Abstract
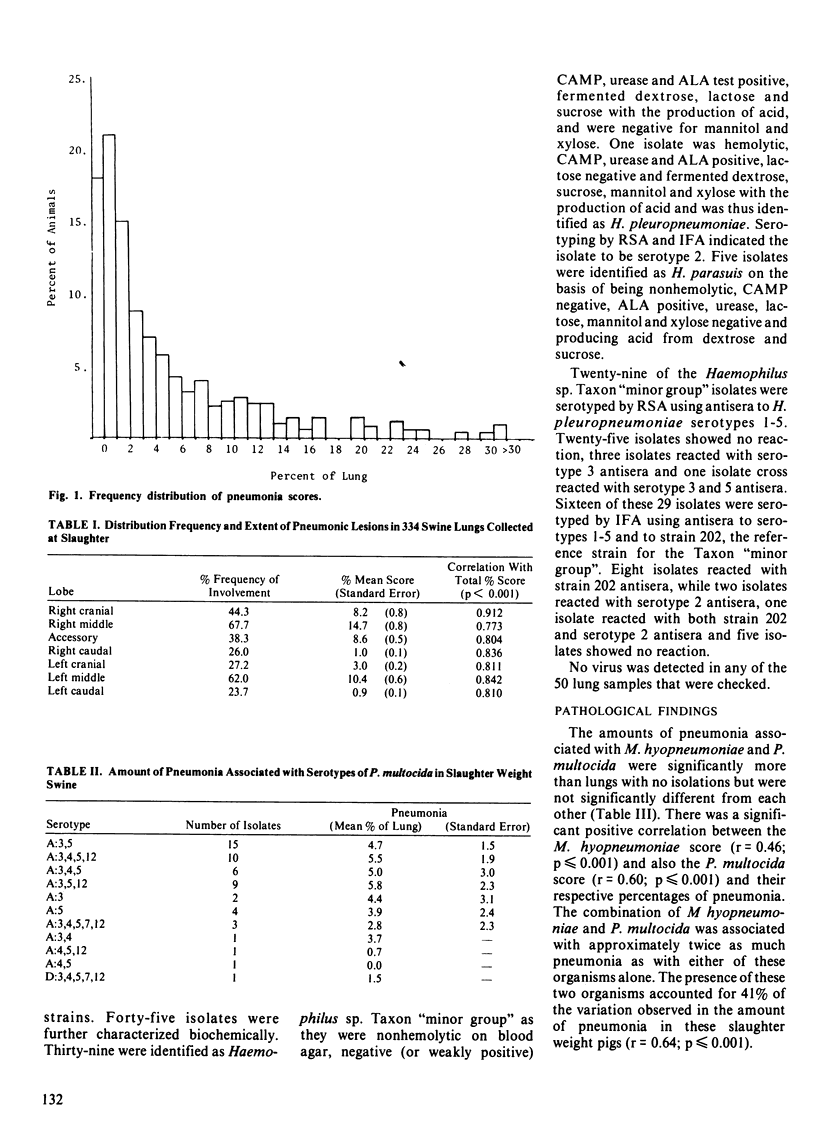
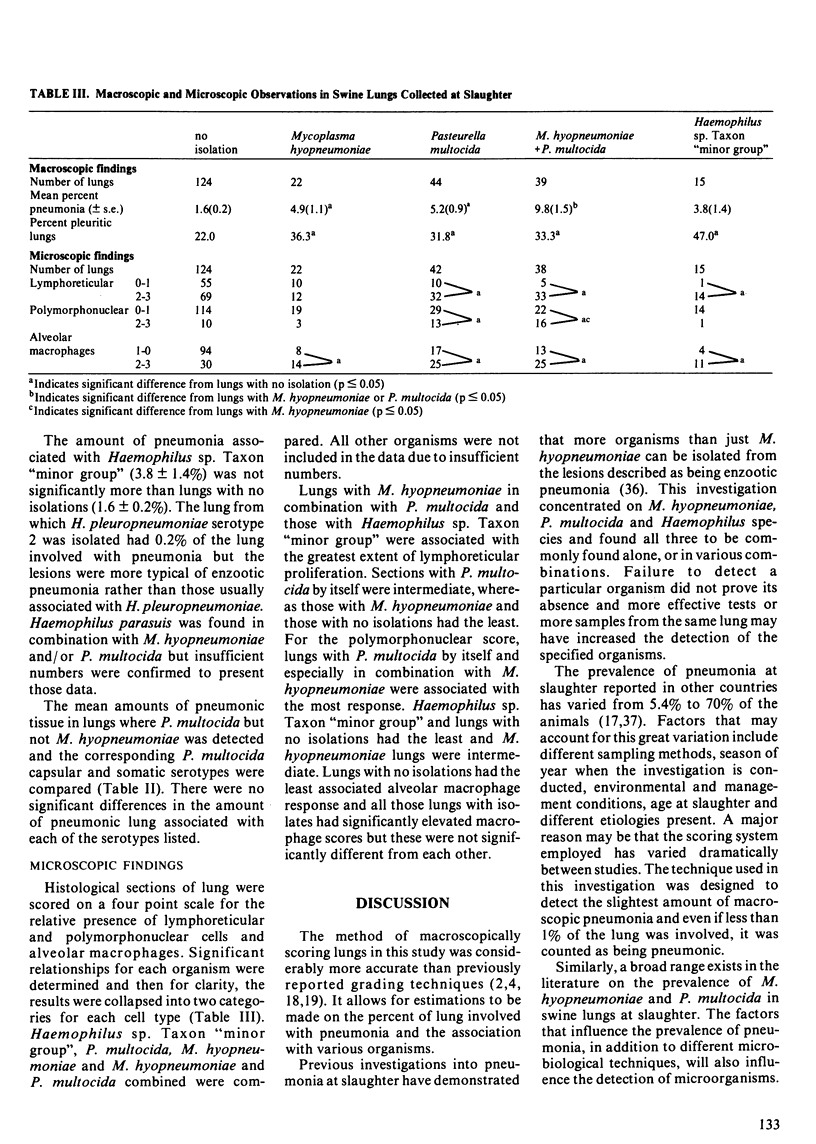
The lungs of 334 pigs were obtained from two slaughter plants in Minnesota and examined in detail. Macroscopic and microscopic evaluation, direct fluorescence for Mycoplasma hyopneumoniae and bacterial culture were done on all of them and a subsample of 50 were selected for virus culture. Mycoplasma hyopneumoniae, Pasteurella multocida and Haemophilus spp. were detected in 24.0%, 34.1% and 27.0% of the lungs, commonly in conjunction with each other. One isolate of Haemophilus pleuropneumoniae serotype 2 was detected and this represents the first report of its presence in the United States. No virus was detected in any of the lungs. Lungs with both M. hyopneumoniae and Pasteurella multocida had the greatest amount of macroscopic pneumonia (9.8% of the lung). Lungs with M. hyopneumoniae or P. multocida alone had 4.9% and 5.2% of the lung involved with pneumonia respectively. Lungs with Haemophilus sp. Taxon "minor group" had 3.8% of the lung involved which was not significantly different from lungs with none of these organisms being detected (1.6%). There was a positive correlation between the extent of M. hyopneumoniae infection, as scored by FAT and the amount of macroscopic pneumonia present (r = 0.46; P less than 0.001). Likewise, there was a positive correlation between the estimated concentration of P. multocida present, as scored by the relative number of colonies on blood agar and the amount of macroscopic pneumonia present (r = 0.60; P less than 0.001). Microscopically, the amount of lymphoreticular proliferation, polymorphonuclear cells and alveolar macrophages were evaluated.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalund O., Willeberg P., Mandrup M., Riemann H. Lung lesions at slaughter: associations to factors in the pig herd. Nord Vet Med. 1976 Oct;28(10):487–495. [PubMed] [Google Scholar]
- Biberstein E. L., Gunnarsson A., Hurvell B. Cultural and biochemical criteria for the identification of haemophilus spp from swine. Am J Vet Res. 1977 Jan;38(1):7–11. [PubMed] [Google Scholar]
- Collins F. M. Mechanisms of acquired resistance to Pasteurella multocida infection: a review. Cornell Vet. 1977 Jan;67(1):103–138. [PubMed] [Google Scholar]
- Degré M., Solberg L. A. Synergistic effect in viral-bacterial infection. 3. Histopathological changes in the trachea of mice following viral and bacterial infection. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):129–136. [PubMed] [Google Scholar]
