Abstract
Background:
The recommended treatment for high-risk non-muscle invasive bladder cancer (NMIBC) is intravesical instillations of Bacillus Calmette-Guérin (BCG). Despite completing BCG therapy, up to 40% of patients experience disease recurrence within five years. T cell exhaustion has been associated with poor outcome following treatment with BCG.
Objective:
In this study, we investigated whether T cell exhaustion, characterized by tumor protein expression of PD-1 and PD-L1 in paired samples obtained before and after BCG treatment could provide further insight into BCG response and help predict outcome in patients with NMIBC.
Methods:
Tumor samples from 104 patients with NMIBC were collected before and after BCG. Sections from tissue microarrays were stained using immunohistochemistry to analyze the protein expression of PD-1 and PD-L1. Data was analyzed using digital pathology software.
Results:
High PD-1 expression was associated with higher tumor stage and grade pre-BCG (p = 0.001 and p = 0.002) and with tumor stage post-BCG (p = 0.005). PD-L1 was associated with higher tumor stage in pre- and post-BCG samples (p = 0.006 and p = 0.048). Patients with low expression of PD-1 and PD-L1 in the pre-BCG tumor had a superior high-grade recurrence-free survival (HG-RFS) compared to patients with high PD-1 (p = 0.008) and PD-L1 (p = 0.006) expression.
Conclusion:
Protein expression of the exhaustion markers PD-1 and PD-L1 in pre-BCG tumor samples were correlated to higher stage and grade as well as worse HG-RFS, indicating that T cell exhaustion may play an important role in resistance to BCG treatment.
Keywords: bladder cancer, BCG, tumor microenvironment, immunohistochemistry, digital image analysis
Introduction
Patients with high-risk non-muscle invasive bladder cancer (NMIBC) receive intravesical instillations of BCG as standard treatment, but up to 40% experience disease recurrence within five years. 1 Many underlying mechanisms can be responsible for this insufficiency and one hypothesis is that the immune landscape in and around the tumor tissue plays a central role in treatment failure.
Hence, further understanding of the tumor microenvironment (TME) may elucidate important characteristics of bladder cancer disease biology and mechanisms of treatment response and resistance. For many cancers, including bladder cancer, the TME composition and its dynamic behavior is known to influence tumorigenesis, progression to metastatic disease and treatment response.2,3
The PD-1/PD-L1 pathway is a key player in antitumor immunity. PD-1 is a regulatory receptor, located on activated T cells, and when engaged with its ligands (PD-L1 or PD-L2) inhibits immune effector functions. 4 This is one of several mechanisms that characterize T cell exhaustion and it presents a way for cancer cells to commandeer the immune system to allow for immune evasion and tumor growth. 4 Several cancers have demonstrated the involvement of the PD-1/PD-L1 axis in tumorigenesis.5–7 Studies investigating the protein expression of PD-1 and/or PD-L1 in BCG-treated NMIBC has found that baseline PD-L1 expression is associated with worse outcome and has opened the conversation of combining Bacillus Calmette-Guérin (BCG) treatment with immune checkpoint inhibitor (ICI) therapy.8–13 Hence, the investigation of the inhibitory receptor and its ligands offers insights into tumor biology and possible BCG and ICI resistance mechanisms.
There is an urgent need for stratification of NMIBC patients to therapeutically improve recurrence and progression outcomes. Identifying patients who are unlikely to benefit from BCG treatment would allow timely initiation of alternative approaches such as combination therapy with BCG and ICI, or ICI alone.
In this study, we aim to delineate the influence of the T cell exhaustion molecules PD-1 and PD-L1 on outcomes and treatment response in patients with NMIBC by examining the TME composition.
Methods
Patients details and sample selection
From a retrospectively selected cohort of 156 patients with NMIBC, 14 who received at least five BCG instillations, 104 patients were included in this study. Clinicopathological information can be found in Table 1. Tumor samples from transurethral resections of bladder tumors (TURBT) were collected from the patients before (pre-BCG), and if patients experienced recurrence, after BCG treatment (post-BCG). Post-BCG samples were only included if there was a pre-BCG sample present. This resulted in 149 tumor samples which were included in tissue microarrays (TMA) with triplicate core biopsies of 1 mm diameter. All patients gave written informed consent to participate in future research, and this study was approved by the National Ethics Committee (#1708266).
Table 1.
