Abstract
Background
In the phase III EV-302 study (NCT04223856), enfortumab vedotin (EV) plus pembrolizumab (P) demonstrated superior efficacy and safety versus platinum-based chemotherapy in patients with previously untreated locally advanced/metastatic urothelial cancer (la/mUC). We report the efficacy of EV+P in prespecified subgroups, including those defined by cisplatin eligibility status, the presence or absence of liver metastases, and metastatic disease sites.
Methods
Patients with previously untreated la/mUC were randomly assigned 1 : 1 to receive either EV 1.25 mg/kg and pembrolizumab 200 mg, or gemcitabine plus cisplatin or carboplatin, all intravenously. The two primary endpoints were progression-free survival (PFS) and overall survival (OS). Confirmed objective response rate was one of the secondary endpoints.
Results
Overall, 886 patients were randomized: 442 to EV+P and 444 to chemotherapy. Baseline characteristics were balanced across treatment groups. Efficacy and safety data for the intention-to-treat (ITT) population, along with PFS and OS data for cisplatin-eligible and -ineligible patients, were previously published (Powles et al. N Eng J Med, 2024). In this analysis, EV+P showed benefit across prespecified subgroups that was consistent with the ITT population. OS benefit in the EV+P arm versus chemotherapy was seen across all subgroups, including patients with liver metastases (OS 19.1 versus 10.1 months), patients without liver metastases [OS not estimable (NE) versus 17.9 months], patients with visceral metastases (OS 25.6 versus 13.6 months), and in patients with lymph node–only disease (OS NE versus 27.5 months). In addition, confirmed objective response rate and PFS benefit with EV+P versus chemotherapy was seen across all examined subgroups.
Conclusion
Along with previously published safety data, EV+P demonstrated benefit compared with chemotherapy across all prespecified subgroups, consistent with the ITT population and supporting EV+P as the standard of care for first-line treatment of la/mUC.
Key words: enfortumab vedotin, pembrolizumab, bladder cancer, metastatic urothelial carcinoma, phase III, subgroup analysis
Highlights
-
•
EV+P nearly doubled median OS in patients with la/mUC; this study explores if this benefit is seen in patient subgroups.
-
•
EV+P demonstrated superior PFS and OS across all specified subgroups versus chemotherapy.
-
•
The consistency of results support EV+P as the new standard of care for 1L treatment of la/mUC across subgroups.
Introduction
Locally advanced or metastatic urothelial carcinoma (la/mUC) is an aggressive and incurable disease.1 Historically, platinum-based chemotherapy has been the standard first-line (1L) treatment for patients with la/mUC; however, 5-year survival rates have remained low.1,2 Previous advances in 1L treatment, such as nivolumab plus gemcitabine-cisplatin and avelumab maintenance therapy, have significantly improved patient outcomes, but only in patients with previously untreated la/mUC who were cisplatin-eligible or patients with la/mUC whose disease had not progressed following platinum-based chemotherapy.3,4
Enfortumab vedotin (EV) is an antibody drug conjugate comprising a fully humanized Nectin-4-directed monoclonal antibody, enfortumab, conjugated to the microtubule-disrupting agent monomethyl auristatin E via a protease-cleavable mc-vc linker.5,6 EV and pembrolizumab (P), a programmed death 1 inhibitor, have both emerged as effective monotherapy treatments in patients with la/mUC.7,8
The phase III EV-302 study (NCT04223856) assessed EV+P compared with platinum-based chemotherapy (gemcitabine plus cisplatin or carboplatin) in patients with previously untreated la/mUC. Treatment with EV+P improved progression-free survival (PFS) [median, 12.5 versus 6.3 months; hazard ratio (HR) for disease progression or death, 0.45; 95% confidence interval (CI), 0.38-0.54; P < 0.001], overall survival (OS; median, 31.5 versus 16.1 months; HR for death, 0.47; 95% CI, 0.38-0.58; P < 0.001), and confirmed overall response [67.7% (95% CI, 63.1% to 72.1%) versus 44.4% (95% CI, 39.7% to 49.2%); P < 0.001] compared with chemotherapy. The safety profile of EV+P was consistent with that reported previously, with no new safety signals identified.9 These pivotal results led to approval of EV+P for treatment of la/mUC in many countries,10, 11, 12, 13 and EV+P has become the new 1L standard of care for la/mUC.14
The EV-302 primary analysis demonstrated that the clinical benefit of EV+P was consistent between the intention-to-treat population (ITT) and all prespecified subgroups.9 Here, we present a detailed analysis from EV-302 on the subgroups of clinical interest, including those defined by cisplatin eligibility status, the presence or absence of liver metastases, and metastatic disease sites.