- Flesjå K. I., Ulvesaeter H. O. Pathological lesions in swine at slaughter. I. Baconers. Acta Vet Scand. 1979;20(4):498–514. doi: 10.1186/BF03546577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gois M., Sisák F., Kuksa F., Sovadina M. Incidence and evaluation of the microbial flora in the lungs of pigs with enzootic pneumonia. Zentralbl Veterinarmed B. 1975 Apr;22(3):205–219. doi: 10.1111/j.1439-0450.1975.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Goodwin R. F. Activity of tiamulin against Mycoplasma suipneumoniae and enzootic pneumonia of pigs. Vet Rec. 1979 Mar 3;104(9):194–195. doi: 10.1136/vr.104.9.194. [DOI] [PubMed] [Google Scholar]
- Houghton S. B., Gourlay R. N. Synergism between Mycoplasma bovis and Pasteurella haemolytica in calf pneumonia. Vet Rec. 1983 Jul 9;113(2):41–42. doi: 10.1136/vr.113.2.41. [DOI] [PubMed] [Google Scholar]
- Jericho K. W., Darcel C. Q. Response of the respiratory tract of calves kept at controlled climatic conditions to bovine Herpesvirus 1 in aerosol. Can J Comp Med. 1978 Apr;42(2):156–167. [PMC free article] [PubMed] [Google Scholar]
- Jericho K. W., Done S. H., Saunders R. W. Pneumonia and efficiency of pig production. Can Vet J. 1975 Feb;16(2):44–49. [PMC free article] [PubMed] [Google Scholar]
- Jericho K. W. Histological changes in the respiratory tract of calves exposed to aerosols of bovine herpesvirus 1 and Pasteurella haemolytica. J Comp Pathol. 1983 Jan;93(1):73–82. doi: 10.1016/0021-9975(83)90044-0. [DOI] [PubMed] [Google Scholar]
- Jericho K. W., Magwood S. E. Histological features of respiratory epithelium of calves held at differing temperature and humidity. Can J Comp Med. 1977 Oct;41(4):369–379. [PMC free article] [PubMed] [Google Scholar]
- Kasza L., Hodges R. T., Betts A. O., Trexler P. C. Pneumonia in gnotobiotic pigs produced by simultaneous inoculation of a swine adenovirus and mycoplasma hyopneumoniae. Vet Rec. 1969 Mar 15;84(11):262–267. doi: 10.1136/vr.84.11.262. [DOI] [PubMed] [Google Scholar]
- L'Ecuyer C., Boulanger P. Enzootic pneumonia in pigs: identification of a causative mycoplasma in infected pigs and in cultures by immunofluorescent staining. Can J Comp Med. 1970 Jan;34(1):38–46. [PMC free article] [PubMed] [Google Scholar]
- Little T. W. Haemophilus infection in pigs. Vet Rec. 1970 Oct 3;87(14):399–402. doi: 10.1136/vr.87.14.399. [DOI] [PubMed] [Google Scholar]
- Little T. W., Harding J. D. The interaction of Haemophilus parahaemolyticus and Pasteurella multocida in the respiratory tract of the pig. Br Vet J. 1980 Jul-Aug;136(4):371–383. doi: 10.1016/s0007-1935(17)32240-6. [DOI] [PubMed] [Google Scholar]