Baseline characteristics.
| All patients | BCG Response | |||
|---|---|---|---|---|
| Characteristic | N = 104 a | Post BCG HG-recurrence N = 50 a |
No post BCG HG-reccurence N = 53 a |
p-value b |
| Sex | 0.3 | |||
| Female | 20 (19%) | 12 (24%) | 8 (15%) | |
| Male | 84 (81%) | 38 (76%) | 45 (85%) | |
| Age at diagnosis | 68 (59, 74) | 68 (59, 74) | 67 (58, 74) | >0.9 |
| Smoking Status | 0.5 | |||
| Current | 43 (41%) | 20 (40%) | 24 (45%) | |
| Former | 48 (46%) | 25 (50%) | 22 (42%) | |
| Never | 12 (12%) | 4 (8.0%) | 7 (13%) | |
| Unknown | 1 (1.0%) | 1 (2.0%) | 0 (0%) | |
| Follow-up time (years) c | 6.3 (3.0, 8.8) | 5.5 (2.8, 9.2) | 6.7 (3.9, 8.5) | 0.3 |
| EAU risk group | 0.7 | |||
| Intermediate Risk | 39 (38%) | 19 (38%) | 21 (40%) | |
| High Risk | 42 (40%) | 18 (36%) | 22 (42%) | |
| Very High Risk | 23 (22%) | 13 (26%) | 10 (19%) | |
| pre-BCG tumor stage | 0.7 | |||
| Ta | 64 (62%) | 30 (60%) | 34 (64%) | |
| T1 | 40 (38%) | 20 (40%) | 19 (36%) | |
| pre-BCG tumor grade | 0.006 | |||
| Low grade | 35 (34%) | 10 (20%) | 24 (45%) | |
| High grade | 69 (66%) | 40 (80%) | 29 (55%) | |
| post-BCG tumor stage | 0.13 | |||
| Ta | 35 (80%) | 21 (72%) | 14 (93%) | |
| T1 | 9 (20%) | 8 (28%) | 1 (6.7%) | |
| post-BCG tumor grade | 0.002 | |||
| Low grade | 21 (49%) | 9 (31%) | 12 (80%) | |
| High grade | 23 (51%) | 20 (69%) | 3 (20%) | |
n (%); Median (Q1, Q3)
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test
From RNA sequenced pre-BCG tumor sample to end of follow-up
Clinical follow-up
End of follow-up (FU) was defined as the latest of the following: last cystoscopy, last tumor detected, cystectomy, progression to MIBC or metastatic disease. High grade recurrence free survival (HG-RFS) was measured from the end of BCG induction to first high grade recurrence, progression to MIBC or development of metastatic disease or end of FU. Patients were defined as BCG HG-recurrent if they had a high grade recurrence within two years after BCG or progressed to MIBC/metastatic disease and BCG non-HG-recurrent if they did not develop a high grade recurrence within two years and did not progress to MIBC/metastatic disease
Immunohistochemistry
Standard bright-field immunohistochemistry (IHC) of the TMA's was performed to visualize the protein expression of PD-1 (clone EP-239) and PD-L1 (clone 22c3). TMA sections of 3 µm thickness were cut sequentially (PD-1 → Pan-CK → PD-L1) and mounted on SuperFrost glass slides. The staining protocol was performed using either BenchMark Ultra (Ventana Medical Systems; PD-1) or EnVision Flex (Dako Omnis; PD-L1 and pan-cytokeratin [PanCK]). The sections were deparaffinized at 72°C followed by heat-induced epitope retrieval (HIER) at 97°C (see further specifications in Supplementary Table 1). Peroxidase activity was inhibited, primary antibody added and the slides were counterstained with hematoxylin II followed by a bluing agent. Next, bright-field imaging was performed using the Hamamatsu NanoZoomer 2.0 HT (Hamamatsu Photonics) at 20x magnification.
Digital image analysis
To identify and calculate the positive stained cells we utilized Digital Image Analysis (DIA) software Visiopharm® (Hørsholm, Denmark) and the IHC stained slides were digitized using the Hamamatsu Nanozoomer 2.0 HT. In the DIA workflow, PanCK was used to distinguish between carcinoma and stromal regions of the tumor tissue. Cells were scored positive for PD-1 or PD-L1 if a chromogenic signal was colocalized with the presence of nuclei. Detailed information on the DIA methods and settings used for this study can be found in Supplementary Methods.
Calculation of fractions
An expression fraction of positivity was calculated per tissue core by dividing the number of positive stained cells with the total number of cells for the defined region in question (carcinoma, stroma and total tissue). The average value of these fractions determined the protein expression per sample. A median split was used for dichotomization of the expression results for survival analyses and association analyses with genomic and transcriptomic data (Supplementary Table 2).