Methods
Study design and patients
The study design and primary analysis of EV-302 have been previously published.9 Briefly, EV-302 was a phase III, global, open-label, randomized trial comparing EV+P with platinum-based chemotherapy in patients with previously untreated la/mUC. Patients were randomly assigned 1 : 1 to receive 3-week cycles of EV [1.25 mg/kg intravenously on days 1 and 8] and pembrolizumab (200 mg intravenously on day 1) or gemcitabine and either cisplatin or carboplatin (determined on the basis of eligibility to receive cisplatin). Patients were stratified by cisplatin eligibility (eligible or ineligible), programmed death-ligand 1 (PD-L1) expression by immunohistochemistry high [combined positive score (CPS) ≥10] or low (CPS <10), and the presence or absence of liver metastases. Eligible patients had radiologically documented, histologically confirmed, unresectable la/mUC of the upper and lower tract; patients with squamous or sarcomatoid differentiation or mixed cell types were eligible. Key exclusion criteria included previous exposure to programmed cell death protein 1 or PD-L1 inhibitors or other systemic therapy (except for neoadjuvant or adjuvant chemotherapy after surgery with recurrence >12 months after the completion of therapy), uncontrolled diabetes defined as hemoglobin A1C ≥8% or 7% to <8% with associated diabetes symptoms (polyuria or polydipsia) that are not otherwise explained, ongoing sensory or motor neuropathy of grade ≥2, and previous autoimmune disease for which the patient had received systemic treatment in the previous 2 years; full eligibility criteria can be found in the previously published protocol.9
Endpoints
The two primary endpoints were PFS and OS. PFS was defined as the time from randomization to the first occurrence of disease progression, as assessed by blinded independent central review (BICR) according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, or death from any cause, whichever occurred first. Select secondary endpoints included confirmed overall response rate (ORR) by BICR per RECIST version 1.1, time to response, duration of response (DOR), and safety.
Statistical analyses
Exploratory subgroup analyses were conducted for selected efficacy endpoints, including PFS, OS, confirmed ORR, and DOR. All subgroups reported here were prespecified.
Estimated treatment effect (HR with a nominal 95% CI) for PFS and OS were provided for each subgroup using a stratified Cox proportional hazards model controlling stratification factors. For confirmed ORR, the difference between treatment arms (with a nominal 95% CI) was calculated for each subgroup. In addition, Kaplan–Meier curves were generated by arm for selected subgroups.
Ethical statement
The trial was approved by the Institutional Review Board or Ethics Committee at each site and was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines (as defined by the International Council for Harmonisation), applicable regulatory requirements, and the policies of the trial sponsors regarding bioethics and human biological samples. All patients provided written informed consent before trial entry. The trial was designed by the sponsors and select members of the steering committee.
Results
A total of 886 patients were randomly assigned to receive EV+P (n = 442) or chemotherapy (n = 444). The data cutoff for this analysis was 8 August 2023. The median (range) duration of follow-up was 17.2 months (0.07-37.16 months) for the overall ITT population, 17.3 months (0.26-37.16 months) in the EV+P arm, and 16.9 months (0.07-36.21 months) in the chemotherapy arm.9 Baseline characteristics were generally balanced across the treatment groups.9
Subgroups defined by cisplatin eligibility
At the time of enrollment, cisplatin eligibility was determined by the investigator per protocol-defined criteria. All cisplatin-eligible patients were required to receive cisplatin; patients ineligible for cisplatin would receive carboplatin. In the cisplatin-eligible subgroup, 244 patients were randomly assigned to receive EV+P and 234 were assigned to receive chemotherapy. In the cisplatin-ineligible subgroup, 198 patients were randomly assigned to receive EV+P and 210 patients were assigned to receive chemotherapy; 94.0% of cisplatin-eligible patients received cisplatin and 97.6% of cisplatin-ineligible patients received carboplatin at cycle 1.
