelling intraoperative hypotension and their association with postoperative complications in patients undergoing non-cardiac surgery. Br J Anaesth. 2018;120(5):1080–1089. doi: 10.1016/j.bja.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 73.Pourafkari L, Arora P, Porhomayon J, et al. Acute kidney injury after non-cardiovascular surgery: risk factors and impact on development of chronic kidney disease and long-term mortality. Curr Med Res Opin. 2018;34(10):1829–1837. doi: 10.1080/03007995.2018.1459527. [DOI] [PubMed] [Google Scholar]
- 74.Hassinger TE, Mehaffey JH, Mullen MG, et al. Ureteral stents increase risk of postoperative acute kidney injury following colorectal surgery. Surg Endosc. 2018;32(7):3342–3348. doi: 10.1007/s00464-018-6054-y. [DOI] [PubMed] [Google Scholar]
- 75.Ham SY, Kim EJ, Kim TH, et al. Comparison of perioperative renal function between epidural and intravenous patient-controlled analgesia after living-donor hepatectomy: a retrospective study. Transplant Proc. 2018;50(5):1365–1371. doi: 10.1016/j.transproceed.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Garnier J, Faucher M, Marchese U, et al. Severe acute kidney injury following major liver resection without portal clamping: incidence, risk factors, and impact on short-term outcomes. HPB (Oxford). 2018;20(9):865–871. doi: 10.1016/j.hpb.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 77.Cata JP, Zavala AM, Van Meter A, et al. Identification of risk factors associated with postoperative acute kidney injury after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a retrospective study. Int J Hyperthermia. 2018;34(5):538–544. doi: 10.1080/02656736.2017.1368096. [DOI] [PubMed] [Google Scholar]
- 78.Briggs A, Havens JM, Salim A, et al. Acute kidney injury predicts mortality in emergency general surgery patients. Am J Surg. 2018;216(3):420–426. doi: 10.1016/j.amjsurg.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Zhou X, Wang L, Wang G, et al. A new plasma biomarker enhance the clinical prediction of postoperative acute kidney injury in patients with hepatocellular carcinoma. Clin Chim Acta. 2017;475:128–136. doi: 10.1016/j.cca.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Wu X, Jiang Z, Ying J, et al. Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients: a randomized study: optimal blood pressure reduces acute kidney injury. J Clin Anesth. 2017;43:77–83. doi: 10.1016/j.jclinane.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Toyonaga Y, Asayama K, Maehara Y.. Impact of systemic inflammatory response syndrome and surgical Apgar score on post-operative acute kidney injury. Acta Anaesthesiol Scand. 2017;61(10):1253–1261. doi: 10.1111/aas.12965. [DOI] [PubMed] [Google Scholar]
- 82.Mizota T, Yamamoto Y, Hamada M, et al. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br J Anaesth. 2017;119(6):1127–1134. doi: 10.1093/bja/aex255. [DOI] [PubMed] [Google Scholar]
- 83.Loftus TJ, Bihorac A, Ozrazgat-Baslanti T, et al. Acute kidney injury following exploratory laparotomy and temporary abdominal closure. Shock. 2017;48(1):5–10. PubMed Central PMCID: PMC5468485. doi: 10.1097/SHK.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grass F, Slieker J, Frauche P, et al. Postoperative urinary retention in colorectal surgery within an enhanced recovery pathway. J Surg Res. 2017;207:70–76. doi: 10.1016/j.jss.2016.08.089. [DOI] [PubMed] [Google Scholar]
- 85.Abrahamsson A, Oras J, Snygg J, et al. Perioperative COX-2 inhibitors may increase the risk of post-operative acute kidney injury. Acta Anaesthesiol Scand. 2017;61(7):714–721. doi: 10.1111/aas.12912. [DOI] [PubMed] [Google Scholar]
- 86.Wu HC, Lee LC, Wang WJ.. Incidence and mortality of postoperative acute kidney injury in non-dialysis patients: comparison between the AKIN and KDIGO criteria. Ren Fail. 2016;38(2):330–339. doi: 10.3109/0886022X.2015.1128790. [DOI] [PubMed] [Google Scholar]
- 87.Serrano AB, Candela-Toha AM, Zamora J, et al. Preoperative hydration with 0.9% normal saline to prevent acute kidney injury after major elective open abdominal surgery: A randomised controlled trial. Eur J Anaesthesiol. 2016;33(6):436–443. doi: 10.1097/EJA.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 88.Romagnoli S, Zagli G, Tuccinardi G, et al. Postoperative acute kidney injury in high-risk patients undergoing major abdominal surgery. J Crit Care. 2016;35:120–125. doi: 10.1016/j.jcrc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 89.Quan S, Pannu N, Wilson T, et al. Prognostic implications of adding urine output to serum creatinine measurements for staging of acute kidney injury after major surgery: a cohort study. Nephrol Dial Transplant. 2016;31(12):2049–2056. doi: 10.1093/ndt/gfw374. [DOI] [PubMed] [Google Scholar]
- 90.Long TE, Helgason D, Helgadottir S, et al. Acute kidney injury after abdominal surgery: incidence, risk factors, and outcome. Anesth Analg. 2016;122(6):1912–1920. doi: 10.1213/ANE.0000000000001323. [DOI] [PubMed] [Google Scholar]