Whole-exome sequencing and RNA sequencing data
From previously-generated whole-exome sequencing (WES) and RNA-sequencing data, 14 we used genomic tumor mutational burden (TMB) and mutational signatures (COSMIC SBS) 15 and the transcriptomic T-cell exhaustion score and UROMOL classifications, respectively. The UROMOL classification system is based on a transcriptomic analysis which has generated four classes (1, 2a, 2b and 3), which reflect tumor biology and disease progression. 16
Statistical analysis
To compare and determine the independence between groups, Wilcoxon rank sum test and Kruskral-Wallis-test were used for numerical data and Fisher's exact test was used for categorical data. For comparison of survival curves between groups, we used log-rank test and to determine the influence of variables on survival times for both uni- and multivariate analyses, we used Cox regression analysis. We performed time-dependent ROC statistics, and compared areas under the curve (AUCs) using DeLong's test for two correlated ROC curves. All p values below 0.05 were considered statistically significant and data analysis was performed using R (version 4.4.1 and Rstudio version 2024.04.2).
Results
Clinicopathological data and PD-1/PD-L1 staining results
A total of 149 FFPE tumor biopsies from 104 patients collected before (n = 104) and after (n = 45) BCG treatment were analyzed by IHC staining of PD-1, PD-L1 (Figure 1(a)) and PanCK. Table 1 lists clinical information and Table 2 IHC results for all patients.
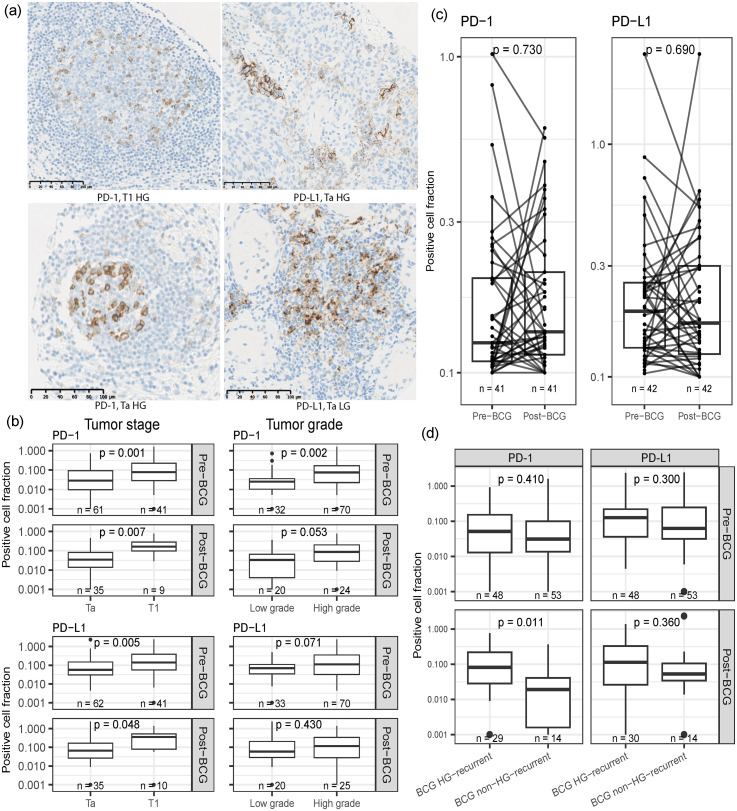
Figure 1.
PD-1 and PD-L1 expression and correlations to clinical features. (a) Examples of IHC images of PD-1 (left) and PD-L1 (right). (b) Correlations between PD-1 and PD-L1 expression and tumor stage and grade (Wilcoxon Mann-Whitney test). (c) Correlation of PD-1 and PD-L1 expression between paired samples before and after BCG treatment (Wilcoxon signed-rank test) (d) Comparison of marker expression in BCG non-HG-recurrence compared to BCG HG-recurrence in pre- and post-BCG samples (Wilcoxon Mann-Whitney test). BCG: Bacillus Calmette-Guérin; HG: High Grade.
Table 2.