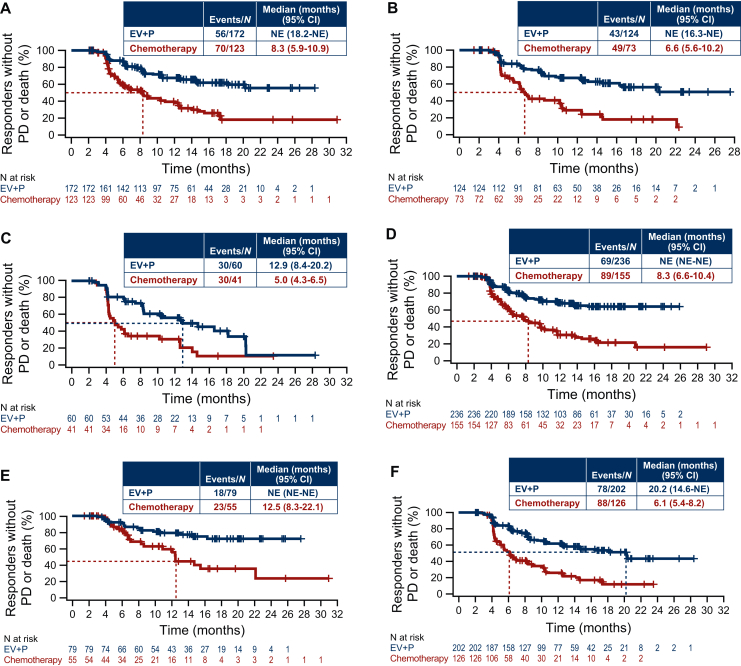
PFS and OS for cisplatin-eligible and -ineligible patients have been previously published and demonstrated a consistent benefit of EV+P regardless of cisplatin eligibility.9 Further analyses showed that confirmed ORR by BICR was 70.8% and 53.0% with EV+P and chemotherapy, respectively, for cisplatin-eligible patients (absolute difference, 17.8%; 95% CI, 9.1% to 26.2%) and 63.9% and 34.9% with EV+P and chemotherapy, respectively, for cisplatin-ineligible patients (absolute difference, 29.0%; 95% CI, 19.4% to 38.0%) (Figure 1, Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2025.105544). Among the cisplatin-eligible population, 32.5% of patients achieved complete response with EV+P versus 15.5% with chemotherapy. Among the cisplatin-ineligible population, 24.7% of patients with EV+P and 9.1% with chemotherapy achieved complete response. The median time to response was 2.1 months with EV+P or chemotherapy for both cisplatin-eligible and -ineligible populations (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2025.105544). In the cisplatin-eligible subgroup, the median DOR by BICR was not estimable (NE) (95% CI, 18.2 months-NE) with EV+P and 8.3 months (95% CI, 5.9-10.9 months) with chemotherapy. In the cisplatin-ineligible subgroup, the median DOR by BICR was NE (95% CI, 16.3-NE) with EV+P and 6.6 months (95% CI, 5.6-10.2 months) with chemotherapy (Figure 2A and B).
Figure 1.
Confirmed ORR by BICR in patients who are cisplatin eligible and ineligible, with and without liver metastases, and who have visceral metastases and lymph node–only disease. BICR, blinded independent review committee; CI, confidence interval; EV, enfortumab vedotin; P, pembrolizumab.
Figure 2.
DOR by BICR. Data are shown for patients who were (A) cisplatin eligible or (B) ineligible, (C) patients with liver metastases, (D) patients without liver metastases, (E) patients with lymph node-only disease, and (F) patients with visceral metastases. BICR, blinded independent central review; CI, confidence interval; DOR, duration of response; EV, enfortumab vedotin; NE, not estimable; P, pembrolizumab; PD, disease progression.
Subgroups defined by the presence or absence of liver metastases
Of the 199 patients with liver metastases, 100 were assigned to receive EV+P and 99 were assigned to receive chemotherapy. Median PFS by BICR was 8.2 months (95% CI, 6.1-12.0 months) with EV+P and 6.0 months (95% CI, 4.4-6.3 months) with chemotherapy (HR, 0.53; 95% CI, 0.38-0.76) in patients with liver metastases (Figure 3A). Median OS was 19.1 months (95% CI, 15.4-NE) in the EV+P arm and 10.1 months (95% CI, 7.9-12.1 months) in the chemotherapy arm (HR, 0.47; 95% CI, 0.32-0.71) in patients with liver metastases (Figure 3B). Confirmed ORR by BICR was 60.0% and 41.4% with EV+P and chemotherapy, respectively, for patients with liver metastases (absolute difference, 18.6%; 95% CI, 4.7% to 31.8%) (Figure 1). The median DOR by BICR was 12.9 months (95% CI, 8.4-20.2 months) with EV+P and 5.0 months (95% CI, 4.3-6.5 months) with chemotherapy in patients with liver metastases (Figure 2C).