- 91.Lim SY, Lee JY, Yang JH, et al. Predictive factors of acute kidney injury in patients undergoing rectal surgery. Kidney Res Clin Pract. 2016;35(3):160–164. PubMed Central PMCID: PMC5025479. doi: 10.1016/j.krcp.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim C, Audureau E, Salloum C, et al. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford). 2016;18(6):540–548. PubMed Central PMCID: PMC4913133. doi: 10.1016/j.hpb.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim S-K, Choi S-S, Sim J-H, et al. Effect of hydroxyethyl starch on acute kidney injury after living donor hepatectomy. Transplant Proc. 2016;48(1):102–106. doi: 10.1016/j.transproceed.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 94.Bang JY, Lee J, Oh J, et al. The Influence of propofol and sevoflurane on acute kidney injury after colorectal surgery: a retrospective cohort study. Anesth Analg. 2016;123(2):363–370. doi: 10.1213/ANE.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 95.Arjona-Sánchez A, Cadenas-Febres A, Cabrera-Bermon J, et al. Assessment of RIFLE and AKIN criteria to define acute renal dysfunction for HIPEC procedures for ovarian and non ovarian peritoneal malignances. Eur J Surg Oncol. 2016;42(6):869–876. doi: 10.1016/j.ejso.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 96.Wu HC, Wang WJ, Chen YW, et al. The association between the duration of postoperative acute kidney injury and in-hospital mortality in critically ill patients after non-cardiac surgery: an observational cohort study. Ren Fail. 2015;37(6):985–993. doi: 10.3109/0886022X.2015.1044755. [DOI] [PubMed] [Google Scholar]
- 97.Morgan DJ, Ho KM.. Acute kidney injury in bariatric surgery patients requiring intensive care admission: a state-wide, multicenter, cohort study. Surg Obes Relat Dis. 2015;11(6):1300–1306. doi: 10.1016/j.soard.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 98.McIlroy DR, Chan MTV, Wallace SK, et al. Is preoperative endothelial dysfunction a potentially modifiable risk factor for renal injury associated with noncardiac surgery? J Cardiothorac Vasc Anesth. 2015;29(5):1220–1228. doi: 10.1053/j.jvca.2015.05.116. [DOI] [PubMed] [Google Scholar]
- 99.Li S, An YZ, Ren JY, et al. Myocardial injury after surgery is a risk factor for weaning failure from mechanical ventilation in critical patients undergoing major abdominal surgery. PLoS One. 2014;9(11):e113410. PubMed Central PMCID: PMC4237423. doi: 10.1371/journal.pone.0113410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weingarten TN, Gurrieri C, McCaffrey JM, et al. Acute kidney injury following bariatric surgery. Obes Surg. 2013;23(1):64–70. doi: 10.1007/s11695-012-0766-1. [DOI] [PubMed] [Google Scholar]
- 101.Slankamenac K, Beck-Schimmer B, Breitenstein S, et al. Novel prediction score including pre- and intraoperative parameters best predicts acute kidney injury after liver surgery. World J Surg. 2013;37(11):2618–2628. doi: 10.1007/s00268-013-2159-6. [DOI] [PubMed] [Google Scholar]
- 102.Wu V-C, Huang T-M, Lai C-F, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011;80(11):1222–1230. doi: 10.1038/ki.2011.259. [DOI] [PubMed] [Google Scholar]
- 103.Kurian A, Suryadevara S, Ramaraju D, et al. In-hospital and 6-month mortality rates after open elective vs open emergent colectomy in patients older than 80 years. Dis Colon Rectum. 2011;54(4):467–471. doi: 10.1007/DCR.0b013e3182060904. [DOI] [PubMed] [Google Scholar]
- 104.Biagioni E, Cavazzuti I, Busani S, et al. Acute renal failure and renal replacement therapy in the postoperative period of orthotopic liver transplant patients versus nonelective abdominal surgery patients. Transplant Proc. 2011;43(4):1145–1147. doi: 10.1016/j.transproceed.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 105.Arhinful E, Jenkins D, Schiller HJ, et al. Outcomes of damage control laparotomy with open abdomen management in the octogenarian population. J Trauma. 2011;70(3):616–621. doi: 10.1097/TA.0b013e31820d19ed. [DOI] [PubMed] [Google Scholar]
- 106.Thakar CV, Kharat V, Blanck S, et al. Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol. 2007;2(3):426–430. doi: 10.2215/CJN.03961106. [DOI] [PubMed] [Google Scholar]
- 107.Marcotte JH, Patel K, Desai R, et al. Acute kidney injury following implementation of an enhanced recovery after surgery (ERAS) protocol in colorectal surgery. Int J Colorectal Dis. 2018;33(9):1259–1267. doi: 10.1007/s00384-018-3084-9. [DOI] [PubMed] [Google Scholar]
- 108.Wiener JGD, Goss L, Wahl TS, et al. The association of enhanced recovery pathway and acute kidney injury in patients undergoing colorectal surgery. Dis Colon Rectum. 2020;63(2):233–241. doi: 10.1097/DCR.0000000000001528. [DOI] [PubMed] [Google Scholar]