IHC results.
| All patients | BCG Response | |||
|---|---|---|---|---|
| Characteristic | N = 104 a | Post BCG
HG-recurrence N = 50 a |
No post BCG
HG-recurrence N = 53 a |
p-value b |
| pre-BCG PD-1 status | 0.2 | |||
| High | 54 (59%) | 29 (66%) | 24 (51%) | |
| Low | 38 (41%) | 15 (34%) | 23 (49%) | |
| post-BCG PD-1 status | 0.5 | |||
| High | 21 (55%) | 16 (59%) | 4 (40%) | |
| Low | 17 (45%) | 11 (41%) | 6 (60%) | |
| pre-BCG PD-L1 status | 0.021 | |||
| High | 55 (53%) | 32 (65%) | 22 (42%) | |
| Low | 48 (47%) | 17 (35%) | 30 (58%) | |
| post-BCG PD-L1 status | 0.2 | |||
| High | 21 (54%) | 15 (60%) | 5 (38%) | |
| Low | 18 (46%) | 10 (40%) | 8 (62%) | |
n (%)
Pearson's Chi-squared test; Fisher's exact test.
We investigated if PD-1/PD-L1 expression was correlated to tumor stage and grade and found that a high PD-1 expression before BCG was associated with higher tumor stage (p = 0.001) and grade (p = 0.002; Figure 1(b)). A high PD-1 expression after BCG was associated with higher tumor stage (p = 0.007) but not with tumor grade (p = 0.053). High PD-L1 pre-BCG was likewise associated with higher tumor stage (p = 0.005) and not tumor grade (p = 0.071) (Figure 1(b)). Post-BCG PD-L1 expression was associated with higher tumor stage (p = 0.048), but not grade (p = 0.43). The correlations according to the separate regions (carcinoma and stroma) can be found in Supplementary Figure 1. When comparing the PD-1 and PD-L1 expression in paired samples collected before and after treatment with BCG (n = 41 and n = 42, respectively), no differences were observed (p = 0.730 and p = 0.690, respectively) (Figure 1(c)). We also wanted to investigate if expression was correlated to post-BCG HG-recurrence and here we found post-BCG samples from BCG non-HG-recurrent patients to have a statistically significant lower expression of PD-1 compared to BCG HG-recurrent patients (p = 0.011; Figure 1(d)). We also investigated whether pre- to post-BCG samples changed within these groups, but no significant change was observed (Supplementary Figure 2)
PD-1 and PD-L1 as prognostic markers in NMIBC
By testing the difference in high-grade recurrence-free survival (HG-RFS) between high and low PD-1/PD-L1 expression in the stroma and carcinoma regions combined (based on median split, see Supplementary Table 2), we observed a significantly lower HG-RFS in patients with high PD-1 and PD-L1 expression (p = 0.006 and p = 0.007; Figure 2(a)). We also observed a worse HG-RFS when combining the PD-1 high samples with PD-L1 high samples compared to other combinations of expression status (p = 0.004; Figure 2(a)).
Figure 2.
Survival analysis and genomic correlates. (a) Kaplan-Meier plots of HG-RFS in pre-BCG samples for PD-1 expression status, PD-L1 expression status and combined PD-1 and PD-L1 expression statuses. Dichotomization is based on the median split of the expression fraction. All p values are calculated with log-rank test. (b) Correlation between PD-1/PD-L1 expression and TMB (Wilcoxon Mann-Whitney test). (c) Correlation between PD-1/PD-L1 expression and mutational signature SBS4 (Wilcoxon Mann-Whitney test). HG-RFS: high grade recurrence free survival; BCG: bacillus Calmette-Guérin; TMB: tumor mutational burden; SBS: single base substitution.
Genomic correlates to PD-1 and PD-L1 expression
Next, we correlated PD-1 and PD-L1 expression to genomic features in pre-BCG samples with matching WES data (n = 104). We observed that high PD-1 expression in the stromal region was significantly associated with a higher tumor mutation burden (TMB; p = 0.038; Figure 2(b)), but no associations were observed for PD-1 in the carcinoma region or PD-L1 in either region (p = 0.2, p = 0.87 and p = 0.4, respectively).
Furthermore, we investigated the association to known mutational signatures (see Strandgaard et al. 2022 14 ), and we observed correlations of high PD-1 expressions to the SBS4 signature in both the stromal (p = 0.014) and carcinoma region (p = 0.048; Figure 2(c)), which is associated with mutational processes driven by tobacco smoking, a behavior reported in 88% of the patients (as either former or current). No additional differences were observed (Supplementary Figure 3).