- Little T. W. Respiratory disease in pigs: a study. Vet Rec. 1975 Jun 21;96(25):540–544. doi: 10.1136/vr.96.25.540. [DOI] [PubMed] [Google Scholar]
- Livingston C. W., Jr, Stair E. L., Underdahl N. R., Mebus C. A. Pathogenesis of mycoplasmal pneumonia in swine. Am J Vet Res. 1972 Nov;33(11):2249–2258. [PubMed] [Google Scholar]
- MATTHEWS P. R., PATTISON I. H. The identification of a Haemophilus-like organism associated with pneumonia and pleurisy in the pig. J Comp Pathol. 1961 Jan;71:44–52. doi: 10.1016/s0368-1742(61)80007-6. [DOI] [PubMed] [Google Scholar]
- Onofrio J. M., Toews G. B., Lipscomb M. F., Pierce A. K. Granulocyte-alveolar-macrophage interaction in the pulmonary clearance of Staphylococcus aureus. Am Rev Respir Dis. 1983 Mar;127(3):335–341. doi: 10.1164/arrd.1983.127.3.335. [DOI] [PubMed] [Google Scholar]
- Osborne A. D., Saunders J. R., K-Sebunya T. An abattoir survey of the incidence of pneumonia in Saskatchewan swine and an investigation of the microbiology of affected lungs. Can Vet J. 1981 Apr;22(4):82–85. [PMC free article] [PubMed] [Google Scholar]
- PATTISON I. H., HOWELL D. G., ELLIOT J. A haemophilus-like organism isolated from pig lung and the associated pneumonic lesions. J Comp Pathol. 1957 Oct;67(4):320–330. doi: 10.1016/s0368-1742(57)80031-9. [DOI] [PubMed] [Google Scholar]
- Pierce A. K., Reynolds R. C., Harris G. D. Leukocytic response to inhaled bacteria. Am Rev Respir Dis. 1977 Oct;116(4):679–684. doi: 10.1164/arrd.1977.116.4.679. [DOI] [PubMed] [Google Scholar]
- Pijoan C., Lastra A., Ramirez C., Leman A. D. Isolation of toxigenic strains of Pasteurella multocida from lungs of pneumonic swine. J Am Vet Med Assoc. 1984 Sep 1;185(5):522–523. [PubMed] [Google Scholar]
- Pijoan C., Morrison R. B., Hilley H. D. Dilution technique for isolation of Haemophilus from swine lungs collected at slaughter. J Clin Microbiol. 1983 Jul;18(1):143–145. doi: 10.1128/jcm.18.1.143-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijoan C., Morrison R. B., Hilley H. D. Serotyping of Pasteurella multocida isolated from swine lungs collected at slaughter. J Clin Microbiol. 1983 Jun;17(6):1074–1076. doi: 10.1128/jcm.17.6.1074-1076.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijoan C., Ochoa G. Interaction between a hog cholera vaccine strain and Pasteurella multocida in the production of porcine pneumonia. J Comp Pathol. 1978 Apr;88(2):167–170. doi: 10.1016/0021-9975(78)90020-8. [DOI] [PubMed] [Google Scholar]
