Abstract
Minor hallucinations (MH), comprising three subtypes (presence hallucinations, passage hallucinations, and visual illusions), typically emerge during the early stages of Parkinson’s disease (PD) and precede the onset of well-structured visual hallucinations (VH). Whether the number of MH is associated with VH in PD patients remains unclear. We investigated the association between the number of MH and VH in 372 PD patients. Log-binomial regressions showed that individuals with multiple MH had a significantly higher prevalence of VH (RR = 2.08, 95% CI: 1.11–3.90 for 2 MH; RR = 6.20, 95% CI: 3.40–11.31 for 3 MH), while those with only one MH had a similar VH prevalence compared to those without MH (RR = 1.08, 95% CI: 0.53–2.21). Women exhibited stronger associations than men. These findings emphasize the importance of quantifying MH subtypes when assessing hallucinations, though their potential utility as predictive markers of disease progression and targets for early intervention requires validation through longitudinal studies.
Subject terms: Parkinson's disease, Risk factors, Signs and symptoms
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, the burden of which has been increasing globally1. It is estimated that by 2050, 25.2 million people worldwide will suffer from PD, placing a heavy economic burden on healthcare systems2. Non-motor symptoms of PD are a significant component of the disease burden, with their impact often surpassing that of motor symptoms3. Hallucinations are among the most common non-motor symptoms in PD and have received increasing attention in recent years4,5. A longitudinal study found that after 20 years of follow-up, up to three-quarters of PD patients developed well-structured visual hallucinations (VH)6. VH not only seriously affect the quality of life of patients but are also associated with a worse disease prognosis, increased healthcare costs, and greater social burdens. Therefore, early identification and intervention for VH are crucial for improving the management of these patients.
Minor hallucinations (MH) generally occur early in PD, often pre-dating motor symptoms, and are specifically categorized into three types: presence hallucinations (i.e., the sensation of someone nearby in the absence of an actual stimulus), passage hallucinations (i.e., the brief perception of a shadow rapidly passing through the peripheral vision without a real stimulus), and visual illusions (i.e., transient and momentary misperceptions of objects or living beings that are inconsistent with objective reality)7. Due to their transient, occasional nature and preserved insight, MH usually do not cause distress to patients and are often overlooked or underrecognized. In contrast, VH involve more realistic and vivid visual experiences, typically consisting of well-formed images of people, animals, objects, or scenes. These hallucinations are characterized by greater frequency, longer duration, and increased severity, and are closely associated with higher care burden, dementia incidence, and mortality8,9.
MH typically precedes the onset of VH, and, as the disease progresses, may either evolve into VH or coexist with it7,10. Functional connectivity analysis demonstrated that both MH and VH involve alterations in visual-attentional networks, with MH specifically characterized by brainstem-visual connectivity disruptions and VH by temporal-parietal-occipital network dysfunction11. Previous epidemiological studies have demonstrated that MH are strongly associated with VH10,12,13. For instance, Fénelon et al. found that patients with presence of hallucinations had a 4.5-fold higher odds of developing VH compared to those without the presence of hallucinations12. However, these studies have primarily focused on the association of the presence or absence of MH with VH. The three subtypes of MH can occur independently or concurrently, but whether the number of MH is associated with VH and whether clinical characteristics differ by the number of MH remain unknown. This is important because the three MH subtypes may be associated with VH via different neurobiological mechanisms and thus have independent associations with VH. For example, the phenomenological and clinical characteristic differences between presence hallucinations, passage hallucinations, and visual illusions suggest potentially functional and metabolic alterations in different brain regions (e.g., the precuneus, V5/MT area, and occipital regions)14. Therefore, our study aims to examine the associations of the number of MH with the prevalence of VH and PD-related clinical features in PD patients.
Results
Demographic and clinical characteristics
A total of 372 patients with PD were included in this study. Among these, 114 patients (30.6%) experienced at least one MH subtype, and 55 patients (14.8%) had VH, including 25 patients who experienced both MH and VH. Participants were categorized into four groups based on the number of MH subtypes: 258 patients without MH, 66 patients with single MH, 32 patients with two MH, and 16 patients with three MH. Table 1 summarizes the demographic and clinical characteristics of PD patients with different numbers of MH subtypes. Age, sex, education level, lifestyle behaviors, history of medical conditions, Unified Parkinson’s Disease Rating Scale (UPDRS) III scores, and Hoehn and Yahr (H-Y) stage were overall comparable between the four groups. Patients with a greater number of MH subtypes appeared to have a longer disease duration and higher levodopa equivalent daily dose (LEDD), though the differences were not statistically significant.
Table 1.