- 109.Lee E-H, Kim HR, Baek S-H, et al. Risk factors of postoperative acute kidney injury in patients undergoing esophageal cancer surgery. J Cardiothorac Vasc Anesth. 2014;28(4):936–942. doi: 10.1053/j.jvca.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Jiang L, Wang B, et al. Epidemiological characteristics of and risk factors for patients with postoperative acute kidney injury: a multicenter prospective study in 30 Chinese intensive care units. Int Urol Nephrol. 2018;50(7):1319–1328. doi: 10.1007/s11255-018-1828-7. [DOI] [PubMed] [Google Scholar]
- 111.Goren O, Levy A, Cattan A, et al. Acute kidney injury in pancreatic surgery; association with urine output and intraoperative fluid administration. Am J Surg. 2017;214(2):246–250. doi: 10.1016/j.amjsurg.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 112.Schmid S, Kapfer B, Heim M, et al. Algorithm-guided goal-directed haemodynamic therapy does not improve renal function after major abdominal surgery compared to good standard clinical care: a prospective randomised trial. Crit Care. 2016;20(1):50. PubMed Central PMCID: PMC4782303. doi: 10.1186/s13054-016-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Trongtrakul K, Sawawiboon C, Wang AY, et al. Acute kidney injury in critically ill surgical patients: epidemiology, risk factors and outcomes. Nephrology (Carlton). 2019;24(1):39–46. doi: 10.1111/nep.13192. [DOI] [PubMed] [Google Scholar]
- 114.Tagawa M, Ogata A, Hamano T.. Pre- and/or intra-operative prescription of diuretics, but not renin-angiotensin-system inhibitors, is significantly associated with acute kidney injury after non-cardiac surgery: a retrospective cohort study. PLoS One. 2015;10(7):e0132507. PubMed Central PMCID: PMC4492997. doi: 10.1371/journal.pone.0132507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Medve L, Gondos T.. Epidemiology of postoperative acute kidney injury in Hungarian intensive care units: an exploratory analysis. Ren Fail. 2012;34(9):1074–1078. doi: 10.3109/0886022X.2012.713254. [DOI] [PubMed] [Google Scholar]
- 116.Paquette IM, Solan P, Rafferty JF, et al. Readmission for dehydration or renal failure after ileostomy creation. Dis Colon Rectum. 2013;56(8):974–979. doi: 10.1097/DCR.0b013e31828d02ba. [DOI] [PubMed] [Google Scholar]
- 117.Toyonaga Y, Kikura M.. Hyperchloremic acidosis is associated with acute kidney injury after abdominal surgery. Nephrology (Carlton). 2017;22(9):720–727. doi: 10.1111/nep.12840. [DOI] [PubMed] [Google Scholar]
- 118.Ren H, Meng L.. Acute kidney injury treatment for elderly patients after esophageal cancer operation. Zhonghua Yi Xue Za Zhi. 2015;95(25):2000–2002. doi: 10.3760/cma.j.issn.0376-2491.2015.25.009. [DOI] [PubMed] [Google Scholar]
- 119.Ida M, Sumida M, Naito Y, et al. Impact of intraoperative hypotension and blood loss on acute kidney injury after pancreas surgery. Braz J Anesthesiol. 2020;70(4):343–348. doi: 10.1016/j.bjan.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vicente-Hernández B, Pérez-Beltrán CF, Rodríguez-Weber F, et al. Acute renal lesion in patients submitted to apendicectomy. Medicina Interna de Mexico. 2017;33(2):151–158. [Google Scholar]
- 121.Collaborative ST. Prognostic model to predict postoperative acute kidney injury in patients undergoing major gastrointestinal surgery based on a national prospective observational cohort study. BJS Open. 2018;2(6):400–410. doi: 10.1002/bjs5.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Connor ME, Hewson RW, Kirwan CJ, et al. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg. 2017;104(7):868–876. doi: 10.1002/bjs.10498. [DOI] [PubMed] [Google Scholar]
- 123.Magyar CTJ, Rajendran L, Babakhani S, et al. Impact of intraoperative hypotension during laparoscopic liver resection on postoperative complications including acute kidney injury. Surgery. 2025;178:108924. doi: 10.1016/j.surg.2024.10.015. [DOI] [PubMed] [Google Scholar]
- 124.Maddy BP, Tischer KM, McGree ME, et al. Implementation of enhanced recovery protocol did not increase rates of acute kidney injury in open gynecologic oncology surgery: A single-institution experience. Gynecol Oncol. 2025;192:181–188. doi: 10.1016/j.ygyno.2024.12.005. [DOI] [PubMed] [Google Scholar]
- 125.Yin L, Wang C, Zhao W, et al. Association between muscular tissue desaturation and acute kidney injury in older patients undergoing major abdominal surgery: a prospective cohort study. J Anesth. 2024;38(4):434–444. PubMed Central PMCID: PMC11284187. doi: 10.1007/s00540-024-03332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun R, Zhou Z, Li X, et al. Prognostic significance of preoperative nutritional status for postoperative acute kidney injury in older patients undergoing major abdominal surgery: a retrospective cohort study. Int J Surg. 2024;110(2):873–883. PubMed Central PMCID: PMC10871641. doi: 10.1097/js9.0000000000000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peng X, Zhu T, Chen Q, et al. A simple machine learning model for the prediction of acute kidney injury following noncardiac surgery in geriatric patients: a prospective cohort study. BMC Geriatr. 