Figure 3.
PFS by BICR and OS in subgroups defined by the presence or absence of liver metastases. Data are shown for patients with liver metastases (A, B) and without liver metastases (C, D). BICR, blinded independent central review; CI, confidence interval; EV, enfortumab vedotin; HR, hazard ratio; NE, not estimable; OS, overall survival; P, pembrolizumab; PFS, progression-free survival. aCalculated using stratified Cox proportional hazards model; an HR <1 favors the EV+P arm.
Among 687 patients without liver metastases, 342 were assigned to receive EV+P and 345 were assigned to receive chemotherapy. Median PFS by BICR was 16.4 months (95% CI, 10.6 months-NE) with EV+P and 6.4 months (95% CI, 6.2-7.4 months) with chemotherapy (HR, 0.43; 95% CI, 0.35-0.52) in patients without liver metastases (Figure 3C). Median OS was NE (95% CI, NE-NE) in the EV+P arm and 17.9 months (95% CI, 15.6-20.6 months) in the chemotherapy arm (HR, 0.47; 95% CI, 0.36-0.61) in patients without liver metastases (Figure 3D). Confirmed ORR was 70.0% and 45.3% with EV+P and chemotherapy, respectively, for patients without liver metastases (absolute difference, 24.7%; 95% CI, 17.4% to 31.8%) (Figure 1). The median DOR by BICR was NE (95% CI, NE-NE) with EV+P and 8.3 months (95% CI, 6.6-10.4 months) with chemotherapy in patients without liver metastases (Figure 2D).
Subgroups defined by sites of metastases
Among 207 patients with lymph node–only disease, 103 were assigned to receive EV+P and 104 were assigned to receive chemotherapy. Median PFS by BICR was NE (95% CI, 16.6-NE) with EV+P and 8.3 months (95% CI, 6.2-12.6 months) with chemotherapy (HR, 0.40; 95% CI, 0.26-0.62) in patients with lymph node–only disease (Figure 4A). Median OS was NE (95% CI, NE-NE) in the EV+P arm and 27.5 months (95% CI, 15.0 months-NE) in the chemotherapy arm (HR, 0.46; 95% CI, 0.27-0.78) in patients with lymph node–only disease (Figure 4B). Confirmed ORR per BICR was 77.5% and 53.4% with EV+P and chemotherapy, respectively, for patients with lymph node–only disease (absolute difference, 24.1%; 95% CI, 11.1% to 36.3%) (Figure 1). The median DOR by BICR was NE (95% CI, NE, NE) with EV+P and 12.5 months (95% CI, 8.3-22.1 months) with chemotherapy in patients with lymph node–only disease (Figure 2E).
Figure 4.
PFS by BICR and OS in subgroups defined by sites of metastases. Data are shown for patients with LN-only disease (A, B) and visceral metastases (C, D). BICR, blinded independent central review; CI, confidence interval; EV, enfortumab vedotin; HR, hazard ratio; LN, lymph node; NE, not estimable; OS, overall survival; P, pembrolizumab; PFS, progression-free survival. aCalculated using stratified Cox proportional hazards model; an HR <1 favors the EV+P arm.
Among 636 patients with visceral metastases, 318 were assigned to receive EV+P and 318 were assigned to receive chemotherapy. Median PFS by BICR was 10.4 months (95% CI, 8.3-12.5 months) with EV+P and 6.2 months (95% CI, 6.0-6.3 months) with chemotherapy (HR, 0.45; 95% CI, 0.37-0.55) in patients with visceral metastases (Figure 4C). Median OS was 25.6 months (95% CI, 22.8 months-NE) in the EV+P arm and 13.6 months (95% CI, 11.8-15.9 months) in the chemotherapy arm (HR, 0.47; 95% CI, 0.37-0.60) in patients with visceral metastases (Figure 4D). Confirmed ORR per BICR was 64.1% and 39.6% with EV+P and chemotherapy, respectively, for patients with visceral metastases (absolute difference, 24.5%; 95% CI, 16.8% to 31.9%) (Figure 1). The median DOR by BICR was 20.2 months (95% CI, 14.6 months-NE) with EV+P and 6.1 months (95% CI, 5.4-8.2 months) with chemotherapy in patients with visceral metastases (Figure 2F).