Participant characteristics in PD patients with different numbers of MH
| No. of MH | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | p value | |
| Participants, n (%) | 258 (69.35) | 66 (17.74) | 32 (8.60) | 16 (4.30) | |
| VH, n (%) | 30 (11.63) | 7 (10.61) | 10 (31.25) | 8 (50.00) | <0.001 |
| Age, y, mean (SD) | 66.54 (9.91) | 67.73 (8.12) | 68.16 (9.72) | 67.19 (8.13) | 0.705 |
| Sex, n (%) | 0.671 | ||||
| Male | 116 (44.96) | 30 (45.45) | 12 (37.50) | 9 (56.25) | |
| Female | 142 (55.04) | 36 (54.55) | 20 (62.50) | 7 (43.75) | |
| Education, n (%) | 0.749 | ||||
| Illiterate | 39 (15.12) | 9 (13.64) | 1 (4.55) | 2 (6.25) | |
| Primary school | 40 (15.50) | 9 (13.64) | 6 (27.27) | 9 (28.13) | |
| Middle school | 126 (48.84) | 36 (54.55) | 10 (45.45) | 14 (43.75) | |
| College or above | 126 (48.84) | 12 (18.18) | 5 (22.73) | 7 (21.88) | |
| BMI, mean (SD) | 23.93 (3.65) | 22.98 (2.94) | 23.90 (3.89) | 23.35 (3.35) | 0.259 |
| Allergies, n (%) | 34 (13.18) | 8 (12.12) | 5 (15.63) | 1 (6.25) | 0.827 |
| Smoker, n (%) | 65 (25.19) | 16 (24.24) | 9 (28.13) | 2 (12.50) | 0.677 |
| Alcohol intake, n (%) | 35 (13.57) | 12 (18.18) | 8 (25.00) | 1 (6.25) | 0.222 |
| Daily exercise, n (%) | 130 (50.39) | 34 (51.52) | 14 (43.75) | 6 (37.50) | 0.704 |
| Hypertension, n (%) | 101 (39.15) | 19 (28.79) | 15 (46.88) | 9 (56.25) | 0.125 |
| Diabetes, n (%) | 28 (10.85) | 12 (18.18) | 3 (9.38) | 1 (6.25) | 0.327 |
| Family history of PD, n (%) | 26 (10.08) | 4 (6.06) | 4 (12.50) | 3 (18.75) | 0.434 |
| Age of onset, y, mean (SD) | 60.75 (10.56) | 61.47 (9.96) | 60.78 (10.24) | 59.88 (8.98) | 0.939 |
| Disease duration, y, mean (SD) | 5.83 (4.86) | 6.35 (4.92) | 7.56 (4.68) | 7.09 (3.17) | 0.200 |
| LEDD, mg, mean (SD) | 450.42 (385.20) | 450.85 (344.50) | 615.92 (304.18) | 547.55 (411.21) | 0.093 |
| UPDRS III, mean (SD) | 33.52 (15.65) | 31.23 (17.76) | 36.53 (16.01) | 30.56 (7.24) | 0.395 |
| H-Y stage, mean (SD) | 2.48 (0.82) | 2.52 (0.70) | 2.66 (0.57) | 2.38 (0.47) | 0.602 |
Categorical variables are presented as n (percentage, %), continuous variables are presented as mean (standard deviation, SD). Group comparisons were performed using chi-square test or analyses of variance as appropriate.
PD Parkinson’s disease, MH minor hallucinations, VH visual hallucinations, SD standard deviation, BMI body mass index, LEDD levodopa equivalent daily dose, UPDRS Unified Parkinson’s Disease Rating Scale, H-Y Hoehn and Yahr.
Bold value indicates statistical significance at a two-tailed p value of 0.05.
Associations of the number of MH subtypes with VH
As shown in Fig. 1, Log-binomial regression analysis showed a positive association between the number of MH and the prevalence of VH (RR = 1.08, 95% CI: 0.53–2.21 for 1 MH; 2.08, 1.11–3.90 for 2 MH; 6.20, 3.40–11.31 for 3 MH). There was no significant interaction between age and MH counts (p = 0.734); the associations between the number of MH and VH prevalence were similar in patients aged <67 years and those aged ≥67 years (Supplementary Table S3). A significant interaction was observed between sex and MH counts in relation to VH prevalence (p = 0.027). In females, a dose-response relationship was observed between the number of MH and VH prevalence: the prevalence of VH increased progressively with higher MH counts (RR = 3.94, 95% CI: 1.35–11.51 for 1 MH; 9.49, 2.30–39.14 for 2 MH; 30.33, 8.09–113.67 for 3 MH). In males, patients with 3 MH showed a significant association with the prevalence of VH (RR = 2.65, 95% CI: 1.13–6.20), while those with a lower number of MH did not have significant associations. Exploratory analyses of the eight-category exposure variable found that all MH categories had a greater VH prevalence compared to those who had no MH (though only the group with 3 MH was statistically significant), except that statistical comparison could not be performed among those who had only presence hallucination (Supplementary Table S4). The frequency of MH appeared to have a dose-dependent association with VH (RR = 1.61, 95% CI: 0.84–3.08 for <1/week; 1.82, 0.89–3.72 for 1–6 times/week; 2.53, 1.34–4.77 for daily) (Supplementary Table S5).