2024;24(1):549. PubMed Central PMCID: PMC11197315. doi: 10.1186/s12877-024-05148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parab SY, Majety SC, Ranganathan P, et al. Incidence of acute kidney injury and its associated risk factors in patients undergoing elective oesophagectomy surgeries at a tertiary care cancer institute - A pilot prospective observational study. Indian J Anaesth. 2024;68(6):572–578. PubMed Central PMCID: PMC11186524. doi: 10.4103/ija.ija_98_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ota E, Watanabe J, Suwa H, et al. Preoperative risk factors for ileostomy-associated kidney injury in colorectal tumor surgery following ileostomy formation. Int J Colorectal Dis. 2024;39(1):160. PubMed Central PMCID: PMC11471691. doi: 10.1007/s00384-024-04732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schmied ID, Rajkumar D, Chang MI, et al. Ureteral stents do not increase the risk for acute kidney injury after colorectal surgery. Dis Colon Rectum. 2024;67(1):151–159. doi: 10.1097/dcr.0000000000002841. [DOI] [PubMed] [Google Scholar]
- 131.McIlroy DR, Feng X, Shotwell M, et al. Candidate kidney protective strategies for patients undergoing major abdominal surgery: a secondary analysis of the RELIEF trial cohort. Anesthesiology. 2024;140(6):1111–1125. doi: 10.1097/aln.0000000000004957. [DOI] [PubMed] [Google Scholar]
- 132.Mannion JD, Rather A, Fisher A, et al. Systemic inflammation and acute kidney injury after colorectal surgery. BMC Nephrol. 2024;25(1):92. PubMed Central PMCID: PMC10929149. doi: 10.1186/s12882-024-03526-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lydon K, Shah S, Mongan KL, et al. Intraoperative fluid management is not predictive of AKI in major pancreatic surgery: a retrospective cohort study. J Anesth Analg Crit Care. 2024;4(1):39. PubMed Central PMCID: PMC11218130. doi: 10.1186/s44158-024-00176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu Y, Xiao Z, Zhao X, et al. Incidence, risk factors, and outcomes of the transition of HIPEC-induced acute kidney injury to acute kidney disease: a retrospective study. Ren Fail. 2024;46(1):2338482. PubMed Central PMCID: PMC11011229. doi: 10.1080/0886022x.2024.2338482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu H, Luo R, Qian L, et al. The effect of dexmedetomidine on acute kidney injury after elective major abdominal surgery: a retrospective single-center propensity score matched study. BMC Anesthesiol. 2024;24(1):456. PubMed Central PMCID: PMC11657137. doi: 10.1186/s12871-024-02845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li S, Ren W, Ye X, et al. An online-predictive model of acute kidney injury after pancreatic surgery. Am J Surg. 2024;228:151–158. doi: 10.1016/j.amjsurg.2023.09.006. [DOI] [PubMed] [Google Scholar]
- 137.Krause M, Mehdipour S, Veerapong J, et al. Development of a predictive model for risk stratification of acute kidney injury in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Sci Rep. 2024;14(1):6630. PubMed Central PMCID: PMC10951241. doi: 10.1038/s41598-024-54979-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao SC, Ma JH, Kong H, et al. Intraoperative hyperthermia is associated with increased acute kidney injury following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a retrospective cohort study. Ren Fail. 2024;46(2):2420835. PubMed Central PMCID: PMC11536636. doi: 10.1080/0886022x.2024.2420835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng Y, Nie S, Zhao X, et al. Incidence, risk factors and outcome of postoperative acute kidney injury in China. Nephrol Dial Transplant. 2024;39(6):967–977. doi: 10.1093/ndt/gfad260. [DOI] [PubMed] [Google Scholar]
- 140.Beltrán D, Pintado M-C, Oñoro A, et al. Side-effects on the renal function of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Int J Med Sci. 2024;21(13):2537–2543. PubMed Central PMCID: PMC11492877. doi: 10.7150/ijms.97774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu Y, Wu H, Liu C, et al. Intraoperative renal desaturation and postoperative acute kidney injury in older patients undergoing liver resection: A prospective cohort study. J Clin Anesth. 2023;87:111084. doi: 10.1016/j.jclinane.2023.111084. [DOI] [PubMed] [Google Scholar]
- 142.Yan Y-T, Liu H-M, Kong Y-F, et al. Association of preoperative neutrophil-lymphocyte ratio with acute kidney injury in patients with non-cardiac surgery: difference among surgical types. Int Urol Nephrol. 2023;55(10):2647–2656. doi: 10.1007/s11255-023-03567-4. [DOI] [PubMed] [Google Scholar]
- 143.Swartling O, Evans M, Larsson P, et al. Risk factors for acute kidney injury after pancreatoduodenectomy, and association with postoperative complications and death. Pancreatology. 2023;23(2):227–233. doi: 10.1016/j.pan.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 144.Rossouw E, Chetty S.. Acute kidney injury after major non-cardiac surgery: incidence and risk factors. S Afr Med J. 2023;113(3):135–140. doi: 10.7196/SAMJ.2023.v113i3.16783. [DOI] [PubMed] [Google Scholar]