Safety
Across all subgroups, treatment-related adverse events (TRAEs) of any grade occurred in 95.9%-97.9% of patients in the EV+P arm and 94.1%-96.9% of patients in the chemotherapy arm (Table 1). In the EV+P arm, TRAEs of special interest for EV and treatment-emergent adverse events of special interest for pembrolizumab were consistent with those in the ITT population (Supplementary Tables S2 and S3, available at https://doi.org/10.1016/j.esmoop.2025.105544).9 TRAEs leading to discontinuation of EV occurred in 26.4%-44.7% of patients, and TRAEs leading to discontinuation of pembrolizumab occurred in 19.2%-30.1% of patients across all subgroups (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2025.105544).
Table 1.
Summary of TRAEs across subgroups
| Cisplatin-eligible |
Cisplatin-ineligible |
Liver metastases present |
Liver metastases absent |
Lymph node–only disease |
Visceral metastases present |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EV+P n = 243 | Chemotherapy n = 228 | EV+P n = 197 | Chemotherapy n = 205 | EV+P n = 99 | Chemotherapy n = 96 | EV+P n = 341 | Chemotherapy n = 337 | EV+P n = 103 | Chemotherapy n = 102 | EV+P n = 316 | Chemotherapy n = 309 | |
| Any TRAE, n (%) | 238 (97.9) | 221 (96.9) | 189 (95.9) | 193 (94.1) | 95 (96.0) | 93 (96.9) | 332 (97.4) | 321 (95.3) | 100 (97.1) | 98 (96.1) | 306 (96.8) | 295 (95.5) |
| Any grade ≥3 TRAE, n (%) | 131 (53.9) | 143 (62.7) | 115 (58.4) | 158 (77.1) | 54 (54.5) | 71 (74.0) | 192 (56.3) | 230 (68.2) | 54 (52.4) | 68 (66.7) | 181 (57.3) | 219 (70.9) |
| Any serious TRAE, n (%) | 62 (25.5) | 40 (17.5) | 60 (30.5) | 45 (22.0) | 32 (32.3) | 16 (16.7) | 90 (26.4) | 69 (20.5) | 24 (23.3) | 18 (17.6) | 92 (29.1) | 64 (20.7) |
Safety data is reported in patients that received at least one dose of study treatment.
EV, enfortumab vedotin; P, pembrolizumab; TRAE, treatment-related adverse event.
Discussion
This analysis of EV-302 demonstrated benefit of EV+P across subgroups of interest, which was consistent with previously published results from the ITT population. In the overall population, EV+P demonstrated a significant and clinically meaningful benefit over chemotherapy for PFS and OS in patients with previously untreated la/mUC, with a 55% reduction in risk of disease progression or death and a 53% reduction in risk of death.9 Previously published safety data for EV+P in the overall population showed a consistent safety profile with that observed previously for this combination, with no new safety signals reported.9
The risk of disease progression or death with EV+P was 52% and 57% lower in patients who were cisplatin-eligible and cisplatin-ineligible compared with the chemotherapy group, respectively, and the risk of death was reduced by 47% and 57%, respectively.9 The higher absolute difference in response rate for EV+P in the cisplatin-ineligible subgroup compared with the cisplatin-eligible subgroup was due to the lower response rate seen in the control arm for patients who were ineligible for cisplatin and treated with carboplatin. Given that almost half of all patients with la/mUC are ineligible for cisplatin, the safety and efficacy of EV+P addresses a significant unmet need and provides a crucial treatment option in patients with la/mUC.15,16 The National Comprehensive Cancer Network® (NCCN) now lists EV+P as a preferred regimen for treatment of la/mUC in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). This recommendation is for patients regardless of their cisplatin eligibility.17
Treatment with EV+P resulted in better clinical outcomes than chemotherapy in patients with and without liver metastases. EV+P reduced the risk of disease progression or death by 47% and 57% versus chemotherapy for patients with and without liver metastases, respectively. The risk of death was also reduced by 53% and 53%, respectively. The presence of liver metastases has been associated with poorer clinical outcomes, including lower ORR and shorter PFS and OS.18,19 Here, we demonstrate that treatment with EV+P resulted in an improved response rate and significantly prolonged survival in this difficult-to-treat patient population.
Clinical benefit of EV+P versus chemotherapy was observed in patients with visceral metastases and lymph node-only disease. The risk of disease progression or death for EV+P was 55% and 60% lower for patients with visceral metastases or lymph node-only metastases compared with the risk in the chemotherapy group, respectively. The risk of death was also reduced by 53% and 54% for EV+P versus chemotherapy, respectively. Patients with lymph node-only metastases have been shown to have better outcomes than patients whose cancer has spread beyond the lymph nodes.18 Here, EV+P was associated with higher response rate and significantly improved PFS and OS compared with chemotherapy in this subgroup with good prognosis.