Fig. 1. Associations between VH and the number of MH, stratified by sex.

RRs for VH risk from log-binomial regression are depicted as blue boxes, and 95% CIs are shown as horizontal lines. All analyses were adjusted for age, sex, education, duration, levodopa equivalent daily dose, Unified Parkinson’s Disease Rating Scale score and Hoehn and Yahr stage. MH minor hallucinations, RR relative risk, CI confidence interval.
Comparison of clinical characteristics scores across different numbers of MH subtypes
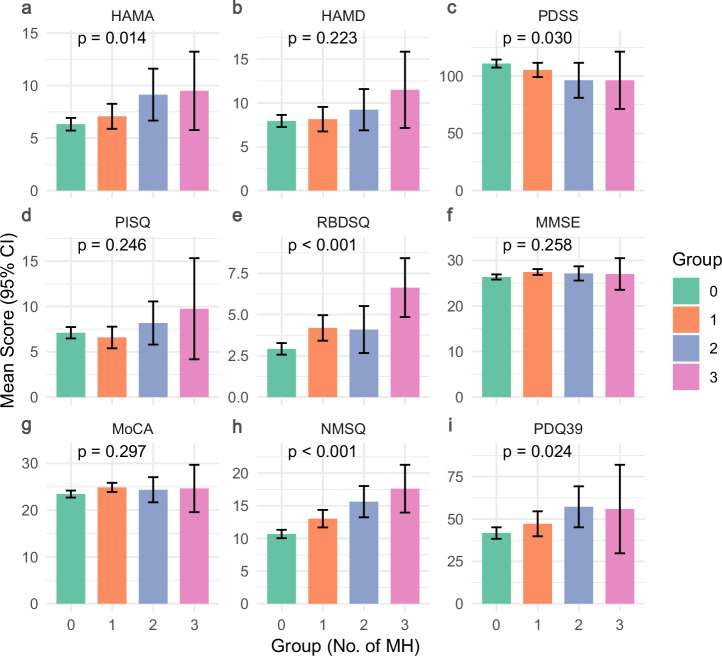
Figure 2 presents mean values with 95% CI for nine PD-related clinical characteristics scores across different numbers of MH subtypes. There were statistically significant differences in scores of Hamilton Anxiety Rating Scale (HAMA), PD Sleep Scale (PDSS), Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Non-Motor Symptoms Questionnaire (NMSQ), and Parkinson’s Disease Questionnaire-39 (PDQ39) between groups with different numbers of MH subtypes; specifically, the groups with more MH subtypes exhibited elevated anxiety scores (HAMA: p = 0.014), more severe sleep disorders (RBDSQ: p < 0.001), increased non-motor symptoms (NMSQ: p < 0.001), and poorer quality of life (PDQ39: p = 0.024). In contrast, global cognitive measures [Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA)] showed no significant variation across MH groups (p = 0.258 and p = 0.297, respectively).
Fig. 2. Comparison of clinical characteristics among groups with different numbers of MH in PD.
Bar charts comparing clinical scale scores (a HAMA, b HAMD, c PDSS, d PISQ, e RBDSQ, f MMSE, g MoCA, h NMSQ, i PDQ39) across different numbers of MH using analyses of variance. Error bars represent 95% confidence intervals. HAMA Hamilton Anxiety Rating Scale, HAMD Hamilton Depression Rating Scale, PDSS PD Sleep Scale, PSQI Pittsburgh Sleep Quality Index, RBDSQ Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, NMSQ Non-Motor Symptoms Questionnaire, PDQ39 Parkinson’s Disease Questionnaire-39, CI confidence interval.
Associations of clinical characteristics scores with the number of MH subtypes
Log-binomial regression analyses showed that most clinical characteristics were associated with the number of MH subtypes in PD patients (Table 2). Anxiety symptoms (HAMA) demonstrated a dose-response association with increasing number of MH subtypes (reference outcome group: no MH), with RRs progressively increasing from 1.25 (95% CI: 0.99–1.58) for 1 MH, to 1.49 (1.01–2.21) for 2 MH, and reaching 2.59 (1.33–5.02) for 3 MH. A similar pattern for associations was also found for PDSS, RBDSQ, NMSQ, and PDQ39, while depression (HAMD) and overall sleep quality (PISQ) scores were only associated with the category of 3 MH subtypes (vs. no MH). Of note, sleep disturbances (RBDSQ) exhibited strong associations, with RRs increasing from 1.37 to 4.20 across an increasing number of MH subtypes. Non-motor symptom burden (NMSQ) had particularly striking associations, with RRs escalating from 1.58 to 6.59 with increasing MH counts. Interestingly, cognitive assessments appeared to have some protective associations with the number of MH subtypes (MMSE: RR = 1.65, 95% CI 1.12–2.42, p = 0.011; MoCA: 1.55, 1.05–2.28, p = 0.028).
Table 2.