- 145.Putowski Z, Majewska K, Gruca K, et al. Intraoperative hypotension and its association with postoperative acute kidney injury in patients undergoing pancreaticoduodenectomy: a 5-year, single-center, retrospective cohort study. Med Sci Monit. 2023;29:e938945. PubMed Central PMCID: PMC10105515. doi: 10.12659/msm.938945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liesenfeld LF, Quiring E, Al-Saeedi M, et al. Extensive peritonectomy is an independent risk factor for cisplatin HIPEC-induced acute kidney injury. Ann Surg Oncol. 2023;30(5):2646–2656. PubMed Central PMCID: PMC10085927. doi: 10.1245/s10434-022-12661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kuang L, Lin W, Chen B, et al. A nomogram for predicting acute kidney injury following hepatectomy: A propensity score matching analysis. J Clin Anesth. 2023;90:111211. doi: 10.1016/j.jclinane.2023.111211. [DOI] [PubMed] [Google Scholar]
- 148.Kim HJ, Kim HJ, Park JH, et al. Association between 20% albumin use and acute kidney injury in major abdominal surgery with transfusion. Int J Mol Sci. 2023;24(3) :2333. PubMed Central PMCID: PMC9916446. doi: 10.3390/ijms24032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kang P, Park J-B, Yoon H-K, et al. Association of the perfusion index with postoperative acute kidney injury: a retrospective study. Korean J Anesthesiol. 2023;76(4):348–356. PubMed Central PMCID: PMC10391075. doi: 10.4097/kja.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ji Y, Zhou Y, Shen Z, et al. Risk factors for and prognostic values of postoperative acute kidney injury after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: A retrospective, propensity score-matched cohort study of 1312 patients. Cancer Med. 2023;12(7):7823–7834. PubMed Central PMCID: PMC10134349. doi: 10.1002/cam4.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fujii T, Takakura M, Taniguchi T, et al. Intraoperative hypotension affects postoperative acute kidney injury depending on the invasiveness of abdominal surgery: A retrospective cohort study. Medicine (Baltimore). 2023;102(48):e36465. PubMed Central PMCID: PMC10695494. doi: 10.1097/md.0000000000036465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Valencia Morales DJ, Plack DL, Kendrick ML, et al. Urine output and acute kidney injury following laparoscopic pancreas operations. HPB (Oxford). 2022;24(11):1967–1974. doi: 10.1016/j.hpb.2022.06.006. [DOI] [PubMed] [Google Scholar]
- 153.Shen J, Chu Y, Wang C, et al. Risk factors for acute kidney injury after major abdominal surgery in the elderly aged 75 years and above. BMC Nephrol. 2022;23(1):224. PubMed Central PMCID: PMC9229523. doi: 10.1186/s12882-022-02822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Putowski Z, Krzych Ł, Czajka S.. High intraoperative pulse pressure is a risk factor for postoperative acute kidney injury in a cohort of abdominal surgery patients: An exploratory study. Adv Clin Exp Med. 2022;31(5):511–517. doi: 10.17219/acem/145946. [DOI] [PubMed] [Google Scholar]
- 155.Mikkelsen TB, Schack A, Oreskov JO, et al. Acute kidney injury following major emergency abdominal surgery - a retrospective cohort study based on medical records data. BMC Nephrol. 2022;23(1):94. PubMed Central PMCID: PMC8897898. doi: 10.1186/s12882-022-02708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murphy CF, Dunne T, Elliott JA, et al. Acute kidney injury after esophageal cancer surgery: incidence, risk factors, and impact on oncologic outcomes. Ann Surg. 2022;275(5):e683–e9. doi: 10.1097/sla.0000000000004146. [DOI] [PubMed] [Google Scholar]
- 157.Loria A, Melucci A, Speranza J, et al. Acute kidney injury is a common and significant complication following ileostomy formation. Colorectal Dis. 2022;24(1):102–110. doi: 10.1111/codi.15917. [DOI] [PubMed] [Google Scholar]
- 158.Imai E, Morohashi Y, Mishima K, et al. A goal-directed therapy protocol for preventing acute kidney injury after laparoscopic liver resection: a retrospective observational cohort study. Surg Today. 2022;52(9):1262–1274. doi: 10.1007/s00595-022-02453-3. [DOI] [PubMed] [Google Scholar]
- 159.Drakeford PA, Tham SQ, Kwek JL, et al. Acute kidney injury within an enhanced recovery after surgery (ERAS) program for colorectal surgery. World J Surg. 2022;46(1):19–33. doi: 10.1007/s00268-021-06343-6. [DOI] [PubMed] [Google Scholar]