Association to clinical and histopathological features
Next, we investigated if PD-1 and PD-L1 expression were associated with clinical outcomes using uni- and multivariable Cox regression analysis for each protein in the pre-BCG samples. The univariate analysis demonstrated that high tumor grade, high PD-1 and PD-L1 level expression and UROMOL2021 Class 2a/2b classification were independently significantly associated with worse HG-RFS after BCG treatment. When adjusting for tumor grade in the multivariable analysis, we identified UROMOL2021 classification (p = 0.009) and PD-L1 status (p = 0.02) as independent predictors of HG-RFS (Figure 3(a)). We further calculated a risk score based on the Cox proportional hazards model which demonstrated a significantly improved HG-RFS in lower risk group (p = 0.0003) (Supplementary Figure 4(a)) and we performed a time-dependent receiver operating characteristics (ROC) analyses. This demonstrated that addition of PD-L1 expression improved the prediction accuracy compared to tumor grade alone (Supplementary Figure 4(b))
Figure 3.
PD1 and PD-L1 expression and association with clinical and molecular features. (a) Uni- and multivariate Cox regression analysis including forest plot. Full circles depict the hazard ratios and the horizontal lines depict the 95 % confidence interval (CI). The vertical dashed line depicts a hazard ratio of 1. p.val: p values (obtained from the Wald test, evaluating the statistical significance of each predictor in the Cox model); p.adjust: adjusted p value for multiple testing calculated using the Benjamini-Hochberg correction method to the false discovery rate; p.multi: p values for the multivariate analysis in which tumor grade was included; N: number of patients/samples in each analysis (b) Comparison of marker expression in samples with a high versus low post-BCG exhaustion score in pre-BCG samples (Wilcoxon signed rank test). (c) Stacked barplot depicting the distribution of high and low expression status (dichotomized based on median split) for pre-BCG samples with either high or low exhaustion scores (Fisher’s exact test). (d) Distribution of UROMOL2021 classes in pre-BCG samples in low or high PD-1 and PD-L1 expression (dichotomized based on median spit, Fisher's exact test). (e) Comparison of PD-1 and PD-L1 expression between the UROMOL2021 classes (Kruskal-Wallis test and Wilcoxon signed-rank test for individual comparisons). CIS: Carcinoma in situ; EAU: European Association of Urology; CI: confidence interval; HG: high grade; LG: low grade.
Transcriptomic correlates
Next, we investigated the correlation between PD-1 and PD-L1 expression in the full tumor area analyzed and different transcriptomic variables that were previously shown to have a correlation to BCG treatment response or outcome.14,16 We observed a higher expression of PD-L1 in pre-BCG samples which exhibited a high exhaustion score 14 (p = 0.006; Figure 3(b)). We also compared the dichotomized data for all samples (pre- and post-BCG) and here we observed a significantly higher number of samples with high PD-L1 status in the group with the higher exhaustion profile (p = 0.005; Figure 3(c)).
Tumor samples from the current cohort have previously been classified using the UROMOL2021 system on bulk RNA-sequencing data. 16 This classification system identified four subtypes of NMIBC (1, 2a, 2b and 3), where classes 2a and 2b denote high-risk groups with worse outcomes compared to classes 1 and 3. 16 When comparing the expression results to these transcriptional classes, we observed a significant difference between expression status of PD-1 and PD-L1 in total tissue and the transcriptomic classes of pre-BCG samples (p = 0.032 and p = 0.034, respectively) with more class 3 contribution in the PD-1 and PD-L1 low groups and more class 2a and class 2b in the PD-1 and PD-L1 high groups (Figure 3(d)). However, the majority of the samples belonged to class 2a regardless of PD-1 and PD-L1 status, which is in alignment with the high risk NMIBC disease status of these patients. When evaluating the distribution of the expression levels of PD-1 and PD-L1, we observed that the class 2a and class 2b samples were significantly higher compared to class 3 (p = 0.012 and p = 0.007 for PD-1 and p = 0.027 and p = 0.009 for PD-L1; Figure 3(e)).
Discussion
In this study we investigated the protein expression of the immune inhibitory molecules PD-1 and PD-L1. We found that a high expression of these molecules was associated with higher stage and grade of the tumors as well as worse HG-RFS after BCG. In a multivariate regression analysis we identified pre-BCG PD-L1 status and UROMOL2021 classification as independent predictors of post-BCG HG-RFS. This suggests that these molecules are indicative of an exhausted phenotype that weakens the anti-tumor response of the immune system. This aligns with and corroborates current knowledge that the molecular mechanism of the PD-1/PD-L1 pathway is downregulating T cell activity.5,17 Of note, the EAU risk score was not predictive of HG-RFS in our cohort, potentially due to the exclusion of low and intermediate risk NMIBC patients and the limited heterogeneity of risk across included cases. This reflects the score's reduced discriminatory ability in uniformly high-risk populations and suggests that additional biomarkers or immune profiling may be needed to refine risk stratification in BCG-treated patients.