Log-binomial regressions for associations between clinical characteristics and the number of MH among PD patients
| No. of MHa | ||||||
|---|---|---|---|---|---|---|
| 1 vs 0 | 2 vs 0 | 3 vs 0 | ||||
| RR (95% CI) | p value | RR (95% CI) | p value | RR (95% CI) | p value | |
| HAMA | 1.25 (0.99, 1.58) | 0.055 | 1.49 (1.01, 2.21) | 0.046 | 2.59 (1.33, 5.02) | 0.005 |
| HAMD | 1.09 (0.86, 1.38) | 0.485 | 1.08 (0.74, 1.58) | 0.678 | 2.09 (1.17, 3.73) | 0.013 |
| PDSS | 0.82 (0.66, 1.01) | 0.061 | 0.71 (0.48, 1.05) | 0.088 | 0.56 (0.34, 0.91) | 0.019 |
| PISQ | 0.91 (0.71, 1.16) | 0.461 | 1.04 (0.66, 1.61) | 0.879 | 1.74 (0.71, 4.21) | 0.223 |
| RBDSQ | 1.37 (1.11, 1.68) | 0.004 | 1.43 (0.90, 2.27) | 0.130 | 4.20 (2.35, 7.52) | <0.001 |
| MMSE | 1.65 (1.12, 2.42) | 0.011 | 1.48 (0.58, 3.77) | 0.408 | 1.83 (0.84, 3.97) | 0.127 |
| MoCA | 1.55 (1.05, 2.28) | 0.028 | 1.38 (0.53, 3.55) | 0.509 | 2.37 (0.59, 9.54) | 0.225 |
| NMSQ | 1.58 (1.23, 2.03) | <0.001 | 2.52 (1.52, 4.16) | <0.001 | 6.59 (1.67, 26.01) | 0.007 |
| PDQ39 | 1.40 (1.06, 1.86) | 0.020 | 1.59 (0.94, 2.70) | 0.085 | 3.47 (1.11, 10.84) | 0.033 |
All clinical characteristics were standardized as z scores for analyses.
All analyses were adjusted for age, sex, education, duration, levodopa equivalent daily dose, Unified Parkinson’s Disease Rating Scale III score and Hoehn and Yahr stage.
MH minor hallucinations, RR relative risk, CI confidence interval, HAMA Hamilton Anxiety Rating Scale, HAMD Hamilton Depression Rating Scale, PDSS PD Sleep Scale, PSQI Pittsburgh Sleep Quality Index, RBDSQ Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, NMSQ Non-Motor Symptoms Questionnaire, PDQ39 Parkinson’s Disease Questionnaire-39.
aIndividuals who had no MH were used as the referent group for the outcome.
Bold value indicates statistical significance at a two-tailed p value of 0.05.
Discussion
This study provides novel insights into the clinical significance of the number of MH in PD patients, demonstrating a strong association between the presence of multiple MH and the risk of VH, as well as other PD-related clinical symptoms. Specifically, by systematically quantifying MH subtypes (presence hallucinations, passage hallucinations, and visual illusions), we extend beyond the conventional binary classification of MH and demonstrated for the first time, that it is not merely the presence of MH, but rather the number of MH subtypes, that was a stronger factor associated with the prevalence of VH. Additionally, an increase in the number of MH was associated with the deterioration of non-motor symptoms such as mood and sleep in PD patients. These findings may have important clinical implications in that patients experiencing multiple MH may benefit from enhanced monitoring, targeted counseling on hallucination management, and early intervention. This is particularly relevant for patients with comorbid mood, sleep disorders, and poorer quality of life, who may derive clinical benefit from treatment at even lower MH subtype counts. These insights emphasize the importance of quantifying MH subtypes when assessing hallucinations in PD patients, though their potential utility as predictive markers of disease progression and targets for early intervention requires validation through longitudinal studies.
Multiple studies have explored the relationship between the binary classification of MH and VH. MH typically occur in the early stage of the disease and serve as a strong predictor of VH. Neuroimaging studies have demonstrated that PD patients with MH and VH exhibit similar patterns of altered brain network connectivity, with VH being associated with more pronounced network disruptions15,16. However, these studies were limited to examining the relationship between the dichotomous (presence or absence) variable of MH and VH, without considering the clinical significance of MH subtype quantification, despite the common phenomenon of coexisting multiple MH subtypes17,18. Our work fills this gap by showing that PD patients with only one MH had a similar prevalence of VH compared to those without MH, while those with multiple MH had a substantially increased prevalence of VH (up to 6-fold in those with 3 MH). Prospective cohort studies and randomized controlled trials are needed to determine whether the presence of only one MH might indicate an early stage of the disease progression, and intervening at this stage may prevent the further increase in the number of MH and thus reduce the risk of VH.