- 160.Chiu C, Fong N, Lazzareschi D, et al. Fluids, vasopressors, and acute kidney injury after major abdominal surgery between 2015 and 2019: a multicentre retrospective analysis. Br J Anaesth. 2022;129(3):317–326. doi: 10.1016/j.bja.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 161.STARSurg Collaborative and EuroSurg Collaborative . Validation of the OAKS prognostic model for acute kidney injury after gastrointestinal surgery. BJS Open. 2022 Jan 6;6(1):zrab150. PubMed Central PMCID: PMC8855527. doi: 10.1093/bjsopen/zrab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saugel B, Sander M, Katzer C, et al. Association of intraoperative hypotension and cumulative norepinephrine dose with postoperative acute kidney injury in patients having noncardiac surgery: a retrospective cohort analysis. Br J Anaesth. 2025;134(1):54–62. 20241212. PubMed Central PMCID: PMC11718363. doi: 10.1016/j.bja.2024.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krone S, Bokoch MP, Kothari R, et al. Association between peripheral perfusion index and postoperative acute kidney injury in major noncardiac surgery patients receiving continuous vasopressors: a post hoc exploratory analysis of the VEGA-1 trial. Br J Anaesth. 2024;132(4):685–694. doi: 10.1016/j.bja.2023.11.054. [DOI] [PubMed] [Google Scholar]
- 164.Zarbock A, Weiss R, Albert F, et al. Epidemiology of surgery associated acute kidney injury (EPIS-AKI): a prospective international observational multi-center clinical study. Intensive Care Med. 2023;49(12):1441–1455. PubMed Central PMCID: PMC10709241. doi: 10.1007/s00134-023-07169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shimada T, Pu X, Kutlu Yalcin E, et al. Association between postoperative hypotension and acute kidney injury after noncardiac surgery: a historical cohort analysis. Can J Anaesth. 2023;70(12):1892–1900. doi: 10.1007/s12630-023-02601-4. [DOI] [PubMed] [Google Scholar]
- 166.Dong L, Takeda C, Kamitani T, et al. Association between intraoperative end-tidal carbon dioxide and postoperative organ dysfunction in major abdominal surgery: A cohort study. PLoS One. 2023;18(3):e0268362. PubMed Central PMCID: PMC10004519. doi: 10.1371/journal.pone.0268362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schiefer J, Bernardi MH, Lichtenegger P, et al. Incidence and outcomes of AKI in postoperative patients admitted to ICU using full KDIGO criteria—a cohort study. J Clin Anesth. 2023;89:111156. doi: 10.1016/j.jclinane.2023.111156. [DOI] [PubMed] [Google Scholar]
- 168.Dai Y, Shi Y, Wang H, et al. Metabolic syndrome for the prognosis of postoperative complications after open pancreatic surgery in Chinese adult: a propensity score matching study. Sci Rep. 2023;13(1):3889. PubMed Central PMCID: PMC9995346. doi: 10.1038/s41598-023-31112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bani Hani A, Abu Abeeleh M, Al-Najjar S, et al. Incidence, risk factors and outcomes of acute kidney injury in surgical intensive care unit octogenarians at the Jordan University Hospital. BMC Geriatr. 2023;23(1):266. PubMed Central PMCID: PMC10158325. doi: 10.1186/s12877-023-03975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang JN, Li Z, Wang ML, et al. Preoperative dipstick albuminuria is associated with acute kidney injury in high-risk patients following non-cardiac surgery: a single-center prospective cohort study. J Anesth. 2022;36(6):747–756. doi: 10.1007/s00540-022-03113-z. [DOI] [PubMed] [Google Scholar]
- 171.Wang J, Dong Y, Zhao B, et al. Preoperative NT-proBNP and LVEF for the prediction of acute kidney injury after noncardiac surgery: a single-centre retrospective study. BMC Anesthesiol. 2022;22(1):196. PubMed Central PMCID: PMC9229082. doi: 10.1186/s12871-022-01727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shimada T, Mascha EJ, Yang D, et al. Intra-operative hypertension and myocardial injury and/or mortality and acute kidney injury after noncardiac surgery: a retrospective cohort analysis. Eur J Anaesthesiol. 2022;39(4):315–323. doi: 10.1097/eja.0000000000001656. [DOI] [PubMed] [Google Scholar]
- 173.Okuda H, Obata Y, Kamijo-Ikemori A, et al. Quantitative and qualitative analyses of urinary L-FABP for predicting acute kidney injury after emergency laparotomy. J Anesth. 2022;36(1):38–45. doi: 10.1007/s00540-021-03003-w. [DOI] [PubMed] [Google Scholar]
- 174.Hu L, Gao L, Zhang D, et al. The incidence, risk factors and outcomes of acute kidney injury in critically ill patients undergoing emergency surgery: a prospective observational study. BMC Nephrol. 2022;23(1):42. PubMed Central PMCID: PMC8782702. doi: 10.1186/s12882-022-02675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.French WB, Shah PR, Fatani YI, et al. Mortality and costs associated with acute kidney injury following major elective, non-cardiac surgery. J Clin Anesth. 2022;82:110933. doi: 10.1016/j.jclinane.2022.110933. [DOI] [PubMed] [Google Scholar]