These analyses are limited by small sample sizes in some subgroups. Additionally, subgroup analyses were exploratory, and the study was not designed or powered to detect differences between treatment arms within each subgroup. Therefore, results of these analyses should be interpreted with caution. Further follow-up is required to fully understand the long-term efficacy outcomes of this study. Promising long-term results of EV+P were demonstrated in Dose Escalation/Cohort A of the EV-103 study (NCT03288545), with an estimated 5-year survival rate of 41.5% in cisplatin-ineligible patients with previously untreated la/mUC.20
In conclusion, this analysis demonstrated the benefit of EV+P compared with chemotherapy across prespecified subgroups consistent with that in the ITT population in patients with previously untreated la/mUC. These findings support the primary analysis and validate EV+P as a standard of care for 1L treatment of la/mUC. Longer-term follow-up from the EV-302 study will provide valuable additional data on the efficacy and safety of EV+P.
Acknowledgements
Medical writing support was provided by Blaise Low, PhD, and Kiran Verma, MSc, of Nucleus Global, an Inizio Company, and was funded by Pfizer and Astellas Pharma Inc.
Funding
This work was supported by Astellas Pharma Inc. (no grant number), Northbrook, IL, USA; Merck Sharp & Dohme LLC (no grant number), a subsidiary of Merck & Co., Inc. (no grant number), Rahway, NJ, USA; and Seagen Inc. (no grant number), Bothell, WA, USA, which was acquired by Pfizer in December 2023.
Disclosure
MSvdH reports research funding from BMS, AstraZeneca, MSD, 4SC, and Roche, and consultancy fees from BMS, Roche, Merck Sharp & Dohme, AstraZeneca, Pfizer, Janssen, Seagen, and Daiichi-Sankyo, which were all paid to the Netherlands Cancer Institute. TP reports consulting or advisory roles with BMS, AstraZeneca, Ipsen, Pfizer, Novartis, Seagen, Roche, Exelixis, MSD, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai, MashupMD, Merck, and Incyte; travel, accommodations, and expenses from Pfizer, MSD, AstraZeneca, Roche, and Ipsen; honoraria from AstraZeneca, Eisai, Merck, Novartis, Pfizer, Roche, Astellas Pharma, BMS GmbH & Co. KG, Exelixis, Incyte, Ipsen, Seagen, Merck Serono, Johnson & Johnson/Janssen, and MashupMD; and research funding from AstraZeneca, Roche, BMS, Exelixis, Ipsen, MSD, Novartis, Pfizer, Seagen, Merck Serono, Astellas Pharma, Johnson & Johnson, and Eisai. SG reports consulting roles for BMS, Merck, Pfizer, Gilead, Bayer, and Seagen; a speaker role for BMS; and institutional research funding from Seagen, Pfizer, Merck, BMS, Roche, Novartis, and Tyra Biosciences. YL reports personal fees and non-financial support from Sanofi, Janssen, Astellas, Seagen, Gilead, AstraZeneca, BMS, MSD, Pfizer, Merck KGaA, Pfizer, and Tahio, and grants from Celsius, Sanofi, Roche, and MSD. MDG reports stock and other ownership interests from Pfizer, Merck, and Gilead Sciences; institutional research funding from Merck, BMS, Mirati Therapeutics, Seagen, Alliance Foundation Trials, Alliance for Clinical Trials in Oncology, Clovis Oncology, Arvinas, ALX Oncology, Hoosier Cancer Research Network, Novartis, Acrivon Therapeutics, Astellas Pharma, Genentech, Accuray, PCCTC, G1 Therapeutics, OncoC4, Flare Therapeutics, Loxo/Lilly, and Roche; other relationships with Elsevier, Medscape, and Research to Practice; and uncompensated relationships with G1 Therapeutics and Loxo/Lilly. BPV reports honoraria from BMS/Medarex, EUSA Pharma, Pfizer, Astellas Pharma, Bayer, Merck/Pfizer, Merck, AAA HealthCare, Ipsen, and MSD Oncology; consulting or advisory roles for BMS/Medarex, MSD Oncology, Astellas Pharma, AstraZeneca, Novartis, Bayer, Advanced Accelerator Applications/Novartis; and