Although the pathophysiological mechanisms underlying MH and VH in PD remain incompletely understood, emerging evidence suggests both distinct and shared neural pathways. Neuroimaging studies indicate that MH primarily involve brainstem dysfunction, whereas VH are associated with temporal-parietal-occipital network impairment11,19. Conversely, recent evidence also suggests overlapping neural substrates and shared network characteristics between MH and VH (e.g., alterations in functional connectivity within the default mode network)15,16. Electroencephalographic studies further demonstrate shared frontal theta oscillations in both MH and VH, implicating common cholinergic dysfunction in frontal—subcortical networks20–22. These shared pathways provide a pathophysiological basis for their association. The independent associations of MH subtypes with VH can be explained by their distinct neural mechanisms: presence hallucinations may reflect precuneus dysfunction within the default mode network23, passage hallucinations could involve motion-processing areas (V5/MT) through disrupted dorsal visual stream connectivity24, while visual illusions may result from primary occipital cortex hyperexcitability combined with top-down parietal disinhibition25. Thus, the co-occurrence of multiple MH subtypes may signify more complex neural network disruption and more severe neurotransmitter disturbances, consequently increasing susceptibility to VH.
This study found that the associations between the number of MH and VH prevalence were much stronger in female patients. In females, there was a clear dose-response relationship, with the prevalence of VH increasing progressively as the number of MH increased, while in males, a significant association was only observed among those with 3 MH subtypes. These sex differences were partly supported by the finding that female sex emerged as a risk factor for hallucination development during the follow-up of PD patients26. Although extensive studies have shown that the prevalence of PD in females is lower than that in males, the disease progresses more quickly in females, and the severity of the disease is associated with the risk of hallucinations26,27. Moreover, different genetic, hormonal and lifestyle factors may also lead to sex differences in the pathogenesis of PD28. Therefore, future research elucidating sex-specific mechanisms underlying VH among PD patients may facilitate the development and implementation of sex-specific prevention and treatment strategies.
We found that multiple PD-related clinical characteristics were associated with the number of MH, especially the coexistence of three subtypes of MH. Higher mood scores, more severe sleep disorders, increased non-motor symptoms, and poorer quality of life were observed in groups with more MH. These factors have all been previously found to be related to the occurrence of VH in PD patients29–31. Our study further confirms and extends on these previous findings. Therefore, it may be worthy screening for these non-motor symptoms in the early stage of PD disease. Cognitive impairment is an important risk factor for VH9,32, but the relationship between cognitive function and the number of MH subtypes remains unclear. A previous study showed no significant differences in overall cognitive function between PD patients with and without MH33. Our study also did not observe a meaningful difference in cognitive function evaluated by the MMSE and MoCA between groups with varying numbers of MH. However, in a longitudinal cohort study, Bejr-Kasem et al. found that MH development in early disease stages predicted cognitive decline after 5 years of follow-up7. Similarly, the research by Bernasconi et al. also showed that the frontal-subcortical cognitive functions declined significantly over 5 years in PD patients with MH compared to those without MH20. This discrepancy may arise because MH primarily affect visual/perceptual systems, whereas standard cognitive assessments (MMSE/MoCA) focus on core domains like executive function and memory, thereby underestimating their correlation. Additionally, compensatory effects from cognitive reserve and specific alterations in brain structure15, visual/attentional networks16 and neurotransmitter systems34–36 may mask abnormalities in global cognitive assessments. Further longitudinal studies using tools to assess PD-specific cognitive deficits are needed to clarify the relationship between the number of MH with cognitive functioning.
Certain limitations are present in our study. First, the cross-sectional design precludes causal inferences regarding the relationship between the number of MH with VH or investigating the progression of other clinical symptoms. Numerous existing studies have demonstrated that MH typically occurs in the early stages of PD7,10,37, whereas VH predominantly manifests in the middle to late stages of the disease38, and longitudinal studies have further confirmed that MH is an independent risk factor for VH10,12. Second, the relatively small sample size limited in-depth analysis of different MH subtype combinations and may affect the accuracy of sex-stratified results. Future studies with longitudinal approaches and larger samples are warranted to confirm our findings. Third, given that the specific phenomenological contents of hallucinations were collected using a semi-structured interview, our results should be validated using more detailed structured assessment tools. The exclusive use of generic cognitive screening tools (MMSE and MoCA) in our study also represents a methodological limitation, as they lack sensitivity to detect PD-specific cognitive deficits, particularly in visuospatial and attentional domains. Thus, our findings about cognitive function should be validated by future studies using the Mini-Mental Parkinson39 scale, which was specifically validated to assess these PD-related cognitive impairments. Fourth, we were not able to accurately capture cumulative frequencies of MH subtypes, and future studies should specifically examine the association of MH frequency with VH. Finally, our findings are based on neuropsychiatric questionnaires, it is necessary to use objective clinical monitoring measures such as magnetic resonance and electroencephalography to analyze structural and functional differences between different subtypes of VH.
In conclusion, our study demonstrated that the number of MH subtypes is associated with the prevalence of VH in PD patients, and PD-related clinical characteristics also differed by the number of MH. These findings emphasize the importance of quantifying MH subtypes when assessing hallucinations in PD patients, though their potential utility as predictive markers of disease progression and targets for early intervention requires validation through longitudinal studies. Further longitudinal studies are also needed to validate these results and further explore the underlying neurobiological mechanisms.