- 176.De Guglielmo W, Rebibou JM, Aho S, et al. Pre-operative factors associated with the occurrence of acute kidney injury in patients aged 65 years and over undergoing non-ambulatory non-cardiac surgery. Healthcare (Basel). 2022;10(3):558. PubMed Central PMCID: PMC8955534. doi: 10.3390/healthcare10030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ripollés-Melchor J, Fernández Dorado F, Rubio Aguilera AI, et al. Association between preoperative baseline pulse pressure and estimated pulse wave velocity and acute renal failure and mortality following colorectal surgery. A single-centre observational study. Rev Esp Anestesiol Reanim (Engl Ed). 2021;68(10):564–575. doi: 10.1016/j.redare.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 178.Mizunoya K, Yagi Y, Kamachi H, et al. Diagnostic timing dependent characteristics of acute kidney injury following hepatectomy: a retrospective historical cohort analysis. HPB (Oxford). 2021;23(12):1897–1905. doi: 10.1016/j.hpb.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 179.Katayama HT, Gomes BC, Lobo SMA, et al. The effects of acute kidney injury in a multicenter cohort of high-risk surgical patients. Ren Fail. 2021;43(1):1338–1348. PubMed Central PMCID: PMC8477947. doi: 10.1080/0886022x.2021.1977318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Córdova-Sánchez BM, Joffre-Torres A, Joachín-Sánchez E, et al. Acute kidney injury in critically ill patients after oncological surgery: risk factors and 1-year mortality. Nephrology (Carlton). 2021;26(12):965–971. doi: 10.1111/nep.13964. [DOI] [PubMed] [Google Scholar]
- 181.Iyer KV, Giri S, Ray BR, et al. Association between intraoperative starch use and postoperative kidney dysfunction in patients undergoing major gastro-intestinal surgery: a propensity score-matched analysis. J Perioper Pract. 2025;35(1-2):14–21. doi: 10.1177/17504589231174967. [DOI] [PubMed] [Google Scholar]
- 182.Shin S, Choi TY, Han DH, et al. An explainable machine learning model to predict early and late acute kidney injury after major hepatectomy. HPB (Oxford). 2024;26(7):949–959. doi: 10.1016/j.hpb.2024.04.005. [DOI] [PubMed] [Google Scholar]
- 183.Matkov TG, Curry LS, Ochoa AL.. Risk stratification of acute kidney injury (AKI) following ureteral stent insertion for colorectal surgery. Surg Endosc. 2024;38(1):312–318. doi: 10.1007/s00464-023-10440-z. [DOI] [PubMed] [Google Scholar]
- 184.Ripollés-Melchor J, Tomé-Roca JL, Zorrilla-Vaca A, et al. Hemodynamic management guided by the hypotension prediction index in abdominal surgery: a multicenter randomized clinical trial. Anesthesiology. 2025;142(4):639–654. doi: 10.1097/ALN.0000000000005355. [DOI] [PubMed] [Google Scholar]
- 185.Pang Z, Liang S, Zhou N, et al. Individualized blood pressure regulation and acute kidney injury in older patients having major abdominal surgery: a pilot randomized trial. Int J Surg. 2025;111(4):2894–2902. PubMed Central PMCID: PMC12175809. doi: 10.1097/JS9.0000000000002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chaudhary RK, Dhir A, Ganesh V, et al. Effect of isotonic sodium bicarbonate infusion on perioperative acid-base status among patients undergoing emergency laparotomy for perforation peritonitis (ISABEL trial): a randomized controlled trial. Eur J Trauma Emerg Surg. 2025;51(1):10. doi: 10.1007/s00068-024-02751-0. [DOI] [PubMed] [Google Scholar]
- 187.Funcke S, Schmidt G, Bergholz A, et al. Cardiac index-guided therapy to maintain optimised postinduction cardiac index in high-risk patients having major open abdominal surgery: the multicentre randomised iPEGASUS trial. Br J Anaesth. 2024;133(2):277–287. PubMed Central PMCID: PMC11282469. doi: 10.1016/j.bja.2024.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim YJ, Kim BR, Kim HW, et al. Effect of driving pressure-guided positive end-expiratory pressure on postoperative pulmonary complications in patients undergoing laparoscopic or robotic surgery: a randomised controlled trial. Br J Anaesth. 2023;131(5):955–965. doi: 10.1016/j.bja.2023.08.007. [DOI] [PubMed] [Google Scholar]
- 189.Nicklas JY, Diener O, Leistenschneider M, et al. Personalised haemodynamic management targeting baseline cardiac index in high-risk patients undergoing major abdominal surgery: a randomised single-centre clinical trial. Br J Anaesth. 2020;125(2):122–132. doi: 10.1016/j.bja.2020.04.094. [DOI] [PubMed] [Google Scholar]
- 190.Ferrando C, Aldecoa C, Unzueta C, et al. Effects of oxygen on post-surgical infections during an individualised perioperative open-lung ventilatory strategy: a randomised controlled trial. Br J Anaesth. 2020;124(1):110–120. doi: 10.1016/j.bja.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 191.Szturz P, Folwarczny P, Kula R, et al. Multi-parametric functional hemodynamic optimization improves postsurgical outcome after intermediate risk open gastrointestinal surgery: a randomized controlled trial. Minerva Anestesiol. 2019;85(3):244–254. doi: 10.23736/S0375-9393.18.12467-9. [DOI] [PubMed] [Google Scholar]
- 192.Jongerius IM, Mungroop TH, Uz Z, et al. Goal-directed fluid therapy vs. low central venous pressure during major open liver resections (GALILEO): a surgeon- and patient-blinded randomized controlled trial. HPB (Oxford). 2021;23(10):1578–1585. doi: 10.1016/j.hpb.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 193.Suzuki T, Koyama K.. Open randomized trial of the effects of 6% hydroxyethyl starch 130/0.4/9 and 5% albumin on safety profile, volume efficacy, and glycocalyx degradation in hepatic and pancreatic surgery. J Anesth. 2020;34(6):912–923. doi: 10.1007/s00540-020-02847-y. [DOI] [PubMed] [Google Scholar]