Previous studies have investigated the PD-1/PD-L1 pathway through IHC in BCG-treated NMIBC,8–11,13 for among other reasons to find alternatives to RC in BCG-unresponsive patients.18,19 Despite similar study designs to ours, there are central differences between the methodologies utilized in these studies that make direct comparisons difficult. These differences include the choice of tissue size (TMA vs. whole slide), antibodies used for IHC, scoring of IHC results and cut-off values. There are also differences in the reported outcomes compared to our study, which include association to BCG failure and disease-free recurrence. Kates et al. 8 concluded that PD-L1 expression predicted an unfavorable response to BCG and Roumigiué et al. 13 found an association between PD-L1 expression and BCG failure. These findings were not found in our study. These differences could be caused by the aforementioned differences in methods or the overall high heterogeneity of bladder cancer tumors.
Other studies have demonstrated an increase in expression of PD-1 9 and PD-L1. 20 A reason for these variations could be differences in scoring of the IHC results and size of tissue. A study by Kates et al. 8 showed that the concordance of PD-L1 scores between TMA and full slide was poor and therefore suggested a transition from TMA to whole slide for future studies. Additionally, there are several PD-L1 antibodies approved for IHC staining of bladder tissue (SP263 [Ventana], SP142 [Ventana], 22c3 [Dako] and 28-8 [Dako]) and others used only for research purposes (E1L3N [CST] and AP142 [Abcam]), which may affect antibody specificity. Comparison of these on the same tissue samples show different expression levels.21,22
To score the PD-1 and PD-L1 staining results, we calculated the fraction of positive cells within the total tissue region. To refine the analysis, we separately assessed the carcinoma and stromal regions in order to assess differences in compartments. In each case, the fraction was determined by dividing the number of positive cells by the total number of cells in the respective region. In the stromal area we assumed that most positive stained cells were immune cells and found that these were often located in tertiary lymphoid structures (exemplified in Figure 1(a)). Without more antibodies in the study, we could not make further distinction between cell types. Other studies have utilized well-known scoring systems for PD-L1, such as combined positive score (CPS), tumor proportion score (TPS) and tumor infiltrating immune cells (IC).8,10,11,22,23 PD-L1 is thoroughly investigated due to its impact in treatment strategies for advanced stages of urothelial cancer, 24 but there is, to our knowledge, no such scoring system for PD-1 in urothelial cancer. For the purposes of using the same scoring system for PD-1 and PD-L1 we performed the fraction calculation as mentioned above. In regards to cut-off values for positivity, these range from 0% in our study to 1%, 5% or 10% for TMA's in other studies and hotspot evaluation with lymphocyte count for whole slides.9,13,25
The limitations of our study include a retrospective study design and an overall low expression fraction of the samples. It is well known that PD-1 and PD-L1 are more highly expressed in tumors from muscle-invasive bladder cancer compared to NMIBC.26,27
Our study utilized standard IHC methods that lack the cellular resolution offered by other methods such as flow cytometry. A study by Cañizo et al. 28 utilizing flow cytometry found PD-L1 to be highly expressed on not just tumor cells but also on immune subsets like macrophages and dendritic cells. This highlights the need for cell-specific analyses. To address this limitation, more advanced technologies are now available that combine high-dimensional protein detection with spatial context. With novel spatial proteomic methods, such as imaging mass cytometry and multiplexed ion beam imaging, it is possible to investigate a multitude of markers at single cell level on the same tissue, thereby enabling colocalization of cellular proteins and further elucidate cell subtype identification. This could eventually lead to more precise classification of the cells and overall tissue. In future work it would be preferable to distinguish the carcinoma cells and TILs to determine which cell types are expressing PD-1 and PD-L1.
Another significant limitation of this study (and other studies of the same topic), is the lack of consensus in regard to IHC methods and scoring system. Consistent use of assays and scoring systems will allow for better cross validation and will minimize subjective evaluations. Moving forward it would be pertinent to reach a common agreement on IHC staining and scoring for urothelial carcinoma since IHC is likely to remain the standard practice for evaluating tissue protein expression in a clinical setting.
Conclusions
This study found that expression of PD-1 and PD-L1 were associated with clinical outcomes. Taken together, these results could further support the addition of immune checkpoint inhibition in addition to conventional treatment. However, further research is needed to determine the predictive value of PD-1/PD-L1 expression and the effect of combination therapies, including the role of the spatial distribution of the different cell types. Comparison of studies requires a consensus on IHC protocols to facilitate cross-study comparisons.