travel, accommodations, and expenses from Merck/Pfizer and BMS. SSS reports consulting or advisory roles for Astellas Pharma, AstraZeneca, Bayer, BMS, Janssen, Merck & Co, Kenilworth, NJ, Pfizer, Roche/Genentech, and Seagen, and institutional research funding from the healthcare business of Merck KGaA, Darmstadt, Germany. EYY reports personal fees from Aadi Bioscience, Advanced Accelerator Applications, Bayer, BMS, Janssen, Lantheus, Loxo, Merck, and Oncternal, and grants from Bayer, Blue Earth, Dendreon, Lantheus, Merck, Oncternal, SeaGen, and Tyra. GVI reports grants and personal fees from Bayer, Janssen, Mirati Therapeutics, Flare Therapeutics, Basilea Pharmaceutica, Gilead Sciences, LOXO Oncology at Lilly, and Lynx Group, and grants from Debiopharm Group, Seagen, and Aadi Biosciences. EK reports consulting or advisory roles for Astellas Pharma, AstraZeneca, BMS-Ono Pharmaceutical, Chugai Pharma, Janssen, Merck, MSD, and Pfizer; speakers bureau participation for Astellas Pharma, AstraZeneca, Bayer, BMS-Ono Pharmaceutical, Chugai Pharma, Janssen, Kissei Pharmaceutical, Kyorin, Kyowa Kirin International, Merck, MSD, Nippon Kayaku, Nippon Shinyaku, Pfizer, Sanofi, Taiho Pharmaceutical, and Takeda; and institutional research funding from Chugai Pharma, Kissei Pharmaceutical, Kyorin, Kyowa Kirin International, Nihonkayaku, Nippon Shinyaku, Otsuka, Sanofi, Taiho Pharmaceutical, and Takeda. DC reports research funding from Astellas; educational grants from Pfizer and Janssen; travel grants from Pfizer and BMS; and participation in advisory boards for Pfizer, Astellas, Janssen, Sanofi, Bayer, and Roche. JHH-C reports honoraria from Roche/Genentech, Clovis Oncology, and Gilead Sciences; consulting or advisory roles from Roche/Genentech, Foundation Medicine, AstraZeneca, Seagen, and Gilead Sciences; research funding from Sanofi, Genentech, Foundation Medicine, Astellas Pharma (Inst), and EMD Serono (Inst); and travel, accommodations, and expenses from Roche/Genentech. AD reports personal fees from BMS, Merck, AZ, Roche, Seagen, Exelixis, and EMD Serono. NM reports honoraria from MSD. JPMR reports consulting or advisory roles for Astellas Pharma, Ipsen, BMS, Merck/Pfizer, Bayer, and Janssen, and travel, accommodations, and expenses from Merck/Pfizer, and Bayer. CV reports consulting or advisory roles for Janssen Cilag, Roche/Genentech, GSK, Atheneum, Astellas Pharma, MSD, BMS, Leo Pharma, Bayer, AstraZeneca, Pfizer, and Ipsen, and travel, accommodations, and expenses from Roche. WA reports consulting or advisory roles for Seagen and Cardinal Health, and institutional research funding from Seagen/Astellas, Merck, Basilea, and AstraZeneca. ID reports advisory board payments from Astellas, BMS, Immunomedics, Janssen, MSD, Roche, Bicycle Therapeutics, and Gilead; payments for invited speakers roles from Astellas, Bayer, BMS, Janssen, Merck, Pfizer, and Roche; and travel, accommodation, and registration expenses from Bayer, AstraZeneca, and Gilead. NAD reports honoraria from Astellas, AstraZeneca, Bayer, Eisai, EMD Serono, Exelixis, Janssen, Lantheus, Merck, Pfizer, Seagen, and Sumitomo Pharma America, Inc. US reports personal fees from Astellas, Exelixis, Seagen, Janssen, Imvax, Sanofi, AstraZeneca, Gilead, Pfizer, and Adaptimmune, and grants from Janssen, Exelixis, and Astellas/Seagen. SG reports employment with Astellas Pharma, Inc. BHM reports employment in Merck & Co. Inc. XY, YTL report employment with and stock ownership in Pfizer. JB reports financial interests from Astellas, AstraZeneca, BMS, Daiichi-Sankyo, Eisai, Ipsen, Janssen, Merck Sorono, MSD, Pfizer, Roche, Nektar, Novartis, and Seagen, and membership in the European Association of Urology and Renal Cell Carcinoma Guidelines Panel (Vice-Chairman).