- 194.Richards T, Baikady RR, Clevenger B, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet. 2020;396(10259):1353–1361. doi: 10.1016/S0140-6736(20)31539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li X-F, Jiang D, Jiang Y-L, et al. Comparison of low and high inspiratory oxygen fraction added to lung-protective ventilation on postoperative pulmonary complications after abdominal surgery: A randomized controlled trial. J Clin Anesth. 2020;67:110009. doi: 10.1016/j.jclinane.2020.110009. [DOI] [PubMed] [Google Scholar]
- 196.Fischer M-O, Fiant A-L, Debroczi S, et al. Perioperative non-invasive haemodynamic optimisation using photoplethysmography: a randomised controlled trial and meta-analysis. Anaesth Crit Care Pain Med. 2020;39(3):421–428. doi: 10.1016/j.accpm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 197.Kabon B, Sessler DI, Kurz A, Crystalloid-Colloid Study T . Effect of intraoperative goal-directed balanced crystalloid versus colloid administration on major postoperative morbidity: a randomized trial. Anesthesiology. 2019;130(5):728–744. doi: 10.1097/ALN.0000000000002601. [DOI] [PubMed] [Google Scholar]
- 198.Joosten A, Delaporte A, Ickx B, et al. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: a randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology. 2018;128(1):55–66. doi: 10.1097/ALN.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 199.Pallesen J, Bhavsar R, Fjølner J, et al. The effects of preoperative focused cardiac ultrasound in high-risk patients: A randomised controlled trial (PREOPFOCUS). Acta Anaesthesiol Scand. 2022;66(10):1174–1184. PubMed Central PMCID: PMC9804977. doi: 10.1111/aas.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lv X, Hou A, Han S, et al. Effect of perioperative rehabilitation exercise on postoperative outcomes in patients aged >/=65 years undergoing gastrointestinal surgery: a multicenter randomized controlled trial. J Clin Anesth. 2024;99:111670. doi: 10.1016/j.jclinane.2024.111670. [DOI] [PubMed] [Google Scholar]
- 201.Diaper J, Schiffer E, Barcelos GK, et al. Goal-directed hemodynamic therapy versus restrictive normovolemic therapy in major open abdominal surgery: a randomized controlled trial. Surgery. 2021;169(5):1164–1174. doi: 10.1016/j.surg.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 202.de Waal EEC, Frank M, Scheeren TWL, et al. Perioperative goal-directed therapy in high-risk abdominal surgery. A multicenter randomized controlled superiority trial. J Clin Anesth. 2021;75:110506. doi: 10.1016/j.jclinane.2021.110506. [DOI] [PubMed] [Google Scholar]
- 203.Calvo-Vecino JM, Ripollés-Melchor J, Mythen MG, et al. Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial). Br J Anaesth. 2018;120(4):734–744. doi: 10.1016/j.bja.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 204.Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 205.Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494–1503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 206.Group OIT. Cardiac output-guided haemodynamic therapy for patients undergoing major gastrointestinal surgery: OPTIMISE II randomised clinical trial. BMJ. 2024;387:e080439. PubMed Central PMCID: PMC12036648. doi: 10.1136/bmj-2024-080439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357. PubMed Central PMCID: PMC5710560. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378(24):2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 209.Karalapillai D, Weinberg L, Peyton P, et al. Effect of intraoperative low tidal volume vs conventional tidal volume on postoperative pulmonary complications in patients undergoing major surgery: a randomized clinical trial. JAMA. 2020;324(9):848–858. PubMed Central PMCID: PMC7489812. doi: 10.1001/jama.2020.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang C, Gao Y, Tian Y, et al. Prediction of acute kidney injury after cardiac surgery from preoperative N-terminal pro-B-type natriuretic peptide. Br J Anaesth. 2021;127(6):862–870. doi: 10.1016/j.bja.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 211.Costa NA, Gut AL, Azevedo PS, et al. Protein carbonyl concentration as a biomarker for development and mortality in sepsis-induced acute kidney injury. Biosci Rep. 2018;38(1):BSR20171238. PubMed Central PMCID: PMC5784177. doi: 10.1042/BSR20171238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this systematic review and meta-analysis are available from the corresponding author upon reasonable request. The data consist of the extracted information from published studies included in our analysis. All primary sources are cited within the manuscript and listed in the references.