Methods
Participants
This cross-sectional study included 372 participants recruited from the geriatric neurology outpatient clinic and inpatient ward of Nanjing Brain Hospital, affiliated with Nanjing Medical University, from April 2020 to September 2024. All participants met the MDS Clinical Diagnostic Criteria for PD40 and were diagnosed by at least two neurologists. Exclusion criteria included: current or previous history of secondary Parkinsonism; history of major psychiatric disorders or use of any antipsychotic medications; participants who experienced only other types of structured hallucinations without concomitant MH or VH; focal brain lesions on MRI scans; severe ocular diseases (e.g., glaucoma, cataracts); other neurological disorders (e.g., stroke, epilepsy) or serious medical diseases. All participants provided written informed consent and contributed clinical data. The study was approved by the local Ethics Committee of Nanjing Brain Hospital Affiliated with Nanjing Medical University and conducted in accordance with the Declaration of Helsinki.
Minor and visual hallucinations assessment
All participants and their caregivers were investigated for MH and VH by experienced clinicians or researchers. Hallucination screening was performed based on Item 1.2 (Hallucinations and psychosis) of the Movement Disorder Society Unified Parkinsons Disease Rating Scale (MDS-UPDRS): 0 (Normal): no hallucinations or psychotic behavior; 1 (Slight): illusions or non-formed hallucinations, but patient recognizes them without loss of insight; 2 (Mild): formed hallucinations independent of environmental stimuli. No loss of insight; 3 (Moderate): formed hallucinations with loss of insight; 4 (Severe): patient has delusions or paranoia41. The A semi-structured questionnaire was subsequently administrated to confirm the subtypes of hallucinations and obtain detailed phenomenological contents (frequency, duration, and other details of phenomena of each subtype; Supplementary Materials). Detailed phenomenological characteristics for participants with each type of hallucinations are presented in Supplementary Table S1; the numbers of patients experiencing presence hallucinations, passage hallucinations, visual illusions, and VH were 46, 55, 77, and 55, respectively (non-mutually exclusive counts, so participants were counted in more than one category when hallucinations co-occurred). MH may occur as a single isolated subtype or in combinations of two or three subtypes, which may also co-occur with VH. For primary analyses, we classified participants into 4 categories by the number of MH subtypes primary exposure: 258 patients with no MH, 66 with 1 MH, 32 with 2 MH, and 16 with 3 MH. In exploratory analyses (see “Statistical Analysis”), we classified participants into: (1) 8 categories based on all possible combinations considering both the number and subtype of MH: no MH, presence hallucination, passage hallucination, visual illusion, presence hallucination and passage hallucination, presence hallucination and visual illusion, passage hallucination and visual illusion, and 3 MH; (2) 4 categories by the frequency of MH: 0, <1/week, 1–6 times/week, Daily (classification was based on the highest frequency subtype observed for patients experiencing multiple MH subtypes).
Clinical assessment
Demographic data and health-related characteristics of the participants were collected through questionnaires and electronic medical records, including age, sex, education, height, weight, smoking status, alcohol consumption, tea consumption, coffee consumption, exercise habits, allergies, medical history, and medications (detailed PD medications are summarized in the Supplementary Table S2). Participants with a history of major psychiatric disorders (e.g., schizophrenia, bipolar disorder, major depressive disorder) or use of antipsychotic medications were excluded from the study. The LEDD was calculated according to the standard method42. PD motor symptom severity was assessed according to the UPDRS part III score and the H-Y stage. PD-related clinical characteristics were assessed as follows: The NMSQ43 and PDQ3944 were used to assess overall non-motor symptoms and quality of life, respectively. We assessed moods using the HAMA45 and the Hamilton Depression Rating Scale (HAMD)46. Sleep quality and the severity of sleep disorders were evaluated using the PDSS47 and the Pittsburgh Sleep Quality Index (PSQI)48. The RBDSQ49 was used to further evaluate RBD. The MMSE and the MoCA were used to assess global cognitive function50. Higher scores on the UPDRS, NMSQ, PDQ39, HAMA, HAMD, PSQI, and RBDSQ scales indicate greater symptom severity, while higher scores on the PDSS, MMSE, and MoCA reflect better functional status. All assessments were conducted in the off-medication state.
Statistical analysis
Descriptive statistics were used to summarize participant characteristics, with continuous variables being presented as mean standard deviation SD, while categorical variables as number (percentage). Comparisons of these variables between participants with different numbers of MH subtypes (the primary exposure) were performed using chi-square tests or Fisher’s exact tests, as appropriate, for categorical variables and analyses of variance for continuous variables.