Declaration of generative AI and AI-assisted technologies in the writing process
A proprietary generative artificial intelligence tool (GPT-4; 28 Aug 2024) was used with author review to develop the first draft; the authors take full responsibility for the content.
Data sharing
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: https://externaldatasharing-msd.com/) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor, or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Contributor Information
M.S. van der Heijden, Email: ms.vd.heijden@nki.nl.
J. Bedke, Email: j.bedke@klinikum-stuttgart.de.
Supplementary data
References
- 1.Bloudek L., Wright P., McKay C., et al. Systematic literature review (SLR) and network meta-analysis (NMA) of first-line therapies (1L) for locally advanced/metastatic urothelial carcinoma (la/mUC) Curr Oncol. 2023;30:3637–3647. doi: 10.3390/curroncol30040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute Cancer stat facts: bladder cancer. https://seer.cancer.gov/statfacts/html/urinb.html Available at.
- 3.van der Heijden M.S., Sonpavde G., Powles T., et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389:1778–1789. doi: 10.1056/NEJMoa2309863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 5.Maiorano B.A., Catalano M., Maiello E., Roviello G. Enfortumab vedotin in metastatic urothelial carcinoma: the solution EVentually? Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1254906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration . USFDA; MD, USA: 2023. PADCEV U.S. full Prescribing Information. [Google Scholar]
- 7.Powles T., Csoszi T., Ozguroglu M., et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 8.Powles T., Rosenberg J.E., Sonpavde G.P., et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powles T., Valderrama B.P., Gupta S., et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390:875–888. doi: 10.1056/NEJMoa2312117. [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer. 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic-urothelial-cancer Available at. Accessed July 30 , 2025.
- 11.Astellas Pharma Inc European Commission Approves Astellas’ PADCEV™ (enfortumab vedotin) in combination with KEYTRUDA® (pembrolizumab) for first-line treatment of advanced urothelial cancer. 2024. https://www.astellas.com/en/news/29371 Available at.
- 12.Canada Pfizer PADCEV® (enfortumab vedotin) in combination with pembrolizumab approved by Health Canada to treat advanced bladder cancer. 2024. https://www.pfizer.ca/en/media-centre/padcev-enfortumab-vedotin-in-combination-with-pembrolizumab-approved-by-health-canada-to-treat-advanced-bladder-cancer Available at.
- 13.Astellas Pharma Inc Japan’s Ministry of Health, Labour and Welfare approves PADCEV (enfortumab vedotin) with KEYTRUDA® (pembrolizumab) for first-line treatment of radically unresectable urothelial carcinoma. 2024. https://newsroom.astellas.us/2024-09-24-Japans-Ministry-of-Health,-Labour-and-Welfare-Approves-PADCEV-TM-enfortumab-vedotin-with-KEYTRUDA-R-pembrolizumab-for-First-Line-Treatment-of-Radically-Unresectable-Urothelial-Carcinoma Available at.
- 14.Powles T., Bellmunt J., Comperat E., et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol. 2024;35:485–490. doi: 10.1016/j.annonc.2024.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Bellmunt J., Mottet N., De Santis M. Urothelial carcinoma management in elderly or unfit patients. EJC Suppl. 2016;14:1–20. doi: 10.1016/j.ejcsup.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galsky M.D., Hahn N.M., Rosenberg J., et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–2438. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 17.NCCN Referencing Guidance . © National Comprehensive Cancer Network, Inc.; Verbatim: 2025. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V.1.2025.https://www.nccn.org/docs/default-source/business-policy/nccn-referencing-guidance.pdf Available at. [Google Scholar]
- 18.Makrakis D., Talukder R., Lin G.I., et al. Association between sites of metastasis and outcomes with immune checkpoint inhibitors in advanced urothelial carcinoma. Clin Genitourin Cancer. 2022;20:e440–e452. doi: 10.1016/j.clgc.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tufano A., Cordua N., Nardone V., et al. Prognostic significance of organ-specific metastases in patients with metastatic upper tract urothelial carcinoma. J Clin Med. 2022;11(18):5310. doi: 10.3390/jcm11185310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg J., Petrylak D., Flaig T., et al. Study EV-103 dose escalation/cohort A (DE/A): 5y follow-up of first-line (1L) enfortumab vedotin (EV) + pembrolizumab (P) in cisplatin (CIS)-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) Ann Oncol. 2024;35(2):S1139–S1140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.