Supplemental Material
Supplemental material, sj-docx-1-blc-10.1177_23523735251368683 for Expression of PD-1 and PD-L1 in BCG-treated NMIBC by Tine Ginnerup Andreasen, Trine Strandgaard, Line Raaby, Jørgen Bjerggaard Jensen and Lars Dyrskjøt in Bladder Cancer
Supplemental material, sj-docx-2-blc-10.1177_23523735251368683 for Expression of PD-1 and PD-L1 in BCG-treated NMIBC by Tine Ginnerup Andreasen, Trine Strandgaard, Line Raaby, Jørgen Bjerggaard Jensen and Lars Dyrskjøt in Bladder Cancer
Acknowledgments
The authors thank the Department of Pathology at Aarhus University Hospital for their contributions to this study.
Footnotes
ORCID iDs: Tine Ginnerup Andreasen https://orcid.org/0000-0002-9633-2987
Lars Dyrskjøt https://orcid.org/0000-0001-7061-9851
Ethical considerations: This study was approved by the National Ethics Committee (#1708266).
Author contributions: Conception: TGA, TS, LD
Interpretation of data: TGA, TS, LR, LD
Drafting the manuscript: TGA, TS, LD
Critical review and editing of the manuscript: all authors
All authors had access to the data. All authors have approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dansk Kræftforskningsfond has funded this work through salary for TGA.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TGA, TS and LR report no relevant conflicts of interest.
JBJ has sponsored research agreements with medac, Photocure ASA, Roche, Ferring, Olympus, Astellas, Cepheid, Nucleix, Urotech, Pfizer, AstraZenica, VingMed, Laborie, AMBU, and Cystotech and serves in an advisory/consulting role for Ferring, Roche/Genentech, Cepheid, Urotech, Olympus, AMBU, Janssen, Cystotech, and Polyceutix and has received speaker honoraria from medac, Olympus, Photocure ASA, and Conmed and is furthermore proctor for Intuitive Surgery.
LD has sponsored research agreements with C2i Genomics, Veracyte, Natera, AstraZeneca, Photocure, and Ferring and serves in an advisory/consulting role for Ferring, MSD, Cystotech, and UroGen. LD has received speaker honoraria from AstraZeneca, Pfizer, and Roche. Lars Dyrskjøt is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Data availability: The current legislation on data sharing requires that sensitive (including pseudonymized) data can only be shared following approval by the National Committee on Health Research Ethics in Denmark of the project and by the Danish Data Registry.
Supplemental material: Supplemental material for this paper is available online.
References
- 1.Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance Bacillus Calmette-Guérin. Eur Urol 2016; 69: 60–69. [DOI] [PubMed] [Google Scholar]
- 2.Bilotta MT, Antignani A, Fitzgerald DJ. Managing the TME to improve the efficacy of cancer therapy. Front Immunol 2022; 13: 954992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y-C, Lam H-M, Rosser C, et al. The dynamic roles of the bladder tumour microenvironment. Nat Rev Urol 2022; 19: 515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezaei N. (ed.) Cancer immunology: A translational medicine context. 2nd ed. Cham, Switzerland: Springer Nature, 2020. [Google Scholar]
- 5.Kornepati AVR, Vadlamudi RK, Curiel TJ. Programmed death ligand 1 signals in cancer cells. Nat Rev Cancer 2022; 22: 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 pathway: signaling, cancer, and beyond. Adv Exp Med Biol 2020; 1248: 33–59. [DOI] [PubMed] [Google Scholar]
- 7.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007; 56: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kates M, Matoso A, Choi W, et al. Adaptive immune resistance to intravesical BCG in non-muscle invasive bladder cancer: implications for prospective BCG-unresponsive trials. Clin Cancer Res 2020; 26: 882–891. [DOI] [PubMed] [Google Scholar]
- 9.Fukumoto K, Kikuchi E, Mikami S, et al. Clinical role of programmed cell death-1 expression in patients with non-muscle-invasive bladder cancer recurring after initial Bacillus Calmette-Guérin therapy. Ann Surg Oncol 2018; 25: 2484–2491. [DOI] [PubMed] [Google Scholar]
- 10.Aydin AM, Baydar DE, Hazir B, et al. Prognostic significance of pre- and post-treatment PD-L1 expression in patients with primary high-grade non-muscle-invasive bladder cancer treated with BCG immunotherapy. World J Urol 2020; 38: 2537–2545. [DOI] [PubMed] [Google Scholar]