We estimated relative risks (RRs) with 95% confidence intervals (CIs) using multivariable log-binomial regression models to examine: (1) the associations between the number of MH and VH prevalence, and (2) the associations between PD-related clinical characteristics (standardized as z scores) and the number of MH (no MH used as the referent group for outcome comparison). All models were adjusted for the following covariates: age, sex, education level, disease duration, LEDD, UPDRS III score, and H-Y stage. We also performed analyses of variance to compare the differences in PD-related clinical characteristics across different numbers of MH subtypes.
We also examined for differences in the associations between the number of MH subtypes and VH prevalence by sex (female/male) and age (<67 years/≥67 years) through interaction (i.e., including a product term of the exposure with sex or age) and stratification analyses by these variables. In exploratory analyses, we examined the associations of both the eight-category variable for all combinations of MH subtypes (see methods above for details) and MH frequency (0, <1/week, 1–6 times/week, and daily) with VH prevalence. All statistical analyses were performed using STATA software (version 17.0, StataCorp LLC, Texas, USA), and data visualizations were conducted using R software (version 4.4.3, R Foundation for Statistical Computing, Vienna, Austria). A 2-sided p value of <0.05 was set as significant.
Supplementary information
Acknowledgements
We appreciate the participation of patients and their families in this study. This work was supported by the National Natural Science Foundation of China (Grant No. 82171249), Nanjing Rehabilitation Medicine Center Project Special Funds of the Jiangsu Provincial Key Research and Development Program (Grant No. BE2022842), Jiangsu Province Elderly Health Project (Grant No. LD2021013) and Jiangsu Provincial Cadre Health Projects (Grant No. BJ20005).
Author contributions
H.Z.: Conceptualization; investigation; writing—original draft; methodology; validation; visualization; writing—review and editing; formal analysis; data curation, Y.Z.: Investigation; validation; writing—review and editing; data curation, Y.C.: Investigation; data curation; writing—review and editing, Y.F.: Investigation; data curation, B.S.: Conceptualization; investigation; methodology; data curation, S.D and J.Z.: Investigation; data curation, X.J. and D.L.: Investigation, Y.C.: Investigation, F.H: Conceptualization; supervision, L.Z: Conceptualization; funding acquisition; project administration; resources; supervision. All authors reviewed the manuscripts.
Data availability
Data are available upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-025-01106-9.
References
- 1.Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet397, 2284–2303 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Su, D. et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: modelling study of Global Burden of Disease Study 2021. BMJ388, e080952 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira, A. H. V., Chaudhuri, K. R. & Jenner, P. Non-motor features of Parkinson's disease. Nat. Rev. Neurosci.18, 435–450 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Pagonabarraga, J., Bejr-Kasem, H., Martinez-Horta, S. & Kulisevsky, J. Parkinson disease psychosis: from phenomenology to neurobiological mechanisms. Nat. Rev. Neurol.20, 135–150 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Collerton, D. et al. Understanding visual hallucinations: a new synthesis. Neurosci. Biobehav. Rev.150, 105208 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M. & Morris, J. G. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov. Disord.23, 837–844 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Lenka, A., Pagonabarraga, J., Pal, P. K., Bejr-Kasem, H. & Kulisevsky, J. Minor hallucinations in Parkinson’s disease: a subtle symptom with major clinical implications. Neurology93, 259–266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetz, C. G. & Stebbins, G. T. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology45, 669–671 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Bronnick, K., Emre, M., Tekin, S., Haugen, S. B. & Aarsland, D. Cognitive correlates of visual hallucinations in dementia associated with Parkinson’s disease. Mov. Disord.26, 824–829 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Pagonabarraga, J. et al. Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov. Disord.31, 45–52 (2016). [DOI] [PubMed] [Google Scholar]
- 11.D’Antonio, F. et al. Visual hallucinations in Lewy body disease: pathophysiological insights from phenomenology. J. Neurol.269, 3636–3652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fénelon, G., Soulas, T., Cleret de Langavant, L., Trinkler, I. & Bachoud-Lévi, A. C. Feeling of presence in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry82, 1219–1224 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bejr-Kasem, H. et al. Minor hallucinations reflect early gray matter loss and predict subjective cognitive decline in Parkinson’s disease. Eur. J. Neurol.28, 438–447 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Nishio, Y. et al. Defining visual illusions in Parkinson’s disease: Kinetopsia and object misidentification illusions. Park. Relat. Disord.55, 111–116 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Baik, K. et al. Functional brain networks of minor and well-structured major hallucinations in Parkinson’s disease. Mov. Disord.39, 318–327 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Bejr-Kasem, H. et al. Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson’s disease. Mov. Disord.34, 78–86 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Zhang, Y. et al. Clinical characteristics of minor hallucinations in Chinese Parkinson’s disease patients. Front. Aging Neurosci.13, 723405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong, M. et al. Prevalence and risk factors for minor hallucinations in patients with Parkinson’s disease. Behav. Neurol.2021, 3469706 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pezzoli, S. et al. Neuroanatomical and cognitive correlates of visual hallucinations in Parkinson’s disease and dementia with Lewy bodies: voxel-based morphometry and neuropsychological meta-analysis. Neurosci. Biobehav. Rev.128, 367–382 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Bernasconi, F. et al. Theta oscillations and minor hallucinations in Parkinson’s disease reveal decrease in frontal lobe functions and later cognitive decline. Nat. Ment. Health1, 477–488 (2023). [Google Scholar]