- ROBERTS E. D., SWITZER W. P., L'ECUYER C. Influence of Pasteurella multocida and Mycoplasma hyorhinis (PPLO) on the histopathology of field case of swine pneumonia. Cornell Vet. 1962 Jul;52:306–327. [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F., Erickson B. Z. Serotyping of Haemophilus pleuropneumoniae by rapid slide agglutination and indirect fluorescent antibody tests in swine. Am J Vet Res. 1985 Jan;46(1):185–192. [PubMed] [Google Scholar]
- Rosendal S., Lombin L., DeMoor J. Serotyping and detection of Haemophilus pleuropneumoniae by indirect fluorescent antibody technique. Can J Comp Med. 1981 Jul;45(3):271–274. [PMC free article] [PubMed] [Google Scholar]
- Sanford S. E., Josephson G. K., Key D. W. An epizootic of Swine influenza in ontario. Can Vet J. 1983 Jun;24(6):167–171. [PMC free article] [PubMed] [Google Scholar]
- Smith I. M., Hodges R. T., Betts A. O., Hayward A. H. Experimental infections of gnotobiotic piglets with Pasteurella septica (sero-group A) alone or with Mycoplasma hyopneumoniae. J Comp Pathol. 1973 Jul;83(3):307–321. doi: 10.1016/0021-9975(73)90055-8. [DOI] [PubMed] [Google Scholar]
- Smith J. E. Analysis of autopsy data on pig respiratory disease by multivariate methods. Br Vet J. 1977 May-Jun;133(3):281–291. doi: 10.1016/s0007-1935(17)34090-3. [DOI] [PubMed] [Google Scholar]
- Snella M. C., Rylander R. Lung cell reactions after inhalation of bacterial lipopolysaccharides. Eur J Respir Dis. 1982 Nov;63(6):550–557. [PubMed] [Google Scholar]
- Straw B. E., Bürgi E. J., Hilley H. D., Leman A. D. Pneumonia and atrophic rhinitis in pigs from a test station. J Am Vet Med Assoc. 1983 Mar 15;182(6):607–611. [PubMed] [Google Scholar]
- Százados I., Kádas I. Identification of Haemophilus parahaemolyticus pleuropneumonia among normal slaughtery pigs. Acta Vet Acad Sci Hung. 1981;29(3):301–316. [PubMed] [Google Scholar]
- Toews G. B., Gross G. N., Pierce A. K. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am Rev Respir Dis. 1979 Sep;120(3):559–566. doi: 10.1164/arrd.1979.120.3.559. [DOI] [PubMed] [Google Scholar]
- Underdahl N. R., Kennedy G. A., Ramos A. S., Jr Duration of Mycoplasma hyopneumoniae infection in gnotobiotic pigs. Can Vet J. 1980 Sep;21(9):258–261. [PMC free article] [PubMed] [Google Scholar]
- Wilkie B. N. Respiratory tract immune response to microbial pathogens. J Am Vet Med Assoc. 1982 Nov 15;181(10):1074–1079. [PubMed] [Google Scholar]
- Winward L. D., Leendertsen L., Shen D. T. Microimmunodiffusion test for diagnosis of ovine progressive pneumonia. Am J Vet Res. 1979 Apr;40(4):564–566. [PubMed] [Google Scholar]