- 11.Wankowicz SAM, Werner L, Orsola A, et al. Differential expression of PD-L1 in high grade T1 vs muscle invasive bladder carcinoma and its prognostic implications. J Urol 2017; 198: 817–823. [DOI] [PubMed] [Google Scholar]
- 12.Chevalier MF, Schneider AK, Cesson V, et al. Conventional and PD-L1-expressing regulatory T cells are enriched during BCG therapy and may limit its efficacy. Eur Urol 2018; 74: 540–544. [DOI] [PubMed] [Google Scholar]
- 13.Roumiguié M, Compérat E, Chaltiel L, et al. PD-L1 expression and pattern of immune cells in pre-treatment specimens are associated with disease-free survival for HR-NMIBC undergoing BCG treatment. World J Urol 2021; 39: 4055–4065. [DOI] [PubMed] [Google Scholar]
- 14.Strandgaard T, Lindskrog SV, Nordentoft I, et al. Elevated T-cell exhaustion and urinary tumor DNA levels are associated with Bacillus Calmette-Guérin failure in patients with non-muscle-invasive bladder cancer. Eur Urol 2022; 82: 646–656. [DOI] [PubMed] [Google Scholar]
- 15.Sondka Z, Dhir NB, Carvalho-Silva D, et al. COSMIC: a curated database of somatic variants and clinical data for cancer. Nucleic Acids Res 2024; 52: D1210–D1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindskrog SV, Prip F, Lamy P, et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun 2021; 12: 2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong FC, Rutten VC, Zuiverloon TCM, et al. Improving anti-PD-1/PD-L1 therapy for localized bladder cancer. Int J Mol Sci 2021; 22: 2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange A, Madiraju S, Petros FG. Therapeutic advances in bladder preservation for BCG-unresponsive non-muscle invasive bladder cancer. Cancers (Basel) 2025; 17: 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannouneh ZA, Hijazi A, Alsaleem AA, et al. Novel immunotherapeutic options for BCG-unresponsive high-risk non-muscle-invasive bladder cancer. Cancer Med 2023; 12: 21944–21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashizume A, Umemoto S, Yokose T, et al. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget 2018; 9: 34066–34078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davick JJ, Frierson HF, Smolkin M, et al. PD-L1 expression in tumor cells and the immunologic milieu of bladder carcinomas: a pathologic review of 165 cases. Hum Pathol 2018; 81: 184–191. [DOI] [PubMed] [Google Scholar]
- 22.Paliogiannis P, Lobrano R, Bella MA, et al. PD-L1 immunohistochemical expression in bladder urothelial cancer with SP263, SP142 and 22C3 antibodies: a comparative study. Ann Diagn Pathol 2024; 69: 152267. [DOI] [PubMed] [Google Scholar]
- 23.Munari E, Querzoli G, Brunelli M, et al. Comparison of three validated PD-L1 immunohistochemical assays in urothelial carcinoma of the bladder: interchangeability and issues related to patient selection. Front Immunol 2022; 13: 954910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 2021; 79: 82–104. [DOI] [PubMed] [Google Scholar]
- 25.Marletta S, Fusco N, Munari E, et al. Atlas of PD-L1 for pathologists: indications, scores, diagnostic platforms and reporting systems. J Pers Med 2022; 12: 1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taber A, Prip F, Lamy P, et al. Immune contexture and differentiation features predict outcome in bladder cancer. Eur Urol Oncol 2022; 5: 203–213. [DOI] [PubMed] [Google Scholar]
- 27.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression: associations with localized stage progression. Cancer 2007; 109: 1499–1505. [DOI] [PubMed] [Google Scholar]
- 28.Cañizo CG, Guerrero-Ramos F, Perez Escavy M, et al. Characterisation of the tumour microenvironment and PD-L1 granularity reveals the prognostic value of cancer-associated myofibroblasts in non-invasive bladder cancer. Oncoimmunology 2025; 14: 2438291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-blc-10.1177_23523735251368683 for Expression of PD-1 and PD-L1 in BCG-treated NMIBC by Tine Ginnerup Andreasen, Trine Strandgaard, Line Raaby, Jørgen Bjerggaard Jensen and Lars Dyrskjøt in Bladder Cancer
Supplemental material, sj-docx-2-blc-10.1177_23523735251368683 for Expression of PD-1 and PD-L1 in BCG-treated NMIBC by Tine Ginnerup Andreasen, Trine Strandgaard, Line Raaby, Jørgen Bjerggaard Jensen and Lars Dyrskjøt in Bladder Cancer