- 21.Dauwan, M. et al. Aberrant resting-state oscillatory brain activity in Parkinson’s disease patients with visual hallucinations: an MEG source-space study. Neuroimage Clin.22, 101752 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baik, K. et al. Implication of EEG theta/alpha and theta/beta ratio in Alzheimer’s and Lewy body disease. Sci. Rep.12, 18706 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba, Y. et al. Clinical profiles of dementia with Lewy bodies with and without Alzheimer’s disease-like hypometabolism. Int. J. Geriatr. Psychiatry30, 316–323 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Born, R. T. & Bradley, D. C. Structure and function of visual area MT. Annu. Rev. Neurosci.28, 157–189 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Watanabe, H. et al. Behavioral and neural correlates of pareidolic illusions in dementia with Lewy bodies. Park. Relat. Disord.113, 105513 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Zhu, K., van Hilten, J. J., Putter, H. & Marinus, J. Risk factors for hallucinations in Parkinson’s disease: results from a large prospective cohort study. Mov. Disord.28, 755–762 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Vaidya, B., Dhamija, K., Guru, P. & Sharma, S. S. Parkinson’s disease in women: mechanisms underlying sex differences. Eur. J. Pharm.895, 173862 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Schaffner, S. L., Tosefsky, K. N., Inskter, A. M., Appel-Cresswell, S. & Schulze-Hentrich, J. M. Sex and gender differences in the molecular etiology of Parkinson’s disease: considerations for study design and data analysis. Biol. Sex. Differ.16, 7 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Nunez, A. E. et al. Clinically probable RBD is an early predictor of malignant non-motor Parkinson’s disease phenotypes. NPJ Parkinsons Dis.11, 25 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang, Y. et al. Minor hallucinations in Parkinson’s disease with probable rapid eye movement sleep behavior disorder. Front. Neurosci.17, 1205439 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fénelon, G., Mahieux, F., Huon, R. & Ziégler, M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain123, 733–745 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Puntambekar, I. & J, A. F. Neuropsychological correlates of visual hallucinatory phenomena in Lewy body disease. Int. J. Geriatr. Psychiatry38, e5950 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Llebaria, G. et al. Neuropsychological correlates of mild to severe hallucinations in Parkinson’s disease. Mov. Disord.25, 2785–2791 (2010). [DOI] [PubMed] [Google Scholar]
- 34.d’Angremont, E. et al. Cholinergic deficiency in Parkinson’s disease patients with visual hallucinations. Brain147, 3370–3378 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huot, P. et al. Increased 5-HT2A receptors in the temporal cortex of Parkinsonian patients with visual hallucinations. Mov. Disord.25, 1399–1408 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Ignatavicius, A., Matar, E. & Lewis, S. J. G. Visual hallucinations in Parkinson’s disease: spotlight on central cholinergic dysfunction. Brain148, 376–393 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fénelon, G., Soulas, T., Zenasni, F. & Cleret de Langavant, L. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov. Disord.25, 763–766 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stang, C. D. et al. Incidence, prevalence, and mortality of psychosis associated with Parkinson’s disease (1991–2010). J. Parkinsons Dis.12, 1319–1327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa, A. et al. Mini mental Parkinson test: standardization and normative data on an Italian sample. Neurol. Sci.34, 1797–1803 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord.30, 1591–1601 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord.23, 2129–2170 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Jost, S. T. et al. Levodopa dose equivalency in Parkinson’s disease: updated systematic review and proposals. Mov. Disord.38, 1236–1252 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Chaudhuri, K. R. et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov. Disord.21, 916–923 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Peto, V., Jenkinson, C., Fitzpatrick, R. & Greenhall, R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual. Life Res.4, 241–248 (1995). [DOI] [PubMed] [Google Scholar]
- 45.Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol.32, 50–55 (1959). [DOI] [PubMed] [Google Scholar]
- 46.Williams, J. B. A structured interview guide for the Hamilton Depression Rating Scale. Arch. Gen. Psychiatry45, 742–747 (1988). [DOI] [PubMed] [Google Scholar]
- 47.Chaudhuri, K. R. et al. The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry73, 629–635 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buysse, D. J., Reynolds, C. F. 3rd, Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res.28, 193–213 (1989). [DOI] [PubMed] [Google Scholar]
- 49.Stiasny-Kolster, K. et al. The REM sleep behavior disorder screening questionnaire-a new diagnostic instrument. Mov. Disord.22, 2386–2393 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Gill, D. J., Freshman, A., Blender, J. A. & Ravina, B. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov. Disord.23, 1043–1046 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the corresponding author.